Coupling Hydrochemistry and Stable Isotopes (δ2H, δ18O and 87Sr/86Sr) to Identify the Major Factors Affecting the Hydrochemical Process of Groundwater and Surface Water in the Lower Reaches of the Yarlung-Zangbo River, Southern Tibet, Southwestern China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Study Area
2.2. Field Samples and Laboratory Analysis
3. Results
3.1. Statistical Results of Hydrochemical Parameters
3.2. Hydrochemical Type
3.3. Hydrogen and Oxygen Isotopes
3.4. Sr Concentration and Sr Isotope
4. Discussion
4.1. Multivariate Statistical Analysis
4.1.1. Correlation Analysis
4.1.2. Principal Component Analysis
4.1.3. Self-Organizing Map
4.2. Governing Factors of Hydrochemical Compositions
4.2.1. Gibbs Analysis
4.2.2. Ion Ratio Analysis
4.2.3. Ion Exchange
4.2.4. Saturation Index Analysis
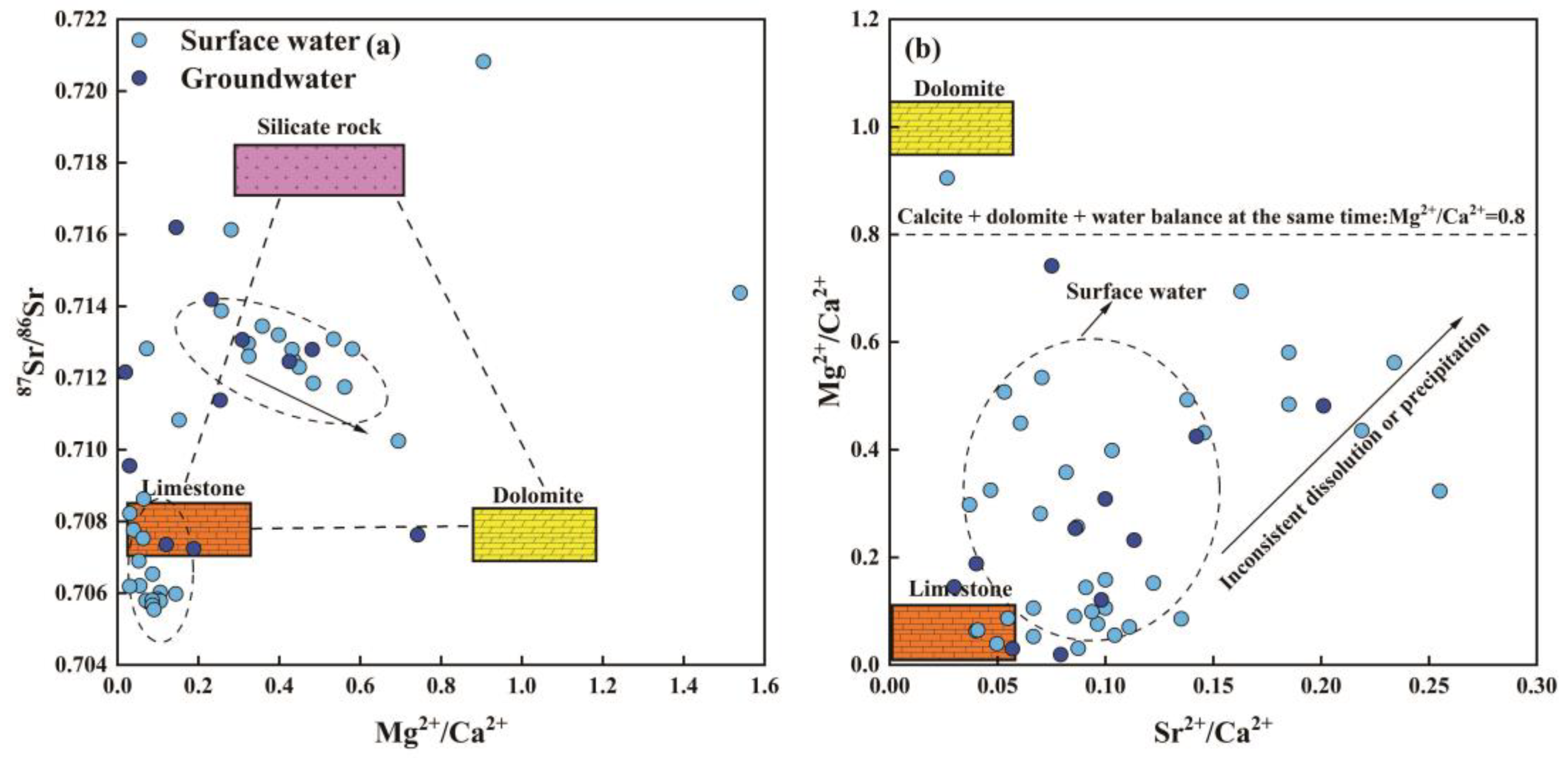
4.2.5. Water–Rock Interaction Implied by Sr Isotope
4.2.6. Recharge Sources Determined by D-O Isotopes
4.3. Assessment of Groundwater Quality Based on EWQI
4.4. Hydrochemical Conceptual Model of the Yarlung-Zangbo River
5. Conclusions
- (1)
- Surface water and groundwater were weakly alkaline and belonged to very soft to moderately hard fresh water. Major cations in surface water and groundwater ranked as Ca2+ > Mg2+ > Na+ > K+. Major anion concentrations in surface water were SO42− > HCO3− > Cl− > F− > NO3−, and major anion concentrations in groundwater were HCO3− > SO42− > Cl− > NO3− > F−, respectively. The hydrochemical type of surface water is mainly Ca-HCO3 (mainstream) and Ca-SO4-HCO3 (tributary), while the hydrochemical type of groundwater was mainly Ca-SO4-HCO3.
- (2)
- Hydrochemical compositions of surface water and groundwater were mainly affected by water–rock interaction. Silicate weathering, calcite dissolution, and cation exchange were involved in water–rock interaction of surface water and groundwater. Evaporite dissolution locally occurred in the tributary area. Surface water and groundwater were recharged by atmosphere precipitation and local snow melting. Stronger evaporation occurred in the middle reaches than that in the upper reaches and lower reaches of the Yarlung-Zangbo River.
- (3)
- The EWQI values indicated most of the surface water and all groundwater samples belonged to excellent water of class 1. Only 2 surface water samples, having higher fluoride concentrations of 1.62 mg/L and 2.02 mg/L, were good water of class 2. Therefore, surface water and groundwater reach the standard of drinking purpose in the lower reaches of the Yarlung-Zangbo River.
- (4)
