Fe3O4 Nanoparticles Loaded Bentonite/Sawdust Interface for the Removal of Methylene Blue: Insights into Adsorption Performance and Mechanism via Experiments and Theoretical Calculations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
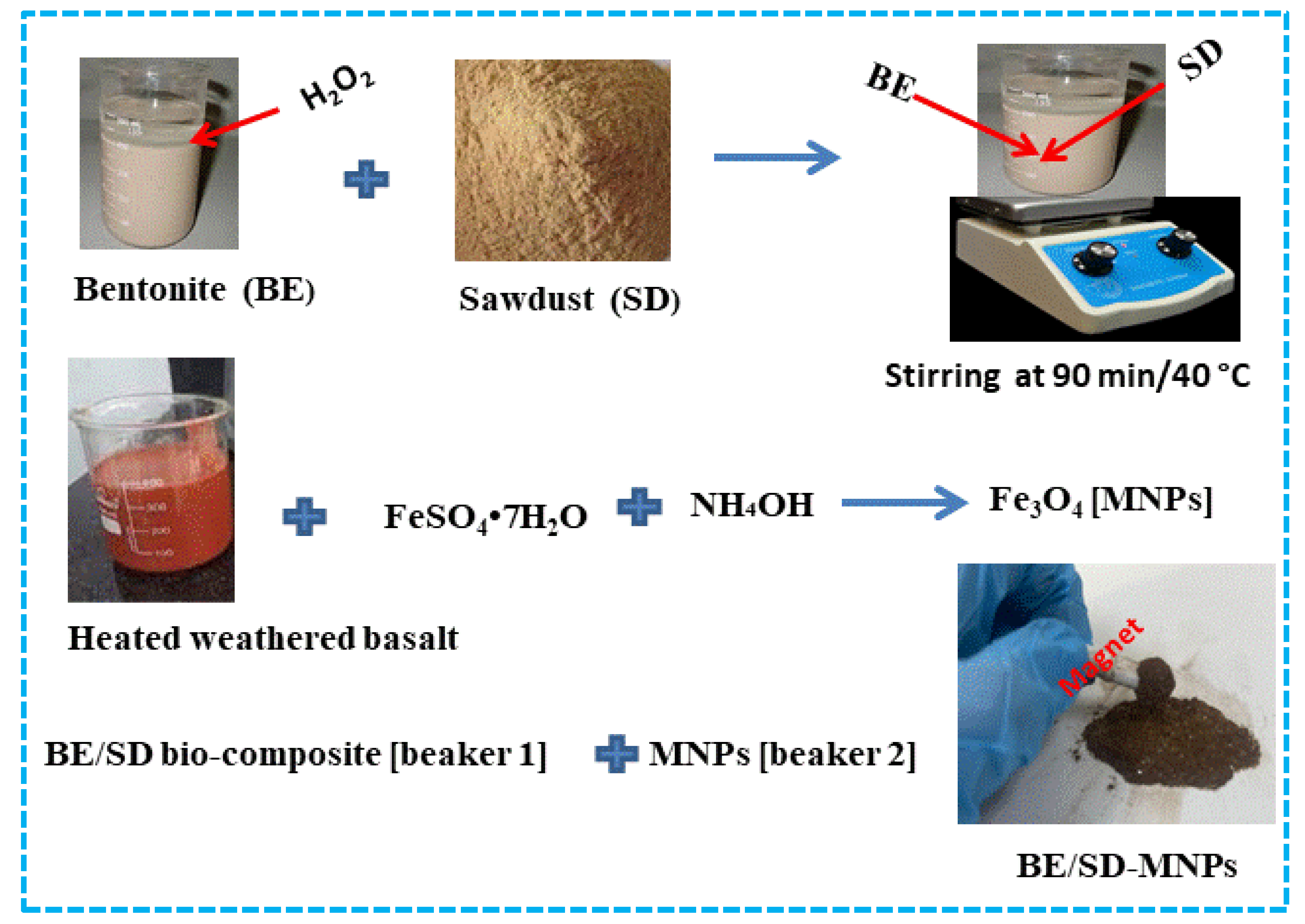
2.2. Preparation of BE/SD–MNPs Composite
2.3. Characterization of BE/SD–MNPs
2.4. Kinetics Studies of MB Adsorption onto BE/SD–MNPs
2.5. Equilibrium Studies of MB Adsorption onto BE/SD–MNPs
2.6. Classical Modeling of MB Adsorption onto BE/SD–MNPs
2.7. Advanced Modeling of MB Adsorption onto BE/SD–MNPs
2.7.1. Monolayer Model (M1)
2.7.2. Double Layer Model (M2)
2.7.3. Multilayer Model (M3)
2.8. Regeneration of BE/SD–MNPs Adsorbent
3. Results and Discussion
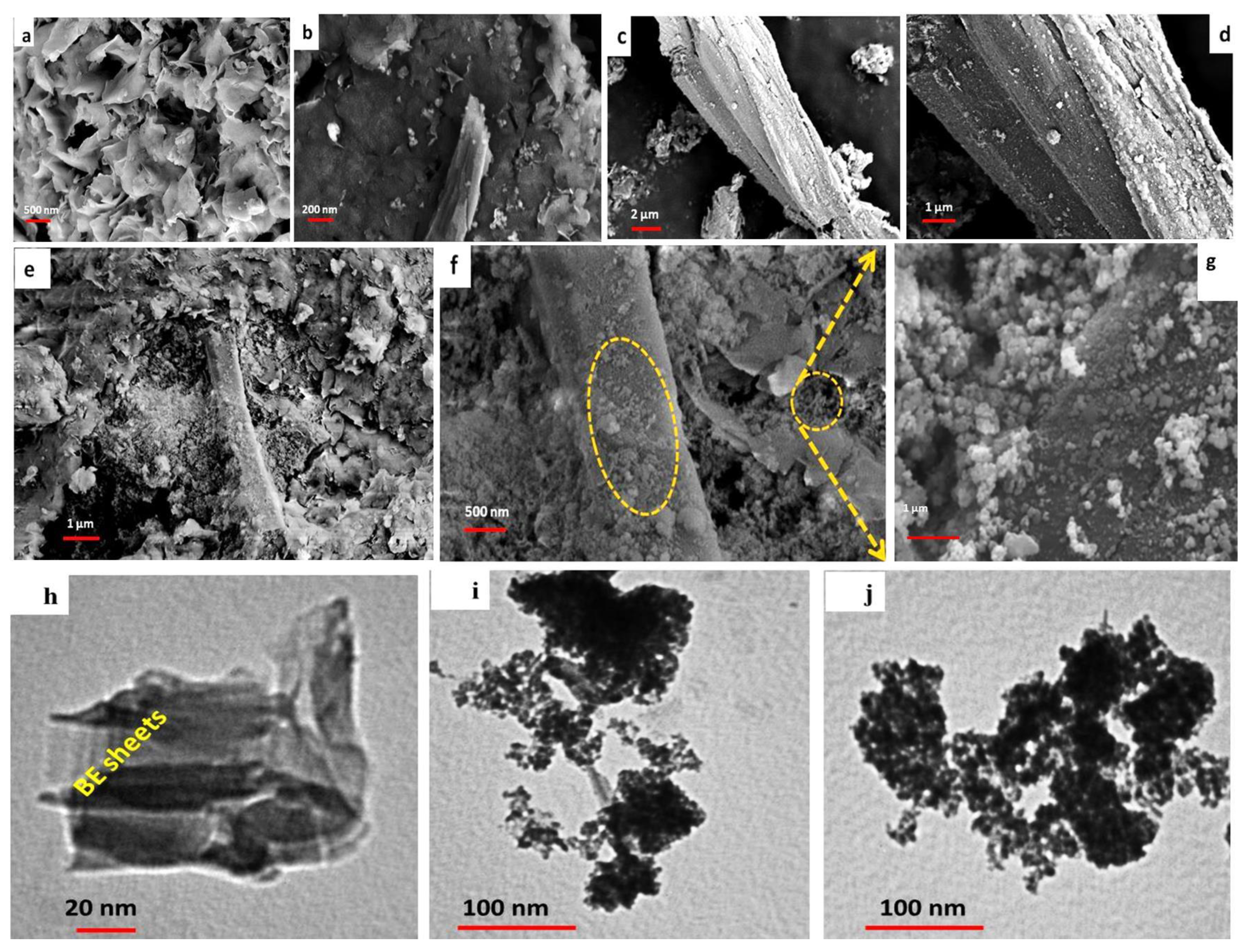
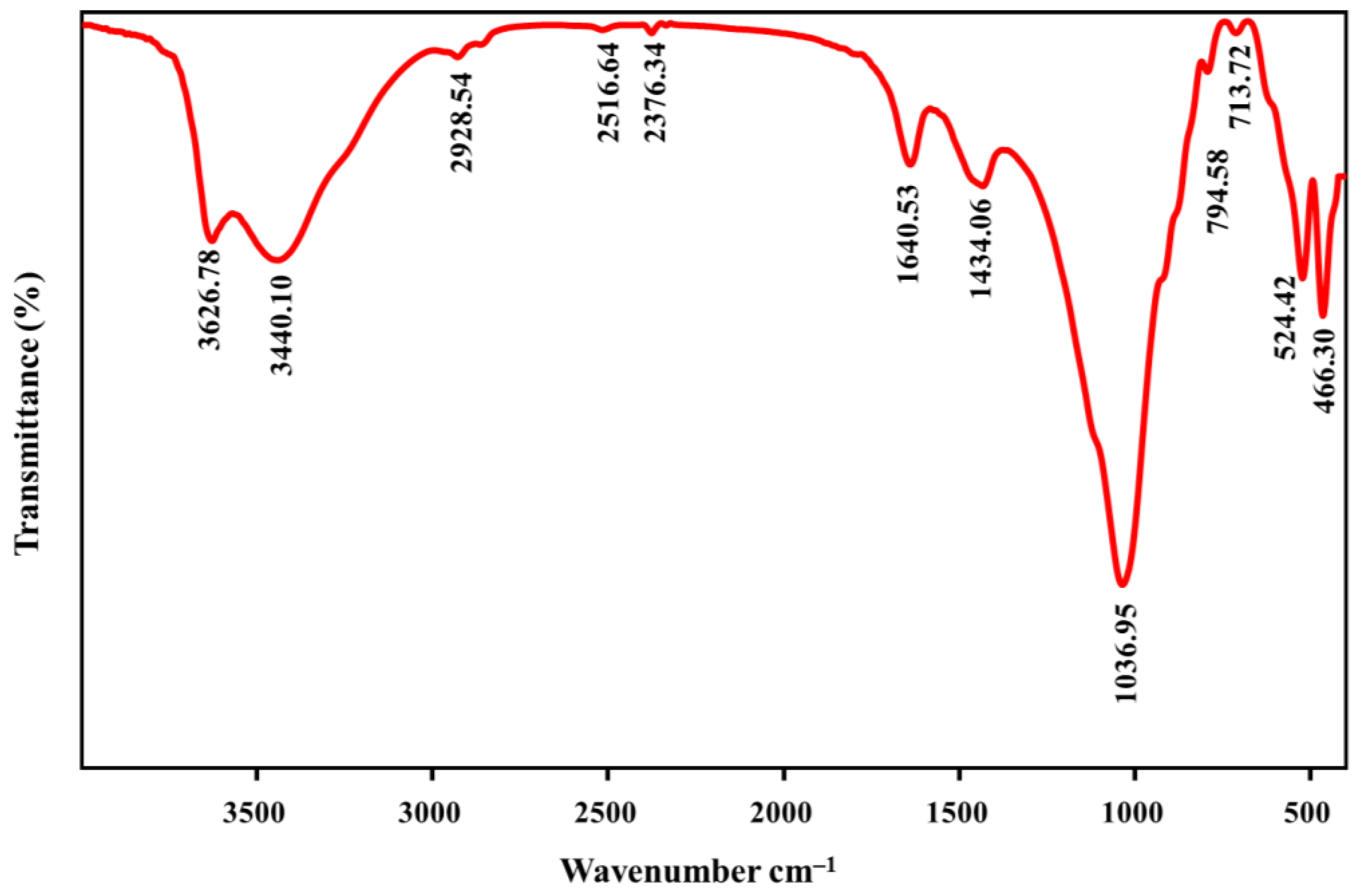
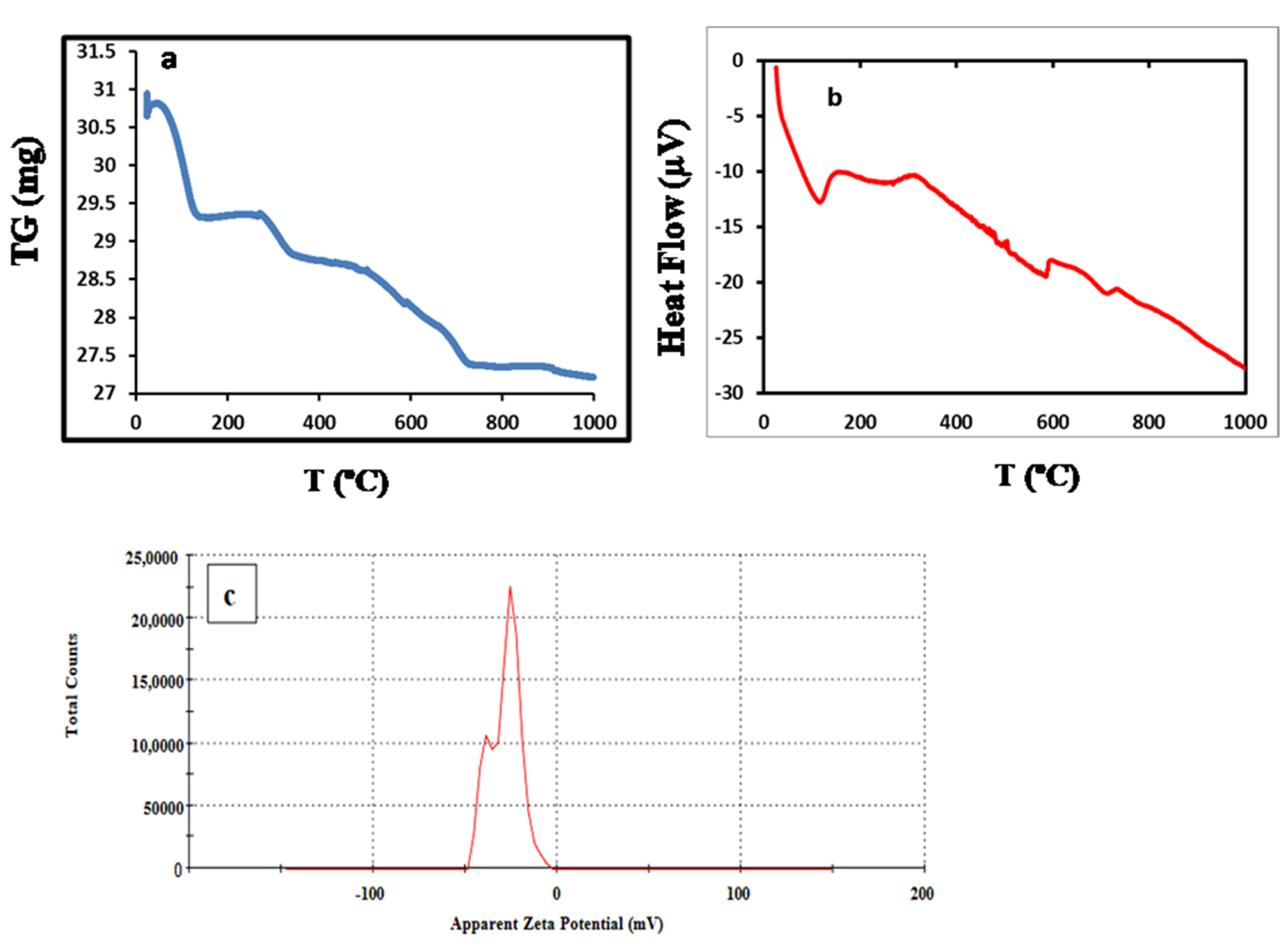
3.1. Characterization of BE/SD–MNPs Composite
3.2. Contact Time Effect on MB Adsorption on BE/SD–MNPs
3.3. MB Adsorption Kinetics
3.4. Diffusion Mechanism
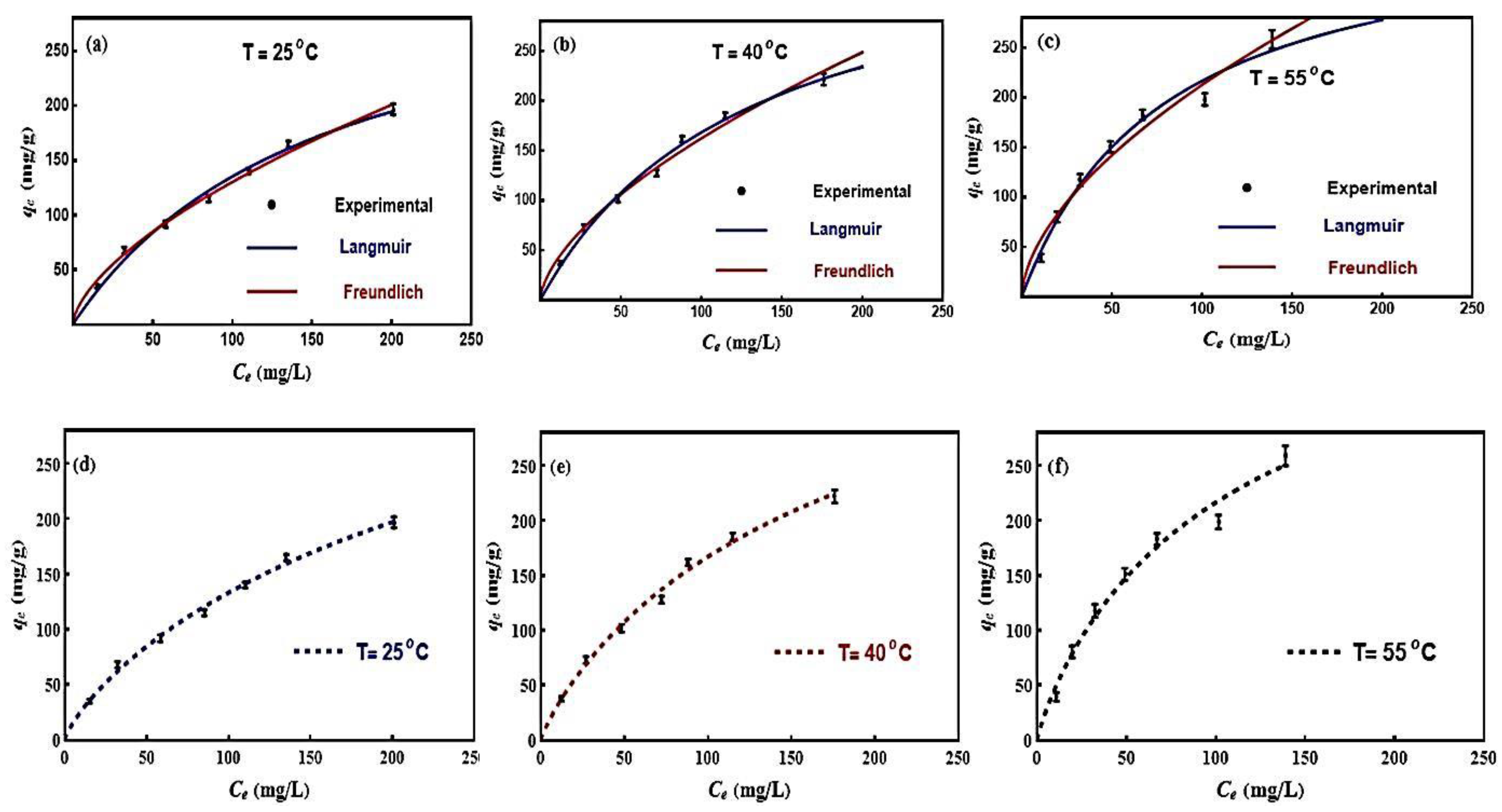
3.5. Classical and Advanced Modeling of MB Adsorption on BE/SD–MNPs
3.5.1. Traditional Isotherm Models
3.5.2. Statistical Physics Models
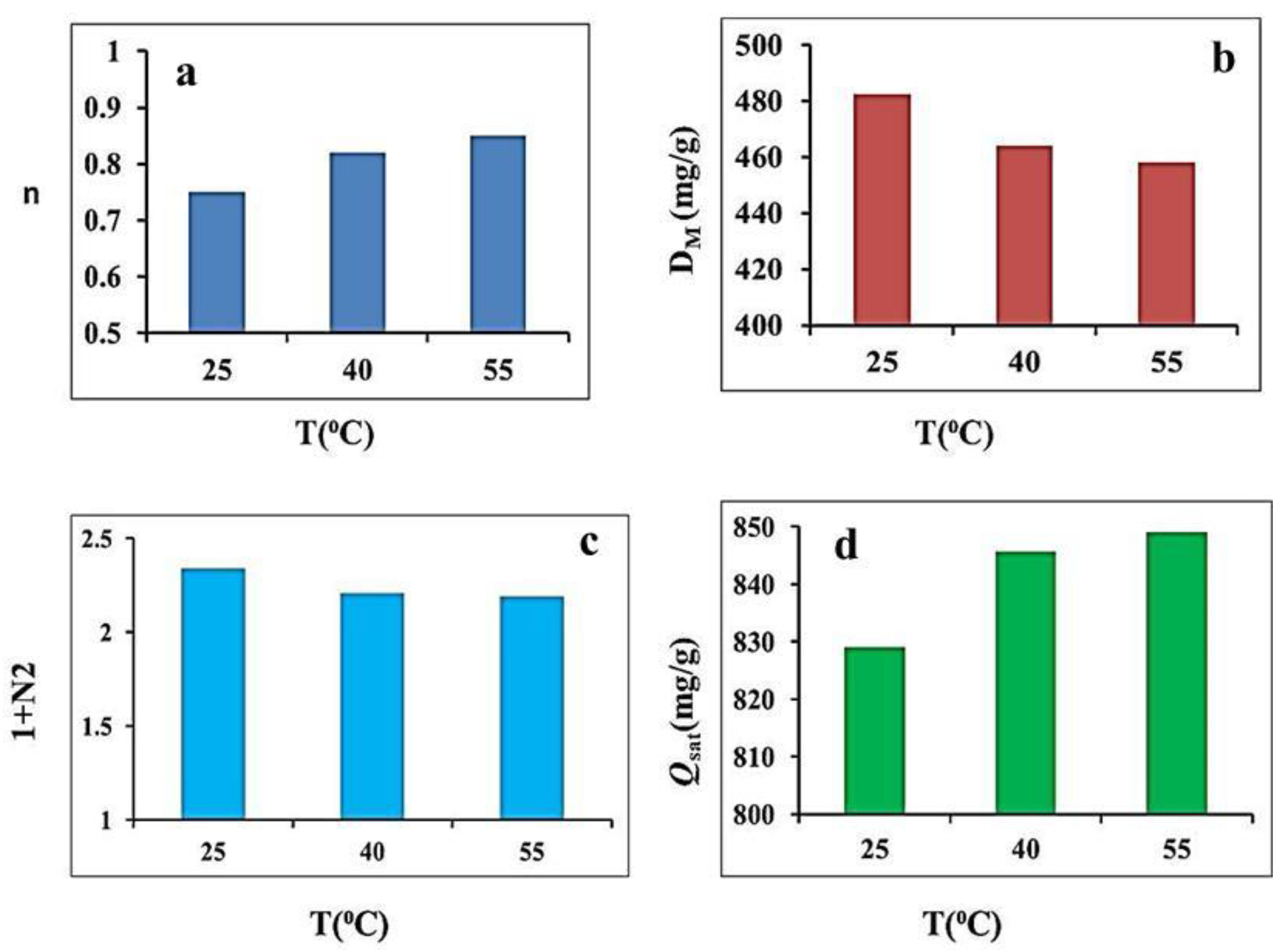
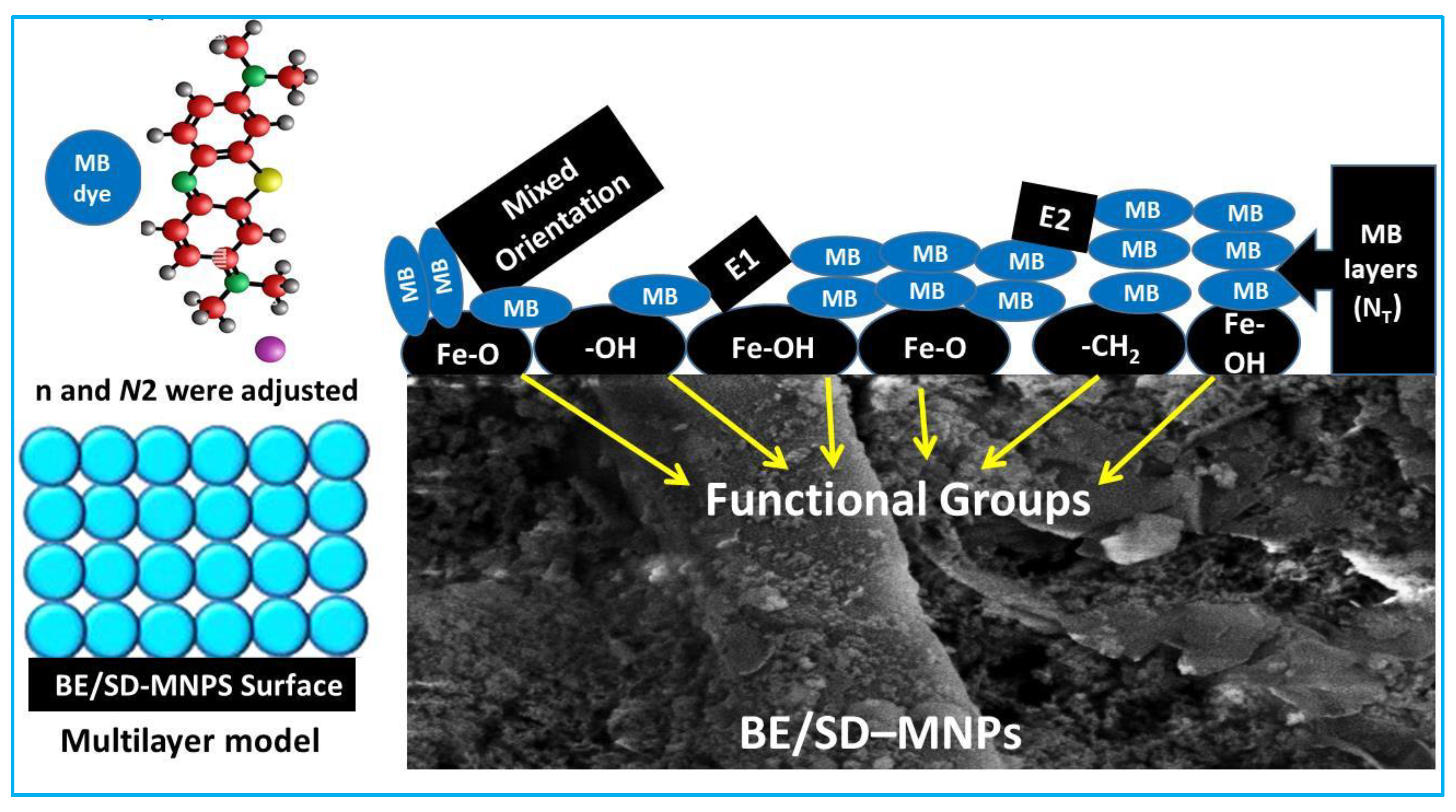
3.6. Steric Parameters Interpretation
3.6.1. The n Parameter
3.6.2. The DM Parameter
3.6.3. Total Number of Formed Layers (Nt = 1 + N2)
3.6.4. Adsorbed MB Quantity at Saturation (Qsat Parameter)
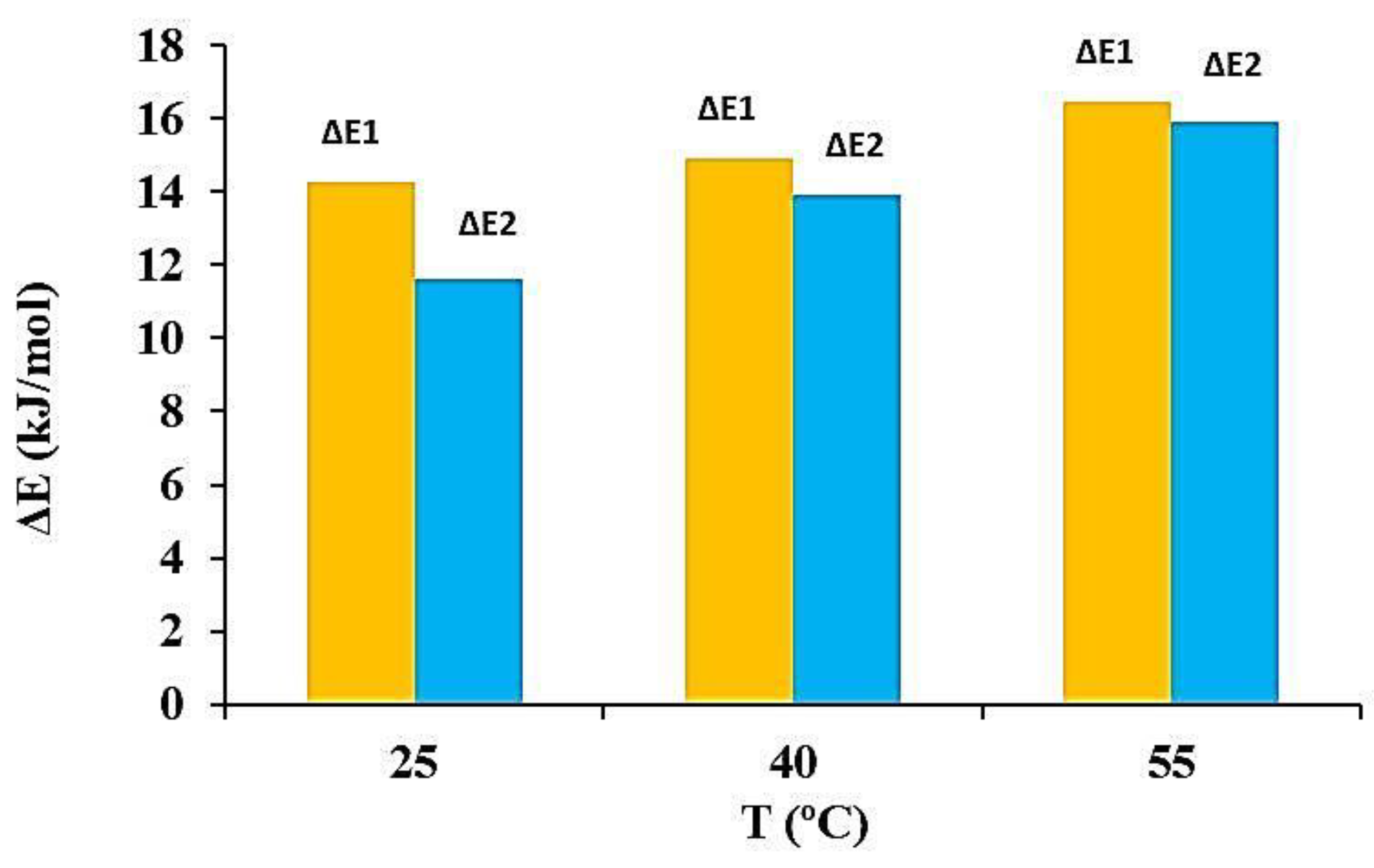
3.7. Interpretation of MB Adsorption on BE/SD–MNPs via Adsorption Energy (ΔE)
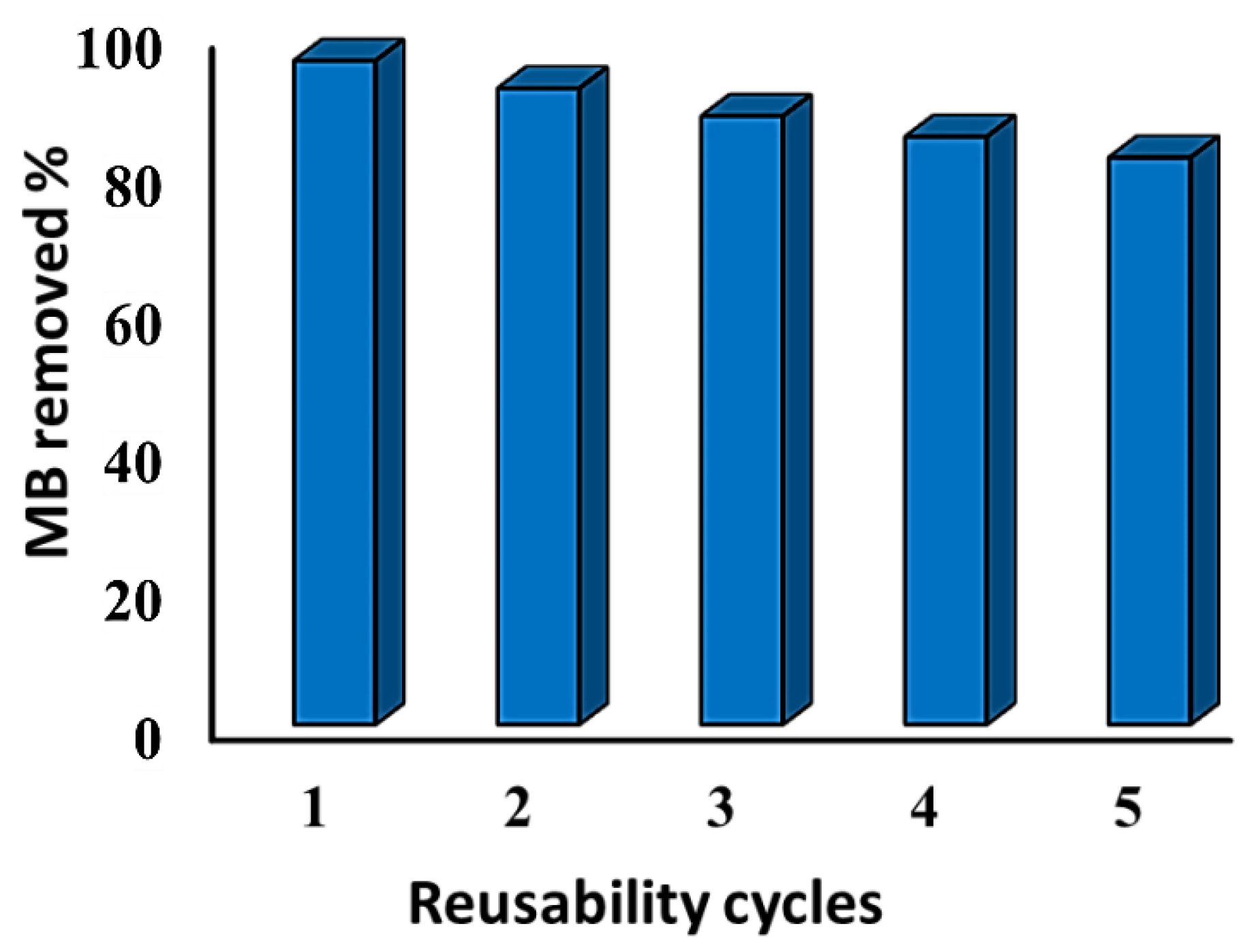
3.8. Regeneration of Rt/BC Adsorbent
3.9. Economic Profit
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pirbazari, A.E.; Saberikhah, E.; Gorabi, N.G.A. Fe3O4 nanoparticles loaded onto wheat straw: An efficient adsorbent for Basic Blue 9 adsorption from aqueous solution. Desalination Water Treat. 2014, 57, 4110–4121. [Google Scholar] [CrossRef]
