Distribution and Demography of Antarctic Krill and Salps in the Atlantic Sector of the Southern Ocean during Austral Summer 2021–2022
Abstract
:1. Introduction
2. Materials and Methods
2.1. Remote Sensing Methods
2.2. CTD Measurements and Sampling
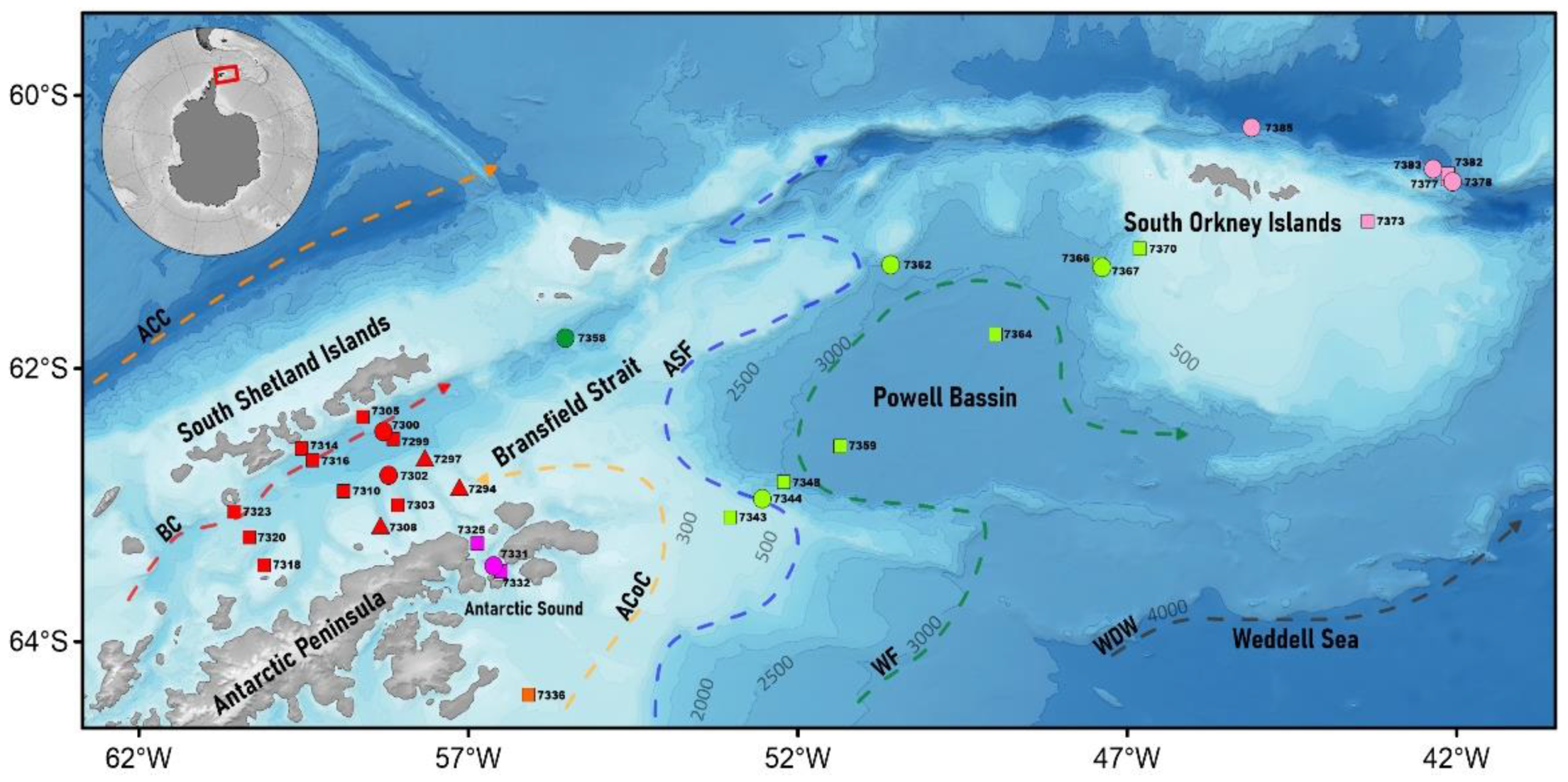
3. Results
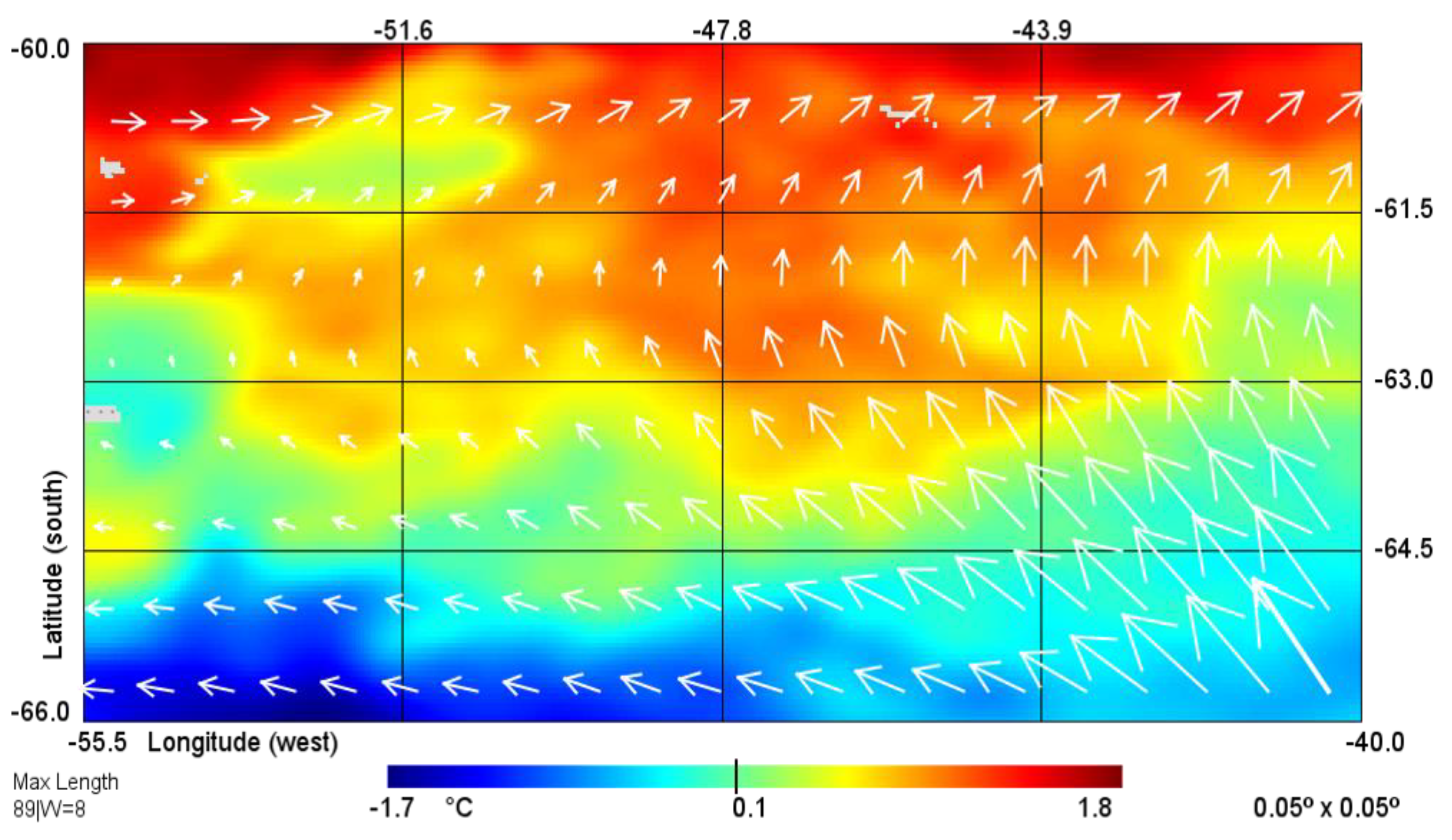
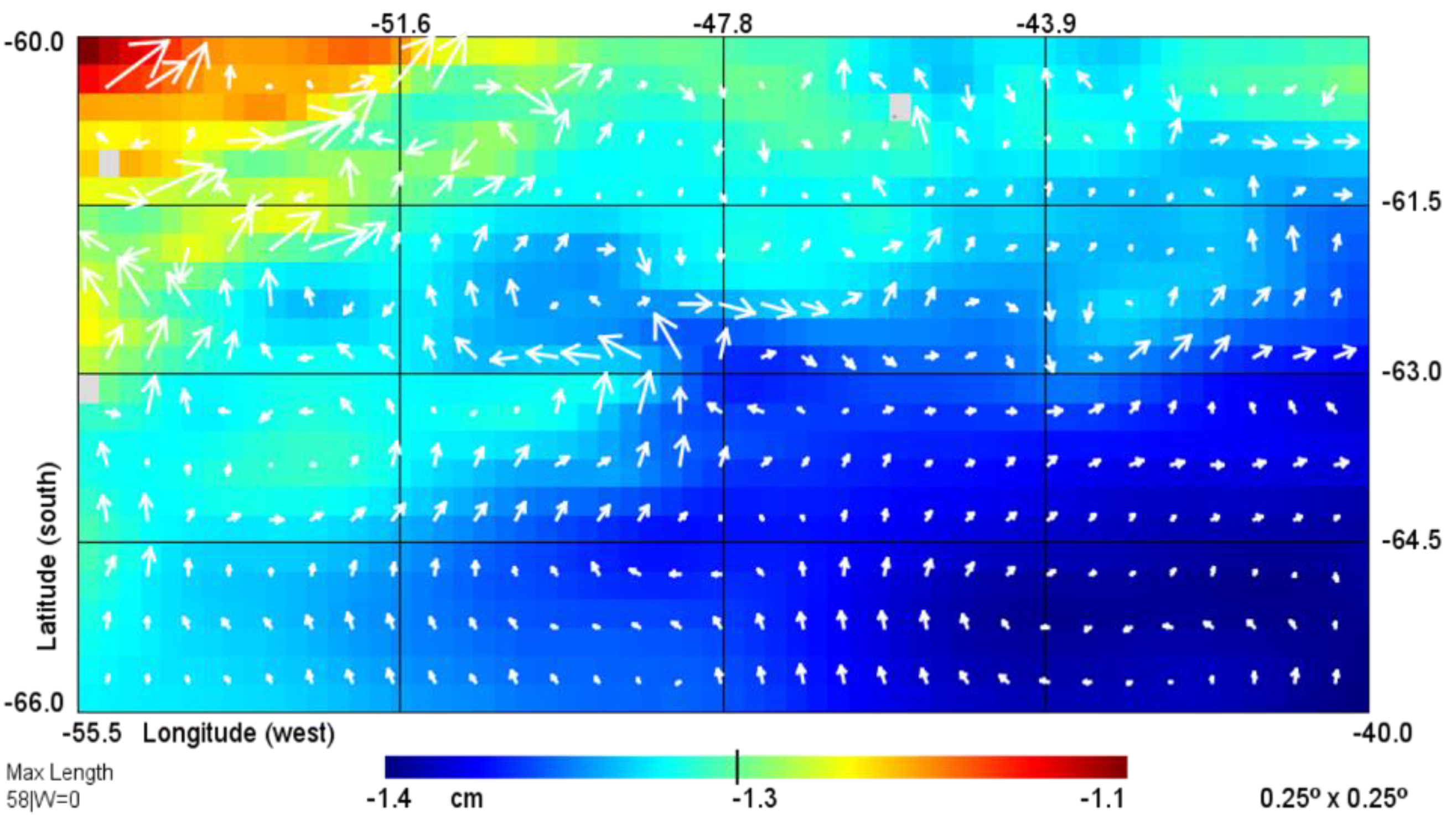
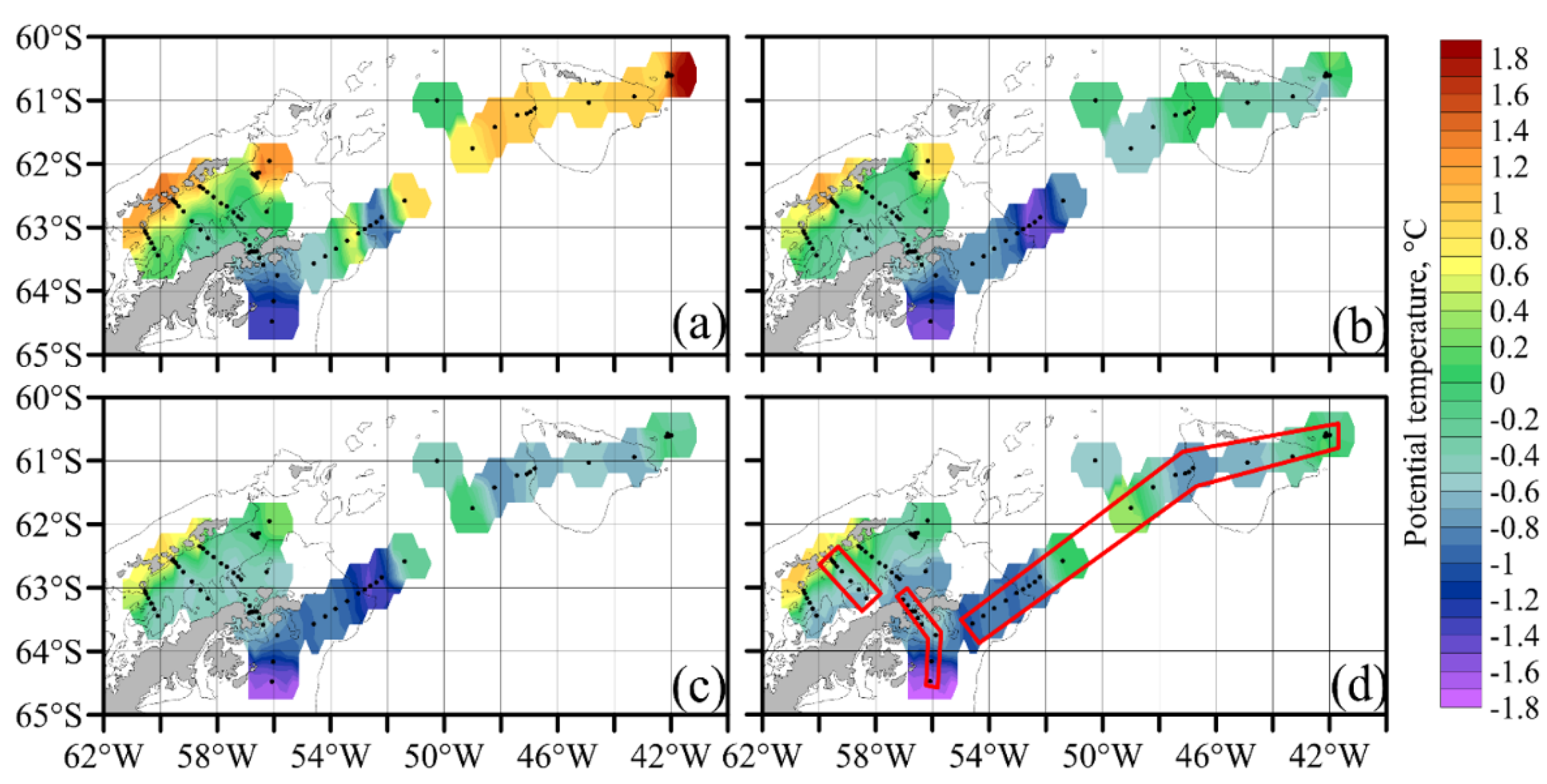
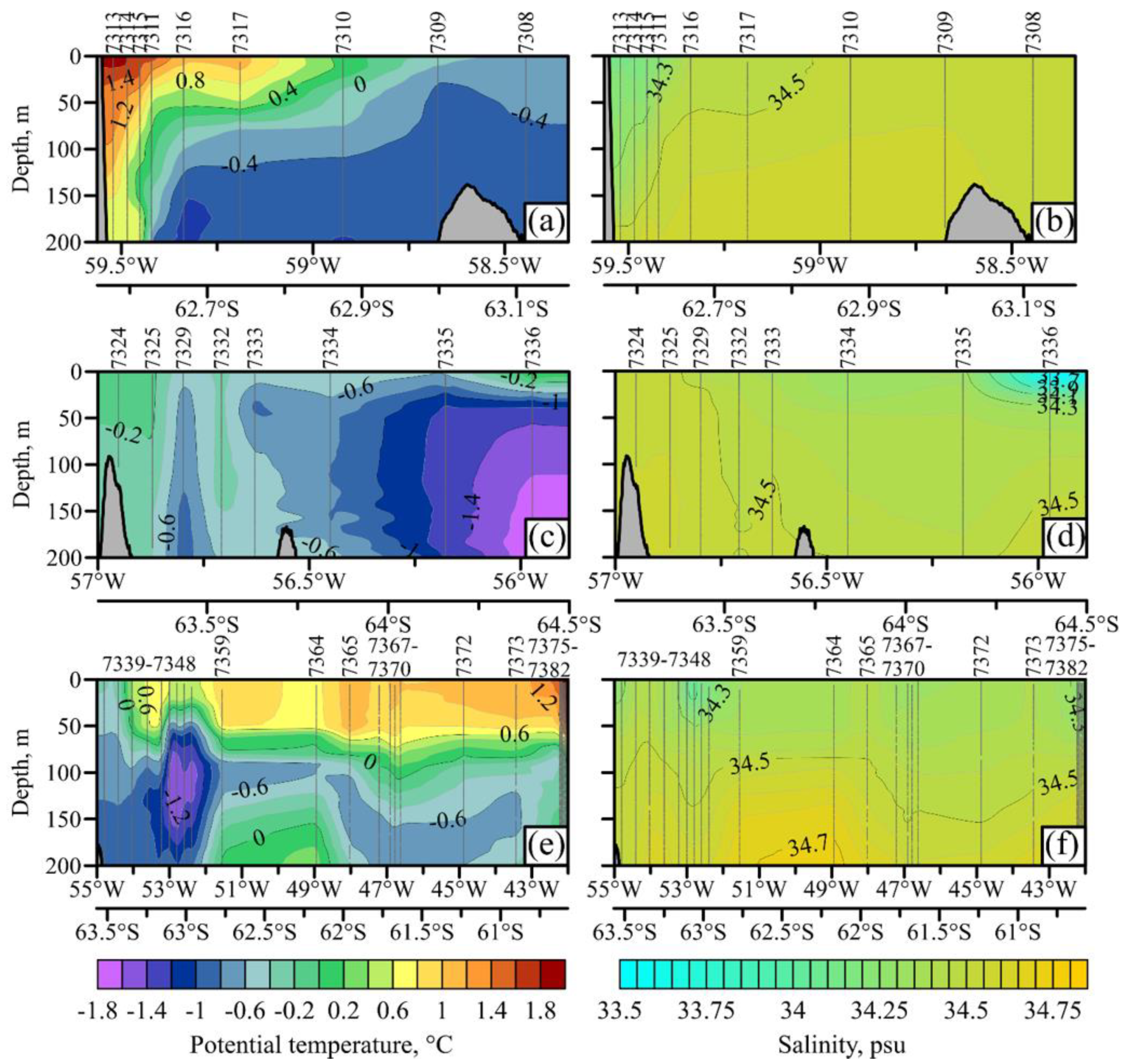
3.1. Hydrology
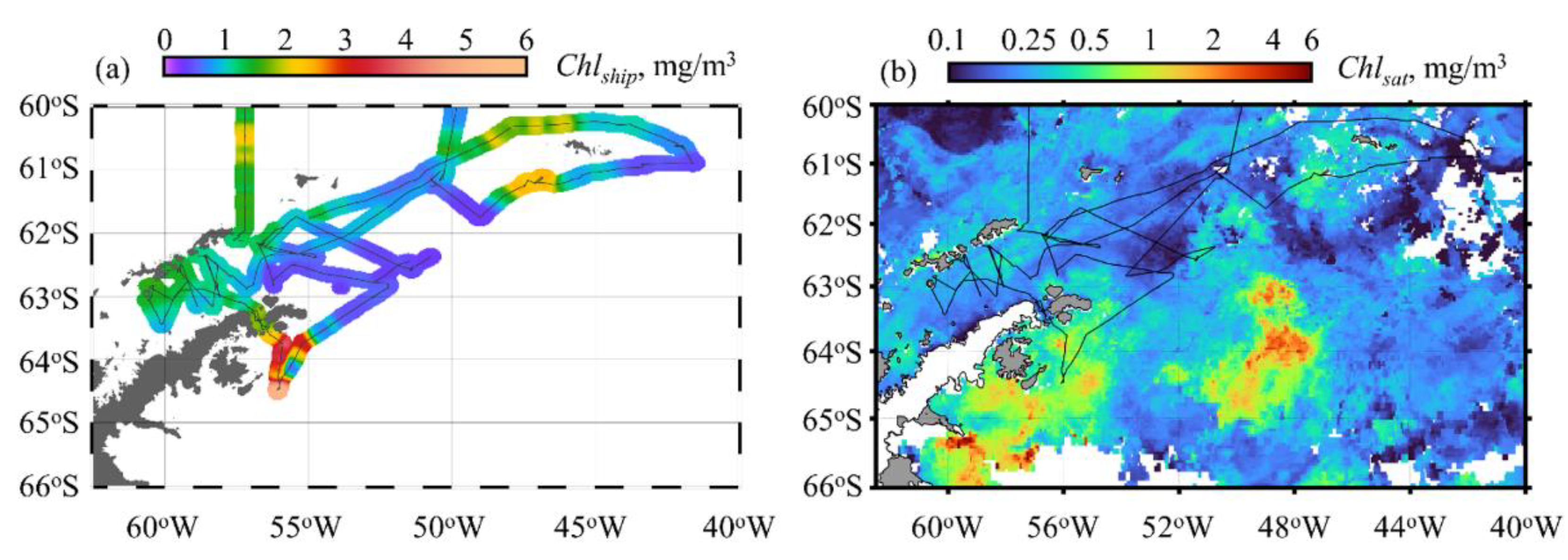
3.2. Chlorophyll a
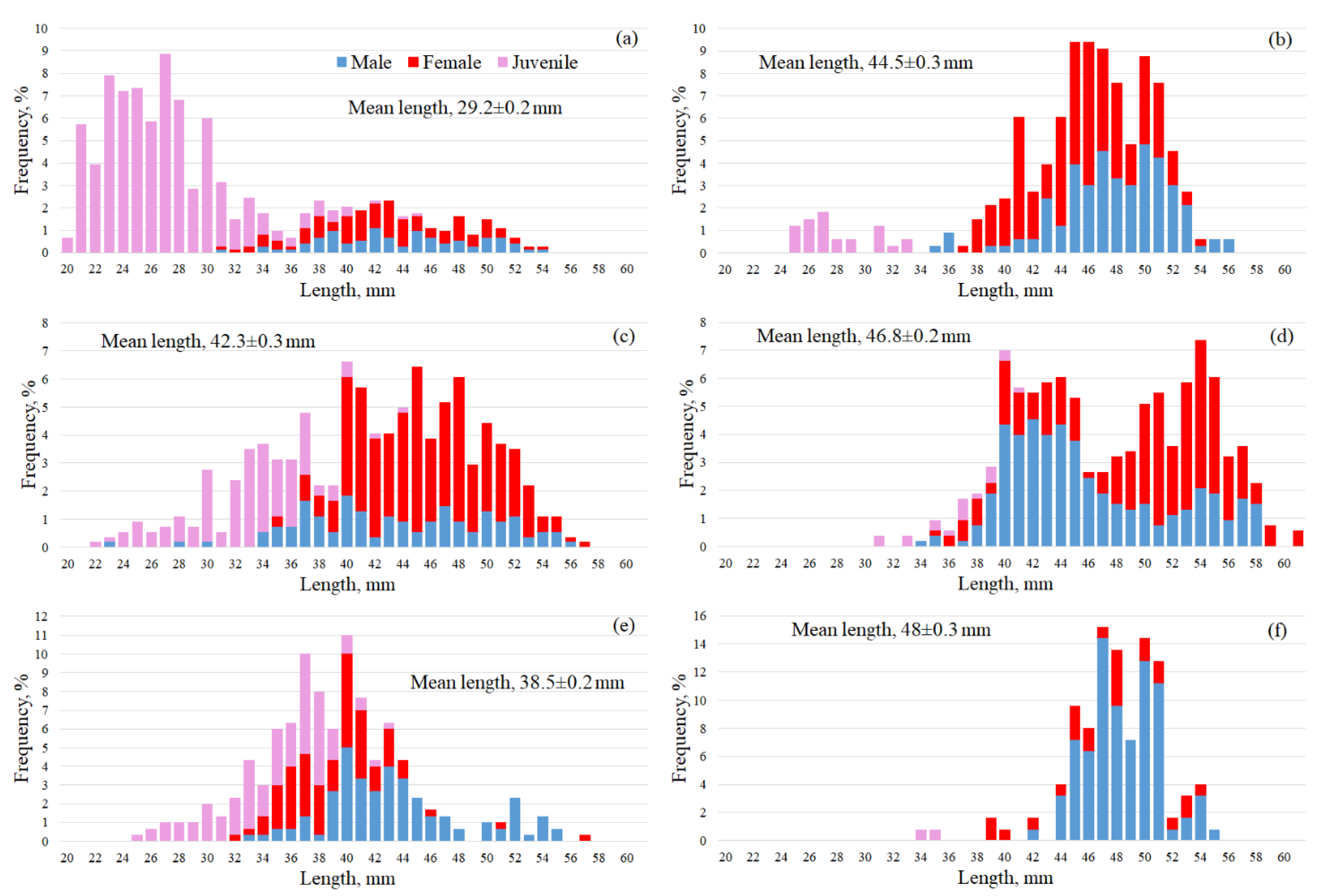
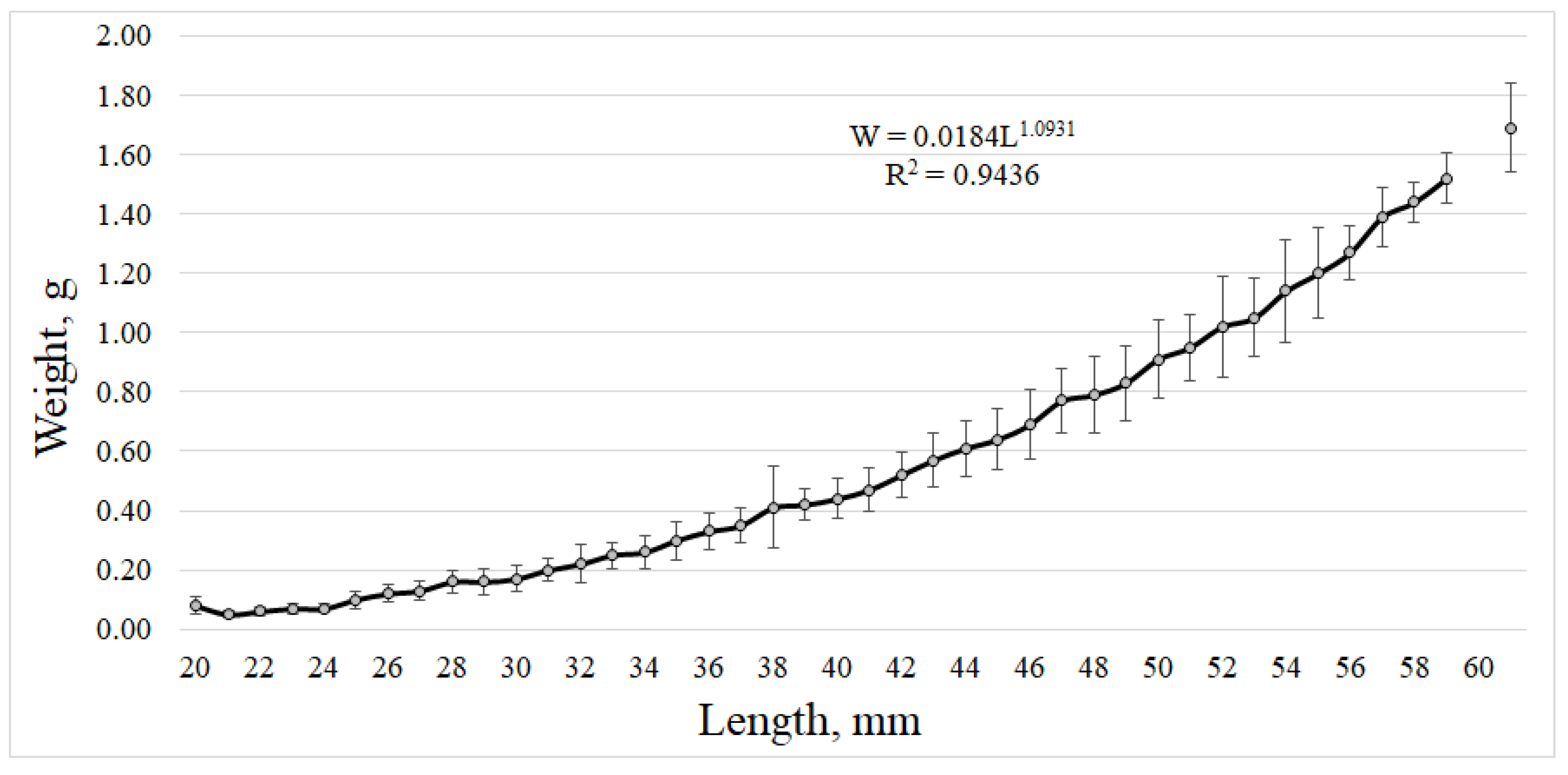
3.3. Macrozooplankton
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethics Approval
References
- Rintoul, S.R.; Sparrow, M.; Meredith, M.P.; Wadley, V.; Speer, K.; Hofmann, E.; Summerhayes, C.; Urban, E.; Bellerby, R. (Eds.) The Southern Ocean Observing System: Initial Science and Implementation Strategy; SCAR: Cambridge, UK, 2012; pp. 1–74. [Google Scholar]
- Trathan, P.N.; Hill, S.L. The Importance of Krill Predation in the Southern Ocean. In Biology and Ecology of Antarctic Krill; Springer: Cham, Switzerland, 2016; pp. 321–350. [Google Scholar]
- Sologub, D.O. Modern Features of Distribution, Biology and Horizontal Migrations of Antarctic Krill (Euphausia superba) in the Atlantic Sector of Antarctica. Ph.D. Thesis, VNIRO Publ. House, Moscow, Russia, 2016; pp. 1–247. [Google Scholar]
- Nicol, S.; Foster, J. The Fishery for Antarctic Krill: Its Current Status and Management Regime. In Biology and Ecology of Antarctic Krill; Springer: Cham, Switzerland, 2016; pp. 387–421. [Google Scholar]
- Atkinson, A.; Siegel, V.; Pakhomov, E.A.; Rothery, P.; Loeb, V.; Ross, R.M.; Quetin, L.B.; Schmidt, K.; Fretwell, P.; Murphy, E.J.; et al. Oceanic Circumpolar Habitats of Antarctic Krill. Mar. Ecol. Prog. Ser. 2008, 362, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Siegel, V. Introducing Antarctic Krill Euphausia superba Dana, 1850. In Biology and Ecology of Antarctic Krill; Springer: Cham, Switzerland, 2016; pp. 1–20. [Google Scholar] [CrossRef]
