Aging Characteristics and Fate Analysis of Liquid Digestate Ammonium Nitrogen Disposal in Farmland Soil
Abstract
:1. Introduction
2. Materials and Methods
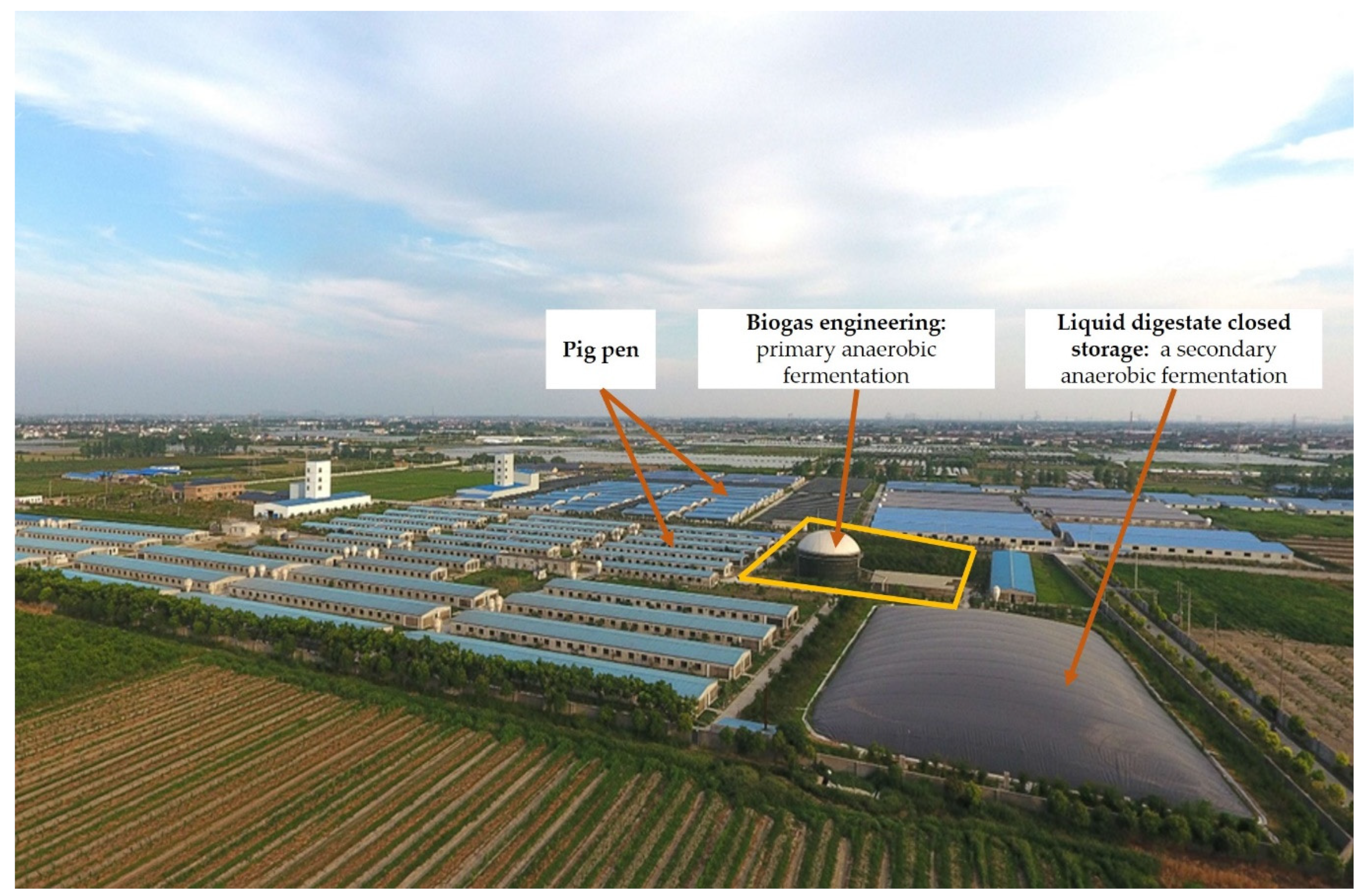
2.1. Materials
2.2. Static Soil Column Fabrication
2.3. Design and Setting
2.4. Sampling and Analysis
2.5. Calculation Formula
2.6. Data Analysis
3. Results
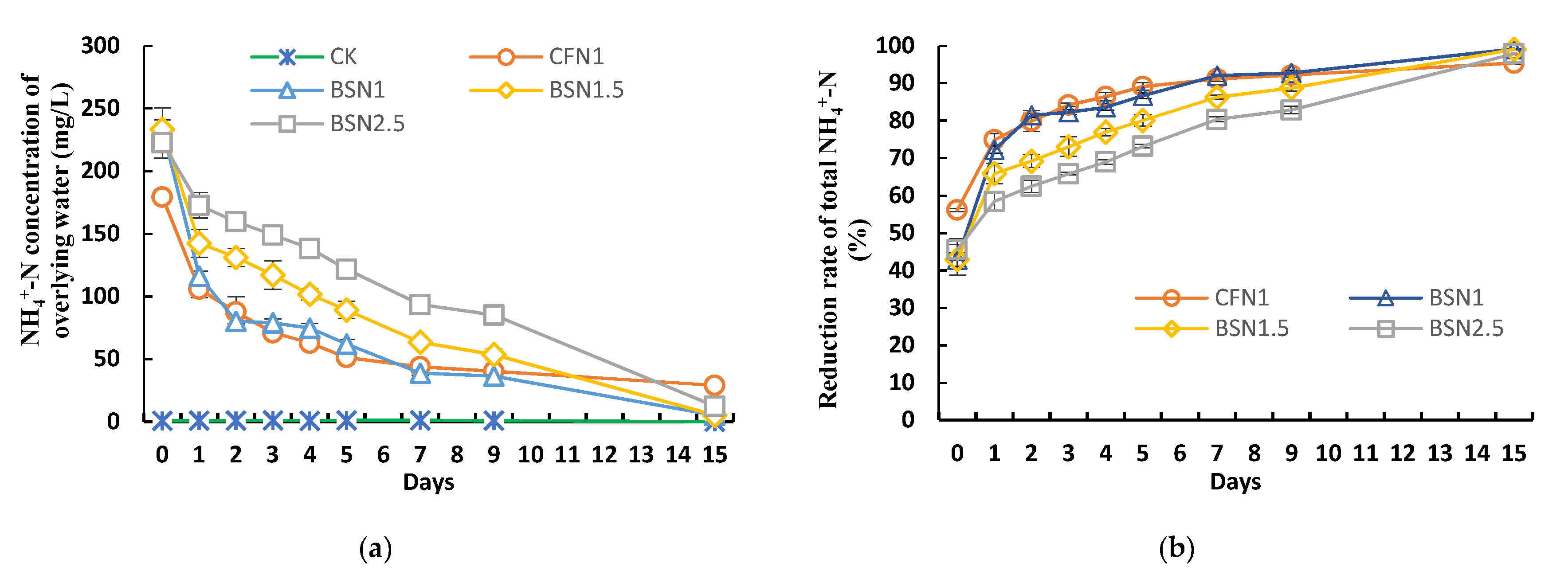
3.1. Variation Characteristics of NH4+-N Concentration and NH4+-N Reduction Rate in Overlying Water
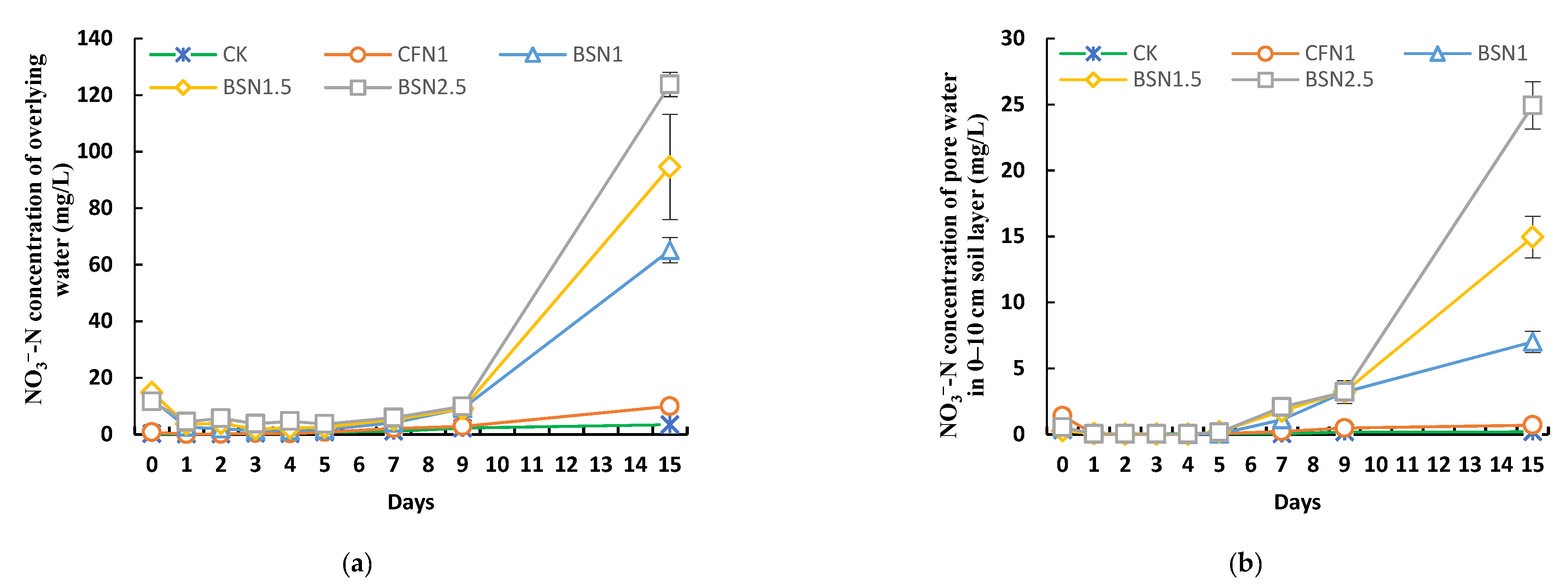
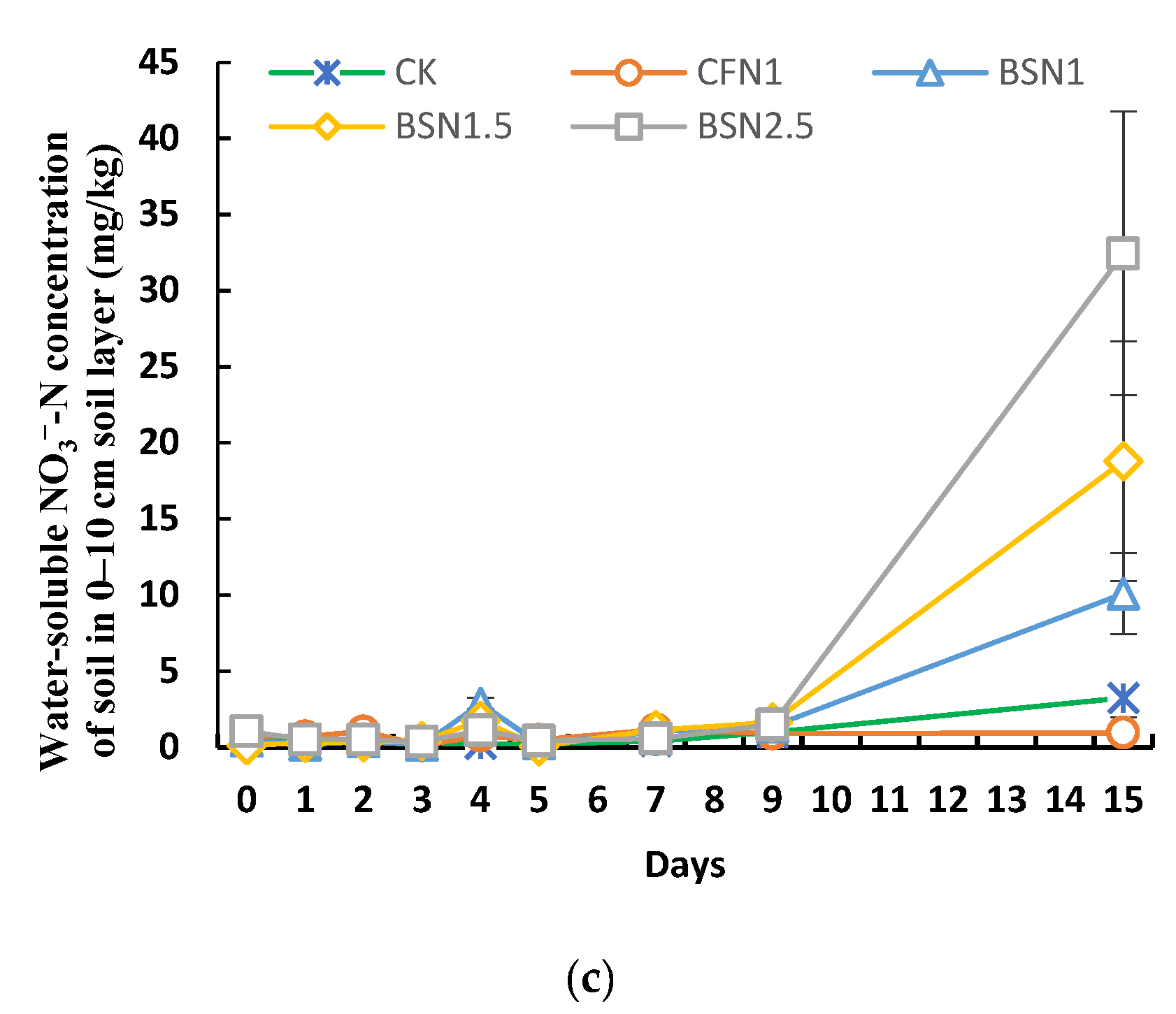
3.2. Migration and Soil Sorption Characteristics of Liquid Digestate NH4+-N
3.3. Characteristics of Liquid Digestate NH4+-N Converted to NO3−-N
