Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation
Abstract
1. Introduction
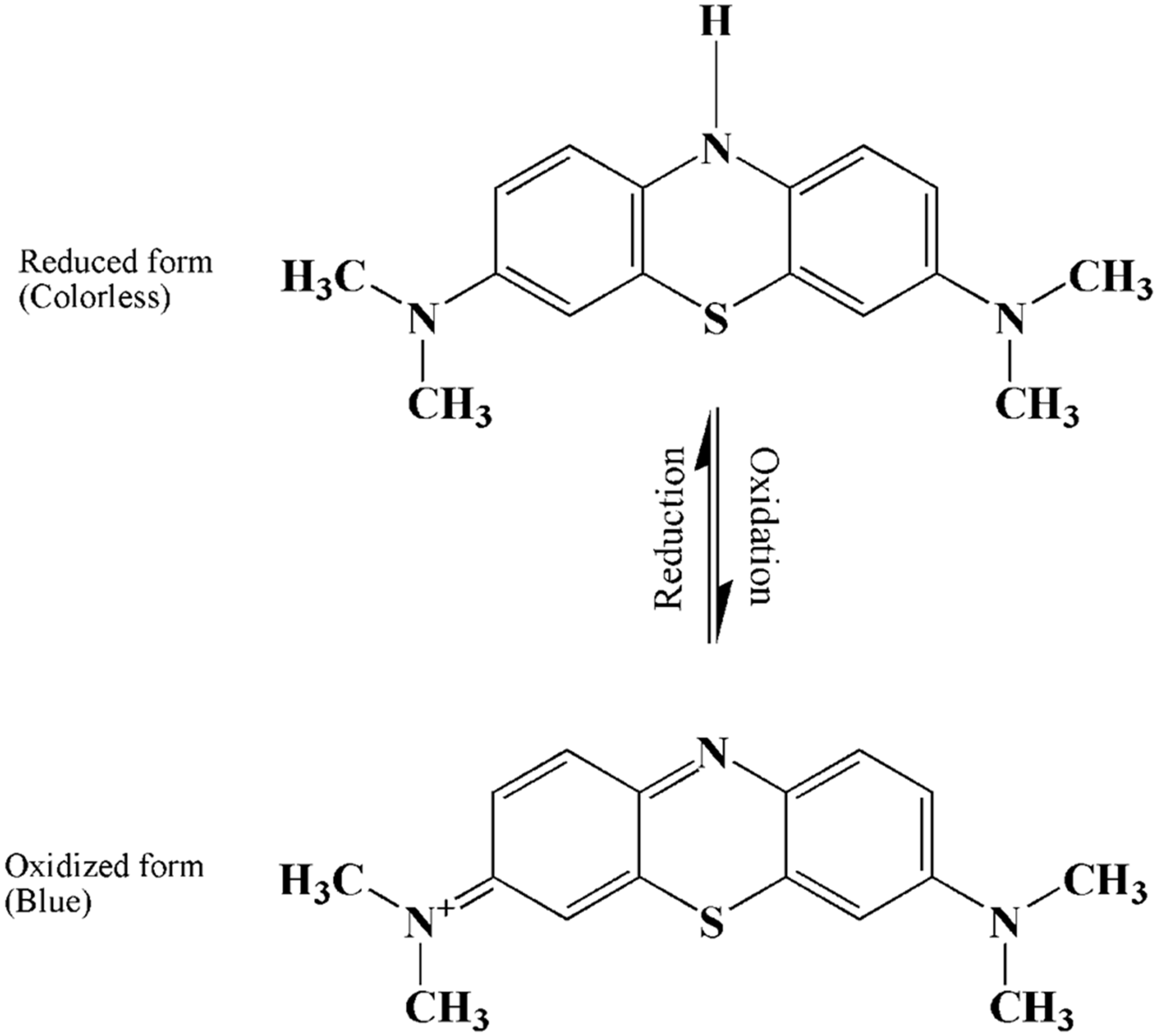
2. Properties of Methylene Blue
3. Uses and Applications of Methylene Blue
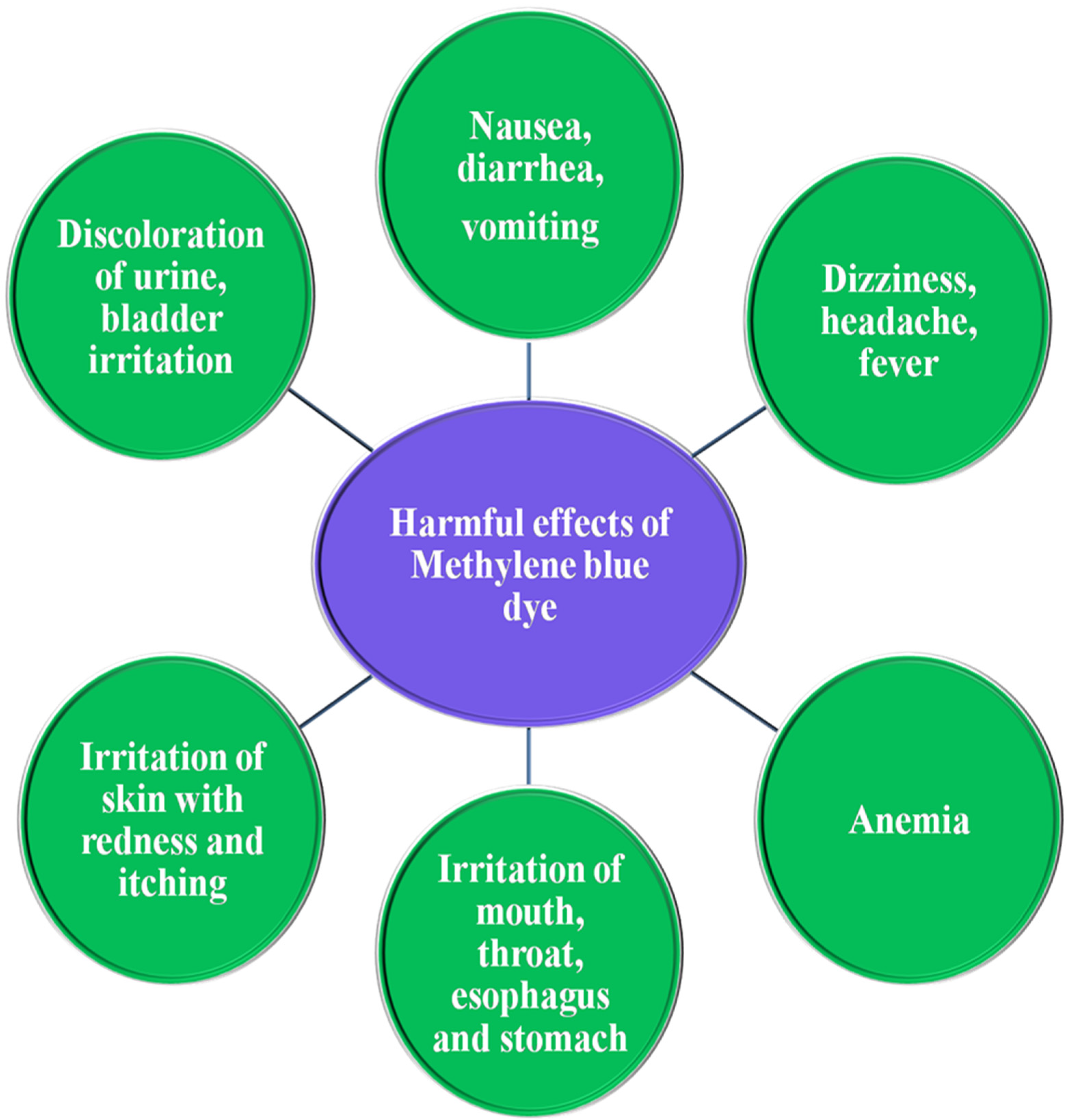
4. Toxicity of Methylene Blue
5. Methods of Removal of Methylene Blue
6. Photodegradation of Methylene Blue
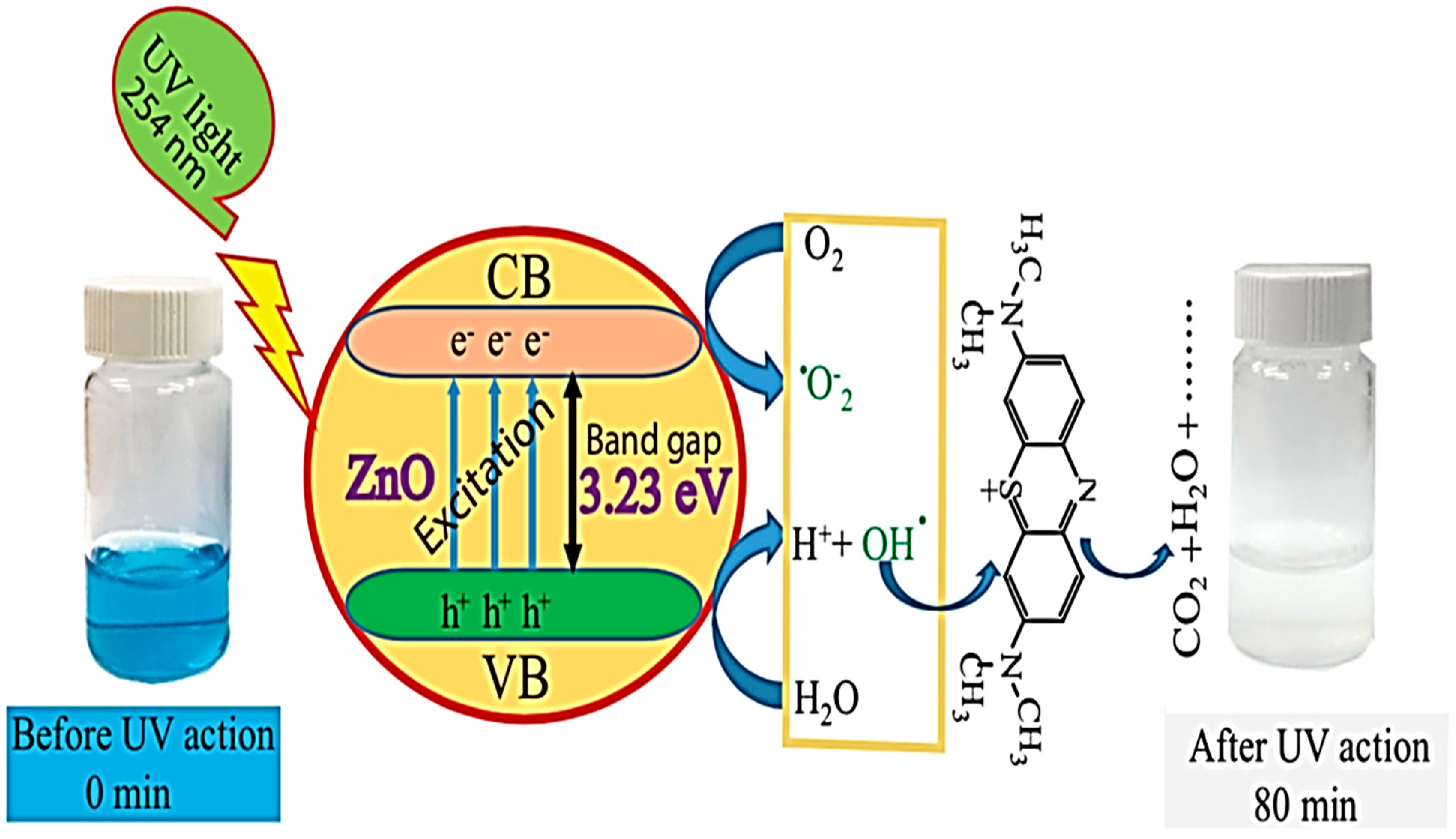
7. Mechanism of Photodegradation of Methylene Blue
8. Parameters Affecting Methylene Blue Photodegradation
8.1. Effect of Irradiation Time
8.2. Light Source and Intensity
8.3. Effect of Initial Dye Concentration
8.4. The pH Effect
8.5. Effect of Oxidants
8.6. Effect of Radical Scavengers and Ions
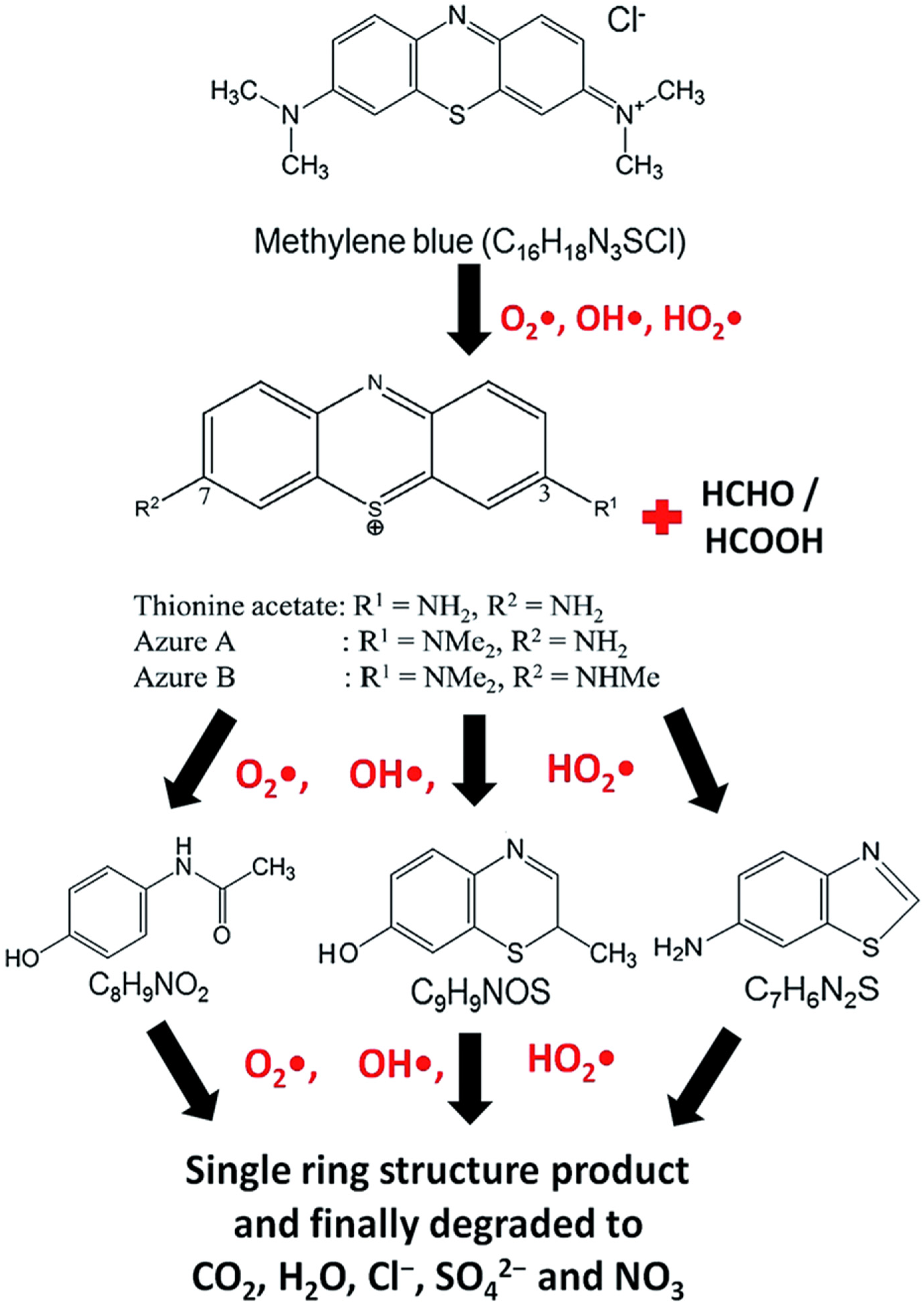
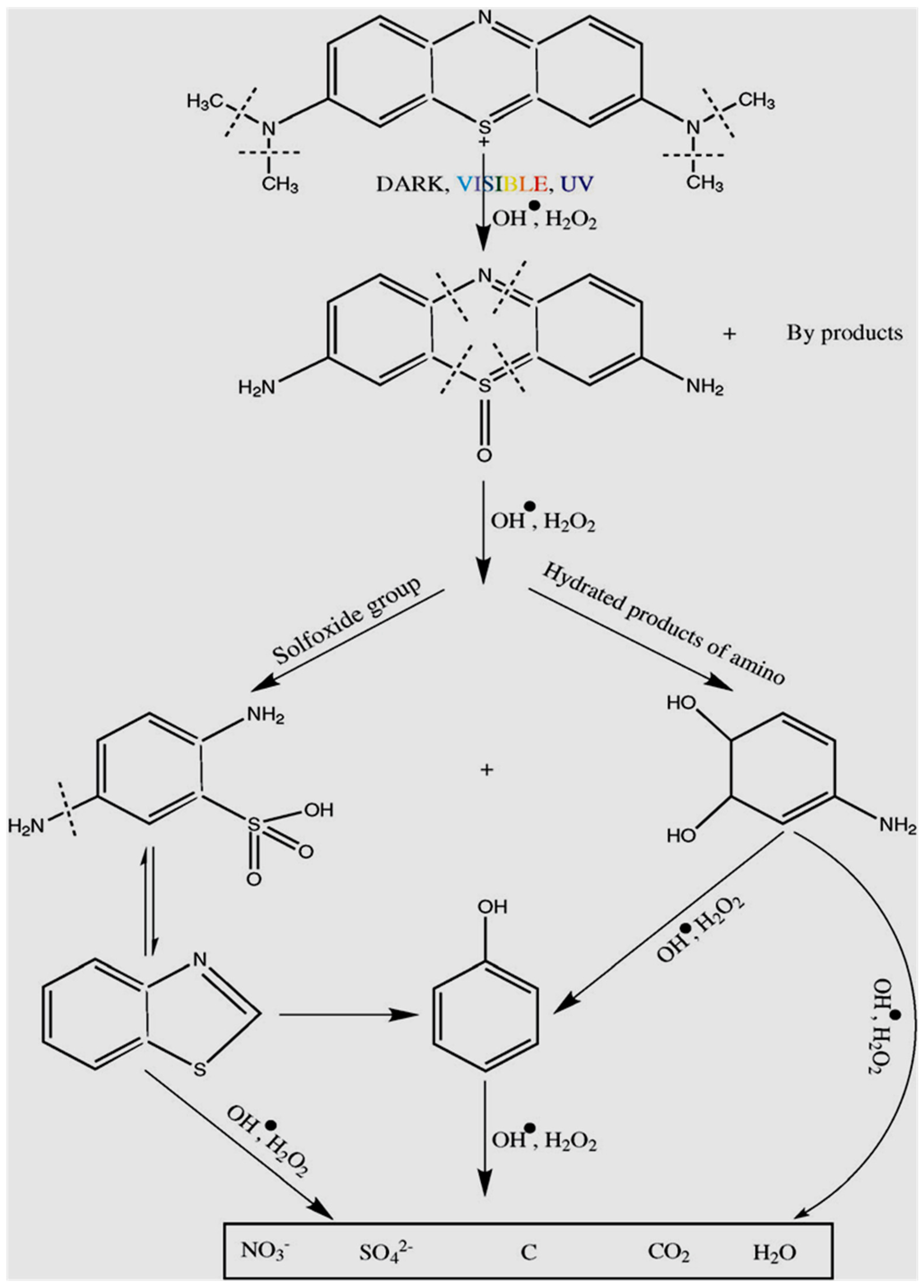
9. Degradation Products of Methylene Blue, Its Identification, and Reaction Pathways
10. Role of Catalysts
- Particle size: when the particle size is reduced to the nanoscale level, the specific surface area and the number of the active sites increases.
- Morphology: morphology is a key that provides the exposed area to sunlight. It is reported that nano rod-like ZnO structures form a high amount of reactive species due to strong absorption and lower recombination [271].
- Crystallinity: higher crystallinity leads to fewer defects for the recombination of photoexcited electron-hole pairs, and hence improves the overall photocatalytic activity of the catalyst [272].
- The high surface area associated with more active sites, and dye adsorption capacity.
- Kinetic directing catalysts, which produce the desired products from the recycled MB degraded products for use, are also important.
11. Summary and Future Perspectives
- The wettability and optical properties of MB suggest its hydrophobic and strong fluorescent nature. Its emission peak is observed at 686 nm (λex 665 nm), and this property can be exploited in multiple advanced applications. Due to these rationalities, MB has recently been used as an extrinsic fluorophore to study the micellization behaviour of drug delivery systems, i.e., bile salts (BS) [289]. The fluorescence response was monitored by fluorescence anisotropy at 686 nm, which indicates the MB–BS (MB-bile salt) association supported by the heat of formation values. This definitive study suggests the future potential of MB dyes as extrinsic fluorescence probes [289]. Moreover, the same property (in combination with various NPs) can be used in future imaging/diagnosis and treatment of tumours and other diagnostic applications [290]. Moreover, these properties can aid with optical sensor fabrication, though limited literature is available on the topic.
- An important unexplored dimension is to utilize modified MB dye in petroleum applications. The fluorescent nature of these materials could be helpful to probe the oil pockets, map the oil transport pathways and investigate various mechanisms, especially at the dead ends of the rock, where the operational conditions and depth hindered the application of the usual investigative techniques.
- Another critical aspect, which can be further investigated, is to convert the MB to beneficial and viable products via in situ bioconversion approaches. These investigations will not only remove the MB from the aqueous medium but also help to generate a variety of lower molecular products.
- Lastly, the simple adsorption approach for removal of MB dye needs to rediscover by utilizing modern concepts and materials. The ultimate goal should be to achieve greater efficiency at a cheaper cost. In this regard, various naturally available supports, especially the plant bio sorbents, still possess enough potential. Recently, the fava bean peels Vicia faba (FBP), were explored for the removal of methylene blue (MB) dye, a novel ultrasonic-assisted shaking sorption. The comparison with conventional shaking indicates that the MB removal efficiency reached 90% at 50 mg/L of the initial dye concentration for the ultrasonic-assisted sorbents in a remarkably shorter time [58,291].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benkhaya, S.; M’rabet, S.; El Harfi, A. A review on classifications, recent synthesis and applications of textile dyes. Inorg. Chem. Commun. 2020, 115, 107891. [Google Scholar] [CrossRef]
- Abd-Elhamid, A.I.; Emran, M.; El-Sadek, M.H.; El-Shanshory, A.A.; Soliman, H.M.A.; Akl, M.A.; Rashad, M. Enhanced removal of cationic dye by eco-friendly activated biochar derived from rice straw. Appl. Water Sci. 2020, 10, 45. [Google Scholar] [CrossRef]
- Bouras, H.D.; Isik, Z.; Arikan, E.B.; Yeddou, A.R.; Bouras, N.; Chergui, A.; Favier, L.; Amrane, A.; Dizge, N. Biosorption characteristics of methylene blue dye by two fungal biomasses. Int. J. Environ. Stud. 2020, 78, 365–381. [Google Scholar] [CrossRef]
- Tara, N.; Siddiqui, S.I.; Rathi, G.; Chaudhry, S.A.; Inamuddin; Asiri, A.M. Nano-engineered Adsorbent for the Removal of Dyes from Water: A Review. Curr. Anal. Chem. 2019, 16, 14–40. [Google Scholar] [CrossRef]
- Khan, I.; Khan, I.; Usman, M.; Imran, M.; Saeed, K. Nanoclay-mediated photocatalytic activity enhancement of copper oxide nanoparticles for enhanced methyl orange photodegradation. J. Mater. Sci. Mater. Electron. 2020, 31, 8971–8985. [Google Scholar] [CrossRef]
- Alencar, L.V.T.D.; Passos, L.M.S.; Soares, C.M.F.; Lima, A.S.; Souza, R.L. Efficiency Method for Methylene Blue Recovery Using Aqueous Two-Phase Systems Based on Cholinium-Ionic Liquids. J. Fash. Technol. Text. Eng. 2020, 6, 13–20. [Google Scholar] [CrossRef]
- Ahmad, A.; Mohd-Setapar, S.H.; Chuong, C.S.; Khatoon, A.; Wani, W.A.; Kumar, R.; Rafatullah, M. Recent advances in new generation dye removal technologies: Novel search for approaches to reprocess wastewater. RSC Adv. 2015, 5, 30801–30818. [Google Scholar] [CrossRef]
- Ahmad, M.; Rehman, W.; Khan, M.M.; Qureshi, M.T.; Gul, A.; Haq, S.; Ullah, R.; Rab, A.; Menaa, F. Phytogenic fabrication of ZnO and gold decorated ZnO nanoparticles for photocatalytic degradation of Rhodamine B. J. Environ. Chem. Eng. 2021, 9, 104725. [Google Scholar] [CrossRef]
- Pandey, S.; Do, J.Y.; Kim, J.; Kang, M. Fast and highly efficient removal of dye from aqueous solution using natural locust bean gum based hydrogels as adsorbent. Int. J. Biol. Macromol. 2020, 143, 60–75. [Google Scholar] [CrossRef]