- Siegel, V.; Watkins, J.L. Distribution, Biomass and Demography of Antarctic Krill, Euphausia superba. In Biology and Ecology of Antarctic Krill; Springer: Cham, Switzerland, 2016; pp. 21–100. [Google Scholar]
- Atkinson, A.; Siegel, V.; Pakhomov, E.; Rothery, P. Long-term Decline in Krill Stock and Increase in Salps within the Southern Ocean. Nature 2004, 432, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Laws, R.M. Ecology of the Southern Ocean. Amer. Sci. 1985, 73, 26–40. [Google Scholar]
- Johnston, N.M.; Murphy, E.J.; Atkinson, A.; Andrew, J.; Constable, A.J.; Cotté, C.; Cox, M.; Daly, K.L.; Driscoll, R.; Flores, H. at al. Status, Change and Futures of Zooplankton in the Southern Ocean. Front. Ecol. Evol. 2022, 9, 624692. [Google Scholar] [CrossRef]
- Samyshev, E.Z. Conclusion on the State of Krill Population and Pelagic Ecosystem in the Western Region of the Atlantic Part of Antarctica in the Pre-winter Period of 1998. Bull. Ukr. Anarct. Center 2000, 3, 231–236. [Google Scholar]
- Samyshev, E.Z. Antarctic Krill and the Structure of Planktonic Community in its Distribution Area; USSR Nauka (Acad. of Sci. of the USSR. All-Union Hydrobiol. Soc.): Moscow, Russia; ECOSEA: Sevastopol, Ukraine, 2002; pp. 1–268. [Google Scholar]
- Perry, F.A.; Atkinson, A.; Sailley, S.F.; Tarling, G.A.; Hill, S.G.; Lucas, C.H. Habitat Partitioning in Antarctic Krill: Spawning Hotspots and Nursery Areas. PLoS ONE 2019, 14, eo219325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, A.; Hill, S.L.; Pakhomov, E.; Siegel, V.; Anadon, R.; Chiba, S.; Daly, K.L.; Downie, R.; Fielding, S.; Fretwell, P.; et al. KRILLBASE: A Circumpolar Database of Antarctic Krill and Salp Numerical Densities, 1926–2016. Earth Syst. Sci. Data. 2017, 9, 193–2107. [Google Scholar] [CrossRef] [Green Version]
- Pakhomov, E.A.; Froneman, P.W.; Perissinoto, R. Salp/Krill Interactions in the Southern Ocean: Spatial Segregation and Implications for the Carbon Flux. Deep Sea Res. II 2002, 2, 1881–1907. [Google Scholar] [CrossRef]
