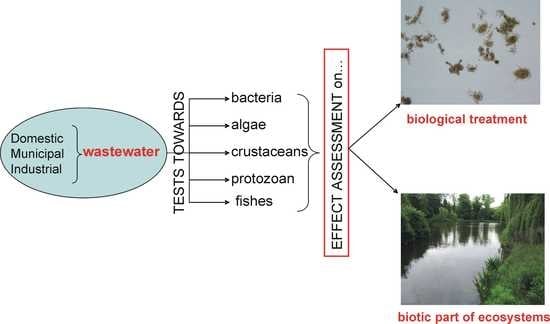
Evaluation of Ecotoxicity of Wastewater from the Full-Scale Treatment Plants
Abstract
:1. Introduction
2. Methods Used in the Review of Literature
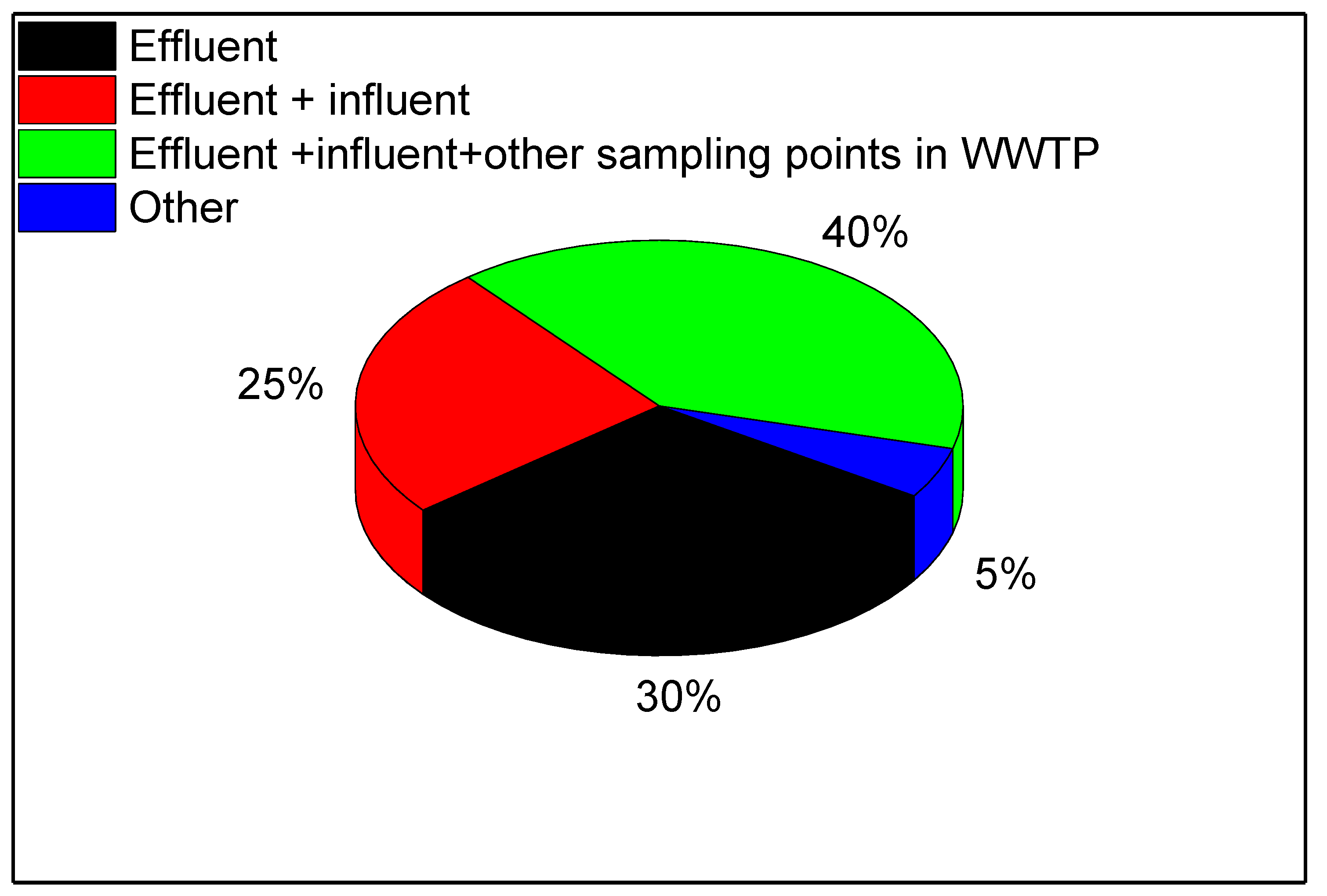
3. Types of Wastewater Tested and Sampling Points
4. Organisms and Tests Used for Evaluation of Wastewater Toxicity
| Wastewater | Species | Experimental Conditions | Method | Endpoint | Result | Remarks | Reference |
|---|---|---|---|---|---|---|---|
| Influent and effluent from 12 WWTPs in industrial parks (China). | Photobacterium phosphoreum T3 spp. | Bioluminescence of photobacterium T3 was measured by a biological toxicity detector. | Bioluminescence inhibition test. | Inhibition percentage | Inhibition degree of influents from 25.9% to 100%, while for effluents it varied from 18.5% to 91%. | Generally, the decrease in inhibition after treatment was found. Only in sequencing batch reactor (SBR) was the increase in inhibition percentage was observed. | [10] |
| Two types of wastewater from the company processing meat: (1) the washing wastewater, (2) the condensate wastewater. The wastewater were treated in SBR. | Vibrio fischeri | Measurement of the emission of light for 15 min with various dilutions of wastewater and a suspension of luminescent bacteria. | Microtox® test made in agreement with DIN ISO 11348–3, 1998. | EC50 | Influents were highly toxic (EC50 < 60%), while the effluents from SBR were low or not toxic (EC50 > 82%). | The correlation between the ammonium nitrogen and the toxicity of wastewater from both the influents and the effluents was found. | [28] |
| Stormwater samples (51) were taken from the urban area of Sydney, Brisbane and Melbourne (Australia). | Vibrio fischeri | Measurement of luminescence of the naturally bioluminescent marine bacteria Vibrio fischeri. | EN ISO 11348–3, 1998. | Toxicity equivalent concentration (TEQ) | TEQ ranged between 0.20 and 2.75 mg × L−1 for most samples. | The results were similar or slightly higher than those obtained for the secondary effluents of WWTPs. Highest effects similar to primary effluents. | [11] |
| The antibiotics wastewater treatment plant treating wastewater from a drug manufacturer (Shijiazhuang City, China); 15 samples from five sampling points incl. SBR, biofilm reactor and secondary clarifier. | Vibrio fischeri | Measurement of luminescence of the naturally bioluminescent marine bacteria Vibrio fischeri. | ISO 21338, 2010. | Toxicity unit (TU50) calculated upon EC50–15 min. | TU50 varied from 1.40 to 49.75% depending on the sampling point. | The raw wastewater samples were highly toxic, and the wastewater toxicity decreased during the treatment process. A significant, positive linear correlation between TU50 and indicators of wastewater contaminations (e.g., chemical oxygen demand (COD), biochemical oxygen demand (BOD5), NH4+ and others). | [23] |
| Samples taken seasonally (four times per year) for 4 years from two WWTPs with conventional activated sludge systems. Each time samples were taken from influent, secondary and tertiary effluent. | Vibrio fischeri (bacteria NRRL B-11177) | Measurement of luminescence of the naturally bioluminescent marine bacteria Vibrio fischeri. | Microtox® test | EC50 transformed to toxic units (TUs) | TU values varied from 3.8 to 40.0 for the influents. TUs varied from below 0.1 to 1.8 for the secondary effluents and for the tertiary effluents. | Toxicity of the effluents was usually lower in autumn and winter than in spring and summer. V. fischeri was less sensitive than P. subcapitata and D. magna. | [16] |
| Samples of 21 sites including three WWTPs (Australia) were taken. | Vibrio fischeri | Measurement of luminescence of the naturally bioluminescent marine bacteria Vibrio fischeri. | EN ISO 11348–3, 1998. | Toxicity equivalent concentration (TEQ) | E.g., in the Oxley Creek, WWTP toxicity decreased from 25.6 mg × L−1 to 1.26 mg × L−1. In the Caboolture Enhanced Wastewater Treatment Plant (EWTP), with ozonation and activated carbon treatment toxicity was reduced to 0.56 mg × L−1. | Decrease in toxicity was found after activated sludge treatment, reverse osmosis, advanced oxidation. Chloramination and microfiltration caused increase in toxicity. | [32] |
| Samples of influent and effluent from the conventional WWTP (Zgierz, Poland). Long-term (13 months) and short-term (two weeks) measurement campaigns were conducted. | Escherichia coli; Activated sludge microorganisms | Method is based on the reduction of resazurin, a redox-active dye, by bacterial respiration. The presence of toxic substances in the sample decreases the rate of resazurin reduction, which can be measured colorimetrically. | ToxTrak™Method 10017, HACH LANGE Manual. | Degree of inhibition (DI), toxicity unit (TU) | DI for raw wastewater varied from 10.2 to 59.8%, whereas for the treated ones from 3.3 to 35.6%. Out of 25 samples of the effluent, 2 belonged to class III and 23 samples were ranked as class IV. | The toxicity of the effluent was always lower than that of influent. The linear correlation between the toxicity of the influent and effluent was found. Lower toxicity of raw wastewater was observed in summer than in winter. | [20] |
