Evaluation of Microalgal Bacterial Dynamics in Pig-Farming Biogas Digestate under Impacts of Light Intensity and Nutrient Using Physicochemical Parameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biogas Digestate and Microalgae Inoculation
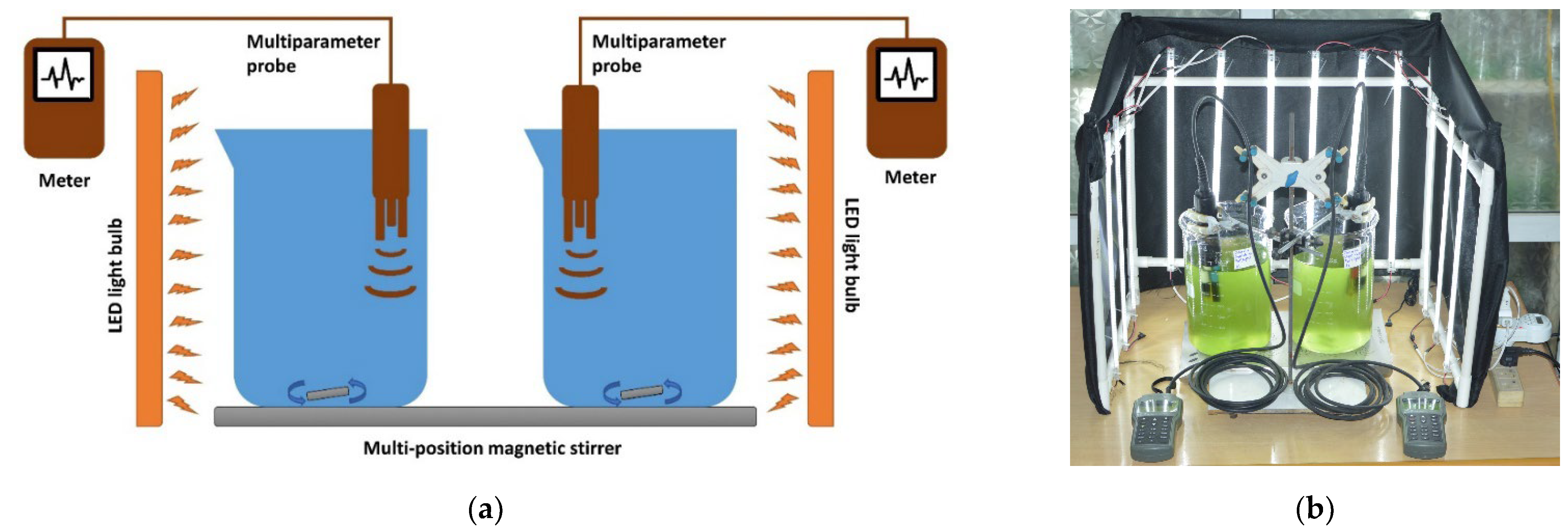
2.2. Experimental Setup
2.3. Sample Collection and Analytical Procedure
2.4. Sample Collection and Analytical Procedure
3. Results and Discussion
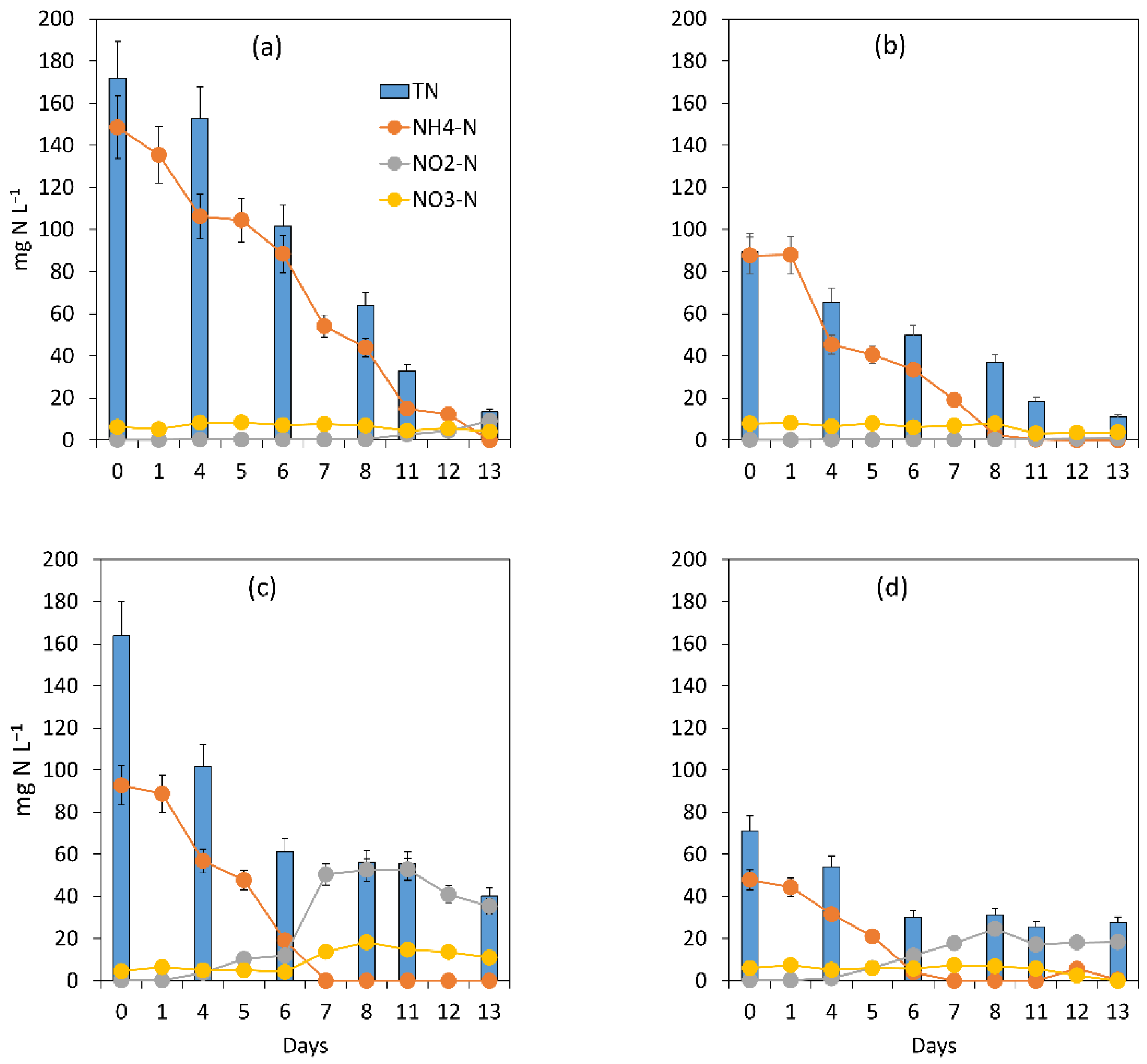
