Photo-Fenton Catalyzed by Cu2O/Al2O3: Bisphenol (BPA) Mineralization Driven by UV and Visible Light
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cu Nanoparticle Preparation and Characterization
2.2. Catalyst Preparation and Characterization
2.3. BPA Degradation Procedure
2.4. Analytical Methods
3. Results
3.1. Cu Nanoparticles and Catalyst Characterization
3.2. BPA Degradation Results
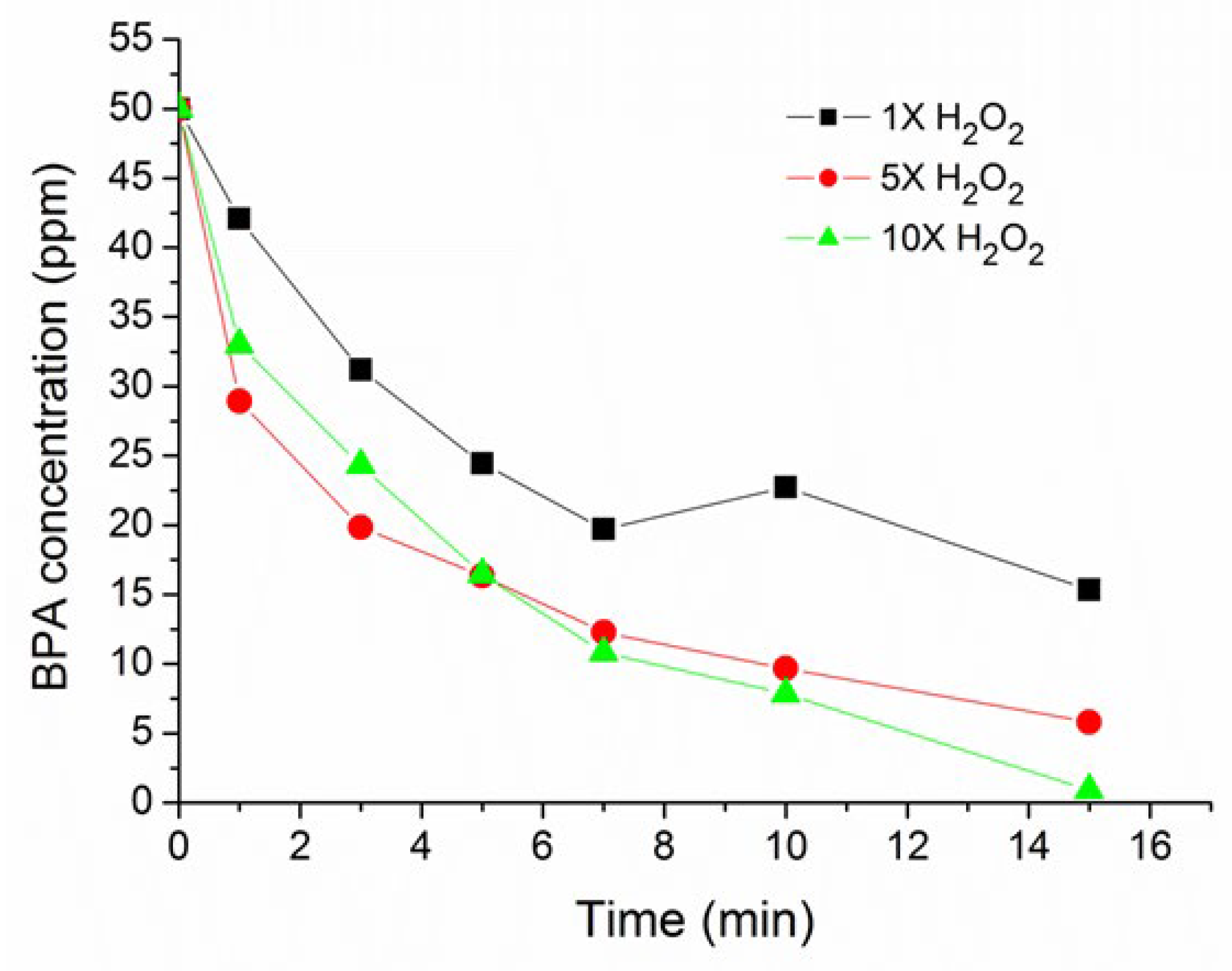
3.3. Effect of H2O2 Concentration
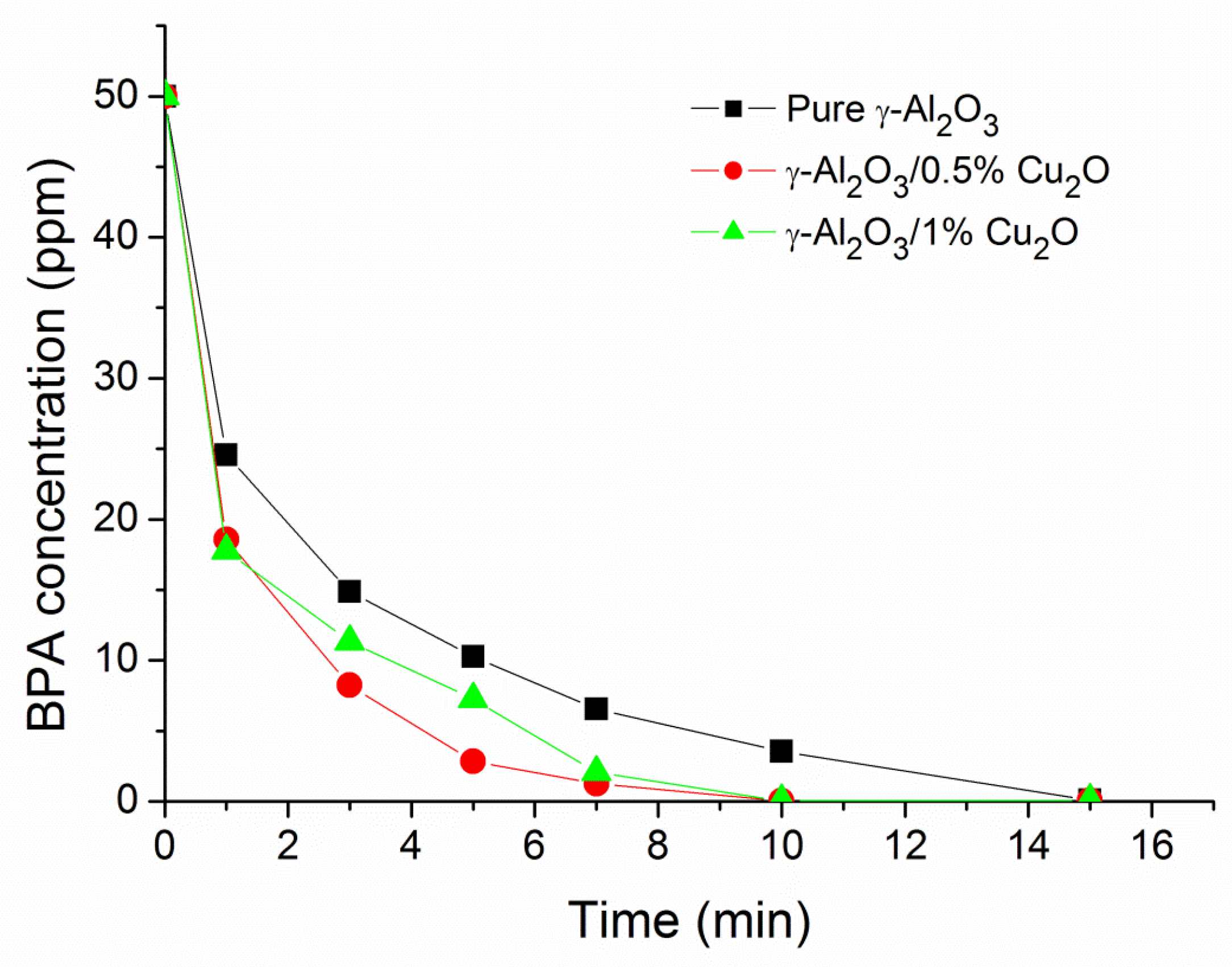
3.4. Effect of Cu2O Concentration on the Catalyst
3.5. Effect of Radiation Source Wavelength
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations List and Nomenclature
| Abbreviations List | |
| AOP | Advanced oxidation process |
| BPA | Bisphenol A |
| BPS | Bisphenol S |
| CE | Capillary electrophoresis |
| EDS | Energy dispersive X-ray spectroscopy |
| HRTEM | High resolution transmission electron microscopy |
| LAL | Laser ablation in liquids |
| LC/MS | Liquid chromatography coupled with mass spectroscopy |
| TEM | Transmission electron microscopy |
| TOC | Total organic carbon |
| SEM | Scanning electron microscopy |
| Nomenclature | |
| −rBPA,o | Initial BPA oxidation rate |
| k | Pseudo-first order kinetic constant |
| R2 | Determination coefficient |
| CBPA,o | Initial BPA concentration |
| pHo | Initial pH |
| Wcat | Catalyst concentration |
References
- Chen, M.-Y.; Ike, M.; Fujita, M. Acute toxicity, mutagenicity, and estrogenicity of bisphenol-A and other bisphenols. Environ. Toxicol. 2002, 17, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Grignard, E.; Lapenna, S.; Bremer, S. Weak estrogenic transcriptional activities of Bisphenol A and Bisphenol S. Toxicol. Vitr. 2012, 26, 727–731. [Google Scholar] [CrossRef]
