Linking the Community and Metacommunity Perspectives: Biotic Relationships Are Key in Benthic Diatom Ecology
Abstract
:1. Introduction
1.1. Community and Metacommunity of Benthic Diatoms: The Scale
1.2. Drivers of BD Distribution: Environmental Factors, Habitat and Substrate
1.3. Goals of This Study
2. Material and Methods
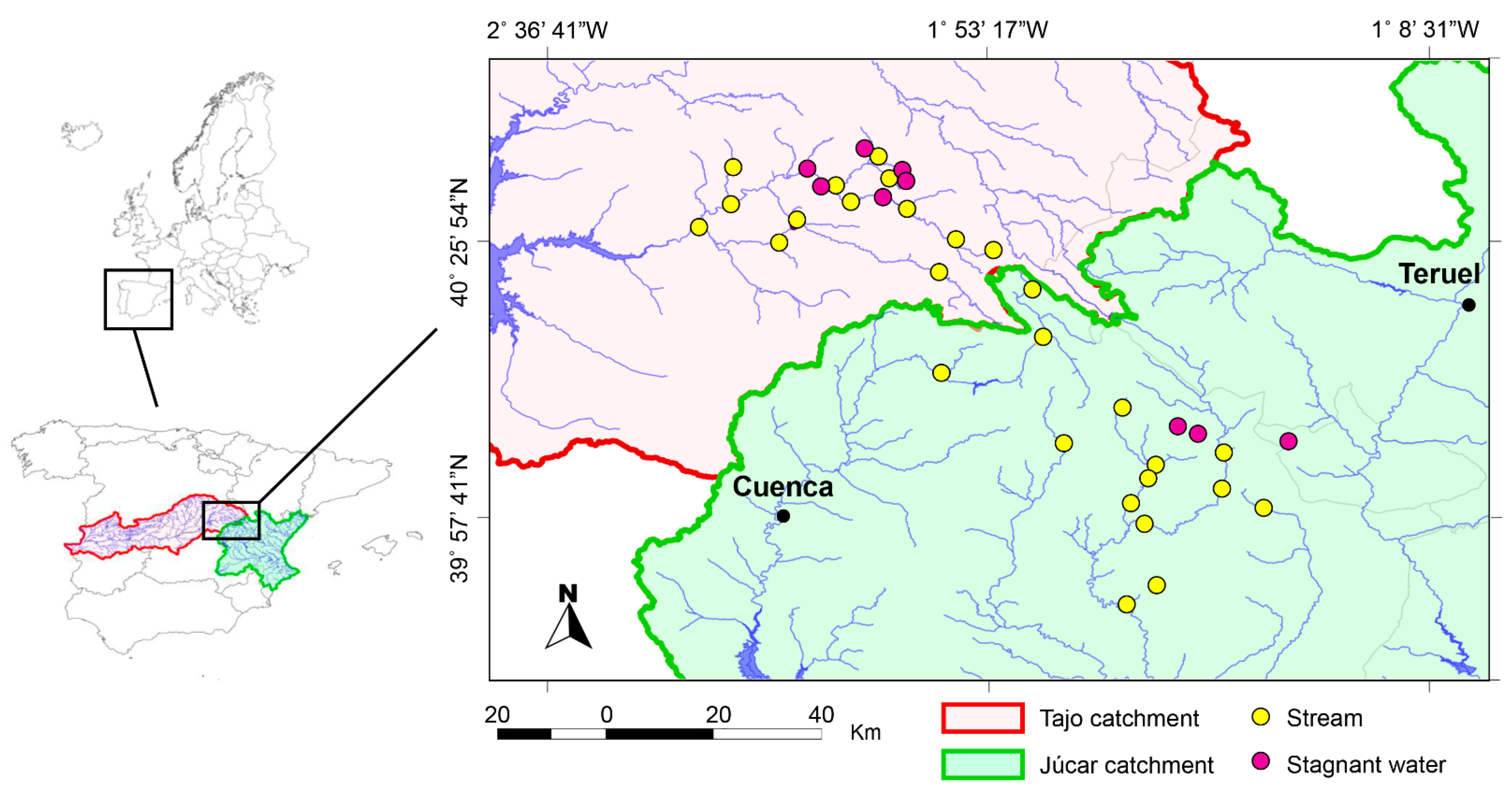
2.1. Field Work
2.2. Data Collection Matrices
2.3. Population–Community Structure and Its Control
2.4. Metacommunity Structure, Its Control and Spatial Scales
3. Results
3.1. Benthic Diatom Assemblages and Their Controlling Factors
3.2. Metacommunity Structure: Beta Diversity
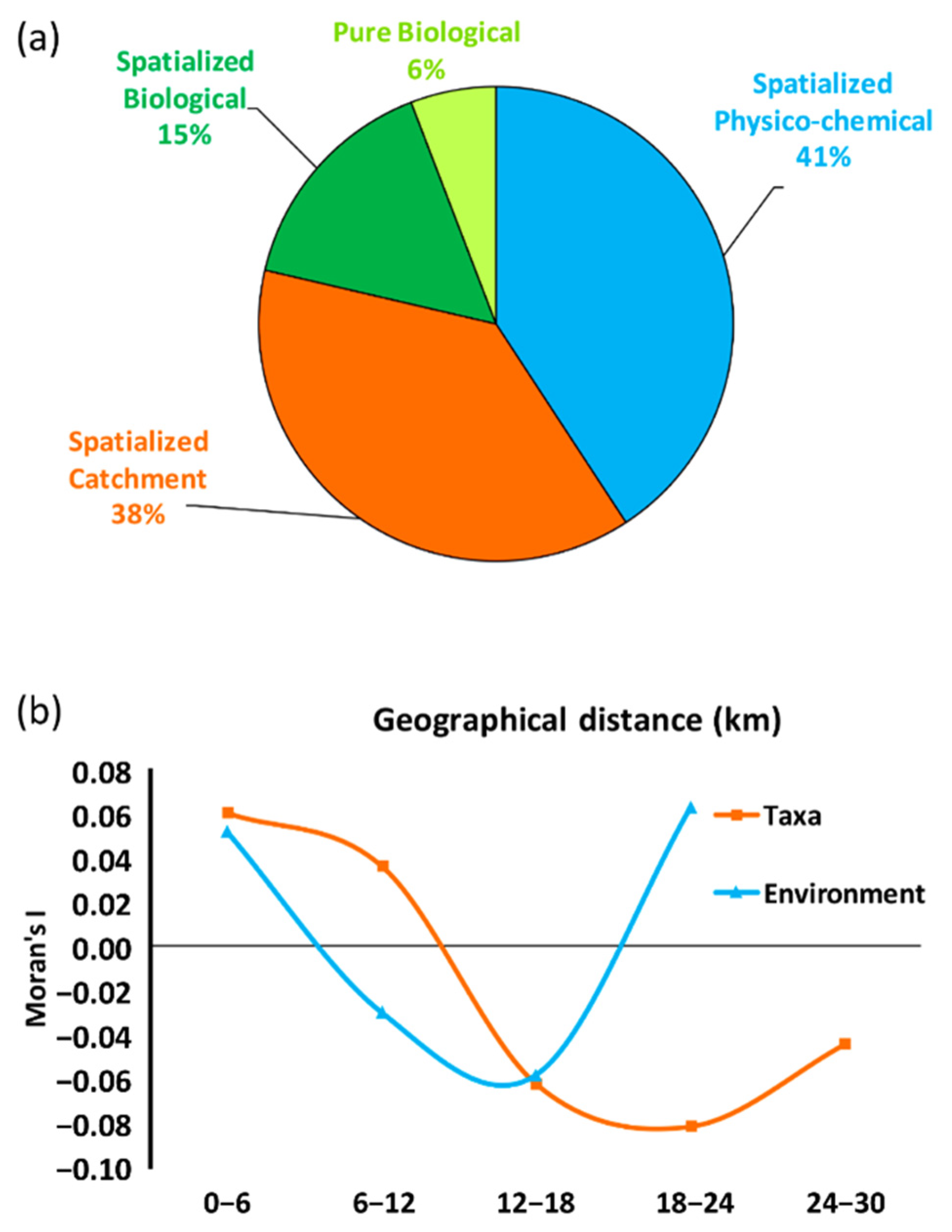
3.3. The Metacommunity: Variance Partition and Spatial Patterns
4. Discussion
4.1. Benthic Diatom Populations–Communities and Their Control Factors
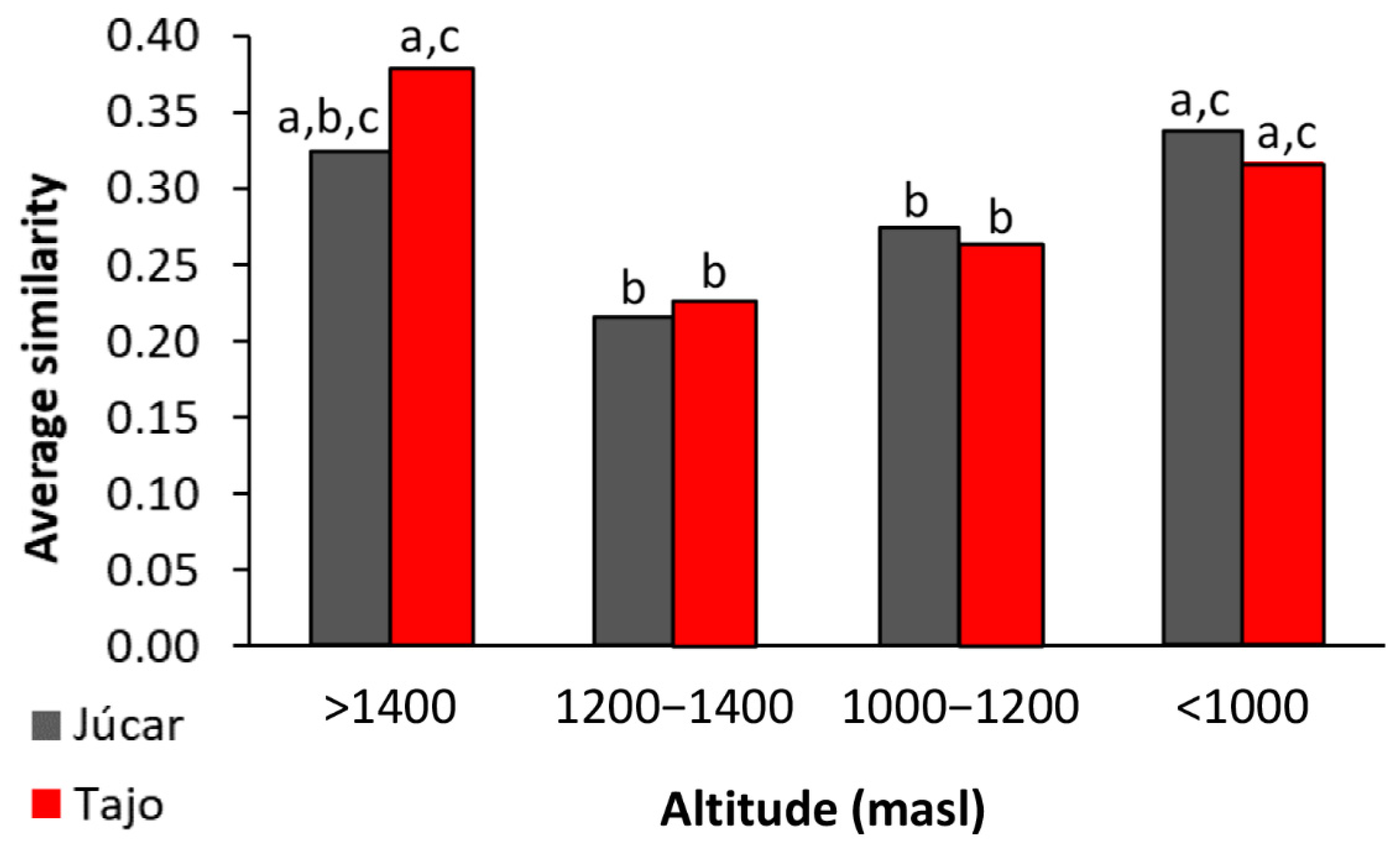
4.2. Metacommunity Structure: βD Patterns
4.3. The Metacommunity: Variance Partition and Spatial Patterns
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rojo, C.; Rodrigo, M.A.; Álvarez-Cobelas, M. Plankton diversity as an outcome of the assembly process. SIL Proc. 1922–2010 2006, 29, 1906–1908. [Google Scholar] [CrossRef]
- Rojo, C. Community assembly: Perspectives from phytoplankton’s studies. Hydrobiologia 2021, 848, 31–52. [Google Scholar] [CrossRef]
- Passy, S.I. Spatial paradigms of lotic diatom distribution: A landscape ecology perspective. J. Phycol. 2001, 37, 370–378. [Google Scholar] [CrossRef]
- Potapova, M.G.; Charles, D.F. Benthic diatoms in USA rivers: Distributions along spatial and environmental gradients. J. Biogeogr. 2002, 29, 167–187. [Google Scholar] [CrossRef]
- Passy, S. Community analysis in stream biomonitoring: What we measure and what we don’t. Environ. Monit. Assess. 2007, 127, 409–417. [Google Scholar] [CrossRef]
- Grenouillet, G.; Brosse, S.; Tudesque, L.; Lek, S.; Baraillé, Y.; Loot, G. Concordance among stream communities and spatial autocorrelation along a fragmented gradient. Divers. Distrib. 2008, 14, 592–603. [Google Scholar] [CrossRef]
- Soininen, J. The ecological characteristics of idiosyncratic and nested diatoms. Protist 2008, 159, 65–72. [Google Scholar] [CrossRef]
- Urrea, G.; Sabater, S. Epilithic diatom assemblages and their relationship to environmental characteristics in an agricultural watershed (Guadiana River, SW Spain). Ecol. Indic. 2009, 9, 693–703. [Google Scholar] [CrossRef]
- Soininen, J.; Kongas, P. Analysis of nestedness in freshwater communities—Patterns across taxa and trophic levels. Freshw. Sci. 2012, 31, 1145–1155. [Google Scholar] [CrossRef]
- Göthe, E.; Angeler, D.G.; Gottschalk, S.; Löfgren, S.; Sandin, L. The influence of environmental, biotic and spatial factors on diatom metacommunity structure in Swedish headwater streams. PLoS ONE 2013, 8, e72237. [Google Scholar] [CrossRef]
- Tornés, E.; Ruhí, A. Flow intermittency decreases nestedness and specialization of diatom communities in Mediterranean rivers. Freshw. Biol. 2013, 58, 2555–2566. [Google Scholar] [CrossRef]
- Bottin, M.; Soininen, J.; Ferrol, M.; Tison-Rosebery, J. Do spatial patterns of benthic diatom communities vary across regions and years? Freshw. Sci. 2014, 33, 402–416. [Google Scholar] [CrossRef]
- Winegardner, A.; Beisner, B.; Legendre, P.; Gregory-Eaves, I. Are the landscape-level drivers of water column and surface sediment diatoms different? Freshw. Biol. 2015, 60, 267–281. [Google Scholar] [CrossRef]
- Bere, T.; Mangadze, T.; Mwedzi, T. Variation partitioning of diatom taxa data matrices: Understanding the influence of multiple factors on benthic diatom communities in tropical streams. Sci. Total Environ. 2016, 566-567, 1604–1613. [Google Scholar] [CrossRef]