- Li, Z.; Sellaoui, L.; Dotto, G.L.; Lamine, A.B.; Petriciolet, A.B.; Hanafy, H.; Belmabrouk, H.; Matias, S.N.; Erto, A. Interpretation of the adsorption mechanism of Reactive Black 5 and Ponceau 4R dyes on chitosan/polyamide nanofibers via advanced statistical physics model. J. Mol. Liq. 2019, 285, 165–170. [Google Scholar] [CrossRef]
- Sellaoui, L.; Mendoza-Castillo, D.I.; Ávila, H.E.R.; Ávila-Camacho, B.A.; Ghalla, H.; Bonilla-Petriciolet, A.; Lamine, A.B. Understanding the adsorption of Pb2+, Hg2+ and Zn2+ from aqueous solution on a lignocellulosic biomass char using advanced statistical physics models and density functional theory simulations. Chem. Eng. J. 2019, 365, 305–316. [Google Scholar] [CrossRef]
- Ivanets, A.; Prozorovich, V.; Roshchina, M.; Sychova, O.; Srivastava, V.; Sillanpa, M. Methylene blue adsorption on magnesium ferrite: Optimization study, kinetics and reusability. Mater. Today Commun. 2022, 31, 103594. [Google Scholar] [CrossRef]
- Sharib, A.A.A.A.; Bonilla-Petriciolet, A.; Selim, A.Q.; Mohamed, E.A.; Seliem, M.K. Utilizing modified weathered basalt as a novel approach in the preparation of Fe3O4 nanoparticles: Experimental and theoretical studies for crystal violet adsorption. J. Environ. Chem. Eng. 2021, 9, 106220. [Google Scholar] [CrossRef]
- Mobarak, M.; Selim, A.Q.; Mohamed, E.; Seliem, M.K. A superior adsorbent of CTAB/H2O2 solution−modified organic carbon rich-clay for hexavalent chromium and methyl orange uptake from solutions. Mol. Liq. J. 2018, 259, 384–397. [Google Scholar] [CrossRef]
- Xue, H.; Wang, X.; Xu, Q.; Dhaouadi, F.; Sellaoui, L.; Seliem, M.K.; Lamine, A.B.; Belmabrouk, H.; Bajahzar, A.; Bonilla-Petricioletf, A.; et al. Adsorption of methylene blue from aqueous solution on activated carbons and composite prepared from an agricultural waste biomass: A comparative study by experimental and advanced modeling analysis. Chem. Eng. J. 2022, 430, 132801. [Google Scholar] [CrossRef]
- Kumar Seera, S.D.; Kundu, D.; Gami, P.; Naik, P.K.; Banerjee, T. Synthesis and characterization of xylan-gelatin cross-linked reusable hydrogel for the adsorption of methylene blue. Carbohydr. Polym. 2021, 256, 117520. [Google Scholar] [CrossRef]
