Abstract
Bioremediation of contaminated solutions has attracted extensive attention in recent years due to its wide range of applicability to various types of contaminants and environmental friendliness. Previous studies adequately confirmed the potential of Sporosarcina pasteurii (i.e., S. pasteurii)-based bioremediation for heavy metal contaminated solutions, but they focused mainly on the bioremediation ability of single-heavy-metal contaminated solutions. This study focuses on S. pasteurii-based bioremediation under more complex pollution conditions. A series of laboratory experiments were performed to explore the efficiency and mechanism of S. pasteurii-based bioremediation to heavy metal contaminated solutions under various conditions, including single-heavy-metal pollution condition, heavy metal pollution under high mineral salinity context and multi-heavy-metal pollution scenarios. The results show that S. pasteurii can effectively remove heavy metals such as Cd, Cr(III), and Zn through biomineralization; for the typical range of mineral salinity (including NaCl and KCl) possibly encountered in practice in some contaminated solutions, such as leachate of landfills, the detrimental influence of high mineral salinity on efficiency of S. pasteurii-based bioremediation can be neglected; more importantly, S. pasteurii-based bioremediation can be considered as a potential option for remedying multi-heavy-metal contaminated solutions, though the addition of some heavy metals tends to produce a substantially detrimental influence on the bioremediation ability of S. pasteurii to other heavy metals.
1. Introduction
Plenty of industrial activities (such as metal plating facilities, fertilizer industries, mining operations, batteries, pesticides, tanneries, and paper industries, etc.) have caused water pollution by heavy metals to become a severe problem on a global scale [1,2,3]. Heavy metals such as Cd, Zn, Ni, Cr and Cu are highly toxic. They can endanger human safety through the food chain [4], damage ecosystems by affecting biological and biochemical properties and components [5], and cause considerable economic losses [6]. Many studies have reported that the concentrations of these heavy metals exceed the control criterion specified in relevant technical standards/specifications [7,8,9,10]. Moreover, the pollution degree generally varies substantially in different contaminated sites and the pollution situations/conditions tend to be more and more complex. For instance, soluble mineral salts (e.g., potassium and sodium salts) possibly co-exist together with heavy metals in some contaminated environments [11,12,13]; and a multi-heavy-metal pollution scenario, on the other hand, also becomes more common than the single heavy metal pollution case [2,3]. These complex situations/conditions should be taken into account when selecting and applying remediation technology for heavy metal contaminated solutions.
Existing studies have paid attention to the application capabilities of physical and chemical remediation technologies, including chemical precipitation, ion exchange, adsorption, membrane filtration technology, flotation, and electrochemical treatment techniques [14,15,16,17]. Although these treatment techniques can be employed to remove heavy metals, they may show some limitations in practice. For instance, chemical precipitation and ion exchange technologies are not economical and tend to produce a large amount of sludge or cause serious secondary pollution, while adsorption, membrane filtration, flotation and electrochemical treatment techniques seem to be relatively expensive [14]. Under this circumstance, bioremediation technology has attracted extensive attention in recent years [18,19,20,21,22,23]. It refers to a technology that changes the mobility of heavy metals by bioleaching, biosorption, biomineralization, bioaccumulation, and redox reactions. This technology can be a good potential option due to its wide range of applicability to various types of contaminants (via diverse bacterial species) and environmental friendliness [24,25,26]. In the past, microorganisms such as Sporosarcina pasteurii (i.e., S. pasteurii) [6], Kocuria flava CR1 [27], Exiguobacteium undae [28] and Terrabacter tumescens [29] have been isolated, and their potential for heavy metal bioremediation has been explored. Among these microorganisms, S. pasteurii seems to be able to show good performance in both urease activity and heavy metal tolerance [6,29].
Previously, S. pasteurii was used more commonly for the repair of concrete cracks [30] and cementation of granular soil [31,32,33]. In contrast, studies directly exploring the performance of S. pasteurii for bioremediation of heavy metal contaminated solutions still remain insufficient. Mugwar and Harbottle [34] studied the tolerance and removal rate of S. pasteurii to Zn, Cd, Cu, and Pb and found that S. pasteurii could effectively remove Cd (at 1.68–168 mg/L) but the remaining rate of Zn (or Cu) increases significantly when the initial concentration exceeds 130 mg/L (or 3.2 mg/L). Jalilvand et al. [6] found that S. pasteurii eliminate 98.71% of Pb (at concentration of 414 mg/L), 97.15% of Cd (at concentration of 224 mg/L), and 94.83% of Zn (at concentration of 130 mg/L). Moreover, Li et al. [29] found that the bioremediation rates of S. pasteurii to heavy metals (such as Ni, Cu, Pb, Zn, and Cd) can reach up to 88% at the concentration of 2000 mg/L. These studies have adequately confirmed the potential of S. pasteurii for the bioremediation of heavy metal contaminated solutions, but the obtained results differ with each other to some extent. Particularly, these studies focused merely on the bioremediation ability for the single-heavy-metal contaminated solution without any other complex conditions. Intuitively, complex conditions such as a high salinity context or multi-heavy-metal pollution scenarios may bring along new challenges or difficulties for S. pasteurii-based bioremediation.
Therefore, this study aims to explore the bioremediation potential of S. pasteurii under more complex conditions, including both the aforementioned high salinity context and a multi-heavy-metal pollution scenario. The bioremediation case for a single-heavy-metal contaminated solution was also covered in this study to provide a reference line. To be specific, the work can be divided into three parts. First, a series of laboratory experiments were performed for different types of single-heavy-metal contaminated solutions with various pollution levels to confirm the bioremediation capability and mechanism of S. pasteurii and to ascertain the influence of heavy metal concentration on the bioremediation efficiency. Second, the effort was made to evaluate the effect of one complex pollution condition, i.e., high mineral salinity context, on the bioremediation efficiency of S. pasteurii to various single-heavy-metal contaminated solutions. Third, particular attention was paid to the multi-heavy-metal pollution scenario to explore the bioremediation potential of S. pasteurii under this complex pollution condition and to compare the bioremediation efficiency of S. pasteurii for the single-heavy-metal contaminated solution and that for the multi-heavy-metal contaminated solution.
2. Materials and Methods
2.1. Bacteria and Culture Medium
Sporosarcina pasteurii (i.e., S. pasteurii, CGMCC 1.3687), a known urease-positive endospore-forming bacteria, was obtained from the China General Microbiological Culture Collection Centre. S. pasteurii was cultivated using the Bacillus pasteurii NH4-YE medium, including 20 g/L yeast extract, 10 g/L ammonium sulfate and 0.13 M Tris buffer (pH 9.0) [35]. Before inoculation, the nutrient broth was sterilized by autoclaving at 121 °C for 30 min. Then, the bacteria were inoculated into the sterilized medium and cultivated for 24 h at 30 °C. The OD600 value of the bacterial solution was about 2.0, and urease activity was 10 U/mL after 24 h.
2.2. Laboratory Experiments for Bioremediation
To explore the bioremediation efficiency of S. pasteurii to Cd, Cr(III), Zn, Ni, and Cu in different conditions, three groups of bioremediation experiments were carried out, including (a) the group for single-heavy-metal-contaminated solution (i.e., single-heavy-metal group), (b) the group for the heavy metal contaminated solution under high salinity context (i.e., high salinity group), and (c) the group for multi-heavy-metal-contaminated solution (i.e., multi-heavy-metal group). The details for the experimental cases covered in the three groups are provided in Table 1.

Table 1.