| Effluent samples from two textile WWTPs (Ksar Hellal, Tunisia). | Vibrio fischeri | Measurement of luminescence of the naturally bioluminescent marine bacteria Vibrio fischeri. | Standard UNI EN ISO 11348–3, 2007. | EC50; LOEC; NOEC; TU | One effluent was toxic: EC50 = 3%, LOEC = 0.9, TU = 33.1, while the second one did not cause any toxicity. | V. fischeri was relatively good bioindicator for testing toxicity of the textile wastewaters. | [22] |
| Samples from five sampling points (incl. influent and effluents) from the pilot plant and the full-scale WWTP in Koblenz (Germany). | Vibrio fischeri | Measurement of luminescence of the naturally bioluminescent marine bacteria Vibrio fischeri. | Standard UNI EN ISO 11348–3, 2007. | EC50 | EC50 for influent was 1.49 ± 0.41 REF (Relative factor of enrichment). Reduction of toxicity by 86.1% in the full-scale WWTP. | Baseline toxicity was effectively removed in the activated sludge systems. | [13] |
| Influent and effluent from three full-scale WWTPs in Tuscany (Italy). | Vibrio fischeri | Measurement of luminescence of the naturally bioluminescent marine bacteria Vibrio fischeri. | Standard UNI EN ISO 11348–3, 2007. | Inhibition percentage | Inhibition percentage varied widely from −40.8% to 95.4%. Reduction of toxicity after treatment processes was usually found. | One of two of the most sensitive bioindicators used in this work; 90% of samples induced a significant bacterial inhibition. | [5] |
| Samples from six sampling points (incl. influent and effluents) from the full-scale WWTP treating pigment- containing wastewater (China). | Photobacterium phosphoreum | Acute toxicity tests of bioluminecent bacteria. | Standard National Environmental Protection Administration, China, NEPA, 1995. | EC50; TU | TU varied from 0 to 5.5. | The highest toxicity was found in the anoxic tank effluent (TU = 5.5). Reduction of toxicity after treatment (to TU = 0) in the final effluent was noticed. | [12] |
| Wastewater | Species | Experimental Condition | Method | Endpoint | Result | Remarks | Reference |
|---|---|---|---|---|---|---|---|
| Influent and effluent from 12 WWTPs in industrial parks (China). | Euglena gracilis | Measurement of absorbance at 610 nm. | Growth inhibition test. Acute toxicity. | Degree of growth inhibition | Degree of inhibition for influents and effluents varied from 42.8% to 77.3%. | For 9 out 12 WWTPs, no significant change in the degree of inhibition between influent and effluent was reported. | [10] |
| Effluents (93 samples) from SBR and MBR treating domestic, municipal and industrial wastewater. WWTPs were located in Venice (Italy). | Phaeodactylum tricornutum Bohlin | P. tricornutum was exposed to increasing concentrations of samples for 72 ± 2 h at 20 ± 1 °C and 6000–10,000 lux. Cell density was measured. | Growth inhibition or stimulation was determined according to ISO 10,253 method. | Toxicity unit (TU50); biostimulation unit (BU50) | 91% of all samples showed a stimulation effect. Among them 7, 30, 31 and 7 samples with low, medium, high and very high effects, respectively, were detected. In general, 90% of samples showed from medium to very high stimulation or toxicity effects. | Toxicity classification system based on inhibition and stimulation of microalgal growth was established. | [27] |
| Stormwater samples (51) were taken from urban areas of Sydney, Brisbane and Melbourne (Australia). | Pseudokirchneriella subcapitata | Inhibition of photosynthesis was assessed after 2 h of exposure using I-PAM (imaging pulse-amplitude-modulated) fluorometry and inhibition of growth rate after 24 h exposure. | The combined algae test integrates the quantification of the inhibition of photosynthesis with specific and non-specific effects on the growth rate. | IPAM: diuron equivalent concentration (DEQ). Algal growth: TEQ | In most samples photosynthesis was more sensitive endpoint than growth inhibition. | Algal toxicity was caused by herbicides in most samples. | [24] |
| Samples taken seasonally (four times per year) for 4 years from two WWTPs with conventional activated sludge systems. Each time samples were taken from influent, secondary and tertiary effluent. | Pseudokirchneriella subcapitata | Algae growth was determined by the measurement of optical density. | Algaltoxkit FTM according to OECD method no. 201, 2011 | EC50 transformed to toxic units (TUs) | TUs > 100 for the influents; TUs varied from 1.3 to above 100 for the secondary effluents; TUs varied from 0.4 to above 100 for the tertiary effluents. | Algae were the most sensitive species out of four species tested in this work. Seasonal decrease in the toxicity of effluents in autumn and winter in comparison to spring and summer. | [16] |
