Foraminiferal Distribution in Two Estuarine Intertidal Mudflats of the French Atlantic Coast: Testing the Marine Influence Index
Abstract
:1. Introduction
2. Material and Methods
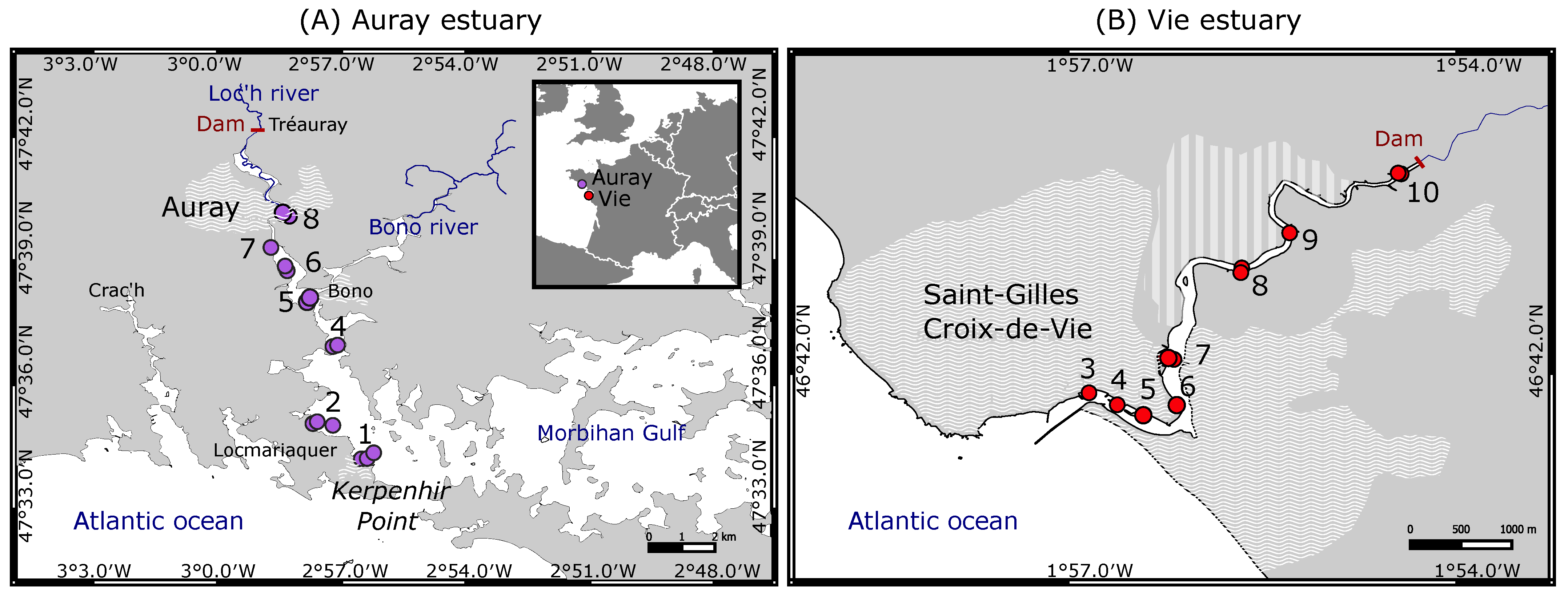
2.1. Study Area
2.2. Sampling Procedure
2.3. Laboratory Analyses
- EF < 1.5: no enrichment;
- 1.5 < EF < 3: minor enrichment;
- 3 < EF < 5: moderate enrichment;
- EF > 5: strong enrichment.
2.4. Marine Influence Index
2.5. Statistical Analyses
3. Results
3.1. Sediment Grain Size
3.2. Trace Metal Distribution
3.3. Organic Matter
3.4. Foraminiferal Distribution
3.4.1. Density
3.4.2. Foraminiferal Diversity
3.4.3. Foraminiferal Communities
3.4.4. Foraminiferal Indices of Environmental Quality
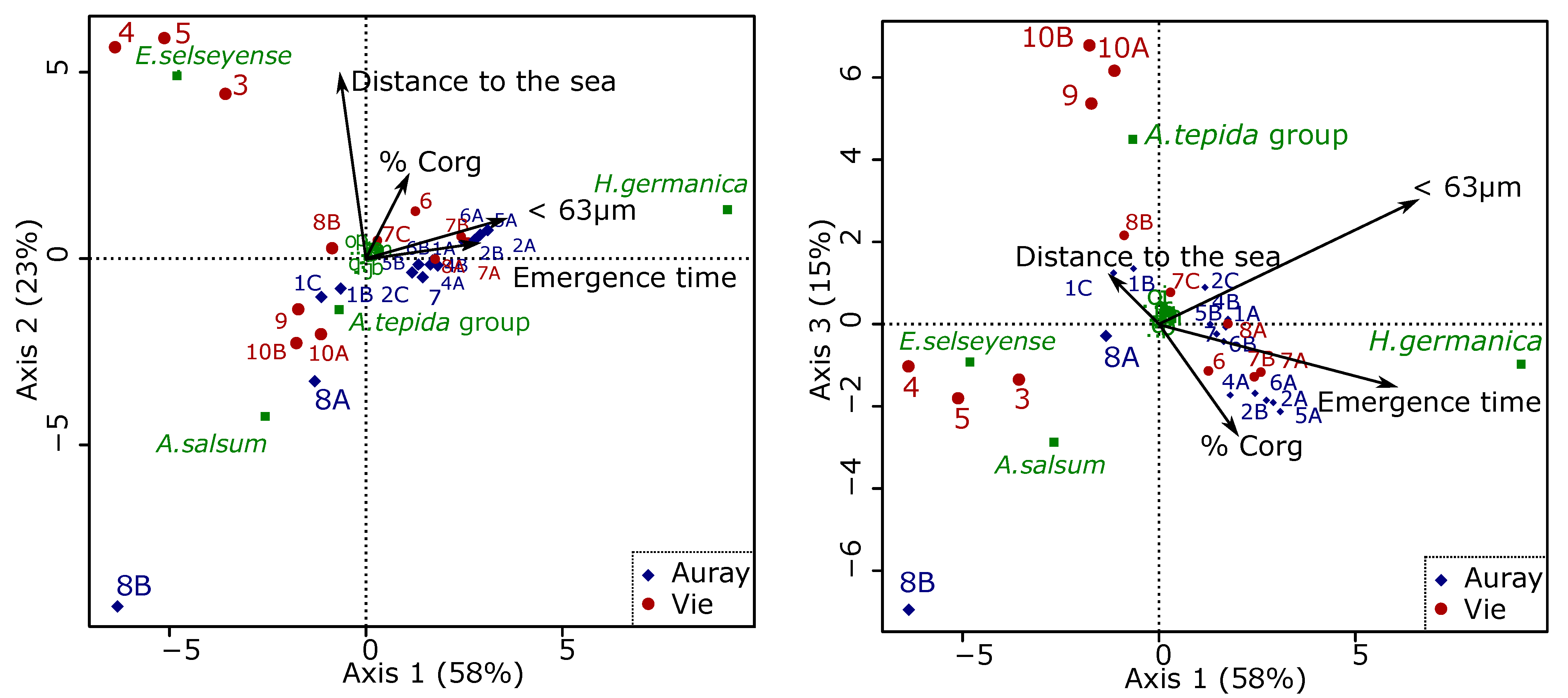
3.4.5. Relations between Foraminiferal Distribution and the Marine Influence Index (MII)
4. Discussion
4.1. Environmental Characteristics
- (I).
