Feasibility and Environmental Impact of NOM Reduction by Microfiltration at a Finnish Surface Water Treatment Plant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feed Water
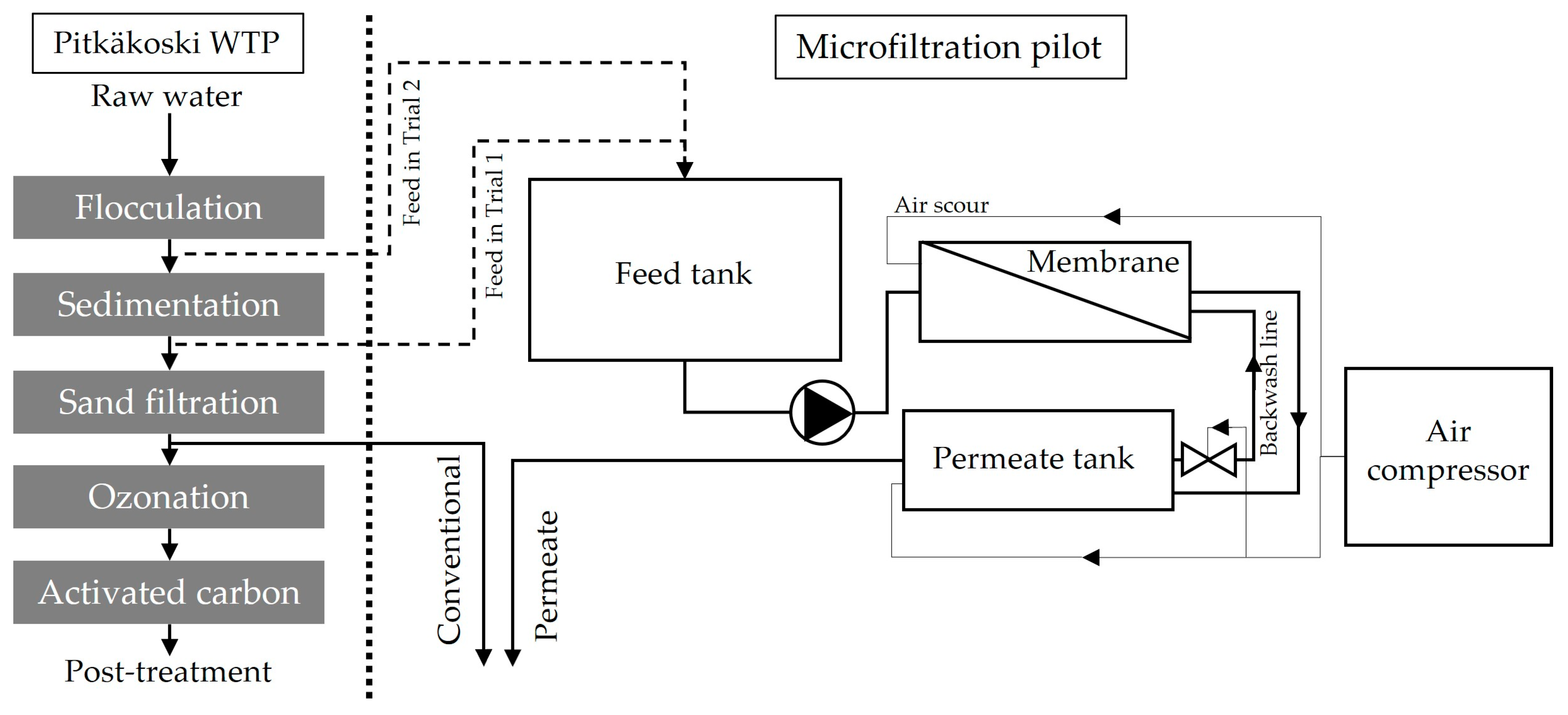
2.2. Pilot Experiment
2.3. Water Quality Analyses
2.4. Cost Calculation and Environmental Impact Assessment Methodology
3. Results and Discussion
3.1. Water Quality
3.1.1. Natural Organic Matter Removal
3.1.2. Physical and Microbiological Quality
3.2. Water Production between Settings
3.3. Cost Comparison
3.3.1. Membrane Treatment Process Composition
3.3.2. Energy Consumption
3.3.3. Chemical Consumption
3.3.4. Required Membrane Surface Area
3.4. Economic Summary
3.5. Environmental Impact of Microfiltration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Isomäki, E.; Britschgi, R.; Gustafsson, J.; Kuusisto, E.; Munsterhjelm, K.; Santala, E.; Suokko, T.; Valve, M. Yhdyskuntien Vedenhankinnan Tulevaisuuden Vaihtoehdot [Future Options for Community Water Supplies]; Finnish Environment Institute: Helsinki, Finland, 2007; p. 86. Available online: http://hdl.handle.net/10138/38390 (accessed on 27 February 2023).