- Dong, X.; Li, B.; He, F.; Gu, Y.; Sun, M.; Zhang, H.; Tan, L.; Xiao, W.; Liu, S.; Cai, Q. Flow directionality, mountain barriers and functional traits determine diatom metacommunity structuring of high mountain streams. Sci. Rep. 2016, 6, 24711. [Google Scholar] [CrossRef] [Green Version]
- Soininen, J.; Jamoneau, A.; Rosebery, J.; Passy, S.I. Global patterns and drivers of taxa and trait composition in diatoms. Global Ecol. Biogeogr. 2016, 25, 940–950. [Google Scholar] [CrossRef]
- Virtanen, L.K.; Soininen, J. Temporal variation in community–environment relationships and stream classifications in benthic diatoms: Implications for bioassessment. Limnologica 2016, 58, 11–19. [Google Scholar] [CrossRef]
- Heino, J.; Soininen, J.; Alahuhta, J.; Lappalainen, J.; Virtanen, R. Metacommunity ecology meets biogeography: Effects of geographical region, spatial dynamics and environmental filtering on community structure in aquatic organisms. Oecologia 2017, 183, 121–137. [Google Scholar] [CrossRef] [Green Version]
- Vilmi, A.; Karjalainen, S.M.; Heino, J. Ecological uniqueness of stream and lake diatom communities shows different macroecological patterns. Divers. Distrib. 2017, 23, 1042–1053. [Google Scholar] [CrossRef] [Green Version]
- Teittinen, A.; Wang, J.; Strömgård, S.; Soininen, J. Local and geographical factors jointly drive elevational patterns in three microbial groups across subarctic ponds. Global Ecol. Biogeogr. 2017, 26, 973–982. [Google Scholar] [CrossRef]
- Virta, L.; Soininen, J. Distribution patterns of epilithic diatoms along climatic, spatial and physicochemical variables in the Baltic Sea. Helgoland Mar. Res. 2017, 71, 16. [Google Scholar] [CrossRef]
- Winegardner, A.; Legendre, P.; Beisner, B.E.; Gregory-Eaves, I. Diatom diversity patterns over the past c. 150 years across the conterminous United States of America: Identifying mechanisms behind beta diversity. Global Ecol. Biogeogr. 2017, 26, 1303–1315. [Google Scholar] [CrossRef]
- Jamoneau, A.; Passy, S.I.; Soininen, J.; Leboucher, T.; Tison-Rosebery, J. Beta diversity of diatom taxa and ecological guilds: Response to environmental and spatial mechanisms along the stream watercourse. Freshw. Biol. 2018, 63, 62–73. [Google Scholar] [CrossRef] [Green Version]
- Jyrkänkallio-Mikkola, J.; Heino, J.; Soininen, J. Beta diversity of stream diatoms at two hierarchical spatial scales: Implications for biomonitoring. Freshw. Biol. 2016, 61, 239–250. [Google Scholar] [CrossRef]
- Passy, S.I.; Larson, C.A.; Jamoneau, A.; Budnick, W. Biogeographical patterns of species richness and abundance distribution in stream diatoms are driven by climate and water chemistry. Am. Nat. 2018, 192, 605–617. [Google Scholar] [CrossRef] [Green Version]
- Szabó, B.; Lengyel, E.; Padisák, J.; Stenger-Kovács, C. Benthic diatom metacommunity across small freshwater lakes: Driving mechanisms, β-diversity and ecological uniqueness. Hydrobiologia 2019, 828, 183–198. [Google Scholar] [CrossRef]
- Rodríguez-Alcalá, O.; Blanco, S.; García-Girón, J.; Jeppesen, E.; Irvine, K.; Nõges, P.; Nõges, T.; Gross, E.M.; Bécares, E. Beta diversity of stream diatoms at two hierarchical spatial scales: Implications for biomonitoring. Sci. Total Environ. 2020, 61, 239–250. [Google Scholar] [CrossRef]
- González-Trujillo, J.D.; Pedraza-Garzón, E.; Donato-Rondon, J.C.; Sabater, S. Ecoregional Characteristics Drive the Distribution Patterns of Neotropical Stream Diatoms. J. Phycol. 2020, 56, 1053–1065. [Google Scholar] [CrossRef]