- Pakhomov, E.A.; Dubischar, C.; Strass, V.; Brichta, M.; Bathmann, U. The Tunicate Salpa Thompsoni Ecology in the Southern Ocean—I. Distribution, Biomass, Demography and Feeding Ecophysiology. Mar. Biol. 2006, 149, 609–623. [Google Scholar] [CrossRef] [Green Version]
- Pakhomov, E.A.; Dubishar, C.; Hunt, B.P.V.; Strass, V.; Cisewski, B.; Siegel, V.; von Harbou, L.; Gurney, L.; Kitchener, J.; Bathmann, U. Biology and Life Cycles of Pelagic Tunicates in the Lazarev Sea, Southern Ocean. Deep Sea Res. II 2011, 58, 1677–1689. [Google Scholar] [CrossRef]
- Lomakin, P.D.; Samyshev, E.Z. Oceanographic Conditions in the Area of the South Shetland Islands in March-April 1997, 1998 and Their Influence on the Distribution of Krill and Salp. Oceanology 2004, 44, 882–891. [Google Scholar]
- Flores, H.; Atkinson, A.; Kawaguchi, S.; Krafft, B.A.; Milinevsky, G.; Nicol, S.; Reiss, C.; Tarling, G.A.; Werner, R.; Rebolledo, E.L.B.; et al. Impact of Climate Change on Antarctic Krill. Mar. Ecol. Prog. Ser. 2012, 458, 1–19. Available online: https://hal.archives-ouvertes.fr/hal-01250922 (accessed on 21 November 2022). [CrossRef]
- Pakhomov, E.A.; Hunt, P.V. Trans-Atlantic Variability in Ecology of the Pelagic Tunicate Salpa thompsoni near the Antarctic Polar Front. Deep–Sea Res. II 2017, 138, 126–140. [Google Scholar] [CrossRef]
- Groeneveld, Y.; Berger, U.; Henschke, N.; Pakhomov, E.; Reiss, C.; Meyer, B. Blooms of a Key Grazer in the Southern Ocean—An Individual-Based Model of Salpa thompsoni. Prog. Oceanogr. 2020, 185, 102339. [Google Scholar] [CrossRef]
- Luo, J.Y.; Stock, C.A.; Henschke, N.; Dunne, J.P.; O’Brien, T.D. Global Ecological and Biogeochemical Impacts of Pelagic Tunicates. BioRxiv 2022, 205, 102822. [Google Scholar] [CrossRef]
- Bombosch, A. Euphausia superba or Salpa thompsoni—Who is Going to Win? 2008. Available online: https://www.coolantarctica.com (accessed on 11 October 2020).