- Hydrochemical process displayed a changing trend along the Yarlung-Zangbo River. Hydrochemical type was Ca-Na-HCO3-SO4 in the upper reaches of the Yarlung-Zangbo River, whose hydrochemical compositions were determined by silicate weathering and carbonate dissolution. Hydrochemical type was Ca-HCO3 and Ca-HCO3-SO4 in the middle reaches of the Yarlung-Zangbo River, and the main ions were derived from carbonate and evaporite dissolution. Hydrochemical type was Ca-HCO3 and Ca-SO4-HCO3 in the lower reaches of the Yarlung-Zangbo River, and the main ions were derived from carbonate and evaporite dissolution. Therefore, the Yarlung-Zangbo River is controlled by the dissolution of carbonate rocks and local silicate weather and evaporate dissolution. In this study, a hydrochemical-type transition of Ca-Na-HCO3-SO4 → Ca-HCO3 and Ca-HCO3-SO4 → Ca-HCO3 and Ca-SO4-HCO3 has been identified along the Yarlung-Zangbo River. The different hydrochemical types would be produced by relevant water-rock interactions. The achievements would be helpful for understanding the hydrochemical processes in the catchment of the Yarlung-Zangbo River, providing a vital reference for water resource management.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, C.; Gao, X.; Li, S.; Bundschuh, J. A review of the distribution, sources, genesis, and environmental concerns of salinity in groundwater. Environ. Sci. Pollut. Res. 2020, 27, 41157–41174. [Google Scholar] [CrossRef] [PubMed]
- Li, P. To Make the Water Safer. Expo. Health 2020, 12, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.G.; Nash, S.; Olbert, A.I. A review of water quality index models and their use for assessing surface water quality. Ecol. Indic. 2021, 122, 107218. [Google Scholar] [CrossRef]
- Elshall, A.S.; Arik, A.D.; El-Kadi, A.I.; Pierce, S.; Ye, M.; Burnett, K.M.; Wada, C.A.; Bremer, L.L.; Chun, G. Groundwater sustainability: A review of the interactions between science and policy. Environ. Res. Lett. 2020, 15, 093004. [Google Scholar] [CrossRef]
- Medici, G.; Langman, J.B. Pathways and Estimate of Aquifer Recharge in a Flood Basalt Terrain; A Review from the South Fork Palouse River Basin (Columbia River Plateau, USA). Sustainability 2022, 14, 11349. [Google Scholar] [CrossRef]
- Qu, S.; Duan, L.; Shi, Z.; Liang, X.; Lv, S.; Wang, G.; Liu, T.; Yu, R. Hydrochemical assessments and driving forces of groundwater quality and potential health risks of sulfate in a coalfield, northern Ordos Basin, China. Sci. Total Environ. 2022, 835, 155519. [Google Scholar] [CrossRef]