| Samples of 21 sites including 3 WWTPs (Australia) were taken. | Pseudokirchneriella subcapitata | Inhibition of photosynthesis was assessed after 2 h of exposure using I-PAM (imaging pulse-amplitude-modulated) fluorometry and inhibition of growth rate after 24 h exposure. | The combined algae test integrates the quantification of the inhibition of photosynthesis with specific and non-specific effects on the growth rate. | IPAM: diuron equivalent concentrations (DEQ). Algal growth: TEQ | E.g., in the Oxley Creek, WWTP DEQ decreased from 2.15 to 0.98 μg × L−1. Caboolture Enhanced Wastewater Treatment Plant (EWTP) reduced toxicity from 0.26 to 0.09 μg × L−1. | Decrease in toxicity was found after activated sludge treatment, reverse osmosis. Enhanced treatment (e.g., UV radiation, microfiltration, ozonation) did not alter the toxicity towards microalgae. | [32] |
| Piggery wastewater effluent samples were collected from a farm in Stellenbosch (Western Cape province, South Africa). | Pseudokirchneriella subcapitata | 72 h growth rate inhibition test | ALGALTOXKIT F, in agreeement with ISO norm 8692 and OECD method no. 201. | Percentage inhibition (%); EC50–48 h | P. subcapitata exposed to 1% unfiltered piggery effluent did not show any toxicity. EC50–48 h values for 10 and 20% unfiltered piggery effluent were 49.3% and 13.9%, respectively. | Unfiltered piggery effluent at concentration 10% and 20% can be regarded as toxic. | [14] |
| Samples of the effluent from the WWTP in the region of the Western Cape (South Africa). | Pseudokirchneriella subcapitata | 72 h growth rate inhibition test | ALGALTOXKIT F, in agreeement with ISO norm 8692 and OECD method no. 201. | Percentage inhibition (%); LC; TU | TU varied from 0.234 to 1.000, indicating low acute toxicity. | The WWTP effluent can be toxic to microalgae. Lower toxicity was observed in summer than in winter and autumn. | [18] |
| Influent and effluent samples from three WWTPs (Bangkok, Thailand) treating hospital wastewater were taken. Conventional activated sludge systems were applied in all WWTPs. In two of them, the effluents were chlorinated. | Chlorella vulgaris and Scenedesmus quadricauda | 72 h growth rate inhibition test | OECD method no. 201. | EC50; TU | The values of EC50 determined for Ch. vulgaris ranged from 13.83 to 17.16% (v/v) for influents and from 41.33 to 51.60% (v/v) for effluents. In the case of S. quadricauda, these were from 9.81 to 13.63% (v/v) for influents and from 45.8 to 87.1% (v/v) for effluents. | All hospital wastewaters showed similar toxic levels to the test algae. Toxicity decreased after treatment. TU of the effluents was from 1.15 to 2.42. S. quadricauda was more sensitive than C. vulgaris to hospital wastewater. | [11] |
| Effluent samples from two textile WWTPs (Ksar Hellal, Tunisia). | Raphidocelis subcapitata | 72 h growth rate inhibition test | Standard UNI EN ISO 8692:2005. | Inhibition percentage | Inhibition percentage varied from 0 to 69.3%. | R. subcapitata is a good bioindicator for testing toxicity of the textile wastewaters. | [22] |
| Samples from five sampling points (incl. influent and effluents) from the pilot plant and the full-scale WWTP in Koblenz (Germany). | Desmodesmus subspicatus | 72 h growth inhibition test | Standard ISO 8692, 2012. | Algae cell number | Increase in algal growth in all treatments was found. | The use of the classic growth inhibition test to determine phytotoxic effects of wastewater should be considered. | [13] |
| Influent and effluent from three full-scale WWTPs in Tuscany (Italy). | Raphidocelis subcapitata | 72 h growth inhibition test | Standard UNI EN ISO 692:2012, 2012. | Inhibition percentage | Inhibition percentage varied from 10.1 to 98.2%. Reduction of toxicity after treatment. | One of two the most sensitive bioindicators used in this work; 90% of samples induced a significantly algal inhibition. | [5] |
| Samples from each stage of treatment (incl. influent and effluents) from three full-scale WWTPs of different treatment systems (SBR, conventional activated sludge and Linpur) were tested. | Scenedesmus obliquus | 72 h growth inhibition test | OECD method no. 201, 2006. | Inhibition percentage (refers to cell density, chlorophyll-A synthesis, superoxidedismutase (SOD) activity). Percentage of cell viability (refers to cell membrane integrity). | The increase in cell growth was observed in all WWTPs studied. Only the effluent from NaClO disinfection units inhibited the cell growth by 131.8%. Analogous results were found for activity of SOD and chlorophyll-A synthesis. Percentage of cell viability decreased from 0.33% to 17.5%. | The acute toxicity of municipal wastewater on chlorophyll-A synthesis in S. obliquus was significantly correlated to phosphorus and organic carbon concentration. SOD activity and chlorophyll-A synthesis were found to be sensitive endpoints for the municipal wastewater studied. | [15] |