3.4. Fate Analysis of Farmland Absorbing Liquid Digestate NH4+-N
4. Discussions
4.1. Time Node of Liquid Digestate NH4+-N Removal in Farmland
4.2. Migration and Transformation Characteristics of NH4+-N in Farmland Consuming Liquid Digestate
4.3. Fate of Farmland Disposal Liquid Digestate NH4+-N
Limitations and Directions for Future Research
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, H.; Wang, Z. Discussions and recommendations about establishing agro-ecological compensation mechanism in Jiangsu Province. Asian Agric. Res. 2014, 6, 108–111. [Google Scholar] [CrossRef]
- Song, Y.; Wang, G.; Li, R.; Chen, G. Research progress of biogas slurry treatment and resource utilization. Trans. CSAE 2021, 37, 237–250. [Google Scholar] [CrossRef]
- Li, H.; Zhang, T.; Shaheen, S.M.; Abdelrahman, H.; Ali, E.F.; Bolan, N.S.; Li, G.; Rinklebe, J. Microbial inoculants and struvite improved organic matter humification and stabilized phosphorus during swine manure composting: Multivariate and multiscale investigations. Bioresour. Technol. 2022, 351, 126976. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wu, X.; Shaheen, S.M.; Rinklebe, J.; Bolan, N.S.; Ali, E.F.; Li, G.; Tsang, D.C.W. Effects of microorganism-mediated inoculants on humification processes and phosphorus dynamics during the aerobic composting of swine manure. J. Hazard. Mater. 2021, 416, 125738. [Google Scholar] [CrossRef]
- Nasir, I.M.; Mohd Ghazi, T.I.; Omar, R. Anaerobic digestion technology in livestock manure treatment for biogas production: A review. Eng. Life Sci. 2012, 12, 258–269. [Google Scholar] [CrossRef]
- Lu, G.; Yang, F.; Chen, H.; Du, T. Research progress on application of biogas slurry. Soil Fertil. Sci. China 2021, 1, 339–345. [Google Scholar] [CrossRef]
- Zou, M.; Dong, H.; Zhu, Z.; Zhan, Y.; Zhang, Y.; Yue, C. Progress and prospect of treatments and resource utilization of biogas slurry on livestock and poultry farms. China Poult. 2020, 42, 103–109. [Google Scholar] [CrossRef]
- Dong, Y.; Liang, D.; Li, D.; Jin, H. Characteristic analysis of main nutrient content in biogas slurry. Jiangsu J. Agr. Sci. 2021, 37, 1206–1214. Available online: http://qikan.cqvip.com/Qikan/Article/Detail?id=7105949915 (accessed on 9 January 2020).