- Zhu, S.; Khan, M.A.; Kameda, T.; Xu, H.; Wang, F.; Xia, M.; Yoshioka, T. New insights into the capture performance and mechanism of hazardous metals Cr3+ and Cd2+ onto an effective layered double hydroxide based material. J. Hazard. Mater. 2022, 426, 128062. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Xia, M.; Chu, Y.; Khan, M.A.; Lei, W.; Wang, F.; Muhmood, T.; Wang, A. Adsorption and Desorption of Pb(II) on l-Lysine Modified Montmorillonite and the simulation of Interlayer Structure. Appl. Clay Sci. 2018, 169, 40–47. [Google Scholar] [CrossRef]
- Munoz, M.; de Pedro, Z.M.; Casas, J.A.; Rodriguez, J.J. Preparation of magnetite-based catalysts and their application in heterogeneous Fenton oxidation—A review. Appl. Catal. B Environ. 2015, 176–177, 249–265. [Google Scholar] [CrossRef] [Green Version]
- Chand, R.; Ince, N.H.; Gogate, P.R.; Bremner, D.H. Phenol degradation using 20, 300 and 520 kHz ultrasonic reactors with hydrogen peroxide, ozone and zero valent metals. Sep. Purif. Technol. 2009, 67, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Torres-Blancas, T.; Roa-Morales, G.; Barrera-Díaz, C.; Ureña-Nuñez, F.; Cruz-Olivares, J.; Balderas-Hernandez, P.; Natividad, R. Ozonation of Indigo Carmine Enhanced by Fe/Pimenta dioica L. Merrill Particles. Int. J. Photoenergy 2015, 2015, 608412. [Google Scholar] [CrossRef] [Green Version]
- Ziylan, A.; Ince, N.H. Catalytic ozonation of ibuprofen with ultrasound and Fe-based catalysts. Catal. Today 2015, 240, 2–8. [Google Scholar] [CrossRef]
- Umar, K.; Haque, M.M.; Mir, N.A.; Muneer, M.; Farooqi, I.H. Titanium Dioxide-mediated Photocatalysed Mineralization of Two Selected Organic Pollutants in Aqueous Suspensions. J. Adv. Oxid. Technol. 2013, 16, 252–260. [Google Scholar] [CrossRef]
- Haque, M.M.; Khan, A.; Umar, K.; Mir, N.A.; Muneer, M.; Harada, T.; Matsumura, M. Synthesis, Characterization and Photocatalytic Activity of Visible Light Induced Ni-Doped TiO2. Energy Environ. Focus 2013, 2, 73–78. [Google Scholar] [CrossRef]
