A Short Cost-Effective Methodology for Tracing the Temporal and Spatial Anthropogenic Inputs of Micropollutants into Ecosystems: Verified Mass-Balance Approach Applied to River Confluence and WWTP Release
Abstract
1. Introduction
2. Sample Collection and Analytical Methods
3. Materials and Methods Developed
3.1. Objective and Constraints of the Sampling Zones
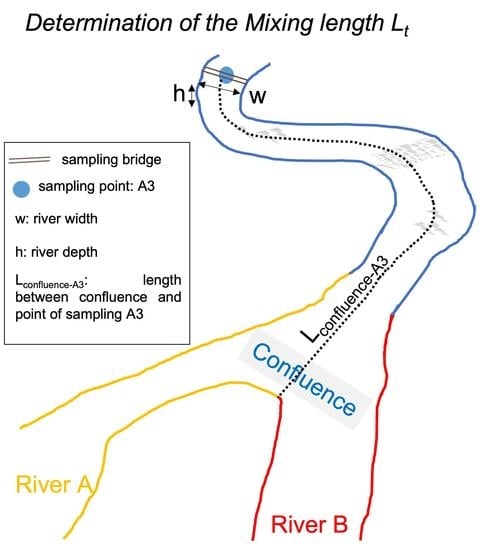
3.2. Mixing Length
| Coef | Coef′ | Coef″ | Model Equation | Lt (Confluence) (km) | Lt (WWTP) (km) | References |
|---|---|---|---|---|---|---|
| 0.4 | 0.6 | 0.667 | 4.3 | 2.1 | [30,31] | |
| 0.125 | 0.16 | 0.78 | 5.0 | 2.5 | [29,32,33] | |
| 0.075 | 0.23 | 0.33 | 2.2 | 1.1 | [34,35,36,37] | |
| 0.0759 | 0.6 | 0.133 | 0.9 | 0.4 | [38] |
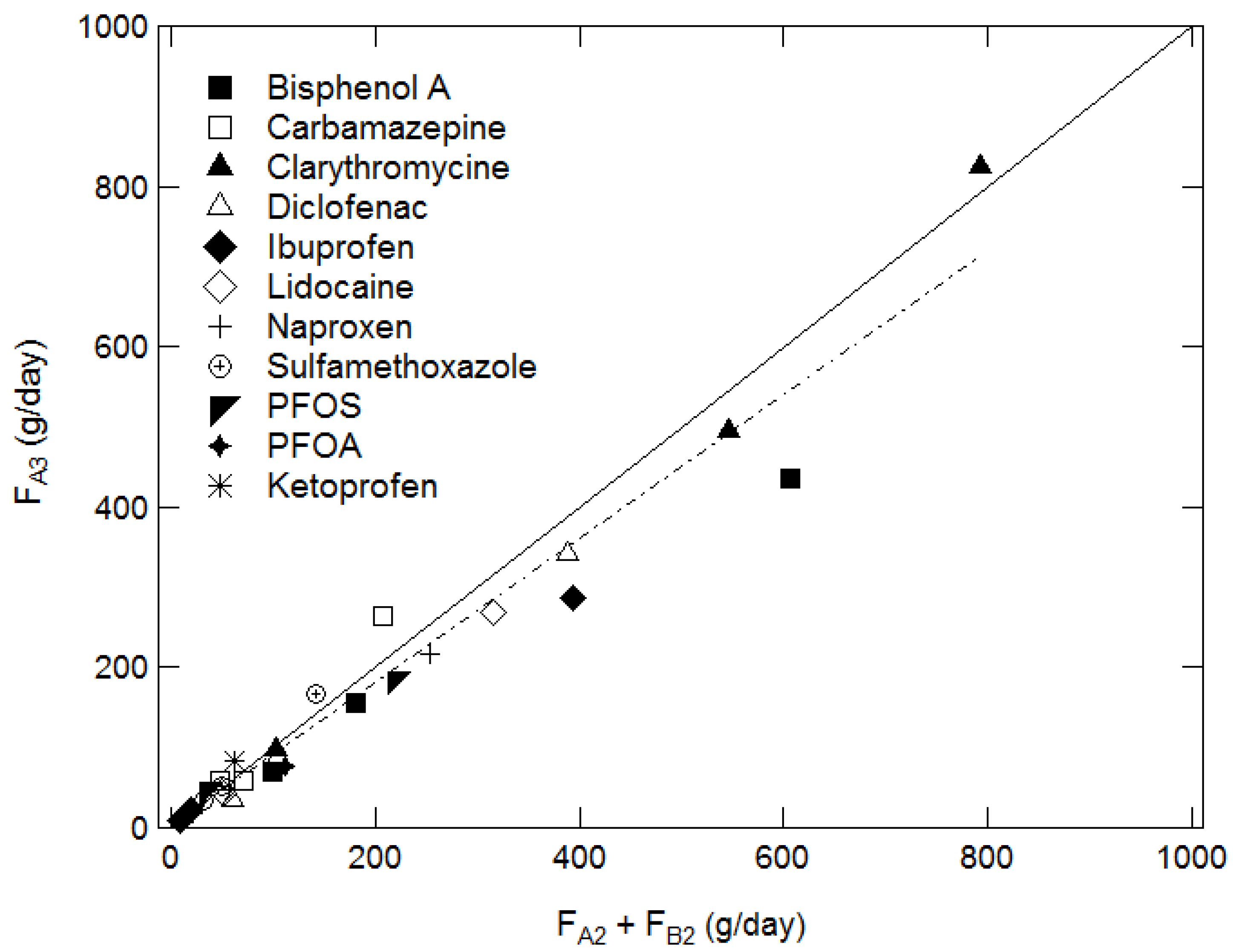
3.3. Verification of the Mixing Length Method by Using Mass Balance at a River Confluence
4. Results and Discussions
4.1. Overall Study of the Results
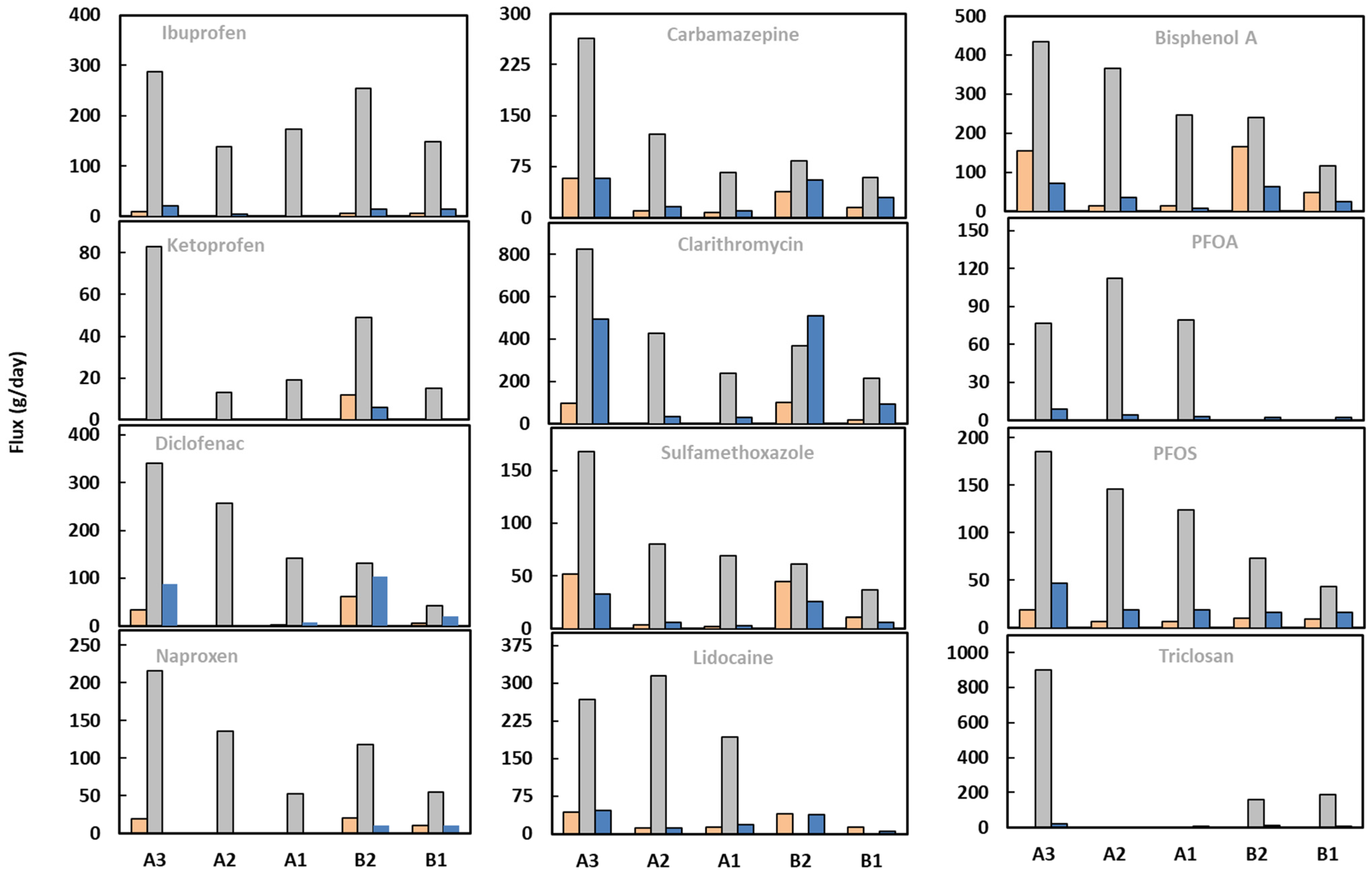
4.2. Release of Micropollutants from WWTP
4.3. Drinking Water Assessment
4.4. Spatial Variations of Micropollutants in Rivers: Effect of Hydrological and Anthropogenic Factors
4.5. Temporal Variations of Micropollutants in Rivers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, H.; Nkundabose, J.P.; Chen, H.; Yang, L.; Meng, C.; Ding, N. Decontamination of ibuprofen micropollutants from water based on visible-light-responsive hybrid photocatalyst. J. Environ. Chem. Eng. 2022, 10, 107154. [Google Scholar] [CrossRef]
- Gomes, I.B.; Simões, L.C.; Simões, M. The effects of emerging environmental contaminants on Stenotrophomonas maltophilia isolated from drinking water in planktonic and sessile states. Sci. Total Environ. 2018, 643, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Vuppaladadiyam, A.K.; Antunes, E.; Whelan, A.; Fearon, R.; Sheehan, M.; Reeves, L. Emerging contaminants in biosolids: Presence, fate and analytical techniques. Emerg. Contam. 2022, 8, 162–194. [Google Scholar] [CrossRef]
- Richardson, S.D. Water Analysis: Emerging Contaminants and Current Issues. Anal. Chem. 2003, 75, 4943. [Google Scholar] [CrossRef][Green Version]