Program for laboratory experiments of bioremediation in this study.
In the single-heavy-metal group, five types of heavy metals (including Cd, Cr(III), Zn, Ni, and Cu), were considered separately. For each type of heavy metal, five different concentrations were covered to identify the influence of heavy metal concentration on the bioremediation efficiency. The case without adding any heavy metal (Case S0) was also included in this group as a reference. The concentrations of each heavy metal were formulated by considering the toxicity and pollution degree of heavy metals [36,37].
In the high salinity group, the bioremediation experiments were performed for four types of single heavy metal contaminated solutions with high salinity context, in which the concentration of mineral salt (NaCl + KCl) varies from (0 + 0) mg/L to (6400 + 6400) mg/L, to explore the effect of salt (i.e., NaCl and KCl) on the bioremediation efficiency of S. pasteurii. The concentration of mineral salt (NaCl + KCl) was considered to represent the typical range of salinity possibly encountered in practice in some contaminated solutions such as leachate of landfills [11,12].
In the multi-heavy-metal group, four combinations of heavy metals (i.e., four types of multi heavy metal contaminated solutions) were taken into account, including (Cd + Cr(III)), (Cd + Cr(III) + Zn), (Cd + Cr(III) + Ni) and (Cd + Cr(III) + Cu), and for each combination, five different pollution levels (with proportionally increased concentrations of heavy metals) were involved to gain an insight into the coupling effect of both heavy metal combination and pollution level.
For each experiment case, the contaminated solution was prepared by dissolving the prescribed amount of CdCl2·2.5H2O (CrCl3·6H2O, ZnCl2, NiCl2·6H2O, or CuCl2·2H2O) and mineral salt (NaCl + KCl) into the 1 M urea-CaCl2 amended solution. All agents were purchased from Wuhan Xinshenshi Chemical Technology Co., Ltd (Wuhan, China). Subsequently, the bioremediation experiment was carried out using a simple mixing method. A total of 50 mL of bacterial solution with urease activity of 10 U/mL was mixed with 50 mL of the mixture of the contaminated solutions, and the final concentrations of heavy metals and mineral salt are shown in Table 1. The bioremediation treatment was completed after 48 h. As a control group, the chemical precipitation test was carried out, and the test cases were the same as those of the single-heavy-metal group (as shown in Table 1). Slowly drop 0.1 M of sodium hydroxide (NaOH) solution into 30 mL of contaminated solutions and continuously measure the pH of the solution until the solution is neutral (pH 7 ± 0.1).
Some key performance indexes were measured in the experiments, including pH of supernatant, the concentrations of heavy metals and calcium ions, and the precipitate masses. The pH value was measured by the pH meter (pH-100A). The supernatant was filtered and diluted, and then the concentrations of heavy metals and calcium ions were measured by an atomic absorption spectrophotometer (AA-6880). The precipitate mass was measured by the subtraction method. The dry masses before and after precipitation were recorded as m0 and m48. Therefore, the mass of the precipitate can be determined as the difference between m48 and m0. Triplicate samples were prepared for each experiment case and the average value was used in the following to reduce the experimental error.
In addition, X-ray diffraction (XRD, model x’pert3 powder and manufactured in PANalytical B.V., Almelo, the Netherlands) analyses were also conducted to obtain the composition of the precipitates, which can facilitate an explanation of the bioremediation mechanism. Before the X-ray diffraction analyses, the collected precipitates were dried, milled, and sieved with a 0.1 mm fine sieve. During the XRD testing, the samples were scanned from 5° to 70° (2θ) and the testing results were processed using the software MDI Jade 6 (The International Centre for Diffraction Data, Newtown Square, PA, USA).
2.3. Key Indicators for Result Analyses
There are some common measurement parameters, including heavy metal concentration, calcium ion concentration, precipitation mass, and pH in the research of bioremediation. These parameters can reflect the changes in the chemical composition and biological metabolism of the system during the bioremediation process. The evolution of heavy metal concentration directly indicates the bioremediation effect. Previous studies have shown that as the bioremediation proceeds, urea is hydrolyzed, and carbonate precipitate is formed, leading to a decrease in the concentration of calcium and an increase in precipitation mass and pH in the solution system [6,34]. Therefore, these three parameters will be used in the following to represent the activity of S. pasteurii.
The percentage of insoluble heavy metals/bioremediation rate and calcium removal rate widely applied in references [6,27,29] were also adopted in this study for characterizing the changes in ion concentration. They can be determined via Equations (1) and (2) below:
where γ, η, α and β represent the percentage of insoluble heavy metals/bioremediation rate, the calcium removal rate, the concentration of heavy metal and the concentration of calcium, respectively; and the subscripts 0 and 48 stand for the initial state and the state after 48 h of bioremediation, respectively. Ignoring the interference of liquid withdrawal on the volume of the solution, γ and η can be calculated directly by the concentration of heavy metals and calcium. Besides, the measured values of heavy metal concentration at 0 h is close to the design values in Table 1, thus, the design values of each heavy metal are adopted for relevant statements in the following paragraphs to avoid confusion.
3. Results and Discussions
3.1. Chemical Precipitation of Single Heavy Metal
The pH of solution can increase in the process of microorganism induced carbonate precipitation, and it is a major factor affecting the morphology of heavy metals. Therefore, the experiment of chemical precipitation of heavy metals was carried out, and the pH values and the morphology change of heavy metals before and after adjusting the pH of solution were measured. Figure 1a shows the pH values of each heavy metal contaminated solutions before titration of sodium hydroxide (NaOH). The result shows that the pH values of all solutions are lower than 7.00. Specifically, the pH values of Cd and Ni contaminated solutions range from 6.43 to 6.84, and both decrease slightly with the increase of initial heavy metal concentration. The pH of Zn contaminated solutions ranges from 6.21 to 6.87. In addition, the pH values of Cr and Cu contaminated solutions range from 2.79–3.65 and 4.62–5.58 respectively. Those heavy metal contaminated solutions are all acidic mainly because the salt chloride used to prepare the contaminated solution is a strong acid and weak base, and the hydrolysis of the salt chloride produced hydrogen ions, leading to the acidic solution. Subsequently, a certain amount of 0.1 M sodium hydroxide (NaOH) solution was titrated in the contaminated solutions. When the pH was adjusted to 7.00 ± 0.10, Cd(II), Zn(II) and Ni(II) almost remained soluble, while Cr(III) and Cu(II) almost completely precipitated (shown as Figure 1b). The results indicate that the change in pH can significantly affect the morphology of Cr(III) and Cu(II) in the solution. The removal mechanism of heavy metal ions by S. pasteurii will be analyzed based on the results.
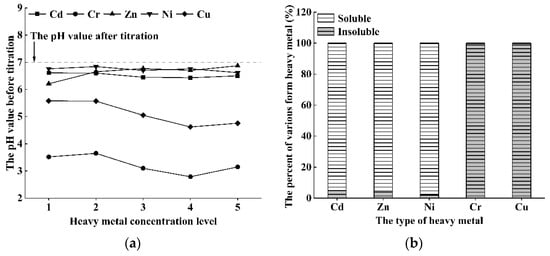
Figure 1.
(a) The pH values of each heavy metal contaminated solutions before titration of sodium hydroxide (NaOH); (b) the percent of various form of each heavy metal after titration of sodium hydroxide (NaOH), and all the heavy metal levels are 5.