- Frankowski, R.; Płatkiewicz, J.; Stanisz, E.; Grześkowiak, T.; Zgoła-Grześkowiak, A. Biodegradation and photo-Fenton degradation of bisphenol A, bisphenol S and fluconazole in water. Environ. Pollut. 2021, 289, 117947. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Jia, X.; Li, J.; Li, M. Dynamics of microbial community in the bioreactor for bisphenol S removal. Sci. Total Environ. 2019, 662, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wang, L.; Liu, Y.; Huang, Z.; He, H.; Wang, X.; Jiang, J.; Gao, D.; Ma, J. Comparative study on ferrate oxidation of BPS and BPAF: Kinetics, reaction mechanism, and the improvement on their biodegradability. Water Res. 2018, 148, 115–125. [Google Scholar] [CrossRef]
- Chai, F.; Li, K.; Song, C.; Guo, X. Synthesis of magnetic porous Fe3O4/C/Cu2O composite as an excellent photo-Fenton catalyst under neutral condition. J. Colloid Interface Sci. 2016, 475, 119–125. [Google Scholar] [CrossRef]
- Davarnejad, R.; Azizi, J. Alcoholic wastewater treatment using electro-Fenton technique modified by Fe2O3 nanoparticles. J. Environ. Chem. Eng. 2016, 4, 2342–2349. [Google Scholar] [CrossRef]
- Mahy, J.G.; Tasseroul, L.; Zubiaur, A.; Geens, J.; Brisbois, M.; Herlitschke, M.; Hermann, R.; Heinrichs, B.; Lambert, S.D. Highly dispersed iron xerogel catalysts for p-nitrophenol degradation by photo-Fenton effects. Microporous Mesoporous Mater. 2014, 197, 164–173. [Google Scholar] [CrossRef]
- Dükkancı, M. Sono-photo-Fenton oxidation of bisphenol-A over a LaFeO3 perovskite catalyst. Ultrason. Sonochemistry 2018, 40, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Noor, S.H.M.; Othman, M.H.D.; Khongnakorn, W.; Sinsamphanh, O.; Abdullah, H.; Puteh, M.H.; Kurniawan, T.A.; Zakria, H.S.; El-Badawy, T.; Ismail, A.F.; et al. Bisphenol A Removal Using Visible Light Driven Cu2O/PVDF Photocatalytic Dual Layer Hollow Fiber Membrane. Membranes 2022, 12, 208. [Google Scholar] [CrossRef]
- Chen, D.D.; Yi, X.H.; Ling, L.; Wang, C.C.; Wang, P. Photocatalytic Cr(VI) sequestration and photo-Fenton bisphenol A decomposition over white light responsive PANI/MIL-88A(Fe). Appl. Organomet. Chem. 2020, 34, e5795. [Google Scholar] [CrossRef]