- Subba Rao, N.; Dinakar, A.; Sun, L. Estimation of groundwater pollution levels and specific ionic sources in the groundwater, using a comprehensive approach of geochemical ratios, pollution index of groundwater, unmix model and land use/land cover—A case study. J. Contam. Hydrol. 2022, 248, 103990. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, K.; Qian, H.; Gao, Y.; Fang, Y.; Xiao, S.; Tang, S.; Zhang, Q.; Qu, W.; Ren, W. Characterization of soil salinization and its driving factors in a typical irrigation area of Northwest China. Sci. Total Environ. 2022, 837, 155808. [Google Scholar] [CrossRef]
- Mao, G.; Zhao, Y.; Zhang, F.; Liu, J.; Huang, X. Spatiotemporal variability of heavy metals and identification of potential source tracers in the surface water of the Lhasa River basin. Environ. Sci. Pollut. Res. 2019, 26, 7442–7452. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Chen, H.; Li, Q.; Yu, C.; Huang, X.; Guo, H. Water environment in the Tibetan Plateau: Heavy metal distribution analysis of surface sediments in the Yarlung Tsangpo River Basin. Environ. Geochem. Health 2020, 42, 2451–2469. [Google Scholar] [CrossRef]
- Tan, H.; Chen, X.; Shi, D.; Rao, W.; Liu, J.; Liu, J.; Eastoe, C.J.; Wang, J. Base flow in the Yarlungzangbo River, Tibet, maintained by the isotopically—depleted precipitation and groundwater discharge. Sci. Total Environ. 2021, 759, 143510. [Google Scholar] [CrossRef]
- Shi, D.; Tan, H.; Chen, X.; Rao, W.; Basang, R. Uncovering the mechanisms of seasonal river–groundwater circulation using isotopes and water chemistry in the middle reaches of the Yarlungzangbo River, Tibet. J. Hydrol. 2021, 603, 127010. [Google Scholar] [CrossRef]
- Cao, H.-W.; Pei, Q.-M.; Santosh, M.; Li, G.-M.; Zhang, L.-K.; Zhang, X.-F.; Zhang, Y.-H.; Zou, H.; Dai, Z.-W.; Lin, B.; et al. Himalayan leucogranites: A review of geochemical and isotopic characteristics, timing of formation, genesis, and rare metal mineralization. Earth. Sci. Rev. 2022, 234, 104229. [Google Scholar] [CrossRef]
- Zhang, Y.-h.; Cao, H.-w.; Hollis, S.P.; Tang, L.; Xu, M.; Jiang, J.-s.; Gao, S.-b.; Wang, Y.-s. Geochronology, geochemistry and Sr-Nd-Pb-Hf isotopes of the Early Paleogene gabbro and granite from Central Lhasa, southern Tibet: Petrogenesis and tectonic implications. Int. Geol. Rev. 2019, 61, 868–894. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Yao, R.-W.; Wang, Y.; Duo, J.; Cao, H.-W. Zircon U–Pb and sericite Ar–Ar geochronology, geochemistry and S–Pb–Hf isotopes of the Zebuxia Pb–Zn deposit, Tibet, southwestern China. Ore. Geol. Rev. 2022, 148, 104999. [Google Scholar] [CrossRef]
- Yan, J.; Chen, J.; Zhang, W. Impact of land use and cover on shallow groundwater quality in Songyuan city, China: A multivariate statistical analysis. Environ. Pollut. 2022, 307, 119532. [Google Scholar] [CrossRef]
- Gaillardet, J.; Dupré, B.; Louvat, P.; Allègre, C.J. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar] [CrossRef]