3.2. Bioremediation of Single-Heavy-Metal Contaminated Solution
This section focuses mainly on analyzing the percent of various forms of heavy metals, calcium removal rate, precipitation mass, and pH value in the single-heavy-metal cases with various heavy metal types and concentrations. The meanings of these indicators have been explained earlier in Section 2.3. As the bioremediation efficiency and feature depend heavily on the type of contaminated solution, the results are discussed separately in accordance with the type of heavy metals. Besides, the bioremediation mechanism is also discussed in conjunction with some XRD spectra.
3.2.1. Bioremediation Efficiency of S. pasteurii
- For Cd Contaminated Solution
Figure 2 depicts the bioremediation ability of S. pasteurii to Cd contaminated solution with the pollution concentration varying in the range of 5–25 mg/L. As can be seen, around 99% of Cd ions are removed (Figure 2a), the calcium removal rates are higher than 95% (Figure 2b), the precipitate masses are close to the precipitation mass in the reference case (S0) (i.e., 5 g) (Figure 2c), and the pH values of the solutions increases slightly after bioremediation (Figure 2d). Furthermore, the values of all indicators (including the percent of various forms of Cd, calcium removal rate, precipitation mass, and pH value) basically do not change with the increase in initial Cd concentration. These results indicate that S. pasteurii has good activity in the solution under the test conditions, and can almost completely induce calcium precipitation and remove Cd. This is consistent with the experimental findings in some previous research, which have confirmed that S. pasteurii has a strong tolerance to Cd and the bioremediation rate of Cd can reach 97% when the initial Cd concentration is in the range of 1.69–224.82 mg/L [6,34,38]. To sum up, it is believed that S. pasteurii is an excellent strain for engineering applications to remedy simple Cd-contaminated solutions.
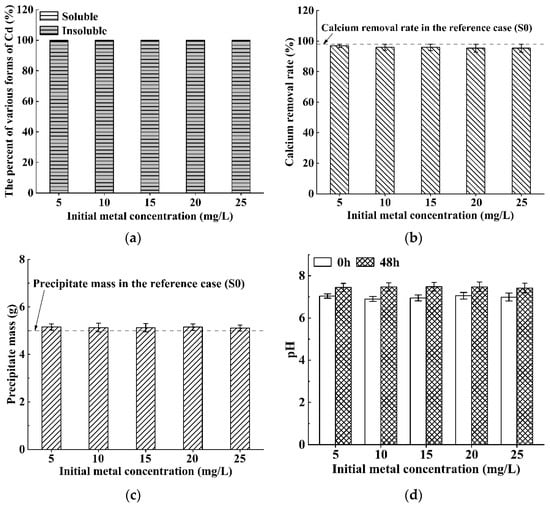
Figure 2.
Bioremediation efficiency of S. pasteurii to Cd contaminated solution: (a) bioremediation rate; (b) calcium removal rate; (c) precipitate mass; and (d) pH values before/after bioremediation (Error bars ± 1 SD).
- For Cr(III) contaminated solution
Figure 3 presents the results for Cr(III) contaminated solution with the concentration range of 250–1250 mg/L. The removal rate reaches up to 96% and the calcium removal rates constantly exceed 90% (Figure 3a,b). It appears that neither are affected by the initial Cr(III) concentration. It can also be found from Figure 3d that, with the initial Cr(III) concentration from 250 mg/L to 1250 mg/L, the pH values before bioremediation decrease evidently from 6.3 to 4.0 whereas the pH values after 48 h of bioremediation are constantly maintained at around 7.5. The above observations are interesting, and suggest that S. pasteurii can still metabolize normally and keep a good biological activity for removing heavy metals in an acidic environment, though it is widely accepted that S. pasteurii shows better activity in a weakly alkaline environment [39,40]. Moreover, it is worth noting that, with the increase in pollution concentration, the change trends of the calcium removal rate and the precipitation mass are opposite (Figure 3b,c). To be specific, when the initial Cr(III) concentration increases from 250 mg/L to 1250 mg/L, the calcium removal rates decrease slightly from 93% to 91% but the precipitation masses increase gradually from 5.68 g to 7.44 g. The opposite variation trends can be attributed to the fact that more precipitate of chromium compounds can be formed in a case with a higher Cr(III) concentration. This phenomenon will be further discussed later in Section 3.2.2 in conjunction with the XRD results. In summary, the results evidently demonstrate that S. pasteurii can also remedy Cr(III) contaminated solution effectively.
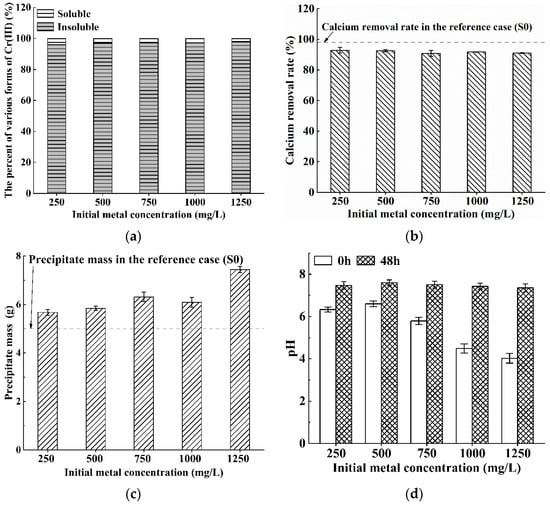
Figure 3.
Bioremediation efficiency of S. pasteurii to Cr(III) contaminated solution: (a) bioremediation rate; (b) calcium removal rate; (c) precipitate mass; and (d) pH values before/after bioremediation (Error bars ± 1 SD).
- For Zn contaminated solution
Figure 4 plots the variation in the percent of various forms of Zn, calcium removal rate, precipitate mass, and pH values before/after bioremediation against the pollution concentration, respectively. As can be seen from Figure 4a,b, when the initial Zn concentration is in the range of 500–1000 mg/L, S. pasteurii can still keep positive activity and has good bioremediation ability, and the percent of insoluble forms of Zn and the calcium removal rate are above 92% and 86%, respectively. However, when the initial Zn concentration increases from 1000 mg/L to 2500 mg/L, the percent of insoluble forms of Zn decrease substantially from 92% to 53% (Figure 4a), the calcium removal rates decline sharply from 86% to 6% (Figure 4b), and the precipitation mass also decreases evidently from 4.5 g to 3.9 g (Figure 4c).
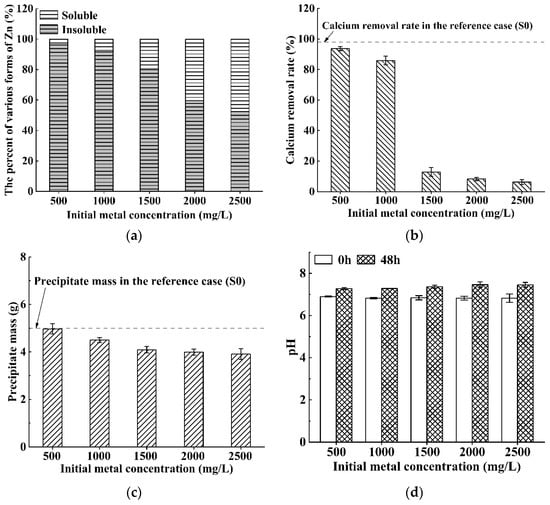
Figure 4.
Bioremediation efficiency of S. pasteurii to Zn contaminated solution: (a) bioremediation rate; (b) calcium removal rate; (c) precipitate mass; and (d) pH values before/after bioremediation (Error bars ± 1 SD).
