Desalination of Irrigation Water Using Metal Polymers
Abstract
:1. Introduction
1.1. ZVI Desalination Process
- Spanish Patent ES2598032B1 assumes that magnetic n-Fe0 surfaces will become charged in water, due to the presence of OH− ions and H+ ions. Functionalization may take the form:
- US patent US8636906B2 assumes that the n-ZVI can be functionalized by the addition of cationic and anionic coatings to allow NaCl removal:
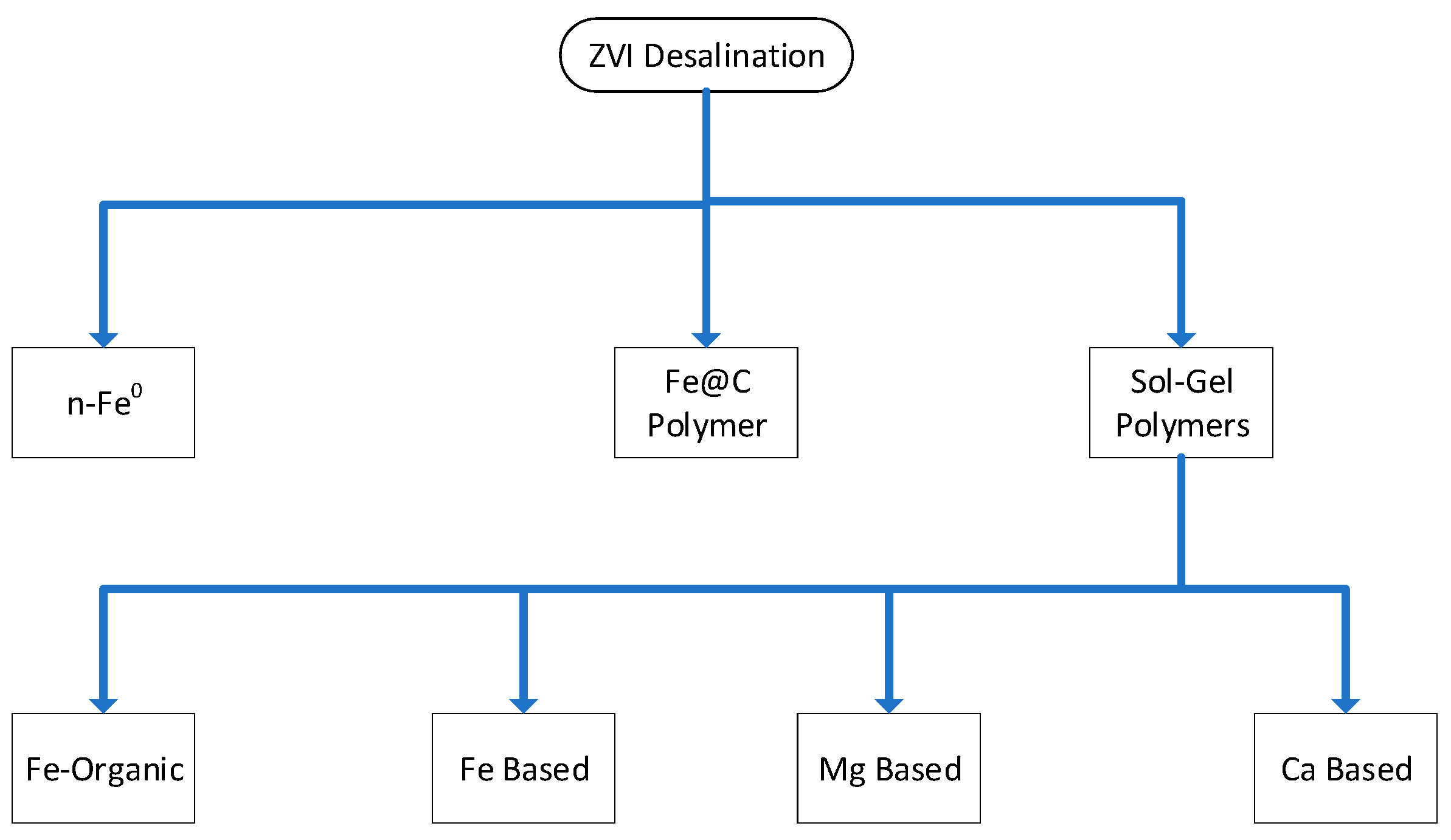
1.2. Past ZVI Approaches Used to Desalinate Water
- Adding ZVI, or Fe0, to saline water to directly remove NaCl from the water [16]. This approach may have a desalination cost within the range USD 2 to USD 500 m−3. This approach results in the formation of Fe0:Fe(a,b,c) polymers on the surfaces of the individual ZVI particles and entrained colloidal and flocculate n-Fe(a,b,c) polymers within the water. Ion removal is by direct reaction and incorporation within the polymers. The addition of ZVI to water, results in an increase in the pH of the water. The ZVI is normally only effective to treat a single volume of water with a concentration of 10 to 100 g Fe0 L−1 water. The wide range in ZVI desalination cost is a function of particle size, ZVI source and the concentration of ZVI added to the water to achieve the required result. Micron sized particles may be purchasable (FOB) for between USD 500 and 5000 t−1 depending on supplier, quantity, and quality. Nano sized particles may be purchasable (FOB) for between USD 10,000 and 1,000,000 t−1 depending on supplier, quantity, and quality. FOB = free of board.
- Using ZVI to manufacture pellets, which, if added to water, removes NaCl from the water [16,17]. These pellets contain a high proportion of dead-end pores, and the ZVI surface, and pore surfaces are lined with Fe0:Fe(a,b,c) polymers. These polymers remove Na+ and Cl− ions from the open water body and deposit them into the dead-end pores contained with the pellets; the addition of pellets to water results in an increase in the pH of the water. The ZVI is normally only effective to treat a single volume of water with a concentration of 20 to 50 g Pellet L−1 water. However, the pellets can be regenerated and reconstituted to allow reuse [17].
- Using ZVI to manufacture Fe0 supported polymers of the form Fe0:Fe(a,b,c) and Fe0:Fe(a,b,c)@urea, where the pH increase is effected by bubbling air through the water [18]. Cyclic pressure fluctuations in the water are used to promote adsorption and desorption from the polymer. Na+ and Cl− ion removal rates decrease with time. Catalyst regeneration is achieved by replacing the partially desalinated water with a fresh saline water charge. A single polymer charge located on 400 g Fe0 was demonstrated to process 70 batches of water (17 m3) without loss of activity [18].
- Using Fen+ ions to manufacture SiO2 supported polymers of the form SiO2@Fe(a,b,c) and SiO2@Fe(a,b,c)@urea, where the pH increase is effected by bubbling air through the water [19]. Cyclic pressure fluctuations in the water are used to promote adsorption and desorption from the polymer. Na+ and Cl− ion removal rates decrease with time. Catalyst regeneration is achieved by replacing the partially desalinated water with a fresh saline water charge. A single polymer charge containing 10 g Fe was demonstrated to process 50 batches of water (43 m3) without loss of activity [19]. It was proposed that the polymer catalyses the formation of entrained polymer particles in the water using metal cations present in the feed water [19]. The water contained Fe, Ca, Mg and Al ions. It is currently unclear which metal (Fe, Ca, Mg, Al) based polymers, can remove Na+ or Cl− ions.
1.3. ZVI Water Remediation
- (i)
- Redox modification of the Eh and pH of the water to force a change in the equilibrium reactant quotient associated with a pollutant, to convert a pollutant into a benign product;
- (ii)
- Direct reaction with the pollutant to either form an iron complex or a benign product
- (iii)
- Physical adsorption, or chemical adsorption, of the pollutant;
- (iv)
- Catalysis to convert a pollutant into a benign product;
- (v)
- Adsorption by, or interaction with, iron polymers (flocculants).
2. Technology, Methodology, Materials and Equipment Used
2.1. Data, Data Terminology and Data Interpretation
2.1.1. Statistical Methodology Used
- PCC = 0.9 to 1.0 (R2 = 0.81 to 1.00): Interpretation = very strong correlation
- PCC = 0.7 to 0.89 (R2 = 0.49 to 0.79): Interpretation = strong correlation
- PCC = 0.4 to 0.69 (R2 = 0.16 to 0.47): Interpretation = moderate correlation
- PCC = 0.1 to 0.39 (R2 = 0.01 to 0.15): Interpretation = weak correlation
- PCC = 0.0 to 0.10 (R2 = 0.00 to 0.01): Interpretation = negligible correlation
2.1.2. Polymer Terminology
2.2. Chemicals
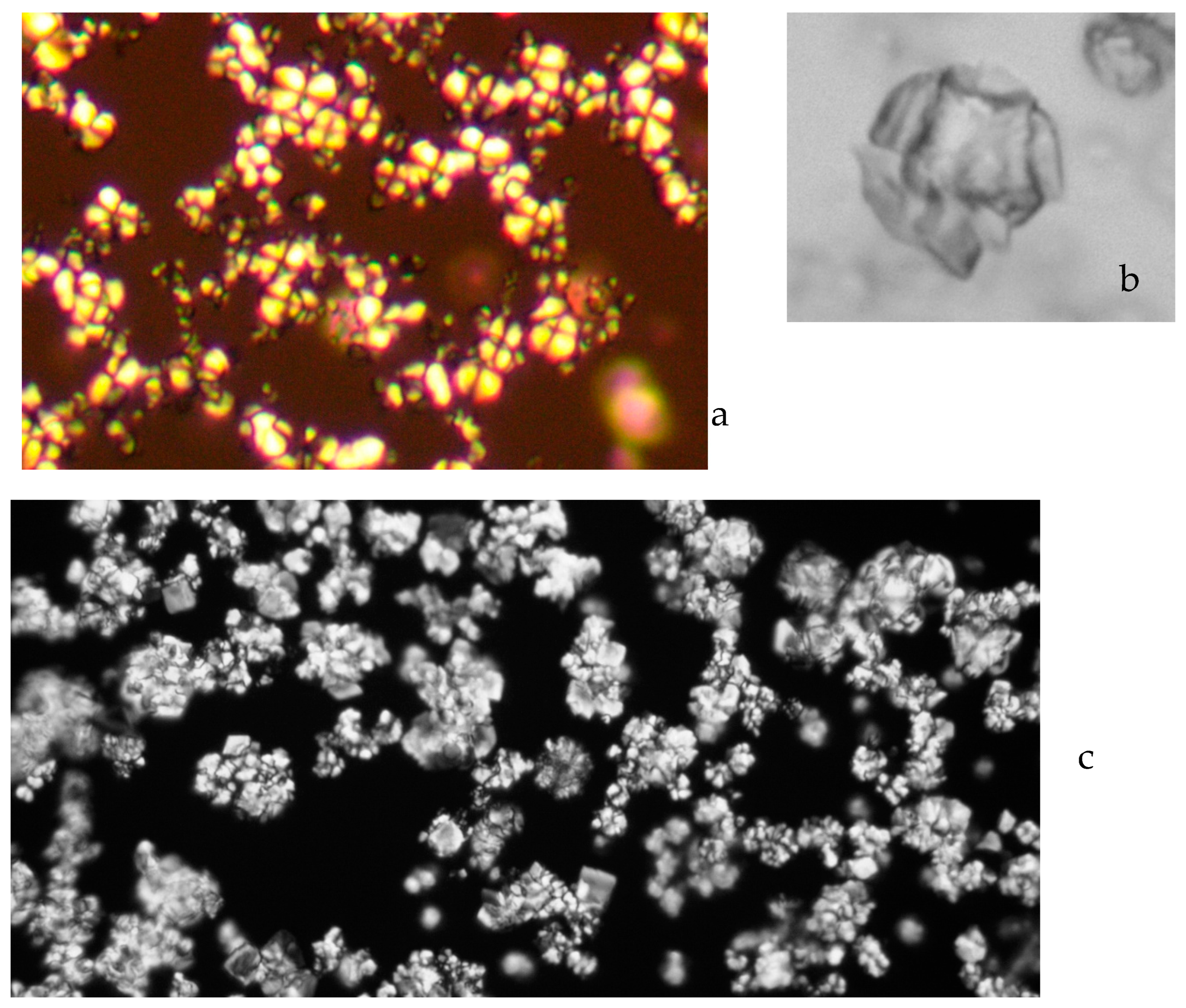
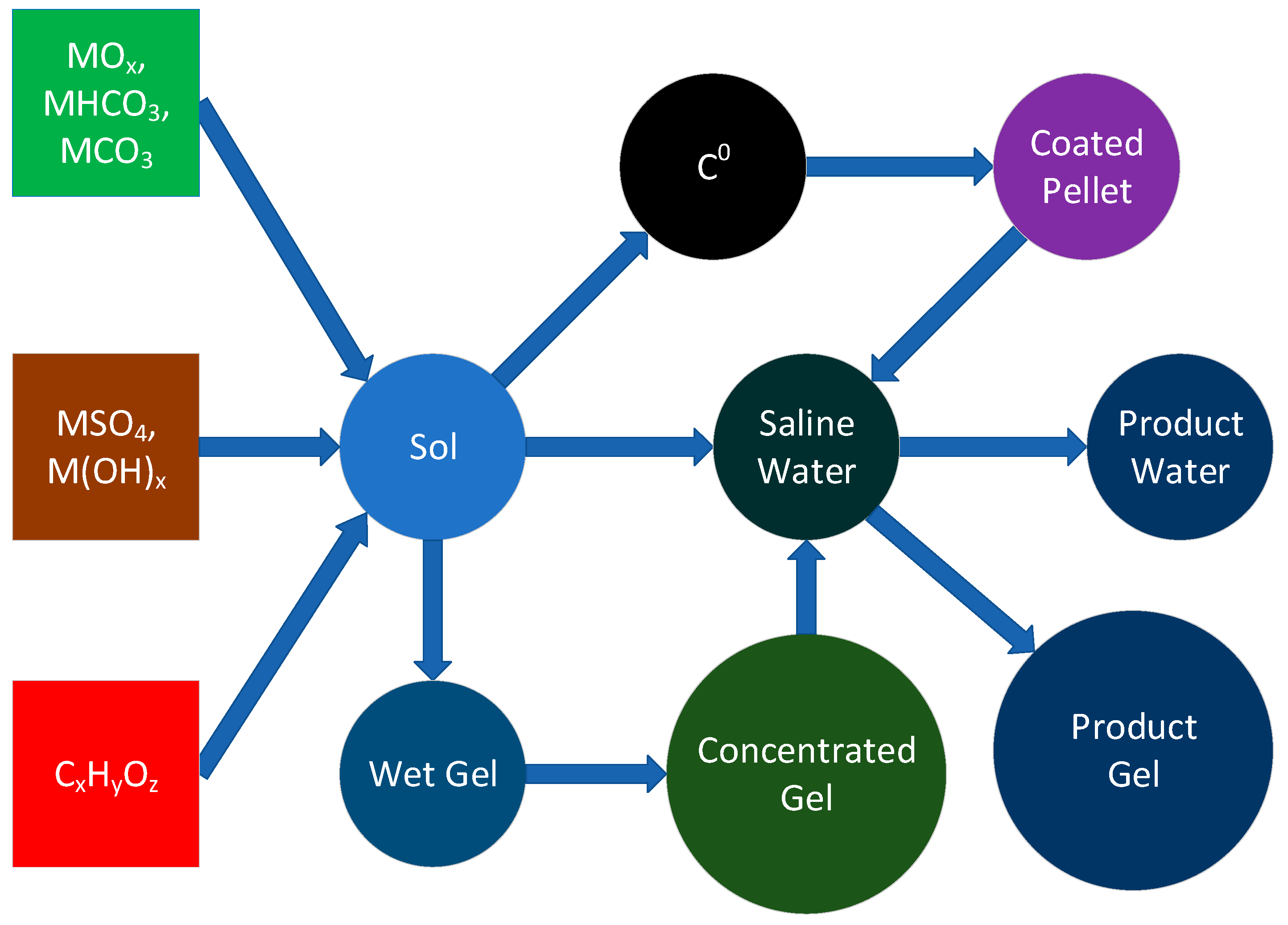
2.3. Construction of Entrained Polymers
- Manufacture of the polymer, with concentration and extraction, prior to placement in water;
- Manufacture of the polymer, in proximity with a support (active carbon), prior to placement of the support in water;
- Manufacture of the polymer, in the target saline water body.
2.3.1. Characterisation
2.3.2. Instant Desalination
2.4. Measurement Equipment
- ORP (oxidation reduction potential) meter (HM Digital) calibrated at ORP = 200 mV; Measured ORP (oxidation reduction potential) values are converted to Eh, mV as: Eh, mV = −65.667pH + 744.67 + ORP (mV), using a quinhydrone calibration at pH = 4 and pH = 7.
- pH meter (HM Digital) calibrated at pH = 4.01; 7.0; 10.0.
- EC (electrical conductivity) meter (HM Digital meter calibrated at EC = 1.431 mScm−1).
- Cl− ISE (Ion Selective Electrode); Bante Cl− ISE, EDT Flow Plus Combination Cl− ISE; Cole Parmer Cl− ISE attached to a Bante 931 Ion meter. Calibration was undertaken using 0.001, 0.01, 0.1, 1.0 M NaCl calibration solutions.
- Na+ ISE (Ion Selective Electrode); Bante Na ISE, Sciquip Na ISE, Cole Parmer Na ISE attached to a Bante 931 Ion meter. Calibration was undertaken using 0.001, 0.01, 0.1, 1.0 M NaCl calibration solutions.
- Temperature measurements, were made using a temperature probe, attached to a Bante 931 Ion meter.
2.5. Salinity Units
3. m-Fe:n-Fe(b)@n-C0 Polymer
- Approach A: Manufacture of the n-C0, n-Fe(b) and m-Fe0:n-Fe(b)@n-C0 desalination catalyst polymer in a bubble column diffusion reactor containing ZVI. This described in detail in reference [17].
- Approach B: Manufacture by passing a gas containing entrained n-C0 into a bubble column diffusion reactor containing ZVI. The manufacturing approach for this desalination pellet type may be published at a future date.
