Importance of Anthropogenic Determinants of Tubastraea coccinea Invasion in the Northern Gulf of Mexico
Abstract
:1. Introduction
2. Materials and Methods
2.1. Focal Species
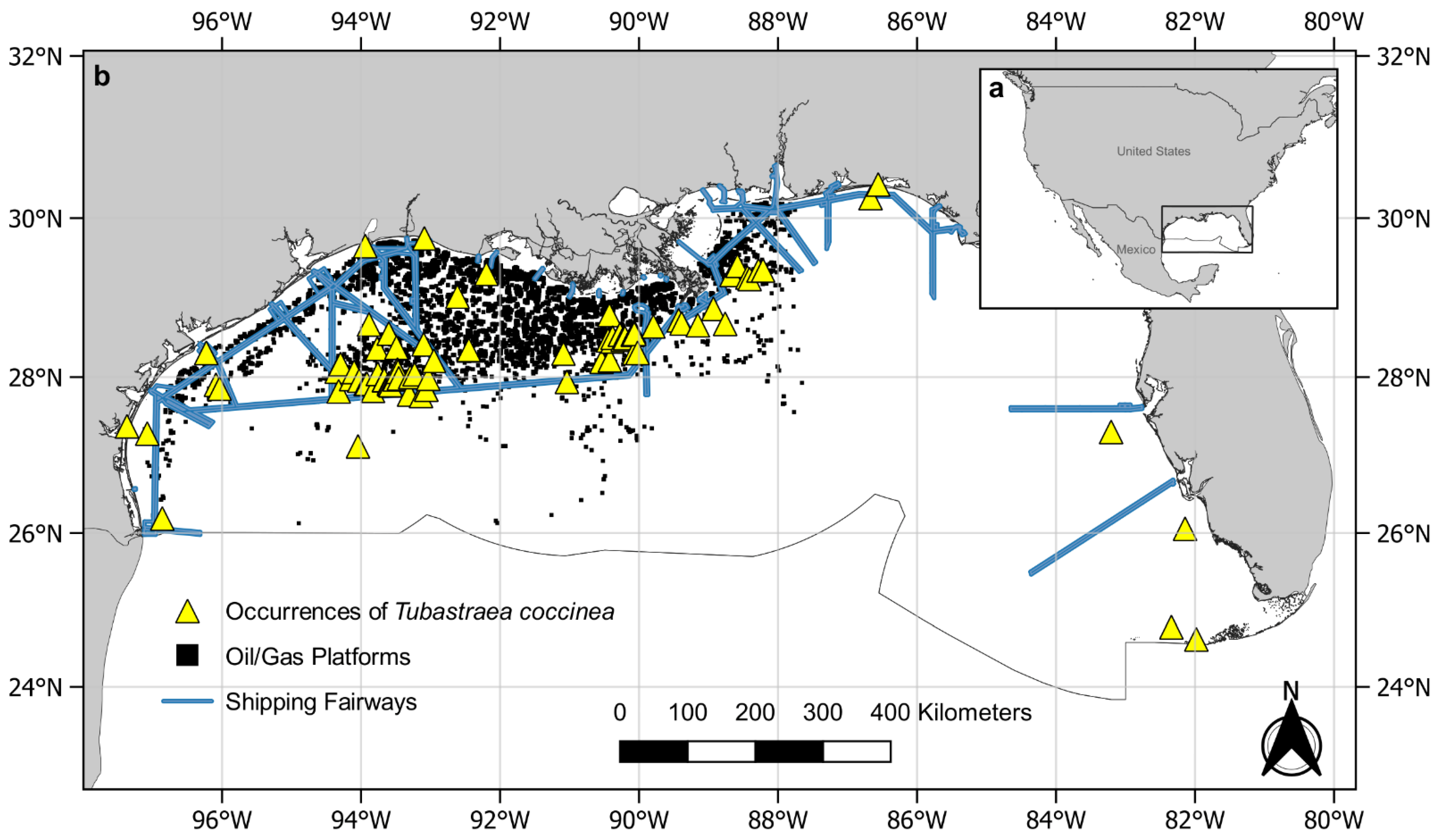