- Bone, Q.; Carre, C.; Rian, K.P. The Endostyle and Feeding Filter in Salps (Tunicata). J. Mar. Biol. Ass. UK 2000, 80, 523–534. [Google Scholar] [CrossRef]
- Heron, A.C.; Benham, E.E. Individual Growth Rates of Salps in Three Populations. J. Plankt. Res. 1984, 6, 811–828. [Google Scholar] [CrossRef]
- Lüskow, F.; Pakhomov, E.A.; Stukel, M.R.; Décima, M. Biology of Salpa thompsoni at the Chatham Rise, New Zeland: Demography, Growth, and Diel Vertical Migration. Mar. Biol. 2020, 167, 175. [Google Scholar] [CrossRef]
- Henschke, N.; Blain, S.; Cherel, Y.; Cotte, C.; Espinasse, B.; Brian, P.V.; Hunt, B.P.V.; Pakhomov, E.A. Population Demographics and Growth Rate of Salpa Thompsoni on the Kerguelen Plateau. J. Mar. Syst. 2021, 214, 103489. [Google Scholar] [CrossRef]
- Everett, J.; Baird, M.; Suthers, I. Three-Dimensional Structure of a Swarm of the Salp Thalia democratica within a Cold-Core Eddy off Southeast Australia. J. Geophys. Res. 2011, 116, C12046. [Google Scholar] [CrossRef] [Green Version]
- Alcaraz, M.; Almeda, R.; Duarte, C.M.; Horstkotte, B.; Lasternas, S.; Agustí, S. Changes in the C, N, and P Cycles by the Predicted Salps-Krill Shift in the Southern Ocean. Front. Mar. Scien. 2014, 1, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Morozov, E.G.; Flint, M.V.; Orlov, A.M.; Frey, D.I.; Molodtsova, T.N.; Krechik, V.A.; Latushkin, A.A.; Salyuk, P.A.; Murzina, S.A.; Minin, K.V.; et al. Oceanographic and Ecosystem Studies in the Atlantic Sector of Antarctica (Cruise 87 of the Research Vessel Akademik Mstislav Keldysh). Oceanology 2022, 62, 721–723. [Google Scholar] [CrossRef]
- Cross-Calibrated Multi-Platform Ocean Surface Wind. PO. DAAC. Available online: https://www.remss.com (accessed on 20 August 2022).
- Copernicus Marine Environment Monitoring Service. CMEMS. Available online: https://www.copernicus.eu (accessed on 20 July 2022).
- Ocean Color WEB. Available online: https://oceancolor.gsfc.nasa.gov/l3/ (accessed on 20 August 2022).
- Hu, C.; Lee, Z.; Franz, B. Chlorophyll a Algorithms for Oligotrophic Oceans: A Novel Approach Based on Three-Band Reflectance Difference. J. Geophys. Res. 2012, 117, C01011. [Google Scholar] [CrossRef] [Green Version]
- O’Reilly, J.E.; Maritorena, S.; Mitchell, B.G.; Siegel, D.A.; Carder, K.L.; Garver, S.A.; Kahru, M.; McClain, C. Ocean Color Chlorophyll Algorithms for SeaWiFS. J. Geophys. Res. 1998, 103, 24937–24953. [Google Scholar] [CrossRef] [Green Version]
- Visbeck, M. Deep Velocity Profiling Using Lowered Acoustic Doppler Current Profiler: Bottom Track and Inverse Solution. J. Atmos. Ocean. Tech. 2002, 19, 794–807. [Google Scholar] [CrossRef]
- Egbert, G.D.; Erofeeva, S.Y. Efficient Inverse Modeling of Barotropic Ocean Tides. J. Atmos. Ocean. Tech. 2002, 19, 183–204. [Google Scholar] [CrossRef]
- Koblents-Mishke, O.I. Extractive and Non-Extractive Methods for the Determination of Photosynthetic Pigments in Samples. In Modern Methods for Quantifying the Distribution of Marine Plankton; Vinogradov, M.E., Ed.; Nauka: Moscow, Russia, 1983; pp. 114–125. [Google Scholar]
- Nagornyi, I.G.; Salyuk, P.A.; Maior, A.Y.; Doroshenkov, I.M. A Mobile Complex for On-Line Studying Water Areas and Surface Atmosphere. Instrum. Exp. Tech. 2014, 57, 68–71. [Google Scholar] [CrossRef]
- Bouchard, C.; Mollard, S.; Suzuki, K.; Robert, D.; Fortier, L. Contrasting the Early Life Histories of Sympatric Arctic Gadids Boreogadus Saida and Arctogadus Glacialis in the Canadian Beaufort Sea. Polar Biol. 2016, 39, 1005–1022. [Google Scholar] [CrossRef]
- Kobyliansky, S.G.; Orlov, A.M.; Gordeeva, N.V. Composition of Deepsea Pelagic Ichthyocenes of the Southern Atlantic, from Waters of the Range of the Mid-Atlantic and Walvis Ridges. J. Ichthyol. 2010, 50, 932–50949. [Google Scholar] [CrossRef]
- Bongo Plankton Net. Available online: https://www.nhbs.com/bongo-plankton-net (accessed on 20 August 2022).