- Stamm, C.; Räsänen, K.; Burdon, F.; Altermatt, F.; Jokela, J.; Joss, A.; Ackermann, M.; Eggen, R. Unravelling the Impacts of Micropollutants in Aquatic Ecosystems. In Advances in Ecological Research; Elsevier: Amsterdam, The Netherlands, 2016; Volume 55, pp. 183–223. [Google Scholar] [CrossRef]
- Gallé, T.; Pittois, D.; Bayerle, M.; Braun, C. An immission perspective of emerging micropollutant pressure in Luxembourgish surface waters: A simple evaluation scheme for wastewater impact assessment. Environ. Pollut. 2019, 253, 992–999. [Google Scholar] [CrossRef]
- Yang, L.; Xu, L.; Bai, X.; Jin, P. Enhanced visible-light activation of persulfate by Ti3+ self-doped TiO2/graphene nanocomposite for the rapid and efficient degradation of micropollutants in water. J. Hazard. Mater. 2019, 365, 107–117. [Google Scholar] [CrossRef]
- Liu, J.; Lu, G.; Yang, H.; Dang, T.; Yan, Z. Ecological impact assessment of 110 micropollutants in the Yarlung Tsangpo River on the Tibetan Plateau. J. Environ. Manag. 2020, 262, 110291. [Google Scholar] [CrossRef]
- Castiglioni, S.; Davoli, E.; Riva, F.; Palmiotto, M.; Camporini, P.; Manenti, A.; Zuccato, E. Mass balance of emerging contaminants in the water cycle of a highly urbanized and industrialized area of Italy. Water Res. 2018, 131, 287–298. [Google Scholar] [CrossRef]
- Boxall, A.B.A.; Rudd, M.A.; Brooks, B.W.; Caldwell, D.J.; Choi, K.; Hickmann, S.; Innes, E.; Ostapyk, K.; Staveley, J.P.; Verslycke, T.; et al. Pharmaceuticals and Personal Care Products in the Environment: What Are the Big Questions? Environ. Health Perspect. 2012, 120, 1221–1229. [Google Scholar] [CrossRef]
- Richardson, S.D.; Kimura, S.Y. Water Analysis: Emerging Contaminants and Current Issues. Anal Chem. 2020, 92, 473–505. [Google Scholar] [CrossRef]
- Salvestrini, S.; Fenti, A.; Chianese, S.; Iovino, P.; Musmarra, D. Diclofenac sorption from synthetic water: Kinetic and thermodynamic analysis. J. Environ. Chem. Eng. 2020, 8, 104105. [Google Scholar] [CrossRef]