2.2. Study Area
2.3. Data Collection
2.4. Data Processing and Analysis
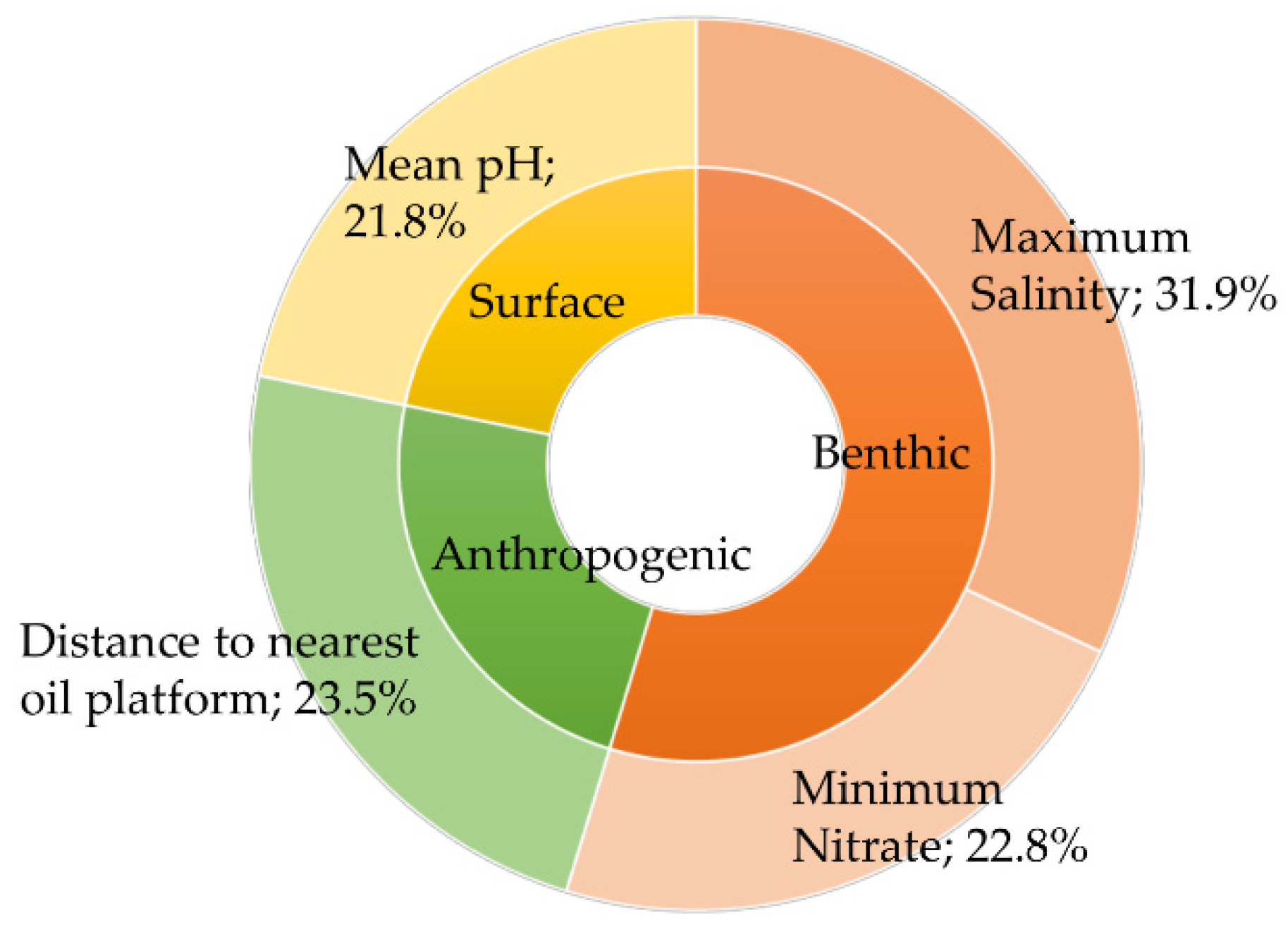
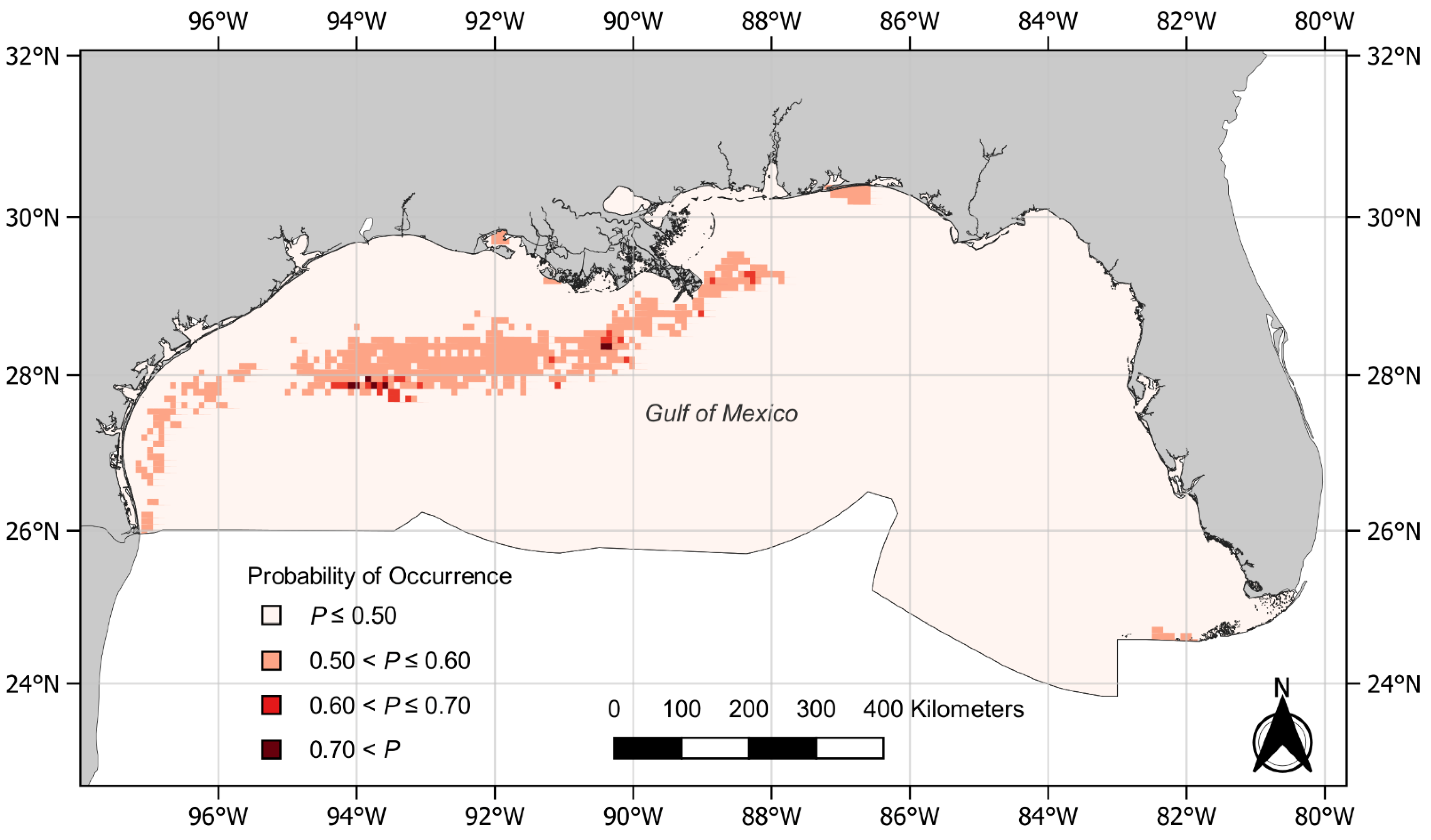
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brander, L.; van Beukering, P. The Total Economic Value of US Coral Reefs: A Review of the Literature; National Oceanic and Atmospheric Administration: Washington, DC, USA, 2013; p. 32.