- Fu, H.; Song, X.X.; Wu, L.; Zhao, C.; Wang, P.; Wang, C.C. Room-temperature preparation of MIL-88A as a heterogeneous photo-Fenton catalyst for degradation of rhodamine B and bisphenol A under visible light. Mater. Res. Bull. 2020, 125, 110806. [Google Scholar] [CrossRef]
- Huang, W.; Luo, M.; Wei, C.; Wang, Y.; Hanna, K.; Mailhot, G. Enhanced heterogeneous photo-Fenton process modified by magnetite and EDDS: BPA degradation. Environ. Sci. Pollut. Res. 2017, 24, 10421–10429. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Moya, M.; Kaisto, T.; Navarro, M.; del Valle, L.J. Study of the degradation performance (TOC, BOD, and toxicity) of bisphenol A by the photo-Fenton process. Environ. Sci. Pollut. Res. 2016, 24, 6241–6251. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.G.R.; Costa, E.P.; Starling, M.C.V.M.; Azevedo, T.d.S.; Bottrel, S.E.C.; Pereira, R.O.; Sanson, A.L.; Afonso, R.J.C.F.; Amorim, C.C. LED irradiated photo-Fenton for the removal of estrogenic activity and endocrine disruptors from wastewater treatment plant effluent. Environ. Sci. Pollut. Res. 2021, 28, 24067–24078. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Zhou, C.; Ye, X.; Lian, Z.; Zhang, N.; Yang, J.; Chen, W.; Li, H. Solid-Phase Microwave Reduction of WO3 by GO for Enhanced Synergistic Photo-Fenton Catalytic Degradation of Bisphenol A. ACS Appl. Mater Inter. 2020, 12, 32604–32614. [Google Scholar] [CrossRef] [PubMed]
- Khandarkhaeva, M.; Batoeva, A.; Sizykh, M.; Aseev, D.; Garkusheva, N. Photo-Fenton-like degradation of bisphenol A by persulfate and solar irradiation. J. Environ. Manag. 2019, 249, 109348. [Google Scholar] [CrossRef]
- Liu, Y.; Mao, Y.; Tang, X.; Xu, Y.; Li, C.; Li, F. Synthesis of Ag/AgCl/Fe-S plasmonic catalyst for bisphenol A degradation in heterogeneous photo-Fenton system under visible light irradiation. Chin. J. Catal. 2017, 38, 1726–1735. [Google Scholar] [CrossRef]