- Trapido, M.; Epold, I.; Bolobajev, J.; Dulova, N. Emerging micropollutants in water/wastewater: Growing demand on removal technologies. Environ. Sci. Pollut. Res. 2014, 21, 12217–12222. [Google Scholar] [CrossRef] [PubMed]
- Reinstorf, F.; Strauch, G.; Schirmer, K.; Gläser, H.-R.; Möder, M.; Wennrich, R.; Osenbrück, K. Mass fluxes and spatial trends of xenobiotics in the waters of the city of Halle, Germany. Environ. Pollut. 2008, 152, 452–460. [Google Scholar] [CrossRef]
- Feitosa-Felizzola, J.; Chiron, S. Occurrence and distribution of selected antibiotics in a small Mediterranean stream (Arc River, Southern France). J. Hydrol. 2009, 364, 50–57. [Google Scholar] [CrossRef]
- Liu, W.-R.; Yang, Y.-Y.; Liu, Y.-S.; Zhang, L.-J.; Zhao, J.-L.; Zhang, Q.-Q.; Zhang, M.; Zhang, J.-N.; Jiang, Y.-X.; Ying, G.-G. Biocides in wastewater treatment plants: Mass balance analysis and pollution load estimation. J. Hazard. Mater. 2017, 329, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Spina, F.; Gea, M.; Bicchi, C.; Cordero, C.; Schilirò, T.; Varese, G.C. Ecofriendly laccases treatment to challenge micropollutants issue in municipal wastewaters. Environ. Pollut. 2020, 257, 113579. [Google Scholar] [CrossRef] [PubMed]
- Barceló, D.; Žonja, B.; Ginebreda, A. Toxicity tests in wastewater and drinking water treatment processes: A complementary assessment tool to be on your radar. J. Environ. Chem. Eng. 2020, 8, 104262. [Google Scholar] [CrossRef]
- Sabri, N.A.; Schmitt, H.; Van Der Zaan, B.; Gerritsen, H.W.; Zuidema, T.; Rijnaarts, H.H.M.; Langenhoff, A.A.M. Prevalence of antibiotics and antibiotic resistance genes in a wastewater effluent-receiving river in the Netherlands. J. Environ. Chem. Eng. 2020, 8, 102245. [Google Scholar] [CrossRef]
- Luo, Y.L.; Guo, W.S.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef]
- Hatoum, R.; Potier, O.; Roques-Carmes, T.; Lemaitre, C.; Hamieh, T.; Toufaily, J.; Horn, H.; Borowska, E. Elimination of Micropollutants in Activated Sludge Reactors with a Special Focus on the Effect of Biomass Concentration. Water 2019, 11, 2217. [Google Scholar] [CrossRef]