- Sediment grain size—two dominant grain size populations coexist, which may correspond to a mix of sand deposits during high river discharge periods and the settling of clay-silt from riverine input during slack waters. There is a significant decrease in grain size towards stations higher on the mudflats where tidal currents are weaker;
- (II).
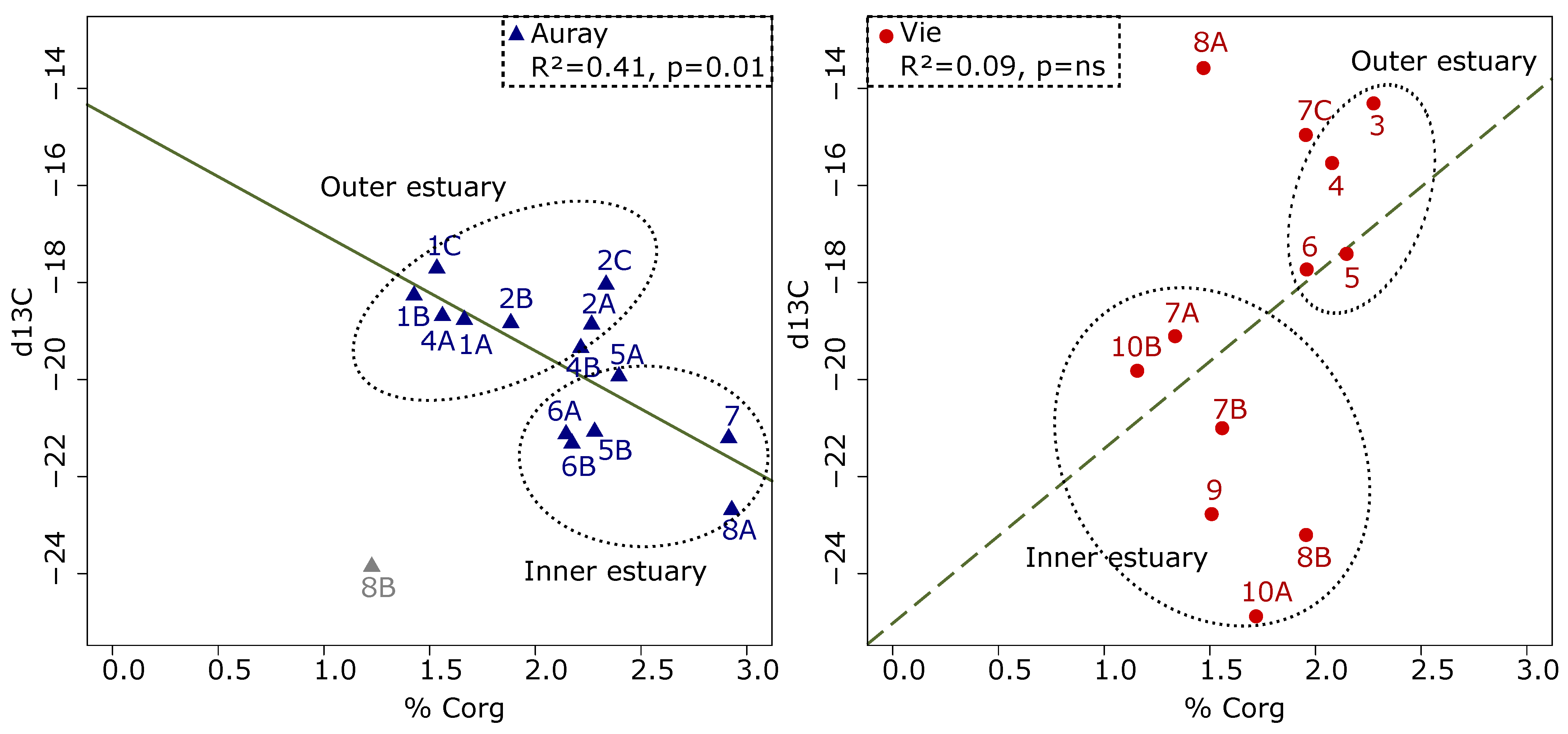
- Organic matter quantity and quality—different %Corg patterns underline the difference between the two estuaries. In the Auray estuary, the %Corg decreases seawards, whereas the opposite is observed in the Vie estuary. In fact, in the outer Auray estuary (sites 1 and 2), open to the sea, organically poor marine waters replace organically rich riverine waters. In both estuaries, δ13C shows an overall seaward decrease, which corresponds to the natural gradual transition of continental to marine organic matter [78,79]. In the outer Auray estuary, δ13C values vary from −18 to −20‰, suggesting a major contribution of microphytobenthos [59]. In the Vie estuary, the low values observed at several stations (between −14 and −16‰) could be related to the presence of macro-algae [59,80], which were observed during the sampling campaign. In summarising, it appears that both the %Corg and the δ13C trends reflect natural patterns that seem not be biased by anthropogenic organic matter input;
- (III).
- Trace metal concentrations—in both estuaries, none of the six trace metals considered relevant for biota attains the strong enrichment limit (as defined by Larrose et al. [54]). Concerning Cu concentrations, the moderate enrichment at stations Auray 1A and Vie 6 and the minor enrichment at stations Vie 4 and 5 may be related to antifouling paint used in the nearby harbours. Only minor Pb and Zn enrichment was detected (mainly at station Auray 8B).
4.2. Foraminifera Communities
- (1).