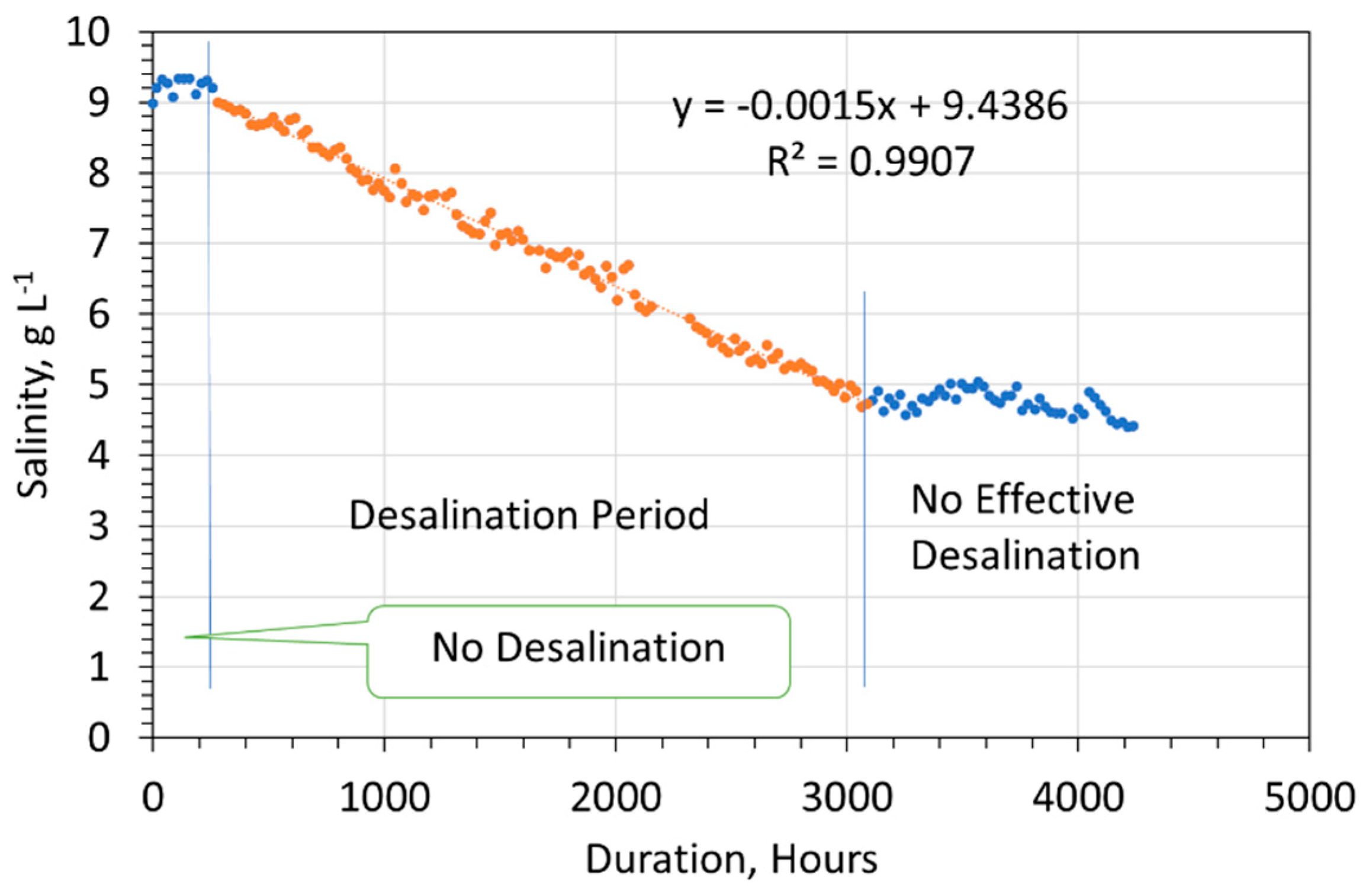
- An initial period (Tp1) where no desalination occurs, or desalination occurs at a slow rate;
- An active period (Tp2) where desalination occurs at a higher rate;
- An inactive period (Tp3) where desalination either ceases, proceeds at a slower rate, or a slow rate of resalination occurs.
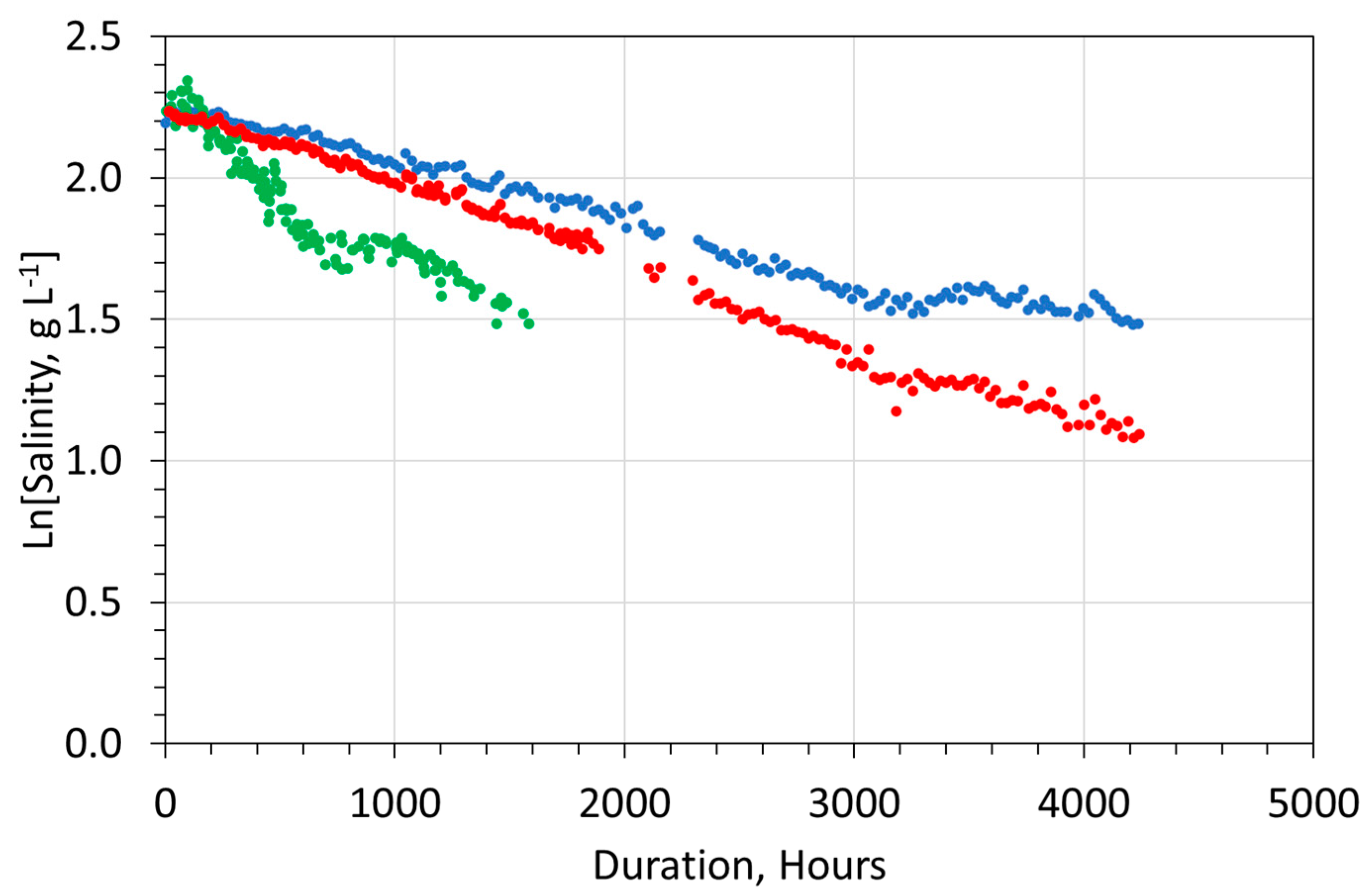
- Salinity removal during the desalination period (Figure 4) follows a zero-order reaction (Equation (11)).
4. Results Associated with Sol-Gel Polymer Formation
5. Discussion
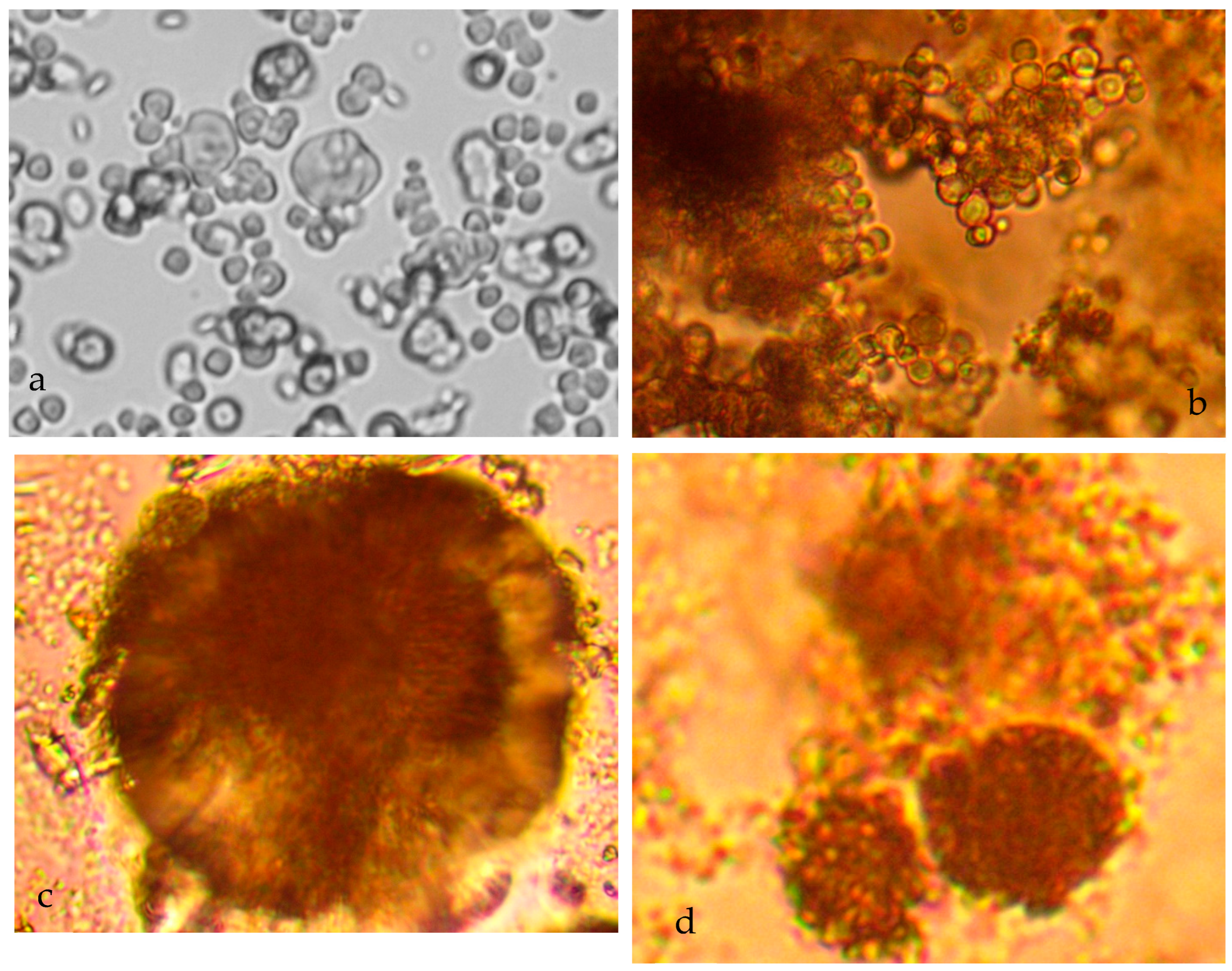
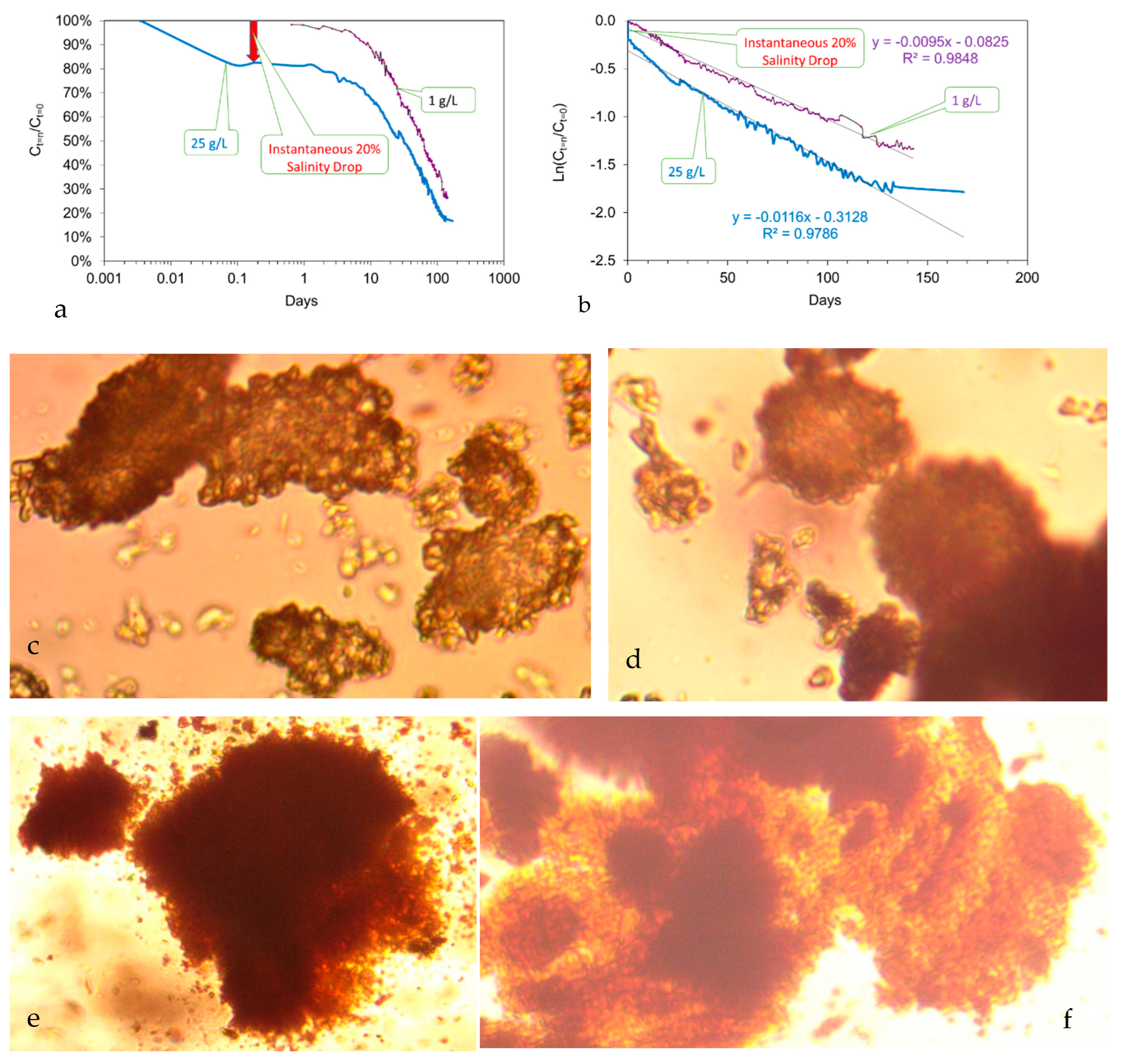
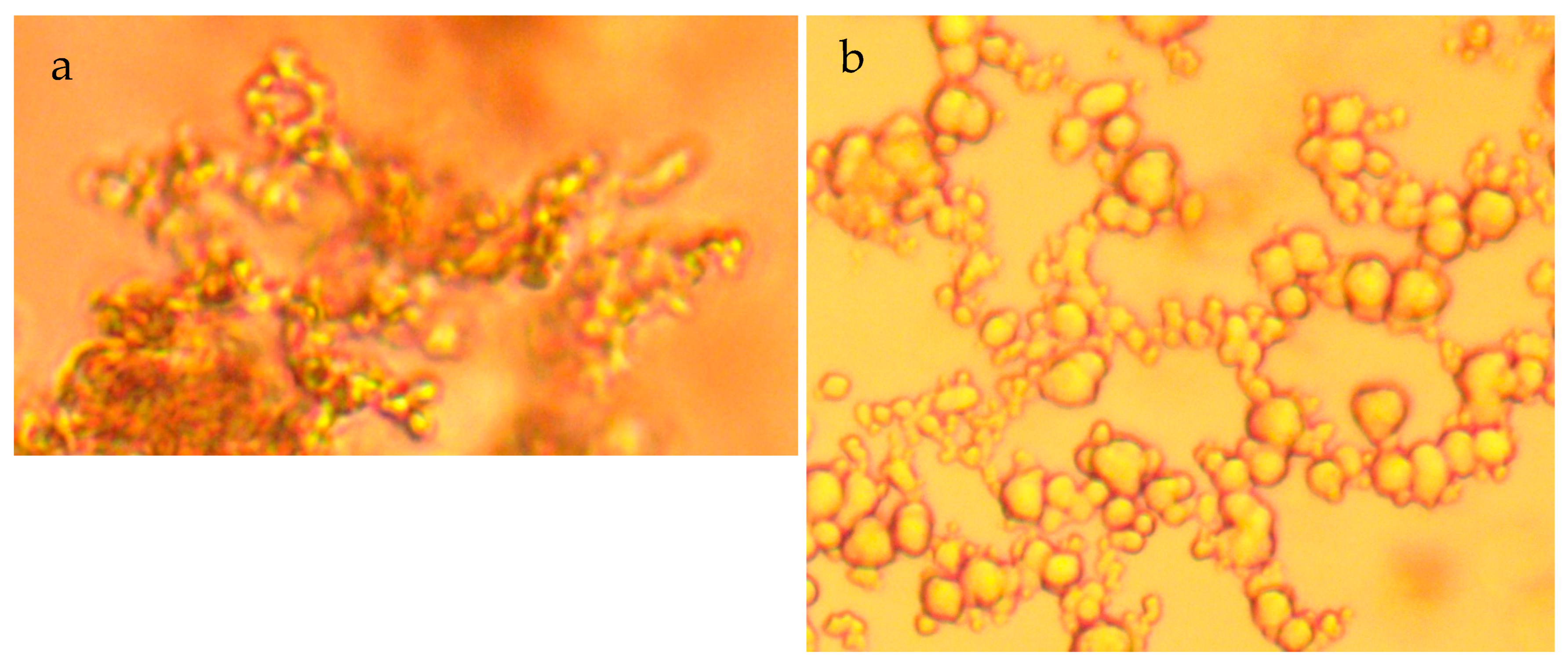
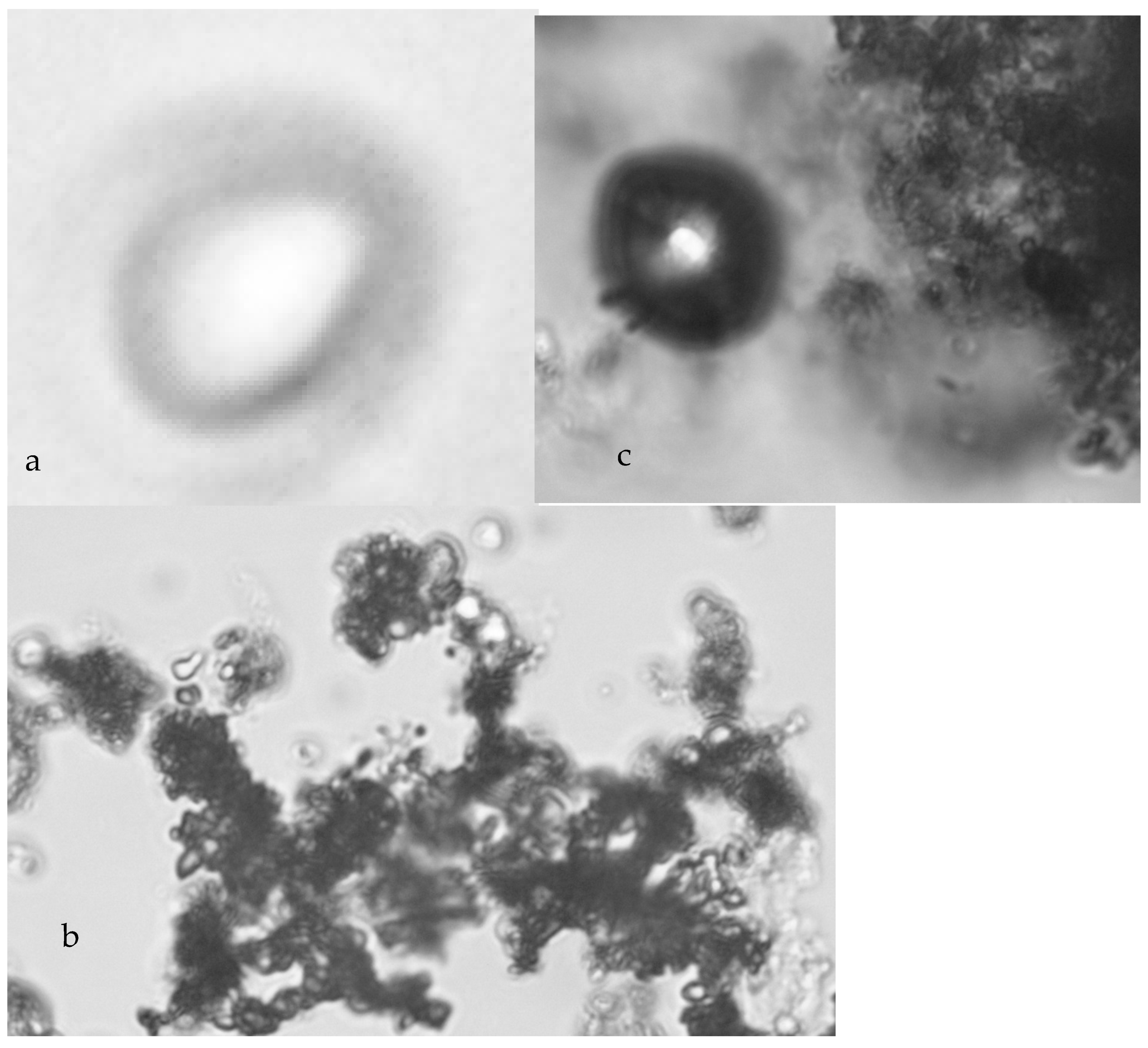
5.1. Colloid Growth
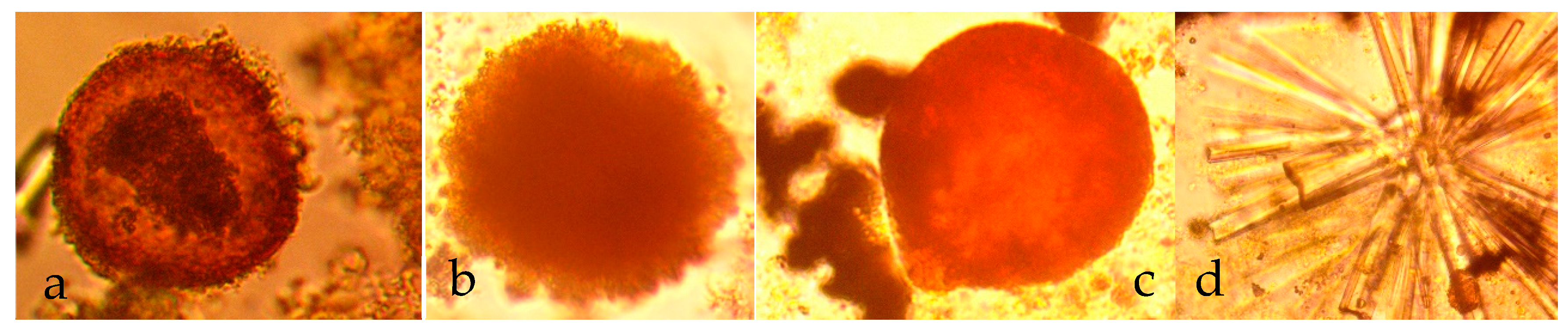
- Placement of n-Fe0 in saline water is accompanied by a near instant drop in salinity, which is associated with the formation of fluid filled micron sized spheres of n-Fe0/n-Fe(a,b) (Figure 7a–c). This instantaneous salinity change may form the underlying basis for the near instant n-ZVI desalination observed in US Patent US8636906B2; French Patent FR2983191A1; and Spanish Patent ES2,598,032, and in reference [43]. The magnitude of the near instantaneous salinity reduction may increase with increasing water salinity [43]:
- ○
- Fe0 particles of 44–80 microns, when placed in saline water, initially release Fen+ ions into the water. This is then followed by a zero-order salinity decline until a critical salinity is achieved. At this point salinity decline follows a zero-order reaction at a slower rate [44].