| Effluents from 17 municipal WWTPs of different sizes located in Poland. | Pseudokirchneriella subcapitata | 72 h chronic growth inhibition biotest | Algaltoxkit, procedure, 1996. | Inhibition percentage | The mean growth inhibition percentage varied from 11 to 100%. | High acute hazard was noted for four WWTPs tested. P. subcapitata was sensitive bioindicator for treated wastewater. | [19] |
| Wastewater | Species | Experimental Condition | Method | Endpoint | Result | Remarks | Reference |
|---|---|---|---|---|---|---|---|
| Influent and effluent from 12 WWTPs in industrial parks (China). | Daphnia magna straus | Determination of the number of immobilized individuals after 24 h and 48 h of exposure. | Immobilization inhibition test. Acute toxicity. | Degree of immobilization inhibition (%) | Degree of inhibition reached 100% after 48 h exposure in 4 out of 12 influents tested. | A large variation of results of tests. Degree of inhibition for influents and effluents varied from several percent to 100%. | [10] |
| Samples taken seasonally (four times per year) for 4 years from two WWTPs with conventional activated sludge systems. Each time samples were taken from influent, secondary and tertiary effluent. | Daphnia magna | Neonates were incubated at the appropriate conditions, and after 24 h and 48 h, the number of dead/immobilized neonates was calculated. | Daphtoxkit FTM according to OECD method no. 202, 2004. | EC50 transformed to toxic units (TUs) | TUs > 100 for the influents; TUs varied from 0.4 to above 100 for the secondary effluents; TUs varied widely from below 0.05 (non-toxic) to above 100 (very toxic) for the tertiary effluents | The toxicity to D. magna (48 h) was at the same level as toxicity to P. subcapitata determined in this work. | [16] |
| Artemia salina | Method based on determination of immobilization of Artemia nauplii after 24 and 48 h. | Methodology from US EPA, 2002. | EC50 transformed to toxic units (TUs) | TU values varied from 2.6 to 5.8 for the influents; A. salina was not affected by secondary and tertiary effluents of either WWTP (TUs < 0.1) | A. salina was the least sensitive indicator out of organisms used in the toxicity tests in this study. | [16] | |
| Piggery wastewater effluent samples were collected from a farm in Stellenbosch (Western Cape province, South Africa). | Daphnia magna | 48 h mortality/immobilization effect test | DAPHTOXKIT F, in agreement with ISO norm 6341 and OECD method no. 202. | Percent immobile (%); LC50 | At concentration higher than 1%, piggery effluent caused significant percentage immobility of D. magna after 24 h exposure. | The different percentage concentration of piggery effluent and a high-level dose of mixtures of veterinary pharmaceutical can also cause acute toxicity to D. magna. | [14] |
| Samples of the effluent from the WWTP in the region of the Western Cape (South Africa). | Daphnia magna | 48 h mortality/immobilization effect test | DAPHTOXKIT F, in agreement with ISO norm 6341 and OECD method no. 202. | Percentage mortality (%); LC; TU | Percentage of mortality after 48 h varied from 5% to 45%. TU varied from 0.944 to 1. | D. magna was the least sensitive organism in this study. | [18] |
| Influent and effluent samples from three WWTPs (Bangkok, Thailand) treating hospital wastewater were taken. Conventional activated sludge systems were applied in all WWTPs. In two of them, the effluents were chlorinated. | Microcrustacean: Moina macrocopa | 48 h mortality/immobilization effect test | OECD method no. 202. | LC50; TU | The values of LC50 were from 32.37 to 38.16% (v/v) for influents, while in the case of effluents it was from 45.91 to 59.25% (v/v). | Treatment reduced the toxic effect on the tested organism. Chlorination did not give a negative effect on this organism. | [11] |
| Effluent samples from two textile WWTPs (Ksar Hellal, Tunisia). | Daphnia magna | 24 h mortality/immobilization effect test | Standard UNI EN ISO 6341:2012 | Mortality; TU | One effluent exhibited 100% mortality, while the second one did not cause any mortality. | Good bioindicator for testing toxicity of the textile wastewaters. | [22] |
| Artemia franciscana | 24 h mortality effect test | ARTOXKIT M in agreement with Ecotoxicological method 8060 of APAT-IRSA, 2003. | Immobilization percentage | Immobilization percentage varied from 0% to 40%. | A. franciscana is not recommended for testing toxicity of textile wastewater. | [22] | |
| Samples from five sampling points (incl. influent and effluents) from the pilot plant and the full-scale WWTP in Koblenz (Germany). | Daphnia magna | 48 h acute immobilization test | Standard ISO 6341, 2012. | Percentage of immobilization | No adverse effect of wastewater on D. magna. | Tests with D. magna occurred to be of limited relevance in evaluation of toxicity of wastewater. | [13] |
| Influent and effluent from three full-scale WWTPs in Tuscany (Italy). | Daphnia magna | 48 h acute immobilization test | Standard UNI EN ISO 6341:2013 | Inhibition percentage | Inhibition percentage was 0% except one sample, when it was 100%. | D. magna almost never responsed to the samples tested. | [5] |