- The foraminiferal assemblage at station Auray 8B is composed by more than 90% agglutinated species (Ammotium salsum, Ammobaculites agglutinans, Trochammina inflata). These agglutinated species, typical of oligohaline conditions, are commonly found in salt marshes [28,84,85] and may also occur in inner estuaries [86]. This assemblage is compatible with the low salinity recorded (about 16 at high spring tide). Station 8B also stands out because of its much higher proportion of medium sand. Very close to station 8B, the estuary artificially narrows to a single channel to pass under a road bridge, leading to a higher current velocity (Figure 3). This brackish and dynamic hydrological setting could explain the very different foraminiferal community observed at this station;
- (2).
- In the Vie estuary, stations 4 and 5 have been sampled on concrete shipping ramps, covered with several-decimetres-thick mud deposits, inside docks in the St-Gilles-Croix-de-Vie harbour, and undergo strong anthropogenic influence. This is partly evidenced by the slightly increased Cu concentrations at these two stations (Figure 4), corresponding to minor enrichment [51]. At these stations the foraminiferal community shows high abundances of E. selseyense. In view of the preference of E. selseyense for the outer estuary [86], and the occurrence of a similar community at station 3, where no increased Cu-content was observed, it seems unlikely that its dominance at these stations reflects strong pollution.
4.3. Comparison with Previous Studies
4.4. Foraminiferal Indices of Environmental Quality
4.5. Foraminiferal Community and the Marine Influence Index (MII)
- (1)
- The uncertainty about the distance to the sea, especially in cases of man-made structures, which artificially block salt intrusion;
- (2)
- The difficulty of measuring altitude (used to calculate emergence time). As explained in the Section 2, different approaches were used for the stations at higher and lower altitudes. This may have led to errors, especially for the higher stations;
- (3)
- Both estuaries have been sampled after dry periods, with exceptionally low runoff volumes in the 30 days before sampling. Consequently, our RD/CS values (river discharge divided by the estuarine channel cross-area) are almost zero, and do not play a role in the calculation of our MII. As shown by Jorissen et al. [40], this will be different in different seasons;
- (4)
- The scaling factors α, β and γ, contributing the three factors making up the MII (distance along the salinity gradient, emergence time, relative importance of river discharge), have not been determined yet. A much larger data set is needed to define these constants.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whitfield, A.; Elliott, M. Ecosystem and biotic classifications of estuaries and coasts. In Treatise on Estuarine and Coastal Science; Wolanski, E., Elliott, M., Eds.; Academic Press: Waltham, MA, USA, 2011; Volume 1, pp. 99–124. ISBN 978-0-08-087885-0. [Google Scholar]
- Day, J.W.; Kemp, W.M.; Yáñez-Arancibia, A.; Crump, B.C. Estuarine Ecology; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 978-1-118-39191-4. [Google Scholar]
- Cave, R.R.; Ledoux, L.; Turner, K.; Jickells, T.; Andrews, J.E.; Davies, H. The Humber Catchment and Its Coastal Area: From UK to European Perspectives. Sci. Total Environ. 2003, 314–316, 31–52. [Google Scholar] [CrossRef]
- Clark, B.M.; Bennett, B.A.; Lamberth, S.J. A Comparison of the Ichthyofauna of Two Estuaries and Their Adjacent Surf Zones, with an Assessment of the Effects of Beach-Seining on the Nursery Function of Estuaries for Fish. S. Afr. J. Mar. Sci. 1994, 14, 121–131. [Google Scholar] [CrossRef]
- Kennish, M.J. Environmental Threats and Environmental Future of Estuaries. Environ. Conserv. 2002, 29, 78–107. [Google Scholar] [CrossRef]
- Belzuncea, M.J.; Solauna, O.; Valenciaa, V.; Péreza, V. Contaminants in Estuarine and Coastal Waters. Forest 2004, 1, 850–893. [Google Scholar]
- Bald, J.; Borja, A.; Muxika, I.; Franco, J.; Valencia, V. Assessing Reference Conditions and Physico-Chemical Status According to the European Water Framework Directive: A Case-Study from the Basque Country (Northern Spain). Mar. Pollut. Bull. 2005, 50, 1508–1522. [Google Scholar] [CrossRef] [PubMed]
- Council of the European Communities. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy. Off. J. Eur. Communities 2000, 327, 1–73. [Google Scholar]
- Hummel, H.; Frost, M.; Juanes, J.A.; Kochmann, J.; Bolde, C.F.C.P.; Aneiros, F.; Vandenbosch, F.; Franco, J.N.; Echavarri, B.; Guinda, X.; et al. A Comparison of the Degree of Implementation of Marine Biodiversity Indicators by European Countries in Relation to the Marine Strategy Framework Directive (MSFD). J. Mar. Biol. Assoc. UK 2015, 95, 1519–1531. [Google Scholar] [CrossRef] [Green Version]
- Murray, J.W. Ecology and Applications of Benthic Foraminifera; Cambridge University Press: Cambridge, UK, 2006; ISBN 978-0-521-82839-0. [Google Scholar]
- Albani, A.; Barbero, R.S.; Donnici, S. Foraminifera as Ecological Indicators in the Lagoon of Venice, Italy. Ecol. Indic. 2007, 7, 239–253. [Google Scholar] [CrossRef]
- WoRMS—World Register of Marine Species. Available online: http://www.marinespecies.org/aphia.php?p=image&pic=76439&tid=114064 (accessed on 17 November 2020).