- Yan, Y.P.; Niu, F.X.; Liu, J.; Liu, X.T.; Li, Y.; Peng, H.; Yan, D.H.; Xiao, S.B. Hydrochemical characteristics and sources of the upper Yarlung Zangbo River in summer. China Environ. Sci. 2022, 42, 815–825, (In Chinese and English Abstract). [Google Scholar]
- Chen, W. Hydrochemical Characteristics in the Upper Reaches of the Yarlung Zangbo River in Summer. Master’s Thesis, China Three Gorges University, Yichang, China, 2021. (In Chinese and English Abstract). [Google Scholar]
- Liu, J.; Li, Y.; Gao, Z.; Wang, M.; Ma, Y.; Shi, M.; Zhang, H. Ionic composition characteristics and their controlling factors in the main stream of the Yarlung Zangbo River. J. Glaciol. Geocryol. 2019, 41, 697–708, (In Chinese and English Abstract). [Google Scholar]
- Li, Y.Z.; Gao, Z.J.; Liu, J.T.; Wang, M.; Han, C. Hydrogeochemical and isotopic characteristics of spring water in the Yarlung Zangbo River Basin, Qinghai-Tibet Plateau, Southwest China. J. Mt. Sci. 2021, 18, 2061–2078. [Google Scholar] [CrossRef]
- Duan, R.; Li, P.; Wang, L.; He, X.; Zhang, L. Hydrochemical characteristics, hydrochemical processes and recharge sources of the geothermal systems in Lanzhou City, northwestern China. Urban Clim. 2022, 43, 101152. [Google Scholar] [CrossRef]
- Li, P.; He, X.; Guo, W. Spatial groundwater quality and potential health risks due to nitrate ingestion through drinking water: A case study in Yan’an City on the Loess Plateau of northwest China. Hum. Ecol. Risk Assess. Int. J. 2019, 25, 11–31. [Google Scholar] [CrossRef]
- Zhang, L.; Li, P.; He, X. Interactions between surface water and groundwater in selected tributaries of the Wei River (China) revealed by hydrochemistry and stable isotopes. Hum. Ecol. Risk Assess. Int. J. 2021, 28, 79–99. [Google Scholar] [CrossRef]
- Seyedrahimi-Niaraq, M.; Mahdiyanfar, H.; Mokhtari, A.R. Integrating principal component analysis and U-statistics for mapping polluted areas in mining districts. J. Geochem. Explor. 2022, 234, 106924. [Google Scholar] [CrossRef]
- Zeinalzadeh, K.; Rezaei, E. Determining spatial and temporal changes of surface water quality using principal component analysis. J. Hydrol. Reg. Stud. 2017, 13, 1–10. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, M.; Li, X.; Qi, J.; Zhang, Q.; Guo, J.; Yu, L.; Zhao, R. Hydrochemical Characteristics and Multivariate Statistical Analysis of Natural Water System: A Case Study in Kangding County, Southwestern China. Water 2018, 10, 80. [Google Scholar] [CrossRef] [Green Version]
- Bigdeli, A.; Maghsoudi, A.; Ghezelbash, R. Application of self-organizing map (SOM) and K-means clustering algorithms for portraying geochemical anomaly patterns in Moalleman district, NE Iran. J. Geochem. Explor. 2022, 233, 106923. [Google Scholar] [CrossRef]