- Fong, W.M.; Affam, A.C.; Chung, W.C. Synthesis of Ag/Fe/CAC for colour and COD removal from methylene blue dye wastewater. Int. J. Environ. Sci. Technol. 2020, 17, 3485–3494. [Google Scholar] [CrossRef]
- Derakhshan, Z.; Baghapour, M.A.; Ranjbar, M.; Faramarzian, M. Adsorption of Methylene Blue Dye from Aqueous Solutions by Modified Pumice Stone: Kinetics and Equilibrium Studies. Health Scope 2013, 2, 136–144. [Google Scholar] [CrossRef]
- Allouche, F.N.; Yassaa, N. Potential adsorption of methylene blue from aqueous solution using green macroalgae Posidonia oceanica. In Proceedings of the IOP Conference Series: Materials Science and Engineering, International Conference on Functional Materials and Chemical Engineering (ICFMCE 2017), Dubai, UAE, 24–26 November 2017; Volume 323, p. 012006. [Google Scholar]
- Han, T.H.; Khan, M.M.; Kalathil, S.; Lee, J.; Cho, M.H. Simultaneous enhancement of methylene blue degradation and power generation in a microbial fuel cell by gold nanoparticles. Ind. Eng. Chem. Res. 2013, 52, 8174–8181. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef]
- Santoso, E.; Ediati, R.; Kusumawati, Y.; Bahruji, H.; Sulistiono, D.O.; Prasetyoko, D. Review on recent advances of carbon based adsorbent for methylene blue removal from waste water. Mater. Today Chem. 2020, 16, 100233. [Google Scholar] [CrossRef]
- Mashkoor, F.; Nasar, A. Magsorbents: Potential candidates in wastewater treatment technology—A review on the removal of methylene blue dye. J. Magn. Magn. Mater. 2020, 500, 166408. [Google Scholar] [CrossRef]
- Zamel, D.; Khan, A.U. Bacterial immobilization on cellulose acetate based nanofibers for methylene blue removal from wastewater: Mini-review. Inorg. Chem. Commun. 2021, 131, 108766. [Google Scholar] [CrossRef]
- Din, M.I.; Khalid, R.; Najeeb, J.; Hussain, Z. Fundamentals and photocatalysis of methylene blue dye using various nanocatalytic assemblies—A critical review. J. Clean. Prod. 2021, 298, 126567. [Google Scholar] [CrossRef]
- Sahu, S.; Pahi, S.; Sahu, J.K.; Sahu, U.K.; Patel, R.K. Kendu (Diospyros melanoxylon Roxb) fruit peel activated carbon—an efficient bioadsorbent for methylene blue dye: Equilibrium, kinetic, and thermodynamic study. Environ. Sci. Pollut. Res. 2020, 27, 22579–22592. [Google Scholar] [CrossRef]
- Amode, J.O.; Santos, J.H.; Md Alam, Z.; Mirza, A.H.; Mei, C.C. Adsorption of methylene blue from aqueous solution using untreated and treated (Metroxylon spp.) waste adsorbent: Equilibrium and kinetics studies. Int. J. Ind. Chem. 2016, 7, 333–345. [Google Scholar] [CrossRef]
- Kuang, Y.; Zhang, X.; Zhou, S. Adsorption of Methylene Blue in Water onto Activated Carbon by Surfactant Modification. Water 2020, 12, 587. [Google Scholar] [CrossRef]
- Makeswari, M.; Saraswathi, P. Photo catalytic degradation of methylene blue and methyl orange from aqueous solution using solar light onto chitosan bi-metal oxide composite. SN Appl. Sci. 2020, 2, 336. [Google Scholar] [CrossRef]
- Sabar, S.; Abdul Aziz, H.; Yusof, N.H.; Subramaniam, S.; Foo, K.Y.; Wilson, L.D.; Lee, H.K. Preparation of sulfonated chitosan for enhanced adsorption of methylene blue from aqueous solution. React. Funct. Polym. 2020, 151, 104584. [Google Scholar] [CrossRef]
- Cheng, J.; Zhan, C.; Wu, J.; Cui, Z.; Si, J.; Wang, Q.; Peng, X.; Turng, L.S. Highly Efficient Removal of Methylene Blue Dye from an Aqueous Solution Using Cellulose Acetate Nanofibrous Membranes Modified by Polydopamine. ACS Omega 2020, 5, 5389–5400. [Google Scholar] [CrossRef]
- Wei, X.; Wang, Y.; Feng, Y.; Xie, X.; Li, X.; Yang, S. Different adsorption-degradation behavior of methylene blue and Congo red in nanoceria/H 2 O 2 system under alkaline conditions. Sci. Rep. 2019, 9, 4964. [Google Scholar] [CrossRef]
- Anushree, C.; Philip, J. Efficient removal of methylene blue dye using cellulose capped Fe3O4 nanofluids prepared using oxidation-precipitation method. Colloids Surf. A Physicochem. Eng. Asp. 2019, 567, 193–204. [Google Scholar] [CrossRef]
- Albayati, T.M.; Sabri, A.A.; Alazawi, R.A. Separation of Methylene Blue as Pollutant of Water by SBA-15 in a Fixed-Bed Column. Arab. J. Sci. Eng. 2016, 41, 2409–2415. [Google Scholar] [CrossRef]
- Saeed, M.; Jamal, M.A.; Haq, A.U.; Ilyas, M.; Younas, M.; Shahzad, M.A. Oxidative Degradation of Methylene Blue in Aqueous Medium Catalyzed by Lab Prepared Nickel Hydroxide. Int. J. Chem. React. Eng. 2016, 14, 45–51. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Lei, Y.; Pan, C. Photocatalytic and degradation mechanisms of anatase TiO2: A HRTEM study. Catal. Sci. Technol. 2011, 1, 273–278. [Google Scholar] [CrossRef]
- Saad, M.E.K.; Mnasri, N.; Mhamdi, M.; Chafik, T.; Elaloui, E.; Moussaoui, Y. Removal of methylene blue onto mineral matrices. Desalin. Water Treat. 2015, 56, 2773–2780. [Google Scholar] [CrossRef]
- Kahlert, H.; Meyer, G.; Albrecht, A. Colour maps of acid–base titrations with colour indicators: How to choose the appropriate indicator and how to estimate the systematic titration errors. ChemTexts 2016, 2, 7. [Google Scholar] [CrossRef]
- Pomicpic, J.; Dancel, G.C.; Cabalar, P.J.; Madrid, J. Methylene blue removal by poly(acrylic acid)-grafted pineapple leaf fiber/polyester nonwoven fabric adsorbent and its comparison with removal by gamma or electron beam irradiation. Radiat. Phys. Chem. 2020, 172, 108737. [Google Scholar] [CrossRef]
- Rao, H.; Lu, Z.; Liu, X.; Ge, H.; Zhang, Z.; Zou, P.; He, H.; Wang, Y. Visible light-driven photocatalytic degradation performance for methylene blue with different multi-morphological features of ZnS. RSC Adv. 2016, 6, 46299–46307. [Google Scholar] [CrossRef]
- Zhu, L.; Hong, M.; Ho, G.W. Fabrication of wheat grain textured TiO2/CuO composite nanofibers for enhanced solar H2 generation and degradation performance. Nano Energy 2015, 11, 28–37. [Google Scholar] [CrossRef]
- Ali, S.; Khan, S.A.; Khan, I.; Yamani, Z.H.; Sohail, M.; Morsy, M.A. Surfactant-free synthesis of ellipsoidal and spherical shaped TiO2 nanoparticles and their comparative photocatalytic studies. J. Environ. Chem. Eng. 2017, 5, 3956–3962. [Google Scholar] [CrossRef]
- Barnes, R.J.; Molina, R.; Xu, J.; Dobson, P.J.; Thompson, I.P. Comparison of TiO2 and ZnO nanoparticles for photocatalytic degradation of methylene blue and the correlated inactivation of gram-positive and gram-negative bacteria. J. Nanopart. Res. 2013, 15, 1432. [Google Scholar] [CrossRef]