| Samples from six sampling points (incl. influent and effluents) from the full-scale WWTP treating pigment containimg wastewater (China). | Daphnia magna | 48 h acute immobilization test | OECD method no. 202. | EC50; TU | TU varied from 1.1 to 13.6. | The acute toxicity to D. magna was reduced by 91.8%, to which the anaerobic and aerobic biological treatment units contributed 65.3% and 12.5%, respectively. | [12] |
| Samples from eight sampling points (incl. influent and effluents) from the full-scale WWTP treated acrylonitrile containing wastewater (China). | Daphnia magna | 48 h acute immobilization test | OECD method no. 202, 2004. | LC50; TU | TU varied from below 0.4 to 125. | Systems anaerobic oxic (A/O) and anaerobic oxic-aerobic biological fluidized tank (A/O-ABFT) used in the WWTP were efficient in removal of toxicity to D. magna. Effluent was not toxic to D. magna. | [21] |
5. Effect of Wastewater on Aquatic Organisms
5.1. Effect on Bacteria
5.2. Effect on Algae
5.3. Effect on Crustaceans and Other Model Organisms
| Wastewater | Species | Experimental Condition | Method | Endpoint | Result | Remarks | Reference |
|---|---|---|---|---|---|---|---|
| Influent and effluent ww from 12 WWTPs in industrial parks (China). | Tetrahymena thermophila | Measurement of absorbance at 492 nm. | Growth inhibition test. Acute toxicity. | Degree of growth inhibition (%). | Degree of inhibition of influents and effluents was between 69.7 and 96.0% and 75.9 and 95.9%, respectively. | For 10 out 12 WWTPs, no change in the inhibition degree between influent and effluent was reported. | [10] |
| Vicia faba | The number of micronucleated cells was determined. | Micronucleus test–genotoxicity evaluation. | Pollution index (PI)—the ratio of the mean rate of micronucleus between the treatment and control groups. | The values of PI ranged from 0.38 to 2.00. | A relatively low level of genetic toxicity on V. faba for most of the wastewater samples was found. | [10] | |
| Piggery wastewater effluent samples were collected from a farm in Stellenbosch (Western Cape province, South Africa). | Tetrahymena thermophila | 24 h reproductive inhibition test | PROTOXKIT F, adhered to OECD method no. 202. | Percentage inhibition (%); EC50 | EC50 values varied from 4.81 to 52.39% depending on the concentration of piggery effluent. | A relationship between the percentage concentrations of toxicants and percentage growth inhibition of protozoa was found. | [14] |
| Samples of the effluent from the WWTP in the region of the Western Cape (South Africa). | Tetrahymena thermophila | 24 h reproductive inhibition test | PROTOXKIT F, adhered to OECD method no. 202. | Percentage inhibition (%); EC50 | TU varied from 84 to 89.6. | T. termophila was the most sensitive organism in this study. The effluents showed high acute toxicity to protozoa. | [18] |
| Effluent samples from two textile WWTPs (Ksar Hellal, Tunisia). | Lemna minor | 7 d growth inhibition rate test | Standard ISO SO/WD 20079, 2001 | Inhibition percentage | Inhibition percentage varied from 10.1 ± 4.2% to 52.7 ± 25% | L. minor was less sensitive than other bioindicators used in this study. | [22] |
| Cucumis sativus and Lepidium sativum | 72 h seeds germination and early growth tests. | Standard Method ISO 1651:2003, 2003. | Inhibition percentage | Inhibition percentage of germination varied from 20.0 ± 1.6% to 100 ± 0%. Inhibition percentage of root elongation varied from 77.0 ± 1.2% to 100 ± 0%. | Both plants were sensitive organisms to the textile wastewaters. | [22] | |
| Samples from five sampling points (incl. influent and effluents) from the pilot plant and the full-scale WWTP in Koblenz (Germany). | Potamopyrgus antipodarum | 28 d reproduction test | OECD method no. 242, 2016. | Mortality; number of embryos | Mortality of snails did not exceed 10%. The mean reproductive output was 17.9 ± 5.6 embryos in the control test. Exposure to wastewater effluents increased the reproduction by 10.4–31.1%. | No reproductive toxicity after direct exposure to conventionally treated wastewater. | [13] |
| Lumbriculus variegatus | 28 d reproduction test | OECD method no. 225, 2007. | Number of worms; biomass of worms | No significant effect on reproduction. Biomass exposed to the wastewater decreased compared to the control from 25.5 to 34.2%. | Decrease in biomass of earthworms but no effect on reproduction. | [13] | |
| Influent and effluent from three full-scale WWTPs in Tuscany (Italy). | Sorghum commune, Lepidium sativum, Cucumis sativus | 72 h germination and early growth test | Standard UNI 11357:2010. | Inhibition percentage; germination index (GI) | A large variation of results. Both toxic and stimulatory effects were found. | Inhibition phenomenon was observed in 37% of samples. | [5] |
| Samples from six sampling points (incl. influent and effluents) from the full-scale WWTP treatimg pigment containimg wastewater (China). | Danio rerio | 96 h static acute toxicity test | Standard ISO 7346–3:1996, 1996. | EC50; TU | TU varied from 2.0 to 3.7. | Only 20% of the acute toxicity was removed. | [12] |