Some previous studies have found that Zn has substantial biological toxicity to S. pasteurii in the range of 32.5–195 mg/L [6,34], leading to poor bioremediation efficiency to Zn contaminated solutions at high concentrations (the removal rate = 10–40%) [34]. However, the removal rate in this study is larger than 52% even when the Zn concentration increases up to 2500 mg/L. In comparison, the tolerance of S. pasteurii to zinc improves significantly in this study, and this may be attributed to the larger volume ratio of the bacterial solution and the contaminated solution adopted in the experiments (1:1 (v/v)). Jalilvand et al. [6] found that the removal rate of heavy metals was significantly improved when increasing the mixing ratio of bacterial solution and contaminated solution from 1:9 (v/v) to 1:1 (v/v). Therefore, the volume ratio still needs to be further optimized to improve environmental performance and economy while ensuring a high bioremediation rate.
- For Ni contaminated solution
Figure 5 plots the results for Ni contaminated solution with the concentration varying from 150 mg/L to 750 mg/L. The results indicate that the calcium removal rates are maintained at around 70–80% (Figure 5b) and precipitate masses increase from 4.0 g to 4.7 g (Figure 5c). This suggests that the removal efficiency to calcium is slightly inhibited, but the microorganisms can still induce the precipitation of most calcium ions under the Ni contamination context. Giovanella et al. [41] also confirmed that the bioremediation efficiency of Hg is barely inhibited in the medium containing Ni. For instance, the removal rates of Hg by Pseudomonas sp. and Alcaligenes faecalis decrease from 90.0% and 83.8% to 87.7% and 76.9%, respectively, when the Ni concentration is in the range of 0.25–1.5 mM. Besides, Qiao et al. [42] also reported that Ni is an important trace element being able to activate urease activity and promote urea hydrolysis. This may further explain why the inhibition of Ni to calcium removal rate is not obvious and the precipitate mass increases with the nickel concentration.
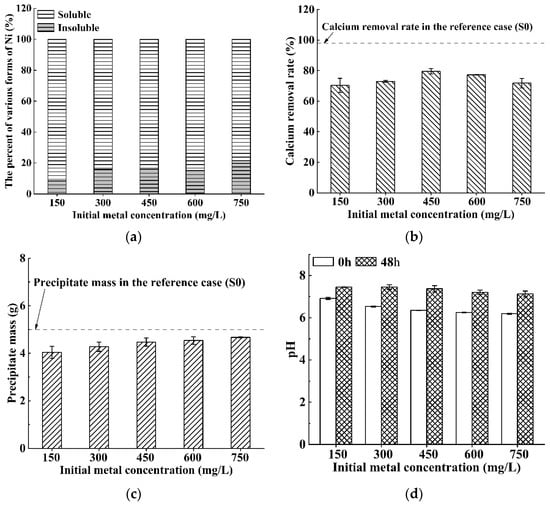
Figure 5.
Bioremediation efficiency of S. pasteurii to Ni contaminated solution: (a) bioremediation rate; (b) calcium removal rate; (c) precipitate mass; and (d) pH values before/after bioremediation (Error bars ± 1 SD).
However, it should be noted that the removal rates of Ni (around 8–20%) are significantly smaller than the calcium removal rates (70–80%) (Figure 5a,b), and the pH values after 48 h of bioremediation decrease slightly from 7.44 to 7.12 as the Ni concentration increases, implying the presence of soluble Ni after bioremediation. This is not totally surprising because Gheethi et al. [43] also found that only 50% nickel can be remedied by S. pasteurii 586 s when Ni concentration is 0.8 mg/L. Hence, the bioremediation ability of S. pasteurii to Ni contaminated solution is limited, and other measures (e.g., complex bacteria agents) may be required.
- For Cu contaminated solution
Figure 6 presents the results for Cu contaminated solution with the Cu concentration varying from 400 mg/L to 2000 mg/L. It can be observed from Figure 6a–c that all of the bioremediation rates, the calcium removal rates and the precipitation masses for Cu contaminated solution are very small or even marginal within the range of interest (i.e., initial Cu concentration = 400–2000 mg/L). The pH values of Cu contaminated solutions in the chemical precipitation test are in the range of 4.76–5.58 (as shown in Figure 1a), and these shown in Figure 6d are between 3.90–6.70. These results indicate that S. pasteurii cannot remedy copper effectively and the urease activity of bacteria may be inactivated.
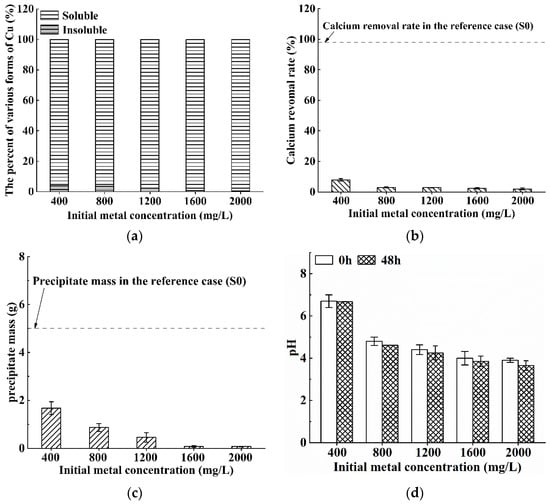
Figure 6.
Bioremediation efficiency of S. pasteurii to Cu contaminated solution: (a) bioremediation rate; (b) calcium removal rate; (c) precipitate mass; and (d) pH values before/after bioremediation (Error bars ± 1 SD).
The above finding is actually consistent with some existing studies which confirmed that Cu exhibits strong biological toxicity to S. pasteurii. For instance, Mugwar and Harbottle [34] found that the removal rate of S. pasteurii is only about 40% when the initial copper concentration is 64 mg/L, and Duarte-Nass et al. [44] concluded that the removal rate of Cu is only 10% when the initial Cu concentration is larger than 32 mg/L. In addition, Chung et al. [45] verified that free Cu inhibited urea hydrolysis. On the one hand, the biological toxicity of copper inhibits microbial activity, and on the other hand, the copper carbonate attached to the surface of the calcium carbonate precipitate prevents the reaction from proceeding further [46]. These two aspects are considered to be the main reasons for the low bioremediation rate for Cu-contaminated solutions. Particularly, the Cu concentration in this study is far beyond the concentration range covered in the existing studies, so the S. pasteurii loses its activity to a very large extent.
3.2.2. Mechanism of S. pasteurii-Based Bioremediation
Figure 7 provides the XRD spectra for the precipitations induced by S. pasteurii-based bioremediation for different types of single-heavy-metal contaminated solutions. The difference among the XRD spectra is analyzed below.
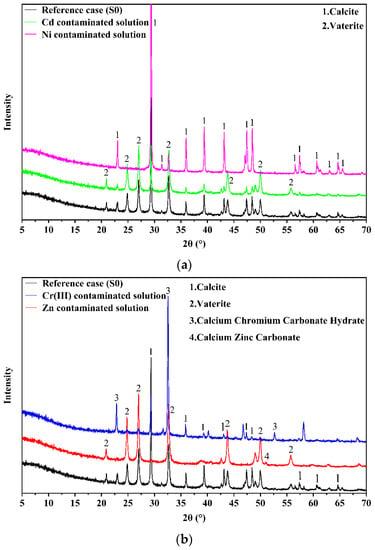
Figure 7.
XRD spectra for confirming precipitation induced by S. pasteurii-based bioremediation: (a) for Cd or Ni contaminated solution; (b) for Zn or Cr(III) contaminated solution.