- Ashraf, M.; Khan, I.; Usman, M.; Khan, A.; Shah, S.S.; Khan, A.Z.; Saeed, K.; Yaseen, M.; Ehsan, M.F.; Nawaz Tahir, M.; et al. Hematite and Magnetite Nanostructures for Green and Sustainable Energy Harnessing and Environmental Pollution Control: A Review. Chem. Res. Toxicol. 2020, 33, 1292–1311. [Google Scholar] [CrossRef]
- Khan, I.; Khalil, A.; Khanday, F.; Shemsi, A.M.; Qurashi, A.; Siddiqui, K.S. Synthesis, Characterization and Applications of Magnetic Iron Oxide Nanostructures. Arab. J. Sci. Eng. 2018, 43, 43–61. [Google Scholar] [CrossRef]
- Khan, I.; Qurashi, A. Sonochemical-Assisted In Situ Electrochemical Synthesis of Ag/α-Fe2O3/TiO2 Nanoarrays to Harness Energy from Photoelectrochemical Water Splitting. ACS Sustain. Chem. Eng. 2018, 6, 11235–11245. [Google Scholar] [CrossRef]
- Sarfraz, N.; Khan, I.; Sarfaraz, N.; Khan, I.; Sarfraz, N.; Khan, I. Plasmonic Gold Nanoparticles (AuNPs): Properties, Synthesis and their Advanced Energy, Environmental and Biomedical Applications. Chem.—Asian J. 2021, 16, 720–742. [Google Scholar] [CrossRef]
- Lu, D.; Wang, H.; Zhao, X.; Kondamareddy, K.K.; Ding, J.; Li, C.; Fang, P. Highly efficient visible-light-induced photoactivity of Z-scheme g-C3N4/Ag/MoS2 ternary photocatalysts for organic pollutant degradation and production of hydrogen. ACS Sustain. Chem. Eng. 2017, 5, 1436–1445. [Google Scholar] [CrossRef]
- Ghafoor, S.; Ata, S.; Mahmood, N.; Arshad, S.N. Photosensitization of TiO2 nanofibers by Ag2S with the synergistic effect of excess surface Ti3+ states for enhanced photocatalytic activity under simulated sunlight. Sci. Rep. 2017, 7, 255. [Google Scholar] [CrossRef]
- Ashraf, M.; Khan, I.; Baig, N.; Hendi, A.H.; Ehsan, M.F.; Sarfraz, N. A Bifunctional 2D Interlayered β-Cu2V2O7/Zn2V2O6 (CZVO) Heterojunction for Solar-Driven Nonsacrificial Dye Degradation and Water Oxidation. Energy Technol. 2021, 9, 2100034. [Google Scholar] [CrossRef]
- Khan, I.; Qurashi, A. Shape Controlled Synthesis of Copper Vanadate Platelet Nanostructures, Their Optical Band Edges, and Solar-Driven Water Splitting Properties. Sci. Rep. 2017, 7, 14370. [Google Scholar] [CrossRef]
- Arumugam, M.; Choi, M.Y. Effect of Operational Parameters on the Degradation of Methylene Blue Using Visible Light Active BiVO4 Photocatalyst. Bull. Korean Chem. Soc. 2020, 41, 304–309. [Google Scholar] [CrossRef]
- Guo, W.; Chemelewski, W.D.; Mabayoje, O.; Xiao, P.; Zhang, Y.; Mullins, C.B. Synthesis and Characterization of CuV2O6 and Cu2V2O7: Two Photoanode Candidates for Photoelectrochemical Water Oxidation. J. Phys. Chem. C 2015, 119, 27220–27227. [Google Scholar] [CrossRef]
- Lamdab, U.; Wetchakun, K.; Phanichphant, S.; Kangwansupamonkon, W.; Wetchakun, N. In VO4–BiVO4 composite films with enhanced visible light performance for photodegradation of methylene blue. Catal. Today 2016, 278, 291–302. [Google Scholar] [CrossRef]
- Tyagi, D.; Wang, H.; Huang, W.; Hu, L.; Tang, Y.; Guo, Z.; Ouyang, Z.; Zhang, H. Recent advances in two-dimensional-material-based sensing technology toward health and environmental monitoring applications. Nanoscale 2020, 12, 3535–3559. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, C.; Lu, X.; Mu, X.; Song, P. Effects of defects in g-C3N4 on excited-state charge distribution and transfer: Potential for improved photocatalysis. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2020, 227, 117687. [Google Scholar] [CrossRef]
- Simon, Y.D.T.; Hadis, B.; Estella, T.N.; Arabamiri, M.; Serges, D.; Arnaud, K.T.; Samuel, L.; Minoo, T.; Michael, S.; Reinhard, S. Urea and green tea like precursors for the preparation of g-C3N4 based carbon nanomaterials (CNMs) composites as photocatalysts for photodegradation of pollutants under UV light irradiation. J. Photochem. Photobiol. A Chem. 2020, 398, 112596. [Google Scholar] [CrossRef]
- Ai, B.; Duan, X.; Sun, H.; Qiu, X.; Wang, S. Metal-free graphene-carbon nitride hybrids for photodegradation of organic pollutants in water. Catal. Today 2015, 258, 668–675. [Google Scholar] [CrossRef]
- de Oliveira Guidolin, T.; Possolli, N.M.; Polla, M.B.; Wermuth, T.B.; Franco de Oliveira, T.; Eller, S.; Klegues Montedo, O.R.; Arcaro, S.; Cechinel, M.A.P. Photocatalytic pathway on the degradation of methylene blue from aqueous solutions using magnetite nanoparticles. J. Clean. Prod. 2021, 318, 128556. [Google Scholar] [CrossRef]
- Chen, H.; Chen, N.; Gao, Y.; Feng, C. Photocatalytic degradation of methylene blue by magnetically recoverable Fe3O4/Ag6Si2O7 under simulated visible light. Powder Technol. 2018, 326, 247–254. [Google Scholar] [CrossRef]
- Chakraborty, B.; Ray, L.; Basu, S. Biochemical degradation of Methylene Blue using a continuous reactor packed with solid waste by E. coli and Bacillus subtilis isolated from wetland soil. Desalin. Water Treat. 2016, 57, 14077–14082. [Google Scholar] [CrossRef]
- Wijaya, R.; Andersan, G.; Permatasari Santoso, S.; Irawaty, W. Green Reduction of Graphene Oxide using Kaffir Lime Peel Extract (Citrus hystrix) and Its Application as Adsorbent for Methylene Blue. Sci. Rep. 2020, 10, 667. [Google Scholar] [CrossRef]
- Mahmoud, M.S.; Farah, J.Y.; Farrag, T.E. Enhanced removal of Methylene Blue by electrocoagulation using iron electrodes. Egypt. J. Pet. 2013, 22, 211–216. [Google Scholar] [CrossRef]
- Jia, P.; Tan, H.; Liu, K.; Gao, W. Removal of Methylene Blue from Aqueous Solution by Bone Char. Appl. Sci. 2018, 8, 1903. [Google Scholar] [CrossRef]
- Bayomie, O.S.; Kandeel, H.; Shoeib, T.; Yang, H.; Youssef, N.; El-Sayed, M.M.H. Novel approach for effective removal of methylene blue dye from water using fava bean peel waste. Sci. Rep. 2020, 10, 7824. [Google Scholar] [CrossRef]
- Sousa, H.R.; Silva, L.S.; Sousa, P.A.A.; Sousa, R.R.M.; Fonseca, M.G.; Osajima, J.A.; Silva-Filho, E.C. Evaluation of methylene blue removal by plasma activated palygorskites. J. Mater. Res. Technol. 2019, 8, 5432–5442. [Google Scholar] [CrossRef]
- Salimi, A.; Roosta, A. Experimental solubility and thermodynamic aspects of methylene blue in different solvents. Thermochim. Acta 2019, 675, 134–139. [Google Scholar] [CrossRef]
- Pham, V.L.; Kim, D.-G.; Ko, S.-O. Mechanisms of Methylene Blue Degradation by Nano-Sized β-MnO2 Particles. KSCE J. Civ. Eng. 2020, 24, 1976–3808. [Google Scholar] [CrossRef]
- Potential Biosorbent Derived from Calligonum Polygonoides for Removal of Methylene Blue Dye from Aqueous Solution. Available online: https://www.hindawi.com/journals/tswj/2015/562693/ (accessed on 1 July 2020).