- Jalil, A.A.; Triwahyono, S.; Adam, S.H.; Rahim, N.D.; Aziz, M.A.A.; Hairom, N.H.H.; Razali, N.A.M.; Abidin, M.A.Z.; Mohamadiah, M.K.A. Adsorption of methyl orange from aqueous solution onto calcined Lapindo volcanic mud. J. Hazard. Mater. 2010, 181, 755–762. [Google Scholar] [CrossRef]
- Mohammadi, N.; Khani, H.; Gupta, V.K.; Amereh, E.; Agarwal, S. Adsorption process of methyl orange dye onto mesoporous carbon material–kinetic and thermodynamic studies. J. Colloid Interface Sci. 2011, 362, 457–462. [Google Scholar] [CrossRef]
- Tran, H.N.; Fen, Y.; You, W.S.J.; Chao, H.P. Insights into the mechanism of cationic dye adsorption on activated charcoal: The importance of π–π interactions. Process Saf. Environ. Prot. 2017, 107, 168–180. [Google Scholar] [CrossRef]
- Fu, J.; Chen, Z.; Wang, M.; Liu, S.; Zhang, J.; Zhang, J.; Han, R.; Xu, Q. Adsorption of methylene blue by a high-efficiency adsorbent (polydopamine microspheres): Kinetics, isotherm, thermodynamics and mechanism analysis. Chem. Eng. J. 2015, 259, 53–61. [Google Scholar] [CrossRef]
- Auta, M.; Hameed, B.H. Modified mesoporous clay adsorbent for adsorption isotherm and kinetics of methylene blue. Chem. Eng. J. 2012, 198–199, 219–227. [Google Scholar] [CrossRef]
- Mobarak, M.; Ali, R.A.M.; Seliem, M.K. Chitosan/activated coal composite as an effective adsorbent for Mn(VII): Modeling and interpretation of physicochemical parameters. Int. J. Biol. Macromol. 2021, 186, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Abukhadra, M.R.; Adlii, A.; Mohamed, B.B. Green fabrication of bentonite/chitosan@cobalt oxide composite (BE/CH@Co) of enhanced adsorption and advanced oxidation removal of Congo red dye and Cr (VI) from water. Int. J. Biol. Macromol. 2019, 126, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Abukhadra, M.R.; Ali, S.M.; Nasr, E.A.; Mahmoud, H.A.A.; Awwad, E.M. Effective Sequestration of Phosphate and Ammonium Ions by the Bentonite/Zeolite Na–P Composite as a Simple Technique to Control the Eutrophication Phenomenon: Realistic Studies. ACS Omega 2020, 5, 14656–14668. [Google Scholar] [CrossRef]