For the case without any heavy metal (reference case S0), the precipitation is dominated by calcite and vaterite (Figure 7a). Previous studies have confirmed that the process of inducing carbonate precipitation by S. pasteurii involves complex biochemical reactions governed by urease, and the urea and calcium sources are utilized as substrates for mineralization [34,47].
For the Cd contaminated solution, the precipitation induced by S. pasteurii-based bioremediation is also mainly vaterite and calcite, and no Cd-related carbonate can be observed (Figure 7a). In contrast, Fang et al. [38] found that CdCO3 and (Ca0·67Cd0·33)CO3 are formed when the initial Cd concentration is 5 mM (562 mg/L), and Li et al. [29] also confirmed that Cd is removed as CdCO3 at the concentration of 2000 mg/L under the condition without any calcium source. Nevertheless, it should be noted that the initial Cd concentration in this research is 20–100 times the concentration considered in this study. Therefore, it is inferred that the low Cd concentration is one possible reason why Cd-related carbonates cannot be detected. Besides, Cd is possibly remedied by the adsorption of calcite [48] or S. pasteurii [38]. The adsorbed Cd does not form a regular crystal structure with calcite, so it cannot be detected by XRD.
Similarly, no Ni related carbonate is detected in the case of Ni-contaminated solution, namely, no regular crystal structure with Ni is formed in the test condition (Figure 7a). The results of the existing research on the bioremediation of Ni are diverse. Li et al. [29] proved that Ni is removed as NiCO3 efficiently by S. pasteurii at the concentration of 2000 mg/L without any calcium source. Nonetheless, Gheethi et al. [43] found that, at the concentration of 0.8 mg/L, less than 60% Ni can be remedied through bioaccumulation by S. pasteurii 586S without the involvement of nutrients. The diverse results regarding the bioremediation efficiency of S. pasteurii to Ni contaminated solution do not mean the relevant experiments are inappropriate, since there are evident differences in the testing methods and conditions (e.g., whether or not adding calcium source or nutrient solution). Nonetheless, it should be acknowledged that the mechanism behind the S. pasteurii based bioremediation to Ni contaminated solution still remains unclear and further investigation is needed.
For the case of Cr(III) contaminated solution, Cr(III) mainly exists in the precipitation in terms of calcium chromium carbonate hydrate under the microbial induction (as shown in Figure 7b). The complex compound (i.e., 3CaO·Cr2O3·CaCO3·11H2O) with higher molar mass are precipitated. This is considered as the main reason why the precipitate masses increase with the growth of Cr(III) concentration (as shown in Figure 3c). In addition, the increase in solution pH caused by urea hydrolysis may promote Cr(III) precipitation to a certain extent according to the results in Figure 1b, which need further investigation.
For the case of the Zn contaminated solution, it can be seen from Figure 7b that the precipitates are mainly calcite, vaterite and CaZn(CO3)2. The existence of CaZn(CO3)2 in the precipitates proves that Zn can be removed by biomineralization, and the heavy metal coprecipitation reaction are shown in Equations (3) and (4). Some other studies also found the zinc carbonate or co-existence of calcium and zinc in the precipitates. For instance, Li et al. [29] found that Zn can be precipitated as zinc carbonate by S. pasteurii without calcium resources; Jalilvand et al. [6] proved that the precipitate induced by S. pasteurii contains zinc; and Buekers et al. [49] also confirmed that zinc can be absorbed and encapsulated during the formation of calcium carbonate crystals, and finally exists in the form of Ca-Zn-CO3.
It can be concluded from above analysis that inducing heavy metal ions to form insoluble precipitates (e.g., regular crystals of 3CaO·Cr2O3·CaCO3·11H2O and CaZn(CO3)2) is the primary bioremediation mechanism of S. pasteurii-based bioremediation. The biosorption of calcium carbonate formed during the MICP (i.e., Microbial induced carbonate precipitation) process and the bioaccumulation of S. pasteurii may be another important means to remove heavy metals. In addition, the pH change of solution during the process of carbonate precipitation induced by microorganisms is also a possible reason to promote the precipitation of some heavy metals.
3.3. Bioremediation of Heavy Metal Contaminated Solution under High Salinity Context
To investigate the influence of high salinity (including NaCl and KCl) on the efficiency of S. pasteurii-based bioremediation, various levels of mineral salinity, with the concentration of (NaCl + KCl) ranging from (0 + 0) mg/L to (6400 + 6400) mg/L, are considered respectively for Cd, Cr(III), Zn, and Ni contaminated solutions. The Cu contaminated solution is not covered in this section because the efficiency of S. pasteurii-based bioremediation for the Cu contaminated solution is very limited.
Figure 8a–d plot the variation of bioremediation rates and calcium removal rates versus the concentration of (NaCl + KCl) for the four types of heavy metal contaminated solutions. As shown in the figures, with the increase in the concentration of (NaCl + KCl) from (0 + 0) mg/L to (6400 + 6400) mg/L, the bioremediation rates and calcium removal rates are maintained at constant values for the Cd or Cr(III) contaminated solution (Figure 8a,b), reduce very slightly for the Zn contaminated solution (Figure 8c) and increase gradually from 16% and 76% to 22% and 87% respectively for the Ni contaminated solution (Figure 8d). In a word, it is demonstrated that NaCl and KCl have a very little detrimental effect on the S. pasteurii-based bioremediation to heavy metal contaminated solution.
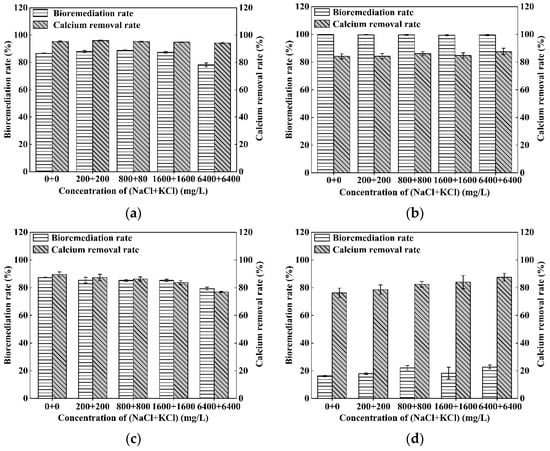
Figure 8.
Variation of bioremediation rate and calcium removal rate versus the salt concentration of (NaCl + KCl): (a) for Cd contaminated solution; (b) for Cr(III) contaminated solution; (c) for Zn contaminated solution; and (d) for Ni contaminated solution (Error bars ± 1 SD).
The high tolerance of S. pasteurii to NaCl and KCl has also been demonstrated in other researches [50,51], and S. pasteurii even induces more calcium carbonate precipitation when the sodium chloride is as high as 3.5% (35,000 mg/L) [50]. However, when the NaCl concentration is too high, the osmotic pressure of the solution will increase, which will also affect the metabolic activities of microorganisms. For instance, Kim et al. [50] found that NaCl inhibits the production of microbial proteins when the NaCl concentration is higher than 100,000 mg/L. As the typical range of mineral salinity possibly encountered in practice in some contaminated solutions such as leachate of landfills is relatively low, the detrimental influence of high mineral salinity (including NaCl and KCl) on the efficiency of S. pasteurii-based bioremediation can be neglected.