- Stoof-Leichsenring, K.R.; Dulias, K.; Biskaborn, B.K.; Pestryakova, L.A.; Herzschuh, U. Lake-depth related pattern of genetic and morphological diatom diversity in boreal Lake Bolshoe Toko, Eastern Siberia. PLoS ONE 2020, 15, e0230284. [Google Scholar] [CrossRef] [Green Version]
- Leibold, M.A.; Holyoak, M.; Mouquet, N.; Amarasekare, P.; Chase, J.M.; Hoopes, M.F.; Holt, R.D.; Shurin, J.B.; Law, R.; Tilman, D.; et al. The metacommunity concept: A framework for multi-scale community ecology. Ecol. Lett. 2004, 7, 601–613. [Google Scholar] [CrossRef]
- Baselga, A. The relationship between species replacement, dissimilarity derived from nestedness, and nestednes. Global Ecol. Biogeogr. 2012, 21, 1223–1232. [Google Scholar] [CrossRef]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Dungan, J.L.; Perry, J.N.; Dale, M.R.T.; Legendre, P.; Citron-Pousty, S.; Fortin, M.-J.; Jakomulska, A.; Miriti, M.; Rosenberg, M.S. A balanced view of scale in spatial statistical analysis. Ecography 2002, 25, 626–640. [Google Scholar] [CrossRef] [Green Version]
- Cottenie, K. Integrating environmental and spatial processes in ecological community dynamics. Ecol. Lett. 2005, 8, 1175–1182. [Google Scholar] [CrossRef]
- Rojo, C.; Mosquera, Z.; Álvarez-Cobelas, M.; Segura, M. Microalgal and cyanobacterial communities on charophytes: A metacommunity perspective. Fund. Appl. Limnol. 2017, 190, 97–115. [Google Scholar] [CrossRef]
- Bailey, R.G. Ecosystem Geography: From Ecoregions to Sites; Springer Science & Business: New York, NY, USA, 2009. [Google Scholar]
- Weiher, E.; Keddy, P. Ecological Assembly Rules: Perspectives, Advances, Retreats; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Chorus, I.; Spijkerman, E. What Colin Reynolds could tell us about nutrient limitation, N:P ratios and eutrophication control. Hydrobiologia 2021, 848, 95–111. [Google Scholar] [CrossRef]
- Naselli-Flores, L.; Dokulil, M.T.; Elliott, J.A.; Padisák, J. New, old and evergreen frontiers in freshwater phytoplankton ecology: The legacy of Colin, S. Reynolds. Hydrobiologia 2021, 848, 1–6. [Google Scholar] [CrossRef]
- Patrick, R.M. Ecology of freshwater diatoms and diatom communities. In The Biology of Diatoms; Werner, D., Ed.; Blackwell Scientific Publications: Oxford, UK, 1977; pp. 284–332. [Google Scholar]
- McCormick, P.V. Resource competition and taxa coexistence in freshwater benthic communities. In Algal Ecology—Freshwater Benthic Ecosystems; Stevenson, R.J., Bothwell, M.L., Lowe, R.L., Eds.; Academic Press: London, UK, 1996; pp. 229–252. [Google Scholar]
- Tang, T.; Wu, N.; Li, F.; Fu, X.; Cai, Q. Disentangling the roles of spatial and environmental variables in shaping benthic algal communities in rivers of central and northern China. Aquat Ecol. 2013, 47, 453–466. [Google Scholar] [CrossRef]
- Romaní, A.M.; Guasch, H.; Balaguer, M.D. Aquatic Biofilms: Ecology, Water Quality and Wastewater Treatment; Caister Academic Press: Norfolk, UK, 2016. [Google Scholar] [CrossRef] [Green Version]
- Tall, L.; Cloutier, L.; Cattaneo, A. Grazer-diatom size relationships in an epiphytic community. Limnol. Oceanogr. 2006, 51, 1211–1216. [Google Scholar] [CrossRef]
- Passy, S.I. Diatom ecological guilds display distinct and predictable behavior along nutrient and disturbance gradients in running waters. Aquat. Bot. 2007, 86, 171–178. [Google Scholar] [CrossRef]
- Rojo, C.; Segura, M.; Rodrigo, M.A. The allelopathic capacity of submerged macrophytes shapes the microalgal communities from a recently restored coastal wetland. Ecol. Eng. 2013, 58, 149–155. [Google Scholar] [CrossRef]
- Soininen, J.; Teittinen, A. Fifteen important questions in the spatial ecology of diatoms. Freshw. Biol. 2019, 64, 2071–2083. [Google Scholar] [CrossRef] [Green Version]