- Ramadan, H.S.; Mobarak, M.; Lima, E.C.; Bonilla-Petriciolet, A.; Li, Z.; Seliem, M.K. Cr(VI) adsorption onto a new composite prepared from Medium black clay and pomegranate peel extract: Experiments and physicochemical interpretations. J. Environ. Chem. Eng. 2021, 9, 105352. [Google Scholar] [CrossRef]
- Barakat, M.A.; Kumar, R.; Lima, E.C.; Seliem, M.K. Facile synthesis of muscovite–supported Fe3O4 nanoparticles as an adsorbent and heterogeneous catalyst for effective removal of methyl orange: Characterization, modelling, and mechanism. J. Taiwan Inst. Chem. Eng. 2021, 119, 146–157. [Google Scholar] [CrossRef]
- Cheng, Z.; Gao, Z.; Ma, W.; Sun, Q.; Wang, B.; Wang, X. Preparation of magnetic Fe3O4 particles modified sawdust as the adsorbent to remove strontium ions. Chem. Eng. J. 2012, 209, 451–457. [Google Scholar] [CrossRef]
- Teixeira, R.A.; Lima, E.C.; Benetti, A.D.; Thue, P.S.; Cunha, M.R.; Cimirro, N.F.G.M.; Sher, F.; Dehghani, M.H.; Reis, G.S.d.; Dotto, G.L. Preparation of hybrids of wood sawdust with 3-aminopropyl-triethoxysilane. Application as an adsorbent to remove Reactive Blue 4 dye from wastewater effluents. J. Taiwan Inst. Chem. Eng. 2021, 125, 141–152. [Google Scholar] [CrossRef]
- Seliem, M.K.; Barczak, M.; Anastopoulos, I.; Giannakoudakis, D.A. A Novel Nanocomposite of Activated Serpentine Mineral Decorated with Magnetic Nanoparticles for Rapid and Effective Adsorption of Hazardous Cationic Dyes: Kinetics and Equilibrium Studies. Nanomaterials 2020, 10, 684. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.A.; Seliem, M.K.; Lima, E.C.; Badawi, M.; Li, Z.; Bonilla-Petriciolet, A.; Anastopoulos, I. Outstanding Performance of a New Exfoliated Clay Impregnated with Rutile TiO2 Nanoparticles Composite for Dyes Adsorption: Experimental and Theoretical Studies. Coatings 2022, 12, 22. [Google Scholar] [CrossRef]
