Hydrochemical Characteristics of Arsenic in Shallow Groundwater in Various Unconsolided Sediment Aquifers: A Case Study in Hetao Basin in Inner Mongolia, China
Abstract
1. Introduction
2. Study Area
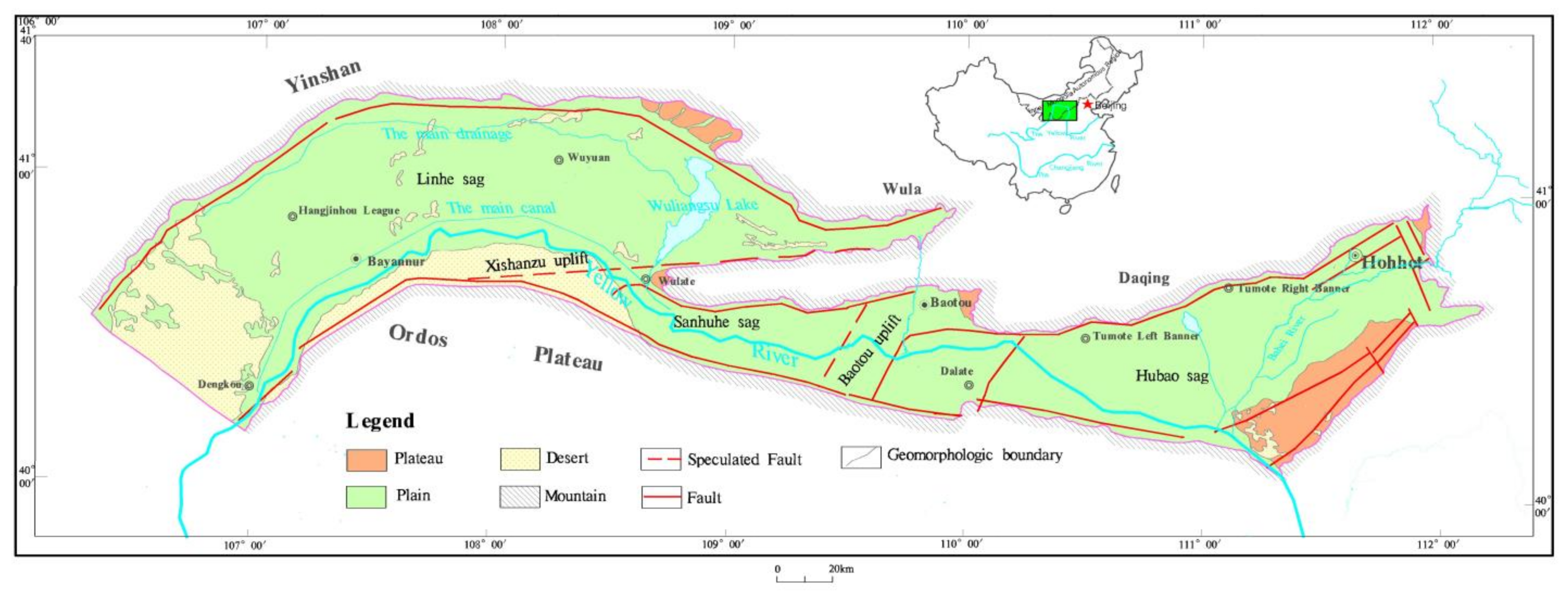
2.1. Geographical Conditions
2.2. Geological Backgrounds
2.3. Hydrogeological Conditions
3. Materials and Methods
3.1. Collection of Samples
3.2. Analytical Method
4. Results
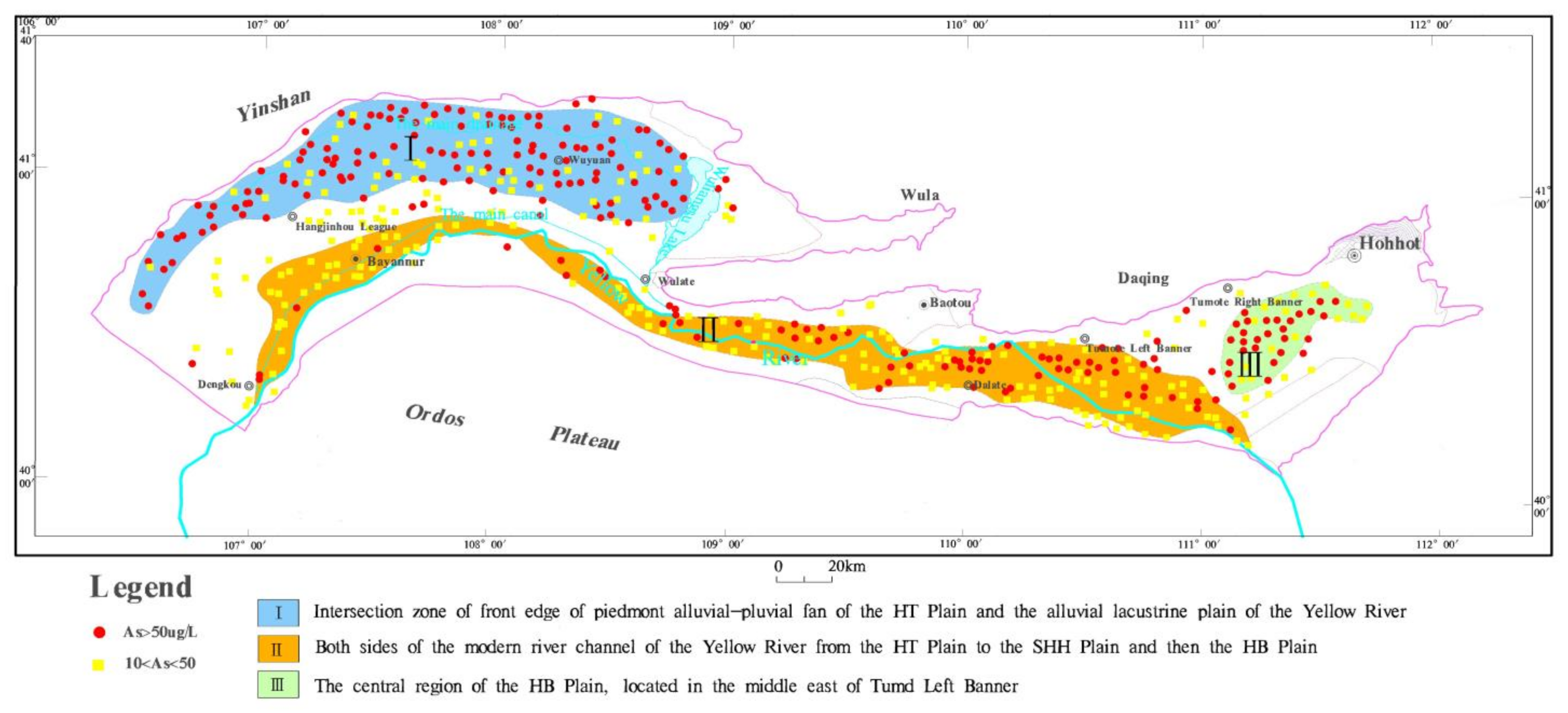
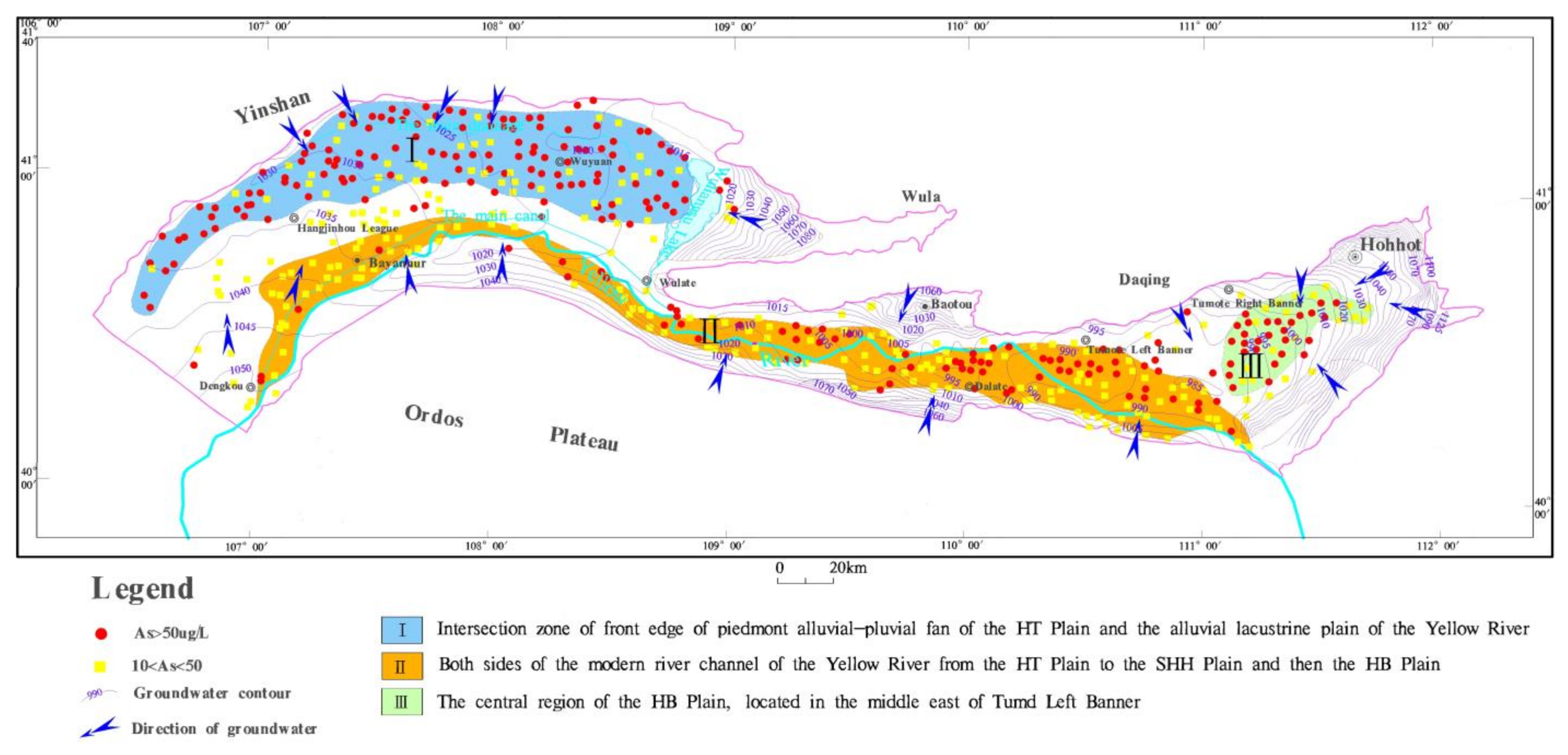
4.1. Content and Regional Distribution of Arsenic in Groundwater
4.2. Hydrochemical Characteristics of Shallow High As Groundwater
4.2.1. Hydrochemical Types
4.2.2. Main Ionic Components
5. Discussion
5.1. The Hydrogeochemical Process of As Mobilization in Aquifers
5.2. Correlation Analysis between Arsenic and Main Oxidation-Reducing Ions
5.3. Correlation between Formation of High As Groundwater and Geological Environment
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, X.; Li, P.; Ji, Y.; Wang, Y.; Su, Z.; Elumalai, V. Groundwater arsenic and fluoride and associated arsenicosis and fluorosis in China: Occurrence, distribution and management. Expo. Health 2020, 12, 355–368. [Google Scholar] [CrossRef]
- He, X.; Li, P.; Wu, J.; Wei, M.; Ren, X.; Wang, D. Poor groundwater quality and high potential health risks in the Datong Basin, northern China: Research from published data. Environ. Geochem. Health 2021, 43, 791–812. [Google Scholar] [CrossRef] [PubMed]
- Nsabimana, A.; Li, P.; He, S.; He, X.; Alam, S.M.K.; Fida, M. Health risk of the shallow groundwater and its suitability for drinking purpose in Tongchuan, China. Water 2021, 13, 3256. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P. Appraisal of shallow groundwater quality with human health risk assessment in different seasons in rural areas of the Guanzhong Plain (China). Environ. Res. 2022, 207, 112210. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, P.; Lyu, Q.; Ren, X.; He, S. Groundwater contamination risk assessment using a modified DRATICL model and pollution loading: A case study in the Guanzhong Basin of China. Chemosphere 2022, 291, 132695. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, P.; Cui, X.; He, S. Groundwater quality, health risk, and major influencing factors in the lower Beiluo River watershed of Northwest China. Hum. Ecol. Risk Assess. 