- Qu, S.; Shi, Z.; Liang, X.; Wang, G.; Han, J. Multiple factors control groundwater chemistry and quality of multi-layer groundwater system in Northwest China coalfield— Using self-organizing maps (SOM). J. Geochem. Explor. 2021, 227, 106795. [Google Scholar] [CrossRef]
- Yao, R.; Yan, Y.; Wei, C.; Luo, M.; Xiao, Y.; Zhang, Y. Hydrochemical Characteristics and Groundwater Quality Assessment Using an Integrated Approach of the PCA, SOM, and Fuzzy c-Means Clustering: A Case Study in the Northern Sichuan Basin. Front. Environ. Sci. 2022, 10, 648. [Google Scholar] [CrossRef]
- Yu, J.; Tian, Y.; Wang, X.; Zheng, C. Using machine learning to reveal spatiotemporal complexity and driving forces of water quality changes in Hong Kong marine water. J. Hydrol. 2021, 603, 126841. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Li, C.; Gao, X.; Liu, Y.; Wang, Y. Impact of anthropogenic activities on the enrichment of fluoride and salinity in groundwater in the Yuncheng Basin constrained by Cl/Br ratio, δ18O, δ2H, δ13C and δ7Li isotopes. J. Hydrol. 2019, 579, 124211. [Google Scholar] [CrossRef]
- Subba Rao, N.; Sunitha, B.; Sun, L.; Deepthi Spandana, B.; Chaudhary, M. Mechanisms controlling groundwater chemistry and assessment of potential health risk: A case study from South India. Geochemistry 2020, 80, 125568. [Google Scholar] [CrossRef]
- Gao, X.; Li, X.; Wang, W.; Li, C. Human Activity and Hydrogeochemical Processes Relating to Groundwater Quality Degradation in the Yuncheng Basin, Northern China. Int. J. Environ. Res. Public Health 2020, 17, 867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Y.; Hao, Q.; Zhang, Y.; Zhu, Y.; Yin, S.; Qin, L.; Li, X. Investigating sources, driving forces and potential health risks of nitrate and fluoride in groundwater of a typical alluvial fan plain. Sci. Total Environ. 2022, 802, 149909. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, K.; Hao, Q.; Xiao, D.; Zhu, Y.; Yin, S.; Zhang, Y. Hydrogeochemical insights into the signatures, genesis and sustainable perspective of nitrate enriched groundwater in the piedmont of Hutuo watershed, China. Catena 2022, 212, 106020. [Google Scholar] [CrossRef]
- Shoedarto, R.M.; Tada, Y.; Kashiwaya, K.; Koike, K.; Iskandar, I.; Malik, D.; Bratakusuma, B. Investigation of meteoric water and parent fluid mixing in a two-phase geothermal reservoir system using strontium isotope analysis: A case study from Southern Bandung, West Java, Indonesia. Geothermics 2021, 94, 102096. [Google Scholar] [CrossRef]