- Langergren, S.; Svenska, B.K. Veternskapsakad Zurtheorie der sogenanntenadsorption geloesterstoffe. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, J.C.; Morris, W.J. Advances in Water Research, Proceedings of International Conference on Water Pollution Symposium; Pergamon: Oxford, UK, 1962; Volume 2, pp. 231–266. [Google Scholar]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Ali, R.A.M.; Mobarak, M.; Badawy, A.M.; Lima, E.C.; Seliem, M.K.; Ramadan, H.S. New insights into the surface oxidation role in enhancing Congo red dye uptake by Egyptian ilmenite ore: Experiments and physicochemical interpretations. Surf. Interfaces J. 2021, 26, 101316. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Saad, I.; Othman, S.I.; Allam, A.A.; Fathallah, W. Synthesis of Co3O4 @ Organo/Polymeric Bentonite Structures as Environmental Photocatalysts and Antibacterial Agents for Enhanced Removal of Methyl Parathion and Pathogenic Bacteria. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2600–2614. [Google Scholar] [CrossRef]
- Mungondori, H.H.; Mtetwa, S.; Tichagwa, L.; Katwire, D.M.; Nyamukamba, P. Synthesis and application of a ternary composite of clay, sawdust and peanut husks in heavy metal adsorption. Water Sci. Technol. 2017, 75, 2443–2453. [Google Scholar] [CrossRef]
- Thakurata, D.G.; Paul, A.; Das, K.C.; Dhar, S.S. Fabrication of hierarchical Bentonite/Chitosan/NiFe2O4 ternary nanocomposite and its efficiency in the removal of Pb(II) from aqueous medium. Environ. Eng. Res. 2021, 26, 200216. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, H.; Wang, H.; Zhang, L.; Liu, P.; Feng, L. Fast adsorption of nickel ions by porous graphene oxide/sawdust composite and reuse for phenol degradation from aqueous solutions. J. Colloid Interface Sci. 2014, 436, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Ogbu, I.C.; Akpomie, K.G.; Osunkunle, A.A.; Eze, S.I. Sawdust-kaolinite composite as efficient sorbent for heavy metal ions. Bangladesh J. Sci. Ind. Resour. 2019, 54, 99–110. [Google Scholar] [CrossRef]