- Katsanevakis, S.; Wallentinus, I.; Zenetos, A.; Leppäkoski, E.; Çinar, M.E.; Oztürk, B.; Grabowski, M.; Golani, D.; Cardoso, A.C. Impacts of invasive alien marine species on ecosystem services and biodiversity: A pan-European review. Aquat. Invasions 2014, 9, 391–423. [Google Scholar] [CrossRef]
- Mack, R.N.; Simberloff, D.; Mark Lonsdale, W.; Evans, H.; Clout, M.; Bazzaz, F.A. Biotic invasions: Causes, epidemiology, global consequences, and control. Ecol. Appl. 2000, 10, 689–710. [Google Scholar] [CrossRef]
- Wang, H.-H.; Grant, W.E.; Gan, J.; Rogers, W.E.; Swannack, T.M.; Koralewski, T.E.; Miller, J.H.; Taylor, J.W. Integrating spread dynamics and economics of timber production to manage Chinese tallow invasions in southern US forestlands. PLoS ONE 2012, 7, e33877. [Google Scholar]
- Hill, C.E.; Lymperaki, M.M.; Hoeksema, B.W. A centuries-old manmade reef in the Caribbean does not substitute natural reefs in terms of species assemblages and interspecific competition. Mar. Pollut. Bull. 2021, 169, 112576. [Google Scholar] [CrossRef]
- Sammarco, P.W.; Atchison, A.D.; Boland, G.S. Expansion of coral communities within the Northern Gulf of Mexico via offshore oil and gas platforms. Mar. Ecol. Prog. Ser. 2004, 280, 129–143. [Google Scholar] [CrossRef]
- López, C.; Clemente, S.; Moreno, S.; Ocaña, O.; Herrera, R.; Moro, L.; Monterroso, O.; Rodríguez, A.; Brito, A. Invasive Tubastraea spp. and Oculina patagonica and other introduced scleractinians corals in the Santa Cruz de Tenerife (Canary Islands) harbor: Ecology and potential risks. Reg. Stud. Mar. Sci. 2019, 29, 100713. [Google Scholar] [CrossRef]
- Cairns, S.D. A revision of the shallow-water azooxanthellate Scleractinia of the Western Atlantic. Stud. Fauna Curacao Other Caribb. Isl. 2000, 75, 1–34. [Google Scholar]
- Hoeksema, B.W.; Hiemstra, A.F.; Vermeij, M.J. The rise of a native sun coral species on southern Caribbean coral reefs. Ecosphere 2019, 10, e02942. [Google Scholar] [CrossRef]
- Hoeksema, B.W.; ten Hove, H.A. The invasive sun coral Tubastraea coccinea hosting a native Christmas tree worm at Curaçao, Dutch Caribbean. Mar. Biodivers. 2017, 47, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Couto, T.D.; Omena, E.P.; Oigman-Pszczol, S.S.; Junqueira, A.O. A method to assess the risk of sun coral invasion in marine protected areas. An. Da Acad. Bras. De Ciências 2021, 93, e20200583. [Google Scholar] [CrossRef]
- Fenner, D. Biogeography of three Caribbean corals (Scleractinia) and the invasion of Tubastraea coccinea into the Gulf of Mexico. Bull. Mar. Sci. 2001, 69, 1175–1189. [Google Scholar]
- Fenner, D.; Banks, K. Orange cup coral Tubastraea coccinea invades Florida and the Flower Garden Banks, northwestern Gulf of Mexico. Coral Reefs 2004, 23, 505–507. [Google Scholar] [CrossRef]
- Derouen, Z.C.; Peterson, M.R.; Wang, H.-H.; Grant, W.E. Determinants of Tubastraea coccinea invasion and likelihood of further expansion in the northern Gulf of Mexico. Mar. Biodivers. 2020, 50, 101. [Google Scholar] [CrossRef]
- Assis, J.; Tyberghein, L.; Bosch, S.; Verbruggen, H.; Serrão, E.A.; De Clerck, O. Bio-ORACLE v2.0: Extending marine data layers for bioclimatic modelling. Glob. Ecol. Biogeogr. 2018, 27, 277–284. [Google Scholar] [CrossRef]
- Lesson, R.P. Voyage Autour Du Monde, Execute Par Ordre Du Roi, Sur la Corvettede Sa Majeste, la Coquille, Pendant Les Annees 1822, 1823, 1824, Et 1825. Par LI Duperrey; Arthus Bertrand: Paris, France, 1830. [Google Scholar]
- de Paula, A.F.; Creed, J.C. Two species of the coral Tubastraea (Cnidaria, Scleractinia) in Brazil: A case of accidental introduction. Bull. Mar. Sci. 2004, 74, 175–183. [Google Scholar]
- Fofonoff, P.; Ruiz, G.; Steves, B.; Simkanin, C.; Carlton, J. National Exotic Marine and Estuarine Species Information System. Available online: http://invasions.si.edu/nemesis/ (accessed on 6 September 2019).