- Bremner, D.H.; Burgess, A.E.; Houllemare, D.; Namkung, K.-C. Phenol degradation using hydroxyl radicals generated from zero-valent iron and hydrogen peroxide. Appl. Catal. B: Environ. 2006, 63, 15–19. [Google Scholar] [CrossRef]
- Cheng, R.; Cheng, C.; Liu, G.-H.; Zheng, X.; Li, G.; Li, J. Removing pentachlorophenol from water using a nanoscale zero-valent iron/H2O2 system. Chemosphere 2015, 141, 138–143. [Google Scholar] [CrossRef]
- Machado, S.; Stawiński, W.; Slonina, P.; Pinto, A.R.; Grosso, J.P.; Nouws, H.P.A.; Albergariaa, J.T.; Delerue-Matosa, C. Application of green zero-valent iron nanoparticles to the remediation of soils contaminated with ibuprofen. Sci. Total Environ. 2013, 461–462, 323–329. [Google Scholar] [CrossRef] [Green Version]
- de la Plata, G.B.O.; Alfano, O.M.; Cassano, A.E. 2-Chlorophenol degradation via photo Fenton reaction employing zero valent iron nanoparticles. J. Photochem. Photobiol. A: Chem. 2012, 233, 53–59. [Google Scholar] [CrossRef]
- Pastrana-Martínez, L.M.; Pereira, N.; Lima, R.; Faria, J.L.; Gomes, H.T.; Silva, A.M.T. Degradation of diphenhydramine by photo-Fenton using magnetically recoverable iron oxide nanoparticles as catalyst. Chem. Eng. J. 2015, 261, 45–52. [Google Scholar] [CrossRef]
- Shahwan, T.; Abu Sirriah, S.; Nairat, M.; Boyacı, E.; Eroğlu, A.; Scott, T.; Hallam, K. Green synthesis of iron nanoparticles and their application as a Fenton-like catalyst for the degradation of aqueous cationic and anionic dyes. Chem. Eng. J. 2011, 172, 258–266. [Google Scholar] [CrossRef]
- Shin, S.; Yoon, H.; Jang, J. Polymer-encapsulated iron oxide nanoparticles as highly efficient Fenton catalysts. Catal. Commun. 2008, 10, 178–182. [Google Scholar] [CrossRef]
- Morales-Leal, F.J.; De la Rosa, J.R.; Lucio-Ortiz, C.J.; Martínez, D.B.; Rio, D.A.D.H.D.; Garza-Navarro, M.A.; Martínez-Vargas, D.X.; Garcia, C.D. Comparison between the catalytic and photocatalytic activities of Cu/Al2O3 and TiO2 in the liquid–phase oxidation of methanol–ethanol mixtures: Development of a kinetic model for the preparation of catalyst. Appl. Catal. A Gen. 2018, 562, 184–197. [Google Scholar] [CrossRef]