2021, 27, 1987–2013. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, H.; He, S.; Zhang, Y. Comprehensive understanding of groundwater quality for domestic and agricultural purposes in terms of health risks in a coal mine area of the Ordos basin, north of the Chinese Loess Plateau. Environ. Earth Sci. 2019, 78, 446. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Y.; Zhou, H. Groundwater chemistry and groundwater quality index incorporating health risk weighting in Dingbian County, Ordos basin of Northwest China. Geochemistry 2020, 80, 125607. [Google Scholar] [CrossRef]
- Hu, G.; Mian, H.R.; Dyck, R.; Mohseni, M.; Jasim, S.; Hewage, K.; Sadiq, R. Drinking water treatments for arsenic and manganese removal and health risk assessment in white rock, Canada. Expo. Health 2020, 12, 793–807. [Google Scholar] [CrossRef]
- Wei, M.; Wu, J.; Li, W.; Zhang, Q.; Su, F.; Wang, Y. Groundwater geochemistry and its impacts on groundwater arsenic enrichment, variation, and health risks in Yongning County, Yinchuan Plain of Northwest China. Expo. Health 2021, 1–20. [Google Scholar] [CrossRef]
- Alam, M.O.; Shaikh, W.A.; Chakraborty, S.; Avishek, K.; Bhattacharya, T. Groundwater arsenic contamination and potential health risk assessment of gangetic plains of Jharkhand, India. Expo. Health 2016, 8, 125–142. [Google Scholar] [CrossRef]
- Li, Y.; Ma, L.; Abuduwaili, J.; Li, Y.; Uulu, S.A. Spatiotemporal distributions of fluoride and arsenic in rivers with the role of mining industry and related human health risk assessments in Kyrgyzstan. Expo. Health 2021, 1–14. [Google Scholar] [CrossRef]
- Fano, D.; Vásquez-Velásquez, C.; Aguilar, J.; Gribble, M.O.; Wickliffe, J.K.; Lichtveld, M.Y.; Steenland, K.; Gonzales, G.F. Arsenic concentrations in household drinking water: A cross-sectional survey of pregnant women in Tacna, Peru, 2019. Expo. Health 2020, 12, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Sathe, S.S.; Mahanta, C.; Subbiah, S. Hydrogeochemical evaluation of intermittent alluvial aquifers controlling arsenic and fluoride contamination and corresponding health risk assessment. Expo. Health 2021, 13, 661–680. [Google Scholar] [CrossRef]