- Kolbe, R.W. Grundlinien einer allgemeinen Ökologie der Diatomeen. In Ergebnisse der Biologie, 8; Von Fritsch, K., Goldschmidt, R., Ruhland, W., Eds.; Verlag von Julius Springer: Berlin, Germany, 1932; pp. 221–348. [Google Scholar]
- Cholnoky, B.J. Okologie der Diatomeen in Binnengewässern; J. Cramer: Lehre, Germany, 1968; p. 699. [Google Scholar]
- Burkholder, J.M. Interactions of benthic algae with their substrata. In Algal Ecology—Freshwater Benthic Ecosystems; Stevenson, R.J., Bothwell, M.L., Lowe, R.L., Eds.; Academic Press: London, UK, 1996; pp. 253–297. [Google Scholar]
- Letáková, M.; Fránková, M.; Poulíčková, A. Ecology and applications of freshwater epiphytic diatoms—Review. Cryptogamie Algol. 2018, 39, 3–22. [Google Scholar] [CrossRef]
- Soininen, J.; Weckström, J. Diatom community structure along environmental and spatial gradients in lakes and streams. Fund. Appl. Limnol. 2009, 174, 205–213. [Google Scholar] [CrossRef]
- Kahlert, M.; Gottschalk, S. Differences in benthic diatom communities between streams and lakes in Sweden and implications for ecological assessment. Freshw. Sci. 2014, 33, 655–669. [Google Scholar] [CrossRef]
- Liu, J.; Soininen, J.; Han, B.-P.; Declerck, S.A.J. Effects of connectivity, dispersal directionality and functional traits on the metacommunity structure of river benthic diatoms. J. Biogeogr. 2013, 40, 2238–2248. [Google Scholar] [CrossRef]
- Mereschkowsky, C. Diatomées du Tibet; Imperial Russkoe Geograficheskoe Obshchestvo: St. Petersburg, Russia, 1906. [Google Scholar]
- Cantonati, M.; Lowe, R.L. Lake benthic algae: Toward an understanding of their ecology. Freshw. Sci. 2014, 33, 475–486. [Google Scholar] [CrossRef]
- Poulíčková, A.; Manoylov, K. Ecology of freshwater diatoms—Current trends and applications. In Diatoms: Fundamentals and Applications; Seckbach, J., Gordon, R., Eds.; Scrivener Publishing LLC: Beverly, MA, USA, 2019; pp. 289–309. [Google Scholar]
- Johnson, L.; Richards, C.; Host, G.; Arthur, J. Landscape influences on water chemistry in Midwestern stream ecosystems. Freshw. Biol. 1997, 37, 193–208. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association/American Water Works Association/Water Environment Federation: Wasington, DC, USA, 2005. [Google Scholar]
- Carrick, H.J.; Lowe, R.L. Nutrient limitation of benthic algae in Lake Michigan: The role of silica. J. Phycol. 2007, 43, 228–234. [Google Scholar] [CrossRef]
- Jost, L.; DeVries, P.; Walla, T.; Greeney, H.; Chao, A.; Ricotta, C. Partitioning diversity for conservation analyses. Divers. Distrib. 2010, 16, 65–76. [Google Scholar] [CrossRef]
- Koleff, P.; Gaston, K.J.; Lennon, J.J. Measuring beta diversity for presence-absence data. J. Ecol. 2003, 72, 367–382. [Google Scholar] [CrossRef] [Green Version]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Global Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package; R Package Version 2.5-6. 2019. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 21 October 2022).
- Almeida-Neto, M.; Guimarães, P.; Guimarães, P.R.; Loyola, R.D.; Ulrich, W. A consistent metric for nestedness analysis in ecological systems: Reconciling concept and measurement. Oikos 2008, 117, 1227–1239. [Google Scholar] [CrossRef]
- Legendre, P.; De Cáceres, M. Beta diversity as the variance of community data: Dissimilarity coefficients and partitioning. Ecol. Lett. 2013, 16, 951–963. [Google Scholar] [CrossRef]
- Dray, S.; Bauman, D.; Blanchet, G.; Borcard, D.; Clappe, S.; Guenard, G.; Jombart, T.; Larocque, G.; Legendre, P.; Madi, N.; et al. Adespatial: Multivariate Multiscale Spatial Analysis; R Package Version 0.3-8. 2020. Available online: https://github.com/sdray/adespatial (accessed on 21 October 2022).