- Sammarco, P.; Lirette, A.; Tung, Y.; Boland, G.; Genazzio, M.; Sinclair, J. Coral communities on artificial reefs in the Gulf of Mexico: Standing vs. toppled oil platforms. ICES J. Mar. Sci. 2014, 71, 417–426. [Google Scholar] [CrossRef] [Green Version]
- Castro, C.B.; Pires, D.O. Brazilian coral reefs: What we already know and what is still missing. Bull. Mar. Sci. 2001, 69, 357–371. [Google Scholar]
- Felder, D.; Camp, D. Gulf of Mexico Origin, Waters, and Biota: Biodiversity; Texas A&M University Press: College Station, TX, USA, 2009. [Google Scholar]
- Shepard, A.N.; Valentine, J.F.; D’Elia, C.F.; Yoskowitz, D.; Dismukes, D.E. Economic impact of Gulf of Mexico ecosystem goods and services and integration into restoration decision-making. Gulf Mex. Sci. 2013, 31, 10–27. [Google Scholar] [CrossRef]
- OBIS. Tubastraea Coccinea Lesson. 1830. Available online: https://obis.org/taxon/291251 (accessed on 6 January 2019).
- GBIF. Occurrence Download. Available online: https://www.gbif.org/occurrence/download/0022600-181108115102211 (accessed on 6 September 2019).
- Clarivate. Web of Science. Available online: www.webofknowledge.com (accessed on 6 September 2019).
- Tyberghein, L.; Verbruggen, H.; Pauly, K.; Troupin, C.; Mineur, F.; De Clerck, O. Bio-ORACLE: A global environmental dataset for marine species distribution modelling. Glob. Ecol. Biogeogr. 2012, 21, 272–281. [Google Scholar] [CrossRef]
- FMI. The Intersect of the Exclusive Economic Zones and IHO Sea Areas, Version 3. Available online: http://www.vliz.be/en/imis?dasid=6042&doiid=324 (accessed on 6 January 2019).
- BSEE. Bureau of Safety and Environmental Enforcement. Available online: https://www.data.bsee.gov/ (accessed on 16 September 2021).
- ESRI. Global Shipping Routes. Available online: https://bobson.maps.arcgis.com/apps/webappviewer/index.html?id=7ba696c12aa34f2f8c19c96c4a70091f (accessed on 12 November 2021).
- Elith, J.; Leathwick, J.R.; Hastie, T. A working guide to boosted regression trees. J. Anim. Ecol. 2008, 77, 802–813. [Google Scholar] [CrossRef]
- Wang, H.-H.; Koralewski, T.E.; McGrew, E.K.; Grant, W.E.; Byram, T.D. Species distribution model for management of an invasive vine in forestlands of eastern Texas. Forests 2015, 6, 4374–4390. [Google Scholar] [CrossRef] [Green Version]
- R Foundation for Statistical Computing. R 3.6.0. 2017. Available online: http://www.R-project.org/ (accessed on 22 February 2022).
- Ridgeway, G. The gbm Package, Version 1.6-3. Available online: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.456.9958&rep=rep1&type=pdf (accessed on 24 February 2019).