- Siegel, V.; Kawaguchi, S.; Ward, P.; Litvinov, F.; Sushin, V.; Loeb, V.; Watkins, J. Krill Demography and Large-Scale Distribution in the Southwest Atlantic During January/February 2000. Deep Sea Res. II 2004, 51, 1253–1273. [Google Scholar] [CrossRef]
- Morris, D.J.; Watkins, J.L.; Ricketts, C.; Buchholz, F.; Priddle, J. An Assessment of the Merits of Length and Weight Measurements of Antarctic Krill Euphausia superba. Brit. Ant. Surv. Bull. 1988, 79, 27–50. [Google Scholar]
- Anonymous. Scientific Observers Manual; CCAMLR: Hobart, Australia, 2011; pp. 1–232. [Google Scholar]
- Petrov, A.F.; Shust, K.V.; Piyanova, S.; Uryupova, E.; Gordeev, I.I.; Sitov, A.M.; Demina, S.N. Guidelines for the Collection and Processing of Fishing and Biological Data on Aquatic Bioresources of Antarctica for the Russian Scientific Observers in the CCAMLR Convention Area; VNIRO Publ. House: Moscow, Russia, 2014; pp. 45–53. [Google Scholar]
- Makarov, R.R.; Denys, C.J. Stages of Sexual Maturity of Euphausia superba Dana. BIOMASS Handbook 1980, 11, 1–11. [Google Scholar]
- Meyer, B.; Auerswald, L.; Siegel, V.; Spahic, S.; Pape, C.; Fach, B.A.; Teschke, M.; Lopata, A.L.; Fuentes, V. Seasonal Variation in Body Composition, Metabolic Activity, Feeding and Growth of Adult Krill Euphausia superba in the Lazarev Sea. Mar. Ecol. Prog. Ser. 2010, 398, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Watkins, J.L.; Morris, D.J.; Ricketts, C. Nocturnal Changes in the Mean Length of a Euphausiid Population: Vertical Migration, Net Avoidance or Experimental Error? Mar. Biol. 1985, 86, 123–127. [Google Scholar] [CrossRef]
- Tokarczyk, R. Classification of Water Masses in the Bransfield Strait and Southern Part of the Drake Passage Using a Method of Statistical Multidimensional Analysis. Polish Polar Res. 1987, 8, 333–336. [Google Scholar]
- Orsi, A.H.; Nowlin, W.D.; Whitworth III, T. On the Circulation and Stratification of the Weddell Gyre. Deep-Sea Res. I 1993, 40, 169–203. [Google Scholar] [CrossRef]
- Heywood, K.J.; Garabato, A.C.N.; Stevens, D.P.; Muench, R.D. On the Fate of the Antarctic Slope Front and the Origin of the Weddell Front. J. Geophys. Res. 2004, 109, C06021. [Google Scholar] [CrossRef] [Green Version]
- Dorschel, B.; Gutt, J.; Huhn, O.; Bracher, A.; Huntemann, M.; Huneke, W.; Gebhardt, C.; Schroder, M.; Herr, H. Environmental Information for a Marine Ecosystem Research Approach for the Northern Antarctic Peninsula (RV Polarstern expedition PS81, ANT-XXIX/3). Polar Biol. 2016, 39, 765–787. [Google Scholar] [CrossRef]
- Krechik, V.A.; Frey, D.I.; Morozov, E.G. Peculiarities of Water Circulation in the Central Part of the Bransfield Strait in January 2020. Dokl. Earth Sci. 2021, 496, 92–95. [Google Scholar] [CrossRef]
- Morozov, E.G.; Flint, M.V.; Spiridonov, V.A. Antarctic Peninsula Region of the Southern Ocean; Springer: Cham, Switzerland, 2021; pp. 1–455. [Google Scholar] [CrossRef]
- Huneke, W.G.; Huhn, O.; Schroeder, M. Water Masses in the Bransfield Strait and Adjacent Seas, Austral Summer 2013. Polar Biol. 2016, 39, 789–798. [Google Scholar] [CrossRef]
- Krek, A.V.; Krek, E.V.; Krechik, V.A. The Circulation and Mixing Zone in the Antarctic Sound in February 2020. In Antarctic Peninsula Region of the Southern Ocean; Springer: Cham, Switzerland, 2021; pp. 83–99. [Google Scholar] [CrossRef]
- van Caspel, M.; Hellmer, H.H.; Mata, M.M. On the Ventilation of Bransfield Strait Deep Basins. Deep-Sea Res. II 2017, 149, 25–30. [Google Scholar] [CrossRef]
- Kang, S.H.; Kang, J.S.; Lee, S.; Chung, K.H.; Kim, D.; Park, M.G. Antarctic Phytoplankton Assemblages in the Marginal Ice Zone of the Northwestern Weddell Sea. J. Plankt. Res. 2001, 23, 333–352. [Google Scholar] [CrossRef] [Green Version]
- Nunes, S.; Latasa, M.; Delgado, M.; Emelianov, M.; Simó, R.; Estrada, M. Phytoplankton Community Structure in Contrasting Ecosystems of the Southern Ocean: South Georgia, South Orkneys and Western Antarctic Peninsula. Deep-Sea Res. I 2019, 151, 103059. [Google Scholar] [CrossRef]
- Mendes, C.R.B.; de Souza, M.S.; Garcia, V.M.T.; Leal, M.C.; Brotas, V.; Garcia, C.A.E. Dynamics of Phytoplankton Communities During Late Summer Around the Tip of the Antarctic Peninsula. Deep-Sea Res. I. 2012, 65, 1–14. [Google Scholar] [CrossRef]
- Ferreira, A.; Brito, A.C.; Mendes, C.R.; Brotas, V.; Costa, R.R.; Guerreiro, C.V.; Sá, C.; Jackson, T. OC4-SO: A New Chlorophyll-a Algorithm for the Western Antarctic Peninsula Using Multi-Sensor Satellite Data. Remote Sens. 2022, 14, 1052. [Google Scholar] [CrossRef]
- Pereira, E.S.; Garcia, C.A.E. Evaluation of Satellite-Derived MODIS Chlorophyll Algorithms in the Northern Antarctic Peninsula. Deep-Sea Res. II 2018, 149, 124–137. [Google Scholar] [CrossRef]
- Meredith, M.P.; Renfrew, I.A.; Clarke, A.; King, J.C.; Brandon, M.A. Impact of the 1997/98 ENSO on the Upper Waters of Marguerite Bay, Western Antarctic Peninsula J. Geophys. Res. 2004, 109, C09013. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, A.; Shreeve, R.; Hirst, A.; Rothery, P.; Tarling, G.; Pond, D.; Korb, R.; Murphy, E.; Watkins, J.L. Natural Growth Rates in Antarctic Krill (Euphausia Superba): II. Predictive Models Based on Food, Temperature, Body Length, Sex, and Maturity Stage. Limnol. Oceanogr. 2006, 51, 973–987. [Google Scholar] [CrossRef] [Green Version]
- Tarling, G.A.; Cuzin-Roudy, J.; Thorpe, S.E.; Shreeve, R.S.; Ward, P.; Murphy, E.J. Recruitment of Antarctic Krill Euphausia Superba in the South Georgia Region: Adult Fecundity and the Fate of Larvae. Mar. Ecol. Prog. Ser. 2007, 331, 161–179. [Google Scholar] [CrossRef] [Green Version]
- Wiedenmann, J.; Cresswell, K.; Mangel, M. Temperature-Dependent Growth of Antarctic Krill: Predictions for a Changing Climate from a Cohort Model. Mar. Ecol. Prog. Ser. 2008, 358, 191–202. [Google Scholar] [CrossRef] [Green Version]
- Spiridonov, V.A.; Zalota, A.K.; Yakovenko, V.A.; Gorbatenko, K.M. Population Composition and Transport of Antarctic Krill Juveniles in the Powell Basin (Northwestern Part of the Weddell Sea) in January 2020. Tr. VNIRO 2020, 181, 33–51. [Google Scholar] [CrossRef]
- Yakovenko, V.A.; Spiridonov, V.A.; Gorbatenko, K.M.; Shadrin, N.V.; Samyshev, E.Z.; Minkina, N.I. Macro- and Mesozooplankton in the Powell Basin (Antarctica): Species Composition and Distribution of Abundance and Biomass in February 2020. In Antarctic Peninsula Region of the Southern Ocean; Springer: Cham, Switzerland, 2021; pp. 131–141. [Google Scholar] [CrossRef]
- Hereu, C.M.; Suárez-Morales, E.; Lavaniegos, B.E. Record of the Rare Oceanic Salp Helicosalpa Komaii (Tunicata: Thaliacea: Salpida) in the Northeast Pacific. Rev. Mex. Biodiver. 2014, 85, 624–629. [Google Scholar] [CrossRef]
- Kasatkina, S.M.; Abramov, A.M.; Sokolov, M.Y. Biomass and Distribution of Antarctic Krill in the Antarctic Atlantic Area in January-February 2020. Tr. AtlantNIRO 2021, 5, 49–61. [Google Scholar]
- Sytov, A.M.; Kozlov, D.A. Dimensional Composition and Biological Characteristics of Antarctic Krill Euphausia Superba in the Antarctic Part of the Atlantic in January-March 2020. Tr. AtlantNIRO 2021, 5, 101–115. [Google Scholar]
- Krag, L.A.; Herrmann, B.; Iversen, S.A.; Engas, A.; Nordrum, S.; Krafft, B.A. Size Selection of Antarctic Krill (Euphausia superba) in Trawls. PLoS ONE 2014, 9, e102168. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Tang, H.; Herrmann, B.; Xu, L. Catch Pattern for Antarctic Krill (Euphausia superba) of Different Commercial Trawls in Similar Times and Overlapping Fishing Grounds. Grounds. Front. Mar. Sci. 2021, 8, 670663. [Google Scholar] [CrossRef]
- Cox, M.J.; Borchers, D.L.; Demer, D.A.; Cutter, G.R.; Brierley, A.S. Estimating the Density of Antarctic Krill (Euphausia Superba) from Multibeam Echo-Sounder Observations Using Distance Sampling Methods. J. R. Stat. Soc. Ser. C App. Statist. 2011, 60, 301–316. [Google Scholar] [CrossRef]
- Kasatkina, S.M. Methodical Aspects of Acoustic Survey for Antarctic Krill in the CCAMLR Convention are. Tr. AtlantNIRO 2021, 5, 39–48, ISSN: 2541-9692. [Google Scholar]
- Bargmann, H.E. The Development and Life History of Adolescent and Adult Krill Euphausia superba. Discovery Rep. 1945, XXIII, 103–178. [Google Scholar]
- Fraser, F.G. On the Development and Distribution of the Young Stages of Krill (Euphausia superba). Discovery Rep. 1936, XIV, 1–192. [Google Scholar] [CrossRef]
- Marr, J.W.S. The Natural History and Geography of the Antarctic Krill (Euphausia superba Dana). Discovery Rep. 1962, XXXII, 33–464. [Google Scholar]
- Shvetsov, V.V.; Makarov, R.R. On the Biology of Antarctic Krill. Tr. VNIRO. 1969, LXVI, 177–206. [Google Scholar]
- Barkley, E. Nahrung und Filterapparat des Walkrebschens Euphausia superba Dana. Z. Fisch. Deren Hilfswiss. 1940, 1, 65–156. [Google Scholar]
- Brotz, L.; Cheung, W.W.L.; Kleisner, K.; Pakhomov, E.; Pauly, D. Increasing Jellyfish Populations: Trends in Large Marine Ecosystems. Hydrobiol. 2012, 690, 3–20. [Google Scholar] [CrossRef] [Green Version]
- Constable, A.J.; Melbourne-Thomas, J.; Corney, S.P.; Arrigo, K.R.; Barbraud, C.; Barnes, D.K.A.; Bindoff, N.L.; Boyd, P.W.; Brandt, A.; Costa, D.P. at al. Climate Change and Southern Ocean Ecosystems I: How Changes in Physical Habitats Directly Affect Marine Biota. Glob. Change Biol. 2014, 20, 3004–3025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henschke, N.; Everett, J.D.; Richardson, A.J.; Suthers, I.M. Rethinking the Role of Salps in the Ocean. Trends Ecol. Evol. 2016, 31, 720–733. [Google Scholar] [CrossRef]
- Shnar, V.N.; Kasatkina, S.M. Long-Term Variability of Environmental Conditions and Distribution of Antarctic Krill Euphausia Superba in the Sub-Region of the Antarctic Peninsula in 1970-2020. Tr. AtlantNIRO 2021, 5, 101–110. [Google Scholar]
- Atkinson, A.; Hill, S.L.; Pakhomov, E.A.; Siegel, V.; Reiss, C.S.; Loeb, V.J.; Steinberg, D.K.; Schmidt, K.; Tarling, G.A.; Gerrish, L.; et al. Krill (Euphausia superba) Distribution Contracts Southward During Rapid Regional Warming. Nat. Clim. Chang. 2019, 9, 142–147. [Google Scholar] [CrossRef]
- Loeb, V.; Siegel, V.; Holm-Hansen, O.; Hewitt, R.; Fraser, W.; Trivelpiece, W.; Trivelpiece, S. Effects of Sea-Ice Extent and Krill or Salp Dominance on the Antarctic Food Web. Nature 1997, 387, 897–900. [Google Scholar] [CrossRef]
- Ross, R.; Quetin, L.; Newberger, T.; Shaw, T.; Jones, J.; Oakes, S.; Moore, K. Trends, Cycles, Interannual Variability for Three Pelagic Species West of the Antarctic Peninsula 1993–2008. Mar. Ecol. Prog. Ser. 2014, 515, 11–32. [Google Scholar] [CrossRef]
- Minkina, N.I.; Samyshev, E.Z.; Pakhomov, E.A.; Melnikov, V.V. Temporal and Satial Variability of Energy Exchange in Antarctic Salps. Res. Square 2022. [Google Scholar] [CrossRef]
- Atkinson, A.; Siegel, V.; Pakhomov, E.A.; Jessopp, M.J.; Loeb, V. A Re-Appraisal of the Total Biomass and Annual Production of Antarctic Krill. Deep-Sea Res. I 2009, 56, 727–740. [Google Scholar] [CrossRef]
- Murphy, E.J.; Watkins, J.L.; Trathan, P.; Reid, K.; Meredith, M.P.; Thorpe, S.E.; Fleming, A.H. Spatial and Temporal Operation of the Scotia Sea Ecosystem: A Review of Large-Scale Links in a Krill Centred Food Web. Philosophical Trans. R. Soc. B. Biol. Sci. 2007, 362, 113–148. [Google Scholar] [CrossRef] [PubMed]
- Loeb, V.J.; Santora, J.A. Population Dynamics of Salpa Thompsoni Near the Antarctic Peninsula: Growth Rates and Interannual Variations in Reproductive Activity (1993–2009). Prog. Oceanogr. 2012, 96, 93–107. [Google Scholar] [CrossRef]
- Kasyan, V.V.; Bitiutskii, D.G.; Voronin, V.P.; Zuev, O.A.; Kalinina, O.Y.; Kolbasova, G.D.; Mishin, A.V.; Murzina, S.A.; Kolbasova, G.D.; Voronin, V.P.; et al. Composition and Distribution of Plankton Communities in the Atlantic Sector of the Southern Ocean. Diversity 2022, 14, 923. [Google Scholar] [CrossRef]
 )—interquartile range,
)—interquartile range,  —95% confidence intervals, ǀ– median).