- Liang, Y.; Guan, Y.; Wu, H.; Wang, Z. Study on measures and policies to reduce pollution in raising livestock and poultry in Jiangsu Province. Asian Agric. Res. 2013, 10, 93–96. Available online: http://qikan.cqvip.com/Qikan/Article/Detail?id=65657182504849514948485050 (accessed on 1 August 2013).
- Jiang, X.; Sommer, S.G.; Christensen, K.V. A review of the biogas industry in China. Energy Policy 2011, 39, 6073–6081. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Sui, P.; Gao, W.; Qin, F.; Wu, X.; Xiong, J. Efficiency and sustainability analysis of biogas and electricity production from a large-scale biogas project in China: An emergy evaluation based on LCA. J. Clean. Prod. 2014, 65, 234–245. [Google Scholar] [CrossRef]
- Nkoa, R. Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: A review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef]
- Hagos, K.; Zong, J.; Li, D.; Liu, C.; Lu, X. Anaerobic co-digestion process for biogas production: Progress, challenges and perspectives. Renew. Sustain. Energy Rev. 2017, 76, 1485–1496. [Google Scholar] [CrossRef]
- He, X.; Zhang, T.; Ren, H.; Li, G.; Ding, L.; Pawlowski, L. Phosphorus recovery from biogas slurry by ultrasound/H2O2 digestion coupled with HFO/biochar adsorption process. Waste Manag. 2017, 60, 219–229. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, T.; Li, P.; Jiang, R.; Wu, S.; Nie, H.; Wang, Y. Phosphorus recovery from biogas fermentation liquid by Ca-Mg loaded biochar. J. Environ. Sci. 2015, 29, 106–114. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, Y.; Sheng, J.; Guan, Y.; Wu, H.; Chen, L.; Zheng, J. Analysis of water environment risk on biogas slurry disposal in paddy field. Trans. CSAE 2016, 32, 213–220. [Google Scholar] [CrossRef]
- Manici, L.M.; Caputo, F.; Cappelli, G.A.; Ceotto, E. Can repeated soil amendment with biogasdigestates increase soil suppressiveness toward non-specific soil-borne pathogensin agricultural lands? Renew. Agric. Food Syst. 2021, 36, 353–364. [Google Scholar] [CrossRef]
- Feng, Z.; Li, P. The genesis and evolution of the concept of carrying capacity: A view of natural resources and environment. J. Nat. Resour. 2018, 33, 1475–1489. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Ficara, E.; Aboulkas, A.; Barakat, A.; Carrere, H. New opportunities for agricultural digestate valorization: Current situation and perspectives. Energy Environ. Sci. 2015, 8, 2600–2621. [Google Scholar] [CrossRef]
- Ternoeven-Urselmans, T.; Scheller, E.; Raubuch, M.; Ludwig, B.; Joergensen, R.G. CO2 evolution and N mineralization after biogas slurry application in the field and its yield effects on spring barley. Appl. Soil Ecol. 2009, 42, 297–302. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, G.; Wang, Z.; Zong, J.; Zhou, W.; Sheng, J. Analysis of rice seedling growth restriction factors under biogas slurry application. J. Agro-Environ. Sci. 2021, 40, 2537–2543. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, X.; Shaheen, S.M.; Zhao, Q.; Liu, X.; Rinklebe, J.; Ren, H. Ammonium nitrogen recovery from digestate by hydrothermal pretreatment followed by activated hydrochar sorption. Chem. Eng. J. 2020, 379, 122254. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Li, Y. Simulation of nitrate leaching under varying drip system uniformities and precipitation patterns during the growing season of maize in the North China Plain. Agric. Water Manag. 2014, 142, 19–28. [Google Scholar] [CrossRef]
- Tan, X.; Shao, D.; Gu, W.; Liu, H. Field analysis of water and nitrogen fate in lowland paddy fields under different water managements using HYDRUS-1D. Agric. Water Manag. 2015, 150, 67–80. [Google Scholar] [CrossRef]
- Pote, D.; Reed, B.A.; Daniel, T.C.; Nichols, D.J. Water-quality effects of infiltration rate and manure application rate for soils receiving swine manure. J. Soil Water Conserv. 2001, 56, 32–37. [Google Scholar]
- Lovejoy, S.B.; Lee, J.G.; Randhir, T.O.; Engel, B.A. Research needs for water quality management in the 21st century: A spatial decision support system. J. Soil Water Conserv. 1997, 52, 18–22. Available online: https://agris.fao.org/agris-search/search.do?recordID=US9708000 (accessed on 15 February 1996).