- Kazemi, F.; Mohamadnia, Z.; Kaboudin, B.; Karimi, Z. Photodegradation of methylene blue with a titanium dioxide/polyacrylamide photocatalyst under sunlight. J. Appl. Polym. Sci. 2016, 133, 43386. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, Y.; Liu, S. Graphene quantum dots enhanced photocatalytic activity of zinc porphyrin toward the degradation of methylene blue under visible-light irradiation. J. Mater. Chem. A 2015, 3, 8552–8558. [Google Scholar] [CrossRef]
- Huang, S.; Li, H.; Wang, Y.; Liu, X.; Li, H.; Zhan, Z.; Jia, L.; Chen, L. Monitoring of oxygen using colorimetric indicator based on graphene/TiO2 composite with first-order kinetics of methylene blue for modified atmosphere packaging. Packag. Technol. Sci. 2018, 31, 575–584. [Google Scholar] [CrossRef]
- Yang, C.; Dong, W.; Cui, G.; Zhao, Y.; Shi, X.; Xia, X.; Tang, B.; Wang, W. Highly efficient photocatalytic degradation of methylene blue by P2ABSA-modified TiO2 nanocomposite due to the photosensitization synergetic effect of TiO2 and P2ABSA. RSC Adv. 2017, 7, 23699–23708. [Google Scholar] [CrossRef]
- Mondal, S.; De Anda Reyes, M.E.; Pal, U. Plasmon induced enhanced photocatalytic activity of gold loaded hydroxyapatite nanoparticles for methylene blue degradation under visible light. RSC Adv. 2017, 7, 8633–8645. [Google Scholar] [CrossRef]
- Lin, J.; Luo, Z.; Liu, J.; Li, P. Photocatalytic degradation of methylene blue in aqueous solution by using ZnO-SnO2 nanocomposites. Mater. Sci. Semicond. Process. 2018, 87, 24–31. [Google Scholar] [CrossRef]
- Xia, Y.; Yao, Q.; Zhang, W.; Zhang, Y.; Zhao, M. Comparative adsorption of methylene blue by magnetic baker’s yeast and EDTAD-modified magnetic baker’s yeast: Equilibrium and kinetic study. Arab. J. Chem. 2019, 12, 2448–2456. [Google Scholar] [CrossRef]
- Xu, A.; Li, X.; Ye, S.; Yin, G.; Zeng, Q. Catalyzed oxidative degradation of methylene blue by in situ generated cobalt (II)-bicarbonate complexes with hydrogen peroxide. Appl. Catal. B Environ. 2011, 102, 37–43. [Google Scholar] [CrossRef]
- Teng, X.; Li, J.; Wang, Z.; Wei, Z.; Chen, C.; Du, K.; Zhao, C.; Yang, G.; Li, Y. Performance and mechanism of methylene blue degradation by an electrochemical process. RSC Adv. 2020, 10, 24712–24720. [Google Scholar] [CrossRef]
- Dao, H.M.; Whang, C.H.; Shankar, V.K.; Wang, Y.H.; Khan, I.A.; Walker, L.A.; Husain, I.; Khan, S.I.; Murthy, S.N.; Jo, S. Methylene blue as a far-red light-mediated photocleavable multifunctional ligand. Chem. Commun. 2020, 56, 1673–1676. [Google Scholar] [CrossRef]
- Hou, C.; Hu, B.; Zhu, J. Photocatalytic Degradation of Methylene Blue over TiO2 Pretreated with Varying Concentrations of NaOH. Catalysts 2018, 8, 575. [Google Scholar] [CrossRef]
- Lu, G.; Nagbanshi, M.; Goldau, N.; Mendes Jorge, M.; Meissner, P.; Jahn, A.; Mockenhaupt, F.P.; Müller, O. Efficacy and safety of methylene blue in the treatment of malaria: A systematic review. BMC Med. 2018, 16, 59. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, R.H.; Coulibaly, B.; Stich, A.; Scheiwein, M.; Merkle, H.; Eubel, J.; Becker, K.; Becher, H.; Müller, O.; Zich, T.; et al. Methylene blue as an antimalarial agent. Redox Rep. 2003, 8, 272–275. [Google Scholar] [CrossRef]
- Uddin, M.K.; Nasar, A. Decolorization of Basic Dyes Solution by Utilizing Fruit Seed Powder. KSCE J. Civ. Eng. 2020, 24, 345–355. [Google Scholar] [CrossRef]
- Saha, B.; Chowdhury, S.; Sanyal, D.; Chattopadhyay, K.; Suresh Kumar, G. Comparative Study of Toluidine Blue O and Methylene Blue Binding to Lysozyme and Their Inhibitory Effects on Protein Aggregation. ACS Omega 2018, 3, 2588–2601. [Google Scholar] [CrossRef] [PubMed]
- Oz, M.; Lorke, D.E.; Hasan, M.; Petroianu, G.A. Cellular and molecular actions of Methylene Blue in the nervous system. Med. Res. Rev. 2011, 31, 93–117. [Google Scholar] [CrossRef]
- Marimuthu, M.; Praveen Kumar, B.; Mariya Salomi, L.; Veerapandian, M.; Balamurugan, K. Methylene Blue-Fortified Molybdenum Trioxide Nanoparticles: Harnessing Radical Scavenging Property. ACS Appl. Mater. Interfaces 2018, 10, 43429–43438. [Google Scholar] [CrossRef]
- Lo, J.C.Y.; Darracq, M.A.; Clark, R.F. A review of methylene blue treatment for cardiovascular collapse. J. Emerg. Med. 2014, 46, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Nedu, M.E.; Tertis, M.; Cristea, C.; Georgescu, A.V. Comparative study regarding the properties of methylene blue and proflavine and their optimal concentrations for in vitro and in vivo applications. Diagnostics 2020, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Koyuncu, H.; Kul, A.R. Removal of methylene blue dye from aqueous solution by nonliving lichen (Pseudevernia furfuracea (L.) Zopf.), as a novel biosorbent. Appl. Water Sci. 2020, 10, 72. [Google Scholar] [CrossRef]
- Mijinyawa, A.H.; Durga, G.; Mishra, A. A sustainable process for adsorptive removal of methylene blue onto a food grade mucilage: Kinetics, thermodynamics, and equilibrium evaluation. Int. J. Phytoremediat. 2019, 21, 1122–1129. [Google Scholar] [CrossRef]
- Parakala, S.; Moulik, S.; Sridhar, S. Effective separation of methylene blue dye from aqueous solutions by integration of micellar enhanced ultrafiltration with vacuum membrane distillation. Chem. Eng. J. 2019, 375, 122015. [Google Scholar] [CrossRef]
- Balarak, D.; Bazzi, M.; Shehu, Z.; Chandrika, K. Application of Surfactant-Modified Bentonite for Methylene Blue Adsorption from Aqueous Solution. Orient. J. Chem. 2020, 36, 293–299. [Google Scholar] [CrossRef]
- Arias Arias, F.; Guevara, M.; Tene, T.; Angamarca, P.; Molina, R.; Valarezo, A.; Salguero, O.; Vacacela Gomez, C.; Arias, M.; Caputi, L.S. The Adsorption of Methylene Blue on Eco-Friendly Reduced Graphene Oxide. Nanomaterials 2020, 10, 681. [Google Scholar] [CrossRef] [PubMed]
- Siddeeg, S.M.; Tahoon, M.A.; Mnif, W.; Ben Rebah, F. Iron Oxide/Chitosan Magnetic Nanocomposite Immobilized Manganese Peroxidase for Decolorization of Textile Wastewater. Processes 2019, 8, 5. [Google Scholar] [CrossRef]
- Manimohan, M.; Pugalmani, S.; Ravichandran, K.; Sithique, M.A. Synthesis and characterisation of novel Cu(II)-anchored biopolymer complexes as reusable materials for the photocatalytic degradation of methylene blue. RSC Adv. 2020, 10, 18259–18279. [Google Scholar] [CrossRef]
- Giannakopoulou, P.P.; Petrounias, P.; Rogkala, A.; Lampropoulou, P.; Gianni, E.; Papoulis, D.; Koutsovitis, P.; Tsikouras, B.; Hatzipanagiotou, K. Does the Methylene Blue Test Give Equally Satisfactory Results in All Studied Igneous Rocks Relative to the Identification of Swelling Clay Minerals? Minerals 2020, 10, 283. [Google Scholar] [CrossRef]
- Zaghbani, N.; Hafiane, A.; Dhahbi, M. Separation of methylene blue from aqueous solution by micellar enhanced ultrafiltration. Sep. Purif. Technol. 2007, 55, 117–124. [Google Scholar] [CrossRef]
- De Crozals, G.; Farre, C.; Sigaud, M.; Fortgang, P.; Sanglar, C.; Chaix, C. Methylene blue phosphoramidite for DNA labelling. Chem. Commun. 2015, 51, 4458–4461. [Google Scholar] [CrossRef]
- Dante, R.C.; Trakulmututa, J.; Meejoo-Smith, S.; Martín-Ramos, P.; Chamorro-Posada, P.; Rutto, D.; Sánchez-Arévalo, F.M. Methylene blue-carbon nitride system as a reusable air-sensor. Mater. Chem. Phys. 2019, 231, 351–356. [Google Scholar] [CrossRef]
- A Novel of Buton Asphalt and Methylene Blue as Dye-Sensitized Solar Cell using TiO2/Ti Nanotubes Electrode—IOPscience. Available online: https://iopscience.iop.org/article/10.1088/1757-899X/267/1/012035 (accessed on 14 May 2020).
- Reda, S.M.; El-Sherbieny, S.A. Dye-sensitized nanocrystalline CdS and ZnS solar cells with different organic dyes. J. Mater. Res. 2010, 25, 522–528. [Google Scholar] [CrossRef]
- Zhang, Y.; An, Y.; Wu, L.; Chen, H.; Li, Z.; Dou, H.; Murugadoss, V.; Fan, J.; Zhang, X.; Mai, X.; et al. Metal-free energy storage systems: Combining batteries with capacitors based on a methylene blue functionalized graphene cathode. J. Mater. Chem. A 2019, 7, 19668–19675. [Google Scholar] [CrossRef]
- López-Carballo, G.; Muriel-Galet, V.; Hernández-Muñoz, P.; Gavara, R. Gavara Chromatic Sensor to Determine Oxygen Presence for Applications in Intelligent Packaging. Sensors 2019, 19, 4684. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Dias, S.L.P.; Rodrigues, J.R.; Pavan, F.A.; Benvenutti, E.V.; Lima, E.C. Methylene blue immobilized on cellulose acetate with titanium dioxide: An application as sensor for ascorbic acid. J. Braz. Chem. Soc. 2008, 19, 943–949. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Najafpour, G.D.; Ghoreyshi, A.A.; Shakeri, M.; Zare, H. Methylene blue as electron promoters in microbial fuel cell. Int. J. Hydrogen Energy 2011, 36, 13335–13341. [Google Scholar] [CrossRef]
- Pang, Y.; Tong, Z.H.; Tang, L.; Liu, Y.N.; Luo, K. Effect of humic acid on the degradation of methylene blue by peroxymonosulfate. Open Chem. 2018, 16, 401–406. [Google Scholar] [CrossRef]
- Sun, L.; Hu, D.; Zhang, Z.; Deng, X. Oxidative degradation of methylene blue via PDS-based advanced oxidation process using natural pyrite. Int. J. Environ. Res. Public Health 2019, 16, 4773. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.; Grande-Tovar, C.D.; Vallejo, W.; Chaves-López, C. Bio-Removal of Methylene Blue from Aqueous Solution by Galactomyces geotrichum KL20A. Water 2019, 11, 282. [Google Scholar] [CrossRef]
- Abdelrahman, E.A.; Hegazey, R.M.; El-Azabawy, R.E. Efficient removal of methylene blue dye from aqueous media using Fe/Si, Cr/Si, Ni/Si, and Zn/Si amorphous novel adsorbents. J. Mater. Res. Technol. 2019, 8, 5301–5313. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S.; Mastuli, M.S. Acid-factionalized biomass material for methylene blue dye removal: A comprehensive adsorption and mechanism study. J. Taibah Univ. Sci. 2020, 14, 305–313. [Google Scholar] [CrossRef]
- Cusioli, L.F.; Quesada, H.B.; Baptista, A.T.A.; Gomes, R.G.; Bergamasco, R. Soybean hulls as a low-cost biosorbent for removal of methylene blue contaminant. Environ. Prog. Sustain. Energy 2020, 39, e13328. [Google Scholar] [CrossRef]
- Staroń, P.; Chwastowski, J.; Banach, M. Sorption behavior of methylene blue from aqueous solution by raphia fibers. Int. J. Environ. Sci. Technol. 2019, 16, 8449–8460. [Google Scholar] [CrossRef]
- Lebron, Y.A.R.; Moreira, V.R.; de Souza Santos, L.V. Biosorption of methylene blue and eriochrome black T onto the brown macroalgae Fucus vesiculosus: Equilibrium, kinetics, thermodynamics and optimization. Environ. Technol. 2019, 42, 279–297. [Google Scholar] [CrossRef]
- Mabel, M.M.; Sundararaman, T.R.; Parthasarathy, N.; Rajkumar, J. Chitin Beads from Peneaus sp. Shells asa Biosorbent for Methylene Blue Dye Removal. Pol. J. Environ. Stud. 2019, 28, 2253–2259. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, Q.; Akhtar, N.; Chen, X.; Huang, Y. Microporous carbon material from fish waste for removal of methylene blue from wastewater. Water Sci. Technol. 2020, 86, 1180–1190. [Google Scholar] [CrossRef] [PubMed]
- Bharti, V.; Vikrant, K.; Goswami, M.; Tiwari, H.; Sonwani, R.K.; Lee, J.; Tsang, D.C.W.; Kim, K.H.; Saeed, M.; Kumar, S.; et al. Biodegradation of methylene blue dye in a batch and continuous mode using biochar as packing media. Environ. Res. 2019, 171, 356–364. [Google Scholar] [CrossRef]
- Shakoor, S.; Nasar, A. Adsorptive treatment of hazardous methylene blue dye from artificially contaminated water using cucumis sativus peel waste as a low-cost adsorbent. Groundw. Sustain. Dev. 2017, 5, 152–159. [Google Scholar] [CrossRef]
- Ebi Ebi, O.; Falilat Taiwo, A.; Tunde Folorunsho, A. Kinetic Modelling of the Biosorption of Methylene Blue onto Wild Melon (Lagenariasphaerica). Am. J. Chem. Eng. 2018, 6, 126–134. [Google Scholar] [CrossRef]