| Samples from eight sampling points (incl. influent and effluents) from the full-scale WWTP treated acrylonitrile containing wastewater (China). | Danio rerio | 96 h static acute toxicity test | OECD method no. 203, 1992. | LC50; TU | TU varied from below 0.4 to 29.6. | After going through the A/O and ABFT wastewater treatment systems, the final effluent showed no acute toxicity to D. rerio. | [21] |
| Samples from five sampling points (incl. influent and effluents) from three full-scale WWTPs treated municipal wastewater using A/O system (China). | Danio rerio | 96 h acute static test | OECD method no. 203, 1992. | Mortality rate (%) | Mortality rate varied from 0% to 50% ± 10%. | Acute toxicity was reduced along with the A/O process of treatment. Acute toxicity on zebrafish decreased in accordance with the COD removal. | [37] |
| Effluents from 17 municipal WWTPs of different sizes located in Poland. | Thamnocephalus platyurus | 24 h mortality acute biotest | Thamnotoxkit procedure, 1995. | Mortality rate (%) | The mean mortality rate varied from 3 to 100%. | T. platyurus mortality demonstrated a very strong positive correlation with NH4+ and a strong with total nitrogen. | [19] |
| Tetrahymena thermophila | 24 h chronic growth inhibition biotest | Protoxkit procedure, 1998. | Inhibition percentage | The mean growth inhibition percentage did not exceed 40%. | The mean toxicity did not exceed acute hazard for all samples from WWTPs. Stimulation was found. | [19] |
| Trophic Level | Group of Organisms | Examples of Species |
|---|---|---|
| Primary producers | Algae | Raphidocelis subcapitata; Chlorella vulgaris; Scenedesmus sp. |
| Aquatic plants | Lemna minor | |
| Terrestrial plants | Lepidium sativum | |
| Reducers | Bacteria | Vibrio fischeri; Photobacterium phosphoreum |
| Protozoa (ciliata) | Tetrahymena thermophila | |
| Consumers | Crustaceans | Daphnia magna Artemia franciscana |
| Fishes | Danio rerio |
6. Conclusions and Perspectives
- Monitoring of physicochemical indicators of wastewater contamination in the full-scale WWTPs should be supplemented with the ecotoxicological assessment. It comprises not only the treated wastewaters (effluents), which are discharged to the environment—i.e., the whole effluent toxicity tests—but also the raw wastewater (influent) entering the WWTPs. Due to testing of the wastewater toxicity in several points of the WWTPs, the treatment processes, in particular the biological treatment, might be carried out more efficiently.
- The application of the ecotoxicological treatment should concern all types of wastewater (municipal and industrial) and all full-scale WWTPs irrespective of their size, because the small WWTPs are also the source of hazard for the aquatic compartment.
- Wastewater is a complex matrix of compounds, and therefore, the determination of their toxicity is a difficult task. The results of ecotoxicity tests for wastewater often vary to a high extent not only due to the complex composition of wastewater but also because they are dependent on the organisms, which are used as model organisms in the bioassays. In order to evaluate the toxic hazard of wastewater towards biota, a battery of ecotoxicity tests using model organisms of different sensitivities and representing different trophic levels should be employed. So far, bacteria (e.g., Vibrio fischeri), green microalgae (e.g., Raphidocelis subcapitata) and crustaceans (e.g., Daphnia magna) have been the most commonly used organisms in the biological assessment of wastewater. They were applied in almost half (bacteria) or more than a half (microalgae, crustaceans) of papers analyzed in this study. They are usually regarded as sensitive organisms towards wastewater; however, only the application of different model organisms allows for the identification of the organisms of the appropriate sensitivity for a specific WWTP and/or a specific purpose.
- The treatment, in particular the biological treatment, of wastewater contributes to the reduction of wastewater ecotoxicity irrespective of the technological solution applied in the WWTPs. The conventional activated sludge systems (e.g., A/O or A/A/O processes) are efficient in the removal of wastewater toxicity. It concerns both municipal and industrial wastewater. At the same time, the tertiary stage of wastewater treatment, in particular chlorination or ozonation, induces the increase in wastewater toxicity.
- The classification of wastewater toxicity based upon the toxicity units proposed several years ago by Persoone et al. [17] was shown to be very useful and commonly applied. Now, it is necessary to take the next steps in the evaluation of the ecological risks of wastewater and linking this classification with the limit values of wastewater toxicity. These limit values should be primarily established for the final effluent discharged to the aquatic or soil ecosystems.