- Prabhu, Y.T.; Rao, K.V.; Kumari, B.S.; Kumar, V.S.S.; Pavani, T. Synthesis of Fe3O4 nanoparticles and its antibacterial application. Int. Nano Lett. 2015, 5, 85–92. [Google Scholar] [CrossRef]
- Petrinic, I.; Stergar, J.; Bukšek, H.; Drofenik, M.; Gyergyek, S.; Hélix-Nielsen, C.; Ban, I. Superparamagnetic Fe3O4@CA Nanoparticles and Their Potential as Draw Solution Agents in Forward Osmosis. Nanomaterials 2021, 11, 2965. [Google Scholar] [CrossRef]
- Horie, M.; Fujita, K. Toxicity of metal oxides nanoparticles Chapter 4. In Advances in Molecular Toxicology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 5. [Google Scholar]
- Ihaddaden, S.; Aberkane, D.; Boukerroui, A.; Robert, D. Removal of methylene blue (basic dye) by coagulation-flocculation with biomaterials (bentonite and Opuntia ficus indica). J. Water Process Eng. 2022, 49, 102952. [Google Scholar] [CrossRef]
- Rida, K.; Bouraoui, S.; Hadnine, S. Adsorption of methylene blue from aqueous solution by kaolin and zeolite. Appl. Clay Sci. 2013, 83–84, 99–105. [Google Scholar] [CrossRef]
- Chang, J.; Ma, J.; Ma, Q.; Zhang, D.; Qiaoa, N.; Hu, M.; Ma, H. Adsorption of methylene blue onto Fe3O4 /activated montmorillonite nanocomposite. Appl. Clay Sci. 2016, 119, 132–140. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Nie, W.; Song, L.; Chen, P. Highly efficient methylene blue dyes removal from aqueous systems by chitosan coated magnetic mesoporous silica nanoparticles. J. Porous Mater. 2015, 22, 1383–1392. [Google Scholar] [CrossRef]
- Franco, D.S.P.; Tanabe, E.H.; Bertuol, D.A.; Reis, G.S.d.; Lima, É.C.; Dotto, G.L. Alternative treatments to improve the potential of rice husk as adsorbent for methylene blue. Water Sci. Technol. 2017, 75, 296–305. [Google Scholar] [CrossRef]
- Cottet, L.; Almeida, C.A.P.; Naidek, N.; Viante, M.F.; Lopes, M.C.; Debacher, N.A. Adsorption characteristics of montmorillonite clay modified with iron oxide with respect to methylene blue in aqueous media. Appl. Clay Sci. 2014, 95, 25–31. [Google Scholar] [CrossRef]