- ESRI. ArcGIS Pro 2.8.3; Environmental Systems Research Institute, Inc.: Redlands, CA, USA, 2021. [Google Scholar]
- Villamizar-Gomez, A.; Wang, H.H.; Peterson, M.R.; Grant, W.E.; Forstner, M.R.J. Environmental determinants of Batrachochytrium dendrobatidis and the likelihood of further dispersion in the face of climate change in Texas, USA. Dis. Aquat. Org. 2021, 146, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Culpepper, L.Z.; Wang, H.-H.; Koralewski, T.E.; Grant, W.E.; Rogers, W.E. Understory upheaval: Factors influencing Japanese stiltgrass invasion in forestlands of Tennessee, United States. Bot. Stud. 2018, 59, 20. [Google Scholar] [CrossRef] [PubMed]
- Randklev, C.R.; Wang, H.-H.; Groce, J.E.; Grant, W.E.; Robertson, S.; Wilkins, N. Land use relationships for a rare freshwater mussel species endemic to central Texas. J. Fish Wildl. Manag. 2015, 6, 327–337. [Google Scholar] [CrossRef] [Green Version]
- Friedman, J.; Hastie, T.; Tibshirani, R. Response to Mease and Wyner, Evidence Contrary to the Statistical View of Boosting, JMLR 9: 131–156, 2008. J. Mach. Learn. Res. 2008, 9, 175–180. [Google Scholar]
- Mease, D.; Wyner, A. Evidence contrary to the statistical view of boosting. J. Mach. Learn. Res. 2008, 9, 131–156. [Google Scholar]
- Wang, H.-H.; Grant, W.E.; Swannack, T.M.; Gan, J.; Rogers, W.E.; Koralewski, T.E.; Miller, J.H.; Taylor, J.W., Jr. Predicted range expansion of Chinese tallow tree (Triadica sebifera) in forestlands of the southern United States. Divers. Distrib. 2011, 17, 552–565. [Google Scholar] [CrossRef]
- Koo, H.; Iwanaga, T.; Croke, B.F.W.; Jakeman, A.J.; Yang, J.; Wang, H.-H.; Sun, X.; Lü, G.; Li, X.; Yue, T.; et al. Position paper: Sensitivity analysis of spatially distributed environmental models- a pragmatic framework for the exploration of uncertainty sources. Environ. Model. Softw. 2020, 134, 104857. [Google Scholar] [CrossRef]
- Storlazzi, C.; Brown, E.; Field, M.; Rodgers, K.; Jokiel, P. A model for wave control on coral breakage and species distribution in the Hawaiian Islands. Coral Reefs 2005, 24, 43–55. [Google Scholar] [CrossRef]
- Riul, P.; Targino, C.H.; Júnior, L.A.; Creed, J.C.; Horta, P.A.; Costa, G.C. Invasive potential of the coral Tubastraea coccinea in the southwest Atlantic. Mar. Ecol. Prog. Ser. 2013, 480, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Carlos-Júnior, L.; Barbosa, N.; Moulton, T.; Creed, J. Ecological Niche Model used to examine the distribution of an invasive, non-indigenous coral. Mar. Environ. Res. 2015, 103, 115–124. [Google Scholar] [CrossRef]
- Carlos-Júnior, L.A.; Neves, D.M.; Barbosa, N.P.U.; Moulton, T.P.; Creed, J.C. Occurrence of an invasive coral in the southwest Atlantic and comparison with a congener suggest potential niche expansion. Ecol. Evol. 2015, 5, 2162–2171. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-H.; Wonkka, C.L.; Treglia, M.L.; Grant, W.E.; Smeins, F.E.; Rogers, W.E. Incorporating local-scale variables into distribution models enhances predictability for rare plant species with biological dependencies. Biodivers. Conserv. 2019, 28, 171–182. [Google Scholar] [CrossRef]
- Wang, H.-H.; Wonkka, C.L.; Treglia, M.L.; Grant, W.E.; Smeins, F.E.; Rogers, W.E. Species distribution modelling for conservation of an endangered endemic orchid. AoB Plants 2015, 7, plv039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.-H.; Wonkka, C.L.; Grant, W.E.; Rogers, W.E. Range expansion of invasive shrubs: Implication for crown fire risk in forestlands of the southern USA. AoB Plants 2016, 8, plw012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, A.B.; Godsoe, W.; Rodríguez-Sánchez, F.; Wang, H.-H.; Warren, D. Niche estimation above and below the species level. Trends Ecol. Evol. 2019, 34, 260–273. [Google Scholar] [CrossRef]