- Clarke, K.R. Non-parametric multivariate analysis of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Connell, J.H. Diversity in Tropical Rain Forests and Coral Reefs: High diversity of trees and corals is maintained only in a nonequilibrium state. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, P.; Kirilova, E. Benthic diatom assemblages from different substrates of the Iskar River, Bulgaria. In Eighteenth International Diatom Symposium 2004; Witkowski, A., Ed.; Biopress Limited: Bristol, UK, 2006; pp. 107–124. [Google Scholar]
- Mendes, T.; Almeida, S.F.P.; Feio, M.J. Assessment of rivers using diatoms: Effect of substrate and evaluation method. Fund. Appl. Limnol. 2012, 179, 267–279. [Google Scholar] [CrossRef]
- Wojtal, A.; Sobczyk, L. The influence of substrates and physicochemical factors on the composition of diatom communities in karst springs and their applicability in water-quality assessment. Hydrobiologia 2012, 695, 97–108. [Google Scholar] [CrossRef] [Green Version]
- Lürling, M. Grazing resistance in phytoplankton. Hydrobiologia 2021, 848, 237–249. [Google Scholar] [CrossRef]
- Heino, J.; Soininen, J. Assembly rules and community models for unicellular organisms: Patterns in diatoms of boreal streams. Freshw. Biol. 2005, 50, 567–577. [Google Scholar] [CrossRef]
- Cantonati, M.; Scola, S.; Angeli, N.; Guella, G.; Frassanito, R. Environmental controls of epilithic diatom depth distribution in an oligotrophic lake characterised by marked water-level fluctuations. Eur. J. Phycol. 2009, 44, 15–29. [Google Scholar] [CrossRef]
- Padisák, J.; Reynolds, C.S. Selection of phytoplankton associations in Lake Balaton, Hungary, in response to eutrophication and restoration measures, with special reference to the cyanoprokaryotes. Hydrobiologia 1998, 384, 41–53. [Google Scholar] [CrossRef]
- Reynolds, C.S.; Dokulil, M.T.; Padisák, J. The Trophic Spectrum Revisited—Developments in Hydrobiology 150; Springer: Dordrecht, The Netherlands, 2000. [Google Scholar]
- Hollingsworth, E.K.; Vis, M.L. The spatial heterogeneity of diatoms in eight southeastern Ohio streams: How far does a single riffle reach? Hydrobiologia 2010, 651, 173–184. [Google Scholar] [CrossRef]
- Heino, J.; Melo, A.S.; Siqueira, T.; Soininen, J.; Valanko, S.; Bini, L.M. Metacommunity organisation, spatial extent and dispersal in aquatic systems: Patterns, processes and prospects. Freshw. Biol. 2015, 60, 845–869. [Google Scholar] [CrossRef]
- Heino, J.; Grönroos, M. Exploring species and site contributions to beta diversity in stream insect communities. Oecologia 2017, 183, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Leibold, M.A.; Mikkelson, G.M. Coherence, species turnover, and boundary clumping: Elements of metacommunity structure. Oikos 2002, 97, 237–250. [Google Scholar] [CrossRef] [Green Version]
- Ulrich, W.; Almeida-Neto, M.; Gotelli, N.J. A consumer’s guide to nestedness analysis. Oikos 2009, 118, 3–17. [Google Scholar] [CrossRef]
- Podani, J.; Schmera, D. A new conceptual and methodological framework for exploring and explaining patterns in presence-absence data. Oikos 2011, 120, 1625–1638. [Google Scholar] [CrossRef]
- Leibold, M.A.; Chase, J.M. Metacommunity Ecology; Princeton University Press: Princeton, NJ, USA, 2018. [Google Scholar]
- Hagerthey, S.E.; Defew, E.C.; Paterson, D.M. Influence of Corophium volutator and Hydrobia ulvae on intertidal benthic diatom assemblages under different nutrient and temperature regimes. Mar. Ecol. Prog. Ser. 2002, 245, 47–59. [Google Scholar] [CrossRef]
- Murdock, J.N.; Dodds, W.K. Linking benthic algal biomass to stream substratum topography. J. Phycol. 2007, 43, 449–460. [Google Scholar] [CrossRef]
- Legendre, P.; Fortin, M.J. Spatial pattern and ecological analysis. Vegetatio 1989, 80, 107–138. [Google Scholar] [CrossRef]
- Gehlke, C.E.; Biehl, K. Certain effects of grouping upon the size of the correlation coefficient in census tract material. J. Am. Stat. Assoc. 1934, 29, 169–170. [Google Scholar] [CrossRef]
- Jelinski, J.G.; Wu, D.E. The modifiable areal unit problem and implications for landscape ecology. Landscape Ecol. 1996, 11, 129–140. [Google Scholar] [CrossRef]
- Dickey, J.R.; Swenie, R.A.; Turner, S.C.; Winfrey, C.C.; Yaffar, D.; Padukone, A.; Beals, K.K.; Sheldon, K.S.; Kivlin, S.N. The Utility of Macroecological Rules for Microbial Biogeography. Front. Ecol. Evol. 2021, 9, 633155. [Google Scholar] [CrossRef]
- Aemet. Atlas Climático Ibérico. Temperatura del aire y Precipitación (1971–2000); Agencia Estatal de Meteorología: Madrid, Spain, 2011. [Google Scholar]
- Soil Survey Staff. Soil Taxonomy: A basic System of Soil Classification for making and interpreting Soil Surveys, 2nd ed.; Handbook 436; Natural Resources Conservation Service. U.S. Department of Agriculture: Washington, DC, USA, 1999.