- Henriksen, A.; Skjelvåle, B.L.; Mannio, J.; Wilander, A.; Harriman, R.; Curtis, C.; Jensen, J.P.; Fjeld, E.; Moiseenko, T. Northern European Lake Survey, 1995: Finland, Norway, Sweden, Denmark, Russian Kola, Russian Karelia, Scotland and Wales. Ambio 1998, 27, 80–91. [Google Scholar]
- Lavonen, E.E.; Gonsior, M.; Tranvik, L.J.; Schmitt-Kopplin, P.; Köhler, S. Selective chlorination of natural organic matter: Identification of previously unknown disinfection byproducts. Environ. Sci. Technol. 2013, 45, 2264–2271. [Google Scholar] [CrossRef]
- Jacangelo, J.; DeMarco, J.; Owen, D.; Randtke, S. Selected processes for removing NOM: An overview. J. Am. Water Works Assoc. 1995, 87, 64–77. [Google Scholar] [CrossRef]
- Owen, D.; Amy, G.; Chowdhury, Z.; Paode, R.; McCoy, G.; Viscosil, K. NOM characterization and treatability. J. Am. Water Works Assoc. 1995, 87, 46–63. [Google Scholar] [CrossRef]
- Hiraide, M. Heavy metals complexed with humic substances in fresh water. Anal. Sci. 1992, 8, 453–459. [Google Scholar] [CrossRef]
- Monteith, D.; Stoddard, J.; Evans, C.; de Wit, H.; Forsius, M.; Høgåsen, T.; Wilander, A.; Skjelkvåle, B.; Jeffries, D.; Vuorenmaa, J.; et al. Dissolved organic carbon trends resulting from changes in atmospheric deposition chemistry. Nature 2007, 450, 537–540. [Google Scholar] [CrossRef]
- Vuorenmaa, J.; Forsius, M.; Mannio, J. Increasing trends in total organic carbon concentrations in small forest lakes in Finland from 1987 to 2003. Sci. Total Environ. 2006, 365, 47–65. [Google Scholar] [CrossRef]
- Evans, C.; Monteith, D.; Cooper, D. Long-term increases in surface water dissolved organic carbon: Observations possible causes and environmental impacts. Environ. Pollut. 2005, 137, 55–71. [Google Scholar] [CrossRef]
- Perrels, A.; Veijalainen, N.; Jylhä, K.; Aaltonen, J.; Molarius, R.; Porthin, M.; Silander, J.; Rosqvist, T.; Tuovinen, T. The Implications of Climate Change for Extreme Weather Events and Their Socio-Economic Consequences in Finland. VATT Research Reports Vol. 158/2010, Helsinki, Finland, 2010. p. 133. Available online: http://urn.fi/URN:ISBN:978-951-561-923-5 (accessed on 27 February 2023).