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Kosma, C.I.; Lambropoulou, D.A.; Albanis, T.A. Investigation of PPCPs in wastewater treatment plants in Greece: Occurrence, removal and environmental risk assessment. Sci. Total Environ. 2014, 466–467, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Paíga, P.; Correia, M.; Fernandes, M.J.; Silva, A.; Carvalho, M.; Vieira, J.; Jorge, S.S.; Silva, J.G.; Freire, C.; Delerue-Matos, C.; et al. Assessment of 83 pharmaceuticals in WWTP influent and effluent samples by UHPLC-MS/MS: Hourly variation. Sci. Total Environ. 2019, 648, 582–600. [Google Scholar] [CrossRef] [PubMed]
- Henze, M.; Harremoës, P.; Jansen, J.; Arvin, E. Wastewater Treatment. In Biological and Chemical Processes, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2002; p. 430. [Google Scholar]
- Palli, L.; Spina, F.; Varese, G.C.; Vincenzi, M.; Aragno, M.; Arcangeli, G.; Mucci, N.; Santianni, D.; Caffaz, S.; Gori, R. Occurrence of selected pharmaceuticals in wastewater treatment plants of Tuscany: An effect-based approach to evaluate the potential environmental impact. Int. J. Hyg. Environ. Health 2019, 222/4, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; De Araujo, C.; Sze, C.C.; Stuckey, D.C. Toxicity measurement in biological wastewater treatment processes: A review. J. Hazard. Mater. 2015, 286, 15–29. [Google Scholar] [CrossRef]
- Grady, C.P.L., Jr.; Daigger, G.T.; Love, N.G.; Filipe, C.D.M. Biological Wastewater Treatment, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar] [CrossRef]
- EU, 2000, Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy. OJ L 327. 22 December 2000, pp. 1–73. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32000L0060 (accessed on 26 May 2022).
- EU, 2013, Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 Amending Directives 2000/60/EC and 2008/105/EC as Regards Priority Substances in the Field of Water Policy. OJ L 226. 24 August 2013, pp. 1–17. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:226:0001:0017:en:PDF (accessed on 20 May 2022).
- Yu, Y.; Wu, B.; Jiang, L.; Zhang, X.-X.; Ren, H.-Q.; Li, M. Comparative analysis of toxicity reduction of wastewater in twelve industrial park wastewater treatment plants based on battery of toxicity assays. Sci. Rep. 2019, 9, 3751. [Google Scholar] [CrossRef] [Green Version]
- Hamjinda, N.S.; Chiemchaisri, W.; Watanabe, T.; Honda, R.; Chiemchaisri, C. Toxicological assessment of hospital wastewater in different treatment processes. Environ. Sci. Pollut. Res. Int. 2018, 25, 7271–7279. [Google Scholar] [CrossRef]
- Deng, M.; Zhang, Y.; Quan, X.; Na, C.; Chen, S.; Liu, W.; Han, S.; Masunaga, S. Acute toxicity reduction and toxicity identification in pigment-contaminated wastewater during anaerobic-anoxic-oxic (A/A/O) treatment process. Chemosphere 2017, 168, 1285–1292. [Google Scholar] [CrossRef]
- Völker, J.; Vogt, T.; Castronovo, S.; Wick, A.; Ternes, T.A.; Joss, A.; Oehlmann, J.; Wagner, M. Extended anaerobic conditions in the biological wastewater treatment: Higher reduction of toxicity compared to target organic micropollutants. Water Res. 2017, 116, 220–230. [Google Scholar] [CrossRef]
- Udebuani, A.C.; Pereao, O.; Akharame, M.O.; Fatoki, O.S.; Opeolu, B.O. Acute toxicity of piggery effluent and veterinary pharmaceutical cocktail on freshwater organisms. Environ. Monit. Assess. 2021, 193, 293. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Q.; Zhou, J.; Masunaga, S.; Ma, F. Reduction in toxicity of wastewater from three wastewater treatment plants to alga (Scenedesmus obliquus) in northeast China. Ecotoxicol. Environ. Saf. 2015, 119, 132–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasquez, M.I.; Fatta-Kassinos, D. Is the evaluation of “traditional” physicochemical parameters sufficient to explain the potential toxicity of the treated wastewater at sewage treatment plants? Environ. Sci. Pollut. Res. 2013, 20, 3516–3528. [Google Scholar] [CrossRef]
- Persoone, G.; Marsalek, B.; Blinova, I.; Törökne, A.; Zarina, D.; Manusadzianas, L.; Nalecz-Jawecki, G.; Tofan, L.; Stepanova, N.; Tothova, L.; et al. A practical and user-friendly toxicity classification system with microbiotests for natural waters and wastewaters. Environ. Toxicol. 2003, 18, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Pereao, O.; Akharame, M.O.; Fatoki, O.S.; Opeolu, B.O. Effects of municipal wastewater treatment plant effluent quality on aquatic ecosystem organisms. J. Environ. Sci. Health Part A 2021, 56, 1480–1489. [Google Scholar] [CrossRef] [PubMed]
- Szklarek, S.; Kiedrzyńska, E.; Kiedrzyński, M.; Mankiewicz-Boczek, J.; Mitsch, W.J.; Zalewski, M. Comparing ecotoxicological and physicochemical indicators of municipal wastewater effluent and river water quality in a Baltic Sea catchment in Poland. Ecol. Indic. 2021, 126, 107611. [Google Scholar] [CrossRef]