- Velthof, G.L.; Nelemans, J.A.; Oenema, O.; Kuikman, P.J. Gaseous nitrogen and carbon losses from pig manure derived from different diets. Waste Manag. 2005, 34, 698–706. [Google Scholar] [CrossRef]
- Paul, J.W.; Dinn, N.E.; Kannangara, T.; Fisher, L.J. Protein content in dairy cattle diets affects ammonia losses and fertilizer nitrogen value. J. Environ. Qual. 1998, 27, 528–534. [Google Scholar] [CrossRef]
- Svoboda, N.; Taube, F.; Wienforth, B.; Kluß, C.; Kage, H.; Herrmann, A. Nitrogen leaching losses after biogas residue application to maize. Soil Tillage Res. 2013, 130, 69–80. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Liu, S.; Liu, Q.; Yan, L. Effects of biogas slurry application on nitrogen loss soil in black soil area during the autumn fallow period. J. Agro-Environ. Sci. 2021, 40, 2528–2536. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Q.; Chen, D.; Li, A.; Qian, S.; Fu, J.; Wang, J. Effects of paddy field disposal of biogas slurry on the rice production, soil quality and environmental safety. J. Agro-Environ. Sci. 2011, 30, 1328–1336. Available online: http://www.cnki.com.cn/Article/CJFDTotal-NHBH201107013.htm (accessed on 6 January 2011).
- Shi, Y. The Potential Capacity for Paddy Field Ecosystem to Decontaminate Biogas Slurry and its Risks Assessment. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2010. [Google Scholar]
- Chen, D.; Jiang, L.; Huang, H.; Toyata, K. Nitrogen dynamics of anaerobically digested slurry used to fertilize paddy fields. Biol. Fertil. Soils 2013, 49, 647–659. [Google Scholar] [CrossRef]
- Huijsmans, J.F.M.; Hol, J.M.G.; Hendriks, M.M.W.B. Effect of application technique, manure characteristics, weather and field conditions on ammonia volatilization from manure applied to grassland. NJAS Wagening. J. Life Sci. 2001, 49, 323–342. [Google Scholar] [CrossRef]
- Nicholson, F.; Bhogal, A.; Cardenas, L.; Chadwick, D.; Misselbrook, T.; Rollett, A.; Taylor, M.; Thorman, R.; Williams, J. Nitrogen losses to the environment following food-based digestate and compost applications to agricultural land. Environ. Pollut. 2017, 228, 504–516. [Google Scholar] [CrossRef]
- Smith, K.A.; Jackson, D.R.; Misselbrook, T.H.; Pain, B.F.; Johnson, R.A. Reduction of ammonia emission by slurry application techniques. J. Agric. Eng. Res. 2000, 77, 277–287. [Google Scholar] [CrossRef]
- Hou, H.; Zhou, S.; Hosomi, M.; Toyota, K.; Yosimura, K.; Mutou, Y.; Nisimura, T.; Takayanagi, M.; Motobayashi, T. Ammonia emissions from anaerobically-digested slurry and chemical fertilizer applied to flooded forage rice. Water Air Soil Pollut. 2007, 183, 37–48. [Google Scholar] [CrossRef]
- Zhou, S.; Nishiyama, K.; Watanabe, Y.; Hosomi, M. Nitrogen budget and ammonia volatilization in paddy fields fertilized with liquid cattle waste. Water Air Soil Pollut. 2009, 201, 135–147. [Google Scholar] [CrossRef]
- Kowalenko, C.G.; Yu, S. Solution, exchangeable and clay-fixed ammonium in south coast British Columbia soils. Can. J. Soil Sci. 1996, 76, 473–483. [Google Scholar] [CrossRef]
- Steffens, D.; Sparks, D.L. Kinetics of nonexchangeable ammonium release from soils. Soil Sci. Soc. Am. J. 1997, 61, 455–462. [Google Scholar] [CrossRef]
- Zhang, Y.X. Ammonia Forms and Adsorption/Desorption Behavior in Soils from the Downstream of Informal Landfill. Master’s Thesis, Chongqing University, Chongqing, China, 2017. [Google Scholar]
- General Administration of Environmental Protection of the People’s Republic of China; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. Discharge Standard of Pollutants for Livestock and Poultry Breeding. National Standards of the People’s Republic of China. 2003, GB 18596-2001. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/shjbh/swrwpfbz/200301/t20030101_66550.shtml (accessed on 1 January 2003).