—95% confidence intervals, ǀ– median).
 )—interquartile range,
)—interquartile range,  —95% confidence intervals, ǀ– median).
—95% confidence intervals, ǀ– median).
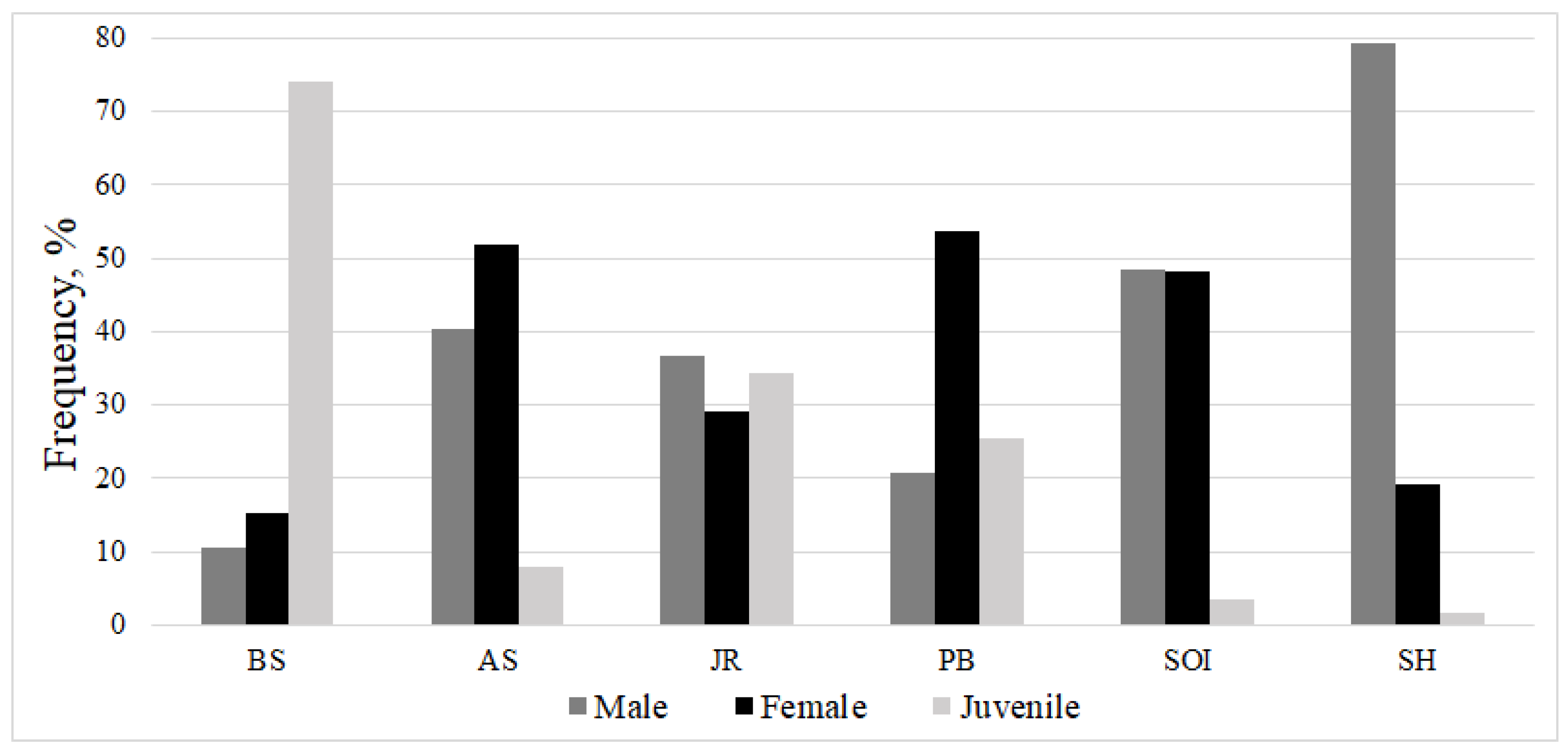
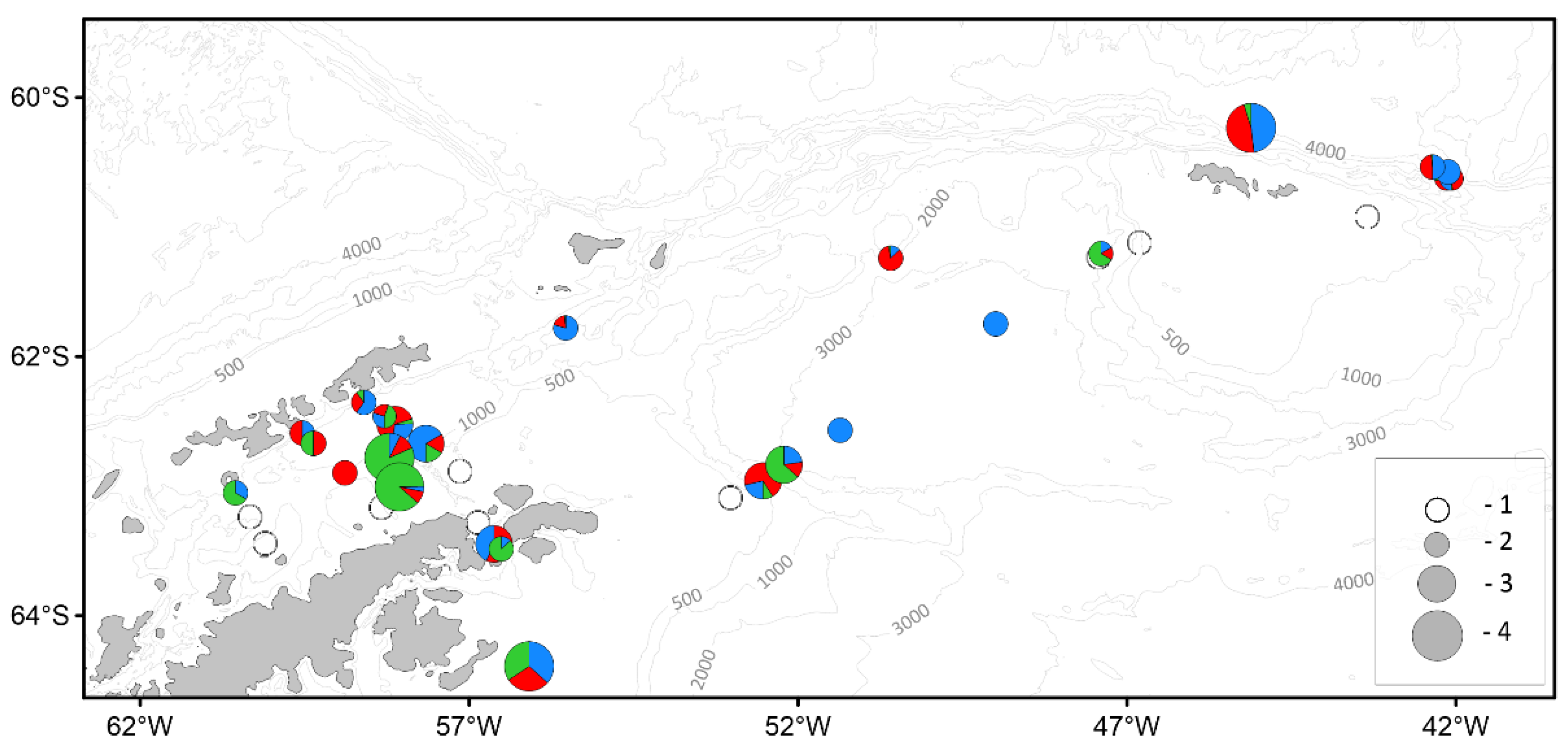
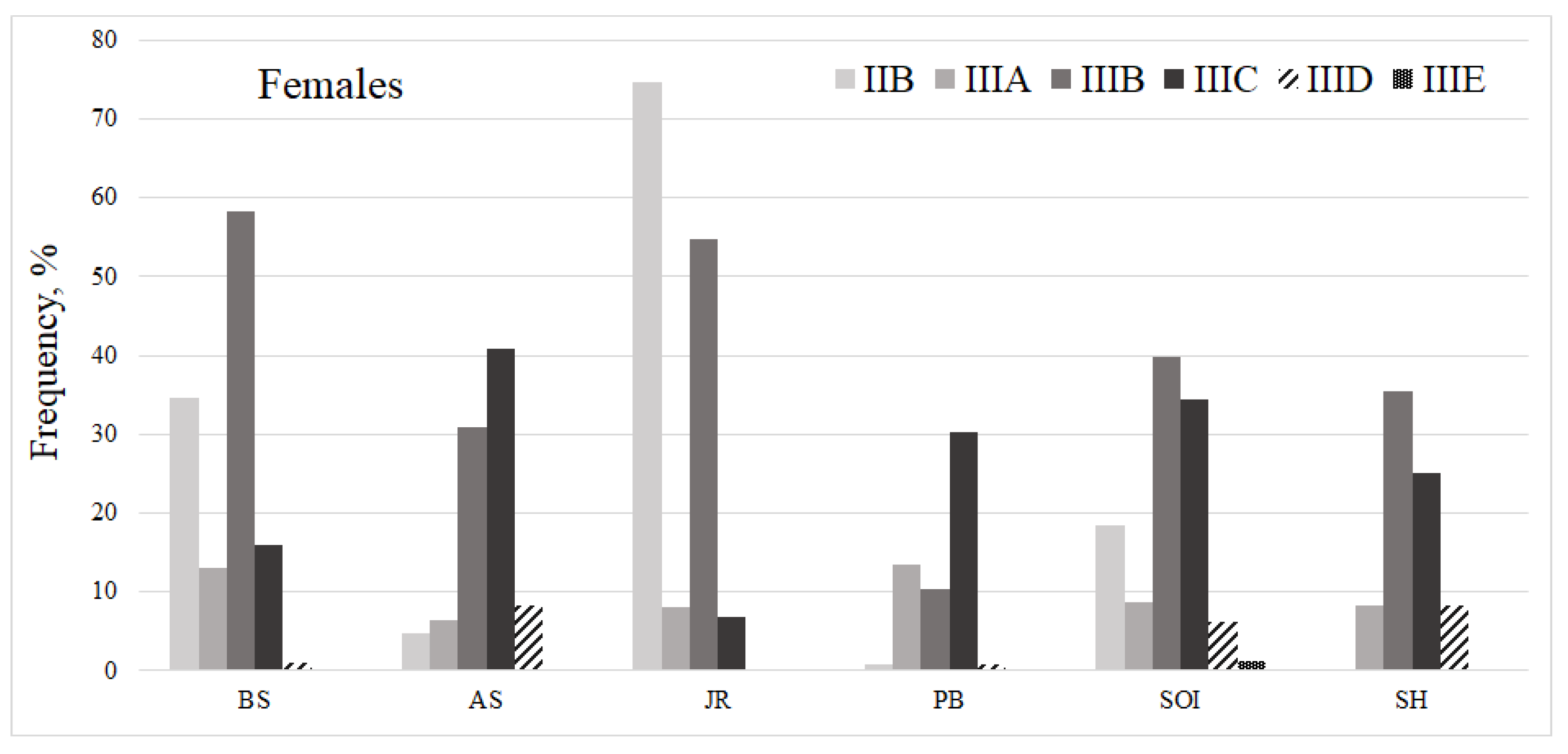
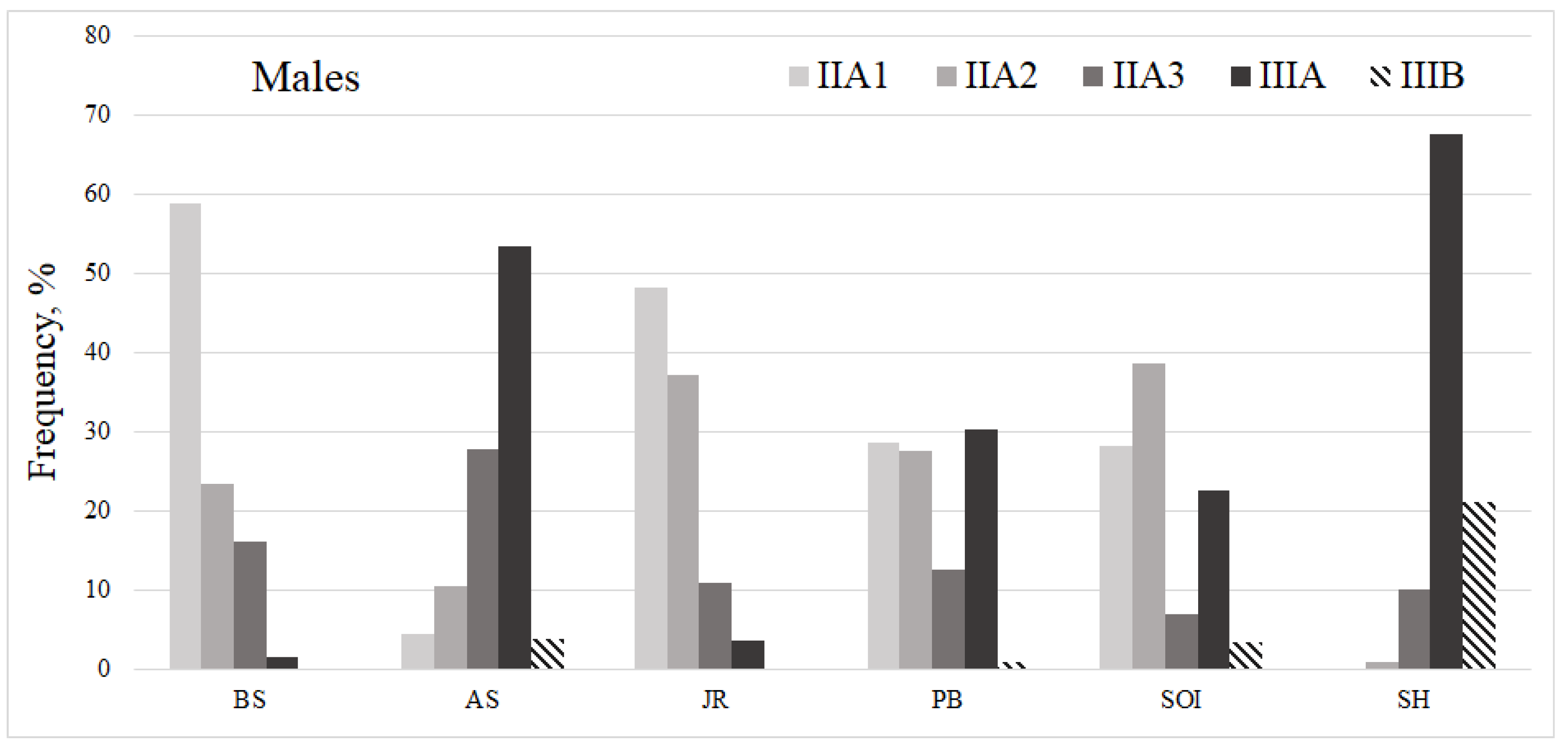
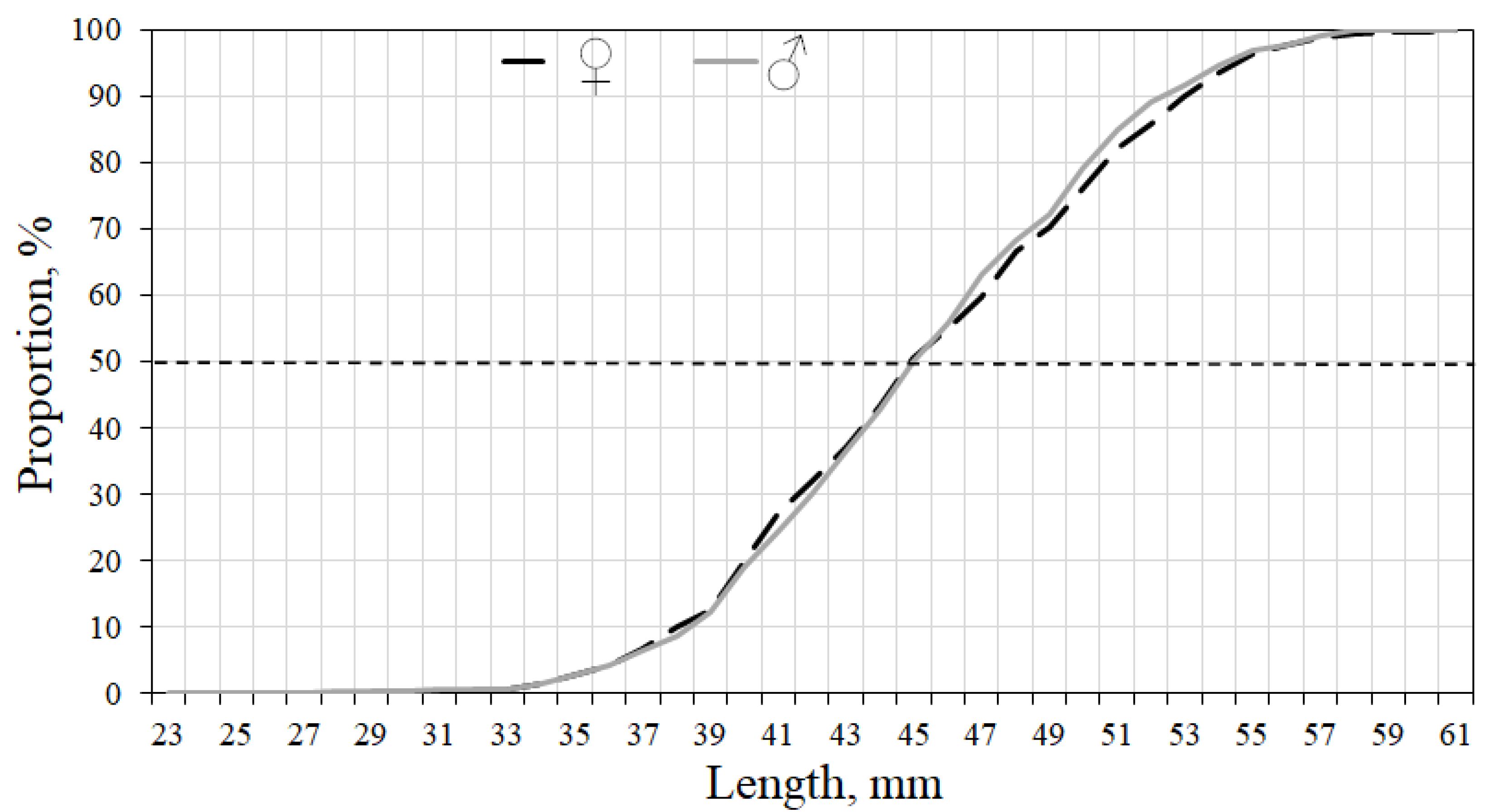
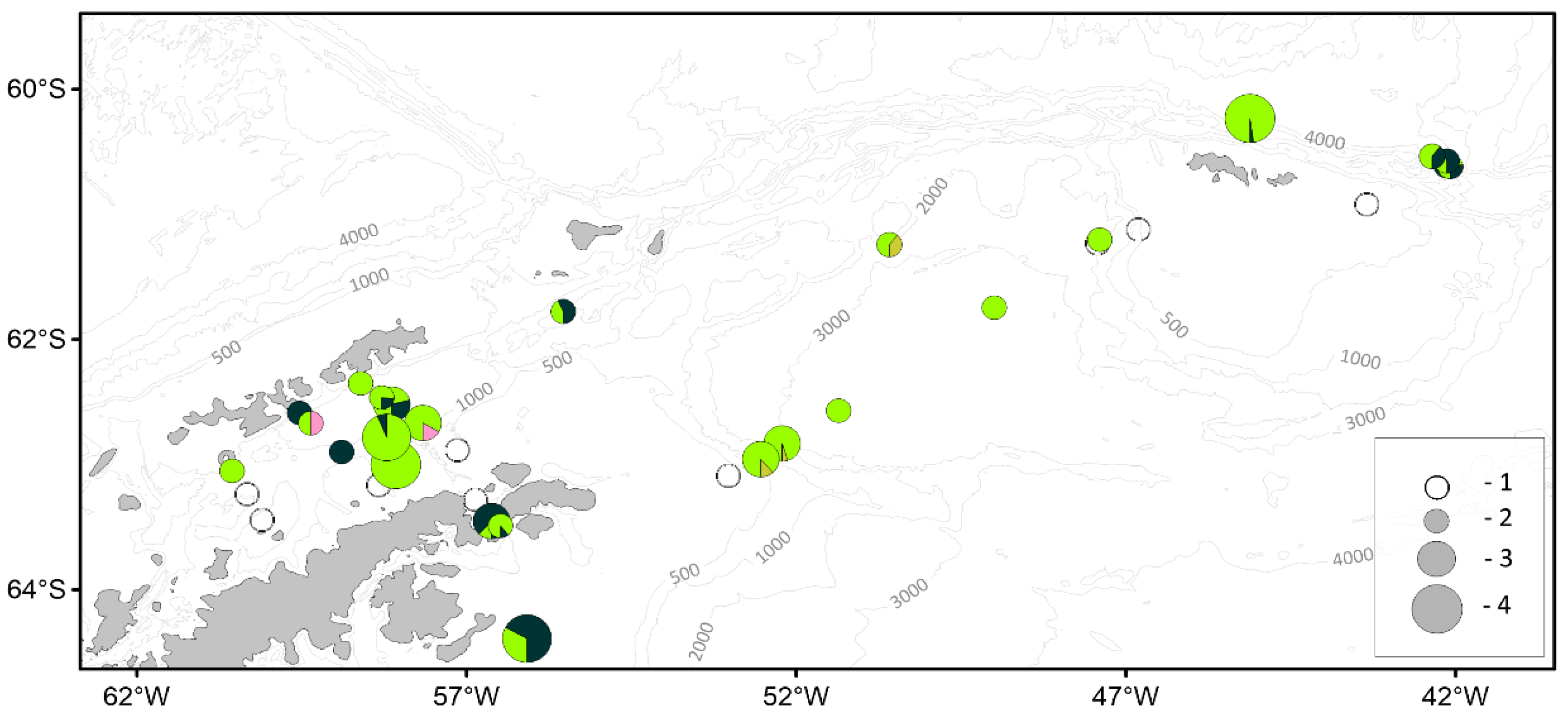
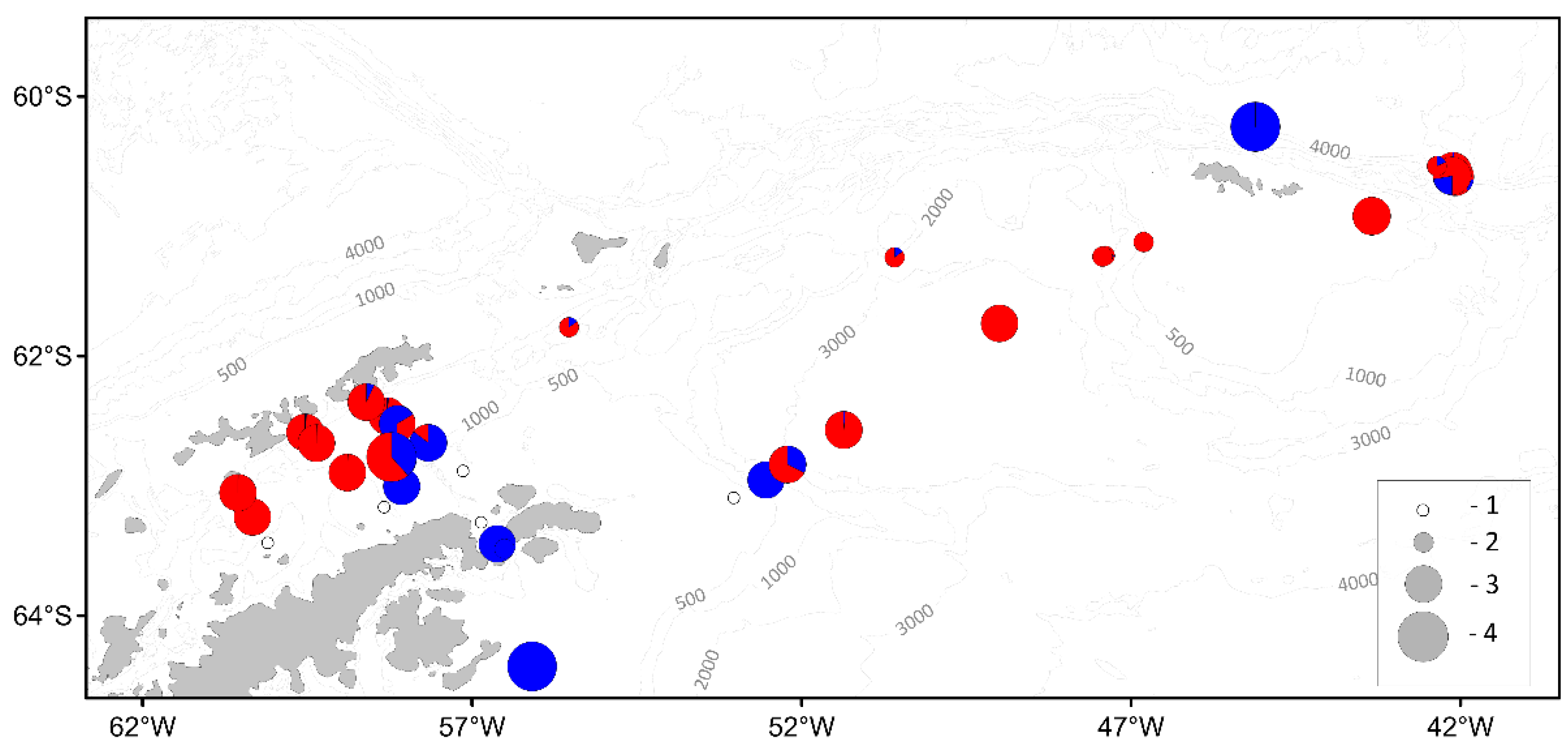
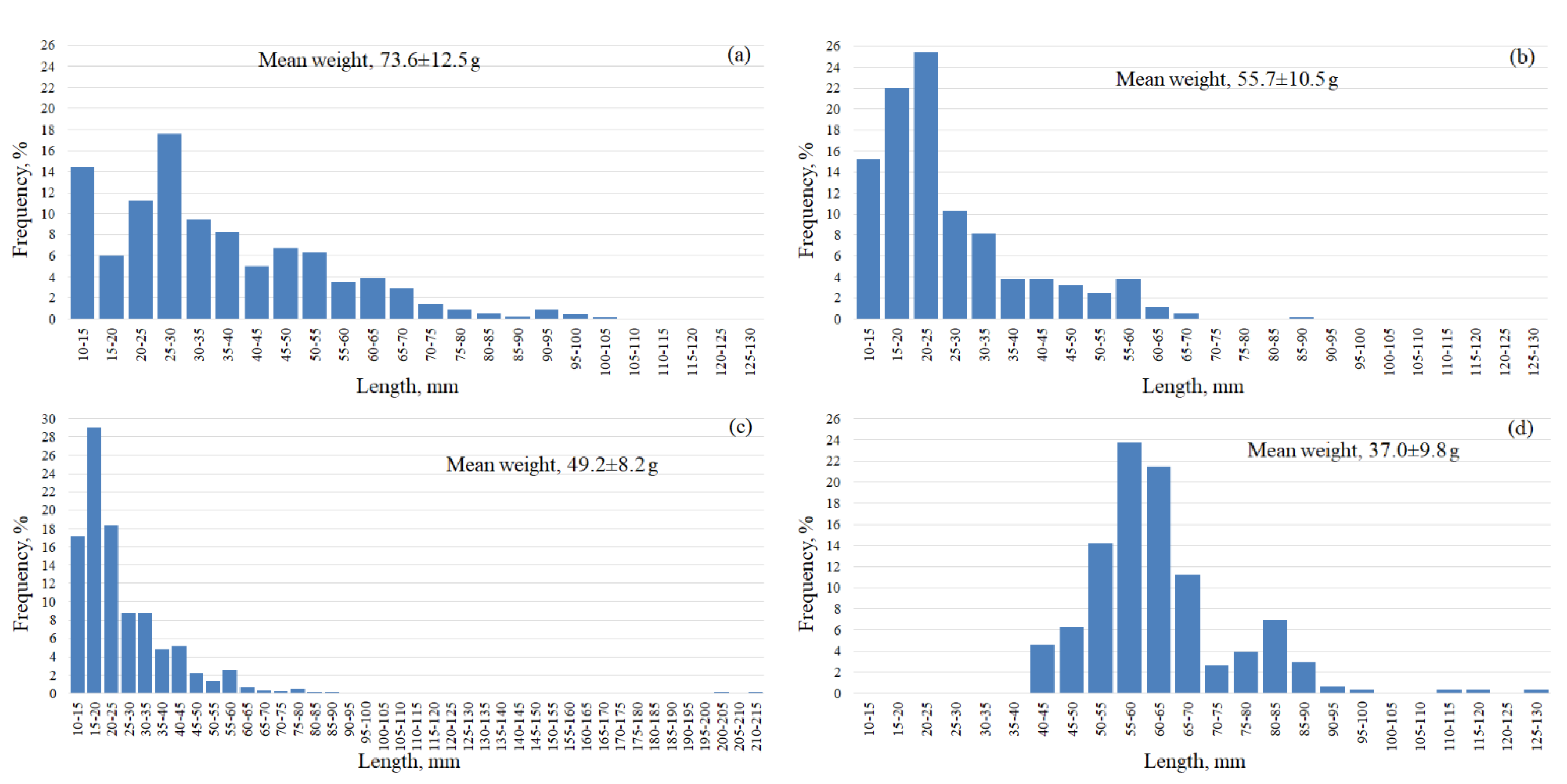
| Parameter | Number of individuals | ||||||
|---|---|---|---|---|---|---|---|
| BS | AS | SOI | PB | JR | SH | Total | |
| Antarctic Krill | |||||||
| Mass measurements | 1140 | 548 | 733 | 743 | 500 | 125 | 3789 |
| Biological analysis | 734 | 330 | 529 | 543 | 300 | 125 | 2561 |