- Hajjaji, M.; Alami, A.; el Bouadili, A. Removal of methylene blue from aqueous solution by fibrous clay minerals. J. Hazard. Mater. 2006, 135, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.A.; Selim, A.Q.; Zayed, A.M.; Komarneni, S.; Mobarak, M.; Seliem, M.K. Enhancing adsorption capacity of Egyptian diatomaceous earth by thermo-chemical purification: Methylene blue uptake. J. Colloid Interface Sci. 2019, 534, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hanafy, H.; Zhang, L.; Sellaoui, L.; Netto, M.S.; Oliveira, M.L.S.; Seliem, M.K.; Dotto, G.L.; Bonilla-Petricioleti, A.; Li, Q. Adsorption of Congo red and methylene blue dyes on an ashitaba waste and a walnut shell-based activated carbon from aqueous solutions Experiments, characterization, and physical interpretations. Chem. Eng. J. 2020, 388, 124263. [Google Scholar] [CrossRef]
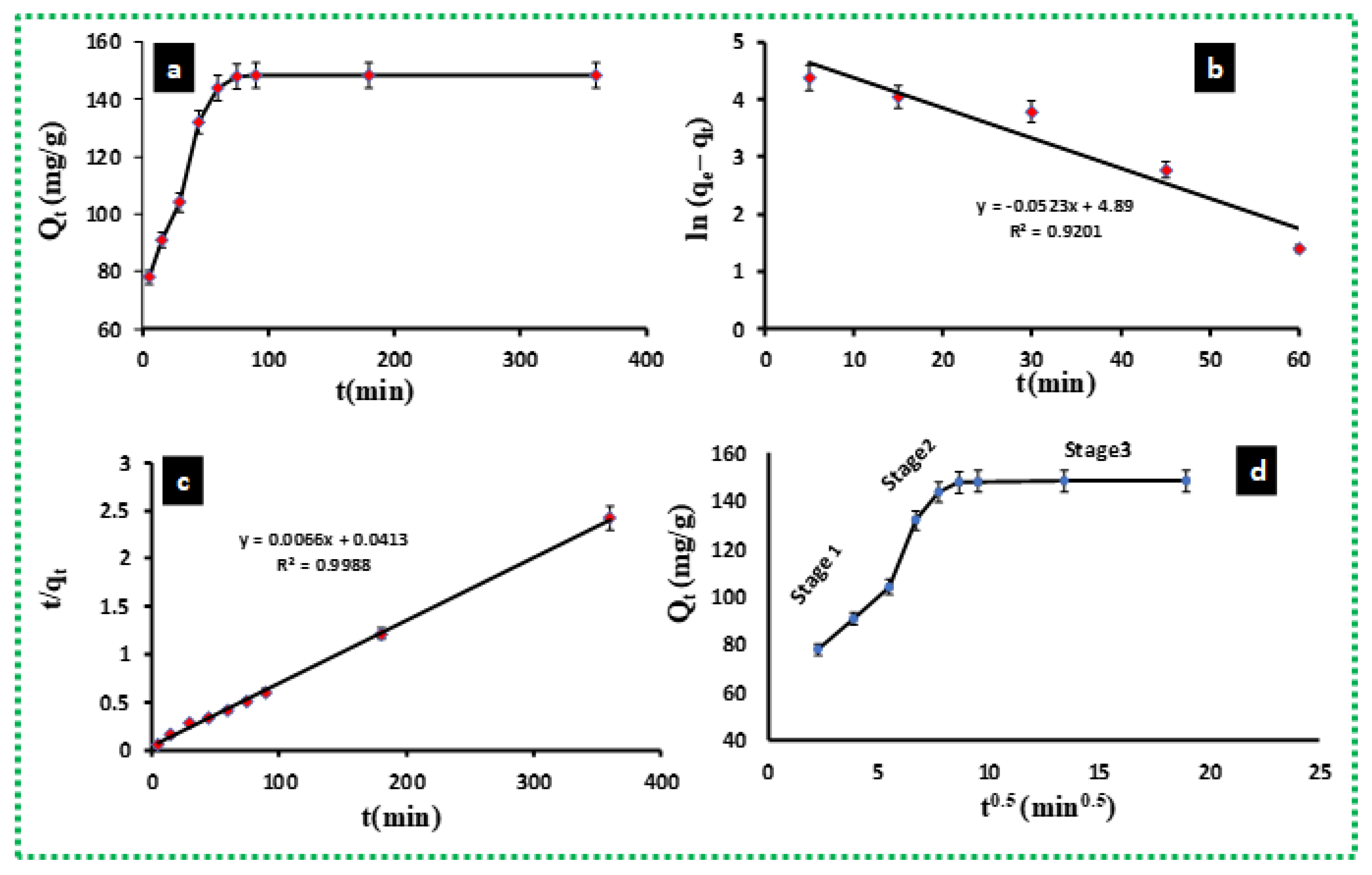
| Kinetics Models | Parameters | ||
|---|---|---|---|
| Pseudo-first-order | |||
| 151.51 | 0.0523 | 0.9201 | |
| Pseudo-second-order | (g/mg min) | ||
| 19.12 | 0.00178 | 0.9988 | |
| Intra-particle diffusion | (mg/g) (1/min) 0.5)] | (mg/g) | |
| 4.2001 | 91.180 | 0.5789 | |
| Isotherm Model | 25 °C | 40 °C | 55 °C |
|---|---|---|---|
| Langmuir | |||
| (g) | 347.99 | 385.27 | 388.12 |
| () | 0.00634 | 0.00776 | 0.01266 |
| 0.9984 | 0.9985 | 0.9966 | |
| 2.258 | 2.121 | 4.639 | |
| Freundlich | |||
| 7.24 | 9.57 | 14.61 | |
| 0.62 | 0.61 | 0.58 | |
| 0.9989 | 0.9975 | 0.9946 | |
| 1.547 | 1.203 | 3.992 |
| Adsorbent | Sorption Capacity (mg/g) | Reference |
|---|---|---|
| Graphene | 153 | [37] |
| Kaolin | 45 | [38] |
| Montmorillonite | 64 | [39] |
| Chitosan/magnetic silica | 201 | [40] |
| Zeolite 4A | 22 | [38] |
| Polydopamine microspheres | 90.7 | [12] |
| Activated rice husk | 65 | [41] |
| Ball clay | 25 | [13] |
| Fe3O4/montmorillonite | 69 | [42] |
| Rt/BC | 214.52 | [22] |
| Fibrous clay minerals | 39–85 | [43] |
| Purified diatomite | 105 | [44] |
| Fe3O4/serpentine composite | 201 | [21] |
| Activated carbon | 289.25 | [45] |
| BE/SD–MNPs composite | 347.99 | This study |
| Statistical Model | T (°C) | Parameters | |
|---|---|---|---|