- Lidén, A.; Persson, K.M. Feasibility study of advanced NOM-reduction by hollow fiber ultrafiltration and nanofiltration at a Swedish surface water treatment plant. Water 2016, 8, 150. [Google Scholar] [CrossRef]
- Ødegaard, H.; Eikebrokk, B.; Storhaug, R. Processes for the removal of humic substances from water—An overview based on Norwegian experiences. Water Sci. Technol. 1999, 40, 37–46. [Google Scholar] [CrossRef]
- Keucken, A.; Heinicke, G.; Persson, K.M.; Köhler, S.J. Combined coagulation and ultrafiltration process to counteract increasing NOM in brown surface water. Water 2017, 9, 697. [Google Scholar] [CrossRef]
- Meyn, T.; Leiknes, T.O. MS2 removal from high NOM content surface water by coagulation—Ceramic microfiltration, for potable water production. AIChE J. 2012, 58, 2270–2281. [Google Scholar] [CrossRef]
- Meyn, T.; Leiknes, T.O. Comparison of optional process configurations and operating conditions for ceramic membrane MF coupled with coagulation/flocculation pre-treatment for the removal of NOM in drinking water production. J. Water Supply Res. Technol. 2010, 59, 81–91. [Google Scholar] [CrossRef]
- Zularisam, A.W.; Ismail, A.F.; Salim, M.R.; Sakinah, M.; Matsuura, T. Application of coagulation–ultrafiltration hybrid process for drinking water treatment: Optimization of operating conditions using experimental design. Sep. Purif. Technol. 2009, 65, 193–210. [Google Scholar] [CrossRef]
- Betancourt, W.Q.; Rose, J.B. Drinking water treatment processes for removal of Cryptosporidium and Giardia. Vet. Parasitol. 2004, 126, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Nieminski, E.C.; Ongerth, J.E. Removing Giardia and Cryptosporidium by conventional treatment and direct filtration. J. Am. Water Works Assoc. 1995, 87, 96–106. [Google Scholar] [CrossRef]
- Redhead, A. Membrane technology at St. Saviours water treatment works, Guernsey, Channel Islands. Water Environ. J. 2008, 22, 75–80. [Google Scholar] [CrossRef]
- Liikanen, R.; Yli-Kuivila, J.; Tenhunen, J.; Laukkanen, R. Cost and environmental impact of nanofiltration in treating chemically pre-treated surface water. Desalination 2006, 201, 58–70. [Google Scholar] [CrossRef]
- Panglisch, S.; Holy, A.; Urban, F.; Dautzenberg, W.; Sous, P.; Ohligschläger, J.; Gimbel, R. Transferring pilot experiments into the planning of Germany's largest two-stage ultrafiltration plant. Desalination 2005, 179, 225–235. [Google Scholar] [CrossRef]
- Hancock, N.T.; Black, N.D.; Cath, T.Y. A comparative life cycle assessment of hybrid osmotic dilution desalination and established seawater desalination and wastewater reclamation processes. Water Res. 2012, 46, 1145–1154. [Google Scholar] [CrossRef]
- Tangsubkul, N.; Parameshwaran, K.; Lundie, S.; Fane, A.G.; Waite, T.D. Environmental life cycle assessment of microfiltration process. J. Membr. Sci. 2006, 284, 214–226. [Google Scholar] [CrossRef]