- ○
- Fe0 particles of 44–80 microns, when placed in saline water, in the presence of Cu0 and Al0, initially release Fen+ ions into the water. Salinity decline then follows a zero-order reaction [44]. In this example the zero-order salinity decline is associated with the removal of Fen+ ions as n-Fe(a,b,c) polymers. The combination of Fe0, Cu0 and Al0 ions slows the zero-order reaction rate when compared with Fe0 [44].
- ○
- A near instant decline in salinity is commonly associated with an initial high rate of Fe0 dissolution to form Fen+ ions [45].
- A general (first-order) decline in salinity occurs as the fluid filled spheres aggregate and coalesce (Figure 7a–c);
- ○
- Coated 50 nm Fe0 commonly shows a second-order reaction rate, in low salinity water, when an initial instant salinity decline is absent [44].
- Cessation of the salinity decline is accompanied by the restructuring of the aggregated colloids to form amorphous flocculates (Figure 7c–f).
5.1.1. Colloid Growth Kinetics
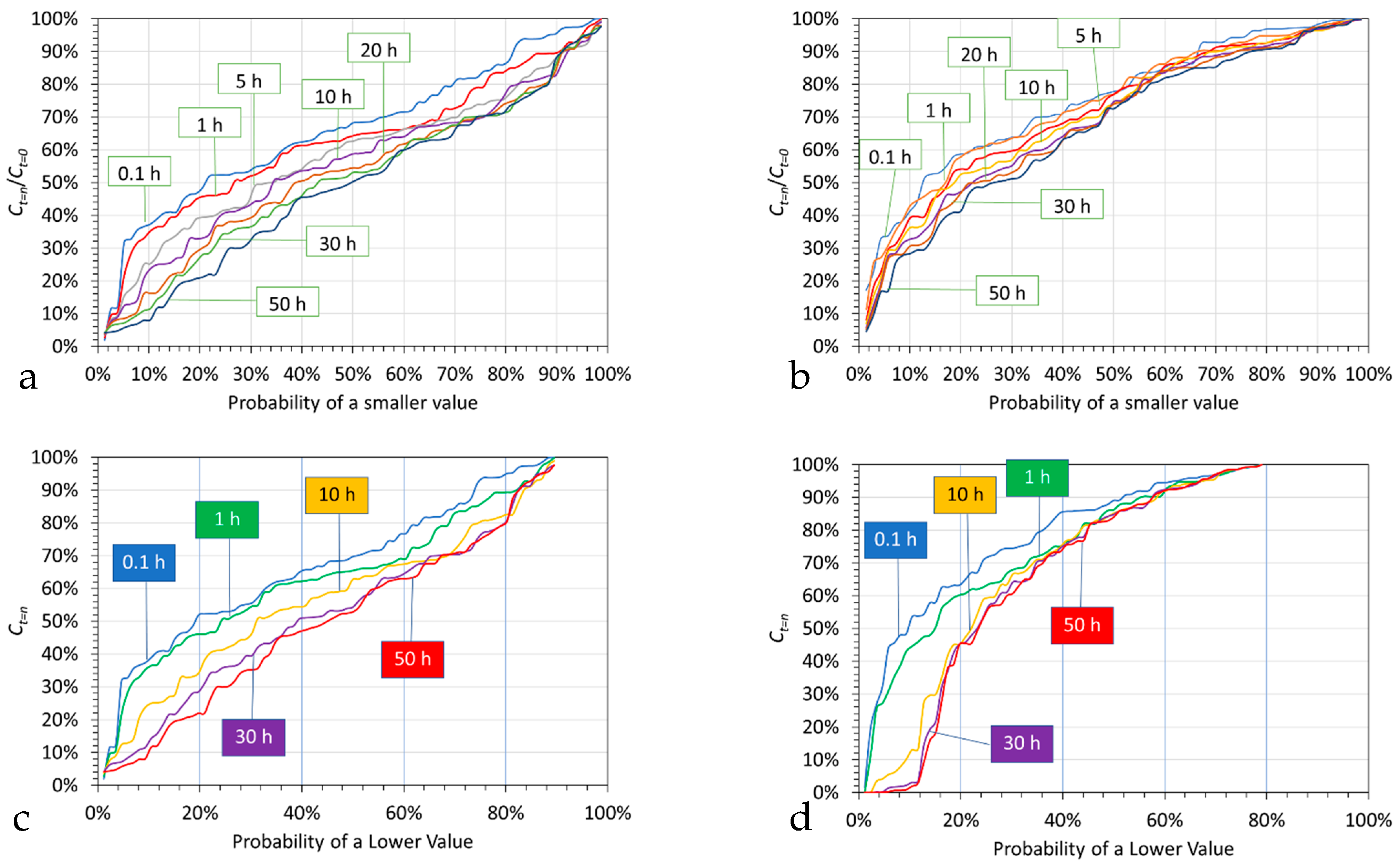
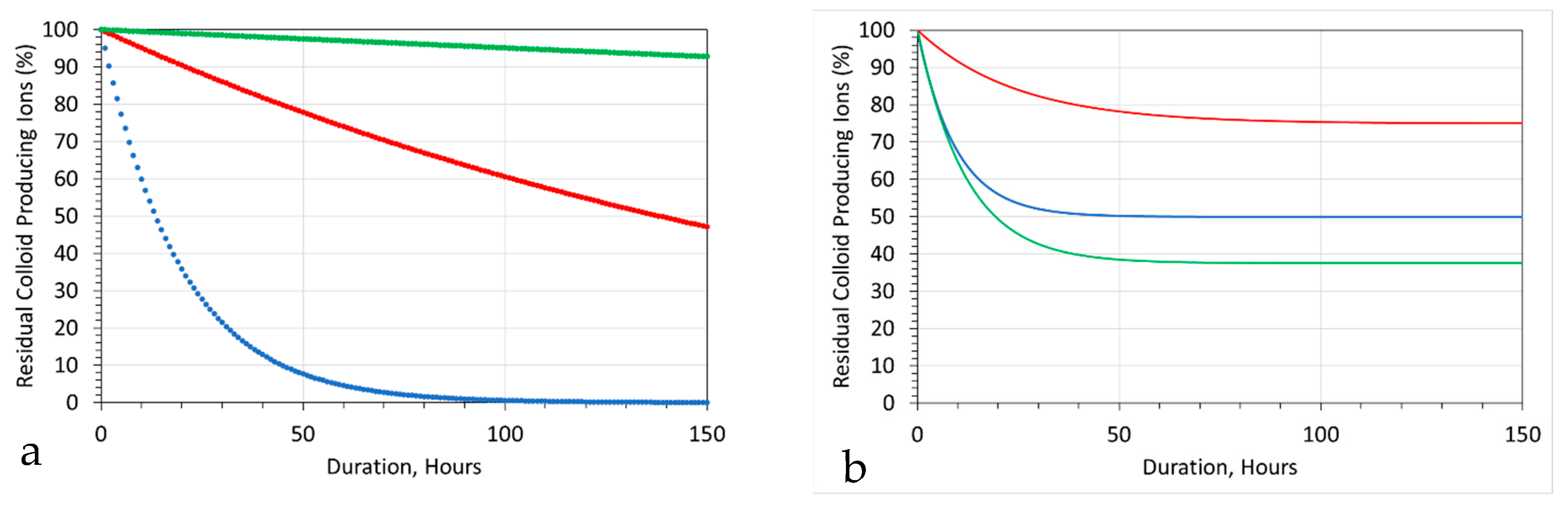
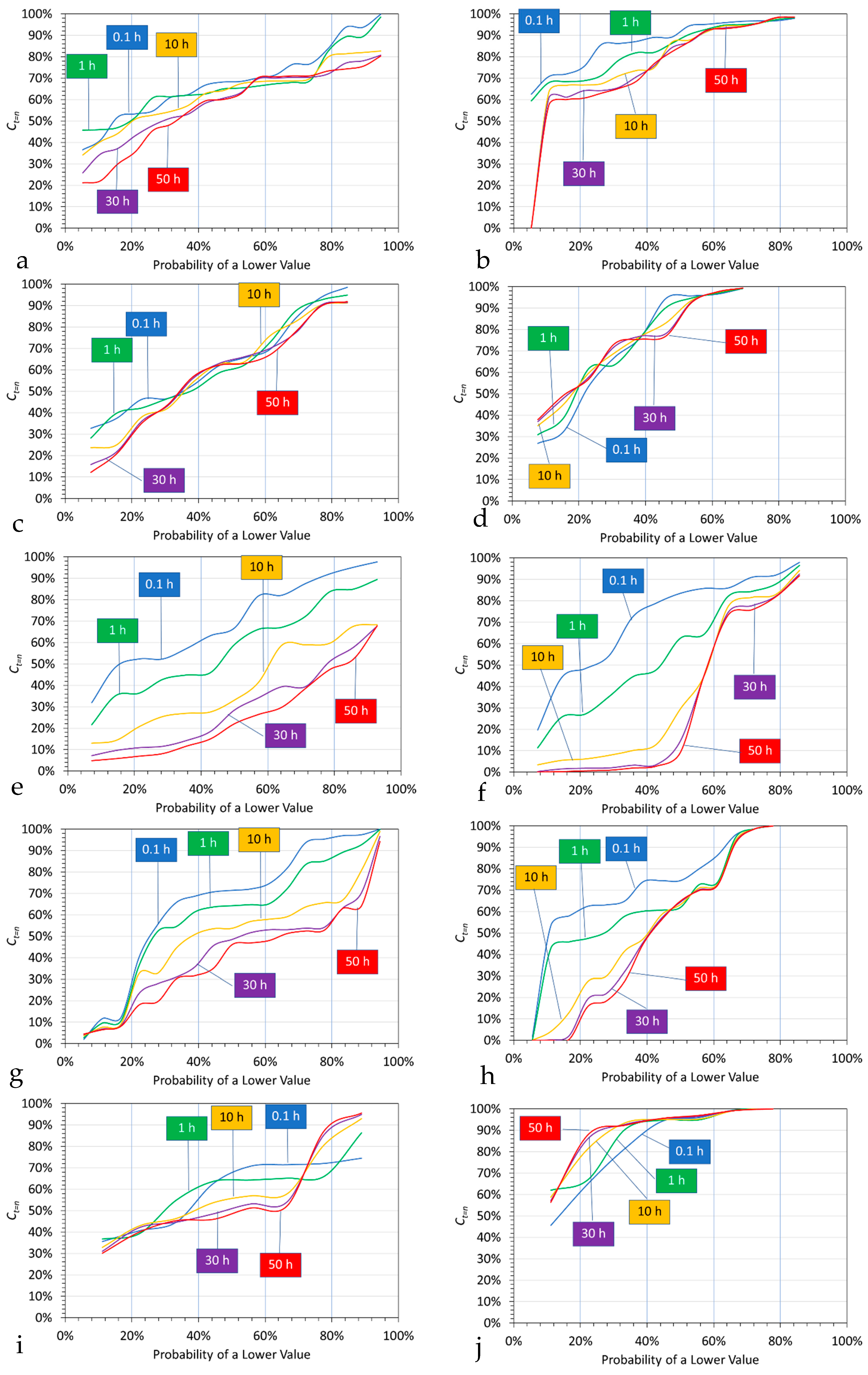
5.1.2. Probability Analysis
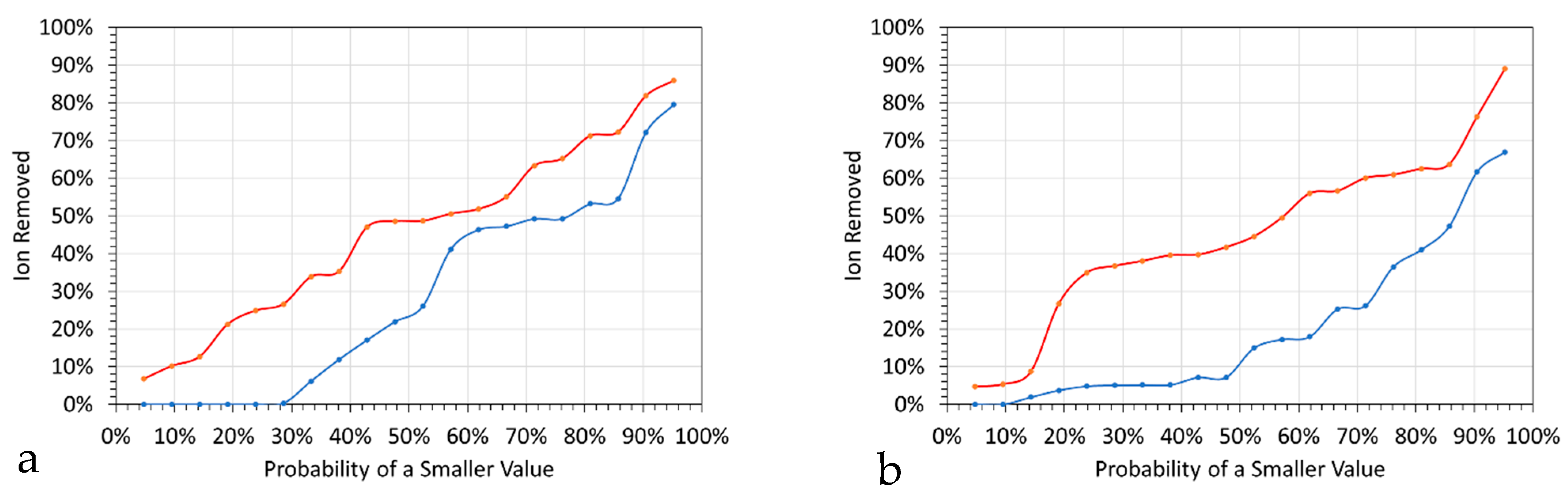
- An initial almost instant ion removal, in the majority of trials;
- An effective cessation of Cl− ion removal after 10 to 30 h;
- An effective cessation of Na+ ion removal after 1 to 10 h;
- An expectation at the 50% probability level, after 0.1 h of 10% Na+ ion removal and 30% Cl− ion removal.
5.1.3. Probability Outcomes as a Function of Polymer Type
- Mg@Al polymer (Figure 11a,b): Cl− ions are removed preferentially relative to Na+ ions. The median expectation is, after 0.1 h, 32% Cl− ion removal and 6% Na+ ion removal. Effective ion removal ceases after 10 to 30 h.
- Fe@Mg@Al polymer (Figure 11c,d): Cl− ions are removed preferentially relative to Na+ ions. The median expectation is, after 0.1 h, 37% Cl− ion removal and 5% Na+ ion removal. Effective ion removal ceases after 1 h.
- Fe@Ca@Zn polymer (Figure 11e,f): Cl− ions are removed preferentially relative to Na+ ions. The median expectation is, after 0.1 h, 33% Cl− ion removal and 17% Na+ ion removal. Effective ion removal ceases after 50 h.
- Fe@Ca polymer (Figure 11g,h): Cl− ions are removed preferentially relative to Na+ ions. The median expectation is, after 0.1 h, 29% Cl− ion removal and 25% Na+ ion removal. Effective ion removal ceases after 50 h.
- Ca polymer (Figure 11i,j): Cl− ions are removed preferentially relative to Na+ ions. The median expectation is, after 0.1 h, 29% Cl− ion removal and 3% Na+ ion removal. Effective ion removal ceases after 0.1 to 10 h.