- Pradhan, A.C.; Parida, K.M.; Nanda, B. Enhanced photocatalytic and adsorptive degradation of organic dyes by mesoporous Cu/Al2O3–MCM-41: Intra-particle mesoporosity, electron transfer and OH radical generation under visible light. Dalton. T. 2011, 40, 7348–7356. [Google Scholar] [CrossRef]
- Javaid, R.; Qazi, U.Y. Catalytic Oxidation Process for the Degradation of Synthetic Dyes: An Overview. Int. J. Environ. Res. Public Health 2019, 16, 2066. [Google Scholar] [CrossRef] [Green Version]
- Kanitz, A.; Kalus, M.-R.; Gurevich, E.L.; Ostendorf, A.; Barcikowski, S.; Amans, D. Review on experimental and theoretical investigations of the early stage, femtoseconds to microseconds processes during laser ablation in liquid-phase for the synthesis of colloidal nanoparticles. Plasma Sources Sci. Technol. 2019, 28, 103001. [Google Scholar] [CrossRef]
- Rehbock, C.; Merk, V.; Gamrad, L.; Streubel, R.; Barcikowski, S. Size control of laser-fabricated surfactant-free gold nanoparticles with highly diluted electrolytes and their subsequent bioconjugation. Phys. Chem. Chem. Phys. 2012, 15, 3057–3067. [Google Scholar] [CrossRef] [Green Version]
- Olea-Mejía, O.; Fernández-Mondragón, M.; Rodríguez-de la Concha, G.; Camacho-López, M. SERS-active Ag, Au and Ag–Au alloy nanoparticles obtained by laser ablation in liquids for sensing methylene blue. Appl. Surf. Sci. 2015, 348, 66–70. [Google Scholar] [CrossRef]
- Eisenberg, G. Colorimetric Determination of Hydrogen Peroxide. Ind. Eng. Chem. Anal. Ed. 1943, 15, 327–328. [Google Scholar] [CrossRef]
- Amado-Piña, D.; Roa-Morales, G.; Molina-Mendieta, M.; Balderas-Hernández, P.; Romero, R.; Díaz, C.E.B.; Natividad, R. E-peroxone process of a chlorinated compound: Oxidant species, degradation pathway and phytotoxicity. J. Environ. Chem. Eng. 2022, 10, 108148. [Google Scholar] [CrossRef]