- Iwanaga, T.; Wang, H.-H.; Hamilton, S.H.; Grimm, V.; Koralewski, T.E.; Salado, A.; Elsawah, S.; Razavi, S.; Yang, J.; Glynn, P.; et al. Socio-technical scales in socio-environmental modeling: Managing a system-of-systems modeling approach. Environ. Model. Softw. 2021, 135, 104885. [Google Scholar] [CrossRef]
- Iwanaga, T.; Wang, H.-H.; Koralewski, T.E.; Grant, W.E.; Jakeman, A.J.; Little, J.C. Toward a complete interdisciplinary treatment of scale: Reflexive lessons from socioenvironmental systems modeling. Elem. Sci. Anthr. 2021, 9, 00182. [Google Scholar] [CrossRef]
- Fern, R.R.; Morrison, M.L.; Wang, H.-H.; Grant, W.E.; Campbell, T.A. Incorporating biotic relationships improves species distribution models: Modeling the temporal influence of competition in conspecific nesting birds. Ecol. Model. 2019, 408, 108743. [Google Scholar] [CrossRef]
- Moustaka, M.; Langlois, T.J.; McLean, D.; Bond, T.; Fisher, R.; Fearns, P.; Dorji, P.; Evans, R.D. The effects of suspended sediment on coral reef fish assemblages and feeding guilds of north-west Australia. Coral Reefs 2018, 37, 659–673. [Google Scholar] [CrossRef]
- Fern, R.R.; Morrison, M.L.; Grant, W.E.; Wang, H.; Campbell, T.A. Modeling the influence of livestock grazing pressure on grassland bird distributions. Ecol. Processes 2020, 9, 42. [Google Scholar] [CrossRef]
- Kleypas, J.A.; McManus, J.W.; Meñez, L.A. Environmental limits to coral reef development: Where do we draw the line? Am. Zool. 1999, 39, 146–159. [Google Scholar] [CrossRef]
- Santos, H.; Silva, F.; Masi, B.; Fleury, B.; Creed, J. Environmental matching used to predict range expansion of two invasive corals (Tubastraea spp.). Mar. Pollut. Bull. 2019, 145, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Coles, S.L.; Jokiel, P.L. Effects of salinity on coral reefs. In Pollution in Tropical Aquatic Systems; CRC Press: Boca Raton, FL, USA, 2018; pp. 147–166. [Google Scholar]
- Kerswell, A.P.; Jones, R.J. Effects of hypo-osmosis on the coral Stylophora pistillata: Nature and cause of low-salinity bleaching. Mar. Ecol. Prog. Ser. 2003, 253, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Röthig, T.; Ochsenkühn, M.A.; Roik, A.; Van Der Merwe, R.; Voolstra, C.R. Long-term salinity tolerance is accompanied by major restructuring of the coral bacterial microbiome. Mol. Ecol. 2016, 25, 1308–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gegner, H.M.; Ziegler, M.; Rädecker, N.; Buitrago-López, C.; Aranda, M.; Voolstra, C.R. High salinity conveys thermotolerance in the coral model Aiptasia. Biol. Open 2017, 6, 1943–1948. [Google Scholar] [CrossRef] [Green Version]
- Rädecker, N.; Pogoreutz, C.; Voolstra, C.R.; Wiedenmann, J.; Wild, C. Nitrogen cycling in corals: The key to understanding holobiont functioning? Trends Microbiol. 2015, 23, 490–497. [Google Scholar] [CrossRef] [Green Version]
- Santos, H.F.; Carmo, F.L.; Duarte, G.; Dini-Andreote, F.; Castro, C.B.; Rosado, A.S.; Van Elsas, J.D.; Peixoto, R.S. Climate change affects key nitrogen-fixing bacterial populations on coral reefs. ISME J. 2014, 8, 2272–2279. [Google Scholar] [CrossRef] [Green Version]
- Wiedenmann, J.; D’Angelo, C.; Smith, E.G.; Hunt, A.N.; Legiret, F.-E.; Postle, A.D.; Achterberg, E.P. Nutrient enrichment can increase the susceptibility of reef corals to bleaching. Nat. Clim. Change 2013, 3, 160–164. [Google Scholar] [CrossRef]