- Pourret, O.; Bollinger, J.C.; Hursthouse, A.; Hullebusch, E.D. Sorption vs adsorption: The words they are a-changin’, not the phenomena. Sci. Total Environ. 2022, 838, 156545. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Q.; Li, A.; Qian, S.; Fu, J.; Ma, J.; Ye, J.; Wang, J. The ecological effects of fallow paddy field disposal biogas slurry and its impact on the following rice safety production. J. Agro-Environ. Sci. 2011, 30, 2483–2490. Available online: http://www.cqvip.com/QK/92252A/201112/40459055.html (accessed on 17 May 2011).
- Wang, Z.; Guan, Y.; Sheng, J.; Liang, Y.; Wu, H.; Chen, L.; Zheng, J. Effects of biogas slurry application on paddy field water nitrogen content at tillering stage. Chin. J. Eco-Agric. 2015, 23, 1544–1551. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, Y.; Sheng, J.; Guan, Y.; Wu, H.; Chen, L.; Zheng, J. Effects of biogas slurry degradation on nitrogen content in paddy field water of panicle fertilizer stage. J. Soil Water Conserv. 2015, 29, 246–251. [Google Scholar] [CrossRef]
- Zhao, W.; Li, Y.; Zhao, Q.; Ning, Z.; Zhou, C.; Wang, H.; Lu, L.; Yang, P.; Zhang, K.; Wang, F.; et al. Adsorption and desorption characteristics of ammonium in eight loams irrigated with reclaimed wastewater from intensive hogpen. Environ. Earth Sci. 2013, 69, 41–49. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Q.; Ma, J.; Chapman, S.; Zou, P.; Ye, J.; Yu, Q.; Sun, W.; Lin, H.; Jiang, L. Soil microbial activity and community composition as influenced by application of pig biogas slurry in paddy field in southeast China. Paddy Water Environ. 2020, 18, 15–25. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Sun, G.; Zhou, W.; Sheng, J.; Ye, X.; Olaniran, A.O.; Kana, E.B.G. Adsorption characteristics of three types of soils on biogas slurry ammonium nitrogen. Front. Environ. Sci. 2022, 10, 942263. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Sun, G.; Zhang, L.; Zhou, W.; Sheng, J.; Ye, X.; Olaniran, A.O.; Kana, E.B.G.; Shao, H. Aging Characteristics and Fate Analysis of Liquid Digestate Ammonium Nitrogen Disposal in Farmland Soil. Water 2022, 14, 2487. https://doi.org/10.3390/w14162487
Wang Z, Sun G, Zhang L, Zhou W, Sheng J, Ye X, Olaniran AO, Kana EBG, Shao H. Aging Characteristics and Fate Analysis of Liquid Digestate Ammonium Nitrogen Disposal in Farmland Soil. Water. 2022; 14(16):2487. https://doi.org/10.3390/w14162487
Chicago/Turabian StyleWang, Zichen, Guofeng Sun, Liping Zhang, Wei Zhou, Jing Sheng, Xiaomei Ye, Ademola O. Olaniran, Evariste B. Gueguim Kana, and Hongbo Shao. 2022. "Aging Characteristics and Fate Analysis of Liquid Digestate Ammonium Nitrogen Disposal in Farmland Soil" Water 14, no. 16: 2487. https://doi.org/10.3390/w14162487