- Bonton, A.; Bouchard, C.; Barbeau, B.; Jedrzejak, S. Comparative life cycle assessment of water treatment plants. Desalination 2012, 284, 42–54. [Google Scholar] [CrossRef]
- Gray, S.; Ritchie, C.; Tran, T.; Bolto, B. Effect of NOM characteristics and membrane type on microfiltration performance. Water Res. 2007, 41, 3833–3841. [Google Scholar] [CrossRef]
- Metsämuuronen, S.; Sillanpää, M.; Bhatnagar, A.; Mänttäri, M. Natural organic matter removal from drinking water by membrane technology. Sep. Purif. Rev. 2014, 43, 1–61. [Google Scholar] [CrossRef]
- Cui, X.; Choo, K. Natural organic matter removal and fouling control in low-pressure membrane filtration for water treatment. Environ. Eng. Res. 2014, 19, 1–8. [Google Scholar] [CrossRef]
- Leiknes, T. The effect of coupling coagulation and flocculation with membrane filtration in water treatment: A review. J. Environ. Sci. 2009, 21, 8–12. [Google Scholar] [CrossRef]
- Walsh, M.; Zhao, N.; Gora, S.; Gagnon, G. Effect of coagulation and flocculation on water quality in an immersed ultrafiltration process. Environ. Technol. 2009, 30, 927–938. [Google Scholar] [CrossRef]
- Huber, S.; Balz, A.; Abert, M.; Pronk, W. Characterisation of aquatic humic and non-humic matter with size-exclusion chromatography—Organic carbon detection—Organic nitrogen detection (LC-OCD-OND). Water Res. 2011, 45, 879–885. [Google Scholar] [CrossRef]
- Greenberg, E.; Franson, M.; Eaton, A.; Clesceri, L. Standard Methods for Examination of Water and Wastewater, 19th ed.; American Public Health Association: Washington, DC, USA, 1995; p. 1100. ISBN 0-87553-223-3. [Google Scholar]
- Miettinen, I.; Vartiainen, T.; Martikainen, P. Determination of assimilable organic carbon in humus-rich drinking waters. Water Res. 1999, 33, 2277–2282. [Google Scholar] [CrossRef]
- Edzwald, J.; Tobiason, J. Enhanced coagulation: US requirements and a broader view. Water Sci. Technol. 1999, 40, 63–70. [Google Scholar] [CrossRef]
- Filella, M. Freshwaters: Which NOM matters? Environ. Chem. Lett. 2009, 7, 21–35. [Google Scholar] [CrossRef]
- Hem, L.; Efraimsen, H. Assimilable organic carbon in molecular weight fractions of natural organic matter. Water Res. 2001, 35, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- Best, G.; Mourato, D.; Singh, M.; Firman, M.; Basu, S. Application of immersed ultrafiltration membranes for colour and TOC removal. Membr. Technol. 1999, 122, 5–12. [Google Scholar] [CrossRef]
- Meier, P.; Salehi, F.; Kazner, C.; Wintgens, T.; Melin, T. Ultrafiltration with Pre-Coagulation in Drinking Water Production—Literature Review; Techneau-project, KWR Water B.V.: Rijswijk, The Netherlands, 2006; pp. 1–81. [Google Scholar]
- Fingrid. Sähköntuotannon ja -Kulutuksen CO2-Päästöarvot. Available online: https://www.fingrid.fi/sahkomarkkinainformaatio/co2/ (accessed on 7 March 2023).