- Rodriguez Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment technologies for emerging contaminants in water: A review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef]
- Gimeno, P.; Severyns, J.; Acuña, V.; Comas, J.; Corominas, L. Balancing environmental quality standards and infrastructure upgrade costs for the reduction of microcontaminant loads in rivers. Water Res. 2018, 143, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Ray, N.M.; Wan, J.; Khan, A.; Chakraborty, T.; Ray, M.B. Micropollutants in Wastewater: Fate and Removal Processes. In Physico-Chemical Wastewater Treatment and Resource Recovery; BoD—Books on Demand: London, UK, 2017. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, W.; Wang, Y.; Jiang, G. Suspect screening analysis of the occurrence and removal of micropollutants by GC-QTOF MS during wastewater treatment processes. J. Hazard. Mater. 2019, 376, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Schwientek, M.; Guillet, G.; Rügner, H.; Kuch, B.; Grathwohl, P. A high-precision sampling scheme to assess persistence and transport characteristics of micropollutants in rivers. Sci. Total Environ. 2016, 540, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, H.; Roques-Carmes, T.; Potier, O.; Koubaissy, B.; Pontvianne, S.; Lenouvel, A.; Guignard, C.; Mousset, E.; Poirot, H.; Toufaily, J.; et al. Iron-impregnated zeolite catalyst for efficient removal of micropollutants at very low concentration from Meurthe river. Environ. Sci. Pollut. Res. 2018, 25, 34950–34967. [Google Scholar] [CrossRef]
- Ayoub, H.; Roques-Carmes, T.; Potier, O.; Koubaissy, B.; Pontvianne, S.; Lenouvel, A.; Guignard, C.; Mousset, E.; Poirot, H.; Toufaily, J.; et al. Comparison of the removal of 21 micropollutants at actual concentration from river water using photocatalysis and photo-Fenton. SN Appl. Sci. 2019, 1, 836. [Google Scholar] [CrossRef]
- Elhadi, N.; Harrington, A.; Hill, I.; Lau, Y.L.; Krishnappan, B.G. River mixing—A state-of-the-art report. Can. J. Civ. Eng. 1984, 11, 585–609. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Mixing Zone for River Confluence; United States Environmental Protection Agency: Washington, DC, USA, 2013.