- Zhou, S.; Du, Z.; Li, X.; Zhang, Y.; He, Y.; Zhang, Y. Degradation of methylene blue by natural manganese oxides: Kinetics and transformation products. R. Soc. Open Sci. 2019, 6, 190351. [Google Scholar] [CrossRef]
- Lawagon, C.P.; Amon, R.E.C. Magnetic rice husk ash “cleanser” as efficient methylene blue adsorbent. Environ. Eng. Res. 2019, 25, 685–692. [Google Scholar] [CrossRef]
- Ahmad Zaini, M.A.; Sudi, R.M. Valorization of human hair as methylene blue dye adsorbents. Green Process. Synth. 2018, 7, 344–352. [Google Scholar] [CrossRef]
- Kosswattaarachchi, A.M.; Cook, T.R. Repurposing the Industrial Dye Methylene Blue as an Active Component for Redox Flow Batteries. ChemElectroChem 2018, 5, 3437–3442. [Google Scholar] [CrossRef]
- Zamel, D.; Hassanin, A.H.; Ellethy, R.; Singer, G.; Abdelmoneim, A. Novel Bacteria-Immobilized Cellulose Acetate/Poly(ethylene oxide) Nanofibrous Membrane for Wastewater Treatment. Sci. Rep. 2019, 9, 18994. [Google Scholar] [CrossRef]
- Thabede, P.M.; Shooto, N.D.; Naidoo, E.B. Removal of methylene blue dye and lead ions from aqueous solution using activated carbon from black cumin seeds. S. Afr. J. Chem. Eng. 2020, 33, 39–50. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, M.; Li, X.; Ning, J.; Zhou, Z.; Li, G. Efficient adsorption of methylene blue from aqueous solution by graphene oxide modified persimmon tannins. Mater. Sci. Eng. C 2020, 108, 110196. [Google Scholar] [CrossRef] [PubMed]
- Andrade Siqueira, T.C.; Zanette da Silva, I.; Rubio, A.J.; Bergamasco, R.; Gasparotto, F.; Aparecida de Souza Paccola, E.; Ueda Yamaguchi, N. Sugarcane Bagasse as an Efficient Biosorbent for Methylene Blue Removal: Kinetics, Isotherms and Thermodynamics. Int. J. Environ. Res. Public Health 2020, 17, 526. [Google Scholar] [CrossRef] [PubMed]
- Regunton, P.C.V.; Sumalapao, D.E.P.; Villarante, N.R. Biosorption of methylene blue from aqueous solution by coconut (Cocos nucifera) shell-derived activated carbon-chitosan composite. Orient. J. Chem. 2018, 34, 115–124. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.; Singh, S.P.; Badhulika, S. Reusable, few-layered-MoS2 nanosheets/graphene hybrid on cellulose paper for superior adsorption of methylene blue dye. New J. Chem. 2020, 44, 5489–5500. [Google Scholar] [CrossRef]
- Hameed, A.M. Synthesis of Si/Cu Amorphous Adsorbent for Efficient Removal of Methylene Blue Dye from Aqueous Media. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2881–2889. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.; Cui, J.; Cui, J.; Wang, F.; Zhang, F. High-efficiency adsorption and regeneration of methylene blue and aniline onto activated carbon from waste edible fungus residue and its possible mechanism. RSC Adv. 2020, 10, 14262–14273. [Google Scholar] [CrossRef]
- Imron, M.F.; Kurniawan, S.B.; Soegianto, A.; Wahyudianto, F.E. Phytoremediation of methylene blue using duckweed (Lemna minor). Heliyon 2019, 5, e02206. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.A.; Morad, N.; Ooi, J.Q. Phytoremediation of Methylene Blue and Methyl Orange Using Eichhornia crassipes. Int. J. Environ. Sci. Dev. 2016, 7, 724–728. [Google Scholar] [CrossRef]
- Lau, Y.Y.; Wong, Y.S.; Teng, T.T.; Morad, N.; Rafatullah, M.; Ong, S.A. Degradation of cationic and anionic dyes in coagulation-flocculation process using bi-functionalized silica hybrid with aluminum-ferric as auxiliary agent. RSC Adv. 2015, 5, 34206–34215. [Google Scholar] [CrossRef]
- Liu, J.; Li, P.; Xiao, H.; Zhang, Y.; Shi, X.; Lü, X.; Chen, X. Understanding flocculation mechanism of graphene oxide for organic dyes from water: Experimental and molecular dynamics simulation. AIP Adv. 2015, 5, 117151. [Google Scholar] [CrossRef]
- Tir, M.; Moulai-Mostefa, N.; Nedjhioui, M. Optimizing decolorization of methylene blue dye by electrocoagulation using Taguchi approach. Desalin. Water Treat. 2015, 55, 2705–2710. [Google Scholar] [CrossRef]
- Banat, F.; Al-Asheh, S.; Qtaishat, M. Treatment of waters colored with methylene blue dye by vacuum membrane distillation. Desalination 2005, 174, 87–96. [Google Scholar] [CrossRef]
- El-Ashtoukhy, E.S.Z.; Fouad, Y.O. Liquid-liquid extraction of methylene blue dye from aqueous solutions using sodium dodecylbenzenesulfonate as an extractant. Alex. Eng. J. 2015, 54, 77–81. [Google Scholar] [CrossRef]
- Zheng, L.; Su, Y.; Wang, L.; Jiang, Z. Adsorption and recovery of methylene blue from aqueous solution through ultrafiltration technique. Sep. Purif. Technol. 2009, 68, 244–249. [Google Scholar] [CrossRef]
- Khosa, M.A.; Shah, S.S.; Nazar, M.F. Application of micellar enhanced ultrafiltration for the removal of methylene blue from aqueous solution. J. Dispers. Sci. Technol. 2011, 32, 260–264. [Google Scholar] [CrossRef]
- Kim, S.; Yu, M.; Yoon, Y. Fouling and Retention Mechanisms of Selected Cationic and Anionic Dyes in a Ti3C2Tx MXene-Ultrafiltration Hybrid System. ACS Appl. Mater. Interfaces 2020, 12, 16557–16565. [Google Scholar] [CrossRef]
- Kong, G.; Pang, J.; Tang, Y.; Fan, L.; Sun, H.; Wang, R.; Feng, S.; Feng, Y.; Fan, W.; Kang, W.; et al. Efficient dye nanofiltration of a graphene oxide membrane: Via combination with a covalent organic framework by hot pressing. J. Mater. Chem. A 2019, 7, 24301–24310. [Google Scholar] [CrossRef]
- Cheng, S.; Oatley, D.L.; Williams, P.M.; Wright, C.J. Characterisation and application of a novel positively charged nanofiltration membrane for the treatment of textile industry wastewaters. Water Res. 2012, 46, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.; Wang, P.; He, Y.; Chen, C.; Li, H.; Yu, H.; Chen, J. Preparation of stable and superior flux GO/LDH/PDA-based nanofiltration membranes through electrostatic self-assembly for dye purification. Polym. Adv. Technol. 2019, 30, 1644–1655. [Google Scholar] [CrossRef]
- García, M.C.; Mora, M.; Esquivel, D.; Foster, J.E.; Rodero, A.; Jiménez-Sanchidrián, C.; Romero-Salguero, F.J. Microwave atmospheric pressure plasma jets for wastewater treatment: Degradation of methylene blue as a model dye. Chemosphere 2017, 180, 239–246. [Google Scholar] [CrossRef]
- Eslami, H.; Sedighi Khavidak, S.; Salehi, F.; Khosravi, R.; Fallahzadeh, R.A.; Peirovi, R.; Sadeghi, S. Biodegradation of methylene blue from aqueous solution by bacteria isolated from contaminated soil. J. Adv. Environ. Health Res. 2017, 5, 10–15. [Google Scholar] [CrossRef]
- Kilany, M. Isolation, screening and molecular identification of novel bacterial strain removing methylene blue from water solutions. Appl. Water Sci. 2017, 7, 4091–4098. [Google Scholar] [CrossRef]
- Van Der Maas, A.S.; Da Silva, N.J.R.; Da Costa, A.S.V.; Barros, A.R.; Bomfeti, C.A. The degradation of methylene blue dye by the strains of pleurotus sp. With potential applications in bioremediation processes. Rev. Ambient. Agua 2018, 13, e2247. [Google Scholar] [CrossRef]
- Naresh Yadav, D.; Anand Kishore, K.; Saroj, D. A Study on removal of Methylene Blue dye by photo catalysis integrated with nanofiltration using statistical and experimental approaches. Environ. Technol. 2020, 42, 2968–2981. [Google Scholar] [CrossRef]
- Nguyet, P.N.; Watari, T.; Hirakata, Y.; Hatamoto, M.; Yamaguchi, T. Adsorption and biodegradation removal of methylene blue in a down-flow hanging filter reactor incorporating natural adsorbent. Environ. Technol. 2019, 42, 410–418. [Google Scholar] [CrossRef]
- Lee, H.; Park, S.H.; Kim, B.H.; Kim, S.J.; Kim, S.C.; Seo, S.G.; Jung, S.C. Contribution of dissolved oxygen to methylene blue decomposition by hybrid advanced oxidation processes system. Int. J. Photoenergy 2012, 2012, 305989. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, S.; Lin, Z.; Yang, J.; Li, C.; Gu, R. Combination of plasma oxidation process with microbial fuel cell for mineralizing methylene blue with high energy efficiency. J. Hazard. Mater. 2020, 384, 121307. [Google Scholar] [CrossRef]
- Liu, Q.-X.; Zhou, Y.-R.; Wang, M.; Zhang, Q.; Ji, T.; Chen, T.-Y.; Yu, D.-C. Adsorption of methylene blue from aqueous solution onto viscose-based activated carbon fiber felts: Kinetics and equilibrium studies. Adsorpt. Sci. Technol. 2019, 37, 312–332. [Google Scholar] [CrossRef]
- Liu, L.; He, D.; Pan, F.; Huang, R.; Lin, H.; Zhang, X. Comparative study on treatment of methylene blue dye wastewater by different internal electrolysis systems and COD removal kinetics, thermodynamics and mechanism. Chemosphere 2020, 238, 124671. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Photocatalysts in Advanced Oxidation Processes for Wastewater Treatment; John Wiley & Sons: Hoboken, NJ, USA, 2020. [CrossRef]
- Khan, I.; Saeed, K.; Ali, N.; Khan, I.; Zhang, B.; Sadiq, M. Heterogeneous photodegradation of industrial dyes: An insight to different mechanisms and rate affecting parameters. J. Environ. Chem. Eng. 2020, 8, 104364. [Google Scholar] [CrossRef]
- Zhang, L.C.; Jia, Z.; Lyu, F.; Liang, S.X.; Lu, J. A review of catalytic performance of metallic glasses in wastewater treatment: Recent progress and prospects. Prog. Mater. Sci. 2019, 105, 100576. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, D.; Zhang, S.; Zhang, X.; Fan, P. Ozonation and Carbon-assisted Ozonation of Methylene Blue as Model Compound: Effect of Solution pH. Procedia Environ. Sci. 2013, 18, 493–502. [Google Scholar] [CrossRef]
- Athikoh, N.; Yulianto, E.; Wibowo Kinandana, A.; Sasmita, E.; Husein Sanjani, A.; Wahyu Mustika, R.; Putra Pratama, A.; Farida Amalia, N.; Gunawan, G.; Nur, M. Reduction of Methylene Blue by Using Direct Continuous Ozone. J. Environ. Earth Sci. 2020, 10, 46–56. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Khaleefa, S.A.; Basheer, M.I. Photolysis of Methylene Blue Dye Using an Advanced Oxidation Process (Ultraviolet Light and Hydrogen Peroxide). J. Eng. Sustain. Dev. 2021, 25, 59–67. [Google Scholar] [CrossRef]
- Jawad, N.H.; Najim, S.T. Removal of Methylene Blue by Direct Electrochemical Oxidation Method Using a Graphite Anode. IOP Conf. Ser. Mater. Sci. Eng. 2018, 454, 012023. [Google Scholar] [CrossRef]
- Kim, J.; Yeom, C.; Kim, Y. Electrochemical degradation of organic dyes with a porous gold electrode. Korean J. Chem. Eng. 2016, 33, 1855–1859. [Google Scholar] [CrossRef]
- Guergueb, M.; Nasri, S.; Brahmi, J.; Loiseau, F.; Molton, F.; Roisnel, T.; Guerineau, V.; Turowska-Tyrk, I.; Aouadi, K.; Nasri, H. Effect of the coordination of π-acceptor 4-cyanopyridine ligand on the structural and electronic properties of: Meso-tetra(para-methoxy) and meso-tetra(para-chlorophenyl) porphyrin cobalt(ii) coordination compounds. Application in the catalytic degradation of methylene blue dye. RSC Adv. 2020, 10, 6900–6918. [Google Scholar] [CrossRef]
- Choquehuanca, A.; Ruiz-Montoya, J.G.; Gómez, A.L.R.-T. Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides. Open Chem. 2021, 19, 1009–1020. [Google Scholar] [CrossRef]
- Ahmed, Y.; Yaakob, Z.; Akhtar, P. Degradation and mineralization of methylene blue using a heterogeneous photo-Fenton catalyst under visible and solar light irradiation. Catal. Sci. Technol. 2016, 6, 1222–1232. [Google Scholar] [CrossRef]
- Dzinun, H.; Ichikawa, Y.; Honda, M.; Zhang, Q. Efficient Immobilised TiO2 in Polyvinylidene fluoride (PVDF) Membrane for Photocatalytic Degradation of Methylene Blue Graphical abstract Keywords. J. Membr. Sci. Res. 2020, 6, 188–195. [Google Scholar] [CrossRef]
- Photocatalytic Degradation of Methylene Blue under Visible Light by Dye Sensitized Titania—IOPscience. Available online: https://iopscience.iop.org/article/10.1088/2053-1591/ab6409 (accessed on 9 May 2020).