3.4. Bioremediation of Multi-Heavy-Metal-Contaminated Solutions
As the bioremediation rates of S. pasteurii to Cd and Cr(III) contaminated solutions are pretty high (larger than 96%, as shown in Figure 2a and Figure 3a), these two types of heavy metals are considered as the fundamental pollutants when exploring the bioremediation potential of S. pasteurii to multi-heavy-metal contaminated solutions. The other three types of heavy metals (i.e., Zn, Ni and Cu) are also included, separately, by adding ZnCl2, NiCl2·6H2O or CuCl2·2H2O into the mixture of CdCl2·2.5H2O and CrCl3·6H2O solutions. The feasibility of S. pasteurii-based bioremediation for multi-heavy-metal-contaminated solutions is assessed based on the bioremediation rates of different heavy metals, and the emphasis will be put on clarifying the influence of the presence of a certain type of heavy metal on the bioremediation efficiency of S. pasteurii to another type of heavy metal (as shown in Figure 9).
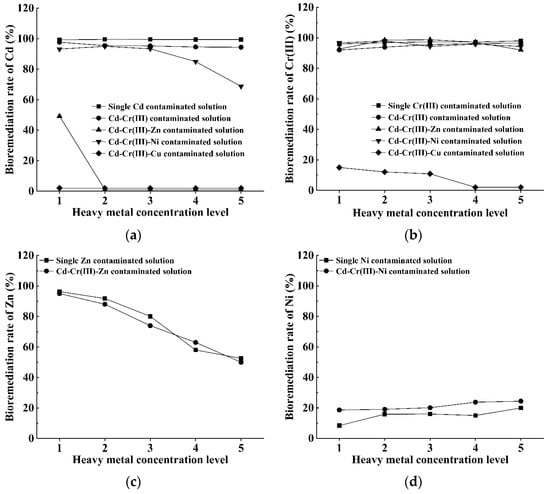
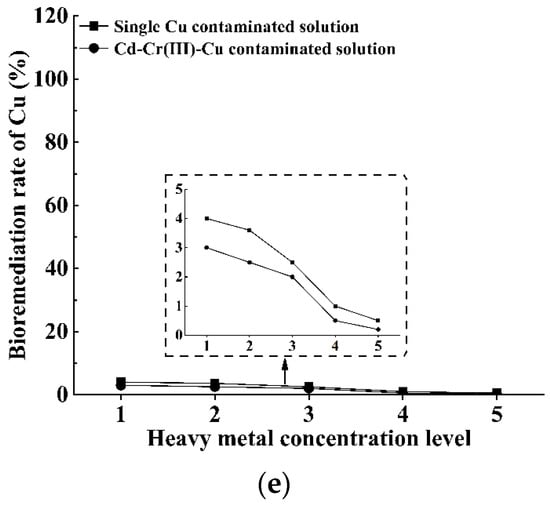
Figure 9.
Variation in bioremediation rate against the heavy metal concentration level under various contaminated conditions: (a) Cd; (b) Cr(III); (c) Zn; (d) Ni and (e) Cu.
Figure 9a shows that the presence of different heavy metals tends to produce a diverse influence on the bioremediation rate of Cd. To be specific, the addition of Cr(III) into Cd-Cr(III) contaminated solution does not show a substantial effect on the bioremediation of Cd, and the bioremediation rate of Cd (around 95%) under the condition of Cd-Cr(III) co-existence is very close to the value (around 99%) for the single Cd contaminated cases in the entire concentration range of interest. Then, the effect produced by further addition of Ni and Zn on the bioremediation rate of Cd is perceptible and depends on the heavy metal concentration. When the Ni concentration increases from 150 mg/L to 750 mg/L, the bioremediation rate of Cd decreases substantially from 93% to 68%. Due to the further addition of Zn, the bioremediation rate of Cd decreases sharply to half of the value for the single Cd contaminated case even when the Zn concentration is 500 mg/L, and more seriously it reduces to a negligible level when the heavy metal concentration exceeds 1000 mg/L. Besides, the addition of Cu makes S. pasteurii lose the bioremediation ability to Cd at the concentration range covered in this study.
From Figure 9b, it can be seen that the addition of Cd, Zn and Ni into Cr(III) contaminated solution hardly affects the bioremediation rate of S. pasteurii to Cr(III), which constantly reaches up to 92–98%. However, the introduction of Cu into Cd-Cr(III) contaminated solution brings along destructive influence on the bioremediation ability of S. pasteurii to Cr(III), and the bioremediation rate for Cr(III) drops extremely to only 2% at the Cu concentration of 2000 mg/L.
Figure 9c,e illustrate that the addition of both Cd and Cr(III) into Zn or Cu contaminated solution does not evidently weaken the bioremediation ability of S. pasteurii to Zn or Cu. It is also very interesting to compare the results presented in Figure 9a,d. While the addition of Ni inhibits the bioremediation ability of S. pasteurii to Cd, the introduction of Cd and Cr(III) into Ni contaminated solution tends to slightly prompt the bioremediation ability of S. pasteurii to Ni.
In summary, the above results confirm that the combination and concentration of involved heavy metals are the main influence factors controlling the bioremediation efficiency under the multi-heavy-metal-contaminated condition. For the heavy metal concentration range covered in this study, the five types of heavy metals can be divided into three groups in accordance with the influence on the bioremediation ability of S. pasteurii to other heavy metals. The first group contains Cd and Cr(III), the addition of which shows a negligible influence on the bioremediation ability of S. pasteurii to other heavy metals. The second group contains Zn and Ni, and their influence on the bioremediation ability of S. pasteurii to other heavy metals is substantial and becomes more and more significant with the increase in concentration. The third group contains Cu only, which tends to produce extremely detrimental effect on (or even completely inhibit) the bioremediation ability of S. pasteurii to other heavy metals.
Some researchers have investigated the multiple heavy metals immobilization based on microbially induced carbonate precipitation by ureolytic bacteria [41,42], but it is difficult to compare the results due to the large differences between the strains, types and concentrations of heavy metals and test methods in diverse test. It is reasonable to conclude that S. pasteurii-based bioremediation can be considered as a practically potential option for remedying multi-heavy-metal contaminated solutions, since the holistic efficiency of S. pasteurii-based bioremediation is relatively good for some combinations of heavy metals.
4. Conclusions
A series of laboratory experiments were performed to explore the efficiency and mechanism of S. pasteurii-based bioremediation to different types of heavy metals under various conditions, including single-heavy-metal pollution conditions, heavy metal pollution under high mineral salinity context and multi-heavy-metal pollution scenarios. The following conclusions can be drawn from the analysis:
- S. pasteurii can effectively remedy single Cd, Cr(III) or Zn contaminated solution. The bioremediation rates for Cd and Cr(III) are as high as 96%, regardless of the heavy metal concentration. The bioremediation rate for Zn is larger than 86% when the initial concentration is 500–1000 mg/L, but it decreases gradually with the increase in initial concentration. The efficiency of S. pasteurii-based bioremediation to Ni or Cu is not good within the concentration range covered in this study (i.e., 150–750 mg/L for Ni and 400–2000 mg/L for Cu).
- High mineral salinity (including NaCl and KCl) has very little detrimental effect on the S. pasteurii-based bioremediation of heavy metal contaminated solution. For the typical range of mineral salinity possibly encountered in practice in some contaminated solutions such as leachate of landfills, the detrimental influence of high mineral salinity on bioremediation efficiency can be neglected.