- Katopodes, N.D. Free-Surface Flow: Environmental Fluid Mechanics; Butterworth-Heinemann: Ann Arbor, MI, USA, 2019. [Google Scholar] [CrossRef]
- Sayre, W.W. Natural mixing processes in rivers. In Environmental Impact on Rivers; Chapter 6; Hsieh Wen Shen: Fort Collins, CO, USA, 1973. [Google Scholar]
- Elder, J.W. The dispersion of marked fluid in turbulent shear flow. J. Fluid Mech. 1959, 5, 544–560. [Google Scholar] [CrossRef]
- Sanders, T.G.; Adrian, D.D.; Joyce, J.M. Mixing length for representative water quality sampling. Water. Pollut. Control. Fed. 1977, 49, 2467–2478. [Google Scholar]
- Ruthven, D. The dispersion of a decaying effluent discharged continuously into a uniformly flowing stream. Water Res. 1971, 5, 343–352. [Google Scholar] [CrossRef]
- Fischer, H.B. Discussion of “Time of travel of soluble contaminants in streams”. J. San. Eng. Div. 1964, 90, 124. [Google Scholar] [CrossRef]
- Fischer, H.B. Dispersion predictions in natural streams. J. San. Eng. Div. 1968, 94, 927. [Google Scholar] [CrossRef]
- Socolofsky, S.; Jirka, G.H. Special Topics in Mixing and Transport Processes in the Environment. Coastal and Ocean Engineering Division. 2004, Texas A&M University, M.S. 3136 College Station, TX 77843-3136. Available online: https://docplayer.net/54848090-Special-topics-in-mixing-and-transport-processes-in-the-environment.html (accessed on 21 September 2020).
- Brisaert, M.; Heylen, M.; Plaizier-Vercammen, J. Investigation on the chemical stability of erythromycin in solutions using an optimization system. Pharm. Weekbl. Sci. Ed. 1996, 18, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Fairbairn, D.J.; Arnold, W.A.; Barber, B.L.; Kaufenberg, E.F.; Koskinen, W.C.; Novak, P.J.; Rice, P.J.; Swackhamer, D.L. Contaminants of Emerging Concern: Mass Balance and Comparison of Wastewater Effluent and Upstream Sources in a Mixed-Use Watershed. Environ. Sci. Technol. 2015, 50, 36–45. [Google Scholar] [CrossRef]
- Musolff, A.; Leschik, S.; Möder, M.; Strauch, G.; Reinstorf, F.; Schirmer, M. Temporal and spatial patterns of micropollutants in urban receiving waters. Environ. Pollut. 2009, 157, 3069–3077. [Google Scholar] [CrossRef]