| Salps | |||||||
| Mass measurements | 1549 | - | 2433 | 2136 | - | 303 | 6421 |
| BS | AS | SOI | PB | JR | SH | Average Score | |
|---|---|---|---|---|---|---|---|
| Juvenile | 3.6/1.8 | 3.4/2.2 | 2.5/0.5 | 0.5/0.2 | 3.4/1.7 | 1.0/0.0 | 2.7/1.3 |
| Females | 3.8/1.7 | 4.0/2.3 | 3.6/1.6 | 2.8/0.9 | 3.4/2.4 | 3.4/0.6 | 3.5/1.5 |
| Males | 3.9/2.3 | 3.9/2.1 | 3.0/1.2 | 1.9/0.9 | 3.8/2.3 | 3.8/1.5 | 3.3/1.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bitiutskii, D.G.; Samyshev, E.Z.; Minkina, N.I.; Melnikov, V.V.; Chudinovskih, E.S.; Usachev, S.I.; Salyuk, P.A.; Serebrennikov, A.N.; Zuev, O.A.; Orlov, A.M. Distribution and Demography of Antarctic Krill and Salps in the Atlantic Sector of the Southern Ocean during Austral Summer 2021–2022. Water 2022, 14, 3812. https://doi.org/10.3390/w14233812
Bitiutskii DG, Samyshev EZ, Minkina NI, Melnikov VV, Chudinovskih ES, Usachev SI, Salyuk PA, Serebrennikov AN, Zuev OA, Orlov AM. Distribution and Demography of Antarctic Krill and Salps in the Atlantic Sector of the Southern Ocean during Austral Summer 2021–2022. Water. 2022; 14(23):3812. https://doi.org/10.3390/w14233812
Chicago/Turabian StyleBitiutskii, Dmitrii G., Ernest Z. Samyshev, Natalia I. Minkina, Victor V. Melnikov, Elena S. Chudinovskih, Sergei I. Usachev, Pavel A. Salyuk, Alexander N. Serebrennikov, Oleg A. Zuev, and Alexei M. Orlov. 2022. "Distribution and Demography of Antarctic Krill and Salps in the Atlantic Sector of the Southern Ocean during Austral Summer 2021–2022" Water 14, no. 23: 3812. https://doi.org/10.3390/w14233812