5.1.4. Formation of Fe0
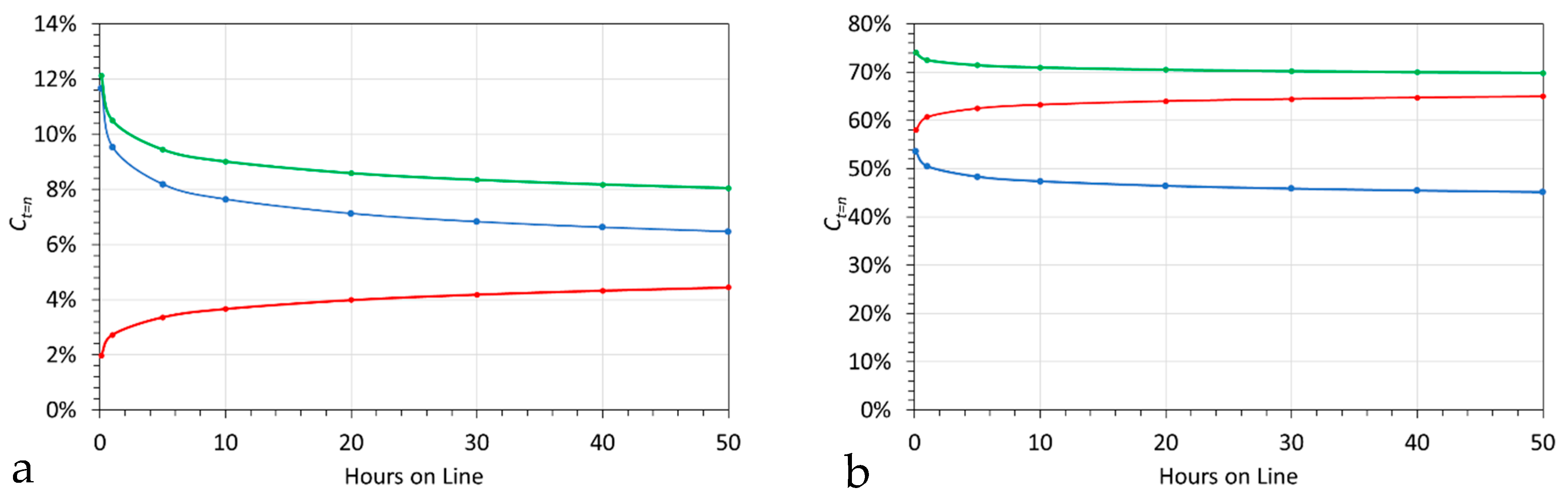
5.2. Dilution
5.2.1. Fe(a,b,c)@Ca@HCOOH Dilution Trials
5.2.2. Dilution Trials: Extracted Product Polymer Slurry Water
6. Applications
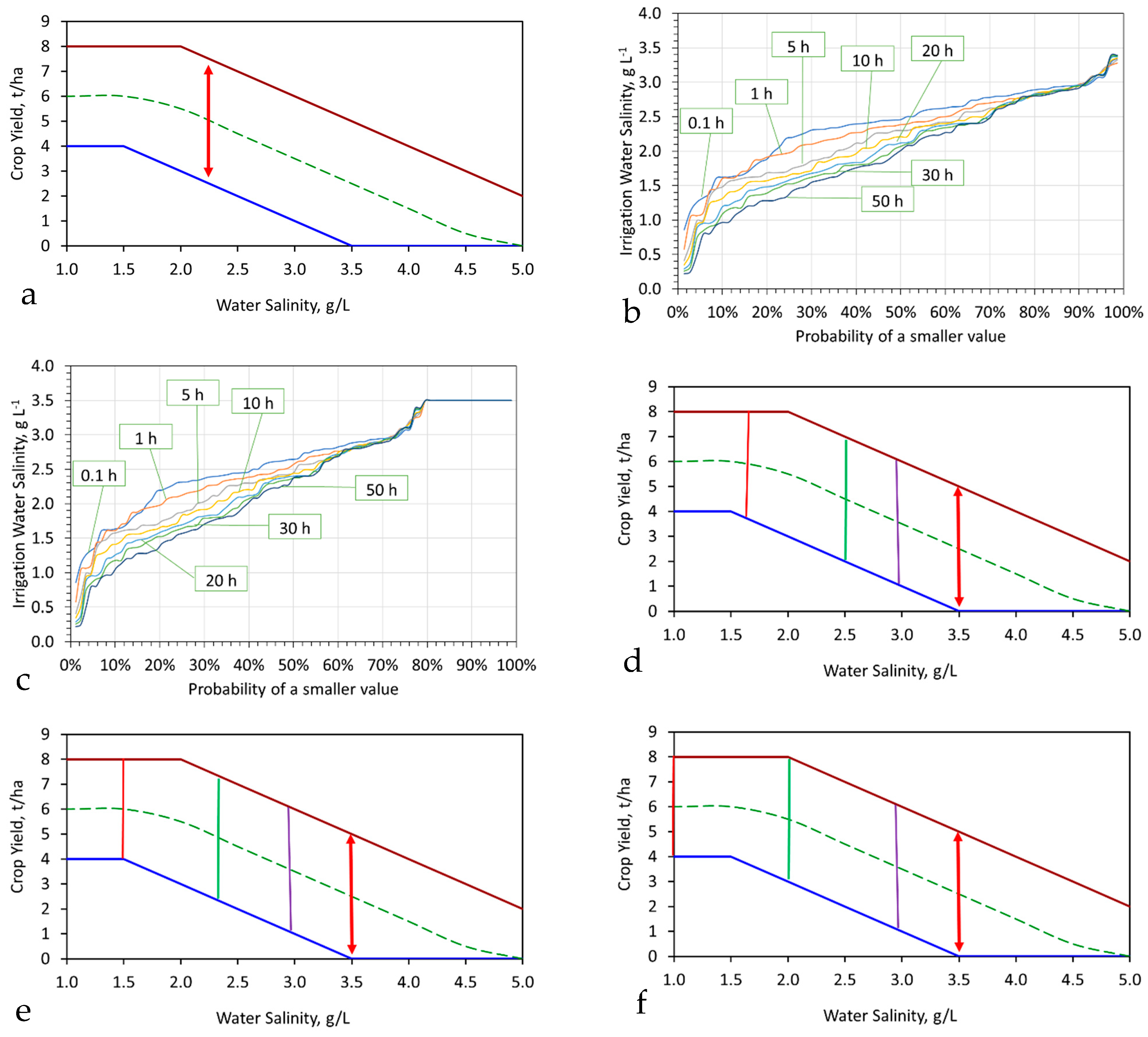
6.1. Irrigation
- Rapidly partially desalinated with a reasonable expectation of a flow line desalination of 10 to 30%;
- Placed in storage for 1 to 50 h to allow polymer formation and a slower partial desalination, with a reasonable expectation of a desalination of 15 to 50%;
- Placed in storage for >1000 h with an expectation of a higher level of desalination.
6.1.1. Example Assessment of the Impact of Desalination on Crop Yield
6.1.2. Leaf and Stem Foliage
6.1.3. Ion Removal
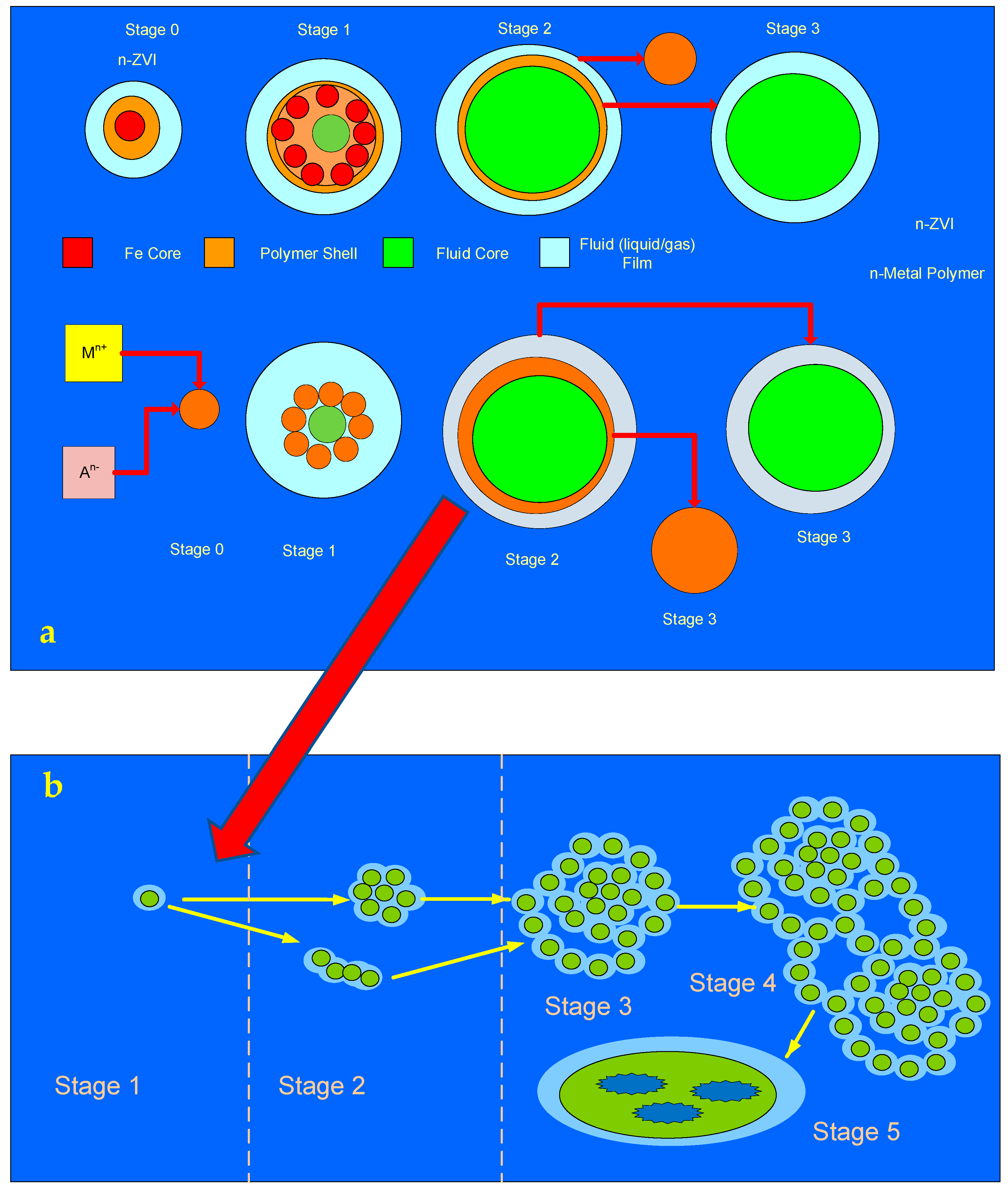
- Stage 0: n-ZVI –formation of a sol-gel nano-particle polymer;
- Stage 1—aggregation of the polymer particles to form a sphere encapsulating a fluid core;
- Stage 2—Expansion of the fluid core volume, with thinning of the polymer rim;
- Stage 3—Dissociation of the polymer rim from the fluid core resulting in collapse of the spheres to form an amorphous flocculate. The fluid core may disassociate into the surrounding water body or remain trapped within an amorphous flocculate body.
- Stage 2—Aggregation of the floating polymer spheres to form small floating clusters or chains or networks of chains;
- Stage 3—Aggregation of the small polymer clusters to form larger floating clusters;
- Stage 4—Aggregation of the larger clusters to form settled aggregated, highly porous clusters;
- Stage 5—Breakdown of the polymer spheres to form an amorphous high porosity polymer colloid or flocculate. A proportion of these pores will be dead end pores which will allow fluid and ion sequestration. Some polymer sequences will move from Stage 3 to Stage 5 without an intervening Stage 4.
| Mean | Standard Deviation | 1st Quartile | Median | 3rd Quartile | Figures | |
|---|---|---|---|---|---|---|
| n-Fe0 | 2.09 | 0.80 | 1.60 | 2.00 | 2.40 | Figure 7c |
| Fe@Ca@Zn polymer | 2.01 | 0.52 | 1.67 | 2.00 | 2.33 | Figure 6a |
| Fe@Ca@Zn polymer | 4.11 | 1.26 | 3.12 | 4.20 | 4.77 | Figure 6b |
| Fe(b)@Ca(a)@Mn(a)@Mg(a)@HCOOH polymer | 1.48 | 0.35 | 1.23 | 1.47 | 1.67 | Figure 6c |
| Fe(b)@Ca(a)@Mn(a)@Mg(a)@HCOOH polymer | 0.89 | 0.24 | 0.70 | 0.85 | 0.98 | Figure 6d |
| Fe@Ca@Mn@HCOOH polymer | 1.11 | 0.32 | 0.90 | 1.06 | 1.34 | Figure 9a |
| Fe@Ca@Mn@HCOOH@Tartaric Acid polymer | 3.61 | 1.05 | 2.95 | 3.62 | 4.16 | Figure 10 |
| Fe@Ca@Mn@HCOOH@Malic Acid polymer | 1.54 | 0.52 | 1.18 | 1.44 | 1.70 | Figure 10 |
| Fe@Ca@Mn@HCOOH@Citric Acid polymer | 1.72 | 0.49 | 1.40 | 1.67 | 1.94 | Figure 10 |
| Ca@urea polymer | 3.05 | 1.28 | 2.03 | 3.07 | 3.60 | Figure 9b |
6.2. Impact on Standard Irrigation Water Quality Indices
6.3. Treatment of Flowback Water to Form Irrigation Water
Assessment of Expected Water Salinity following Desalination
| 0.1 h | 50 h | |||||
|---|---|---|---|---|---|---|
| Cl− | Na+ | NaCl | Cl− | Na+ | NaCl | |
| Feed | 14.9 | 6.96 | 21.86 | 14.9 | 6.96 | 21.86 |
| Product | ||||||
| Mean | 10.27 | 4.97 | 15.24 | 4.05 | 3.88 | 7.92 |
| Standard Deviation | 3.35 | 1.89 | 3.89 | 3.47 | 11.09 | 11.59 |
| 1st Quartile | 7.99 | 3.96 | 12.91 | 1.04 | 0.71 | 3.89 |
| Median | 10.21 | 5.68 | 15.11 | 2.95 | 3.05 | 6.98 |
| 3rd Quartile | 13.73 | 6.45 | 18.55 | 6.07 | 5.85 | 10.03 |
| Removed | ||||||
| Mean | 31.1% | 28.6% | 30.3% | 72.9% | 44.3% | 63.8% |
| 1st Quartile | 46.4% | 43.1% | 40.9% | 93.0% | 89.8% | 82.2% |
| Median | 31.5% | 18.4% | 30.9% | 80.2% | 56.2% | 68.1% |
| 3rd Quartile | 7.8% | 7.4% | 15.1% | 59.3% | 16.0% | 54.1% |

7. Novelty
8. Conclusions
- The polymer monomers, once formed, coalesce to form colloidal fluid filled spheres;
- The colloidal fluid filled spheres act as scavenging agents and sequester Na+ and Cl− ions from the water within their structure and fluids;
- The colloidal fluid filled spheres coalesce by aggregation and capture to form chains, net structures and colloidal aggregates, with a cellular structure;
- The cellular structured colloidal aggregates restructure to form amorphous structures, and in doing so either release the contents of the fluid filled cells into the water body or aggregate them into dead-end porosity within the amorphous aggregate. Ca based cellular structures may form an outer shell of CaCO3 or CaSO4 crystals.
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Example Sol-Gel Formulations and Desalination Outcomes
Appendix A.1. Manufacture of n-Fe(b)@Ca@Mn@formate Polymer

Appendix A.1.1. Fe(b)@Ca(a)@Mn(a)@HCOOH Polymer
- Example A1a: An entrained Fe(b)@Ca(a)@Mn(a)@HCOOH polymer was constructed by mixing 1g FeSO4 + 1.67 g CaO + 2.52 g MnO2 + 1 cm3 (40%) HCOOH L−1 with 2.3 L of feed water (Cl− = 26.55 g L−1; Na+ = 17.21 g L−1 (sea water)). The water was allowed to rest for 241 h at a temperature of 4.8 to 7.0 °C. The product water contained Cl− = 1.35 g L−1; Na+ = 0.78 g L−1; Removal: Cl− = 94.91%; Na+ = 95.46%.
- Example A1b: This trial constructed the polymer from 1g FeSO4 + 3.34 g CaO + 2.52 g MnO2 + 1 cm3 (40%) HCOOH L−1 with the feed water (Cl− = 26.55 g L−1; Na+ = 17.21 g L−1 (seawater)). The water (2.3 L) was allowed to rest for 241 h at a temperature of 4.8 to 7.0 °C. The product water contained Cl− = 9.64 g L−1; Na+ = 2.50 g L−1; Removal: Cl− = 63.19%; Na+ = 85.47%.
Appendix A.1.2. Fe(b)@Ca(a)@Mn(a)@K@HCOOH Polymer
- Example A2a: An entrained Fe(b)@Ca(a)@Mn(a)@K@HCOOH polymer was constructed by mixing 1g FeSO4 + 1.67 g CaO + 2.52 g MnO2 + 1.22 g K2CO3 + 1 cm3 (40%) HCOOH L−1 with the feed water (Cl− = 26.55 g L−1; Na+ = 17.21 g L−1; (seawater)). The water (2.3 L) was allowed to rest for 241 h at a temperature of 4.8 to 7.0 °C. The product water contained Cl− = 5.66 g L−1; Na+ = 2.97 g L−1. Removal: Cl− = 78.68%; Na+ = 82.74%.
- Example A2b: The entrained polymer was constructed from 1g FeSO4 + 3.34 g CaO + 2.52 g MnO2 + 1.22 g K2CO3 + 1.36 g CaCO3 + 0.9 g C4H6O6 + 1 cm3 (40%) HCOOH L−1 + feed water (Cl− = 22.11 g L−1; Na+ = 14.43 g L−1; (seawater)). The water (2.3 L) was allowed to rest for 216 h at a temperature of 4.0 to 7.1 °C. The product water contained Cl− = 1.04 g L−1; Na+ = 5.93 g L−1. Removal: Cl− = 95.29%; Na+ = 58.90%.
- Example A2c: The entrained polymer was constructed from 1g FeSO4 + 1.67 g CaO + 2.52 g MnO2 + 1.22 g K2CO3 + 2.72 g CaCO3 + 0.8 g C4H6O5 + 1 cm3 (40%) HCOOH L−1 + feed water (Cl− = 22.11 g L−1; Na+ = 14.43 g L−1). The water (2.3 L) was allowed to rest for 216 h at a temperature of 4.6 to 7.1 °C. The product water contained Cl− = 1.00 g L−1; Na+ = 3.25 g L−1. Removal: Cl− = 95.47%; Na+ = 77.48%.
- Example A2d: The entrained polymer was constructed from 1g FeSO4 + 1.67 g CaO + 2.52 g MnO2 + 1.22 g K2CO3 + 4.07 g CaCO3 + 0.83 g C6H8O7 + 0.83 g C6H8O7 + 1 cm3 (40%) HCOOH L−1 + feed water (Cl− = 22.11 g L−1; Na+ = 14.43 g L−1). The water (2.3 L) was allowed to rest for 216 h at a temperature of 4.6 to 7.1 °C. The product water contained Cl− = 1.99 g L−1; Na+ = 3.18 g L−1. Removal: Cl− = 90.99%; Na+ = 77.96%.