- Dolven, J.K.; Alve, E.; Rygg, B.; Magnusson, J. Defining Past Ecological Status and in Situ Reference Conditions Using Benthic Foraminifera: A Case Study from the Oslofjord, Norway. Ecol. Indic. 2013, 29, 219–233. [Google Scholar] [CrossRef]
- Alve, E.; Hess, S.; Bouchet, V.M.P.; Dolven, J.K.; Rygg, B. Intercalibration of Benthic Foraminiferal and Macrofaunal Biotic Indices: An Example from the Norwegian Skagerrak Coast (NE North Sea). Ecol. Indic. 2019, 96, 107–115. [Google Scholar] [CrossRef]
- Bouchet, V.M.P.; Alve, E.; Rygg, B.; Telford, R.J. Benthic Foraminifera Provide a Promising Tool for Ecological Quality Assessment of Marine Waters. Ecol. Indic. 2012, 23, 66–75. [Google Scholar] [CrossRef]
- Barras, C.; Jorissen, F.J.; Labrune, C.; Andral, B.; Boissery, P. Live Benthic Foraminiferal Faunas from the French Mediterranean Coast: Towards a New Biotic Index of Environmental Quality. Ecol. Indic. 2014, 36, 719–743. [Google Scholar] [CrossRef] [Green Version]
- Alve, E.; Korsun, S.; Schönfeld, J.; Dijkstra, N.; Golikova, E.; Hess, S.; Husum, K.; Panieri, G. Foram-AMBI: A Sensitivity Index Based on Benthic Foraminiferal Faunas from North-East Atlantic and Arctic Fjords, Continental Shelves and Slopes. Mar. Micropaleontol. 2016, 122, 1–12. [Google Scholar] [CrossRef]
- Dimiza, M.D.; Triantaphyllou, M.V.; Koukousioura, O.; Hallock, P.; Simboura, N.; Karageorgis, A.P.; Papathanasiou, E. The Foram Stress Index: A New Tool for Environmental Assessment of Soft-Bottom Environments Using Benthic Foraminifera. A Case Study from the Saronikos Gulf, Greece, Eastern Mediterranean. Ecol. Indic. 2016, 60, 611–621. [Google Scholar] [CrossRef]
- Jorissen, F.; Nardelli, M.P.; Almogi-Labin, A.; Barras, C.; Bergamin, L.; Bicchi, E.; El Kateb, A.; Ferraro, L.; McGann, M.; Morigi, C.; et al. Developing Foram-AMBI for Biomonitoring in the Mediterranean: Species Assignments to Ecological Categories. Mar. Micropaleontol. 2018, 140, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Elliott, M.; Quintino, V. The Estuarine Quality Paradox, Environmental Homeostasis and the Difficulty of Detecting Anthropogenic Stress in Naturally Stressed Areas. Mar. Pollut. Bull. 2007, 54, 640–645. [Google Scholar] [CrossRef]
- Dauvin, J.-C. Paradox of Estuarine Quality: Benthic Indicators and Indices, Consensus or Debate for the Future. Mar. Pollut. Bull. 2007, 55, 271–281. [Google Scholar] [CrossRef]
- Elliott, M.; Whitfield, A.K. Challenging Paradigms in Estuarine Ecology and Management. Estuar. Coast. Shelf Sci. 2011, 94, 306–314. [Google Scholar] [CrossRef]
- Tweedley, J.R.; Warwick, R.M.; Potter, I.C. Can Biotic Indicators Distinguish between Natural and Anthropogenic Environmental Stress in Estuaries? J. Sea Res. 2015, 102, 10–21. [Google Scholar] [CrossRef] [Green Version]
- Bouchet, V.M.P.; Telford, R.J.; Rygg, B.; Oug, E.; Alve, E. Can Benthic Foraminifera Serve as Proxies for Changes in Benthic Macrofaunal Community Structure? Implications for the Definition of Reference Conditions. Mar. Environ. Res. 2018, 137, 24–36. [Google Scholar] [CrossRef] [Green Version]
- Bouchet, V.M.P.; Frontalini, F.; Francescangeli, F.; Sauriau, P.-G.; Geslin, E.; Martins, M.V.A.; Almogi-Labin, A.; Avnaim-Katav, S.; di Bella, L.; Cearreta, A.; et al. Indicative Value of Benthic Foraminifera for Biomonitoring: Assignment to Ecological Groups of Sensitivity to Total Organic Carbon of Species from European Intertidal Areas and Transitional Waters. Mar. Pollut. Bull. 2021, 164, 112071. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.B.; Schafer, C.T.; Medioli, F.S. Eastern Canadian Estuarine Foraminifera; a Framework for Comparison. J. Foraminifer. Res. 1980, 10, 205–234. [Google Scholar] [CrossRef]
- Cearreta, A. Population dynamics of benthic foraminifera in the Santona estuary, Spain. Rev. Paléobiologie 1988, 2, 721–724. [Google Scholar]
- De Rijk, S. Salinity Control on the Distribution of Salt Marsh Foraminifera (Great Marshes, Massachusetts). J. Foraminifer. Res. 1995, 25, 156–166. [Google Scholar] [CrossRef]
- Horton, B.P.; Murray, J.W. The Roles of Elevation and Salinity as Primary Controls on Living Foraminiferal Distributions: Cowpen Marsh, Tees Estuary, UK. Mar. Micropaleontol. 2007, 63, 169–186. [Google Scholar] [CrossRef]
- Hayward, B.W.; Hollis, C.J. Brackish Foraminifera in New Zealand; a Taxonomic and Ecologic Review. Micropaleontology 1994, 40, 185–222. [Google Scholar] [CrossRef]
- Armynot du Châtelet, E.; Francescangeli, F.; Bouchet, V.M.P.; Frontalini, F. Benthic Foraminifera in Transitional Environments in the English Channel and the Southern North Sea: A Proxy for Regional-Scale Environmental and Paleo-Environmental Characterisations. Mar. Environ. Res. 2018, 137, 37–48. [Google Scholar] [CrossRef]
- Armynot du Châtelet, É.; Bout-Roumazeilles, V.; Riboulleau, A.; Trentesaux, A. Sediment (Grain Size and Clay Mineralogy) and Organic Matter Quality Control on Living Benthic Foraminifera. Rev. Micropaléontologie 2009, 52, 75–84. [Google Scholar] [CrossRef]
- Mojtahid, M.; Geslin, E.; Coynel, A.; Gorse, L.; Vella, C.; Davranche, A.; Zozzolo, L.; Blanchet, L.; Bénéteau, E.; Maillet, G. Spatial Distribution of Living (Rose Bengal Stained) Benthic Foraminifera in the Loire Estuary (Western France). J. Sea Res. 2016, 118, 1–16. [Google Scholar] [CrossRef]
- Alve, E. Benthic Foraminifera in Sediment Cores Reflecting Heavy Metal Pollution in Sorfjord, Western Norway. J. Foraminifer. Res. 1991, 21, 1–19. [Google Scholar] [CrossRef]
- Armynot du Châtelet, E.; Debenay, J.-P.; Soulard, R. Foraminiferal Proxies for Pollution Monitoring in Moderately Polluted Harbors. Environ. Pollut. 2004, 127, 27–40. [Google Scholar] [CrossRef]
- Frontalini, F.; Buosi, C.; da Pelo, S.; Coccioni, R.; Cherchi, A.; Bucci, C. Benthic Foraminifera as Bio-Indicators of Trace Element Pollution in the Heavily Contaminated Santa Gilla Lagoon (Cagliari, Italy). Mar. Pollut. Bull. 2009, 58, 858–877. [Google Scholar] [CrossRef] [PubMed]
- Eichler, P.P.B.; Eichler, B.B.; Gupta, B.S.; Rodrigues, A.R. Foraminifera as Indicators of Marine Pollutant Contamination on the Inner Continental Shelf of Southern Brazil. Mar. Pollut. Bull. 2012, 64, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Tyson, R.V.; Pearson, T.H. Modern and Ancient Continental Shelf Anoxia: An Overview. Geol. Soc. Lond. Spec. Publ. 1991, 58, 1–24. [Google Scholar] [CrossRef]
- Romano, E.; Bergamin, L.; Ausili, A.; Pierfranceschi, G.; Maggi, C.; Sesta, G.; Gabellini, M. The Impact of the Bagnoli Industrial Site (Naples, Italy) on Sea-Bottom Environment. Chemical and Textural Features of Sediments and the Related Response of Benthic Foraminifera. Mar. Pollut. Bull. 2009, 59, 245–256. [Google Scholar] [CrossRef]
- Jorissen, F.J.; Fouet, M.P.A.; Singer, D.; Howa, H. MII: An Index to Quantify Marine Influence on Estuarine Intertidal Mudflat Environments for the Purpose of Foraminiferal Biomonitoring. Water, in press.