- Alvarado-Rolon, O.; Natividad, R.; Ramírez-García, J.; Orozco-Velazco, J.; Hernandez-Servin, J.; Ramírez-Serrano, A. Kinetic modelling of paracetamol degradation by photocatalysis: Incorporating the competition for photons by the organic molecule and the photocatalyst. J. Photochem. Photobiol. A Chem. 2021, 412, 113252. [Google Scholar] [CrossRef]
- Brillas, E.; Sirés, I.; Oturan, M.A. Electro-Fenton Process and Related Electrochemical Technologies Based on Fenton’s Reaction Chemistry. Chem. Rev. 2009, 109, 6570–6631. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, L.; Romero, R.; Mendoza, A.; Brewer, S.; Donkor, K.; Gómez-Espinosa, R.M.; Natividad, R. Paracetamol mineralization by Photo Fenton process catalyzed by a Cu/Fe-PILC under circumneutral pH conditions. J. Photochem. Photobiol. A Chem. 2019, 373, 162–170. [Google Scholar] [CrossRef]
- Hurtado, L.; Avilés, O.; Brewer, S.; Donkor, K.K.; Romero, R.; Gómez-Espinosa, R.M.; Alvarado, O.; Natividad, R. Al/Cu-PILC as a Photo-Fenton Catalyst: Paracetamol Mineralization. ACS Omega 2022, 7, 23821–23832. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Horikoshi, S.; Kawabe, H.; Sugie, Y.; Zhao, J.; Hidaka, H. Photodegradation mechanism for bisphenol A at the TiO2/H2O interfaces. Chemosphere 2003, 52, 851–859. [Google Scholar] [CrossRef]
- Bechambi, O.; Jlaiel, L.; Najjar, W.; Sayadi, S. Photocatalytic degradation of bisphenol A in the presence of Ce–ZnO: Evolution of kinetics, toxicity and photodegradation mechanism. Mat. Chem. Phys. 2016, 173, 95–105. [Google Scholar] [CrossRef]
- Young, T.; Geng, M.; Thagard, S.M.; Lin, L. Oxidative Degradation of Bisphenol A: A Comparison Between Fenton Reagent, UV, UV/H2O2 and Ultrasound. J. Adv. Oxid. Technol. 2013, 16, 89–101. [Google Scholar] [CrossRef]
- Mendoza, A.; Romero, R.; Gutiérrez-Cedillo, G.P.; López-Tellez, G.; Lorenzo-González, O.; Gómez-Espinosa, R.M.; Natividad, R. Selective production of dihydroxyacetone and glyceraldehyde by photo-assisted oxidation of glycerol. Catal. Today 2019, 358, 149–154. [Google Scholar] [CrossRef]