Appendix A.1.3. Fe(b)@Ca(a)@Mn(a)@Mg(a)@HCOOH Polymer
Appendix A.1.4. Fe(b)@Ca(a)@Mn(a)@Mg(a)@Zn(a)@HCOOH Polymer
Appendix A.1.5. Fe(b)@Ca(a)@Zn(a)@HCOOH Polymer
Appendix A.2. Manufacture of @ C4H6O6 Polymers
Appendix A.2.1. Fe(b)@ C4H6O6 Polymer
Appendix A.2.2. Fe(b)@Ca(a)@K@C4H6O6 Polymer
Appendix A.2.3. Fe(b)@Ca(a)@Mn(a)@K@C4H6O6 Polymer
Appendix A.2.4. Fe(b)@Ca(a)@Zn(a)@C4H6O6 Polymer
Appendix A.3. Manufacture of M@ C6H8O7 Polymers
Appendix A.3.1. Fe(b)@C6H8O7 polymer
Appendix A.3.2. Fe(b)@Ca(a)@K@C6H8O7 Polymer
Appendix A.3.3. Fe(b)@Ca(a)@Zn(a)@C6H8O7 Polymer
Appendix A.3.4. Fe(b)@Ca(a)Mn(a)@Mg(a)@Zn(a)@C6H8O7 Polymer
Appendix A.4. Manufacture of @ C6H8O7 Polymers
Appendix A.4.1. Fe(b)@Ca(a)@Zn(a)@C2H4O2 Polymer
Appendix A.4.2. Fe(b)@C4H6O5 Polymer
Appendix A.4.3. Fe(b)@Ca(a)@K@C4H6O5 Polymer
Appendix A.4.4. Fe(b)@Ca(a)@Mn(a)@K@C4H6O5 Polymer
Appendix A.4.5. Fe(b)@Ca(a)@Zn(a)@C4H6O5 Polymer
Appendix A.5. Manufacture of n-Fe(b)@bluecrop Polyphenol Polymer
- An initial first-order desalination reaction, over the initial 10 to 20 h, which removed Na+ and Cl− ions at a slow rate. [A] = ln(Ct=0) or [A] < ln(Ct=0).
- A first-order desalination reaction, over the period [10 to 300 h], which removed Na+ and Cl− ions at a relatively fast rate. [A] > ln(Ct=0).
- After 300 h:
- ○
- Berry juice polyphenol: A first-order desalination reaction, over the period [>300 h], which removed Na+ and Cl− ions at a relatively slow rate. [A] < ln(Ct=0).
- ○
- Leaf or stem polyphenol: A first-order desalination reaction, over the period [>300 h], which added Na+ and Cl− ions at a relatively slow rate. [A] < ln(Ct=0).

Appendix A.5.1. n-Fe(b)@bluecrop Polyphenol Polymer Supported on Activated Carbon Pellets
- Initially no desalination, or a low level of desalination occurs. Desalination, when present can be described using a first-order reaction where [A] = ln(Ct=0) or [A] < ln(Ct=0);
- After a critical time period, Tc, the pellets start to desalinate the water. The desalination process appears to be a faster first-order reaction where [A] > ln(Ct=0);
- ○
- Tc increases with decreasing water salinity;
- ○
- The desalination rate constant decreases with decreasing water salinity;
- After a critical time period, Td, the pellets cease desalinating the water.

Appendix A.5.2. Comparison of n-Fe(b)@bluecrop Polyphenol Polymer Pellets with m-Fe0:n-Fe(b)@n-C0 Pellets
Appendix A.6. n-Fe(b)@tomato Polyphenol Polymer
- Initially no desalination, or a low level of desalination occurs (Supplementary Information, Figure S7);
- After a critical time period, Tc, the pellets start to desalinate the water. Tc is about 200 h. The desalination process appears to be a first-order reaction (Supplementary Information Figure S7).
Appendix A.7. n-Fe(b)@urea Polymers
Appendix A.7.1. Activated n-Fe(b)@urea@Ca(b) Polymer
Appendix A.7.2. Activated n-Ca(b)@urea Polymer
Appendix A.8. n-Al(b) Polymers
Appendix A.8.1. n-Al(b)@Mg(a) Polymer
Appendix A.8.2. n-Ca(a)@Mn(a)@Al(b) Polymer
Appendix A.8.3. n-Ca(a)@Al(b) Polymer
Appendix A.8.4. n-Ca(a)@Zn(a)@Al(b) Polymer
Appendix A.8.5. n-Ca(a)@Mg(a)@Al(b) Polymer
Appendix A.8.6. Mg(a)@K@Al(b) Polymer
Appendix A.8.7. Mn(a)@Zn(a)@Al(b) Polymer
Appendix A.8.8. Mg(a)@Mn(a)@K@Al(b) Polymer
Appendix A.8.9. Mg(a)@Mn(a)@Al(b) Polymer
Appendix A.8.10. Zn(a)@Al(b) Polymer
Appendix A.9. Fe(b) Polymers
Appendix A.9.1. Fe(b)@Ca(a)@Zn(a)@Al(b) Polymer
Appendix A.9.2. Fe(b)@Mg(a)@K@Al(b) Polymer
Appendix A.9.3. Fe(b)@Mg(a)@Al(b) Polymer
Appendix A.9.4. Fe(b)@Mg(a@Zn(a))@Al(b) Polymer
Appendix A.9.5. Fe(b)@Mg(a)@Ca(a)@Mn(a)@Zn(a)@Al(b) Polymer
Appendix A.9.6. Fe(b)@Mg(a)@Ca(a)@Al(b) Polymer
Appendix A.9.7. Fe(b)@Mg(a)@Urea Polymer
Appendix A.9.8. Fe(b)@Mg(a)@K@Urea Polymer
Appendix A.9.9. Fe(b)@Mg(a) Polymer
Appendix A.9.10. Fe(b) Polymer
Appendix A.9.11. Fe(b)@Mg(a)@Ca(a)@K feldspar Polymer
Appendix A.9.12. Fe(b)@Mg(a)@Ca(a) Polymer
Appendix A.9.13. Fe(b)@Mg(a)@Ca(a)@Zn(a) Polymer
Appendix A.9.14. Fe(b)@Mg(a)@Ca(a)@K@Zn(a) Polymer
Appendix A.9.15. Fe(b)@Zn(a) Polymer
Appendix A.9.16. Fe(b)@Ca(a)@Al0@Zn(a) Polymer
Appendix A.10. Fe(b)@Ca(a)@gallic Acid Polymers
Appendix B. Polymer Manufacture and Operation Tables
| Trial | hrs | pH | Eh, mV | T, °C | Cl−, g/L | Na+, g/L | NaCl, g/L |
|---|---|---|---|---|---|---|---|
| 1 | 0 | 8.31 | 372 | 4.8 | 26.55 | 17.21 | 43.76 |
| 2 | 0 | 8.31 | 372 | 4.8 | 26.55 | 17.21 | 43.76 |
| 3 | 0 | 8.31 | 372 | 4.8 | 26.55 | 17.21 | 43.76 |
| 4 | 0 | 8.31 | 372 | 4.8 | 26.55 | 17.21 | 43.76 |
| 5 | 0 | 8.69 | 39 | 4.0 | 22.11 | 14.43 | 36.54 |
| 6 | 0 | 8.69 | 39 | 4.0 | 22.11 | 14.43 | 36.54 |
| 7 | 0 | 8.69 | 39 | 4.0 | 22.11 | 14.43 | 36.54 |
| 8 | 0 | 8.69 | 39 | 4.5 | 22.11 | 14.43 | 36.54 |
| 9 | 0 | 8.69 | 39 | 4.6 | 22.11 | 14.43 | 36.54 |
| 10 | 0 | 8.69 | 39 | 4.6 | 22.11 | 14.43 | 36.54 |
| 11 | 0 | 8.69 | 39 | 5.1 | 22.11 | 14.43 | 36.54 |
| 12 | 0 | 8.69 | 39 | 5.5 | 22.11 | 14.43 | 36.54 |
| 13 | 0 | 8.69 | 39 | 4.6 | 22.11 | 14.43 | 36.54 |
| 14 | 0 | 8.69 | 39 | 4.6 | 22.11 | 14.43 | 36.54 |
| 15 | 0 | 8.57 | 249 | 5.2 | 23.22 | 19.83 | 43.05 |
| 16 | 0 | 8.75 | 246 | 6.1 | 19.52 | 13.36 | 32.88 |
| 17 | 0 | 8.70 | 233 | 6.4 | 26.40 | 15.83 | 42.23 |
| 18 | 0 | 9.33 | 177 | 10.6 | 26.11 | 19.57 | 45.68 |
| 19 | 0 | 9.49 | 154 | 10.9 | 101.48 | 10.51 | 111.99 |
| 20 | 0 | 9.37 | 265 | 10.0 | 47.76 | 21.49 | 69.25 |
| 21 | 0 | 9.17 | 229 | 8.7 | 25.47 | 23.95 | 49.42 |
| 22 | 0 | 9.58 | 173 | 7.8 | 17.40 | 18.73 | 36.13 |
| 23 | 0 | 9.54 | 228 | 7.4 | 19.94 | 8.47 | 28.41 |
| 24 | 0 | 9.51 | 181 | 6.9 | 10.44 | 6.33 | 16.77 |
| 25 | 0 | 9.78 | 152 | 6.3 | 2.76 | 1.23 | 3.99 |
| 26 | 0 | 9.76 | 144 | 7.4 | 3.49 | 1.85 | 5.34 |
| 27 | 0 | 9.54 | 162 | 7.6 | 4.40 | 1.79 | 6.19 |
| 28 | 0 | 9.06 | 196 | 12.0 | 9.40 | 4.98 | 14.38 |
| 29 | 0 | 9.05 | 215 | 9.1 | 12.14 | 3.13 | 15.27 |
| 30 | 0 | 8.69 | 255 | 9.2 | 22.11 | 14.33 | 36.44 |
| 31 | 0 | 8.69 | 255 | 9.2 | 22.11 | 14.33 | 36.44 |
| 32 | 0 | 8.69 | 255 | 9.2 | 22.11 | 14.33 | 36.44 |
| 33 | 0 | 8.81 | 344 | 12.5 | 30.14 | 18.38 | 48.52 |
| 34 | 0 | 8.81 | 344 | 12.5 | 30.14 | 18.38 | 48.52 |
| 35 | 0 | 8.81 | 344 | 12.5 | 30.14 | 18.38 | 48.52 |
| 36 | 0 | 10.35 | 84 | 15.0 | 11.92 | 5.41 | 17.33 |
| 37 | 0 | 10.42 | 74 | 13.7 | 3.07 | 1.41 | 4.48 |
| 38 | 0 | 9.07 | 159 | 11.6 | 8.14 | 4.31 | 12.45 |
| 39 | 0 | 8.56 | 289 | 13.8 | 30.47 | 10.30 | 40.77 |
| 40 | 0 | 8.56 | 289 | 13.8 | 30.47 | 10.30 | 40.77 |
| 41 | 0 | 8.55 | 259 | 13.8 | 25.36 | 16.44 | 41.80 |
| 42 | 0 | 10.31 | 81 | 13.8 | 14.50 | 9.99 | 24.49 |
| 43 | 0 | 10.17 | 85 | 13.8 | 14.50 | 9.99 | 24.49 |
| 44 | 0 | 9.13 | 155 | 14.1 | 1.22 | 1.52 | 2.74 |
| 45 | 0 | 9.86 | 187 | 13.9 | 10.57 | 10.10 | 20.67 |
| 46 | 0 | 8.64 | 303 | 13.7 | 57.04 | 45.57 | 102.61 |
| 47 | 0 | 11.35 | 36 | 18.4 | 2.19 | 7.01 | 9.20 |
| 48 | 0 | 12.06 | −301 | 18.1 | 33.17 | 19.96 | 53.13 |
| 49 | 0 | 12.68 | −474 | 16.3 | 11.54 | 16.77 | 28.31 |
| 50 | 0 | 12.68 | −474 | 16.3 | 11.54 | 16.77 | 28.31 |
| 51 | 0 | 9.78 | 172 | 16.8 | 24.72 | 19.50 | 44.22 |
| 52 | 0 | 10.67 | 72 | 17.2 | 1.48 | 0.70 | 2.18 |
| 53 | 0 | 9.91 | 166 | 18.2 | 2.06 | 1.53 | 3.59 |
| 54 | 0 | 9.96 | 201 | 17.3 | 19.50 | 14.75 | 34.25 |
| 55 | 0 | 9.96 | 201 | 17.3 | 19.50 | 14.75 | 34.25 |
| 56 | 0 | 9.53 | 226 | 17.5 | 57.26 | 44.14 | 101.40 |
| 57 | 0 | 9.53 | 226 | 17.5 | 57.26 | 44.14 | 101.40 |
| 58 | 0 | 8.86 | 274 | 14.5 | 19.17 | 9.76 | 28.93 |
| 59 | 0 | 8.86 | 274 | 15.5 | 19.17 | 9.76 | 28.93 |
| 60 | 0 | 8.29 | 326 | 16.3 | 1.24 | 0.82 | 2.06 |
| 61 | 0 | 8.29 | 326 | 16.3 | 1.24 | 0.82 | 2.06 |
| 62 | 0 | 8.49 | 360 | 14.6 | 24.67 | 13.23 | 37.90 |
| 63 | 0 | 8.49 | 360 | 14.6 | 24.67 | 13.23 | 37.90 |
| 64 | 0 | 8.13 | 405 | 15.2 | 19.30 | 11.18 | 30.48 |
| 65 | 0 | 8.13 | 405 | 15.2 | 19.30 | 11.18 | 30.48 |
| 66 | 0 | 9.80 | 194 | 14.2 | 49.31 | 15.40 | 64.71 |
| 67 | 0 | 9.80 | 194 | 14.2 | 49.31 | 15.40 | 64.71 |
| 68 | 0 | 8.84 | 317 | 15.2 | 21.87 | 17.74 | 39.61 |
| 69 | 0 | 8.84 | 317 | 15.2 | 21.87 | 17.74 | 39.61 |
| 70 | 0 | 8.72 | 321 | 17.2 | 14.50 | 16.04 | 30.54 |
| 71 | 0 | 8.72 | 321 | 17.2 | 14.50 | 16.04 | 30.54 |
| 72 | 0 | 8.68 | 308 | 14.2 | 28.10 | 14.73 | 42.83 |
| 73 | 0 | 8.68 | 308 | 14.2 | 28.10 | 14.73 | 42.83 |
| 74 | 0 | 9.60 | 230 | 15.2 | 10.92 | 7.57 | 18.49 |
| 75 | 0 | 9.60 | 230 | 15.2 | 10.92 | 7.57 | 18.49 |
| 76 | 0 | 8.96 | 297 | 14.9 | 6.41 | 3.96 | 10.37 |
| 77 | 0 | 8.96 | 297 | 14.9 | 6.41 | 3.96 | 10.37 |
| 78 | 0 | 8.92 | 343 | 15.7 | 10.59 | 9.47 | 20.06 |
| 79 | 0 | 8.92 | 343 | 15.7 | 10.59 | 9.47 | 20.06 |
| 80 | 0 | 9.06 | 423 | 15.6 | 11.48 | 15.61 | 27.09 |
| 81 | 0 | 9.06 | 423 | 15.6 | 11.48 | 15.61 | 27.09 |
| 82 | 0 | 9.79 | 360 | 16.0 | 9.60 | 7.54 | 17.14 |
| 83 | 0 | 9.79 | 360 | 16.0 | 9.60 | 7.54 | 17.14 |
| 84 | 0 | 9.67 | 361 | 17.9 | 9.68 | 9.50 | 19.18 |
| 85 | 0 | 9.67 | 361 | 17.9 | 9.68 | 9.50 | 19.18 |
| 86 | 0 | 9.80 | 347 | 16.8 | 7.96 | 7.74 | 15.70 |
| 87 | 0 | 9.80 | 347 | 16.8 | 7.96 | 7.74 | 15.70 |
| Product | Removed | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Trial | hrs | pH | Eh, mV | T, °C | Cl−, g/L | Na+, g/L | NaCl, g/L | Cl− | Na+ | NaCl |
| 1 | 241 | 11.85 | −45 | 7.0 | 1.35 | 0.78 | 2.13 | 94.92% | 95.47% | 95.13% |
| 2 | 241 | 12.23 | −74 | 7.0 | 5.66 | 2.97 | 8.63 | 78.68% | 82.74% | 80.28% |
| 3 | 241 | 9.31 | 193 | 7.0 | 1.55 | 8.75 | 10.30 | 94.16% | 49.16% | 76.46% |
| 4 | 241 | 12.54 | −98 | 7.0 | 9.64 | 2.50 | 12.14 | 63.69% | 85.47% | 72.26% |
| 5 | 0.1 | 2.9 | 868 | 3.8 | 1.51 | 14.43 | 15.94 | 93.17% | 0.00% | 56.38% |
| 6 | 0.1 | 6.2 | 24 | 4.3 | 1.93 | 12.07 | 14.00 | 91.27% | 16.35% | 61.69% |
| 7 | 216 | 9.37 | 172 | 7.1 | 1.04 | 5.93 | 6.97 | 95.30% | 58.91% | 80.93% |
| 8 | 0.1 | 3.42 | 805 | 4.5 | 11.67 | 14.43 | 26.10 | 47.22% | 0.00% | 28.57% |
| 9 | 0.1 | 6.91 | 29 | 4.6 | 6.61 | 7.84 | 14.45 | 70.10% | 45.67% | 60.45% |
| 10 | 216 | 10.11 | 61 | 7.1 | 1.00 | 3.25 | 4.25 | 95.48% | 77.48% | 88.37% |
| 11 | 0.1 | 3.83 | 723 | 5.1 | 1.33 | 14.33 | 15.66 | 93.98% | 0.69% | 57.14% |
| 12 | 0.1 | 6.57 | 117 | 5.5 | 1.29 | 12.82 | 14.11 | 94.17% | 11.16% | 61.38% |
| 13 | 216 | 10.34 | 37 | 7.1 | 1.99 | 3.18 | 5.17 | 91.00% | 77.96% | 85.85% |
| 14 | 96 | 11.18 | 26 | 3.8 | 3.76 | 3.77 | 7.53 | 82.99% | 73.87% | 79.39% |
| 15 | 24 | 12.28 | −103 | 5.2 | 0.97 | 3.32 | 4.29 | 95.82% | 83.26% | 90.03% |
| 16 | 24 | 12.16 | −98 | 6.1 | 6.12 | 3.46 | 9.58 | 68.65% | 74.10% | 70.86% |
| 17 | 24 | 12.15 | −345 | 6.4 | 3.03 | 1.09 | 4.12 | 88.52% | 93.11% | 90.24% |
| 18 | 48 | 11.96 | −26 | 7.8 | 18.10 | 3.75 | 21.85 | 30.68% | 80.84% | 52.17% |
| 19 | 24 | 9.91 | 284 | 8.8 | 7.27 | 6.46 | 13.73 | 92.84% | 38.53% | 87.74% |
| 20 | 24 | 12.33 | −45 | 8.4 | 9.15 | 9.42 | 18.57 | 80.84% | 56.17% | 73.18% |
| 21 | 24 | 11.72 | −35 | 10.7 | 25.18 | 9.00 | 34.18 | 1.14% | 62.42% | 30.84% |
| 22 | 24 | 11.22 | 63 | 6.1 | 8.71 | 9.37 | 18.08 | 49.94% | 49.97% | 49.96% |
| 23 | 24 | 13.1 | −81 | 4.8 | 6.01 | 5.57 | 11.58 | 69.86% | 34.24% | 59.24% |
| 24 | 24 | 11.87 | −4 | 4.1 | 4.51 | 4.91 | 9.42 | 56.80% | 22.43% | 43.83% |
| 25 | 24 | 13.08 | −80 | 6.0 | 0.80 | 1.23 | 2.03 | 71.01% | 0.00% | 49.12% |
| 26 | 24 | 12.42 | −37 | 8.6 | 1.39 | 1.60 | 2.99 | 60.17% | 13.51% | 44.01% |
| 27 | 24 | 12.51 | −23 | 8.3 | 2.32 | 1.74 | 4.06 | 47.27% | 2.79% | 34.41% |
| 28 | 24 | 12.82 | −77 | 11.1 | 5.86 | 3.30 | 9.16 | 37.66% | 33.73% | 36.30% |
| 29 | 24 | 12.87 | −66 | 9.2 | 6.50 | 3.13 | 9.63 | 46.46% | 0.00% | 36.94% |
| 30 | 1344 | 7.97 | 354 | 6.2 | 1.34 | 6.65 | 7.99 | 93.94% | 53.59% | 78.07% |
| 31 | 1344 | 8.66 | 282 | 6.2 | 2.32 | 3.76 | 6.08 | 89.51% | 73.76% | 83.32% |
| 32 | 1398 | 9.62 | 184 | 6.2 | 2.93 | 3.29 | 6.22 | 86.75% | 77.04% | 82.93% |
| 33 | 0.08 | 6.19 | 326 | 12.3 | 12.46 | 18.38 | 30.84 | 58.66% | 0.00% | 36.44% |
| 34 | 0.08 | 6.61 | 170 | 12.3 | 24.67 | 11.38 | 36.05 | 18.15% | 38.08% | 25.70% |