- Owusu, C.; Silverman, G.S.; Vinson, D.S.; Bobyarchick, A.; Paul, R.; Delmelle, E. A spatial autologistic model to predict the presence of arsenic in private wells across Gaston County, North Carolina using geology, well depth, and pH. Expo. Health 2021, 13, 195–206. [Google Scholar] [CrossRef]
- Joardar, M.; Das, A.; Mridha, D.; De, A.; Chowdhury, N.R.; Roychowdhury, T. Evaluation of acute and chronic arsenic exposure on school children from exposed and apparently control areas of West Bengal, India. Expo. Health 2021, 13, 33–50. [Google Scholar] [CrossRef]
- Rehman, U.; Khan, S.; Muhammad, S. Ingestion of arsenic-contaminated drinking water leads to health risk and traces in human biomarkers (hair, nails, blood, and urine), Pakistan. Expo. Health 2020, 12, 243–254. [Google Scholar] [CrossRef]
- Wu, C.; Fang, C.; Wu, X.; Zhu, G. Health-risk assessment of arsenic and groundwater quality classification using random forest in the yanchi region of Northwest China. Expo. Health 2020, 12, 761–774. [Google Scholar] [CrossRef]
- Apollaro, C.; Di Curzio, D.; Fuoco, I.; Buccianti, A.; Dinelli, E.; Vespasiano, G.; De Rosa, R. A multivariate non-parametric approach for estimating probability of exceeding the local natural background level of arsenic in the aquifers of Calabria region (Southern Italy). Sci. Total Environ. 2022, 806, 150345. [Google Scholar] [CrossRef]
- Figoli, A.; Fuoco, I.; Apollaro, C.; Chabane, M.; Criscuoli, A. Arsenic-contaminated groundwaters remediation by nanofiltration. Sep. Purif. Technol. 2019, 238, 116461. [Google Scholar] [CrossRef]
- Matschullat, J. Arsenic in the geosphere—A review. Sci. Total Environ. 2000, 249, 297–312. [Google Scholar] [CrossRef]
- Polizzotto, M.L.; Harvey, C.F.; Li, G.; Badruzzman, B.; Ali, A.; Newville, M.; Sutton, S.; Fendorf, S. Solid-phases and desorption processes of arsenic within bangladesh sediments. Chem. Geol. 2006, 228, 97–111. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Drahota, P.; Filippi, M. Secondary arsenic minerals in the environment: A review. Environ. Int. 2009, 35, 1243–1255. [Google Scholar] [CrossRef] [PubMed]
- Fuoco, I.; De Rosa, R.; Barca, D.; Figoli, A.; Gabriele, B.; Apollaro, C. Arsenic polluted waters: Application of geochemical modelling as a tool to understand the release and fate of the pollutant in crystalline aquifers. J. Environ. Manag. 2022, 301, 113796. [Google Scholar] [CrossRef]