- Office Français De La Biodiversité Découvrir Les Estuaires de La Façade Manche/Atlantique | Le Portail Technique De l’OFB. Available online: https://professionnels.ofb.fr/fr/node/276 (accessed on 23 September 2021).
- Banque Hydro Ministère De L’Ecologie, du Développement Durable Et De L’Energie. Available online: http://hydro.eaufrance.fr/indexd.php (accessed on 5 October 2021).
- Envlit Bassin Loire-Bretagne. Available online: https://wwz.ifremer.fr/envlit/DCE/La-DCE-par-bassin/Bassin-Loire-Bretagne (accessed on 23 September 2021).
- Schönfeld, J.; Alve, E.; Geslin, E.; Jorissen, F.; Korsun, S.; Spezzaferri, S. The FOBIMO (FOraminiferal BIo-MOnitoring) Initiative—Towards a Standardised Protocol for Soft-Bottom Benthic Foraminiferal Monitoring Studies. Mar. Micropaleontol. 2012, 94–95, 1–13. [Google Scholar] [CrossRef]
- SHOM. Horaires De Marées Gratuits Du SHOM. Available online: https://maree.shom.fr/ (accessed on 23 September 2021).
- Vail, C.A.; Walker, A.K. Vertical Zonation of Some Crustose Lichens (Verrucariaceae) in Bay of Fundy Littoral Zones of Nova Scotia. Nena 2021, 28, 311–326. [Google Scholar] [CrossRef]
- Diz, P.; Jorissen, F.J.; Reichart, G.J.; Poulain, C.; Dehairs, F.; Leorri, E.; Paulet, Y.-M. Interpretation of Benthic Foraminiferal Stable Isotopes in Subtidal Estuarine Environments. Biogeosciences 2009, 6, 7453–7480. [Google Scholar] [CrossRef] [Green Version]
- Attrill, M.J. A Testable Linear Model for Diversity Trends in Estuaries. J. Anim. Ecol. 2002, 71, 262–269. [Google Scholar] [CrossRef] [Green Version]
- Farrell, K.M.; Harris, W.B.; Mallinson, D.J.; Culver, S.J.; Riggs, S.R.; Pierson, J.; Self-Trail, J.M.; Lautier, J.C. Standardizing Texture and Facies Codes for A Process-Based Classification of Clastic Sediment and RockSTANDARDIZING TEXTURE. J. Sediment. Res. 2012, 82, 364–378. [Google Scholar] [CrossRef]
- Coynel, A.; Gorse, L.; Curti, C.; Schafer, J.; Grosbois, C.; Morelli, G.; Ducassou, E.; Blanc, G.; Maillet, G.M.; Mojtahid, M. Spatial Distribution of Trace Elements in the Surface Sediments of a Major European Estuary (Loire Estuary, France): Source Identification and Evaluation of Anthropogenic Contribution. J. Sea Res. 2016, 118, 77–91. [Google Scholar] [CrossRef]
- Coynel, A.; Schäfer, J.; Blanc, G.; Bossy, C. Scenario of Particulate Trace Metal and Metalloid Transport during a Major Flood Event Inferred from Transient Geochemical Signals. Appl. Geochem. 2007, 22, 821–836. [Google Scholar] [CrossRef]
- Larrose, A.; Coynel, A.; Schäfer, J.; Blanc, G.; Massé, L.; Maneux, E. Assessing the Current State of the Gironde Estuary by Mapping Priority Contaminant Distribution and Risk Potential in Surface Sediment. Appl. Geochem. 2010, 25, 1912–1923. [Google Scholar] [CrossRef]
- Mason, R.P. Trace Metals in Aquatic Systems: Mason/Trace Metals in Aquatic Systems; John Wiley & Sons, Ltd: Chichester, UK, 2013; ISBN 978-1-118-27457-6. [Google Scholar]
- Audry, S.; Schäfer, J.; Blanc, G.; Jouanneau, J.-M. Fifty-Year Sedimentary Record of Heavy Metal Pollution (Cd, Zn, Cu, Pb) in the Lot River Reservoirs (France). Environ. Pollut. 2004, 132, 413–426. [Google Scholar] [CrossRef]
- Grosbois, C.; Meybeck, M.; Lestel, L.; Lefèvre, I.; Moatar, F. Severe and Contrasted Polymetallic Contamination Patterns (1900–2009) in the Loire River Sediments (France). Sci. Total Environ. 2012, 435, 290–305. [Google Scholar] [CrossRef]
- Dendievel, A.-M.; Mourier, B.; Coynel, A.; Evrard, O.; Labadie, P.; Ayrault, S.; Debret, M.; Koltalo, F.; Copard, Y.; Faivre, Q.; et al. Spatio-Temporal Assessment of the Polychlorinated Biphenyl (PCB) Sediment Contamination in Four Major French River Corridors (1945–2018). Earth Syst. Sci. Data 2020, 12, 1153–1170. [Google Scholar] [CrossRef]
- Gardes, T.; Debret, M.; Copard, Y.; Patault, E.; Winiarski, T.; Develle, A.-L.; Sabatier, P.; Dendievel, A.-M.; Mourier, B.; Marcotte, S.; et al. Reconstruction of Anthropogenic Activities in Legacy Sediments from the Eure River, a Major Tributary of the Seine Estuary (France). Catena 2020, 190, 104513. [Google Scholar] [CrossRef]
- Perez-Belmonte, L. Caractérisation Environnementale, Morphosédimentaire Et Stratigraphique Du Golfe Du Morbihan Pendant L’Holocène Terminal: Implications Évolutives. Doctoral Thesis, Université de Bretagne Sud, Lorient, France, 2008. [Google Scholar]