- Castiglioni, S.; Valsecchi, S.; Polesello, S.; Rusconi, M.; Melis, M.; Palmiotto, M.; Manenti, A.; Davoli, E.; Zuccato, E. Sources and fate of perfluorinated compounds in the aqueous environment and in drinking water of a highly urbanized and industrialized area in Italy. J. Hazard. Mater. 2015, 282, 51–60. [Google Scholar] [CrossRef]
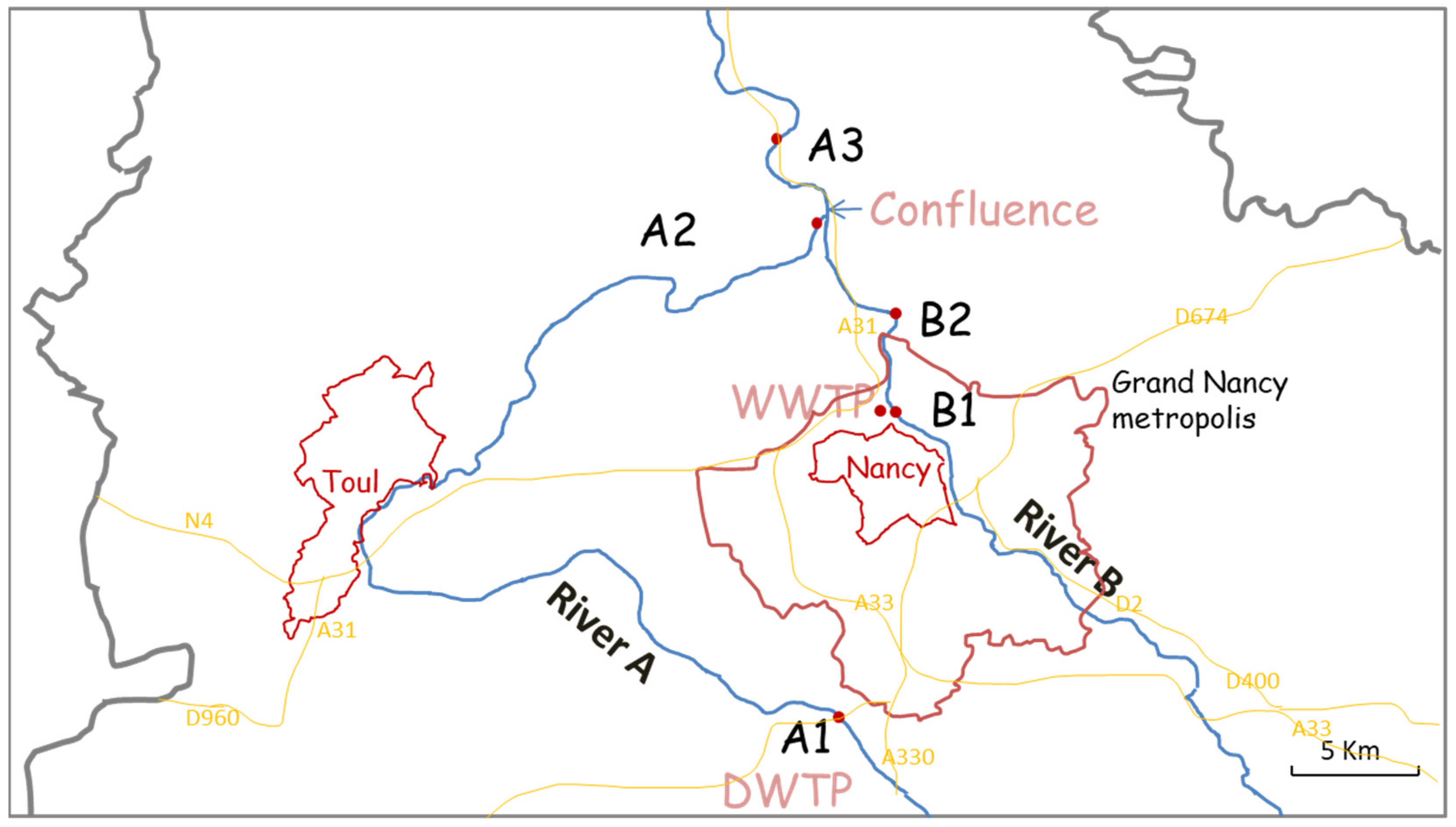
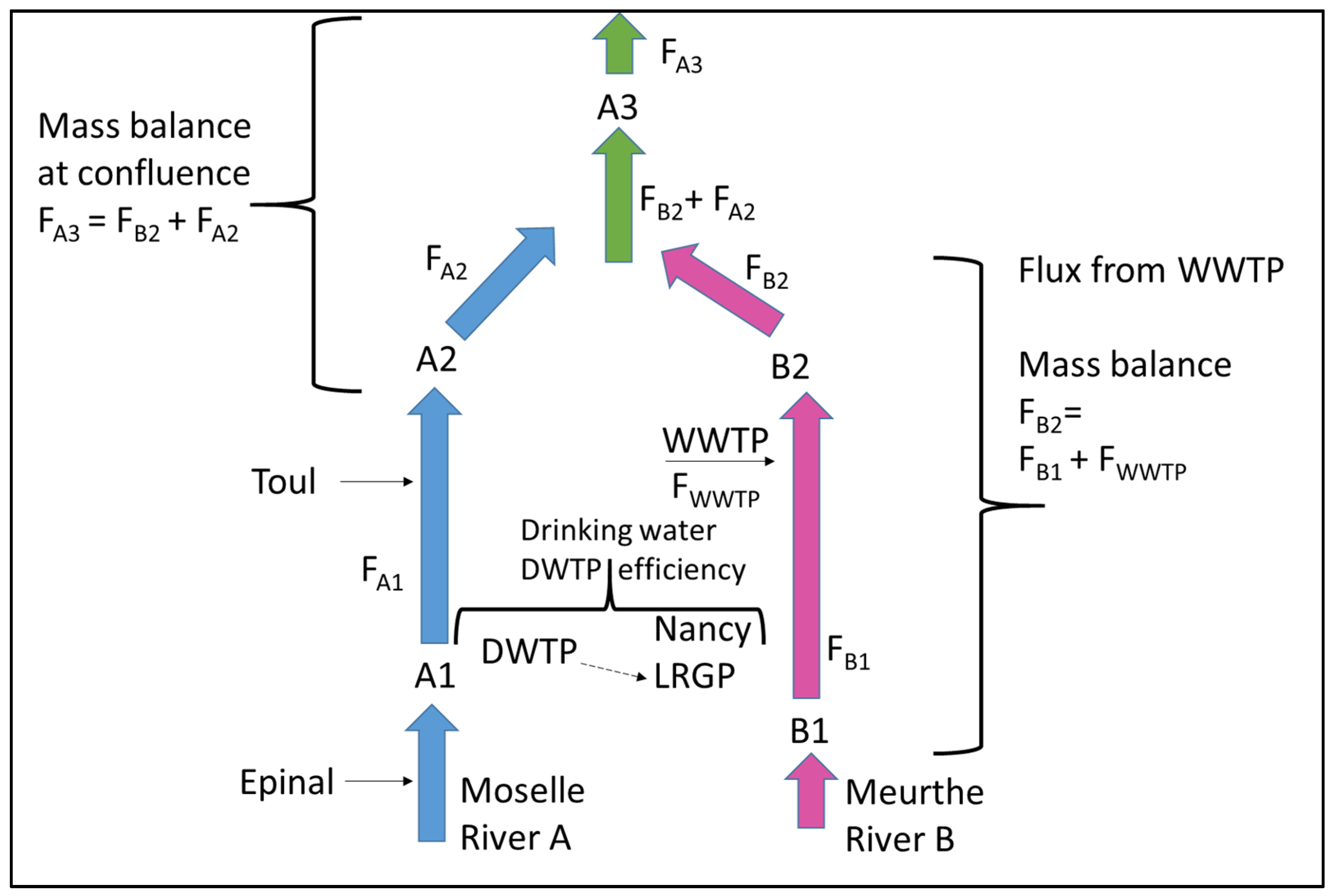
| Codes | Meaning |
|---|---|
| River A | Moselle River |
| River B | Meurthe River |
| Location A1 | Messein: on the Moselle River, place of water catchment (to produce drinking water) |
| Location A2 | Pompey: on the Moselle River, upstream of the confluence |
| Location A3 | Belleville: on the Moselle River, downstream of the confluence of the Meurthe River and the Moselle River |
| Location B1 | Malzéville: on the Meurthe River, upstream of the wastewater treatment plant |
| Location B2 | Moulin Noir: on the Meurthe River, downstream of the wastewater treatment plant and upstream of the confluence |
| FiA1t | Mass flux of micropollutant i at location A1 on the Moselle River and time t (add for all) |
| FiA2t | Mass flux of micropollutant i at location A2 on the Moselle River and time t |
| FiA3t | Mass flux of micropollutant i at location A3 on the Moselle River and time t |
| FiB1t | Mass flux of micropollutant i at location B1 on the Meurthe River and time t |
| FiB2t | Mass flux of micropollutant i at location B2 on the Meurthe River and time t |
| CiA1t | Concentration of micropollutant i at location A1 on the Moselle River and time t |
| CiA2t | Concentration of micropollutant i at location A2 on the Moselle River and time t |
| CiA3t | Concentration of micropollutant i at location A3 on the Moselle River and time t |
| CiB1t | Concentration of micropollutant i at location B1 on the Meurthe River and time t |
| CiB2t | Concentration of micropollutant i at location B2 on the Meurthe River and time t |
| FiWWTPt | Mass flux of micropollutant i released from WWTP and time t |
| CiDWt | Concentration of micropollutant i in the drinking water and time t |
| Family | Class | Compound | LoQ (ng/L) | SPE Recovery % | IM | IS |
|---|---|---|---|---|---|---|
| Addit. | Plastic additive | Bisphenol A | 21.1 | 30 | - | BPA-d16 |
| EDCs and Phs | Hormone | Estrone | 3.14 | 20 | - | Estrone-d4 |
| Hormone | 17β-Estradiol | 12.8 | 20 | - | Estrone-d4 | |