- Sheppard, C.; Davy, S.; Pilling, G.; Graham, N. The Biology of Coral Reefs; Oxford University Press: New York, NY, USA, 2017. [Google Scholar]
- Marubini, F.; Atkinson, M. Effects of lowered pH and elevated nitrate on coral calcification. Mar. Ecol. Prog. Ser. 1999, 188, 117–121. [Google Scholar] [CrossRef]
- Margolin, C.L. Interactive Effects of Water Flow and Light Levels with Decreasing pH on the Growth and Survival of Tropical Cnidarians; University of Miami: Coral Gables, FL, USA, 2012. [Google Scholar]
- Strychar, K.B.; Hauff-Salas, B.; Haslun, J.A.; DeBoer, J.; Cryer, K.; Keith, S.; Wooten, S. Stress resistance and adaptation of the aquatic invasive species Tubastraea coccinea (Lesson, 1829) to climate change and ocean acidification. Water 2021, 13, 3645. [Google Scholar] [CrossRef]
- Kolian, S.R.; Sammarco, P.W.; Porter, S.A. Abundance of corals on offshore oil and gas platforms in the Gulf of Mexico. Environ. Manag. 2017, 60, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Richmond, R.H.; Tisthammer, K.H.; Spies, N.P. The effects of anthropogenic stressors on reproduction and recruitment of corals and reef organisms. Front. Mar. Sci. 2018, 5, 226. [Google Scholar] [CrossRef] [Green Version]
- Braga, M.D.A.; Paiva, S.V.; de Gurjão, L.M.; Teixeira, C.E.P.; Gurgel, A.L.A.R.; Pereira, P.H.C.; de Oliveira Soares, M. Retirement risks: Invasive coral on old oil platform on the Brazilian equatorial continental shelf. Mar. Pollut. Bull. 2021, 165, 112156. [Google Scholar] [CrossRef] [PubMed]
- BOEM. Bureau of Ocean Energy Management Governing Statutes. Available online: http://www.boem.gov/Regulations/BOEM-Governing-Statutes.aspx (accessed on 9 November 2021).

| Variable | Unit | Maximum | Mean | Minimum |
|---|---|---|---|---|
| Benthic | ||||
| Maximum current velocity | m−1 | 1.2980 | 0.1915 | 0.0319 |
| Minimum dissolved oxygen | mol m−3 | 210.0137 | 181.9234 | 119.4268 |
| Minimum light at bottom | - | 19.0054 | 1.4978 | 0 |
| Maximum salinity | PSS | 36.7947 | 35.6285 | 30.8558 |
| Minimum iron | μmol m−3 | 0.0073 | 0.0011 | 0.0004 |
| Minimum nitrate | mol m−3 | 27.5709 | 12.4456 | 0.0000 |
| Maximum primary productivity | g m−3 day−1 | 0.1313 | 0.0114 | 0 |
| Surface | ||||
| Mean calcite | mol m−3 | 0.0560 | 0.0026 | 0.0001 |
| Mean pH | - | 8.2139 | 8.1195 | 7.9690 |
| Anthropogenic | ||||
| Distance to nearest oil platform | m | 782,115 | 167.220 | 25 |
| Distance to nearest shipping fairway | m | 309.949 | 81.067 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brockinton, E.E.; Peterson, M.R.; Wang, H.-H.; Grant, W.E. Importance of Anthropogenic Determinants of Tubastraea coccinea Invasion in the Northern Gulf of Mexico. Water 2022, 14, 1365. https://doi.org/10.3390/w14091365
Brockinton EE, Peterson MR, Wang H-H, Grant WE. Importance of Anthropogenic Determinants of Tubastraea coccinea Invasion in the Northern Gulf of Mexico. Water. 2022; 14(9):1365. https://doi.org/10.3390/w14091365
Chicago/Turabian StyleBrockinton, Emily E., Miranda R. Peterson, Hsiao-Hsuan Wang, and William E. Grant. 2022. "Importance of Anthropogenic Determinants of Tubastraea coccinea Invasion in the Northern Gulf of Mexico" Water 14, no. 9: 1365. https://doi.org/10.3390/w14091365
APA StyleBrockinton, E. E., Peterson, M. R., Wang, H.-H., & Grant, W. E. (2022). Importance of Anthropogenic Determinants of Tubastraea coccinea Invasion in the Northern Gulf of Mexico. Water, 14(9), 1365. https://doi.org/10.3390/w14091365