- Guerra, M.A. Fuentes y manantiales de la Serranía conquense; Diputación de Cuenca: Cuenca, Spain, 1999; 241p. [Google Scholar]
- Cava, L.E. La Serranía alta de Cuenca. Evolución de los usos del suelo y problemática socioterritorial; Universidad Internacional Menéndez Pelayo y Programa LEADER Serranía de Cuenca: Cuenca, Spain, 1994; 588p. [Google Scholar]
- Álvarez-Cobelas, M.; Rojo, C.; Sánchez-Carrillo, S. Nutrient export from largely pristine catchments (Serranía de Cuenca, Central Spain). Bol. Geol. Min. 2020, 131, 559–580. [Google Scholar] [CrossRef]
- Mayoral, O. Estudio Florístico y Aportaciones a la Conservación del alto Cabriel (Cuenca). Doctoral Thesis, University of Valencia, Valencia, Spain, 2011; 554p. [Google Scholar]
- Buil, J.R.; Fernández Yuste, A.; Lozano, J.; Nicolás, I. Datos sobre la distribución de peces en los ríos de la provincia de Cuenca. Ecología 1987, 1, 231–245. [Google Scholar]
- González Guerrero, P. Novedades biológicas en algas de Cuenca. Anales Jard. Bot. Madrid 1940, 1, 107–139. [Google Scholar]
- Blanco, S.; Álvarez, I.; Cejudo, C. A test on different aspects of diatom processing techniques. J. Appl. Phycol. 2008, 20, 445–450. [Google Scholar] [CrossRef]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae 1. Teil: Naviculaceae; 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae; 3. Teil: Centrales, Fragilariaceae, Eunotiaceae; 4. Teil: Achnanthaceae. Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema; G. Fischer Verlag: Frankfurt am Main, Germany, 1986–1991. [Google Scholar]
- Jackson, D.A.; Walker, S.C.; Poos, M.S. Cluster analysis of fish community data: “New” tools for determining meaningful groupings of sites and taxa assemblages. Am. Fish. Soc. Symp. 2010, 73, 503–527. [Google Scholar]
| Site | Area Extent, km2; (Spacing, km); [Altitude, masl] | Substrate; (Water Trophic Status) | Dependent Variable (Number of Taxa) | Statistics Used | Concluding Remarks | Reference |
|---|---|---|---|---|---|---|
| White Creek (NY, USA) | 1.6 × 10−5 (4.9 × 10−5) | Cobble (-) | Taxa relative abundances (41) | Moran’s correlograms on dominant species; CCA on spatial features and current velocity and taxa | Patch length and width of BDA were >3.1 × 0.5–1 m; space explained much lower variability in diatom distribution than current velocity | [3] |
| USA (whole country) | 8.1 × 106 (3.2) | Soft sediment and stone (whole range) | Taxa absolute abundances (433) | RDA on spatial and environmental factors and taxa | The environment plays the most important role in structuring stream BDA, but spatial factors also explain some variation in diatom distribution, especially at the more coarse scale (i.e., continental) | [4] |
| Mesta river (Bulgaria) | 5.0 × 103 (6.5) | Cobble (whole range) | Taxa relative abundances | RDAs on environmental, temporal and spatial factors and taxa | All three independent matrices explain variability in BDA | [5] |
| River Viaur (France) | 1.5 × 103 (3.0) [150–1090] | Cobble | Taxa relative abundances (196) | Mantel correlograms | BDA are spatially autocorrelated; man-made barriers are important for fragmentation of BDA | [6] |
| Finland (whole country) | 3.4 × 105 (5.5) | Cobble (whole range) | Richness of taxa (248) | Nestedness and partial Mantel tests on environmental and spatial factors | Idiosyncratic species show faster turnover and are more widely distributed than nested species | [7] |
| Guadiana basin (Spain) | 6.8 × 104 (1.1) [550–1000] | Cobble (whole range) | Taxa relative abundances (248) | CCAs on environmental, temporal and spatial factors and taxa | Environmental factors mostly structure BDA, but purely spatial control also takes place | [8] |
| Two Finland catchments | (-) | Cobble (whole range) | Richness of taxa | MRM regressions of taxa nestedness on environmental and spatial factors | Nestedness mostly adheres to the local environment, but a minor variability can be attributed to the geographical longitude | [9] |
| Dalälven catchment (Sweden) | 1.4 × 104 (3.9) [146–631] | Cobble | Taxa absolute abundances (186) | RDAs on PCNMs and taxa | Environmental factors mostly structure BDA | [10] |
| 122 stream sites at NE Spain | 3.2 × 104 (1.5) | Cobble (whole range) | Richness and nestedness | LM of taxa richness and ß diversity on local features | BDA inhabiting hydrologically stable rivers present a higher level of order in spatial pattern and a proportion of specialist taxa than communities in intermittent streams | [11] |
| France (whole country) | 5.5 × 105 (0.5) [0–500] | Cobble (whole range) | Taxa relative abundances (1091) | MRM of taxa and environmental factors; Mantel correlograms | Environmental factors mostly structure BDA, but purely spatial control also takes place. Some ecoregions are neatly separated on account of geographical barriers | [12] |
| USA (whole country) | 8.1 × 106 (6.1) | Sediment and water column (whole range) | Taxa relative abundances | RDAs of taxa on environmental and spatial data; co-inertial analysis | Water column and sediment assemblages are congruent and correlated regarding drivers of community composition | [13] |
| Manyame catchment (Zimbabwe) | 4.4 × 104 (10.1) | Cobble (polluted water) | Taxa relative abundances (156) | CCA of hydromorphological factors and organic and heavy metal pollution and taxa | Hydromorphology and pollution partly explained the matrix of relative abundances of BD | [14] |
| Canshang Erhai N. N. Reserve (China) | 9.5 × 102 (0.5) | Cobble (pristine water) | Taxa absolute abundances (149) | RDAs on PCNMs and taxa | Mountain barriers limit dispersal, which occurs through corridor streams | [15] |
| Six data sets worldwide | [1400–4100] | Cobble | Taxa and T-type richness | NMDS and RDAs of Diatom taxa and T-types on environmental and spatial factors | Taxa composition discriminated the geographical regions better, while T-type composition detected the environmental gradients better | [16] |
| Four southern Finland catchments | (-) | Cobble (eutrophic water) | Taxa relative abundances | ANOSIM, DCA and Mantel tests on environmental, spatial and temporal factors and taxa | Three-yearly temporal variation is negligible in the diatom–environment relationship | [17] |