| Monolayer (M1) | 25 | 0.9978 | |
| RMSE | 2.613 | ||
| 40 | 0.9976 | ||
| RMSE | 2.596 | ||
| 55 | 0.9959 | ||
| RMSE | 3.747 | ||
| Double layer (M2) | 25 | 0.9970 | |
| RMSE | 3.487 | ||
| 40 | 0.9931 | ||
| RMSE | 9.698 | ||
| 55 | 0.9969 | ||
| RMSE | 3.014 | ||
| Multilayer (M3) | 25 | 0.9991 | |
| RMSE | 0.9834 | ||
| 40 | 0.999 | ||
| RMSE | 1.426 | ||
| 55 | 0.9964 | ||
| RMSE | 2.701 |
| T (°C) | n | DM (mg/g) | 1 + N2 | Qsat (mg/g) | ΔE1 (kJ/mol) | ΔE2 (kJ/mol) |
|---|---|---|---|---|---|---|
| 25 | 0.75 | 482.73 | 2.34 | 829.10 | 14.30 | 11.62 |
| 40 | 0.82 | 464.16 | 2.21 | 845.75 | 15.44 | 13.90 |
| 55 | 0.85 | 458.24 | 2.19 | 848.99 | 16.04 | 15.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barakat, M.A.; Kumar, R.; Halawani, R.F.; Al-Mur, B.A.; Seliem, M.K. Fe3O4 Nanoparticles Loaded Bentonite/Sawdust Interface for the Removal of Methylene Blue: Insights into Adsorption Performance and Mechanism via Experiments and Theoretical Calculations. Water 2022, 14, 3491. https://doi.org/10.3390/w14213491
Barakat MA, Kumar R, Halawani RF, Al-Mur BA, Seliem MK. Fe3O4 Nanoparticles Loaded Bentonite/Sawdust Interface for the Removal of Methylene Blue: Insights into Adsorption Performance and Mechanism via Experiments and Theoretical Calculations. Water. 2022; 14(21):3491. https://doi.org/10.3390/w14213491
Chicago/Turabian StyleBarakat, Mohamed A., Rajeev Kumar, Riyadh F. Halawani, Bandar A. Al-Mur, and Moaaz K. Seliem. 2022. "Fe3O4 Nanoparticles Loaded Bentonite/Sawdust Interface for the Removal of Methylene Blue: Insights into Adsorption Performance and Mechanism via Experiments and Theoretical Calculations" Water 14, no. 21: 3491. https://doi.org/10.3390/w14213491
APA StyleBarakat, M. A., Kumar, R., Halawani, R. F., Al-Mur, B. A., & Seliem, M. K. (2022). Fe3O4 Nanoparticles Loaded Bentonite/Sawdust Interface for the Removal of Methylene Blue: Insights into Adsorption Performance and Mechanism via Experiments and Theoretical Calculations. Water, 14(21), 3491. https://doi.org/10.3390/w14213491