- Cao, W.; Guo, H.; Zhang, Y.; Rong, M.; Zhao, R. Controls of paleochannels on groundwater arsenic distribution in shallow aquifers of alluvial plain in the Hetao Basin, China. Sci. Total Environ. 2017, 613–614, 958. [Google Scholar] [CrossRef]
- Guo, H.M.; Wen, D.G.; Liu, Z.Y.; Jia, Y.F.; Guo, Q. A review of arsenic-rich groundwater in Mainland and Taiwan, China: Distribution, characteristics and geochemical processes. Appl. Geochem. 2014, 41, 196–217. [Google Scholar] [CrossRef]
- Shen, Y.F.; Sun, D.J.; Zhao, X.H.; Yu, G.Q. Screening report in areas of endemic arsenism and high content of arsenic in China. Chin. J. Endem. 2005, 24, 172–175. Available online: http://en.cnki.com.cn/Article_en/CJFDTOTAL-ZDFB20050200N.htm (accessed on 22 October 2020).
- Jin, Y.L.; Liang, C.K.; He, G.L.; Cao, J.X.; Ji, R.C. Study on distribution of endemic arsenism in China. J. Hyg. Res. 2003, 32, 519–540. [Google Scholar] [CrossRef]
- Smedley, P.L.; Zhang, M.; Zhang, G.; Luo, Z. Mobilisation of arsenic and other trace elements in fluviolacustrine aquifers of the Huhhot Basin, Inner Mongolia. Appl. Geochem. 2003, 18, 1453–1477. [Google Scholar] [CrossRef]
- Guo, H.; Yang, S.; Tang, X.; Li, Y.; Shen, Z. Groundwater geochemistry and its implications for arsenic mobilization in shallow aquifers of the Hetao Basin, Inner Mongolia. Sci. Total Environ. 2008, 393, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wang, Y.; Teng, M. Isotope and minor element geochemistry of arsenic-rich groundwater from Hangjinhouqi, the Hetao Plain, Inner Mongolia. Appl. Geochem. 2009, 24, 587–599. [Google Scholar] [CrossRef]
- Gong, Z.; Lu, X.; Watt, C.; Bei, W.; He, B.; Mumford, J.; Ning, Z.; Xia, Y.; Le, X.C. Speciation analysis of arsenic in groundwater from Inner Mongolia with an emphasis on acid-leachable particulate arsenic. Anal. Chim. Acta 2006, 555, 181–187. [Google Scholar] [CrossRef]
- Jun, H.E.; Teng, M.A.; Deng, Y.; Yang, H.; Wang, Y. Environmental geochemistry of arsenic-rich groundwater at western Hetao plain, Inner Mongolia. Front. Earth Sci. China 2009, 3, 63. [Google Scholar] [CrossRef]
- Lin, N.F.; Tang, J.; Bian, J.M. The study on environmental geo-chemical characteristics in Arseniasis Area in the Inner Mongolia. World Geol. 1999, 18, 83–88. Available online: http://en.cnki.com.cn/Article_en/CJFDTOTAL-SJDZ902.011.htm (accessed on 22 October 2020).