- Krishnan, S.; Rawindran, H.; Sinnathambi, C.M.; Lim, J.W. Comparison of various advanced oxidation processes used in remediation of industrial wastewater laden with recalcitrant pollutants. IOP Conf. Ser. Mater. Sci. Eng. 2017, 206, 012089. [Google Scholar] [CrossRef]
- Anglada, Á.; Urtiaga, A.; Ortiz, I. Contributions of electrochemical oxidation to waste-water treatment: Fundamentals and review of applications. J. Chem. Technol. Biotechnol. 2009, 84, 1747–1755. [Google Scholar] [CrossRef]
- Balakumara, R.; Sathya, K.; Saravanathamizhan, R. Decolorization of Methylene Blue Dye Using Sonocatalytic Followed by Photocatalytic Process. Water Conserv. Sci. Eng. 2016, 1, 161–166. [Google Scholar] [CrossRef][Green Version]
- Sandoval, A.; Hernández-Ventura, C.; Klimova, T.E. Titanate nanotubes for removal of methylene blue dye by combined adsorption and photocatalysis. Fuel 2017, 198, 22–30. [Google Scholar] [CrossRef]
- Xiong, J.; Guo, S.; Zhao, T.; Liang, Y.; Liang, J.; Wang, S.; Zhu, H.; Zhang, L.; Zhao, J.R.; Chen, G. Degradation of methylene blue by intimate coupling photocatalysis and biodegradation with bagasse cellulose composite carrier. Cellulose 2020, 27, 3391–3404. [Google Scholar] [CrossRef]
- Chandra, R.; Nath, M. Controlled synthesis of AgNPs@ZIF-8 composite: Efficient heterogeneous photocatalyst for degradation of methylene blue and congo red. J. Water Process Eng. 2020, 36, 101266. [Google Scholar] [CrossRef]
- Khaing, K.K.; Yin, D.; Ouyang, Y.; Xiao, S.; Liu, B.; Deng, L.; Li, L.; Guo, X.; Wang, J.; Liu, J.; et al. Fabrication of 2D–2D Heterojunction Catalyst with Covalent Organic Framework (COF) and MoS 2 for Highly Efficient Photocatalytic Degradation of Organic Pollutants. Inorg. Chem. 2020, 59, 6942–6952. [Google Scholar] [CrossRef] [PubMed]
- Madkour, M.; Allam, O.G.; Abdel Nazeer, A.; Amin, M.O.; Al-Hetlani, E. CeO2-based nanoheterostructures with p–n and n–n heterojunction arrangements for enhancing the solar-driven photodegradation of rhodamine 6G dye. J. Mater. Sci. Mater. Electron. 2019, 30, 10857–10866. [Google Scholar] [CrossRef]
- Muhd Julkapli, N.; Bagheri, S.; Bee Abd Hamid, S. Recent advances in heterogeneous photocatalytic decolorization of synthetic dyes. Sci. World J. 2014, 2014, 692307. [Google Scholar] [CrossRef] [PubMed]
- Shaban, M.; Elwahab, F.A.; Ghitas, A.E.; El Zayat, M.Y. Efficient and recyclable photocatalytic degradation of methylene blue dye in aqueous solutions using nanostructured Cd1−xCoxS films of different doping levels. J. Sol-Gel Sci. Technol. 2020, 95, 276–288. [Google Scholar] [CrossRef]
- Saeed, K.; Khan, I. Efficient photodegradation of neutral red chloride dye in aqueous medium using graphene/cobalt–manganese oxides nanocomposite. Turk. J. Chem. 2017, 41, 391–398. [Google Scholar] [CrossRef]
- Khan, I.; Sadiq, M.; Khan, I.; Saeed, K. Manganese dioxide nanoparticles/activated carbon composite as efficient UV and visible-light photocatalyst. Environ. Sci. Pollut. Res. 2019, 26, 5140–5154. [Google Scholar] [CrossRef]
- Saeed, K.; Khan, I.; Gul, T.; Sadiq, M. Efficient photodegradation of methyl violet dye using TiO2/Pt and TiO2/Pd photocatalysts. Appl. Water Sci. 2017, 7, 3841–3848. [Google Scholar] [CrossRef]
- Rashad, M.; Shaalan, N.M.; Abd-Elnaiem, A.M. Degradation enhancement of methylene blue on ZnO nanocombs synthesized by thermal evaporation technique. Desalin. Water Treat. 2016, 57, 26267–26273. [Google Scholar] [CrossRef]
- Siong, V.L.E.; Lee, K.M.; Juan, J.C.; Lai, C.W.; Tai, X.H.; Khe, C.S. Removal of methylene blue dye by solvothermally reduced graphene oxide: A metal-free adsorption and photodegradation method. RSC Adv. 2019, 9, 37686–37695. [Google Scholar] [CrossRef]
- León, E.R.; Rodríguez, E.L.; Beas, C.R.; Plascencia-Villa, G.; Palomares, R.A.I. Study of Methylene Blue Degradation by Gold Nanoparticles Synthesized within Natural Zeolites. J. Nanomater. 2016, 2016, 9541683. [Google Scholar] [CrossRef]
- Saraswati, T.E.; Andhika, I.F.; Patiha; Purnawan, C.; Wahyuningsih, S.; Anwar, M. Photocatalytic Degradation of Methylene Blue Using TiO2/Carbon Nanoparticles Fabricated by Electrical Arc Discharge in Liquid Medium. Adv. Mater. Res. 2015, 1123, 285–288. [Google Scholar] [CrossRef]
- Hou, L.; Yang, L.; Li, J.; Tan, J.; Yuan, C. Efficient sunlight-induced methylene blue removal over one-dimensional mesoporous monoclinic BiVO4 nanorods. J. Anal. Methods Chem. 2012, 2012, 345247. [Google Scholar] [CrossRef] [PubMed]
- Soltani, T.; Entezari, M.H. Photolysis and photocatalysis of methylene blue by ferrite bismuth nanoparticles under sunlight irradiation. J. Mol. Catal. A Chem. 2013, 377, 197–203. [Google Scholar] [CrossRef]
- Rajendran, S.; Khan, M.M.; Gracia, F.; Qin, J.; Gupta, V.K.; Arumainathan, S. Ce3+-ion-induced visible-light photocatalytic degradation and electrochemical activity of ZnO/CeO2 nanocomposite. Sci. Rep. 2016, 6, 31641. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Ahmad, A.; Ri, H.; Khan, A.U.; Khan, U.A.; Yuan, Q. Green synthesis of catalytic Zinc Oxide nano-flowers and their bacterial infection therapy. Appl. Organomet. Chem. 2020, 34, e5298. [Google Scholar] [CrossRef]
- Sáenz-Trevizo, A.; Pizá-Ruiz, P.; Chávez-Flores, D.; Ogaz-Parada, J.; Amézaga-Madrid, P.; Vega-Ríos, A.; Miki-Yoshida, M. On the Discoloration of Methylene Blue by Visible Light. J. Fluoresc. 2019, 29, 15–25. [Google Scholar] [CrossRef]
- Shahabuddin, S.; Sarih, N.M.; Mohamad, S.; Ching, J.J. SrTiO3 nanocube-doped polyaniline nanocomposites with enhanced photocatalytic degradation of methylene blue under visible light. Polymers 2016, 8, 27. [Google Scholar] [CrossRef]
- Saeed, K.; Khan, I.; Park, S.Y. TiO2/amidoxime-modified polyacrylonitrile nanofibers and its application for the photodegradation of methyl blue in aqueous medium. Desalin. Water Treat. 2015, 54, 3146–3151. [Google Scholar] [CrossRef]
- Balu, S.; Uma, K.; Pan, G.T.; Yang, T.C.K.; Ramaraj, S.K. Degradation of methylene blue dye in the presence of visible light using SiO2@α-Fe2O3 nanocomposites deposited on SnS2 flowers. Materials 2018, 11, 1030. [Google Scholar] [CrossRef]
- Yu, Z.; Chuang, S.S.C. Probing methylene blue photocatalytic degradation by adsorbed ethanol with in situ IR. J. Phys. Chem. C 2007, 111, 13813–13820. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Ji, Y.; Ma, T.; Zhang, F.; Wang, Y.; Ci, M.; Chen, D.; Jiang, A.; Wang, W. Photocatalytic degradation of methylene blue with ZnO@C nanocomposites: Kinetics, mechanism, and the inhibition effect on monoamine oxidase A and B. NanoImpact 2019, 15, 100174. [Google Scholar] [CrossRef]
- Dagher, S.; Soliman, A.; Ziout, A.; Tit, N.; Hilal-Alnaqbi, A.; Khashan, S.; Alnaimat, F.; Qudeiri, J.A. Photocatalytic removal of methylene blue using titania- and silica-coated magnetic nanoparticles. Mater. Res. Express 2018, 5, 65518. [Google Scholar] [CrossRef]
- Kuila, S.K.; Sarkar, R.; Kumbhakar, P.; Kumbhakar, P.; Tiwary, C.S.; Kundu, T.K. Photocatalytic dye degradation under sunlight irradiation using cerium ion adsorbed two-dimensional graphitic carbon nitride. J. Environ. Chem. Eng. 2020, 8, 103942. [Google Scholar] [CrossRef]
- Hanif, M.; Lee, I.; Akter, J.; Islam, M.; Zahid, A.; Sapkota, K.; Hahn, J. Enhanced Photocatalytic and Antibacterial Performance of ZnO Nanoparticles Prepared by an Efficient Thermolysis Method. Catalysts 2019, 9, 608. [Google Scholar] [CrossRef]
- Nuengmatcha, P.; Porrawatkul, P.; Chanthai, S.; Sricharoen, P.; Limchoowong, N. Enhanced photocatalytic degradation of methylene blue using Fe2O3/graphene/CuO nanocomposites under visible light. J. Environ. Chem. Eng. 2019, 7, 103438. [Google Scholar] [CrossRef]
- Trandafilović, L.V.; Jovanović, D.J.; Zhang, X.; Ptasińska, S.; Dramićanin, M.D. Enhanced photocatalytic degradation of methylene blue and methyl orange by ZnO:Eu nanoparticles. Appl. Catal. B Environ. 2017, 203, 740–752. [Google Scholar] [CrossRef]
- Zuo, R.; Du, G.; Zhang, W.; Liu, L.; Liu, Y.; Mei, L.; Li, Z. Photocatalytic degradation of methylene blue using TiO2 impregnated diatomite. Adv. Mater. Sci. Eng. 2014, 2014, 170148. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Singh, J.; Dhaliwal, A.S. Plasmon-induced photocatalytic degradation of methylene blue dye using biosynthesized silver nanoparticles as photocatalyst. Environ. Technol. 2020, 41, 1520–1534. [Google Scholar] [CrossRef]
- Smazna, D.; Shree, S.; Polonskyi, O.; Lamaka, S.; Baum, M.; Zheludkevich, M.; Faupel, F.; Adelung, R.; Mishra, Y.K. Mutual interplay of ZnO micro- and nanowires and methylene blue during cyclic photocatalysis process. J. Environ. Chem. Eng. 2019, 7, 103016. [Google Scholar] [CrossRef]
- da Silva, L.F.; Lopes, O.F.; de Mendonça, V.R.; Carvalho, K.T.G.; Longo, E.; Ribeiro, C.; Mastelaro, V.R. An Understanding of the Photocatalytic Properties and Pollutant Degradation Mechanism of SrTiO3 Nanoparticles. Photochem. Photobiol. 2016, 92, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Teng, F. SPR-promoted visible-light photocatalytic activity of Bi/ZIF hybrids. J. Photochem. Photobiol. A Chem. 2020, 400, 112679. [Google Scholar] [CrossRef]
- Rahmat, M.; Rehman, A.; Rahmat, S.; Bhatti, H.N.; Iqbal, M.; Khan, W.S.; Bajwa, S.Z.; Rahmat, R.; Nazir, A. Highly efficient removal of crystal violet dye from water by MnO2 based nanofibrous mesh/photocatalytic process. J. Mater. Res. Technol. 2019, 8, 5149–5159. [Google Scholar] [CrossRef]
- Ashrafi, H.; Akhond, M.; Absalan, G. Adsorption and Photocatalytic Degradation of Aqueous Methylene Blue using Nanoporous Carbon Nitride. J. Photochem. Photobiol. A Chem. 2020, 396, 112533. [Google Scholar] [CrossRef]
- Prasert, A.; Sontikaew, S.; Sriprapai, D.; Chuangchote, S. Polypropylene/ZnO Nanocomposites: Mechanical Properties, Photocatalytic Dye Degradation, and Antibacterial Property. Materials 2020, 13, 914. [Google Scholar] [CrossRef]
- Isai, K.A.; Shrivastava, V.S. Photocatalytic degradation of methylene blue using ZnO and 2%Fe–ZnO semiconductor nanomaterials synthesized by sol–gel method: A comparative study. SN Appl. Sci. 2019, 1, 1247. [Google Scholar] [CrossRef]
- Janani, B.; Gayathri, G.; Syed, A.; Raju, L.L.; Marraiki, N.; Elgorban, A.M.; Thomas, A.M.; Khan, S.S. The Effect of Various Capping Agents on Surface Modifications of CdO NPs and the Investigation of Photocatalytic Performance, Antibacterial and Anti-biofilm Activities. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1865–1876. [Google Scholar] [CrossRef]
- Ali Baig, A.B.; Rathinam, V.; Palaninathan, J. Photodegradation activity of yttrium-doped SnO2 nanoparticles against methylene blue dye and antibacterial effects. Appl. Water Sci. 2020, 10, 76. [Google Scholar] [CrossRef]
- Chen, Y.; Xiang, Z.; Wang, D.; Kang, J.; Qi, H. Effective photocatalytic degradation and physical adsorption of methylene blue using cellulose/GO/TiO2 hydrogels. RSC Adv. 2020, 10, 23936–23943. [Google Scholar] [CrossRef]
- Alkaykh, S.; Mbarek, A.; Ali-Shattle, E.E. Photocatalytic degradation of methylene blue dye in aqueous solution by MnTiO3 nanoparticles under sunlight irradiation. Heliyon 2020, 6, e03663. [Google Scholar] [CrossRef]
- Enesca, A.; Isac, L. The Influence of Light Irradiation on the Photocatalytic Degradation of Organic Pollutants. Materials 2020, 13, 2494. [Google Scholar] [CrossRef]
- Zhang, J.; Su, C.; Xie, X.; Liu, P.; Huq, M.E. Enhanced visible light photocatalytic degradation of dyes in aqueous solution activated by HKUST-1: Performance and mechanism. RSC Adv. 2020, 10, 37028–37034. [Google Scholar] [CrossRef]
- Du, Z.; Cui, C.; Zhang, S.; Xiao, H.; Ren, E.; Guo, R.; Jiang, S. Visible-light-driven photocatalytic degradation of rhodamine B using Bi2WO6/GO deposited on polyethylene terephthalate fabric. J. Leather Sci. Eng. 2020, 2, 16. [Google Scholar] [CrossRef]
- Acosta-Esparza, M.A.; Rivera, L.P.; Pérez-Centeno, A.; Zamudio-Ojeda, A.; González, D.R.; Chávez-Chávez, A.; Santana-Aranda, M.A.; Santos-Cruz, J.; Quiñones-Galván, J.G. UV and Visible light photodegradation of methylene blue with graphene decorated titanium dioxide. Mater. Res. Express 2020, 7, 035504. [Google Scholar] [CrossRef]
- Zeleke, M.A.; Kuo, D.H. Synthesis and application of V2O5-CeO2 nanocomposite catalyst for enhanced degradation of methylene blue under visible light illumination. Chemosphere 2019, 235, 935–944. [Google Scholar] [CrossRef]
- Salama, A.; Mohamed, A.; Aboamera, N.M.; Osman, T.A.; Khattab, A. Photocatalytic degradation of organic dyes using composite nanofibers under UV irradiation. Appl. Nanosci. 2018, 8, 155–161. [Google Scholar] [CrossRef]