| Three northern Finland catchments | 6.4 × 104 (5.6) | Cobble (near-pristine water) | Taxa relative abundances | PCA to define metacommunities visually; RDAs on environmental and spatial factors and taxa; MEM on taxa; beta diversity assessment on taxa occurrence | Basin identity was a slightly better predictor of BDA than local environment; beta diversity of regions is high | [18] |
| Southern half of Finland | (-) | Cobble (whole range) | Taxa absolute abundances | Richness, LCBD, SCBD; DBMEMs, RDAs, spatial autocorrelation of beta diversity; landscape features as independent variables | While richness and beta diversity of streams are related to the regional environment, those of lakes are related to spatial measurements; differential hydrological connectivity is the key factor of these diatom variables | [19] |
| 146 subarctic ponds (Finland and Norway) | [10–1080] | Cobble | Richness | LM and RDAs of richness and beta diversity on local features, a terrestrial vegetation index and elevation | Richness and beta diversity are mainly determined by local factors, loosely linked to elevation | [20] |
| Finland Baltic coast | (-) | Cobble | Richness (230) | RDAs of taxa richness on environmental and spatial factors | Richness primarily regulated by local factors, while climatic and spatial variables have little impact on richness | [21] |
| 169 (for genus) and 52 (for species) USA lakes | (-) | Sediment | ß diversity (LCBD) before 1850 and in 2007 | LM of genus and species beta diversity on environmental and spatial factors | Beta diversity does not appear to have changed in the last 150 years; temporal beta diversity was related to land cover changes in watersheds | [22] |
| France (whole country) | 5.5 × 105 (0.5) [0–500] | Cobble (whole range) | ß diversity on diatom presence | Partial Mantel tests on environment and beta diversity matrices | Environmental filtering is more important to beta diversity than space, which gains importance in middle and lower parts of catchments | [23] |
| 21 catchments in SW Finland | 1.7 × 105 (4.0) | Cobble (whole range) | Richness (347) | RDAs on environmental and spatial factors and taxa | Biogeographical variation of BDA results from the interplay of local, catchment and climatic variables, but also it is likely that dispersal limitation plays a role | [24] |
| USA and Finland (whole countries) | 8.1 × 106 (5.4) [0–2448] and 3.4 × 105 (5.8) [0–302] | Cobble (whole range) | Richness and distribution of absolute abundance | LM of taxa richness and SAD on climatic and chemical features | The spatial patterns of richness and abundance defined primarily by the covariance of climate and chemistry with space | [25] |
| 38 Carpathian lakes (Hungary) | [73–311] | Reed, stone, mud | ß diversity (LCBD, SCBD) and relative abundance | LM and RDAs of dependent variables on spatial and environmental heterogeneity | Spatial and environmental variables affect diatom features | [26] |
| 34 lakes in whole of Europe | (-) | Reed (whole range) | Richness | RDAs of beta diversity on environmental and spatial factors | Taxa richness is mainly due to environmental factors | [27] |
| 26 streams in the Orinoco catchment (Colombia) | 4.0 × 104 (7.7) [300–3400] | Cobble and other rocks (whole range) | Taxa and trait relative abundances (297) | ANOSIM of BDA in ecoregions; RDAs on environmental, spatial and historical factors and taxa and traits | Constraints on taxa occurrence and dispersal, as well as legacies of historical events, explain contemporary distribution of diatoms in the area | [28] |
| Lake Bolshoe Toko (Yakutia, Russia) | 8.3 × 101 (0.5) [903] | (-) | DNA and morphological taxa relative abundances | Estimation of alpha and beta diversity (LCBD and SCBD); RDAs on vertical space and morphotaxa and DNA taxa | Genetic diversity was higher than morphodiversity; alpha and beta diversity responded differently to lake depth | [29] |
| Location Name | Lat. (Decim.) | Long. (Decim.) | Habitat |
|---|---|---|---|
| Júcar Catchment | |||
| Algarra river at Algarra | 40.000389 | 1.440572 | F |
| Cabriel river at Alcalá de la Vega | 40.031719 | 1.514378 | F |
| Cabriel river at Boniches | 39.983250 | 1.641311 | F |
| Cabriel river at Salvacañete | 40.097005 | 1.508172 | F |
| Cabriel river at Villar del Humo | 39.840572 | 1.663689 | F |
| Cabriel springs | 40.235075 | 1.554969 | S |
| Guadarroyo river upstream Valdemoro Sierra | 40.104069 | 1.754736 | F |
| Júcar river at Huélamo | 40.279128 | 1.814058 | F |
| Júcar river at Uña | 40.221797 | 1.978241 | F |
| Júcar river close to its spring | 40.364077 | 1.829153 | F |
| La Toba reservoir | 40.211447 | 1.922105 | S |
| Laguna river downstream Laguna del Marquesado | 40.169611 | 1.672333 | F |
| Marquesado lake | 40.187522 | 1.666727 | S |
| Mayor river downstream Cañete | 40.010319 | 1.657928 | F |
| Mayor river upstream Cañete | 40.053000 | 1.631094 | F |
| Tejadillos river at Cañete | 40.066125 | 1.623122 | F |
| Uña lake | 40.224167 | 1.977777 | S |
| Valdemoro Sierra spring and cascade | 40.078117 | 1.776656 | F |
| Vencherque river at Villar del Humo | 40.053000 | 1.631094 | F |
| Tajo Catchment | |||
| Alcantud river downstream Alcantud | 40.512253 | 2.329152 | F |
| Beteta wetland | 40.566338 | 2.072000 | S |
| Cuervo river at Solán de Cabras | 40.512906 | 2.127116 | F |
| Cuervo river at the spring | 40.428511 | 1.889386 | F |
| Cuervo river at Vega del Codorno | 40.422828 | 1.913333 | F |
| Cuervo river upstream Santa María | 40.499953 | 2.035769 | F |
| El Tobar lake | 40.545714 | 2.048944 | S |
| El Tobar lake spring | 40.546547 | 2.044671 | S |
| Escabas river at Tejadillos | 40.394575 | 1.983691 | F |
| Escabas river downstream Cañamares | 40.447552 | 2.247922 | F |
| Escabas river downstream Fuertescusa | 40.468480 | 2.222775 | F |
| Escabas river upstream Guadiela junction | 40.447552 | 2.247922 | F |
| Guadiela river at Beteta | 40.576058 | 2.043937 | F |
| Guadiela river upstream La Ruidera reservoir | 40.509641 | 2.323303 | F |
| Guadiela river upstream Puente Vadillos | 40.532544 | 2.149589 | F |
| La Ruidera reservoir | 40.478502 | 2.376797 | S |
| La Tosca reservoir | 40.517600 | 2.058514 | S |
| Masegar creek at El Tobar | 40.551572 | 2.063969 | F |
| Molino de Chincha reservoir | 40.538200 | 2.161030 | S |
| Overall | Catchments | Habitats | Substrates | ||||
|---|---|---|---|---|---|---|---|