- Gao, C.R.; Chao-Xin, L.I.; Zhou, X.H.; Liu, B.; Liu, W.B.; Cai, L.I.; Feng, D.Y. Occurrence and hydrochemical characteristics of As-rich groundwater in the Linhe district of the Hetao Plain. Hydrogeol. Eng. Geol. 2008, 35, 22–28. [Google Scholar] [CrossRef]
- Gao, C.; Liu, W.; Feng, C.E.; Chen, Y.; Zhang, G.; Song, J. Research on the formation mechanism of arsenic-rich groundwater in arid and semi-arid regions: A case study of Hetao Plain in Inner Mongolia, China. Earth Sci. Front. 2014, 21, 13–29. [Google Scholar] [CrossRef]
- Jia, L.; Zhang, X.; Ye, P.; Zhao, X.; He, Z.; He, X.; Zhou, Q.; Li, J.; Ye, M.; Wang, Z.; et al. Development of the alluvial and lacustrine terraces on the northern margin of the Hetao Basin, Inner Mongolia, China: Implications for the evolution of the Yellow River in the Hetao area since the Late Pleistocene. Geomorphology 2016, 263, 87–98. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Y.; Xing, L.; Jia, Y. Spatial variation in arsenic and fluoride concentrations of shallow groundwater from the town of Shahai in the Hetao basin, Inner Mon-golia. Appl. Geochem. 2012, 27, 2187–2196. [Google Scholar] [CrossRef]
- Guo, H.; Liu, Z.; Ding, S.; Hao, C.; Xiu, W.; Hou, W. Arsenate reduction and mobilization in the presence of indigenous aerobic bacteria obtained from high arsenic aquifers of the hetao basin, inner mongolia. Environ. Pollut. 2015, 203, 50–59. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, Y.; Ma, T.; Gan, Y. Speciation and enrichment of arsenic in strongly reducing shallow aquifers at western hetao plain, northern china. Environ. Geol. 2009, 56, 1467–1477. [Google Scholar] [CrossRef]
- Ni, P.; Guo, H.; Cao, Y.; Jia, Y.; Jiang, Y.; Zhang, D. Aqueous geochemistry and its influence on the partitioning of arsenic between aquifer sediments and groundwater: A case study in the northwest of the Hetao Basin. Environ. Earth Sci. 2016, 75, 356.1–356.13. [Google Scholar] [CrossRef]
- Wang, M. Quaternary Sedimentary and Structure Features of the Hohholt-Baotou Basin, Inner Mongolia. Master’s Thesis, China University of Geosciences, Beijing, China, 2006. (In Chinese with English Abstract). [Google Scholar]
- Xiang, H.; Deng, H.; Zheng, C.; Cao, G. Hydrogeochemical signatures and evolution of groundwater impacted by the bayan obo tailing pond in northwest china. Sci. Total Environ. 2016, 543, 357–372. [Google Scholar]
- Fang, L. Groundwater Monitoring Comprehensive Report from 2001 to 2005 in Baotou City; Inner Mongolia Institute of Geological Environment Monitoring: Hohhot, China, 2006. [Google Scholar]
- Guo, H.; Zhang, B.; Li, Y.; Berner, Z.; Tang, X.; Norra, S.; Stüben, D. Hydrogeological and biogeochemical constrains of arsenic mobilization in shallow aquifers from the Hetao Basin, Inner Mongolia. Environ. Pollut. 2011, 159, 876–883. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, H.; Yan, S. Geochemical Evolution of Shallow Groundwater Systems and Their Vulnerability to Contaminants: A Case Study at Datong Basin, Shanxi Province; Science Press: Beijing, China, 2004; p. 62. (In Chinese) [Google Scholar]
- Goldberg, S. Chemistry of the solid-water interface: Processes at the mineral-water and particle-water interface in natural systems. Geochim. Cosmochim. Acta 1992, 57, 205. [Google Scholar] [CrossRef][Green Version]
- Smedley, P.L.; Kinniburgh, D.G.; Macdonald, D.; Nicolli, H.B.; Barros, A.J.; Tullio, J.O. Arsenic associations in sediments from the loess aquifer of la pampa, argentina. Appl. Geochem. 2005, 20, 989–1016. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Y.; Yang, N.; Jing, L.; Yu, P. Major ion chemistry and quality assessment of groundwater in and around a mountainous tourist town of China. Expo. Health 2016, 8, 239–252. [Google Scholar] [CrossRef]