- Elsayed, E.M.; Elnouby, M.S.; Gouda, S.M.H.; Elessawy, N.A.; Santos, D.M.F. Effect of the morphology of tungsten oxide embedded in sodium alginate/polyvinylpyrrolidone composite beads on the photocatalytic degradation of methylene blue dye solution. Materials 2020, 13, 1905. [Google Scholar] [CrossRef]
- Anju Chanu, L.; Joychandra Singh, W.; Jugeshwar Singh, K.; Nomita Devi, K. Effect of operational parameters on the photocatalytic degradation of Methylene blue dye solution using manganese doped ZnO nanoparticles. Results Phys. 2019, 12, 1230–1237. [Google Scholar] [CrossRef]
- Pandey, A.; Kalal, S.; Ameta, C.; Ameta, R.; Kumar, S.; Punjabi, P.B. Synthesis, characterization and application of naïve and nano-sized titanium dioxide as a photocatalyst for degradation of methylene blue. J. Saudi Chem. Soc. 2015, 19, 528–536. [Google Scholar] [CrossRef]
- Xu, C.; Rangaiah, G.P.; Zhao, X.S. Photocatalytic degradation of methylene blue by titanium dioxide: Experimental and modeling study. Ind. Eng. Chem. Res. 2014, 53, 14641–14649. [Google Scholar] [CrossRef]
- Chowdhury, P.R.; Bhattacharyya, K.G. Ni/Ti layered double hydroxide: Synthesis, characterization and application as a photocatalyst for visible light degradation of aqueous methylene blue. Dalton Trans. 2015, 44, 6809–6824. [Google Scholar] [CrossRef] [PubMed]
- Abdellah, M.H.; Nosier, S.A.; El-Shazly, A.H.; Mubarak, A.A. Photocatalytic decolorization of methylene blue using TiO2/UV system enhanced by air sparging. Alex. Eng. J. 2018, 57, 3727–3735. [Google Scholar] [CrossRef]
- Alkaim, A.F.; Aljeboree, A.M.; Alrazaq, N.A.; Baqir, S.J.; Hussein, F.H.; Lilo, A.J. Effect of pH on adsorption and photocatalytic degradation efficiency of different catalysts on removal of methylene blue. Asian J. Chem. 2014, 26, 8445–8448. [Google Scholar] [CrossRef]
- Singh, R.K.; Behera, S.S.; Singh, K.R.; Mishra, S.; Panigrahi, B.; Sahoo, T.R.; Parhi, P.K.; Mandal, D. Biosynthesized gold nanoparticles as photocatalysts for selective degradation of cationic dye and their antimicrobial activity. J. Photochem. Photobiol. A Chem. 2020, 400, 112704. [Google Scholar] [CrossRef]
- Azeez, F.; Al-Hetlani, E.; Arafa, M.; Abdelmonem, Y.; Nazeer, A.A.; Amin, M.O.; Madkour, M. The effect of surface charge on photocatalytic degradation of methylene blue dye using chargeable titania nanoparticles. Sci. Rep. 2018, 8, 7104. [Google Scholar] [CrossRef]
- Saeed, K.; Zada, N.; Khan, I.; Sadiq, M. Synthesis, characterization and photodegradation application of Fe-Mn and F-MWCNTs supported Fe-Mn oxides nanoparticles. Desalin. Water Treat. 2018, 108, 362–368. [Google Scholar] [CrossRef]
- Nguyen Thi Thu, T.; Nguyen Thi, N.; Tran Quang, V.; Nguyen Hong, K.; Nguyen Minh, T.; Le Thi Hoai, N. Synthesis, characterisation, and effect of pH on degradation of dyes of copper-doped TiO2. J. Exp. Nanosci. 2016, 11, 226–238. [Google Scholar] [CrossRef]
- Hejazi, R.; Mahjoub, A.R.; Khavar, A.H.C.; Khazaee, Z. Fabrication of novel type visible-light-driven TiO2@MIL-100 (Fe) microspheres with high photocatalytic performance for removal of organic pollutants. J. Photochem. Photobiol. A Chem. 2020, 400, 112644. [Google Scholar] [CrossRef]
- Hendekhale, N.R.; Mohammad-Khah, A. A novel synthesis of Co2ZrO5 and m-ZrO2 nanoparticles by sono-precipitation and hydrothermal methods and their application in UV/Visible-photocatalytic studies. J. Environ. Chem. Eng. 2020, 8, 104065. [Google Scholar] [CrossRef]
- Di Mauro, A.; Cantarella, M.; Nicotra, G.; Pellegrino, G.; Gulino, A.; Brundo, M.V.; Privitera, V.; Impellizzeri, G. Novel synthesis of ZnO/PMMA nanocomposites for photocatalytic applications. Sci. Rep. 2017, 7, 40895. [Google Scholar] [CrossRef]
- Jia, Z.; La, L.B.T.; Zhang, W.C.; Liang, S.X.; Jiang, B.; Xie, S.K.; Habibi, D.; Zhang, L.C. Strong enhancement on dye photocatalytic degradation by ball-milled TiO2: A study of cationic and anionic dyes. J. Mater. Sci. Technol. 2017, 33, 856–863. [Google Scholar] [CrossRef]
- Mohammadzadeh, A.; Khoshghadam-Pireyousefan, M.; Shokrianfard-Ravasjan, B.; Azadbeh, M.; Rashedi, H.; Dibazar, M.; Mostafaei, A. Synergetic photocatalytic effect of high purity ZnO pod shaped nanostructures with H2O2 on methylene blue dye degradation. J. Alloys Compd. 2020, 845, 156333. [Google Scholar] [CrossRef]
- Reza, K.M.; Kurny, A.; Gulshan, F. Parameters affecting the photocatalytic degradation of dyes using TiO2: A review. Appl. Water Sci. 2017, 7, 1569–1578. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, L.; Zeng, T.; Shang, Q.; Zhou, H.; Pan, Z.; Cheng, Q. Two pure MOF-photocatalysts readily prepared for the degradation of methylene blue dye under visible light. Dalton Trans. 2018, 47, 4251–4258. [Google Scholar] [CrossRef]
- Tadkar, P.S.; Borkar, P.K.; Gholap, S.N.; Gole, V.L. Treatment of Methylene Blue Dye Using Immersed Lamp Photocatalytic Reactor: 5 L Scale Study. J. Inst. Eng. Ser. E 2019, 100, 199–204. [Google Scholar] [CrossRef]
- Singh, J.; Chang, Y.Y.; Koduru, J.R.; Yang, J.K. Potential degradation of methylene blue (MB) by nano-metallic particles: A kinetic study and possible mechanism of MB degradation. Environ. Eng. Res. 2018, 23, 1–9. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Liu, S.; Li, R. Efficient decolorization of Methylene Blue catalyzed by MgFe-layered double hydroxides in the presence of hydrogen peroxide. Water Sci. Technol. 2020, 81, 781–789. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z.; Rajendran, R.; Kandaramath, T. Photodegradation of methylene blue over novel 3D ZnO microflowers with hexagonal pyramid-like petals. React. Kinet. Mech. Catal. 2014, 112, 527–542. [Google Scholar] [CrossRef]
- Wang, X.; Liu, P.; Fu, M.; Ma, J.; Ning, P. Novel sequential process for enhanced dye synergistic degradation based on nano zero-valent iron and potassium permanganate. Chemosphere 2016, 155, 39–47. [Google Scholar] [CrossRef]
- Sharma, C.P.; Karim, A.V.; Shriwastav, A. Decolorization of methylene blue using Fe(III)-citrate complex in a solar photo-Fenton process: Impact of solar variability on process optimization. Water Sci. Technol. 2019, 80, 2047–2057. [Google Scholar] [CrossRef]
- Ngullie, R.C.; Alaswad, S.O.; Bhuvaneswari, K.; Shanmugam, P.; Pazhanivel, T.; Arunachalam, P. Synthesis and Characterization of Efficient ZnO/g-C3N4 Nanocomposites Photocatalyst for Photocatalytic Degradation of Methylene Blue. Coatings 2020, 10, 500. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, G.; Fu, H.; Wang, P.; Liao, J.; Wang, A. Photocatalytic degradation of methylene blue by a cocatalytic PDA/TiO2 electrode produced by photoelectric polymerization. RSC Adv. 2020, 10, 26133–26141. [Google Scholar] [CrossRef]
- Zheng, Y.; Cao, L.; Xing, G.; Bai, Z.; Huang, J.; Zhang, Z. Microscale flower-like magnesium oxide for highly efficient photocatalytic degradation of organic dyes in aqueous solution. RSC Adv. 2019, 9, 7338–7348. [Google Scholar] [CrossRef]
- Salgado, B.C.B.; Valentini, A. Evaluation of the photocatalytic activity of SiO2@TiO2 hybrid spheres in the degradation of methylene blue and hydroxylation of benzene: Kinetic and mechanistic study. Braz. J. Chem. Eng. 2019, 36, 1501–1518. [Google Scholar] [CrossRef]
- Lee, S.; Park, J.-W. Hematite/Graphitic Carbon Nitride Nanofilm for Fenton and Photocatalytic Oxidation of Methylene Blue. Sustainability 2020, 12, 2866. [Google Scholar] [CrossRef]
- Shelar, S.G.; Mahajan, V.K.; Patil, S.P.; Sonawane, G.H. Effect of doping parameters on photocatalytic degradation of methylene blue using Ag doped ZnO nanocatalyst. SN Appl. Sci. 2020, 2, 820. [Google Scholar] [CrossRef]
- Saeed, M.; Muneer, M.; Khosa, M.K.K.; Akram, N.; Khalid, S.; Adeel, M.; Nisar, A.; Sherazi, S. Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium. Green Process. Synth. 2019, 8, 659–666. [Google Scholar] [CrossRef]
- Yang, C.; Dong, W.; Cui, G.; Zhao, Y.; Shi, X.; Xia, X.; Tang, B.; Wang, W. Highly-efficient photocatalytic degradation of methylene blue by PoPD-modified TiO2 nanocomposites due to photosensitization-synergetic effect of TiO2 with PoPD. Sci. Rep. 2017, 7, 3973. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Cheng, S.; Su, T.; Zuo, G.; Zhao, W.; Qi, X.; Wei, W.; Dong, W. Fenton-like catalyst Fe3O4@polydopamine-MnO2 for enhancing removal of methylene blue in wastewater. Colloids Surf. B Biointerfaces 2019, 181, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Li, K.; Yu, H.; Cohen Stuart, M.A.; Wang, J.; Zhou, S. One-Pot Syntheses of Porous Hollow Silica Nanoreactors Encapsulating Rare Earth Oxide Nanoparticles for Methylene Blue Degradation. Ind. Eng. Chem. Res. 2019, 58, 3726–3734. [Google Scholar] [CrossRef]
- Rodríguez-Chueca, J.; Alonso, E.; Singh, D. Photocatalytic Mechanisms for Peroxymonosulfate Activation through the Removal of Methylene Blue: A Case Study. Int. J. Environ. Res. Public Health 2019, 16, 198. [Google Scholar] [CrossRef]
- Saleh, R.; Taufik, A. Photo-Fenton degradation of methylene blue in the presence of Au-Fe3O4/graphene composites under UV and visible light at near neutral pH: Effect of coexisting inorganic anion. Environ. Nanotechnol. Monit. Manag. 2019, 11, 100221. [Google Scholar] [CrossRef]
- Cheng, X.; Chong, R.; Cao, Y.; Li, D.; Chang, Z.; Zhang, L. Influence of inorganic anions on photocatalytic degeneration of methylene blue on Ag3PO4. J. Nanosci. Nanotechnol. 2016, 16, 12489–12497. [Google Scholar] [CrossRef]
- Gupta, N.K.; Ghaffari, Y.; Kim, S.; Bae, J.; Kim, K.S.; Saifuddin, M. Photocatalytic Degradation of Organic Pollutants over MFe2O4 (M = Co, Ni, Cu, Zn) Nanoparticles at Neutral pH. Sci. Rep. 2020, 10, 4942. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, H.; Lv, Z.; Zhan, S.; Yang, J.; Peng, X.; Ren, Y.; Wu, X. Simulated-sunlight-activated photocatalysis of Methylene Blue using cerium-doped SiO2/TiO2 nanostructured fibers. J. Environ. Sci. 2012, 24, 1867–1875. [Google Scholar] [CrossRef]
- Sahoo, C.; Gupta, A.K.; Sasidharan Pillai, I.M. Photocatalytic degradation of methylene blue dye from aqueous solution using silver ion-doped TiO2 and its application to the degradation of real textile wastewater. J. Environ. Sci. Health—Part A Toxic/Hazard. Subst. Environ. Eng. 2012, 47, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Shao, Q.; Zhang, Y.; Jiang, H.; Ge, S.; Lou, S.; Lin, J.; Zhang, J.; Wu, S.; Dong, M.; et al. N self-doped ZnO derived from microwave hydrothermal synthesized zeolitic imidazolate framework-8 toward enhanced photocatalytic degradation of methylene blue. J. Colloid Interface Sci. 2020, 565, 142–155. [Google Scholar] [CrossRef]
- Tang, S.; Wang, Z.; Yuan, D.; Zhang, Y.; Qi, J.; Rao, Y.; Lu, G.; Li, B.; Wang, K.; Yin, K. Enhanced photocatalytic performance of BiVO4 for degradation of methylene blue under LED visible light irradiation assisted by peroxymonosulfate. Int. J. Electrochem. Sci. 2020, 15, 2470–2480. [Google Scholar] [CrossRef]
- Das, G.S.; Shim, J.P.; Bhatnagar, A.; Tripathi, K.M.; Kim, T.Y. Biomass-derived Carbon Quantum Dots for Visible-Light-Induced Photocatalysis and Label-Free Detection of Fe(III) and Ascorbic acid. Sci. Rep. 2019, 9, 15084. [Google Scholar] [CrossRef]
- Atta, A.M.; Moustafa, Y.M.; Al-Lohedan, H.A.; Ezzat, A.O.; Hashem, A.I. Methylene Blue Catalytic Degradation Using Silver and Magnetite Nanoparticles Functionalized with a Poly(ionic liquid) Based on Quaternized Dialkylethanolamine with 2-Acrylamido-2-methylpropane Sulfonate- co-Vinylpyrrolidone. ACS Omega 2020, 5, 2829–2842. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Shao, Y.; Liu, J.; Ye, Z.; Zhang, H.; Ma, J.; Jia, Y.; Gao, W.; Li, Y. Preparation and characterization of magnetic porous carbon microspheres for removal of methylene blue by a heterogeneous fenton reaction. ACS Appl. Mater. Interfaces 2014, 6, 7275–7285. [Google Scholar] [CrossRef] [PubMed]
- Antoniadou, M.; Arfanis, M.K.; Ibrahim, I.; Falaras, P. Bifunctional g-C3N4/WO3 thin films for photocatalyticwater purification. Water 2019, 11, 2439. [Google Scholar] [CrossRef]
- Rauf, M.A.; Meetani, M.A.; Khaleel, A.; Ahmed, A. Photocatalytic degradation of Methylene Blue using a mixed catalyst and product analysis by LC/MS. Chem. Eng. J. 2010, 157, 373–378. [Google Scholar] [CrossRef]
- Sithole, R.K.; Machogo, L.F.E.; Moloto, M.J.; Gqoba, S.S.; Mubiayi, K.P.; Van Wyk, J.; Moloto, N. One-step synthesis of Cu3N, Cu2S and Cu9S5 and photocatalytic degradation of methyl orange and methylene blue. J. Photochem. Photobiol. A Chem. 2020, 397, 112577. [Google Scholar] [CrossRef]