- Liwarska-Bizukojc, E.; Ślęzak, R.; Klink, M. Study on wastewater toxicity using ToxTrak™ method. Environ. Sci. Pollut. Res. 2016, 23, 9105–9113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Na, C.; Zhang, Y.; Deng, M.; Quan, X.; Chen, S.; Zhang, Y. Evaluation of the detoxication efficiencies for acrylonitrile wastewater treated by a combined anaerobic oxic-aerobic biological fluidized tank (A/O-ABFT) process: Acute toxicity and zebrafish embryo toxicity. Chemosphere 2016, 154, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedoui, A.; Tigini, V.; Ghedira, K.; Varese, G.C.; Chekir Ghedira, L. Evaluation of an eventual ecotoxicity induced by textile effluents using a battery of biotests. Environ. Sci. Pollut. Res. Int. 2015, 22, 16700–16708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Zuo, J.; Li, R.; Gan, L.; Li, Z.; Zhang, F. A combined evaluation of the characteristics and acute toxicity of antibiotic wastewater. Ecotoxicol. Environm. Saf. 2014, 106, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.Y.; Aryal, R.; Deletic, A.; Gernjak, W.; Glenn, E.; McCarthy, D.; Escher, B.I. Toxicity characterization of urban stormwater with bioanalytical tools. Water Res. 2013, 47, 5594–5606. [Google Scholar] [CrossRef] [PubMed]
- Rayaroth, M.P.; Aravindakumar, C.T.; Shah, N.S.; Boczkaj, G. Advanced oxidation processes (AOPs) based wastewater treatment–unexpected nitration side reactions—A serious environmental issue: A review. Chem. Eng. J. 2022, 430 Pt 4, 133002. [Google Scholar] [CrossRef]
- Yi, X.; Kim, E.; Jo, H.; Schlenk, D.; Jung, J. A toxicity monitoring study on identification and reduction of toxicants from a wastewater treatment plant. Ecotoxicol. Environ. Saf. 2009, 72, 1919–1924. [Google Scholar] [CrossRef]
- Libralato, G.; Gentile, E.; Ghirardini, A.V. Wastewater effects on Phaedodactylum tricornutum (Bohlin): Setting up a clasification system. Ecol. Indic. 2016, 60, 31–37. [Google Scholar] [CrossRef]
- Rodríguez-Loaiza, D.C.; Ramírez-Henao, O.; Peńuela-Mesa, G.A. Assessment of toxicity in industrial wastewater treated by biological processes using luminescent bacteria. Actual. Biol. 2016, 38, 211–216. [Google Scholar] [CrossRef]
- ISO 11348–3; Water Quality—Determination of the Inhibitory Effect of Water Samples on the Light Emission of Vibrio fischeri (Luminescent Bacteria Test)—Part 3: Method Using Freeze-Dried Bacteria. International Organization for Standardization: Geneva, Switzerland, 2007.
- ISO 21338; Water Quality—Kinetic Determination of the Inhibitory Effects of Sediment, Other Solids and Coloured Samples on the Light Emission of Vibrio fischeri (Kinetic Luminescent Bacteria Test). International Organization for Standardization: Geneva, Switzerland, 2010.
- Arslan-Alaton, I.; Insel, G.; Eremektar, G.; Germirli-Babuna, F.; Orhon, D. Effect of textile auxiliaries on the biodegradation of dyehouse effluent in activated sludge. Chemosphere 2006, 62, 1549–1557. [Google Scholar] [CrossRef]
- Macova, M.; Toze, S.; Hodgers, L.; Mueller, J.F.; Bartkow, M.; Escher, B.I. Bioanalytical tools for the evaluation of organic micropollutants during sewage treatment, water recycling and drinking water generation. Water Res. 2011, 45, 4238–4247. [Google Scholar] [CrossRef] [PubMed]
- ISO 8692; Water Quality—Fresh Water Algal Growth Inhibition Test with Unicellular Green Algae. International Organization for Standardization: Geneva, Switzerland, 2012.
- OECD Guidelines for the Testing of Chemicals. Test No. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test. 2011. Available online: https://www.oecd-ilibrary.org/environment/test-no-201-alga-growth-inhibition-test_9789264069923-en (accessed on 20 May 2022).
- OECD Guidelines for the Testing of Chemicals. Test No. 202: Daphnia sp. Acute Immobilisation Test. 2004. Available online: https://www.oecd-ilibrary.org/environment/test-no-202-daphnia-sp-acute-immobilisation-test_9789264069947-en (accessed on 20 May 2022).
- OECD Guidelines for the Testing of Chemicals. Test No. 203: Fish, Acute Toxicity Test. 2019. Available online: https://www.oecd-ilibrary.org/environment/test-no-203-fish-acute-toxicity-test_9789264069961-en (accessed on 20 May 2022).
- ISO 7346–3; Water Quality—Determination of the Acute Lethal Toxicity of Substances to a Freshwater Fish [Brachydanio Rerio Hamilton-Buchanan (Teleostei, Cyprinidae)]—Part 3: Flow-through Method. International Organization for Standardization: Geneva, Switzerland, 1996.
- Zhang, J.; Zhang, Y.; Liu, W.; Quan, X.; Chen, S.; Zhao, H.; Jin, Y.; Zhang, W. Evaluation of removal efficiency for acute toxicity and genotoxicity on zebrafish in anoxic–oxic process from selected municipal wastewater treatment plants. Chemosphere 2013, 90/11, 2662–2666. [Google Scholar] [CrossRef]
- Jarošová, B.; Erseková, A.; Hilscherová, K.; Loos, R.; Gawlik, B.M.; Giesy, J.P.; Bláha, L. Europe-wide survey of estrogenicity in wastewater treatment plant effluents: The need for the effect-based monitoring. Environ. Sci. Pollut. Res. 2014, 21, 10970–10982. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liwarska-Bizukojc, E. Evaluation of Ecotoxicity of Wastewater from the Full-Scale Treatment Plants. Water 2022, 14, 3345. https://doi.org/10.3390/w14203345
Liwarska-Bizukojc E. Evaluation of Ecotoxicity of Wastewater from the Full-Scale Treatment Plants. Water. 2022; 14(20):3345. https://doi.org/10.3390/w14203345
Chicago/Turabian StyleLiwarska-Bizukojc, Ewa. 2022. "Evaluation of Ecotoxicity of Wastewater from the Full-Scale Treatment Plants" Water 14, no. 20: 3345. https://doi.org/10.3390/w14203345
APA StyleLiwarska-Bizukojc, E. (2022). Evaluation of Ecotoxicity of Wastewater from the Full-Scale Treatment Plants. Water, 14(20), 3345. https://doi.org/10.3390/w14203345