- Wang, J.; Cui, Z.; Li, Y.; Cao, L.; Lu, Z. Techno-economic analysis and environmental impact assessment of citric acid production through different recovery methods. J. Clean. Prod. 2020, 249, 119315. [Google Scholar] [CrossRef]
- Alvarez-Gaitan, J.; Peters, G.; Rowley, H.; Moore, S.; Short, M. A hybrid life cycle assessment of water treatment chemicals: An Australian experience. Int. J. Life Cycle Assess. 2013, 18, 1291–1301. [Google Scholar] [CrossRef]
- Australian Government, Department of Industry, Science, Energy and Resources. National Greenhouse Accounts Factors, August 2021. Available online: https://www.dcceew.gov.au/sites/default/files/documents/national-greenhouse-accounts-factors-2021.pdf (accessed on 7 March 2023).
- Mölsä, K. Life Cycle Assessment of a Wastewater Treatment and a Sludge Handling Process—Current State and Future Scenarios. Master’s Thesis, Aalto University, Helsinki, Finland, 2019. [Google Scholar]
- Ribera, G.; Clarens, F.; Martínez-Lladó, X.; Jubany, I.; Martí, V.; Rovira, M. Life cycle and human health risk assessments as tools for decision making in the design and implementation of nanofiltration in drinking water treatment plants. Sci. Total Environ. 2014, 466-467, 377–386. [Google Scholar] [CrossRef]
- Prézélus, F.; Tiruta-Barna, L.; Remigy, J.-C.; Guigui, C. Process-based LCA of ultrafiltration for drinking water production. Water Res. 2021, 199, 117159. [Google Scholar] [CrossRef] [PubMed]
- Vince, F.; Aoustin, E.; Bréant, P.; Marechal, F. LCA tool for the environmental evaluation of potable water production. Desalination 2008, 220, 37–56. [Google Scholar] [CrossRef]


| Parameter | Setting 1 | Setting 2 | Setting 3 | Setting 4 | Setting 5 | Setting 6 | Setting 7 † | Setting 8 † |
|---|---|---|---|---|---|---|---|---|
| Flux (L/m2h) at 20 °C | 90 | 90 | 90 | 90 | 130/110 * | 130/110 * | 130 | 130 |
| Backwash int. (min) | 20 | 30 | 20 | 30 | 20 | 30 | 20 | 30 |
| Backwash flux (L/m2h) | 1000 | 1000 | 1300 | 1300 | 1000 | 1000 | 1300 | 1300 |
| Analysis | Trial 1 | Trial 2 | n | ||||
| NOM | Feed water | Permeate | Conventional | Feed water | Permeate | Conventional | |
| TOC (mg/L) | 3.3 ± 0.17 | 2.6 ± 0.20 | 2.5 ± 0.20 | 7.0 ± 0.10 | 2.5 ± 0.07 | 2.4 ± 0.10 | 13 |
| DOC (mg/L) | 2.7 ± 0.14 | 2.7 ± 0.19 | 2.5 ± 0.13 | 2.7 ± 0.19 | 2.7 ± 0.08 | 2.4 ± 0.07 | 13 |
| UVA254 (cm−1) | 0.117 ± 0.006 | 0.042 ± 0.002 | 0.043 ± 0.005 | 0.096 ± 0.035 | 0.044 ± 0.003 | 0.044 ± 0.005 | 23 |
| SUVA254 (L/mg∙m) | 4.9 ± 0.27 | 1.8 ± 0.09 | 2.0 ± 0.21 | 4.0 ± 0.80 | 1.7 ± 0.12 | 1.8 ± 0.20 | 13 |
| Microbiological | Trial 1 | Trial 2 | n | ||||
| HPC (cfu/mL) | 115 ± 47 | 7.1 ± 0.0 | 71 ± 18 | 120 ± 42 | 9.9 ± 6.8 | 20 ± 16 | 3 |
| ATP (pg/L) | 5.7 ± 2.7 | 2.2 ± 1.1 | 3.4 ± 1.0 | 25 ± 8.7 | 5.3 ± 3.8 | 7.8 ± 7.5 | 3 |
| AOC (μg AOC-C/L) | 32 ± 16 | 50 ± 24 | 31 ± 21 | - | 17 ± 0.8 | 20 ± 4.6 | 3 |