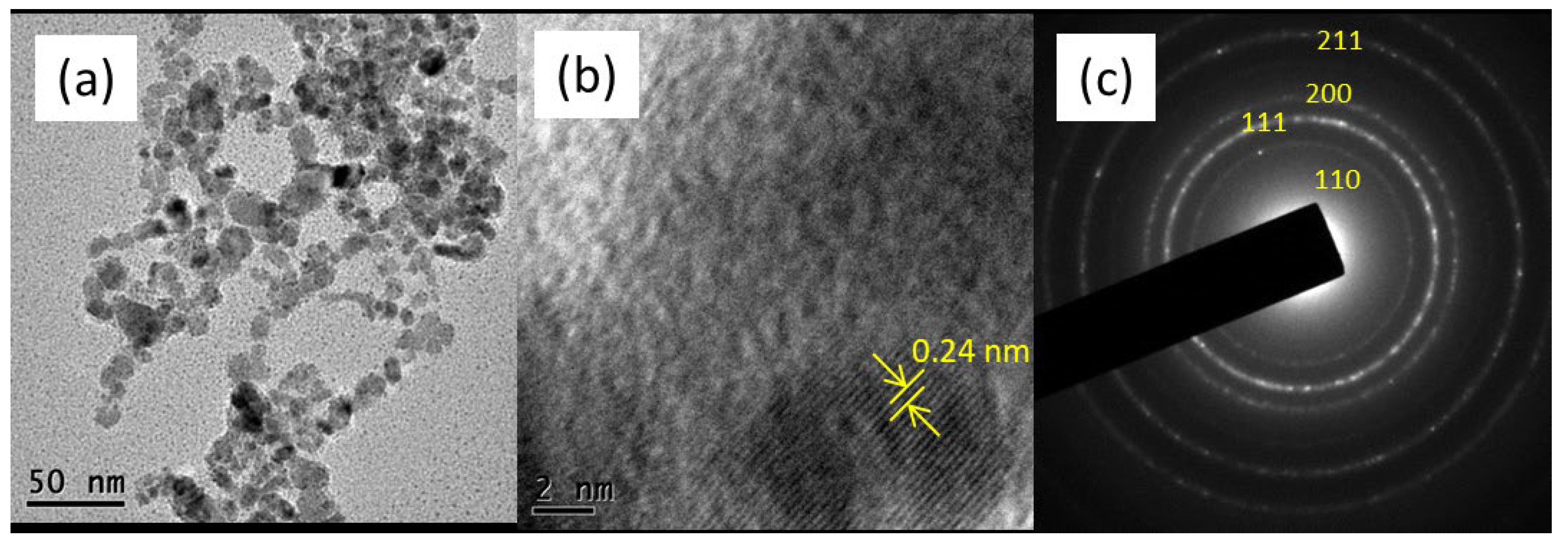

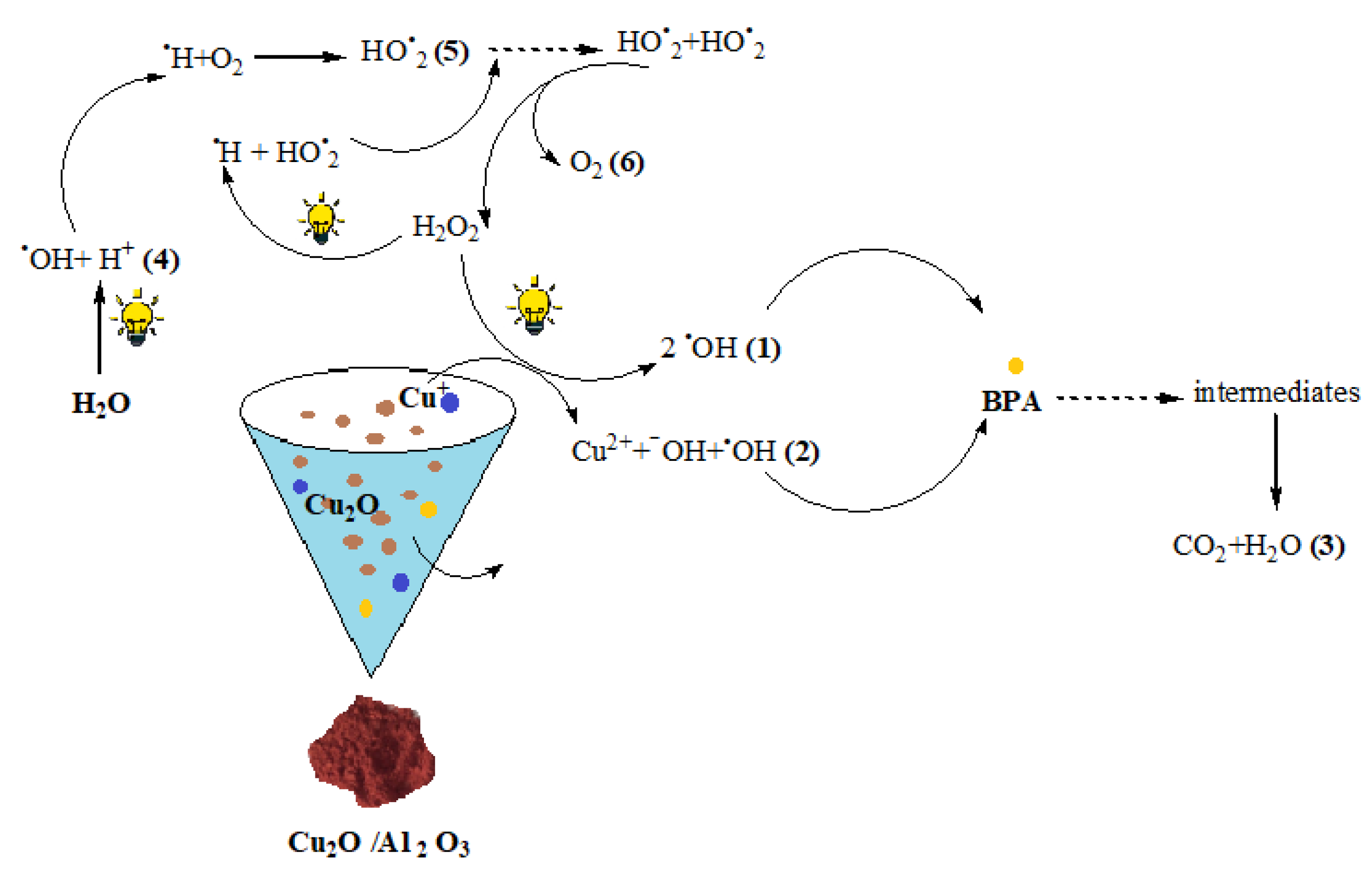

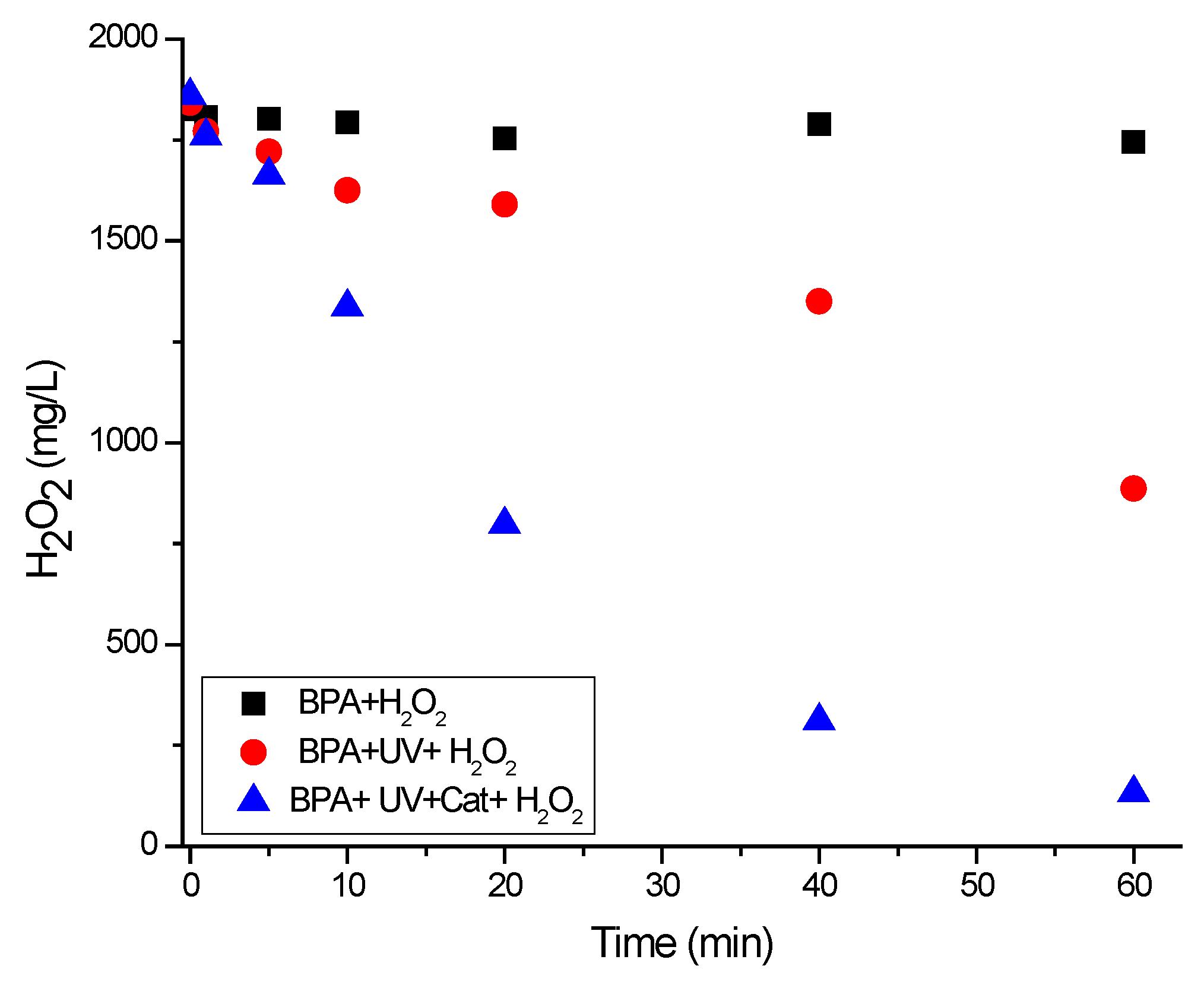
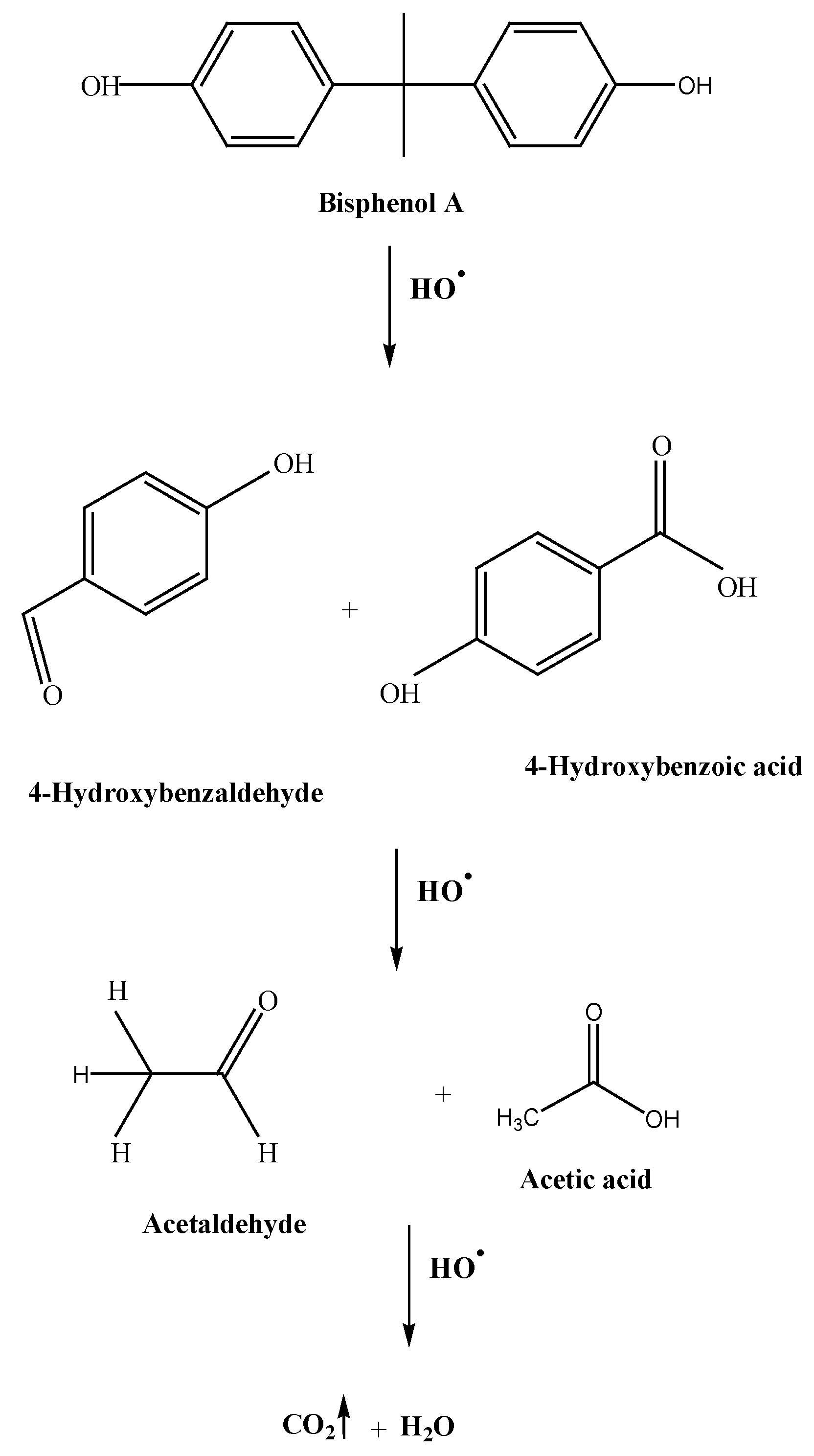
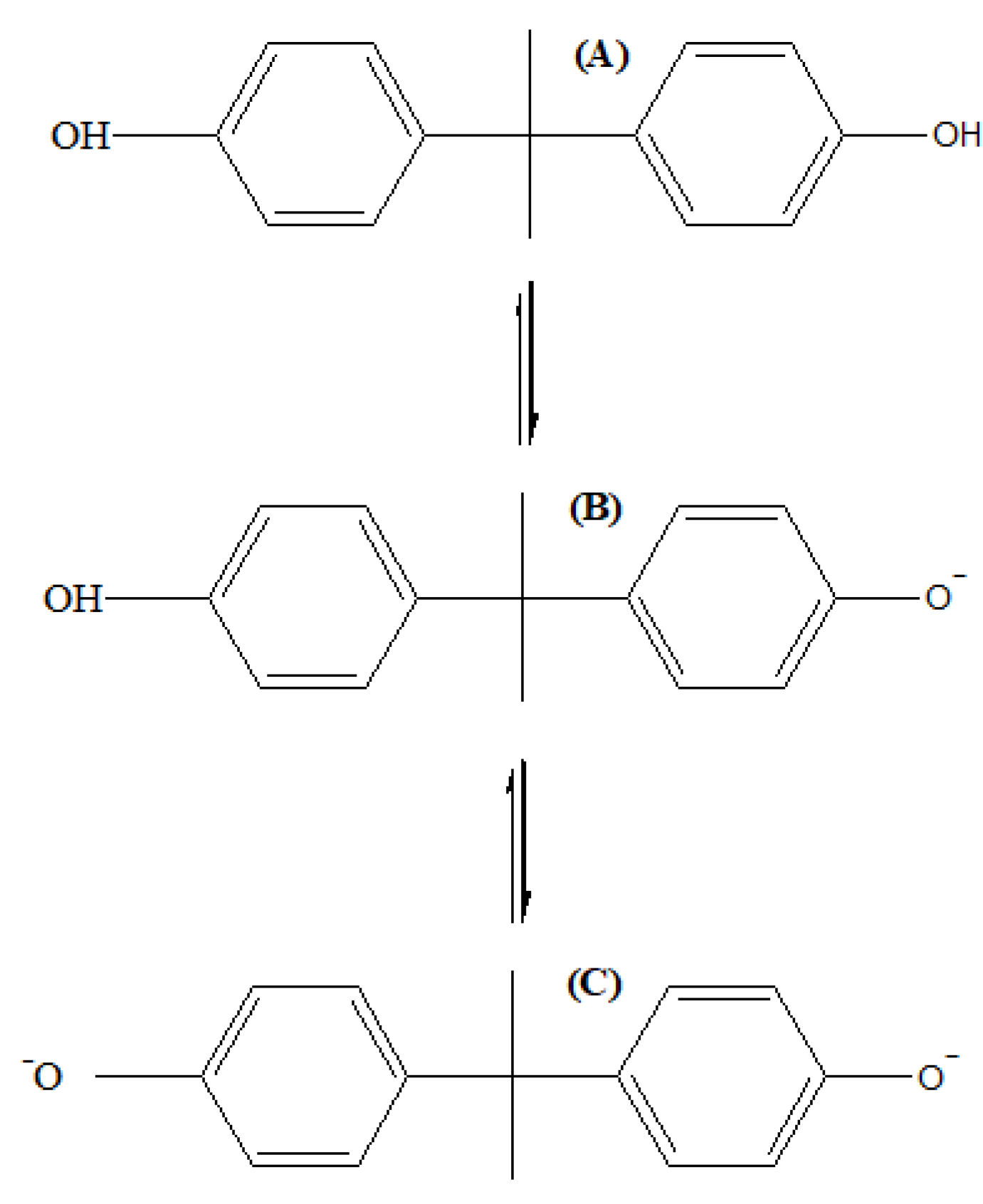


| BPA Mineralization Percentage (%) | ||||
|---|---|---|---|---|
| Time (min) | Photolysis (UV Only) | UV + H2O2 | UV + H2O2 + 1% Cu2O/α-Al2O3 | UV + H2O2 + 1% Cu2O/γ-Al2O3 |
| 20 | 2.4 | 17.5 | 19.6 | 24.4 |
| 40 | 4.7 | 50.5 | 70.1 | 79.6 |
| 60 | 7.1 | 80.9 | 86.5 | 90.7 |
| Kinetic Parameters and Determination Coefficient | ||||
| −rBPA,o (molBPA/L·min) | 1.09 × 10−5 | 1.23 × 10−5 | 1.09 × 10−4 | 1.4 × 10−4 |
| k (min−1) | 0.074 | 0.1982 | 0.3432 | 0.4511 |
| R2 | 0.991 | 0.9969 | 0.9874 | 0.9685 |
| Time (min) | 1X H2O2 | 5X H2O2 | 10X H2O2 |
|---|---|---|---|
| 20 | 2.3 | 16.8 | 17.5 |
| 40 | 15.5 | 25.1 | 50.5 |
| 60 | 20.6 | 50.0 | 80.9 |
| Time (min) | Pure γ-Al2O3 | 0.5% Cu2O/γ-Al2O3 | 1% Cu2O/γ-Al2O3 |
|---|---|---|---|
| 20 | 19.8 | 24.0 | 24.5 |
| 40 | 46.6 | 60.2 | 79.6 |
| 60 | 79.6 | 88.3 | 90.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olea-Mejia, O.; Brewer, S.; Donkor, K.; Amado-Piña, D.; Natividad, R. Photo-Fenton Catalyzed by Cu2O/Al2O3: Bisphenol (BPA) Mineralization Driven by UV and Visible Light. Water 2022, 14, 3626. https://doi.org/10.3390/w14223626
Olea-Mejia O, Brewer S, Donkor K, Amado-Piña D, Natividad R. Photo-Fenton Catalyzed by Cu2O/Al2O3: Bisphenol (BPA) Mineralization Driven by UV and Visible Light. Water. 2022; 14(22):3626. https://doi.org/10.3390/w14223626
Chicago/Turabian StyleOlea-Mejia, Oscar, Sharon Brewer, Kingsley Donkor, Deysi Amado-Piña, and Reyna Natividad. 2022. "Photo-Fenton Catalyzed by Cu2O/Al2O3: Bisphenol (BPA) Mineralization Driven by UV and Visible Light" Water 14, no. 22: 3626. https://doi.org/10.3390/w14223626



_Dionysiou.jpg)