- Dubois, S.; Savoye, N.; Grémare, A.; Plus, M.; Charlier, K.; Beltoise, A.; Blanchet, H. Origin and Composition of Sediment Organic Matter in a Coastal Semi-Enclosed Ecosystem: An Elemental and Isotopic Study at the Ecosystem Space Scale. J. Mar. Syst. 2012, 94, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Somerfield, P.J.; Warwick, R.M. Meiofauna Techniques. In Methods for the Study of Marine Benthos; Eleftheriou, A., Ed.; John Wiley & Sons, Ltd: Oxford, UK, 2013; pp. 253–284. ISBN 978-1-118-54239-2. [Google Scholar]
- Burgess, R. An Improved Protocol for Separating Meiofauna from Sediments Using Colloidal Silica Sols. Mar. Ecol. Prog. Ser. 2001, 214, 161–165. [Google Scholar] [CrossRef]
- Parent, B.; Barras, C.; Jorissen, F. An Optimised Method to Concentrate Living (Rose Bengal-Stained) Benthic Foraminifera from Sandy Sediments by High Density Liquids. Mar. Micropaleontol. 2018, 144, 1–13. [Google Scholar] [CrossRef]
- Charrieau, L.M.; Bryngemark, L.; Hansson, I.; Filipsson, H.L. Improved Wet Splitter for Micropalaeontological Analysis, and Assessment of Uncertainty Using Data from Splitters. J. Micropalaeontol. 2018, 37, 191–194. [Google Scholar] [CrossRef] [Green Version]
- Patterson, R.T.; Fishbein, E. Re-Examination of the Statistical Methods Used to Determine the Number of Point Counts Needed for Micropaleontological Quantitative Research. J. Paleontol. 1989, 63, 245–248. [Google Scholar] [CrossRef]
- Fatela, F.; Taborda, R. Confidence Limits of Species Proportions in Microfossil Assemblages. Mar. Micropaleontol. 2002, 45, 169–174. [Google Scholar] [CrossRef]
- Murray, J.W. British Nearshore Foraminiferids; Published for the Linnean Society of London and the Estuarine and Brackish-water Sciences Association; Academic Press: London, UK, 1979; ISBN 9780125118507. [Google Scholar]
- Hansen, H.J.; Lykke-Andersen, A.L. Wall Structure and Classification of Fossil and Recent Elphidiid and Nonionid Foraminifera. Foss. Strat. 1976, 10, 1–37. [Google Scholar]
- Feyling-Hanssen, R.W. The Foraminifer Elphidium Excavatum (Terquem) and Its Variant Forms. Micropaleontology 1972, 18, 337–354. [Google Scholar] [CrossRef]
- Scott, D.B.; Medioli, F.S. Quantitative Studies of Marsh Foraminiferal Distributions in Nova Scotia: Implications for Sea Level Studies. Spec. Publ Cushman Found. Foram. 1980, 17, 58. [Google Scholar]
- Camacho, S.; Moura, D.; Connor, S.; Scott, D.; Boski, T. Taxonomy, Ecology and Biogeographical Trends of Dominant Benthic Foraminifera Species from an Atlantic-Mediterranean Estuary (the Guadiana, Southeast Portugal). Palaeontol. Electron. 2015, 18, 1–27. [Google Scholar] [CrossRef]
- Schweizer, M.; Polovodova, I.; Nikulina, A.; Schönfeld, J. Molecular Identification of Ammonia and Elphidium Species (Foraminifera, Rotaliida) from the Kiel Fjord (SW Baltic Sea) with RDNA Sequences. Helgol. Mar. Res. 2011, 65, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Pillet, L.; Voltski, I.; Korsun, S.; Pawlowski, J. Molecular Phylogeny of Elphidiidae (Foraminifera). Mar. Micropaleontol. 2013, 103, 1–14. [Google Scholar] [CrossRef]
- Darling, K.F.; Schweizer, M.; Knudsen, K.L.; Evans, K.M.; Bird, C.; Roberts, A.; Filipsson, H.L.; Kim, J.-H.; Gudmundsson, G.; Wade, C.M.; et al. The Genetic Diversity, Phylogeography and Morphology of Elphidiidae (Foraminifera) in the Northeast Atlantic. Mar. Micropaleontol. 2016, 129, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Hayward, B.W.; Holzmann, M.; Grenfell, H.R.; Pawlowski, J.; Triggs, C.M. Morphological Distinction of Molecular Types in Ammonia—Towards a Taxonomic Revision of the World’s Most Commonly Misidentified Foraminifera. Mar. Micropaleontol. 2004, 50, 237–271. [Google Scholar] [CrossRef]
- Richirt, J.; Schweizer, M.; Bouchet, V.M.P.; Mouret, A.; Quinchard, S.; Jorissen, F.J. Morphological Distinction of Three Ammonia Phylotypes Occurring Along European Coasts. J. Foraminifer. Res. 2019, 49, 76–93. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Package “Vegan”. Community Ecology Package, R Package Version 2.0. Available online: https://www.researchgate.net/publication/258996451_Vegan_Community_Ecology_Package_R_Package_Version_20-10 (accessed on 5 October 2021).