- Wolski, L.; Ziolek, M. Insight into pathways of methylene blue degradation with H2O2 over mono and bimetallic Nb, Zn oxides. Appl. Catal. B Environ. 2018, 224, 634–647. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, S.; Ning, P. Degradation mechanism of methylene blue in a heterogeneous fenton-like reaction catalyzed by ferrocene. Ind. Eng. Chem. Res. 2014, 53, 643–649. [Google Scholar] [CrossRef]
- Chithambararaj, A.; Sanjini, N.S.; Bose, A.C.; Velmathi, S. Flower-like hierarchical h-MoO3: New findings of efficient visible light driven nano photocatalyst for methylene blue degradation. Catal. Sci. Technol. 2013, 3, 1405–1414. [Google Scholar] [CrossRef]
- Lee, J.E.; Khoa, N.T.; Kim, S.W.; Kim, E.J.; Hahn, S.H. Fabrication of Au/GO/ZnO composite nanostructures with excellent photocatalytic performance. Mater. Chem. Phys. 2015, 164, 29–35. [Google Scholar] [CrossRef]
- Mohamadi, S.; Ghorbanali, M. Adsorption and UV-assisted photodegradation of methylene blue by CeO2 -decorated graphene sponge. Sep. Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Azzam, E.M.S.; Fathy, N.A.; El-Khouly, S.M.; Sami, R.M. Enhancement the photocatalytic degradation of methylene blue dye using fabricated CNTs/TiO2 /AgNPs/Surfactant nanocomposites. J. Water Process Eng. 2019, 28, 311–321. [Google Scholar] [CrossRef]
- Hoan, N.T.V.; Minh, N.N.; Nhi, T.T.K.; Van Thang, N.; Tuan, V.A.; Nguyen, V.T.; Thanh, N.M.; Van Hung, N.; Khieu, D.Q. TiO2/Diazonium/graphene oxide composites: Synthesis and visible-light-driven photocatalytic degradation of methylene blue. J. Nanomater. 2020, 2020, 4350125. [Google Scholar] [CrossRef]
- Atique Ullah, A.K.M.; Fazle Kibria, A.K.M.; Akter, M.; Khan, M.N.I.; Tareq, A.R.M.; Firoz, S.H. Oxidative Degradation of Methylene Blue Using Mn3O4 Nanoparticles. Water Conserv. Sci. Eng. 2017, 1, 249–256. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Chen, H.; Shao, G.; Zhang, R.; Lu, H. Synthesis and properties of Ag/ZnO/g-C3N4 ternary micro/nano composites by microwave-assisted method. Mater. Res. Express 2018, 5, 015021. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Kumar, N.; Mittal, H.; Reddy, L.; Nair, P.; Ngila, J.C.; Parashar, V. Morphogenesis of ZnO nanostructures: Role of acetate (COOH−) and nitrate (NO3−) ligand donors from zinc salt precursors in synthesis and morphology dependent photocatalytic properties. RSC Adv. 2015, 5, 38801–38809. [Google Scholar] [CrossRef]
- He, D.; Lin, F. Preparation and photocatalytic activity of anatase TiO2 nanocrystallites with high thermal stability. Mater. Lett. 2007, 61, 3385–3387. [Google Scholar] [CrossRef]
- Khan, I.; Khan, A.A.Z.; Sufyan, A.; Khan, M.Y.; Inayath Basha, S.; Khan, A.A.Z. Ultrasonically controlled growth of monodispersed octahedral BiVO4 microcrystals for improved photoelectrochemical water oxidation. Ultrason. Sonochem. 2020, 68, 105233. [Google Scholar] [CrossRef]
- Khan, I. Strategies for Improved Electrochemical CO2 Reduction to Value-added Products by Highly Anticipated Copper-based Nanoarchitectures. Chem. Rec. 2021, 22, e202100219. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Jalilov, A.; Fujii, K.; Qurashi, A. Quasi-1D Aligned Nanostructures for Solar-Driven Water Splitting Applications: Challenges, Promises, and Perspectives. Sol. RRL 2021, 5, 2000741. [Google Scholar] [CrossRef]
- Ehsan, M.F.; Fazal, A.; Hamid, S.; Arfan, M.; Khan, I.; Usman, M.; Shafiee, A.; Ashiq, M.N. CoFe2O4 decorated g-C3N4 nanosheets: New insights into superoxide anion mediated photomineralization of methylene blue. J. Environ. Chem. Eng. 2020, 8, 104556. [Google Scholar] [CrossRef]
- Ismael, M.; Wu, Y. A mini-review on the synthesis and structural modification of g-C3N4-based materials, and their applications in solar energy conversion and environmental remediation. Sustain. Energy Fuels 2019, 3, 2907–2925. [Google Scholar] [CrossRef]
- Shang, M.; Wang, W.; Zhou, L.; Sun, S.; Yin, W. Nanosized BiVO4 with high visible-light-induced photocatalytic activity: Ultrasonic-assisted synthesis and protective effect of surfactant. J. Hazard. Mater. 2009, 172, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Poorsajadi, F.; Sayadi, M.H.; Hajiani, M.; Rezaei, M.R. Synthesis of CuO/Bi2O3 nanocomposite for efficient and recycling photodegradation of methylene blue dye. Int. J. Environ. Anal. Chem. 2020. [Google Scholar] [CrossRef]
- Albiss, B.; Abu-Dalo, M. Photocatalytic Degradation of Methylene Blue Using Zinc Oxide Nanorods Grown on Activated Carbon Fibers. Sustainability 2021, 13, 4729. [Google Scholar] [CrossRef]
- Honarmand, M.; Golmohammadi, M.; Naeimi, A. Green synthesis of SnO2-bentonite nanocomposites for the efficient photodegradation of methylene blue and eriochrome black-T. Mater. Chem. Phys. 2020, 241, 122416. [Google Scholar] [CrossRef]
- Faisal, M.; Harraz, F.A.; Jalalah, M.; Alsaiari, M.; Al-Sayari, S.A.; Al-Assiri, M.S. Polythiophene doped ZnO nanostructures synthesized by modified sol-gel and oxidative polymerization for efficient photodegradation of methylene blue and gemifloxacin antibiotic. Mater. Today Commun. 2020, 24, 101048. [Google Scholar] [CrossRef]
- Wang, W.; Lin, F.; Yan, B.; Cheng, Z.; Chen, G.; Kuang, M.; Yang, C.; Hou, L. The role of seashell wastes in TiO2/Seashell composites: Photocatalytic degradation of methylene blue dye under sunlight. Environ. Res. 2020, 188, 109831. [Google Scholar] [CrossRef]
- Sanad, M.M.S.; Farahat, M.M.; El-Hout, S.I.; El-Sheikh, S.M. Preparation and characterization of magnetic photocatalyst from the banded iron formation for effective photodegradation of methylene blue under UV and visible illumination. J. Environ. Chem. Eng. 2021, 9, 105127. [Google Scholar] [CrossRef]
- Wei, X.; Wang, X.; Pu, Y.; Liu, A.; Chen, C.; Zou, W.; Zheng, Y.; Huang, J.; Zhang, Y.; Yang, Y.; et al. Facile ball-milling synthesis of CeO2/g-C3N4 Z-scheme heterojunction for synergistic adsorption and photodegradation of methylene blue: Characteristics, kinetics, models, and mechanisms. Chem. Eng. J. 2021, 420, 127719. [Google Scholar] [CrossRef]
- Vavilapalli, D.S.; Peri, R.G.; Sharma, R.K.; Goutam, U.K.; Muthuraaman, B.; Ramachandra Rao, M.S.; Singh, S. g-C3N4/Ca2Fe2O5 heterostructures for enhanced photocatalytic degradation of organic effluents under sunlight. Sci. Rep. 2021, 11, 19639. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Wang, X.; Zhang, Z.; Shen, Y.; Chen, K.; Guo, Y.; Zhou, X.; Bai, R. Synthesis of flower-like Bi2O4/ZnO heterojunction and mechanism of enhanced photodegradation for organic contaminants under visible light. Res. Chem. Intermed. 2018, 44, 6569–6590. [Google Scholar] [CrossRef]
- Drmosh, Q.A.; Hezam, A.; Hendi, A.H.Y.; Qamar, M.; Yamani, Z.H.; Byrappa, K. Ternary Bi2S3/MoS2/TiO2 with double Z-scheme configuration as high performance photocatalyst. Appl. Surf. Sci. 2020, 499, 143938. [Google Scholar] [CrossRef]
- Selvam, S.; Sarkar, I. Bile salt induced solubilization of methylene blue: Study on methylene blue fluorescence properties and molecular mechanics calculation. J. Pharm. Anal. 2017, 7, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Pahang, F.; Parvin, P.; Ghafoori-Fard, H.; Bavali, A.; Moafi, A.A.; Tio, K.; Zno, K.; Al, K. Fluorescence properties of methylene blue molecules coupled with metal oxide nanoparticles. OSA Contin. 2020, 3, 688–697. [Google Scholar] [CrossRef]
- Geçgel, Ü.; Özcan, G.; Gürpnar, G.Ç. Removal of methylene blue from aqueous solution by activated carbon prepared from pea shells (Pisum sativum). J. Chem. 2013, 2013, 614083. [Google Scholar] [CrossRef]


| FTIR Transmission Wavenumbers (cm−1) | Assignments |
|---|---|
| 3410 | -NH/-OH overlapped stretching vibration |
| 2928 | symmetrical stretching C-H of -CH2 band |
| 1600 | C=N central ring stretching |
| 1482 | C=C side ring stretching |
| 1384 | multiple ring stretching |
| 1590 | skeleton stretching vibration of the benzene ring |
| 1486.4 and 1389 | stretching vibration of C–N in aromatic amines |
| 1320 | CAr–N stretching |
| 1572 | stretching band of C=O, C-N of amide II |
| 1240 and 1182 | N–CH3 stretching |
| 1143 | stretching vibration of C–N in the aliphatic chain |
| 1442 | symmetrical stretching band of –COOH |
| 1140 and 854 | bending band of N-H and C-N from the amide III |
| 880 | absorption of C–H in-plane bending vibration |
| 665 | skeleton vibration mode of C–S–C |
| Photocatalyst | Inorganic Anions | Positive Effect | Negative Effect | Dual Effect | Negligible Effect | Reference |
|---|---|---|---|---|---|---|
| Au-Fe3O4/ graphene composites | NaCl, Na2SO4, NaH2PO4, NaNO3, and Na2CO3 | SO42−, Cl−, H2PO4−, NO3−, CO32− | Na+ | [248] | ||
| Ag3PO4 | NO3−, OH−, NO2−, HCO3−, Cl−, Br−, CO32−, SO42−, SO32−, S2− and PO43− | OH−, Cl−, Br−, HCO−, CO32−, SO42−, SO32−, S2− | NO2−, | HCO3−, Cl−, SO32−, PO43−, Br− | NO3− | [249] |
| ZnFe2O4 | SO42−, NO3−, Cl−, CO32− | SO42−, NO3−, Cl−, CO32− | [250] | |||
| cerium-doped SiO2/TiO2 | NO3−, SO42−, Cl− | NO3−, SO42−, Cl− | [251] | |||
| silver ion-doped TiO2 | Cl−, NO3−, SO42−, CO32− | Cl−, NO3−, SO42−, CO32− | [252] |
| Optimized Material and Morphology | Synthesis Method | Light Source | %MB Degraded@Time | Favourable Features | Ref |
|---|---|---|---|---|---|
| β-Cu2V2O7/Zn2V2O6 (1 wt%:5 wt%) Layered morphology | Ultrasonic assisted hydrothermal synthesis | 300 Xenon Lamp | 98.7%@65 min | Due to the layered morphology of Cu2V2O7, the Zn2V2O6pelets are distributed evenly, hence facilitate the charge transfer. A large surface area provides more catalytic sites and exposure to light. | [43] |
| CuO/Bi2O3 nanocomposite | Impregnation calcination method | UV-C irradiation | 88.32%@120 min | Probably due to the synergistic effect between the components of the nanocomposite | [279] |
| ZnO-NR/ACF nanocomposites | Hydrothermal method | UV irradiation | 99%%@120 min | Synergistic effects between ZnO nanorods and activated carbon fibers (ACFs) | [280] |
| SnO2-bentonite nanocomposites | Green synthesis | Solar irradiation | 100%@300 min | Efficient dye adsorption on bentonite and high surface of immobilized SnO2 on bentonite surface | [281] |
| 5% PTh/ZnO | Sol-gel and oxidative polymerization techniques | 250 W high-pressure mercury lamp | 95%@180 min | Perfect synchronization and synergistic effect of both PTh and ZnO | [282] |
| TiO2/Seashell composites (23.4% TCAS) | Simple grinding and calcination, followed by the sol–gel process | Natural sunlight | 100%@140 min | The elements presents in abalone shell doped into the substitutional sites of TiO2 and act as semiconductors that improved the charge separation efficiency of TiO2. | [283] |
| γ-Fe3/ Fe3O4/ SiO2 (Ar modified) | Single-stage heat-treatment process | UV-light | 87.5%@120 min | Combined effects of structure defects, oxygen vacancies, and the formed carbon sheets after PVA decomposition | [284] |
| 70% CeO2/g-C3N4 Z-scheme heterojunction | Ball milling | UV light irradiation | 90.1%@180 min | Stronger UV light response, higher charge carrier separation efficiency and the synergy between adsorption and photocatalysis. | [285] |
| g-C3N4/Ca2Fe2O5 heterostructures | Solid-state reaction route | Natural sunlight | 95.4%@70 min | Enhance photodegradation efficient due to the mitigation of recombination of photogenerated charge carriers by Type-II heterojunction | [286] |
| Flower-like Bi2O4/ZnO heterojunction | Hydrothermal method | Xenon lamp of power 300 W | 98.5%@30 min | The product exibited prferable morphology for the photocatalytic activity | [287] |
| Ternary MoS2/Bi2S3/TiO2 heterostructure | Microwave-assisted hydrothermal method | 250 W Xenon lamp | 99%@4 min | Ultrafst Mb phododegradation is duto introducing multiple pathways of electrons transfer that efficiently suppressed the photoelectrons-holes recombination in the heterostructured composite | [288] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. https://doi.org/10.3390/w14020242
Khan I, Saeed K, Zekker I, Zhang B, Hendi AH, Ahmad A, Ahmad S, Zada N, Ahmad H, Shah LA, et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water. 2022; 14(2):242. https://doi.org/10.3390/w14020242
Chicago/Turabian StyleKhan, Idrees, Khalid Saeed, Ivar Zekker, Baoliang Zhang, Abdulmajeed H. Hendi, Ashfaq Ahmad, Shujaat Ahmad, Noor Zada, Hanif Ahmad, Luqman Ali Shah, and et al. 2022. "Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation" Water 14, no. 2: 242. https://doi.org/10.3390/w14020242
APA StyleKhan, I., Saeed, K., Zekker, I., Zhang, B., Hendi, A. H., Ahmad, A., Ahmad, S., Zada, N., Ahmad, H., Shah, L. A., Shah, T., & Khan, I. (2022). Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water, 14(2), 242. https://doi.org/10.3390/w14020242