| 35 | 72 | 12.96 | −131 | 16.4 | 9.43 | 11.22 | 20.65 | 68.71% | 38.96% | 57.44% |
| 36 | 48 | 13.07 | −139 | 14.5 | 4.86 | 4.27 | 9.13 | 59.23% | 21.07% | 47.32% |
| 37 | 48 | 12.36 | −104 | 14.2 | 1.50 | 1.13 | 2.63 | 51.14% | 19.86% | 41.29% |
| 38 | 48 | 7.17 | 153 | 15.7 | 5.22 | 2.06 | 7.28 | 35.87% | 52.20% | 41.53% |
| 39 | 24 | 7.34 | 13 | 14.3 | 30.47 | 6.78 | 37.25 | 0.00% | 34.17% | 8.63% |
| 40 | 24 | 7.35 | 88 | 14.3 | 23.49 | 6.58 | 30.07 | 22.91% | 36.12% | 26.24% |
| 41 | 96 | 11.98 | −71 | 15.0 | 13.35 | 13.95 | 27.30 | 47.36% | 15.15% | 34.69% |
| 42 | 96 | 12.75 | −111.6 | 15.0 | 7.36 | 8.65 | 16.01 | 49.24% | 13.41% | 34.63% |
| 43 | 24 | 12.78 | −108.6 | 14.2 | 7.34 | 9.36 | 16.70 | 49.38% | 6.31% | 31.81% |
| 44 | 24 | 10.49 | 126.8 | 15.1 | 0.64 | 0.92 | 1.56 | 47.54% | 39.47% | 43.07% |
| 45 | 72 | 11.35 | 36.3 | 18.4 | 2.19 | 7.01 | 9.20 | 79.28% | 30.59% | 55.49% |
| 46 | 72 | 12.06 | −301.3 | 18.1 | 33.17 | 19.96 | 53.13 | 41.85% | 56.20% | 48.22% |
| 47 | 48 | 6.77 | 158.1 | 18.1 | 0.23 | 5.48 | 5.71 | 89.50% | 21.83% | 37.93% |
| 48 | 24 | 6.51 | 175.2 | 16.3 | 13.38 | 11.78 | 25.16 | 59.66% | 40.98% | 52.64% |
| 49 | 24 | 9.23 | 236.6 | 17.2 | 10.36 | 15.29 | 25.65 | 10.23% | 8.83% | 9.40% |
| 50 | 24 | 10.58 | 107.9 | 17.5 | 9.13 | 14.28 | 23.41 | 20.88% | 14.85% | 17.31% |
| 51 | 72 | 12.41 | −71.3 | 18.6 | 0.21 | 13.99 | 14.20 | 99.15% | 28.26% | 67.89% |
| 52 | 24 | 11.47 | 35.5 | 19.5 | 0.17 | 0.68 | 0.85 | 88.51% | 2.86% | 61.01% |
| 53 | 24 | 10.31 | 187.6 | 19.3 | 1.35 | 1.19 | 2.54 | 34.47% | 22.22% | 29.25% |
| 54 | 24 | 9.58 | 211.6 | 17.6 | 10.97 | 11.31 | 22.28 | 43.74% | 23.32% | 34.95% |
| 55 | 24 | 10.13 | 158.5 | 17.8 | 12.16 | 8.35 | 20.51 | 37.64% | 43.39% | 40.12% |
| 56 | 24 | 6.11 | 593.4 | 15.6 | 26.66 | 37.97 | 64.63 | 53.44% | 13.98% | 36.26% |
| 57 | 24 | 12.55 | −30.5 | 15.7 | 41.19 | 26.95 | 68.14 | 28.06% | 38.94% | 32.80% |
| 58 | 24 | 5.51 | 655.8 | 15.4 | 11.61 | 5.19 | 16.80 | 39.44% | 46.82% | 41.93% |
| 59 | 24 | 6.55 | 555.6 | 15.7 | 7.87 | 4.34 | 12.21 | 58.95% | 55.53% | 57.79% |
| 60 | 24 | 8.12 | 368.5 | 14.2 | 0.76 | 0.26 | 1.02 | 38.71% | 68.29% | 50.49% |
| 61 | 24 | 9.1 | 294.1 | 13.3 | 0.67 | 0.23 | 0.90 | 45.97% | 71.95% | 56.31% |
| 62 | 48 | 7.46 | 469.8 | 14.2 | 18.86 | 11.25 | 30.11 | 23.55% | 14.97% | 20.55% |
| 63 | 48 | 7.56 | 461.2 | 14.3 | 22.60 | 8.56 | 31.16 | 8.39% | 35.30% | 17.78% |
| 64 | 72 | 6.68 | 529 | 14.4 | 14.88 | 9.73 | 24.61 | 22.90% | 12.97% | 19.26% |
| 65 | 72 | 12.86 | −61.8 | 14.4 | 9.60 | 10.56 | 20.16 | 50.26% | 5.55% | 33.86% |
| 66 | 24 | 8.92 | 329.9 | 15.3 | 44.17 | 15.11 | 59.28 | 10.42% | 1.88% | 8.39% |
| 67 | 24 | 8.89 | 334.9 | 15.4 | 47.47 | 14.85 | 62.32 | 3.73% | 3.57% | 3.69% |
| 68 | 24 | 5.42 | 686.8 | 16.3 | 9.33 | 17.74 | 27.07 | 57.34% | 0.00% | 31.66% |
| 69 | 24 | 11.12 | 186.5 | 16.4 | 7.32 | 14.99 | 22.31 | 66.53% | 15.50% | 43.68% |
| 70 | 24 | 5.64 | 625.3 | 16.6 | 10.88 | 14.22 | 25.10 | 24.97% | 11.35% | 17.81% |
| 71 | 24 | 12.68 | −8 | 16.6 | 7.12 | 11.95 | 19.07 | 50.90% | 25.50% | 37.56% |
| 72 | 24 | 6.34 | 458.3 | 15.4 | 22.86 | 14.28 | 37.14 | 18.65% | 3.05% | 13.29% |
| 73 | 24 | 12.92 | −41.7 | 15.6 | 19.61 | 14.12 | 33.73 | 30.21% | 4.14% | 21.25% |
| 74 | 96 | 7.12 | −67.9 | 14.6 | 10.92 | 5.91 | 16.83 | 0.00% | 21.93% | 8.98% |
| 75 | 96 | 12.69 | −635.6 | 14.9 | 4.58 | 5.64 | 10.22 | 58.06% | 25.50% | 44.73% |
| 76 | 24 | 7.83 | 415.5 | 14.4 | 4.69 | 3.85 | 8.54 | 26.83% | 2.78% | 17.65% |
| 77 | 24 | 12.27 | 57.9 | 14.5 | 2.30 | 3.68 | 5.98 | 64.12% | 7.07% | 42.33% |
| 78 | 24 | 7.43 | 75.8 | 14.7 | 10.59 | 8.84 | 19.43 | 0.00% | 6.65% | 3.14% |
| 79 | 24 | 7.45 | 162.5 | 14.8 | 6.66 | 7.12 | 13.78 | 37.11% | 24.82% | 31.31% |
| 80 | 24 | 7.41 | 84.1 | 16.2 | 10.94 | 13.70 | 24.64 | 4.70% | 12.24% | 9.04% |
| 81 | 24 | 9.69 | −3.6 | 16.5 | 10.57 | 9.06 | 19.63 | 7.93% | 41.96% | 27.54% |
| 82 | 24 | 9.49 | 378.5 | 17.0 | 7.34 | 6.86 | 14.20 | 23.54% | 9.02% | 17.15% |
| 83 | 24 | 10.37 | 297.7 | 17.1 | 7.19 | 6.85 | 14.04 | 25.10% | 9.15% | 18.09% |
| 84 | 24 | 9.54 | 364.2 | 19.0 | 6.79 | 8.81 | 15.60 | 29.86% | 7.26% | 18.67% |
| 85 | 24 | 10.26 | 280.9 | 19.0 | 6.85 | 8.80 | 15.65 | 29.24% | 7.37% | 18.40% |
| 86 | 24 | 9.13 | 385.1 | 17.5 | 5.66 | 7.43 | 13.09 | 28.89% | 4.01% | 16.62% |
| 87 | 24 | 10.53 | 239.2 | 17.5 | 5.39 | 7.74 | 13.13 | 32.29% | 0.00% | 16.37% |
| Trial | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1.67 | 2.52 | ||||||||||
| 2 | 1 | 1.67 | 2.52 | 1.22 | |||||||||
| 3 | 1 | 1.67 | 2.52 | 2.96 | |||||||||
| 4 | 1 | 3.34 | 2.52 | ||||||||||
| 5 | 1 | ||||||||||||
| 6 | 1 | 1.67 | 1.22 | 1.36 | |||||||||
| 7 | 1 | 3.34 | 2.52 | 1.22 | 1.36 | ||||||||
| 8 | 1 | ||||||||||||
| 9 | 1 | 1.67 | 1.22 | 1.36 | |||||||||
| 10 | 1 | 1.67 | 2.52 | 1.22 | 2.71 | ||||||||
| 11 | 1 | ||||||||||||
| 12 | 1 | 1.67 | 1.22 | 1.36 | |||||||||
| 13 | 1 | 1.67 | 2.52 | 1.22 | 4.07 | ||||||||
| 14 | 1 | 1.67 | 2.52 | 1.22 | 1.36 | ||||||||
| 15 | 2 | 3.34 | 11.22 | ||||||||||
| 16 | 2 | 3.34 | 11.22 | ||||||||||
| 17 | 2 | 3.34 | 11.22 | ||||||||||
| 18 | 2 | 3.34 | 5.61 | ||||||||||
| 19 | 2 | 3.34 | 5.61 | ||||||||||
| 20 | 2 | 3.34 | 2.71 | 5.61 | |||||||||
| 21 | 2 | 3.34 | 2.52 | 1.48 | 2.71 | 2.81 | |||||||
| 22 | 2 | 3.34 | 2.52 | 1.48 | 2.71 | 5.61 | |||||||
| 23 | 2 | 3.34 | 5.61 | ||||||||||
| 24 | 2 | 3.34 | 5.61 | ||||||||||
| 25 | 2 | 3.34 | 5.61 | ||||||||||
| 26 | 2 | 3.34 | 5.61 | ||||||||||
| 27 | 2 | 3.34 | 5.61 | ||||||||||
| 28 | 2 | 3.34 | |||||||||||
| 29 | 1 | 1.67 | |||||||||||
| 30 | 0.69 | 3.34 | 2.52 | 1.48 | 0.61 | ||||||||
| 31 | 0.8 | 3.34 | 2.52 | ||||||||||
| 32 | 0.8 | 3.34 | 2.71 | ||||||||||
| 33 | 1.42 | 3.34 | |||||||||||
| 34 | 1.42 | 1.67 | |||||||||||
| 35 | 1.42 | 3.34 | |||||||||||
| 36 | 3.34 | ||||||||||||
| 37 | 3.34 | ||||||||||||
| 38 | 1.42 | 5.61 | |||||||||||
| 39 | 1.42 | 5.61 | |||||||||||
| 40 | 1.42 | 1.36 | 3.34 | 8.42 | |||||||||
| 41 | 1.67 | 2.52 | 1.99 | ||||||||||
| 42 | 1.67 | 2.52 | 1.99 | ||||||||||
| 43 | 1.67 | 1.99 | |||||||||||
| 44 | 1.51 | 1.22 | 1.99 | ||||||||||
| 45 | 1.42 | 1.51 | 1.67 | 2.81 | 1.99 | ||||||||
| 46 | 1.42 | 0.76 | 1.67 | 1.22 | 2.81 | ||||||||
| 47 | 1.42 | ||||||||||||
| 48 | 1.42 | ||||||||||||
| 49 | 1.42 | 1.51 | |||||||||||
| 50 | 1.42 | 1.51 | 1.22 | ||||||||||
| 51 | 2.84 | 1.51 | 3.34 | 2.71 | 2.5 | ||||||||
| 52 | 5.68 | 1.51 | 1.67 | ||||||||||
| 53 | 1.51 | 1.2 | |||||||||||
| 54 | 3.02 | 2.4 | |||||||||||
| 55 | 3.02 | 1.67 | 3.6 | ||||||||||
| 56 | 1.51 | 1.34 | 1.2 | ||||||||||
| 57 | 1.51 | 1.67 | 1.34 | 1.2 | |||||||||
| 58 | 3.02 | 1.34 | 2.4 | ||||||||||
| 59 | 3.02 | 1.67 | 1.34 | 2.4 | |||||||||
| 60 | 1.42 | 3.02 | 1.67 | 2.52 | 1.36 | 2.81 | 1.34 | 3.6 | |||||
| 61 | 1.42 | 3.02 | 3.34 | 2.52 | 1.36 | 2.81 | 1.34 | 3.6 | |||||
| 62 | 2.81 | 1.34 | 1.2 | ||||||||||
| 63 | 1.67 | 2.81 | 1.34 | 1.2 | |||||||||
| 64 | 4.22 | 2 | 1.8 | ||||||||||
| 65 | 3.34 | 4.22 | 2 | 1.8 | |||||||||
| 66 | 1.67 | 1.36 | 2.81 | 1.34 | 1.2 | ||||||||
| 67 | 2.81 | 0 | 1.2 | ||||||||||
| 68 | 1.51 | 1.34 | 1.2 | ||||||||||
| 69 | 1.51 | 1.67 | 1.34 | 1.2 | |||||||||
| 70 | 1.51 | 1.34 | 1.2 | ||||||||||
| 71 | 1.51 | 1.67 | 1.34 | 1.2 | |||||||||
| 72 | 1.51 | 1.34 | 1.2 | ||||||||||
| 73 | 1.51 | 1.67 | 1.34 | 1.2 | |||||||||
| 74 | 1.42 | 1.51 | 0 | 1.2 | |||||||||
| 75 | 1.42 | 1.51 | 1.67 | 0 | 1.2 | ||||||||
| 76 | 1.42 | 1.51 | 2.81 | 1.34 | 0 | ||||||||
| 77 | 1.42 | 1.51 | 1.67 | 1.36 | 2.81 | 1.34 | 1.2 | ||||||
| 78 | 1.42 | 1.51 | 1.2 | ||||||||||
| 79 | 1.42 | 1.51 | 1.22 | 1.2 | |||||||||
| 80 | 1.42 | 1.51 | 1.2 | ||||||||||
| 81 | 1.42 | 1.51 | 3.66 | 1.2 | |||||||||
| 82 | 2.27 | 1.2 | |||||||||||
| 83 | 2.27 | 1.22 | 1.2 | ||||||||||
| 84 | 1.51 | 2.52 | 1.2 | ||||||||||
| 85 | 1.51 | 2.52 | 3.66 | 1.2 | |||||||||
| 86 | 2.52 | 2.81 | 1.34 | ||||||||||
| 87 | 1.42 | 1.67 | 2.81 | 1.34 |
| Trial | Formic Acid | Acetic Acid | Malic Acid | Citric Acid | Tartaric Acid | Tea (Gallic Acid) | Urea |
|---|---|---|---|---|---|---|---|
| 1 | 1 | ||||||
| 2 | 1 | ||||||
| 3 | 1 | ||||||
| 4 | 1 | ||||||
| 5 | 0.9 | ||||||
| 6 | 0.9 | ||||||
| 7 | 1 | 0.9 | |||||
| 8 | 0.8 | ||||||
| 9 | 0.8 | ||||||
| 10 | 1 | 0.8 | |||||
| 11 | 0.83 | ||||||
| 12 | 0.83 | ||||||
| 13 | 1 | 0.83 | |||||
| 14 | 0.8 | 0.83 | 0.9 | ||||
| 15 | 0.9 | ||||||
| 16 | 0.8 | ||||||
| 17 | 1.67 | ||||||
| 18 | 1.79 | ||||||
| 19 | 0.83 | ||||||
| 20 | |||||||
| 21 | 2 | ||||||
| 22 | |||||||
| 23 | 0.83 | ||||||
| 24 | 1 | ||||||
| 25 | 0.8 | ||||||
| 26 | 2 | ||||||
| 27 | |||||||
| 28 | |||||||
| 29 | |||||||
| 30 | 0.94 | ||||||
| 31 | 0.94 | ||||||
| 32 | 0.94 | ||||||
| 33 | 1.32 | ||||||
| 34 | 1.32 | ||||||
| 35 | 1.32 | ||||||
| 36 | 1.32 | ||||||
| 37 | 1.32 | ||||||
| 38 | |||||||
| 39 | |||||||
| 40 | |||||||
| 41 | |||||||
| 42 | |||||||
| 43 | |||||||
| 44 | |||||||
| 45 | |||||||
| 46 | |||||||
| 47 | |||||||
| 48 | 1.32 | ||||||
| 49 | |||||||
| 50 | 1.32 |
| Cl− | Na+ | |||||||
|---|---|---|---|---|---|---|---|---|
| Trial | [a] | [b] | R2 | PPC | [a] | [b] | R2 | PPC |
| 1 | 0.00004980 | −0.4236 | 0.918 | 0.958 | 0.00023480 | −1.05551 | 0.842 | 0.918 |
| 2 | 0.00003149 | −0.4312 | 0.732 | 0.856 | 0.00015187 | −0.92883 | 0.906 | 0.952 |
| 3 | 0.00017958 | −0.7637 | 0.990 | 0.995 | 0.00014111 | −0.94756 | 0.996 | 0.998 |
| 4 | 0.00013545 | −0.7804 | 0.975 | 0.987 | 0.00021644 | −0.88604 | 0.955 | 0.977 |
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| 7 | 0.00065307 | −0.9610 | 0.985 | 0.992 | 0.00018974 | −0.96922 | 0.999 | 0.999 |
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| 10 | 0.00062608 | −0.9715 | 0.996 | 0.998 | 0.00008924 | −0.85519 | 0.968 | 0.984 |
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| 13 | 0.00100064 | −1.0372 | 0.998 | 0.999 | 0.00013866 | −0.85425 | 0.971 | 0.985 |
| 14 | 0.00008985 | −0.7902 | 0.935 | 0.967 | 0.00020695 | −0.86526 | 1.000 | 1.000 |
| 15 | 0.00028994 | −0.7276 | 0.963 | 0.981 | 0.00037814 | −0.86185 | 0.988 | 0.994 |
| 16 | 0.00004954 | −0.6694 | 0.665 | 0.816 | 0.00036220 | −0.92115 | 0.982 | 0.991 |
| 17 | 0.00042454 | −0.8737 | 0.999 | 0.999 | 0.00060368 | −0.91001 | 0.997 | 0.998 |
| 18 | 0.00011063 | −1.0061 | 1.000 | 1.000 | 0.00022458 | −0.91708 | 0.982 | 0.991 |
| 19 | 0.00014622 | −0.5851 | 0.876 | 0.936 | 0.00013026 | −0.88899 | 0.933 | 0.966 |
| 20 | 0.00011476 | −0.5061 | 0.931 | 0.965 | 0.00020437 | −0.94213 | 0.997 | 0.998 |
| 21 | 0.00004542 | −0.4884 | 0.862 | 0.928 | 0.00028932 | −0.92967 | 0.974 | 0.987 |
| 22 | 0.00003102 | −0.3341 | 0.463 | 0.680 | 0.00012206 | −0.89222 | 0.997 | 0.998 |
| 23 | 0.00023506 | −0.8838 | 0.962 | 0.981 | 0.00005493 | −0.87502 | 0.964 | 0.982 |
| 24 | 0.00028050 | −0.8072 | 0.970 | 0.985 | 0.00003633 | −0.33088 | 0.718 | 0.847 |
| 25 | 0.00021541 | −0.7717 | 0.969 | 0.985 | 0.00000998 | −1.69972 | 1.000 | 1.000 |
| 26 | 0.00022242 | −0.9562 | 0.999 | 0.999 | 0.00004715 | −0.93281 | 0.920 | 0.959 |
| 27 | 0.00008986 | −0.7856 | 1.000 | 1.000 | n/a | n/a | n/a | n/a |
| 28 | 0.00012054 | −0.9842 | 0.999 | 1.000 | 0.00008506 | −0.92801 | 0.997 | 0.998 |
| 29 | 0.00016821 | −0.9867 | 0.999 | 0.999 | 0.00000387 | −1.61133 | 1.000 | 1.000 |
| 30 | 0.00004453 | −0.6028 | 1.000 | 1.000 | 0.00287146 | −1.36095 | 1.000 | 1.000 |
| 31 | 0.00000022 | 0.1043 | 1.000 | 1.000 | n/a | n/a | n/a | n/a |
| 32 | 0.00002082 | −0.5451 | 1.000 | 1.000 | n/a | n/a | n/a | n/a |
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| 35 | 0.00027845 | −0.9542 | 0.954 | 0.977 | 0.00013306 | −1.01025 | 1.000 | 1.000 |
| 36 | 0.00016647 | −0.8917 | 1.000 | 1.000 | 0.00000137 | −0.15570 | 1.000 | 1.000 |
| 37 | 0.00013302 | −0.8999 | 0.999 | 0.999 | 0.00001813 | −0.69694 | 0.999 | 0.999 |
| 38 | 0.00006524 | −0.8094 | 0.990 | 0.995 | 0.00012418 | −0.88951 | 0.999 | 0.999 |