- Dubois, S.; Jean-Louis, B.; Bertrand, B.; Lefebvre, S. Isotope Trophic-Step Fractionation of Suspension-Feeding Species: Implications for Food Partitioning in Coastal Ecosystems. J. Exp. Mar. Biol. Ecol. 2007, 351, 121–128. [Google Scholar] [CrossRef]
- Dan, S.F.; Liu, S.-M.; Yang, B.; Udoh, E.C.; Umoh, U.; Ewa-Oboho, I. Geochemical Discrimination of Bulk Organic Matter in Surface Sediments of the Cross River Estuary System and Adjacent Shelf, South East Nigeria (West Africa). Sci. Total Environ. 2019, 678, 351–368. [Google Scholar] [CrossRef]
- Riera, P.; Richard, P.; Gremare, A.; Blanchard, G. Food Source of Intertidal Nematodes in the Bay of Marennes-Oleron (France), as Determined by Dual Stable Isotope Analysis. Oceanogr. Lit. Rev. 1997, 4, 361. [Google Scholar] [CrossRef] [Green Version]
- Castignetti, P. A Time-Series Study of Foraminiferal Assemblages of the Plym Estuary, South-West England. J. Mar. Biol. Assoc. UK 1996, 76, 569–578. [Google Scholar] [CrossRef]
- Alve, E.; Murray, J.W. Ecology and Taphonomy of Benthic Foraminifera in a Temperate Mesotidal Inlet. J. Foraminifer. Res. 1994, 24, 18–27. [Google Scholar] [CrossRef]
- Francescangeli, F.; Portela, M.; Armynot du Chatelet, E.; Billon, G.; Andersen, T.J.; Bouchet, V.M.P.; Trentesaux, A. Infilling of the Canche Estuary (Eastern English Channel, France): Insight from Benthic Foraminifera and Historical Pictures. Mar. Micropaleontol. 2018, 142, 1–12. [Google Scholar] [CrossRef]
- Bradshaw, J.S. Environmental Parameters and Marsh Foraminifera. Limnol. Oceanogr. 1968, 13, 26–38. [Google Scholar] [CrossRef]
- Ellison, R.L.; Murray, J.W. Geographical Variation in the Distribution of Certain Agglutinated Foraminifera along the North Atlantic Margins. J. Foraminifer. Res. 1987, 17, 123–131. [Google Scholar] [CrossRef]
- Debenay, J.-P.; Guillou, J.-J. Ecological Transitions Indicated by Foraminiferal Assemblages in Paralic Environments. Estuaries 2002, 25, 1107–1120. [Google Scholar] [CrossRef]
- Redois, F.; Rebois, F.; Debenay, J.-P. Les Foraminifères Benthiques Actuels Bioindicateurs Du Milieu Marin Exemples Du Plateau Continental Sénégalais Et De L’estran Du Golfe Du Morbihan (France). Ph.D. Dissertation, Université d’Angers, Angers, France, 1996. [Google Scholar]
- Debenay, J.P.; Carbonel, P.; Morzadec-Kerfourn, M.-T.; Cazaubon, A.; Denèfle, M.; Lézine, A.-M. Multi-Bioindicator Study of a Small Estuary in Vendée (France). Estuar. Coast. Shelf Sci. 2003, 58, 843–860. [Google Scholar] [CrossRef]
- Debenay, J.-P.; Bicchi, E.; Goubert, E.; Armynot du Châtelet, E. Spatio-Temporal Distribution of Benthic Foraminifera in Relation to Estuarine Dynamics (Vie Estuary, Vendée, W France). Estuar. Coast. Shelf Sci. 2006, 67, 181–197. [Google Scholar] [CrossRef]
- Martins, V.A.; Frontalini, F.; Tramonte, K.M.; Figueira, R.C.L.; Miranda, P.; Sequeira, C.; Fernández-Fernández, S.; Dias, J.A.; Yamashita, C.; Renó, R.; et al. Assessment of the Health Quality of Ria de Aveiro (Portugal): Heavy Metals and Benthic Foraminifera. Mar. Pollut. Bull. 2013, 70, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.W. Population Dynamics of Benthic Foraminifera; Results from the Exe Estuary, England. J. Foraminifer. Research 1983, 13, 1–12. [Google Scholar] [CrossRef]
- Horton, B.P. The Distribution of Contemporary Intertidal Foraminifera at Cowpen Marsh, Tees Estuary, UK: Implications for Studies of Holocene Sea-Level Changes. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1999, 149, 127–149. [Google Scholar] [CrossRef]
- Murray, J.W.; Alve, E. Major Aspects of Foraminiferal Variability (Standing Crop and Biomass) on a Monthly Scale in an Intertidal Zone. J. Foraminifer. Res. 2000, 30, 177–191. [Google Scholar] [CrossRef]
- Martins, M.V.A.; Silva, F.; Laut, L.L.M.; Frontalini, F.; Clemente, I.M.M.M.; Miranda, P.; Figueira, R.; Sousa, S.H.M.; Dias, J.M.A. Response of Benthic Foraminifera to Organic Matter Quantity and Quality and Bioavailable Concentrations of Metals in Aveiro Lagoon (Portugal). PLoS ONE 2015, 10, e0118077. [Google Scholar] [CrossRef] [Green Version]
- Buzas-Stephens, P.; Buzas, M.A.; Price, J.D.; Courtney, C.H. Benthic Superheroes: Living Foraminifera from Three Bays in the Mission-Aransas National Estuarine Research Reserve, USA. Estuaries Coasts 2018, 41, 2368–2377. [Google Scholar] [CrossRef]
- Bouchet, V.M.P.; Goberville, E.; Frontalini, F. Benthic Foraminifera to Assess Ecological Quality Statuses in Italian Transitional Waters. Ecol. Indic. 2018, 84, 130–139. [Google Scholar] [CrossRef]
- Borja, A.; Franco, J.; Pérez, V. A Marine Biotic Index to Establish the Ecological Quality of Soft-Bottom Benthos Within European Estuarine and Coastal Environments. Mar. Pollut. Bull. 2000, 40, 1100–1114. [Google Scholar] [CrossRef]
- Debenay, J.-P.; Guillou, J.-J.; Redois, F.; Geslin, E. Distribution Trends of Foraminiferal Assemblages in Paralic Environments. In Environmental Micropaleontology: The Application of Microfossils to Environmental Geology; Martin, R.E., Ed.; Topics in Geobiology; Springer: Boston, MA, USA, 2000; pp. 39–67. ISBN 978-1-4615-4167-7. [Google Scholar]