- Temizel, E.H.; Gültekin, F.; Ersoy, A.F.; Gülbay, R.K. Multi-isotopic (O, H, C, S, Sr, B, Li) characterization of waters in a low-enthalpy geothermal system in Havza (Samsun), Turkey. Geothermics 2021, 97, 102240. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, X.; Liao, L.; Tian, J.; Li, Y.; Shi, Z.; Liu, F.; Ouyang, S. Hydrogeochemical Characteristic of Geothermal Water and Precursory Anomalies along the Xianshuihe Fault Zone, Southwestern China. Water 2022, 14, 550. [Google Scholar] [CrossRef]
- Cherchali, M.E.H.; Liégeois, J.P.; Mesbah, M.; Daas, N.; Amrous, K.; Ouarezki, S.A. Central Hoggar groundwaters and the role of shear zones: 87Sr/86Sr, δ18O, δ2H and 14C isotopes, geochemistry and water-rock interactions. Appl. Geochem. 2021, 137, 105179. [Google Scholar] [CrossRef]
- Fantong, W.Y.; Kamtchueng, B.T.; Ishizaki, Y.; Fru, E.C.; Fantong, E.B.; Wirmvem, M.J.; Aka, F.T.; Nlend, B.; Harman, D.; Ueda, A.; et al. Major ions, δ18O, δ13C and 87Sr/86Sr compositions of water and precipitates from springs along the Cameroon volcanic line (Cameroon, west Africa): Implications for provenance and volcanic hazards. J. Afr. Earth Sci. 2019, 150, 12–22. [Google Scholar] [CrossRef]
- Song, H.W.; Meng, Y.C.; Jiang, F.T.; Li, L.X.; Li, Y.Q.; Du, C.H. Isotope characteristics of surface water and groundwater in the middle reaches of Yarlung Zangbo river and their indicators. J. Arid Land Resour. Environ. 2021, 35, 122–128, (In Chinese and English Abstract). [Google Scholar]
- Craig, H. Isotopic variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef]
- Adimalla, N. Application of the Entropy Weighted Water Quality Index (EWQI) and the Pollution Index of Groundwater (PIG) to Assess Groundwater Quality for Drinking Purposes: A Case Study in a Rural Area of Telangana State, India. Arch. Environ. Contam. Toxicol. 2021, 80, 31–40. [Google Scholar] [CrossRef]
- Amiri, V.; Rezaei, M.; Sohrabi, N. Groundwater quality assessment using entropy weighted water quality index (EWQI) in Lenjanat, Iran. Environ. Earth Sci. 2014, 72, 3479–3490. [Google Scholar] [CrossRef]
- Unigwe, C.O.; Egbueri, J.C. Drinking water quality assessment based on statistical analysis and three water quality indices (MWQI, IWQI and EWQI): A case study. Environ. Dev. Sustain. 2022, 1–22. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, Y.; Wang, Y.; Huang, X.; Xiao, Y.; Pei, Q. Hydrochemistry, quality and potential health risk appraisal of nitrate enriched groundwater in the Nanchong area, southwestern China. Sci. Total Environ. 2021, 784, 147186. [Google Scholar] [CrossRef]



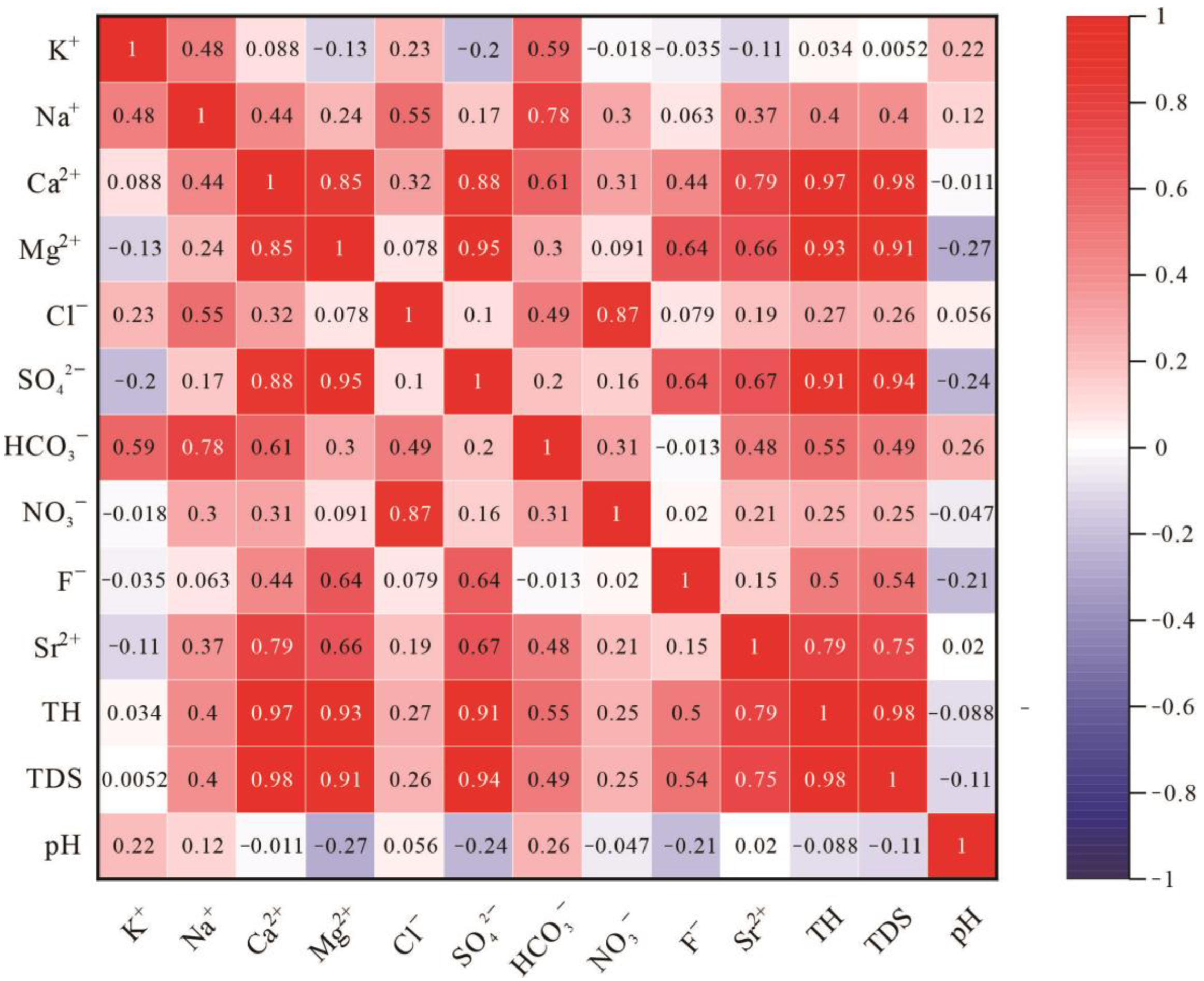