- Li, P.; Tian, R.; Liu, R. Solute geochemistry and multivariate analysis of water quality in the Guohua Phosphorite Mine, Guizhou Province, China. Expo. Health 2019, 11, 81–94. [Google Scholar] [CrossRef]
- Ren, X.; Li, P.; He, X.; Su, F.; Elumalai, V. Hydrogeochemical processes affecting groundwater chemistry in the central part of the Guanzhong Basin, China. Arch. Environ. Contam. Toxicol. 2021, 80, 74–91. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, P.; Wang, D.; Ren, X.; Wei, M. Statistical and multivariate statistical techniques to trace the sources and affecting factors of groundwater pollution in a rapidly growing city on the Chinese Loess Plateau. Hum. Ecol. Risk Assess. 2020, 26, 1603–1621. [Google Scholar] [CrossRef]
- Wu, J.; Li, P.; Qian, H.; Duan, Z.; Zhang, X. Using correlation and multivariate statistical analysis to identify hydrogeochemical processes affecting the major ion chemistry of waters: Case study in Laoheba phosphorite mine in Sichuan, China. Arab. J. Geosci. 2014, 7, 3973–3982. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, W.; Wang, W.; Dong, Q. Distribution of groundwater arsenic and hydraulic gradient along the shallow groundwater flow-path in hetao plain, northern china. J. Geochem. Explor. 2013, 135, 31–39. [Google Scholar] [CrossRef]
- Wang, Y.X.; Su, C.L.; Xie, X.J.; Xie, Z.M. The genesis of arsenic-rich groundwater:a case study in Datong basin. Geol. China 2010, 37, 771–780. [Google Scholar] [CrossRef]





| Area (km2) | Number | 0.1 μg/L (%) | >10 μg/L (%) | Mean (μg/L) | Medium (μg/L) | Max (μg/L) | |
|---|---|---|---|---|---|---|---|
| HT Plain | 13,880 | 450 | 99.33 | 53.78 | 64.13 | 14.44 | 916.7 |
| SHH Plain | 1623 | 56 | 100 | 51.79 | 40.64 | 12.48 | 615.4 |
| HB Plain | 11,382 | 278 | 95.32 | 38.49 | 32.31 | 3.76 | 398.9 |
| SBYR Plain | 4995 | 190 | 99.47 | 43.68 | 22.51 | 6.31 | 238.3 |
| Hetao Baisn | 31,880 | 974 | 98.15 | 47.33 | 45.58 | 7.95 | 916.7 |
| Item | As (μg/L) | As(III) (μg/L) | As(V) (μg/L) | HCO3− (mg/L) | ||||||||
| Mean | Median | Max | Mean | Median | Max | Mean | Median | Max | Mean | Median | Max | |
| HT Plain | 116.93 | 58.21 | 916.70 | 100.69 | 50.99 | 719.40 | 16.24 | 6.95 | 224.60 | 521.34 | 480.70 | 1327.00 |
| SHH Plain | 76.20 | 30.17 | 615.40 | 58.01 | 26.81 | 376.80 | 18.19 | 6.66 | 238.60 | 548.77 | 524.70 | 1347.00 |
| HB Plain | 80.65 | 48.16 | 398.90 | 68.74 | 41.02 | 383.70 | 11.92 | 7.03 | 120.10 | 704.32 | 620.40 | 2123.00 |
| SBYR Plain | 47.60 | 35.41 | 238.30 | 40.02 | 32.29 | 227.10 | 7.57 | 4.98 | 39.10 | 547.50 | 504.10 | 2220.00 |
| Hetao Baisn | 93.46 | 49.64 | 916.70 | 79.67 | 41.02 | 719.40 | 13.80 | 6.52 | 238.60 | 570.25 | 505.00 | 2220.00 |
| Item | Fe (mg/L) | Fe2+ (mg/L) | Fe3+ (mg/L) | SO42− (mg/L) | ||||||||
| Mean | Median | Max | Mean | Median | Max | Mean | Median | Max | Mean | Median | Max | |
| HT Plain | 2.63 | 1.60 | 17.00 | 1.26 | 0.75 | 11.80 | 1.37 | 0.53 | 16.00 | 306.27 | 235.35 | 2494.00 |
| SHH Plain | 5.84 | 2.40 | 40.00 | 2.59 | 1.20 | 30.00 | 3.25 | 0.80 | 24.00 | 291.90 | 227.90 | 2004.00 |
| HB Plain | 2.60 | 1.32 | 25.00 | 1.17 | 0.48 | 17.00 | 1.43 | 0.52 | 22.00 | 360.65 | 263.20 | 2419.00 |
| SBYR Plain | 2.33 | 0.88 | 34.00 | 1.02 | 0.28 | 17.00 | 1.32 | 0.52 | 17.00 | 264.77 | 172.20 | 2544.00 |
| Hetao Baisn | 2.77 | 1.50 | 40.00 | 1.28 | 0.60 | 30.00 | 1.49 | 0.54 | 24.00 | 310.51 | 225.60 | 2544.00 |
| Item | As < 10 μg/L | As ≥ 10 μg/L | ||||
|---|---|---|---|---|---|---|