| 39 | 0.00002758 | −1.1817 | 1.000 | 1.000 | 0.00007301 | −0.86425 | 0.999 | 0.999 |
| 40 | 0.00012969 | −1.1434 | 0.994 | 0.997 | 0.00009155 | −0.93490 | 0.995 | 0.997 |
| 41 | 0.00012274 | −0.8943 | 0.996 | 0.998 | 0.00001469 | −0.75229 | 1.000 | 1.000 |
| 42 | 0.00011897 | −0.8951 | 0.999 | 1.000 | 0.00000002 | 0.61712 | 1.000 | 1.000 |
| 43 | 0.00012533 | −0.8612 | 0.999 | 1.000 | n/a | n/a | n/a | n/a |
| 44 | 0.00000394 | 0.2002 | 1.000 | 1.000 | 0.00014470 | −1.00597 | 1.000 | 1.000 |
| 45 | 0.00035246 | −0.9451 | 1.000 | 1.000 | 0.00012584 | −1.03805 | 0.999 | 0.999 |
| 46 | 0.00017440 | −0.9769 | 0.995 | 0.997 | 0.00016058 | −0.96516 | 0.996 | 0.998 |
| 47 | 0.00030808 | −0.9051 | 0.987 | 0.994 | 0.00002837 | −0.86593 | 0.985 | 0.992 |
| 48 | 0.00018884 | −0.8827 | 0.996 | 0.998 | 0.00003072 | −0.46577 | 0.962 | 0.981 |
| 49 | n/a | n/a | n/a | n/a | 0.00002289 | −0.96488 | 1.000 | 1.000 |
| 50 | 0.00006452 | −0.9979 | 1.000 | 1.000 | 0.00004202 | −0.98071 | 1.000 | 1.000 |
| 51 | 0.00021200 | −0.7400 | 0.778 | 0.882 | 0.00002835 | −0.76823 | 0.984 | 0.992 |
| 52 | 0.00031988 | −0.7421 | 0.967 | 0.983 | 0.00007991 | −1.71288 | 1.000 | 1.000 |
| 53 | 0.00010941 | −0.9289 | 0.987 | 0.993 | 0.00010748 | −1.11038 | 0.998 | 0.999 |
| 54 | 0.00010665 | −0.8374 | 0.992 | 0.996 | 0.00005539 | −0.90984 | 1.000 | 1.000 |
| 55 | 0.00011384 | −0.9658 | 0.999 | 1.000 | 0.00010375 | −0.89297 | 0.996 | 0.998 |
| 56 | 0.00013233 | −0.8722 | 0.997 | 0.999 | 0.00001208 | −0.61099 | 1.000 | 1.000 |
| 57 | 0.00013563 | −1.0919 | 0.996 | 0.998 | 0.00010545 | −0.97158 | 0.986 | 0.993 |
| 58 | 0.00021719 | −1.1093 | 0.997 | 0.998 | n/a | n/a | n/a | n/a |
| 59 | 0.00020969 | −0.9204 | 0.996 | 0.998 | 0.00009402 | −0.72548 | 1.000 | 1.000 |
| 60 | 0.00014835 | −1.0751 | 0.992 | 0.996 | 0.00026394 | −0.93524 | 1.000 | 1.000 |
| 61 | 0.00018920 | −1.0488 | 0.999 | 0.999 | 0.00032635 | −0.97755 | 1.000 | 1.000 |
| 62 | 0.00010253 | −1.4081 | 0.905 | 0.952 | 0.00007970 | −1.14889 | 1.000 | 1.000 |
| 63 | 0.00011499 | −1.2897 | 0.989 | 0.994 | 0.00011197 | −1.05830 | 0.991 | 0.995 |
| 64 | 0.00008288 | −0.8907 | 0.959 | 0.979 | 0.00003477 | −1.06312 | 0.945 | 0.972 |
| 65 | 0.00026005 | −1.0435 | 0.999 | 1.000 | 0.00001502 | −0.84279 | 0.922 | 0.960 |
| 66 | 0.00004073 | −1.3033 | 0.888 | 0.942 | 0.00002833 | −1.41765 | 0.972 | 0.986 |
| 67 | 0.00000697 | −1.0130 | 0.914 | 0.956 | 0.00003494 | −1.29302 | 0.974 | 0.987 |
| 68 | 0.00012969 | −0.7576 | 0.977 | 0.988 | n/a | n/a | n/a | n/a |
| 69 | 0.00018281 | −0.7875 | 0.979 | 0.990 | 0.00006728 | −1.10058 | 0.999 | 0.999 |
| 70 | 0.00010252 | −1.0152 | 0.982 | 0.991 | 0.00004124 | −1.07313 | 1.000 | 1.000 |
| 71 | 0.00021541 | −1.0602 | 0.995 | 0.998 | 0.00002797 | −0.50468 | 0.682 | 0.826 |
| 72 | 0.00005290 | −0.9648 | 1.000 | 1.000 | 0.00001498 | −1.17100 | 1.000 | 1.000 |
| 73 | 0.00013789 | −1.1211 | 0.999 | 0.999 | 0.00000739 | −1.00488 | 0.899 | 0.948 |
| 74 | 0.00008728 | −1.0597 | 0.998 | 0.999 | 0.00000864 | −0.54435 | 1.000 | 1.000 |
| 75 | 0.00025650 | −1.0282 | 1.000 | 1.000 | 0.00000211 | −0.17304 | 0.872 | 0.934 |
| 76 | 0.00001435 | −0.4328 | 1.000 | 1.000 | n/a | n/a | n/a | n/a |
| 77 | 0.00023930 | −0.9511 | 1.000 | 1.000 | n/a | n/a | n/a | n/a |
| 78 | n/a | n/a | n/a | 0.00002120 | −0.99668 | 0.994 | 0.997 | |
| 79 | 0.00013118 | −1.0060 | 1.000 | 1.000 | 0.00007453 | −0.97234 | 1.000 | 1.000 |
| 80 | 0.00002086 | −0.9102 | 0.760 | 0.872 | 0.00001505 | −0.72404 | 1.000 | 1.000 |
| 81 | 0.00003655 | −1.1122 | 0.995 | 0.998 | 0.00013304 | −0.96749 | 1.000 | 1.000 |
| 82 | 0.00003131 | −0.7526 | 0.995 | 0.997 | 0.00002059 | −0.91397 | 0.999 | 0.999 |
| 83 | 0.00003074 | −0.7632 | 0.966 | 0.983 | 0.00005497 | −1.18850 | 0.995 | 0.997 |
| 84 | 0.00011783 | −1.0472 | 1.000 | 1.000 | 0.00001430 | −0.87934 | 1.000 | 1.000 |
| 85 | 0.00011954 | −1.0602 | 1.000 | 1.000 | 0.00000510 | −0.50974 | 0.972 | 0.986 |
| 86 | 0.00006956 | −0.9105 | 1.000 | 1.000 | n/a | n/a | n/a | n/a |
| 87 | 0.00011565 | −1.0153 | 1.000 | 1.000 | 0.00000258 | −0.79701 | 1.000 | 1.000 |
| Trial | hrs | pH | Eh, mV | T °C | Cl−, g/L | Na+ g/L | hrs | pH | Eh, mV | T °C | Cl−, g/L | Na+ g/L |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 8.69 | 255 | 9.2 | 22.11 | 14.43 | 2880 | 7.94 | 362 | 14.8 | 12.16 | 3.67 |
| 2 | 0 | 8.69 | 255 | 9.2 | 22.11 | 14.43 | 2880 | 6.62 | 461 | 14.2 | 20.54 | 6.18 |
| 3 | 0 | 8.69 | 255 | 9.2 | 22.11 | 14.43 | 2880 | 7.58 | 406 | 14.3 | 22.11 | 8.68 |
| 4 | 0 | 8.69 | 255 | 9.2 | 22.11 | 14.43 | 2880 | 8.01 | 376 | 14.2 | 2.28 | 0.67 |
| 5 | 0 | 8.69 | 255 | 9.2 | 22.11 | 14.43 | 2880 | 8.99 | 285 | 14.2 | 7.36 | 3.50 |
| 6 | 0 | 8.69 | 255 | 9.2 | 22.11 | 14.43 | 2880 | 8.21 | 341 | 14.2 | 22.11 | 7.05 |
| 7 | 0 | 8.69 | 255 | 9.2 | 22.11 | 14.43 | 2880 | 8.15 | 361 | 14.9 | 22.11 | 7.75 |
| 8 | 0 | 8.69 | 255 | 9.2 | 22.11 | 14.43 | 2880 | 8.11 | 366 | 15.3 | 21.80 | 13.84 |
| 9 | 0 | 8.69 | 255 | 9.2 | 22.11 | 14.43 | 2880 | 7.98 | 382 | 14.9 | 22.11 | 11.78 |
| Trial | hrs | Cl−, g/L | Na+ g/L | hrs | pH | Eh, mV | T °C | Cl−, g/L | Na+ g/L | Cl− | Na+ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2880 | 2.03 | 0.61 | 2904 | 8.33 | 359 | 13.1 | 0.82 | 0.49 | 77.8% | 79.7% |
| 2 | 2880 | 3.42 | 1.03 | 2904 | 8.32 | 357 | 13.2 | 1.57 | 0.73 | 57.4% | 69.6% |
| 3 | 2880 | 3.69 | 1.45 | 2904 | 8.34 | 355 | 13.4 | 2.03 | 1.06 | 44.8% | 55.9% |
| 4 | 2880 | 0.38 | 0.11 | 2904 | 8.63 | 339 | 13.4 | 0.19 | 0.11 | 95.0% | 95.4% |
| 5 | 2880 | 1.23 | 0.58 | 2904 | 8.76 | 326 | 13.6 | 0.66 | 0.53 | 82.0% | 78.0% |
| 6 | 2880 | 3.69 | 1.18 | 2904 | 8.43 | 353 | 13.4 | 1.99 | 1.18 | 46.0% | 50.9% |
| 7 | 2880 | 3.69 | 1.29 | 2904 | 8.55 | 348 | 13.6 | 2.30 | 0.83 | 37.6% | 65.4% |
| 8 | 2880 | 3.63 | 2.31 | 2904 | 8.44 | 365 | 13.8 | 2.03 | 0.83 | 44.8% | 65.4% |
| 9 | 2880 | 3.69 | 1.96 | 2904 | 8.36 | 371 | 13.9 | 3.10 | 1.20 | 16.0% | 49.9% |
| Cl− | Na+ | |||||
|---|---|---|---|---|---|---|
| Trial | [a] | [b] | R2 | [a] | [b] | R2 |
| 1 | 0.000159 | −0.846301 | 0.999879 | 0.000100 | −1.149606 | 0.999996 |
| 2 | 0.000150 | −0.889702 | 0.999968 | 0.000119 | −1.110224 | 0.999616 |
| 3 | 0.000094 | −0.862291 | 0.998579 | 0.000143 | −1.140650 | 0.999858 |
| 4 | 0.000122 | −0.873837 | 0.999197 | 0.000031 | −1.400760 | 0.991862 |
| 5 | 0.000139 | −0.974937 | 0.999223 | 0.000071 | −1.302737 | 0.999987 |
| 6 | 0.000137 | −0.934201 | 0.999987 | n/a | n/a | n/a |
| 7 | 0.000102 | −0.901164 | 0.999373 | 0.000154 | −1.068848 | 0.999994 |
| 8 | 0.000138 | −0.947561 | 0.999991 | 0.000334 | −1.040462 | 0.999927 |
| 9 | n/a | n/a | n/a | 0.000176 | −1.065346 | 0.999834 |
| Trial | hrs | pH | Eh, mV | T °C | Cl−, g/L | Na+ g/L | hrs | pH | Eh, mV | T °C | Cl−, g/L | Na+ g/L |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 9.06 | 423 | 15.6 | 11.48 | 15.61 | 768 | 8.32 | 428 | 18.1 | 11.48 | 14.49 |
| 2 | 0 | 9.06 | 423 | 15.6 | 11.48 | 15.61 | 768 | 12.54 | −15 | 18.4 | 10.77 | 14.82 |
| 3 | 0 | 8.72 | 321 | 17.2 | 14.50 | 16.04 | 744 | 4.92 | 716 | 17.7 | 12.03 | 14.89 |
| 4 | 0 | 8.72 | 321 | 17.2 | 14.50 | 16.04 | 744 | 12.71 | 14 | 16.8 | 7.65 | 11.98 |
| 5 | 0 | 8.68 | 308 | 14.2 | 28.10 | 14.73 | 720 | 5.76 | 570 | 17.6 | 12.76 | 14.73 |
| 6 | 0 | 8.68 | 308 | 14.2 | 28.10 | 14.73 | 720 | 12.64 | 2 | 17.4 | 14.26 | 14.18 |
| 7 | 0 | 9.60 | 230 | 15.2 | 10.92 | 7.57 | 792 | 5.64 | 249 | 17.7 | 9.62 | 7.57 |
| 8 | 0 | 9.60 | 230 | 15.2 | 10.92 | 7.57 | 792 | 12.33 | −22 | 17.5 | 5.11 | 6.21 |
| 9 | 0 | 8.49 | 360 | 14.6 | 24.67 | 13.23 | 744 | 7.03 | 73 | 16.0 | 14.53 | 10.95 |
| 10 | 0 | 8.49 | 360 | 14.6 | 24.67 | 13.23 | 744 | 7.65 | 433 | 15.2 | 13.24 | 6.98 |
| 11 | 0 | 8.84 | 317 | 15.2 | 21.87 | 17.74 | 720 | 5.70 | 589 | 15.8 | 16.18 | 17.40 |
| 12 | 0 | 8.84 | 317 | 15.2 | 21.87 | 17.74 | 720 | 11.91 | −18 | 15.7 | 11.10 | 15.09 |
| 13 | 0 | 9.53 | 226 | 17.5 | 57.26 | 44.14 | 720 | 6.85 | 382 | 15.7 | 11.67 | 14.57 |
| 14 | 0 | 9.53 | 226 | 17.5 | 57.26 | 44.14 | 720 | 11.73 | 10 | 15.8 | 15.96 | 16.90 |
| 15 | 0 | 9.67 | 361 | 17.9 | 9.68 | 9.50 | 720 | 6.12 | 221 | 15.3 | 9.68 | 9.04 |
| 16 | 0 | 9.67 | 361 | 17.9 | 9.68 | 9.50 | 720 | 6.90 | 268 | 15.5 | 9.68 | 7.01 |
| 17 | 0 | 9.67 | 361 | 17.9 | 9.68 | 9.50 | 720 | 9.18 | 344 | 14.0 | 7.56 | 5.60 |
| 18 | 0 | 9.67 | 361 | 17.9 | 9.68 | 9.50 | 720 | 9.54 | 292 | 14.0 | 9.68 | 6.03 |
| 19 | 0 | 9.80 | 347 | 16.8 | 7.96 | 7.74 | 720 | 7.90 | 465 | 13.9 | 7.94 | 7.34 |
| 20 | 0 | 9.80 | 347 | 16.8 | 7.96 | 7.74 | 720 | 7.92 | 458 | 13.9 | 7.96 | 7.34 |
| Trial | hrs | Cl− g/L | Na+ g/L | hrs | pH | Eh, mV | T °C | Cl− g/L | Na+ g/L | Cl− | Na+ | Dilution Ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 768 | 3.34 | 4.21 | 24 | 9.57 | 287 | 17.5 | 2.45 | 2.29 | 26.6% | 49.5% | 0.29 |
| 2 | 768 | 1.36 | 1.88 | 24 | 11.76 | 104 | 17.5 | 0.77 | 1.25 | 47.0% | 36.8% | 0.13 |
| 3 | 744 | 2.01 | 2.48 | 48 | 7.79 | 356 | 17.6 | 1.24 | 0.97 | 48.7% | 63.7% | 0.17 |
| 4 | 744 | 0.94 | 1.47 | 48 | 11.90 | 77 | 17.6 | 0.88 | 1.22 | 50.6% | 38.1% | 0.12 |
| 5 | 720 | 2.98 | 3.44 | 72 | 7.59 | 363 | 15.8 | 5.90 | 2.24 | 10.2% | 34.9% | 0.23 |
| 6 | 720 | 2.23 | 2.21 | 72 | 12.53 | −35 | 15.9 | 1.26 | 2.19 | 71.3% | 4.8% | 0.16 |
| 7 | 792 | 2.51 | 1.98 | 72 | 7.52 | 186 | 15.9 | 2.14 | 1.19 | 24.9% | 39.7% | 0.26 |
| 8 | 792 | 1.63 | 1.99 | 72 | 12.13 | −5 | 15.9 | 1.28 | 2.21 | 63.3% | 8.7% | 0.32 |
| 9 | 744 | 4.84 | 3.65 | 24 | 7.02 | 367 | 14.9 | 3.96 | 2.57 | 51.8% | 41.7% | 0.33 |
| 10 | 744 | 2.56 | 1.35 | 24 | 7.45 | 457 | 14.8 | 1.66 | 0.96 | 65.2% | 62.5% | 0.19 |
| 11 | 720 | 7.66 | 8.24 | 24 | 6.32 | 521 | 14.9 | 9.66 | 7.95 | 6.8% | 5.4% | 0.47 |
| 12 | 720 | 1.61 | 2.19 | 24 | 10.48 | 206 | 14.8 | 0.88 | 1.03 | 72.3% | 60.0% | 0.15 |
| 13 | 720 | 1.61 | 2.01 | 24 | 7.97 | 377 | 14.7 | 1.11 | 0.67 | 85.9% | 89.0% | 0.14 |
| 14 | 720 | 3.54 | 3.75 | 24 | 9.53 | 308 | 14.8 | 2.30 | 2.32 | 81.9% | 76.3% | 0.22 |
| 15 | 720 | 2.86 | 2.67 | 24 | 7.28 | 225 | 14.0 | 2.50 | 2.06 | 12.7% | 26.7% | 0.30 |
| 16 | 720 | 2.67 | 1.93 | 24 | 7.37 | 264 | 14.8 | 2.10 | 1.45 | 21.2% | 44.6% | 0.28 |
| 17 | 720 | 0.96 | 0.71 | 24 | 10.10 | 209 | 13.7 | 0.55 | 0.47 | 55.1% | 60.9% | 0.13 |
| 18 | 720 | 1.81 | 1.13 | 24 | 10.00 | 216 | 13.8 | 0.93 | 0.77 | 48.6% | 56.7% | 0.19 |
| 19 | 720 | 1.10 | 1.01 | 24 | 9.96 | 210 | 13.7 | 0.71 | 0.47 | 35.3% | 56.0% | 0.14 |
| 20 | 720 | 2.31 | 2.13 | 24 | 9.69 | 234 | 13.6 | 1.53 | 1.36 | 33.9% | 39.6% | 0.29 |
References
- Potapov, P.; Turubanova, S.; Hansen, M.C.; Tyukavina, A.; Zalles, V.; Khan, A.; Song, X.-P.; Pickens, A.; Shen, Q.; Cortez, J. Global maps of cropland extent and change show accelerated cropland expansion in the twenty-first century. Nat. Food 2022, 3, 19–28. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Zhang, G.J.; Wei, L.; Wang, B.; Yu, L. Contrasting influences of biogeophysical and biogeochemical impacts of historical land use on global economic inequality. Nat. Commun. 2022, 13, 2479. [Google Scholar] [CrossRef] [PubMed]
- Siebert, S.; Burke, J.; Faures, J.-M.; Frenken, K.; Hoogeveen, J.; Döll, P.; Portmann, F.T. Groundwater use for irrigation–a global inventory. Hydrol. Earth Syst. Sci. 2010, 14, 1863–1880. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Di, L.; Sun, Z. WaterSmart-GIS: A Web Application of a Data Assimilation Model to Support Irrigation Research and Decision Making. ISPRS Int. J. Geo-Inf. 2022, 11, 271. [Google Scholar] [CrossRef]
- Arboleda, P.; Ducharne, A.; Yin, Z.; Ciais, P. Tuning an improved irrigation scheme inside ORCHIDEE land surface model and assessing its sensitivity over land surface hydrology and energy budget. In Proceedings of the EGU General Assembly 2022, Vienna, Austria, 23–27 May 2022. EGU22-1984. [Google Scholar] [CrossRef]