- Bouchet, V.M.P.; Debenay, J.-P.; Sauriau, P.-G.; Radford-Knoery, J.; Soletchnik, P. Effects of Short-Term Environmental Disturbances on Living Benthic Foraminifera during the Pacific Oyster Summer Mortality in the Marennes-Oléron Bay (France). Mar. Environ. Res. 2007, 64, 358–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akers, W.H. Estuarine Foraminiferal Associations of the Beaufort Area, North Carolina. Tulane Stud. Geol. Paleontol. 1971, 8, 147–165. [Google Scholar]
- Poag, C.W. Paired Foraminiferal Ecophenotypes in Gulf Coast Estuaries: Ecological and Paleoecological Implications. Gulf Coast Association of Geological Societies Transactions. 1978, 28, 395–421. [Google Scholar]
- Ellison, R.L. Ammobaculites, Foraminiferal Proprietor of Chesapeake Bay Estuaries. Environ. Framew. Coast. Plain Estuaries 1972, 133, 247. [Google Scholar]






| Estuary | Station | Species Richness | Shannon | Exp(H’BC) |
|---|---|---|---|---|
| Auray | 1A | 8.0 (±2.0) | 0.68 (±0.16) | 2.01 (±0.31) |
| 1B | 10.7 (±1.2) | 1.44 (±0.08) | 4.22 (±0.30) | |
| 1C | 12.7 (±2.5) | 1.65 (±0.13) | 5.23 (±0.72) | |
| 2A | 2.0 (±1.7) | 0.15 (±0.28) | 1.37 (±0.63) | |
| 2B | 4.3 (±4.9) | 0.40 (±0.53) | 1.80 (±1.02) | |
| 2C | 8.0 (±1.0) | 0.80 (±0.16) | 2.26 (±0.33) | |
| 4A | 8.7 (±1.5) | 0.64 (±0.26) | 1.95 (±0.48) | |
| 4B | 8.0 (±1.0) | 0.62 (±0.17) | 1.92 (±0.31) | |
| 5A | 5.0 (±1.7) | 0.12 (±0.03) | 1.14 (±0.05) | |
| 5B | 11.7 (±2.3) | 0.86 (±0.24) | 2.49 (±0.57) | |
| 6A | 7.3 (±0.6) | 0.30 (±0.17) | 1.37 (±0.24) | |
| 6B | 11.7 (±0.6) | 0.73 (±0.26) | 2.14 (±0.54) | |
| 7 | 5.7 (±1.2) | 0.69 (±0.04) | 2.02 (±0.09) | |
| 8A | 8.3 (±1.2) | 1.27 (±0.14) | 3.73 (±0.34) | |
| 8B | 5.3 (±2.3) | 0.68 (±0.29) | 2.07 (±0.56) | |
| Vie | 3 | 14.3 (±1.5) | 1.14 (±0.20) | 3.17 (±0.27) |
| 4 | 12.3 (±2.5) | 1.09 (±0.35) | 3.10 (±1.07) | |
| 5 | 9.3 (±2.3) | 0.87 (±0.04) | 2.45 (±0.30) | |
| 6 | 9.0 (±2.6) | 0.68 (±0.17) | 2.00 (±0.31) | |
| 7A | 8.3 (±1.2) | 0.33 (±0.01) | 1.40 (±0.01) | |
| 7B | 8.0 (±0.0) | 0.40 (±0.11) | 1.50 (±0.15) | |
| 7C | 10.7 (±0.6) | 0.99 (±0.16) | 2.72 (±0.41) | |
| 8A | 9.7 (±0.6) | 1.19 (±0.07) | 3.30 (±0.19) | |
| 8B | 7.3 (±0.6) | 0.65 (±0.11) | 1.92 (±0.20) | |
| 9 | 9.7 (±0.6) | 1.20 (±0.12) | 3.33 (±0.06) | |
| 10A | 8.7 (±0.6) | 1.03 (±0.10) | 2.84 (±0.34) | |
| 10B | 7.3 (±0.6) | 0.71 (±0.22) | 2.05 (±0.15) |
| PCA1 | PCA2 | PCA3 | R2 | p-Value | |
|---|---|---|---|---|---|
| % Corg | 0.37 | 0.78 | −0.51 | 0.15 | 0.29 |
| <63 µm grain size | 0.88 | 0.26 | 0.40 | 0.30 | 0.05 * |
| Distance to the sea | −0.13 | 0.98 | 0.12 | 0.46 | 0.001 * |
| Emergence time | −0.96 | −0.15 | 0.24 | 0.16 | 0.24 |
| Auray | Station | Foram-AMBI | Ecological Quality Status | Vie | Station | Foram-AMBI | Ecological Quality Status |
| 1A | 2.92 ± 0.01 | Good | 3 | 1.49 ± 0.14 | Good | ||
| 1B | 2.83 ± 0.05 | Good | 4 | 0.95 ± 0.51 | High | ||
| 1C | 2.56 ± 0.10 | Good | 5 | 1.04 ± 0.04 | High | ||
| 2A | 2.97 ± 0.05 | Good | 6 | 2.68 ± 0.12 | Good | ||
| 2B | 2.90 ± 0.09 | Good | 7A | 3.00 ± 0.01 | Good | ||
| 2C | 3.01 ± 0.06 | Good | 7B | 2.94 ± 0.03 | Good | ||
| 4A | 2.95 ± 0.20 | Good | 7C | 2.75 ± 0.16 | Good | ||
| 4B | 2.98 ± 0.00 | Good | 8A | 2.60 ± 0.04 | Good | ||
| 5A | 3.02 ± 0.02 | Good | 8B | 3.03 ± 0.03 | Good | ||
| 5B | 2.95 ± 0.06 | Good | 9 | 2.77 ± 0.08 | Good | ||
| 6A | 3.00 ± 0.03 | Good | 10A | 2.93 ± 0.05 | Good | ||
| 6B | 2.94 ± 0.02 | Good | 10B | 2.99 ± 0.00 | Good | ||
| 7 | 2.93 ± 0.06 | Good | |||||
| 8A | 2.57 ± 0.16 | Good | |||||
| 8B | 1.41 ± 0.07 | Good |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fouet, M.P.A.; Singer, D.; Coynel, A.; Héliot, S.; Howa, H.; Lalande, J.; Mouret, A.; Schweizer, M.; Tcherkez, G.; Jorissen, F.J. Foraminiferal Distribution in Two Estuarine Intertidal Mudflats of the French Atlantic Coast: Testing the Marine Influence Index. Water 2022, 14, 645. https://doi.org/10.3390/w14040645
Fouet MPA, Singer D, Coynel A, Héliot S, Howa H, Lalande J, Mouret A, Schweizer M, Tcherkez G, Jorissen FJ. Foraminiferal Distribution in Two Estuarine Intertidal Mudflats of the French Atlantic Coast: Testing the Marine Influence Index. Water. 2022; 14(4):645. https://doi.org/10.3390/w14040645
Chicago/Turabian StyleFouet, Marie P. A., David Singer, Alexandra Coynel, Swann Héliot, Hélène Howa, Julie Lalande, Aurélia Mouret, Magali Schweizer, Guillaume Tcherkez, and Frans J. Jorissen. 2022. "Foraminiferal Distribution in Two Estuarine Intertidal Mudflats of the French Atlantic Coast: Testing the Marine Influence Index" Water 14, no. 4: 645. https://doi.org/10.3390/w14040645
APA StyleFouet, M. P. A., Singer, D., Coynel, A., Héliot, S., Howa, H., Lalande, J., Mouret, A., Schweizer, M., Tcherkez, G., & Jorissen, F. J. (2022). Foraminiferal Distribution in Two Estuarine Intertidal Mudflats of the French Atlantic Coast: Testing the Marine Influence Index. Water, 14(4), 645. https://doi.org/10.3390/w14040645