| Parameters | pH | TH | TDS | K+ | Na+ | Ca2+ | Mg2+ | Cl− | SO42− | HCO3− | NO3− | F− | Sr2+ | 87Sr/86Sr | δ18O | δD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Max | 9.20 | 343.00 | 470.00 | 1.24 | 6.16 | 82.70 | 44.90 | 2.87 | 409.00 | 92.10 | 0.36 | 2.02 | 0.72 | 0.721 | −14.21 | −97.12 |
| Min | 7.20 | 9.00 | 11.00 | 0.06 | 0.24 | 4.00 | 0.38 | 0.00 | 0.01 | 2.50 | 0.00 | 0.00 | 0.02 | 0.706 | −17.55 | −131.82 |
| Mean | 8.35 | 92.77 | 118.42 | 0.48 | 1.62 | 28.28 | 7.31 | 0.39 | 67.52 | 43.08 | 0.12 | 0.14 | 0.17 | 0.710 | −15.59 | −112.43 |
| SD | 0.43 | 83.86 | 113.72 | 0.25 | 1.52 | 22.31 | 9.66 | 0.49 | 88.31 | 23.48 | 0.08 | 0.42 | 0.20 | - | - | - |
| CV | 0.05 | 0.90 | 0.96 | 0.52 | 0.94 | 0.79 | 1.32 | 1.28 | 1.31 | 0.54 | 0.69 | 2.91 | 1.18 | - | - | - |
| Skewness | −0.94 | 1.00 | 1.22 | 1.03 | 1.54 | 0.92 | 1.77 | 2.93 | 1.77 | 0.46 | 0.68 | 3.89 | 1.35 | - | - | - |
| Kurtosis | 1.09 | 0.23 | 0.83 | 1.16 | 1.45 | −0.24 | 3.80 | 13.87 | 3.77 | −0.68 | 0.02 | 14.80 | 0.49 | - | - | - |
| Max | 8.90 | 259.00 | 357.00 | 3.07 | 28.20 | 82.10 | 20.80 | 6.74 | 213.00 | 238.00 | 4.19 | 0.19 | 0.71 | 0.716 | −14.73 | −108.65 |
| Min | 7.90 | 54.00 | 67.00 | 0.19 | 1.02 | 21.10 | 0.38 | 0.00 | 6.44 | 49.70 | 0.00 | 0.00 | 0.04 | 0.707 | −17.33 | −132.04 |
| Mean | 8.47 | 158.18 | 196.91 | 1.46 | 6.27 | 51.91 | 9.35 | 1.59 | 80.07 | 117.19 | 0.68 | 0.09 | 0.27 | 0.711 | −16.21 | −122.53 |
| SD | 0.31 | 75.54 | 102.43 | 1.11 | 7.18 | 22.60 | 7.34 | 1.86 | 72.76 | 49.98 | 1.14 | 0.07 | 0.21 | - | - | - |
| CV | 0.04 | 0.48 | 0.52 | 0.76 | 1.14 | 0.44 | 0.78 | 1.16 | 0.91 | 0.43 | 1.67 | 0.74 | 0.79 | - | - | - |
| Skewness | −0.61 | −0.22 | 0.30 | 0.20 | 2.91 | −0.04 | 0.29 | 2.25 | 0.72 | 1.20 | 3.00 | −0.10 | 0.98 | - | - | - |
| Kurtosis | −0.69 | −1.81 | −1.42 | −1.93 | 9.10 | −1.95 | −1.39 | 5.14 | −1.20 | 1.82 | 9.42 | −1.64 | −0.14 | - | - | - |
| River | pH | TDS | K+ | Na+ | Ca2+ | Mg2+ | Cl− | SO42− | HCO3− | NO3− | F− |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Global River | 8.00 | 120.00 | 2.30 | 6.30 | 15.00 | 4.10 | 7.80 | 11.20 | 58.40 | 1.00 | - |
| Upper reaches | 7.77 | 102.50 | 1.08 | 17.67 | 20.24 | 4.37 | 3.25 | 20.60 | 70.48 | 0.17 | - |
| Middle reaches | 8.05 | 304.82 | 1.29 | 8.86 | 41.53 | 17.97 | 6.35 | 98.17 | 107.98 | 1.80 | - |
| Lower reaches (this study) | 8.35 | 118.42 | 0.48 | 1.62 | 28.28 | 7.31 | 0.39 | 67.52 | 43.08 | 0.12 | 0.14 |
| Index | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| TH | 0.981 | −0.099 | 0.080 | −0.054 |
| TDS | 0.975 | −0.139 | 0.055 | −0.023 |
| Ca2+ | 0.974 | −0.002 | 0.084 | −0.096 |
| Mg2+ | 0.894 | −0.380 | 0.055 | 0.082 |
| SO42− | 0.889 | −0.420 | −0.030 | 0.007 |
| Sr2+ | 0.790 | −0.026 | 0.095 | −0.449 |
| HCO3− | 0.596 | 0.687 | 0.275 | 0.001 |