| Hormone | Ethynylestradiol | 20.6 | 9 | - | Estrone-d4 | |
| Anti-epileptic | Carbamazepine | 7.72 | 47 | + | Carbamazepine-d10 | |
| Anti-epileptic | Carbamazepine-10,11-epoxide | 2.80 | 105 | + | Carbamazepine-d10 | |
| Antibiotic | Clarithromycin | 31.3 | 11 | + | Sulfadimethoxine-d6 | |
| Chemotherapy Drug | Cyclophosphamide | 1.21 | 47 | + | Sulfadimethoxine-d6 | |
| Anti-inflammatory and pain killer | Diclofenac | 5.09 | 60 | + | Diclofenac-d4 | |
| Antibiotic | Erythromycin | 18.4 | 17 | + | Carbamazepine-d10 | |
| Anti-inflammatory | Ibuprofen | 0.92 | 75 | - | Ibuprofen-d3 | |
| Anti-inflammatory | Ketoprofen | 0.72 | 84 | + | Carbamazepine-d10 | |
| Anesthetic | Lidocaine | 18.0 | 39 | + | Sulfadimethoxine-d6 | |
| Anti-inflammatory | Naproxen | 4.00 | 66 | + | Diclofenac-d4 | |
| Antibiotic | Sulfadimethoxine | 9.50 | 72 | + | Sulfadimethoxine-d6 | |
| Antibiotic | Sulfadimidine | 9.00 | 65 | + | Sulfadimethoxine-d6 | |
| Antibiotic | Sulfamethoxazole | 1.50 | 64 | + | Sulfadimethoxine-d6 | |
| Antibiotic | Sulfathiazole | 1.20 | 61 | + | Sulfadimethoxine-d6 | |
| PCP | Antiseptic | Triclosan | 39.0 | 8 | - | Estrone-d4 |
| PFCs | PFC | PFOA | 6.54 | 74 | - | Ibuprofen-d3 |
| PFC | PFOS | 4.29 | 35 | - | Ibuprofen-d3 |
| Date Micropollutant (i) | September fit% | January fit% | October fit% | Average fit% |
|---|---|---|---|---|
| Clarithromycin | 6 | −4 | 9 | 6.3 |
| Sulfamethoxazole | −6 | −20 | −6 | 10.7 |
| Lidocaine | 15 | 15 | 8 | 12.6 |
| Ibuprofen | −13 | 27 | −10 | 16.7 |
| PFOS | −12 | 16 | −34 | 20.7 |
| Carbamazepine | −19 | −28 | 18 | 21.7 |
| Bisphenol A | 14 | 28 | 28 | 23.3 |
| Diclofenac | 46 | 12 | 15 | 24.3 |
| Date Micropollutant (i) | September FiWWTPt (g/day) | January FiWWTPt (g/day) | October FiWWTPt (g/day) | Average FiWWTPt (g/day) |
|---|---|---|---|---|
| Estrone | - | 13 | 5 | 6 |
| PFOS | 1 | 30 | 0 | 10 |
| Ketoprofen | 12 | 34 | 6 | 17 |
| Lidocaine | 27 | - | 33 | 20 |
| Naproxen | 10 | 63 | 0 | 24 |
| Carbamazepine | 23 | 25 | 25 | 24 |
| Sulfamethoxazole | 34 | 24 | 20 | 26 |
| Ibuprofen | 1 | 107 | 1 | 36 |
| Diclofenac | 56 | 88 | 85 | 76 |
| Bisphenol A | 117 | 124 | 39 | 93 |
| Clarithromycin | 84 | 153 | 417 | 218 |
| Date | September | January | October | March |
|---|---|---|---|---|
| Micropollutant (i) | C (ng/L) | C (ng/L) | C (ng/L) | C (ng/L) |