| As(III) (μg/L) | As(V) (μg/L) | As(III)/As(V) | As(III) (μg/L) | As(V) (μg/L) | As(III)/As(V) | |
| HT Plain | 1.74 | 0.94 | 1.85 | 100.69 | 16.24 | 6.20 |
| SHH Plain | 1.61 | 0.84 | 1.91 | 60.93 | 19.27 | 3.16 |
| HB Plain | 1.2 | 0.56 | 2.14 | 71.89 | 12.71 | 5.66 |
| SBYR plain | 2.12 | 0.97 | 2.19 | 39.35 | 7.38 | 5.33 |
| As | Fe | As(III) | As(V) | Fe2+ | Fe3+ | HCO3− | NO2− | NO3− | SO42− | |
|---|---|---|---|---|---|---|---|---|---|---|
| As | 1 | 0.242 ** | 0.992 ** | 0.797 ** | 0.170 ** | 0.231 ** | 0.177 ** | −0.007 | −0.093 ** | −0.044 |
| Fe | 0.242 ** | 1 | 0.213 ** | 0.315 ** | 0.812 ** | 0.843 ** | 0.139 ** | −0.013 | −0.080 * | 0.171 ** |
| As(III) | 0.992 ** | 0.213 ** | 1 | 0.712 ** | 0.167 ** | 0.188 ** | 0.171 ** | −0.010 | −0.095 ** | −0.050 |
| As(V) | 0.797 ** | 0.315 ** | 0.712 ** | 1 | 0.140 ** | 0.375 ** | 0.159 ** | 0.007 | −0.062 | −0.004 |
| Fe ** | 0.170 ** | 0.812 ** | 0.167 ** | 0.140 ** | 1 | 0.378 ** | 0.054 | −0.011 | −0.093 ** | 0.105 ** |
| Fe ** | 0.231 ** | 0.843 ** | 0.188 ** | 0.375 ** | 0.378 ** | 1 | 0.175 ** | −0.010 | −0.042 | 0.178 ** |
| HCO3 * | 0.177 ** | 0.139 ** | 0.171 ** | 0.159 ** | 0.054 | 0.175 ** | 1 | 0.068 * | 0.081 * | 0.382 ** |
| NO2 * | −0.007 | −0.013 | −0.010 | 0.007 | −0.011 | −0.010 | 0.068 * | 1 | 0.270 ** | 0.011 |
| NO3 * | −0.093 ** | −0.080 * | −0.095 ** | −0.062 | −0.093 ** | −0.042 | 0.081 * | 0.270 ** | 1 | 0.155 ** |
| SO4 ** | −0.044 | 0.171 ** | −0.050 | −0.004 | 0.105 ** | 0.178 ** | 0.382 ** | 0.011 | 0.155 ** | 1 |
| As | Fe | As(III) | As(V) | Fe ** | Fe ** | HCO3 * | NO2 * | NO3 * | SO4 ** | |
|---|---|---|---|---|---|---|---|---|---|---|
| As | 1 | 0.182 ** | 0.993 ** | 0.797 ** | 0.115 * | 0.181 ** | 0.212 ** | 0.137 ** | 0.056 | −0.124 ** |
| Fe | 0.182 ** | 1 | 0.161 ** | 0.243 ** | 0.821 ** | 0.796 ** | 0.166 ** | 0.025 | −0.020 | 0.240 ** |
| As(III) | 0.993 ** | 0.161 ** | 1 | 0.720 ** | 0.121 * | 0.141 ** | 0.210 ** | 0.144 ** | 0.057 | −0.137 ** |
| As(V) | 0.797 ** | 0.243 ** | 0.720 ** | 1 | 0.057 | 0.345 ** | 0.174 ** | 0.066 | 0.040 | −0.027 |
| Fe ** | 0.115 * | 0.821 ** | 0.121 * | 0.057 | 1 | 0.308 ** | 0.030 | 0.045 | −0.024 | 0.191 ** |
| Fe ** | 0.181 ** | 0.796 ** | 0.141 ** | 0.345 ** | 0.308 ** | 1 | 0.246 ** | −0.007 | −0.008 | 0.198 ** |
| HCO3 * | 0.212 ** | 0.166 ** | 0.210 ** | 0.174 ** | 0.030 | 0.246 ** | 1 | 0.125 ** | −0.041 | 0.283 ** |
| NO2 * | 0.137 ** | 0.025 | 0.144 ** | 0.066 | 0.045 | −0.007 | 0.125 ** | 1 | 0.042 | 0.092 |
| NO3 * | 0.056 | −0.020 | 0.057 | 0.040 | −0.024 | −0.008 | −0.041 | 0.042 | 1 | 0.189 ** |
| SO4 ** | −0.124 ** | 0.240 ** | −0.137 ** | −0.027 | 0.191 ** | 0.198 ** | 0.283 ** | 0.092 | 0.189 ** | 1 |
| As | Fe | As(III) | As(V) | Fe ** | Fe ** | HCO3 * | NO2 * | NO3 * | SO4 ** | |
|---|---|---|---|---|---|---|---|---|---|---|
| As | 1 | 0.574 ** | 0.988 ** | 0.958 ** | 0.348 ** | 0.629 ** | 0.368 ** | 0.476 ** | −0.167 | −0.043 |
| Fe | 0.574 ** | 1 | 0.560 ** | 0.563 ** | 0.826 ** | 0.849 ** | 0.117 | 0.174 | −0.218 | −0.073 |
| As(III) | 0.988 ** | 0.560 ** | 1 | 0.901 ** | 0.359 ** | 0.597 ** | 0.378 ** | 0.495 ** | −0.203 | −0.040 |
| As(V) | 0.958 ** | 0.563 ** | 0.901 ** | 1 | 0.305 * | 0.648 ** | 0.327 * | 0.411 ** | −0.091 | −0.044 |
| Fe ** | 0.348 ** | 0.826 ** | 0.359 ** | 0.305 * | 1 | 0.433 ** | 0.096 | 0.131 | −0.182 | −0.062 |
| Fe ** | 0.629 ** | 0.849 ** | 0.597 ** | 0.648 ** | 0.433 ** | 1 | 0.131 | 0.180 | −0.170 | −0.040 |
| HCO3 * | 0.368 ** | 0.117 | 0.378 ** | 0.327 * | 0.096 | 0.131 | 1 | 0.489 ** | 0.020 | 0.687 ** |
| NO2 * | 0.476 ** | 0.174 | 0.495 ** | 0.411 ** | 0.131 | 0.180 | 0.489 ** | 1 | −0.141 | 0.210 |