| Physical Quality | Trial 1 | Trial 2 | n | ||||
| Turbidity (FTU) | 1.17 ± 0.1 | 0.06 ± 0.01 | 0.18 ± 0.05 | - | 0.06 ± 0.01 | 0.18 ± 0.17 | 22 |
| Iron (μg Fe/L) | 920 ± 46 | 42 ± 6.3 | 109 ± 23 | 6250 | 26 ± 6.6 | 74 ± 83 | 11 |
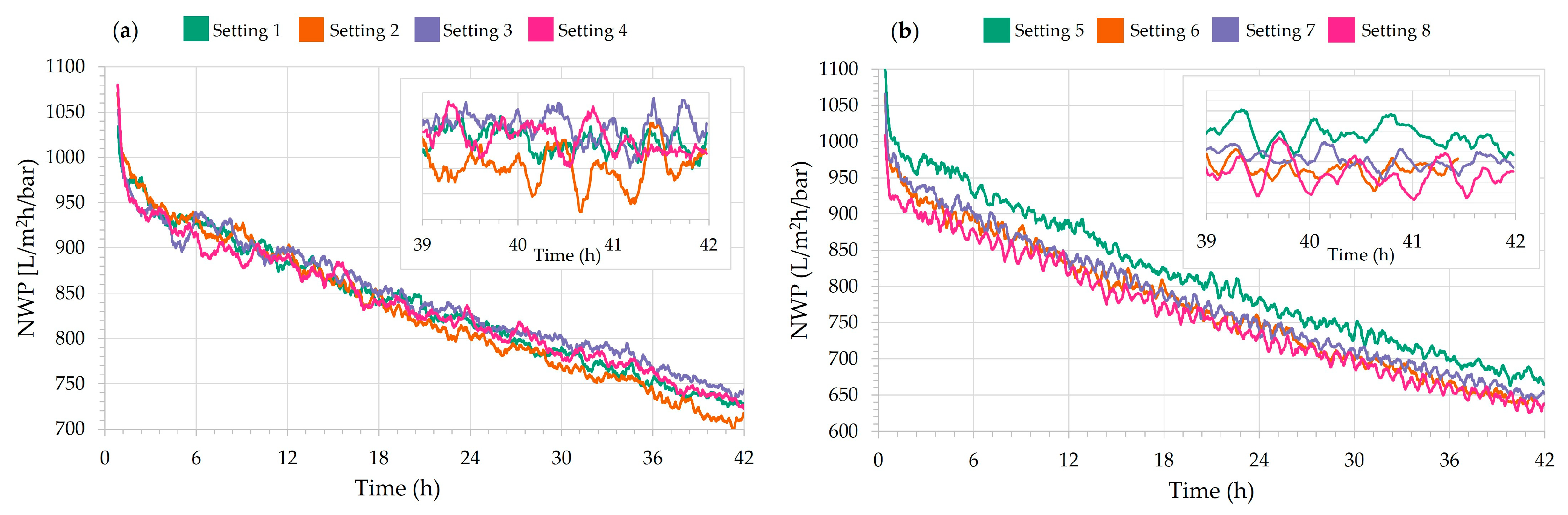
| Setting | Average Flux (L/m2h) | Average TMP (bar) | Average NWP (L/m2h/bar) | NWP Loss (%) | Average Permeate in 42 h (L/h) | Average Permeate in 24 h (L/h) |
| Trial 1 (Clarified Feed Water) | ||||||
| 1 | 91.2 | 0.110 | 836 | 40.5 | 10.6 | 10.9 |
| 2 | 91.2 | 0.111 | 830 | 41.1 | 12.1 | 12.5 |
| 3 | 90.4 | 0.108 | 844 | 39.4 | 10.7 | 10.8 |
| 4 | 91.9 | 0.111 | 835 | 41.4 | 12.3 | 12.5 |
| 5 | 128.1 | 0.159 | 812 | 43.4 | 16.3 | 17.2 |
| 6 | 127.9 | 0.167 | 775 | 43.9 | 17.8 | 18.6 |
| 7 | 129.4 | 0.167 | 781 | 44.8 | 16.7 | 17.5 |
| 8 | 130.2 | 0.173 | 760 | 43.0 | 18.4 | 19.2 |
| Setting | Trial 2 (Flocculated Feed Water) | |||||
| 1 | 87.5 | 0.174 | 505 | 37.8 | 10.0 | 10.1 |
| 2 | 87.8 | 0.180 | 491 | 40.8 | 11.6 | 11.8 |
| 3 | 88.3 | 0.188 | 472 | 41.8 | 10.1 | 10.2 |
| 4 | 86.2 | 0.188 | 459 | 44.5 | 11.3 | 11.7 |
| 5 | 107.9 | 0.248 | 438 | 42.8 | 13.2 | 13.5 |
| 6 | 105.3 | 0.266 | 397 | 45.6 | 14.3 | 14.7 |
| Trial | Setting | Citric Acid | NaOCl | Energy | Modules | Total | Total (2024 Energy) |
|---|---|---|---|---|---|---|---|
| 1 | 2 | 0.0124 | 0.0090 | 0.0033 | 0.0105 | 0.035 | 0.038 |
| 1 | 6 | 0.0084 | 0.0061 | 0.0029 | 0.0071 | 0.025 | 0.027 |
| 2 | 6 | 0.0105 | 0.0076 | 0.0035 | 0.0125 | 0.034 | 0.037 |
| 1 | 6 (24 h) | 0.0141 | 0.0102 | 0.0029 | 0.0068 | 0.034 | 0.037 |
| Trial | Setting | Citric Acid | NaOCl | Energy | Modules | Wastewater Treatment | Full NaOCl Impact | Half NaOCl Impact |
|---|---|---|---|---|---|---|---|---|
| Total | ||||||||
| 1 | 2 | 6.46 | 11.62 | 4.40 | 2.00 | 1.91 | 26.40 | 20.59 |
| 1 | 6 | 4.41 | 7.93 | 3.87 | 2.00 | 1.32 | 19.54 | 15.57 |
| 2 | 6 | 5.48 | 9.85 | 4.61 | 3.00 | 1.28 | 24.60 | 19.68 |
| 1 | 6 (24 h) | 7.36 | 13.23 | 3.79 | 2.00 | 1.65 | 27.65 | 21.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laurell, P.; Poutanen, H.; Hesampour, M.; Tuutijärvi, T.; Vahala, R. Feasibility and Environmental Impact of NOM Reduction by Microfiltration at a Finnish Surface Water Treatment Plant. Water 2023, 15, 1822. https://doi.org/10.3390/w15101822
Laurell P, Poutanen H, Hesampour M, Tuutijärvi T, Vahala R. Feasibility and Environmental Impact of NOM Reduction by Microfiltration at a Finnish Surface Water Treatment Plant. Water. 2023; 15(10):1822. https://doi.org/10.3390/w15101822
Chicago/Turabian StyleLaurell, Panu, Heikki Poutanen, Mehrdad Hesampour, Tanja Tuutijärvi, and Riku Vahala. 2023. "Feasibility and Environmental Impact of NOM Reduction by Microfiltration at a Finnish Surface Water Treatment Plant" Water 15, no. 10: 1822. https://doi.org/10.3390/w15101822