- S. pasteurii-based bioremediation can be considered as a potential option for remedying multi-heavy-metal contaminated solutions. The combination and concentration of involved heavy metals are the main factors controlling the bioremediation efficiency under the multi-heavy-metal contaminated condition. The addition of Cd or Cr(III) shows the negligible influence on the bioremediation ability of S. pasteurii to other heavy metals; the addition of Zn or Ni tends to produce a substantial influence on the bioremediation ability of S. pasteurii to other heavy metals, which becomes more and more significant with the increase in concentration level; whereas the addition of Cu is very likely to produce an extremely detrimental effect on (or even completely inhibit) the bioremediation ability of S. pasteurii to other heavy metals.
Author Contributions
X.H.: conceptualization, investigation, formal analysis and writing—original draft; R.Z.: conceptualization, resources, formal analysis, supervision, and writing—review and editing; M.C.: formal analysis, supervision and writing—review and editing; H.L.: formal analysis, supervision and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study has been supported by the National Key R&D Program of China (No. 2018YFC1802302). The financial support is gratefully acknowledged.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rahman, R.O.A.; Hung, Y.T. Application of Ionizing Radiation in Wastewater Treatment: An Overview. Water 2020, 12, 19. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.H.; Zhang, X.; Yang, Q.; Zhang, K.; Zheng, Y.; Zhou, G.H. Pollution characteristics of atmospheric dustfall and heavy metals in a typical inland heavy industry city in China. J. Environ. Sci. 2018, 71, 283–291. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Li, Z.Y.; Lu, X.N.; Duan, Q.N.; Huang, L.; Bi, J. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 14. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Zhou, P.; Mao, L.A.; Zhi, Y.E.; Zhang, C.H.; Shi, W.J. Effects of plant species coexistence on soil enzyme activities and soil microbial community structure under Cd and Pb combined pollution. J. Environ. Sci. 2010, 22, 1040–1048. [Google Scholar] [CrossRef]
- Jalilvand, N.; Akhgar, A.; Alikhani, H.A.; Rahmani, H.A.; Rejali, F. Removal of Heavy Metals Zinc, Lead, and Cadmium by Biomineralization of Urease-Producing Bacteria Isolated from Iranian Mine Calcareous Soils. J. Soil Sci. Plant Nutr. 2020, 20, 206–219. [Google Scholar] [CrossRef]
- Chileshe, M.N.; Syampungani, S.; Festin, E.S.; Tigabu, M.; Daneshvar, A.; Oden, P.C. Physico-chemical characteristics and heavy metal concentrations of copper mine wastes in Zambia: Implications for pollution risk and restoration. J. For. Res. 2020, 31, 1283–1293. [Google Scholar] [CrossRef] [Green Version]
- Kanakaraju, D.; Shahdad, N.; Lim, Y.C.; Pace, A. Concurrent removal of Cr(III), Cu(II), and Pb(II) ions from water by multifunctional TiO2/Alg/FeNPs beads. Sustain. Chem. Pharm. 2019, 14, 12. [Google Scholar] [CrossRef]
- Lazaridis, N.K.; Charalambous, C. Sorptive removal of trivalent and hexavalent chromium from binary aqueous solutions by composite alginate-goethite beads. Water Res. 2005, 39, 4385–4396. [Google Scholar] [CrossRef]
- Liu, Z.P.; Zhang, Q.F.; Han, T.Q.; Ding, Y.F.; Sun, J.W.; Wang, F.J.; Zhu, C. Heavy Metal Pollution in a Soil-Rice System in the Yangtze River Region of China. Int. J. Environ. Res. Public Health 2016, 13, 63. [Google Scholar] [CrossRef] [Green Version]
- Kamaruddin, M.A.; Yusoff, M.S.; Rui, L.M.; Isa, A.M.; Zawawi, M.H.; Alrozi, R. An overview of municipal solid waste management and landfill leachate treatment: Malaysia and Asian perspectives. Environ. Sci. Pollut. Res. 2017, 24, 26988–27020. [Google Scholar] [CrossRef] [PubMed]
- Naveen, B.P.; Mahapatra, D.M.; Sitharam, T.G.; Sivapullaiah, P.V.; Ramachandra, T.V. Physico-chemical and biological characterization of urban municipal landfill leachate. Environ. Pollut. 2017, 220, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Di Iaconi, C.; Ramadori, R.; Lopez, A. Combined biological and chemical degradation for treating a mature municipal landfill leachate. Biochem. Eng. J. 2006, 31, 118–124. [Google Scholar] [CrossRef]
- Fu, F.L.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Kijjanapanich, P.; Annachhatre, A.P.; Esposito, G.; Lens, P.N.L. Chemical sulphate removal for treatment of construction and demolition debris leachate. Environ. Technol. 2014, 35, 1989–1996. [Google Scholar] [CrossRef]
- Wang, J.; Huang, T.F.; Zhang, L.; Yu, Q.J.; Hou, L.A. Dopamine crosslinked graphene oxide membrane for simultaneous removal of organic pollutants and trace heavy metals from aqueous solution. Environ. Technol. 2018, 39, 3055–3065. [Google Scholar] [CrossRef]
- Wu, S.S.; Xie, F.C.; Chen, S.J.; Fu, B.B. The removal of Pb(II) and Cd(II) with hydrous manganese dioxide: Mechanism on zeta potential and adsorption behavior. Environ. Technol. 2020, 41, 3219–3232. [Google Scholar] [CrossRef]
- Nag, M.; Lahiri, D.; Dutta, B. Biodegradation of used polyethylene bags by a new marine strain of Alcaligenes faecalis LNDR-1. Environ. Sci. Pollut. Res. 2021, 28, 41365–41379. [Google Scholar] [CrossRef]
- Dash, S.; Lahiri, D.; Nag, M.; Das, D.; Ray, R.R. Probing the Surface-Attached In Vitro Microbial Biofilms with Atomic Force (AFM) and Scanning Probe Microscopy (SPM). In Analytical Methodologies for Biofilm Research; Springer: New York, NY, USA, 2021; pp. 223–241. [Google Scholar] [CrossRef]
- Nag, M.; Lahiri, D.; Banerjee, R.; Chatterjee, A.; Ghosh, A.; Banerjee, P.; Ray, R.R. Analysing Microbial Biofilm Formation at a Molecular Level: Role of Fourier Transform Infrared and Raman Spectroscopy. In Analytical Methodologies for Biofilm Research; Springer: New York, NY, USA, 2021; pp. 69–93. [Google Scholar] [CrossRef]
- Dutta, B.; Nag, M.; Lahiri, D.; Ray, R.R. Analysis of Biofilm Matrix by Multiplex Fluorescence In Situ Hybridization (M-FISH) and Confocal Laser Scanning Microscopy (CLSM) during Nosocomial Infections. In Analytical Methodologies for Biofilm Research; Springer: New York, NY, USA, 2021; pp. 183–203. [Google Scholar] [CrossRef]
- Mukherjee, D.; Garai, S.; Lahiri, D.; Nag, M.; Ray, R.R. Monitoring Cell Distribution and Death in Sessile Forms of Microbial Biofilm: Flow Cytometry-Fluorescence Activated Cell Sorting (FCM-FACS). In Analytical Methodologies for Biofilm Research; Springer: New York, NY, USA, 2021; pp. 299–316. [Google Scholar] [CrossRef]