| Júcar | Tajo | Stagnant w. | Streams | Epilithic | Epiphytic | ||
| Number of samples | 132 | 69 | 63 | 24 | 108 | 44 | 88 |
| α diversity indices | |||||||
| Richness range | 3–29 | 3–22 | 5–29 | 6–29 | 3–26 | 6–27 | 3–29 |
| Average | 12.7 | 10.8 | 14.8 | 15.5 | 12.1 | 12.5 | 12.9 |
| SD | 5.0 | 4.1 | 5.2 | 5.7 | 4.7 | 5.6 | 4.8 |
| p | 0.0001 | 0.0004 | 0.4283 | ||||
| Shannon index range | 0.11–2.77 | 0.12–2.28 | 0.14–2.77 | 0.64–2.32 | 0.11–2.77 | 0.64–2.77 | 0.11–2.28 |
| Effective number range | 1.12–15.89 | 1.12–9.78 | 1.15–15.89 | 1.91–10.13 | 1.12–15.89 | 1.89–15.89 | 1.12–9.78 |
| Average | 4.23 | 3.98 | 4.51 | 4.17 | 4.25 | 4.59 | 4.06 |
| SD | 2.21 | 1.85 | 2.52 | 2.17 | 2.22 | 2.72 | 1.89 |
| p | 0.172 | 0.961 | 0.193 | ||||
| Main taxa | |||||||
| Achnanthidium minutissimum | 76 (73%) | 76 (63%) | 70 (84%) | 76 (79%) | 70 (71%) | 69 (64%) | 76 (77%) |
| Cocconeis placentula | 91 (67%) | 91 (76%) | 63 (59%) | 19 (58%) | 91 (69%) | 80 (64%) | 91 (69%) |
| Cymbella affinis | 64 (60%) | 42 (54%) | 64 (67%) | 64 (50%) | 42 (63%) | 64 (55%) | 38 (63%) |
| Cymbella delicatula | 64 (34%) | 64 (24%) | 54 (46%) | 29 (38%) | 64 (33%) | 42 (34%) | 64 (34%) |
| Cymbopleura amphicephala | 61 (53%) | 38 (38%) | 61 (84%) | 61 (79%) | 0 (0%) | 46 (55%) | 61 (63%) |
| Diatoma vulgaris | 98 (46%) | 98 (44%) | 22 (49%) | 4 (29%) | 98 (50%) | 22 (36%) | 98 (51%) |
| Gomphonema angustatum | 50 (77%) | 49 (75%) | 50 (81%) | 46 (75%) | 50 (78%) | 49 (77%) | 50 (77%) |
| Navicula cryptotenella | 55 (42%) | 55 (47%) | 32 (37%) | 1 (21%) | 55 (46%) | 39 (36%) | 55 (44%) |
| β diversity indices | |||||||
| Harrison index | 0.09 | 0.13 | 0.12 | 0.2 | 0.09 | 0.18 | 0.11 |
| NODF (nestedness) | 26.3 | 23.9 | 26.1 | 29.7 | 27.5 | 22.7 | 25.6 |
| Spatial turnover | 0.96 | 0.94 | 0.9 | 0.73 * | 0.95 | 0.9 | 0.93 |
| Dissimilarity due to nestedness | 0.02 | 0.03 | 0.05 | 0.11 * | 0.02 | 0.04 | 0.03 |
| Dependent Variable | Independent Variables | Slope | p | Adj R2 | N |
|---|---|---|---|---|---|
| Taxa In Stagnant Waters | |||||
| Cymbella minuta | Nitrate | 0.247 | 0.040 | 0.25 | 14 |
| Achnanthidium minutissimum | Epithemia goeppertiana | −1.101 | 0.008 | 0.31 | 24 |
| Rhopalodia gibba | −1.079 | 0.025 | |||
| Epithemia goeppertiana | 1.221 | 0.000 | |||
| Cymbella affinis | Cymbella minuta | −1.203 | 0.000 | 0.77 | 24 |
| Achnanthidium minutissimum | −0.146 | 0.034 | |||
| Epithemia goeppertiana | 0.857 | 0.000 | |||
| Cymbella minuta | Cymbella affinis | −0.587 | 0.000 | 0.8 | 24 |
| Achnanthidium minutissimum | −0.109 | 0.023 | |||
| Epithemia goeppertiana | Cymbella minuta | 0.807 | 0.000 | 0.82 | 24 |
| Cymbella affinis | 0.567 | 0.000 | |||
| TAXA IN STREAMS | |||||
| Achnanthidium minutissimum | pH | −0.364 | 0.000 | 0.17 | 73 |
| Cocconeis placentula | pH | 0.388 | 0.001 | 0.28 | 73 |
| Water temperature | −0.036 | 0.000 | |||
| Cymbella affinis | Water temperature | 0.008 | 0.006 | 0.16 | 73 |
| Nitrite | −1.575 | 0.016 | |||
| Cymbella cesatii | % Oxygen | 0.004 | 0.000 | 0.51 | 73 |
| Cymbella helvetica | Water temperature | 0.003 | 0.019 | 0.11 | 73 |
| Stream velocity | −0.060 | 0.018 | |||
| Cymbopleura amphicephala | Water temperature | 0.008 | 0.011 | 0.15 | 73 |
| DIN | −0.060 | 0.011 | |||
| Fragilaria dilatata | Ammonia | −0.386 | 0.002 | 0.23 | 73 |
| Melosira varians | Ammonia | 2.172 | 0.000 | 0.25 | 73 |
| Navicula cryptotenella | pH | 0.247 | 0.000 | 0.25 | 73 |
| Substrate type | 0.011 | 0.016 | |||
| Fish herbivory | −0.083 | 0.002 | |||
| POC | −0.219 | 0.015 | |||
| Achnanthidiium minutissimum | Melosira varians | −0.385 | 0.000 | 0.40 | 108 |
| Diatoma vulgaris | −0.446 | 0.000 | |||
| Gomphonema angustatum | −0.586 | 0.000 | |||
| Gyrosigma attenuatum | −0.586 | 0.005 | |||
| Cymbella cesatii | −0.448 | 0.002 | |||
| Ulnaria ulna | −1.121 | 0.005 | |||
| Cymbella helvetica | −1.401 | 0.007 | |||
| Cocconeis placentula | Achnanthidium minutissimum | −0.577 | 0.000 | 0.44 | 108 |
| Melosira varians | −0.514 | 0.000 | |||
| Cymbella cesatii | −0.562 | 0.001 | |||
| Diatoma vulgaris | −0.469 | 0.002 | |||
| Gyrosigma attenuatum | −0.699 | 0.005 | |||
| Fragilaria dilatata | −1.149 | 0.006 | |||
| Cymbella delicatula | −0.580 | 0.005 | |||
| Navicula cryptotenella | −0.531 | 0.008 | |||
| Cymbella affinis | Gomphonema angustatum | 0.211 | 0.002 | 0.13 | 108 |
| Fragilaria delicatissima | Ulnaria ulna | 0.627 | 0.000 | 0.32 | 108 |
| Fragilaria dilatata | Gomphonema angustatum | 0.145 | 0.000 | 0.14 | 108 |
| Cymbella helvetica | 0.334 | 0.013 | |||
| Gomphonema angustatum | Fragilaria dilatata | 0.801 | 0.000 | 0.2 | 108 |
| Ellerbeckia arenaria | 0.471 | 0.029 | |||
| Cymbella affinis | 0.343 | 0.004 | |||
| Ulnaria ulna | Fragilaria delicatissima | 0.545 | 0.000 | 0.37 | 108 |
| Cymbella affinis | 0.080 | 0.039 | |||
| Achnanthidium minutissimum | −0.037 | 0.018 | |||
| TAXA | Overall | Júcar Catchment | Tajo Catchment | Stagnant Water Bodies | Streams | Epilithic | Epiphytic |
|---|---|---|---|---|---|---|---|
| Number of Samples | 132 | 69 | 63 | 24 | 108 | 44 | 88 |
| Two Independent Matrices | |||||||
| Spatialized Environment | 47 | 49 | 37 | 33 | 48 | 45 | 51 |
| Pure Space | 3 | 2 | 0 | 0 | 3 | 0 | 3 |
| Pure Environment | 6 | 6 | 6 | 1 | 9 | 6 | 7 |
| Unexplained | 51 | 48 | 62 | 68 | 49 | 55 | 49 |
| Four Independent Matrices | |||||||
| Spatialized Physico-chemical | 42 | 44 | 31 | 34 | 40 | 39 | 43 |
| Spatialized Catchment | 39 | 45 | 31 | 34 | 39 | 35 | 40 |
| Spatialized Biological | 16 | 25 | 18 | 22 | 11 | 14 | 18 |
| Pure Biological | 6 | 9 | 7 | 8 | 4 | 14 | 6 |
| Unexplained | 52 | 46 | 62 | 58 | 54 | 47 | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Cobelas, M.; Rojo, C. Linking the Community and Metacommunity Perspectives: Biotic Relationships Are Key in Benthic Diatom Ecology. Water 2022, 14, 3805. https://doi.org/10.3390/w14233805
Álvarez-Cobelas M, Rojo C. Linking the Community and Metacommunity Perspectives: Biotic Relationships Are Key in Benthic Diatom Ecology. Water. 2022; 14(23):3805. https://doi.org/10.3390/w14233805
Chicago/Turabian StyleÁlvarez-Cobelas, Miguel, and Carmen Rojo. 2022. "Linking the Community and Metacommunity Perspectives: Biotic Relationships Are Key in Benthic Diatom Ecology" Water 14, no. 23: 3805. https://doi.org/10.3390/w14233805