| Na+ | 0.512 | 0.661 | 0.133 | 0.165 |
| K+ | 0.072 | 0.657 | 0.447 | 0.484 |
| Cl− | 0.394 | 0.656 | −0.592 | 0.070 |
| NO3− | 0.356 | 0.462 | −0.770 | −0.097 |
| F− | 0.528 | −0.394 | −0.054 | 0.542 |
| pH | −0.097 | 0.433 | 0.411 | −0.466 |
| Eigenvalue | 6.270 | 2.678 | 1.438 | 1.008 |
| Variance/% | 48.229 | 20.600 | 11.065 | 7.754 |
| Cumulative % of variance | 48.229 | 68.829 | 79.894 | 87.648 |
| Reach | Upper Reaches | Middle Reaches | Lower Reaches |
|---|---|---|---|
| Geographical position | Jiema yangzongqu—Lizi section | Shigatse—Gyaca section | Gyaca—Millin section |
| Hydrochemical type | Ca-Na-HCO3-SO4 | Ca-HCO3 and Ca-HCO3-SO4 | Ca-HCO3 and Ca-SO4-HCO3 |
| Controlling factors | Water–rock interaction | Water–rock interaction | Water–rock interaction, Atmospheric precipitation |
| Ion source | Silicate rock, Carbonate rock, Evaporite rock | Carbonate rock, Evaporite rock, Silicate rock | Carbonate rock, Silicate rock, Evaporite rock |
| Influence of human factors | Very low | Very low | Low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Yuan, X.; Guo, H.; Zhang, Y.; Cao, H.; Luo, T.; Gong, Z.; Huang, H. Coupling Hydrochemistry and Stable Isotopes (δ2H, δ18O and 87Sr/86Sr) to Identify the Major Factors Affecting the Hydrochemical Process of Groundwater and Surface Water in the Lower Reaches of the Yarlung-Zangbo River, Southern Tibet, Southwestern China. Water 2022, 14, 3906. https://doi.org/10.3390/w14233906
Yu X, Yuan X, Guo H, Zhang Y, Cao H, Luo T, Gong Z, Huang H. Coupling Hydrochemistry and Stable Isotopes (δ2H, δ18O and 87Sr/86Sr) to Identify the Major Factors Affecting the Hydrochemical Process of Groundwater and Surface Water in the Lower Reaches of the Yarlung-Zangbo River, Southern Tibet, Southwestern China. Water. 2022; 14(23):3906. https://doi.org/10.3390/w14233906
Chicago/Turabian StyleYu, Xiao, Xingcheng Yuan, Hongyang Guo, Yunhui Zhang, Huawen Cao, Tongming Luo, Zhaocheng Gong, and Haoqing Huang. 2022. "Coupling Hydrochemistry and Stable Isotopes (δ2H, δ18O and 87Sr/86Sr) to Identify the Major Factors Affecting the Hydrochemical Process of Groundwater and Surface Water in the Lower Reaches of the Yarlung-Zangbo River, Southern Tibet, Southwestern China" Water 14, no. 23: 3906. https://doi.org/10.3390/w14233906
APA StyleYu, X., Yuan, X., Guo, H., Zhang, Y., Cao, H., Luo, T., Gong, Z., & Huang, H. (2022). Coupling Hydrochemistry and Stable Isotopes (δ2H, δ18O and 87Sr/86Sr) to Identify the Major Factors Affecting the Hydrochemical Process of Groundwater and Surface Water in the Lower Reaches of the Yarlung-Zangbo River, Southern Tibet, Southwestern China. Water, 14(23), 3906. https://doi.org/10.3390/w14233906