| Sulfamethoxazole | 64 | 32 | 23 | 6 |
| Bisphenol A | 236 | 92 | 80 | 54 |
| Ibuprofen | 9 | 97 | 18 | 22 |
| Naproxen | 30 | 45 | 13 | 20 |
| Ketoprofen | 17 | 18 | 7 | 7 |
| Triclosan | - | 60 | 13 | 13 |
| Estrone | - | 7 | 6 | - |
| Clarithromycin | 148 | 140 | 639 | 16 |
| Diclofenac | 87 | 50 | 120 | 51 |
| Carbamazepine | 54 | 32 | 68 | 19 |
| Lidocaine | 57 | - | 47 | - |
| Carbamazepine-10,11-epoxide | 3 | - | 3 | - |
| PFOS | 14 | 27 | 20 | 6 |
| PFOA | - | - | 3 | 0.8 |
| Estrone | - | 7 | 6 | 4 |
| Erythromycin | - | 914 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayoub, H.; Potier, O.; Koubaissy, B.; Pontvianne, S.; Lenouvel, A.; Guignard, C.; Poirot, H.; Toufaily, J.; Hamieh, T.; Roques-Carmes, T. A Short Cost-Effective Methodology for Tracing the Temporal and Spatial Anthropogenic Inputs of Micropollutants into Ecosystems: Verified Mass-Balance Approach Applied to River Confluence and WWTP Release. Water 2022, 14, 4100. https://doi.org/10.3390/w14244100
Ayoub H, Potier O, Koubaissy B, Pontvianne S, Lenouvel A, Guignard C, Poirot H, Toufaily J, Hamieh T, Roques-Carmes T. A Short Cost-Effective Methodology for Tracing the Temporal and Spatial Anthropogenic Inputs of Micropollutants into Ecosystems: Verified Mass-Balance Approach Applied to River Confluence and WWTP Release. Water. 2022; 14(24):4100. https://doi.org/10.3390/w14244100
Chicago/Turabian StyleAyoub, Hawraa, Olivier Potier, Bachar Koubaissy, Steve Pontvianne, Audrey Lenouvel, Cédric Guignard, Hélène Poirot, Joumana Toufaily, Tayssir Hamieh, and Thibault Roques-Carmes. 2022. "A Short Cost-Effective Methodology for Tracing the Temporal and Spatial Anthropogenic Inputs of Micropollutants into Ecosystems: Verified Mass-Balance Approach Applied to River Confluence and WWTP Release" Water 14, no. 24: 4100. https://doi.org/10.3390/w14244100
APA StyleAyoub, H., Potier, O., Koubaissy, B., Pontvianne, S., Lenouvel, A., Guignard, C., Poirot, H., Toufaily, J., Hamieh, T., & Roques-Carmes, T. (2022). A Short Cost-Effective Methodology for Tracing the Temporal and Spatial Anthropogenic Inputs of Micropollutants into Ecosystems: Verified Mass-Balance Approach Applied to River Confluence and WWTP Release. Water, 14(24), 4100. https://doi.org/10.3390/w14244100