- Jehan, S.; Iqbal, M.; Samreen, T.; Liaquat, M.; Kanwal, S.; Naseem, M. Effect of Deficit Irrigation Practice on Nitrogen Mineralization and Nitrate Nitrogen Leaching under Semi-Arid Conditions. J. Water Resour. Prot. 2022, 14, 385–394. [Google Scholar] [CrossRef]
- Wang, X. Managing Land Carrying Capacity: Key to Achieving Sustainable Production Systems for Food Security. Land 2022, 11, 484. [Google Scholar] [CrossRef]
- Rosa, L. Adapting agriculture to climate change via sustainable irrigation: Biophysical potentials and feedbacks. Environ. Res. Lett. 2022, 17, 063008. [Google Scholar] [CrossRef]
- Negacz, K.; Malek, Z.; Vos, A.; Vellinga, P. Saline soils worldwide: Identifying the most promising areas for saline agriculture. J. Arid Environ. 2022, 203, 104775. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Serralheiro, R.P. Soil Salinity: Effect on Vegetable Crop Growth. Management Practices to Prevent and Mitigate Soil Salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; Fao Irrigation and Drainage Paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 1985; Volume 29. [Google Scholar]
- Wei, C.; Li, F.; Yang, P.; Ren, S.; Wang, S.; Wang, Y.; Xu, Z.; Xu, Y.; Wei, R.; Zhang, Y. Effects of Irrigation Water Salinity on Soil Properties, N2O Emission and Yield of Spring Maize under Mulched Drip Irrigation. Water 2019, 11, 1548. [Google Scholar] [CrossRef] [Green Version]
- Amer, R. Spatial Relationship between Irrigation Water Salinity, Waterlogging, and Cropland Degradation in the Arid and Semi-Arid Environments. Remote Sens. 2021, 13, 1047. [Google Scholar] [CrossRef]
- Slater, Y.; Reznik, A.; Finkelshtain, I.; Kan, I. Blending Irrigation Water Sources with Different Salinities and the Economic Damage of Salinity: The Case of Israel. Water 2022, 14, 917. [Google Scholar] [CrossRef]
- Silber, A.; Israeli, Y.; Elingold, I.; Levi, M.; Levkovitch, I.; Russo, D.; Assouline, S. Irrigation with desalinated water: A step toward increasing water saving and crop yields. Water Resour. Res. 2015, 51, 450–464. [Google Scholar] [CrossRef]
- Antia, D.D.J. Desalination of Water Using ZVI (Fe0). Water 2015, 7, 3671–3831. [Google Scholar] [CrossRef] [Green Version]
- Antia, D.D.J. Purification of Saline Water Using Desalination Pellets. Water 2022, 14, 2639. [Google Scholar] [CrossRef]
- Antia, D.D.J. Provision of Desalinated Irrigation Water by the Desalination of Groundwater Abstracted from a Saline Aquifer. Hydrology 2022, 9, 128. [Google Scholar] [CrossRef]
- Antia, D.D.J. Catalytic Partial Desalination of Saline Water. Water 2022, 14, 2893. [Google Scholar] [CrossRef]
- Antia, D.D.J. Water Treatment and Desalination Using the Eco-materials n-Fe0 (ZVI), n-Fe3O4, n-FexOyHz[mH2O], and n-Fex[Cation]nOyHz[Anion]m [rH2O]. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications, 1st ed.; Kharissova, O.V., Torres-Martínez, L.M., Kharisov, B.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 3159–3242. [Google Scholar]
- Antia, D.D.J. Remediation of Saline Wastewater Producing a Fuel Gas Containing Alkanes and Hydrogen Using Zero Valent Iron (Fe0). Water 2022, 14, 1926. [Google Scholar] [CrossRef]
- Konadu-Amoah, B.; Hu, R.; Cao, V.; Tao, R.; Yang, H.; Ndé-Tchoupé, A.I.; Gwenzi, W.; Ruppert, H.; Noubactep, C. Realizing the potential of metallic iron for the mitigation of toxics: Flee or adapt? Appl. Water Sci. 2022, 12, 1–11. [Google Scholar] [CrossRef]
- Xiao, M.; Hu, R.; Ndé-Tchoupé, A.I.; Gwenzi, W.; Noubactep, C. Metallic Iron for Water Remediation: Plenty of Room for Collaboration and Convergence to Advance the Science. Water 2022, 14, 1492. [Google Scholar] [CrossRef]
- Hu, R.; Gwenzi, W.; Sipowo-Tala, V.R.; Noubactep, C. Water Treatment Using Metallic Iron: A Tutorial Review. Processes 2019, 7, 622. [Google Scholar] [CrossRef] [Green Version]
- Noubactep, C. Should the term ‘metallic iron’ appear in the title of a research paper? Chemosphere 2022, 287, 132314. [Google Scholar] [CrossRef]
- Hu, R.; Ndé-Tchoupé, A.I.; Cao, V.; Gwenzi, W.; Noubactep, C. Metallic iron for environmental remediation: The fallacy of the electron efficiency concept. Front. Environ. Chem. 2021, 2, 677813. [Google Scholar] [CrossRef]
- Hu, R.; Noubactep, C. Iron Corrosion: Scientific Heritage in Jeopardy. Sustainability 2018, 10, 4138. [Google Scholar] [CrossRef] [Green Version]
- British Standards Institute. Quality management systems, BSI Handbook 25. In Statistical Interpretation of Data; British Standards Institute: London, UK, 1985; p. 318. ISBN 0580150712/9780580150715. [Google Scholar]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Taylor, R. Interpretation of the Correlation Coefficient: A Basic Review. J. Diagn. Med. Sonogr. 1990, 6, 35–39. [Google Scholar] [CrossRef]
- Bottero, J.Y.; Manceau, A.; Villieras, F.; Tchoubar, D. Structure and mechanisms of formation of iron oxide hydroxide (chloride) polymers. Langmuir 1994, 10, 316–319. [Google Scholar] [CrossRef]
- Spiro, T.G.; Allerton, S.E.; Renner, J.; Terzis, A.; Bils, R.; Saltman, P. The Hydrolytic Polymerization of Iron(III). J. Am. Chem. Soc. 1966, 88, 2721–2726. [Google Scholar] [CrossRef]
- Dong, H.; Gao, B.; Yue, Q.; Sun, S.; Wang, Y.; Li, Q. Floc properties and membrane fouling of different monomer and polymer Fe coagulants in coagulation–ultrafiltration process: The role of Fe (III) species. Chem. Eng. J. 2014, 258, 442–449. [Google Scholar] [CrossRef]
- Chen, D.-W.; Liu, C.; Lu, J.; Mehmood, T.; Ren, Y.-Y. Enhanced phycocyanin and DON removal by the synergism of H2O2 and micro-sized ZVI: Optimization, performance, and mechanisms. Sci. Total Environ. 2020, 738, 140134. [Google Scholar] [CrossRef]
- Ward, D.A.; Ko, E.I. Preparing catalytic materials by the sol-gel method. Ind. Eng. Chem. Res. 1995, 34, 421–433. [Google Scholar] [CrossRef]
- Sakka, S.; Kozuka, H. Handbook of Sol-Gel Science and Technology 1. Sol-Gel Processing; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2005; Volume 1. [Google Scholar]
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Klein, L.; Aparicio, M.; Jitianu, A. (Eds.) Handbook of Sol-Gel Science and Technology; Springer International Publishing: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Ebbing, D.D.; Gammon, S.D. General Chemistr, 8th ed.; Houghton Mifflin Company: New York, NY, USA, 2005; ISBN 0618399429. [Google Scholar]
- Pilling, M.J.; Seakins, P.W. Reaction Kinetics; Oxford Science Publications; Oxford University Press: Oxford, UK, 1995; Volume 305, ISBN 019855527X. [Google Scholar]
- Castellan, G.W. Physical Chemistry, 3rd ed.; Addison Wesley: Boston, MA, USA, 2004; ISBN 0201103850. [Google Scholar]
- Shiyan, L.N.; Tropina, E.A.; Machekhina, K.I.; Gryaznova, E.N.; An, V.V. Colloid stability of iron compounds in groundwater of Western Siberia. Springerplus 2014, 22, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, Y.; Kim, D.; Shin, H.-S. Inhibition of nitrate reduction by NaCl adsorption on a nano-zero valent iron surface during concentrate treatment for water reuse. Environ. Technol. 2015, 36, 1178–1187. [Google Scholar] [CrossRef]
- Antia, D.D.J. Desalination of groundwater and impoundments using Nano-Zero Valent Iron, n-Fe°. Meteorol. Hydrol. Water Management. Res. Oper. Appl. 2015, 3, 21–38. [Google Scholar] [CrossRef]
- Fronczyk, J.; Pawluk, K.; Michniak, M. Application of permeable reactive barriers near roads for chloride ions removal. Ann. Wars. Univ. Life Sci.-SGGW Land Reclam. 2010, 42, 249–259. [Google Scholar] [CrossRef]
- Kovalchuk, N.M.; Starov, V.M. Aggregation in colloidal suspensions: Effect of colloidal forces and hydrodynamic interactions. Adv. Colloid Interface Sci. 2012, 179, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions, 2nd ed.; Library of Congress Catalog Card No 65-11670; Franklin, J.A., Translator; NACE International: Houston, TX, USA, 1974; p. 644. [Google Scholar]
- Misstear, B.; Banks, D.; Clark, L. Water Wells and Boreholes; John Wiley & Sons: Hoboken, NY, USA, 2006; ISBN 978-0-470-84989-7. [Google Scholar]
- Oetjen, K.; Chan, K.E.; Gulmark, K.; Christensen, J.H.; Blotevogel, J.; Borch, T.; Spear, J.R.; Cath, T.Y.; Higgins, C.P. Temporal characterization and statistical analysis of flowback and produced waters and their potential for reuse. Sci. Total Environ. 2018, 619, 654–664. [Google Scholar] [CrossRef]
- Owen, J.; Bustin, R.M.; Bustin, A.M.M. Insights from mixing calculations and geochemical modeling of Montney Formation post hydraulic fracturing flowback water chemistry. J. Pet. Sci. Eng. 2020, 195, 107589. [Google Scholar] [CrossRef]
- Golding, L.A.; Kumar, A.; Adams, M.S.; Binet, M.T.; Gregg, A.; King, J.; McKnight, K.S.; Nidumolu, B.; Spadaro, D.A.; Kirby, J.K. The influence of salinity on the chronic toxicity of shale gas flowback wastewater to freshwater organisms. J. Hazard. Mater. 2022, 428, 128219. [Google Scholar] [CrossRef]
- Luo, M.; Zhang, H.; Zhou, P.; Xiong, Z.; Huang, B.; Peng, J.; Liu, R.; Liu, W.; Lai, B. Efficient activation of ferrate (VI) by colloid manganese dioxide: Comprehensive elucidation of the surface-promoted mechanism. Water Res. 2022, 215, 118243. [Google Scholar] [CrossRef]
- Manquián-Cerda, K.; Cruces, E.; Rubio, M.A.; Reyes, C.; Arancibia-Miranda, N. Preparation of nanoscale iron (oxide, oxyhydroxides and zero-valent) particles derived from blueberries: Reactivity, characterization and removal mechanism of arsenate. Ecotoxicol. Environ. Saf. 2017, 145, 69–77. [Google Scholar] [CrossRef] [PubMed]



| Well | Ca | K | Mg | Na | Cl | HCO3 | SO4 | NaCl | SAR |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.4200 | 0.2420 | 0.3430 | 6.9600 | 14.9000 | 0.3900 | 0.1000 | 21.86 | 60.65 |
| 2 | 22.7000 | 3.9200 | 4.8100 | 40.3000 | 117.0000 | 0.0610 | 0.0400 | 157.30 | 89.42 |
| 3 | 0.1717 | 0.0215 | 0.0153 | 4.3850 | 6.5240 | 1.3030 | 0.0749 | 10.91 | 121.43 |
| 4 | 0.1979 | 0.0239 | 0.0207 | 4.8580 | 7.8860 | 0.8535 | 0.0640 | 12.74 | 123.92 |
| 5 | 0.1633 | 0.0327 | 0.0262 | 4.6590 | 7.6400 | 1.0360 | 0.0656 | 12.30 | 125.94 |
| 6 | 0.2292 | 0.0490 | 0.0345 | 5.7990 | 9.4350 | 0.6951 | 0.0426 | 15.23 | 133.19 |
| 7 | 0.2566 | 0.0547 | 0.0384 | 6.0950 | 10.3590 | 0.5367 | 0.0389 | 16.45 | 132.38 |
| 8 | 0.1851 | 0.0278 | 0.0268 | 5.7170 | 8.8060 | 0.7464 | 0.0144 | 14.52 | 146.67 |
| 9 | 0.2265 | 0.0302 | 0.0268 | 6.1280 | 10.0360 | 0.6744 | 0.0211 | 16.16 | 144.72 |
| 10 | 0.2431 | 0.0265 | 0.0343 | 6.1870 | 9.9270 | 0.5841 | 0.0316 | 16.11 | 138.85 |
| 11 | 0.2669 | 0.0302 | 0.0381 | 6.9340 | 11.6500 | 0.5793 | 0.0087 | 18.58 | 148.35 |
| 0.1 h | 50 h | |||||
|---|---|---|---|---|---|---|
| Cl− | Na+ | NaCl | Cl− | Na+ | NaCl | |
| Feed | 14.9 | 6.96 | 21.86 | 14.9 | 6.96 | 21.86 |
| Product | ||||||
| Mean | 9.87 | 5.23 | 15.10 | 7.20 | 4.65 | 11.85 |
| Standard Deviation | 3.37 | 1.50 | 3.67 | 4.05 | 1.51 | 4.39 |
| 1st Quartile | 8.08 | 4.37 | 13.00 | 3.71 | 3.80 | 8.33 |
| Median | 9.78 | 5.42 | 15.34 | 7.54 | 4.63 | 11.99 |
| 3rd Quartile | 12.59 | 6.59 | 17.83 | 10.17 | 5.93 | 15.03 |
| Removed | ||||||
| Mean | 33.7% | 24.9% | 30.9% | 51.7% | 33.1% | 45.8% |
| 1st Quartile | 45.8% | 37.3% | 40.5% | 75.1% | 45.4% | 61.9% |
| Median | 34.4% | 22.2% | 29.8% | 49.4% | 33.5% | 45.1% |
| 3rd Quartile | 15.5% | 5.3% | 18.4% | 31.8% | 14.8% | 31.3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antia, D.D.J. Desalination of Irrigation Water Using Metal Polymers. Water 2022, 14, 3224. https://doi.org/10.3390/w14203224
Antia DDJ. Desalination of Irrigation Water Using Metal Polymers. Water. 2022; 14(20):3224. https://doi.org/10.3390/w14203224
Chicago/Turabian StyleAntia, David D. J. 2022. "Desalination of Irrigation Water Using Metal Polymers" Water 14, no. 20: 3224. https://doi.org/10.3390/w14203224
APA StyleAntia, D. D. J. (2022). Desalination of Irrigation Water Using Metal Polymers. Water, 14(20), 3224. https://doi.org/10.3390/w14203224