| NO3 * | −0.167 | −0.218 | −0.203 | −0.091 | −0.182 | −0.170 | 0.020 | −0.141 | 1 | 0.406 ** |
| SO4 ** | −0.043 | −0.073 | −0.040 | −0.044 | −0.062 | −0.040 | 0.687 ** | 0.210 | 0.406 ** | 1 |
| As | Fe | As(III) | As(V) | Fe ** | Fe ** | HCO3 * | NO2 * | NO3 * | SO4 ** | |
|---|---|---|---|---|---|---|---|---|---|---|
| As | 1 | 0.288 ** | 0.993 ** | 0.810 ** | 0.237 ** | 0.229 ** | 0.217 ** | −0.045 | −0.175 ** | 0.020 |
| Fe | 0.288 ** | 1 | 0.285 ** | 0.238 ** | 0.704 ** | 0.876 ** | 0.182 ** | −0.044 | −0.087 | 0.277 ** |
| As(III) | 0.993 ** | 0.285 ** | 1 | 0.733 ** | 0.242 ** | 0.221 ** | 0.208 ** | −0.049 | −0.181 ** | 0.018 |
| As(V) | 0.810 ** | 0.238 ** | 0.733 ** | 1 | 0.157 ** | 0.216 ** | 0.214 ** | −0.018 | −0.107 | 0.027 |
| Fe ** | 0.237 ** | 0.704 ** | 0.242 ** | 0.157 ** | 1 | 0.275 ** | 0.119 * | −0.043 | −0.145 * | 0.156 ** |
| Fe ** | 0.229 ** | 0.876 ** | 0.221 ** | 0.216 ** | 0.275 ** | 1 | 0.167 ** | −0.030 | −0.019 | 0.269 ** |
| HCO3 * | 0.217 ** | 0.182 ** | 0.208 ** | 0.214 ** | 0.119 * | 0.167 ** | 1 | 0.056 | 0.101 | 0.417 ** |
| NO2 * | −0.045 | −0.044 | −0.049 | −0.018 | −0.043 | −0.030 | 0.056 | 1 | 0.298 ** | 0.013 |
| NO3 * | −0.175 ** | −0.087 | −0.181 ** | −0.107 | −0.145 * | −0.019 | 0.101 | 0.298 ** | 1 | 0.278 ** |
| SO4 ** | 0.020 | 0.277 ** | 0.018 | 0.027 | 0.156 ** | 0.269 ** | 0.417 ** | 0.013 | 0.278 ** | 1 |
| As | Fe | As(III) | As(V) | Fe ** | Fe ** | HCO3 * | NO2 * | NO3 * | SO4 ** | |
|---|---|---|---|---|---|---|---|---|---|---|
| As | 1 | 0.320 ** | 0.993 ** | 0.823 ** | 0.315 ** | 0.283 ** | 0.231 ** | −0.074 | −0.175 * | 0.006 |
| Fe | 0.320 ** | 1 | 0.303 ** | 0.334 ** | 0.903 ** | 0.945 ** | 0.186 * | −0.046 | −0.096 | 0.195 ** |
| As(III) | 0.993 ** | 0.303 ** | 1 | 0.750 ** | 0.321 ** | 0.251 ** | 0.227 ** | −0.073 | −0.176 * | −0.004 |
| As(V) | 0.823 ** | 0.334 ** | 0.750 ** | 1 | 0.223 ** | 0.376 ** | 0.201 ** | −0.063 | −0.133 | 0.050 |
| Fe ** | 0.315 ** | 0.903 ** | 0.321 ** | 0.223 ** | 1 | 0.713 ** | 0.118 | −0.034 | −0.118 | 0.099 |
| Fe ** | 0.283 ** | 0.945 ** | 0.251 ** | 0.376 ** | 0.713 ** | 1 | 0.214 ** | −0.049 | −0.067 | 0.243 ** |
| HCO3 * | 0.231 ** | 0.186 * | 0.227 ** | 0.201 ** | 0.118 | 0.214 ** | 1 | 0.043 | −0.011 | 0.491 ** |
| NO2 * | −0.074 | −0.046 | −0.073 | −0.063 | −0.034 | −0.049 | 0.043 | 1 | 0.075 | 0.036 |
| NO3 * | −0.175 * | −0.096 | −0.176 * | −0.133 | −0.118 | −0.067 | −0.011 | 0.075 | 1 | 0.072 |
| SO4 ** | 0.006 | 0.195 ** | −0.004 | 0.050 | 0.099 | 0.243 ** | 0.491 ** | 0.036 | 0.072 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Z.; Liu, L.; Xu, W.; Wu, P.; Lu, C. Hydrochemical Characteristics of Arsenic in Shallow Groundwater in Various Unconsolided Sediment Aquifers: A Case Study in Hetao Basin in Inner Mongolia, China. Water 2022, 14, 669. https://doi.org/10.3390/w14040669
Cai Z, Liu L, Xu W, Wu P, Lu C. Hydrochemical Characteristics of Arsenic in Shallow Groundwater in Various Unconsolided Sediment Aquifers: A Case Study in Hetao Basin in Inner Mongolia, China. Water. 2022; 14(4):669. https://doi.org/10.3390/w14040669
Chicago/Turabian StyleCai, Zizhao, Lingxia Liu, Wei Xu, Ping Wu, and Chuan Lu. 2022. "Hydrochemical Characteristics of Arsenic in Shallow Groundwater in Various Unconsolided Sediment Aquifers: A Case Study in Hetao Basin in Inner Mongolia, China" Water 14, no. 4: 669. https://doi.org/10.3390/w14040669
APA StyleCai, Z., Liu, L., Xu, W., Wu, P., & Lu, C. (2022). Hydrochemical Characteristics of Arsenic in Shallow Groundwater in Various Unconsolided Sediment Aquifers: A Case Study in Hetao Basin in Inner Mongolia, China. Water, 14(4), 669. https://doi.org/10.3390/w14040669