- Jasu, A.; Lahiri, D.; Nag, M.; Ray, R.R. Biofilm-Associated Metal Bioremediation. In Biotechnology for Sustainable; Springer: Singapore, 2021; pp. 201–221. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Y.; Tang, Q.; Shi, S.J. Bioremediation of metal-contaminated soils by microbially-induced carbonate precipitation and its effects on ecotoxicity and long-term stability. Biochem. Eng. J. 2021, 166, 107856. [Google Scholar] [CrossRef]
- Tang, Q.; Shi, P.X.; Yuan, Z.; Shi, S.J.; Xu, X.J.; Katsumi, T. Potential of zero-valent iron in remediation of Cd(II) contaminated soil: From laboratory experiment, mechanism study to field application. Soils Found. 2019, 59, 2099–2109. [Google Scholar] [CrossRef]
- Verma, S.; Kuila, A. Bioremediation of heavy metals by microbial process. Environ. Technol. Innov. 2019, 14, 100369. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X.L.; Zhang, D.Y. Remediation of copper-contaminated soil by Kocuria flava CR1, based on microbially induced calcite precipitation. Ecol. Eng. 2011, 37, 1601–1605. [Google Scholar] [CrossRef]
- Kumari, D.; Pan, X.L.; Lee, D.J.; Achal, V. Immobilization of cadmium in soil by microbially induced carbonate precipitation with Exiguobacterium undae at low temperature. Int. Biodeterior. Biodegrad. 2014, 94, 98–102. [Google Scholar] [CrossRef]
- Li, M.; Cheng, X.H.; Guo, H.X. Heavy metal removal by biomineralization of urease producing bacteria isolated from soil. Int. Biodeterior. Biodegrad. 2013, 76, 81–85. [Google Scholar] [CrossRef]
- Phillips, A.J.; Gerlach, R.; Lauchnor, E.; Mitchell, A.C.; Cunningham, A.B.; Spangler, L. Engineered applications of ureolytic biomineralization: A review. Biofouling 2013, 29, 715–733. [Google Scholar] [CrossRef] [Green Version]
- Cui, M.; Zheng, J.; Lai, H.; Zhang, R. Microscopic mechanism of shear behavior of sand with different roughness of particles. J. Huazhong Univ. Sci. Technol. Nat. Sci. 2015, 43, 1–6. [Google Scholar]
- Lai, H.J.; Wu, S.F.; Cui, M.J.; Chu, J. Recent development in biogeotechnology and its engineering applications. Front. Struct. Civ. Eng. 2021, 15, 1073–1096. [Google Scholar] [CrossRef]
- Cheng, L.; Cord-Ruwisch, R.; Shahin, M.A. Cementation of sand soil by microbially induced calcite precipitation at various degrees of saturation. Can. Geotech. J. 2013, 50, 81–90. [Google Scholar] [CrossRef]
- Mugwar, A.J.; Harbottle, M.J. Toxicity effects on metal sequestration by microbially-induced carbonate precipitation. J. Hazard. Mater. 2016, 314, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Cui, M.J.; Zheng, J.J.; Lai, H.J. Effect of method of biological injection on dynamic behavior for bio-cemented sand. Rock Soil Mech. 2017, 38, 3173–3178. [Google Scholar] [CrossRef]
- Chai, X.L.; Shimaoka, T.; Cao, X.Y.; Guo, Q.; Zhao, Y.C. Characteristics and mobility of heavy metals in an MSW landfill: Implications in risk assessment and reclamation. J. Hazard. Mater. 2007, 144, 485–491. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.H.; Babel, S. Physico-chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Fang, L.Y.; Niu, Q.J.; Cheng, L.; Jiang, J.X.; Yu, Y.Y.; Chu, J.; Achal, V.; You, T.Y. Ca-mediated alleviation of Cd2+ induced toxicity and improved Cd2+ biomineralization by Sporosarcina pasteurii. Sci. Total Environ. 2021, 787, 147627. [Google Scholar] [CrossRef]
- Cuzman, O.A.; Rescic, S.; Richter, K.; Wittig, L.; Tiano, P. Sporosarcina pasteurii use in extreme alkaline conditions for recycling solid industrial wastes. J. Biotechnol. 2015, 214, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.M.; Zhu, W.J.; Long, H.Z.; Chai, L.Y.; Wang, Q.W. Chromate reduction by resting cells of Achromobacter sp Ch-1 under aerobic conditions. Process Biochem. 2007, 42, 1028–1032. [Google Scholar] [CrossRef]
- Giovanella, P.; Cabral, L.; Costa, A.P.; Camargo, F.A.D.; Gianello, C.; Bento, F.M. Metal resistance mechanisms in Gram-negative bacteria and their potential to remove Hg in the presen ce of other metals. Ecotoxicol. Environ. Saf. 2017, 140, 162–169. [Google Scholar] [CrossRef]
- Qiao, S.Y.; Zeng, G.Q.; Wang, X.T.; Dai, C.G.; Sheng, M.P.; Chen, Q.; Xu, F.; Xu, H. Multiple heavy metals immobilization based on microbially induced carbonate precipitation by ureolytic bacteria and the precipitation patterns exploration. Chemosphere 2021, 274, 129661. [Google Scholar] [CrossRef]
- Gheethi, A.A.; Efaq, A.N.; Mohamed, R.M.; Abdel-Monem, M.O.; Abduah, A.; Hashim, M.A. Bio-removal of Nickel ions by Sporosarcina pasteurii and Bacillus megaterium, A Comparative Study. IOP Conf. Ser. Mater. Sci. Eng. 2017, 226, 012044. [Google Scholar] [CrossRef]
- Duarte-Nass, C.; Rebolledo, K.; Valenzuela, T.; Kopp, M.; Jeison, D.; Rivas, M.; Azocar, L.; Torres-Aravena, A.; Ciudad, G. Application of microbe-induced carbonate precipitation for copper removal from copper-enriched waters: Challenges to future industrial application. J. Environ. Manag. 2020, 256, 109938. [Google Scholar] [CrossRef]
- Chung, H.; Kim, S.H.; Nam, K. Inhibition of urea hydrolysis by free Cu concentration of soil solution in microbially induced calcium carbonate precipitation. Sci. Total Environ. 2020, 740, 140194. [Google Scholar] [CrossRef]
- Schosseler, P.M.; Wehrli, B.; Schweiger, A. Uptake of Cu2+ by the calcium carbonates vaterite and calcite as studied by continuous wave (CW) and pulse electron paramagnetic resonance. Geochim. Cosmochim. Acta 1999, 63, 1955–1967. [Google Scholar] [CrossRef]
- Achal, V.; Mukherjee, A. A review of microbial precipitation for sustainable construction. Constr. Build. Mater. 2015, 93, 1224–1235. [Google Scholar] [CrossRef]
- Garcia-Sanchez, A.; Alvarez-Ayuso, E. Sorption of Zn, Cd and Cr on calcite. Application to purification of industrial wastewaters. Miner. Eng. 2002, 15, 539–547. [Google Scholar] [CrossRef]
- Buekers, J.; Van Laer, L.; Amery, F.; Van Buggenhout, S.; Maes, A.; Smolders, E. Role of soil constituents in fixation of soluble Zn, Cu, Ni and Cd added to soils. Eur. J. Soil Sci. 2007, 58, 1514–1524. [Google Scholar] [CrossRef]
- Kim, H.; Son, H.M.; Park, S.; Seo, J.; Lee, H.K. Effects of Temperature and Salinity on Concrete-Surface Treatment by Bacteria in Marine Environment. ACI Mater. J. 2020, 117, 57–65. [Google Scholar] [CrossRef]
- Mortensen, B.M.; Haber, M.J.; DeJong, J.T.; Caslake, L.F.; Nelson, D.C. Effects of environmental factors on microbial induced calcium carbonate precipitation. J. Appl. Microbiol. 2011, 111, 338–349. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).