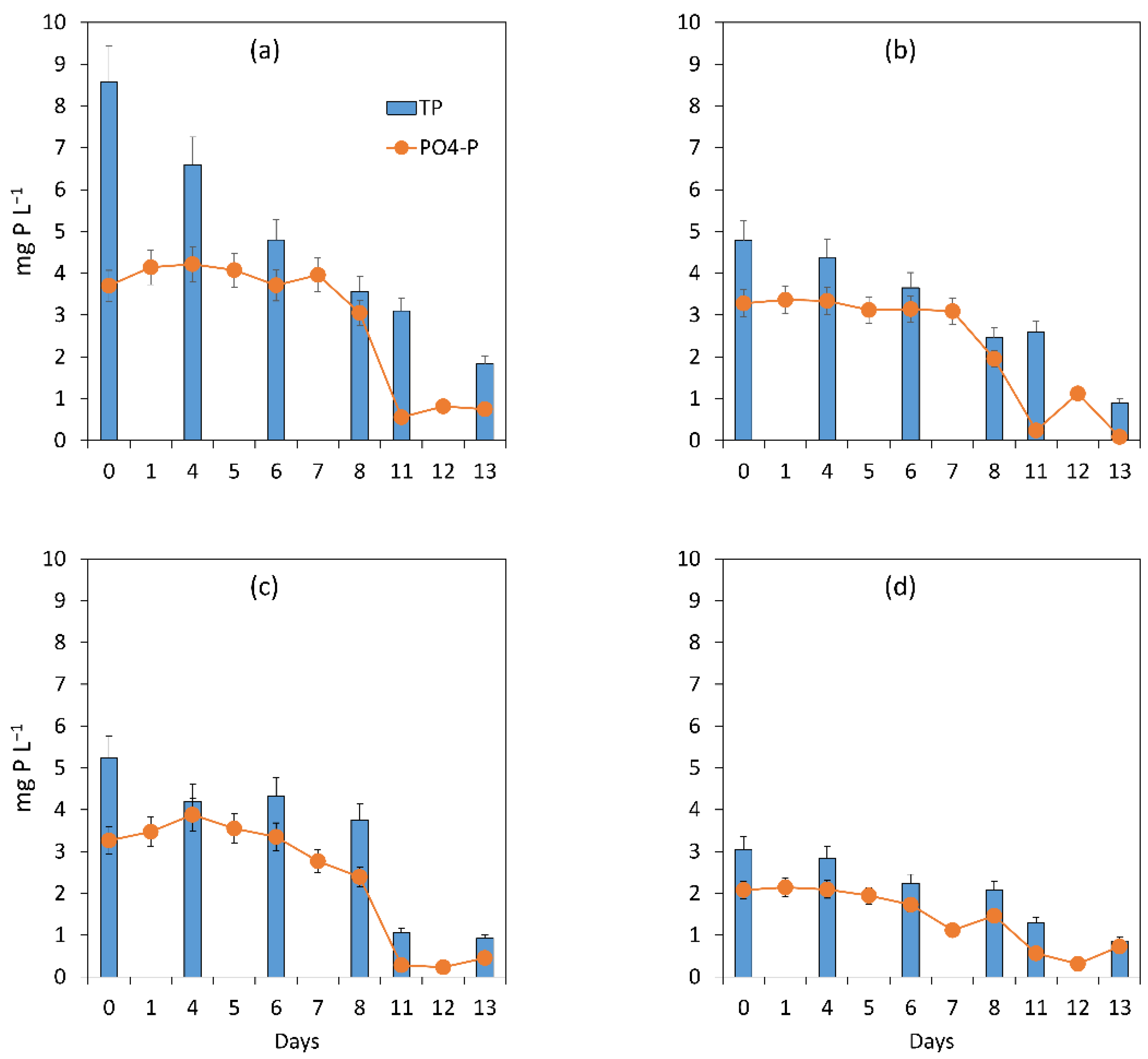
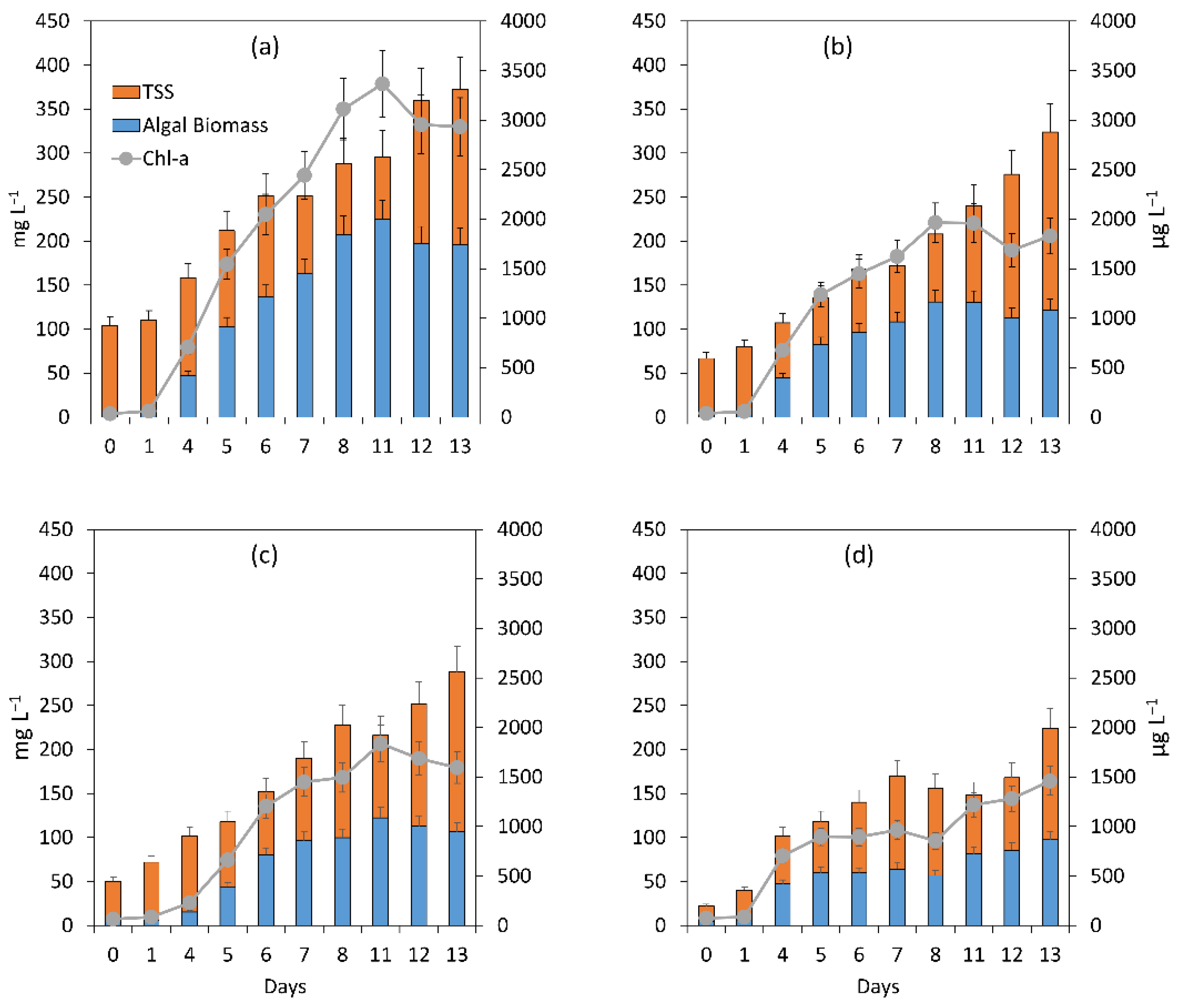
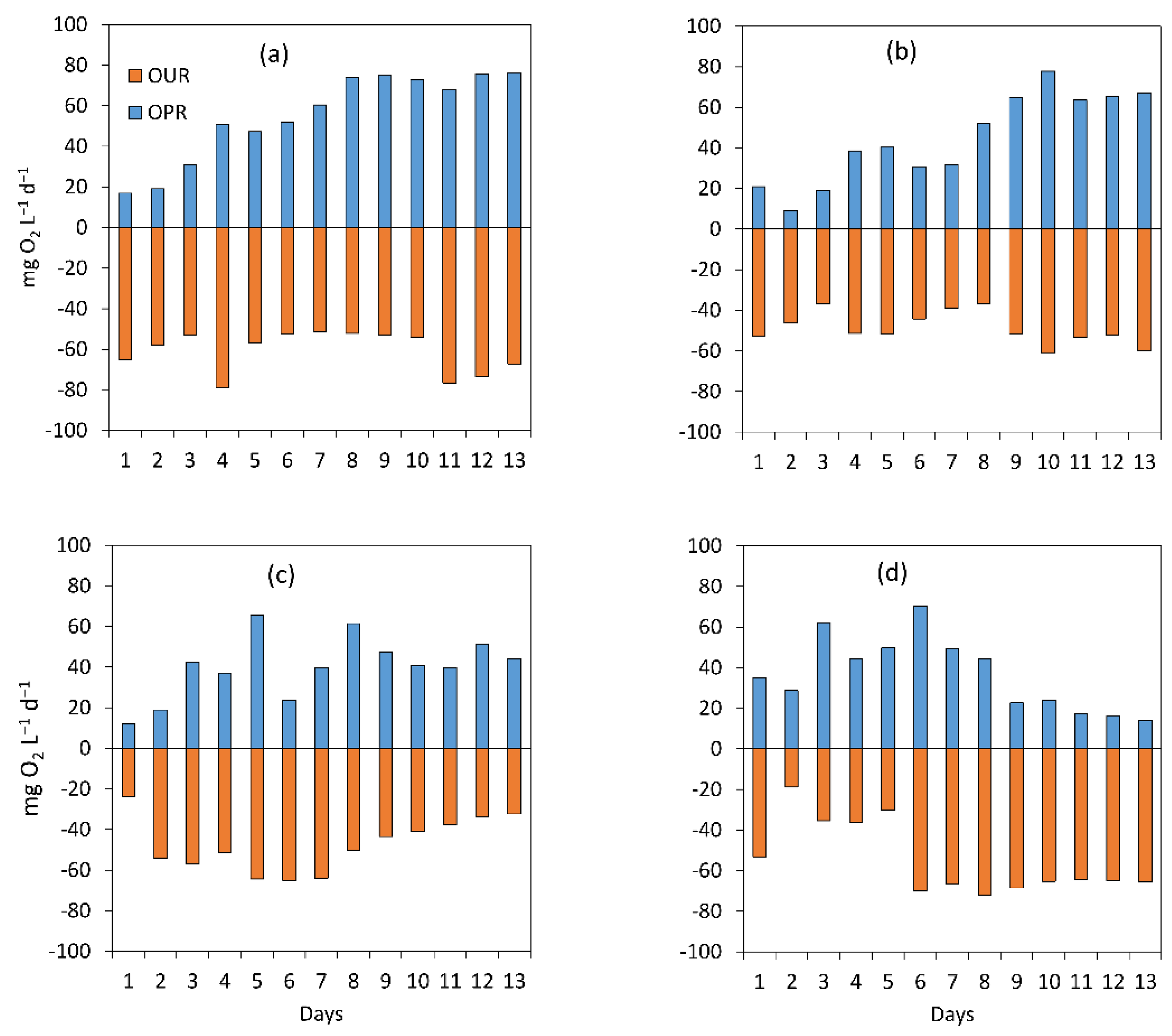
3.1. Evaluation of the Microalgal Bacterial Dynamics under the Impacts of Light and Nutrient Load
3.1.1. General Assessment of the Main Processes Governing Treatment Performance
3.1.2. Assessment of the Treatment Process Dynamics between Microalgae and Bacteria
3.1.3. Microalgal Bacterial Dynamics Assessment under the Impacts of Light and Nutrient Load
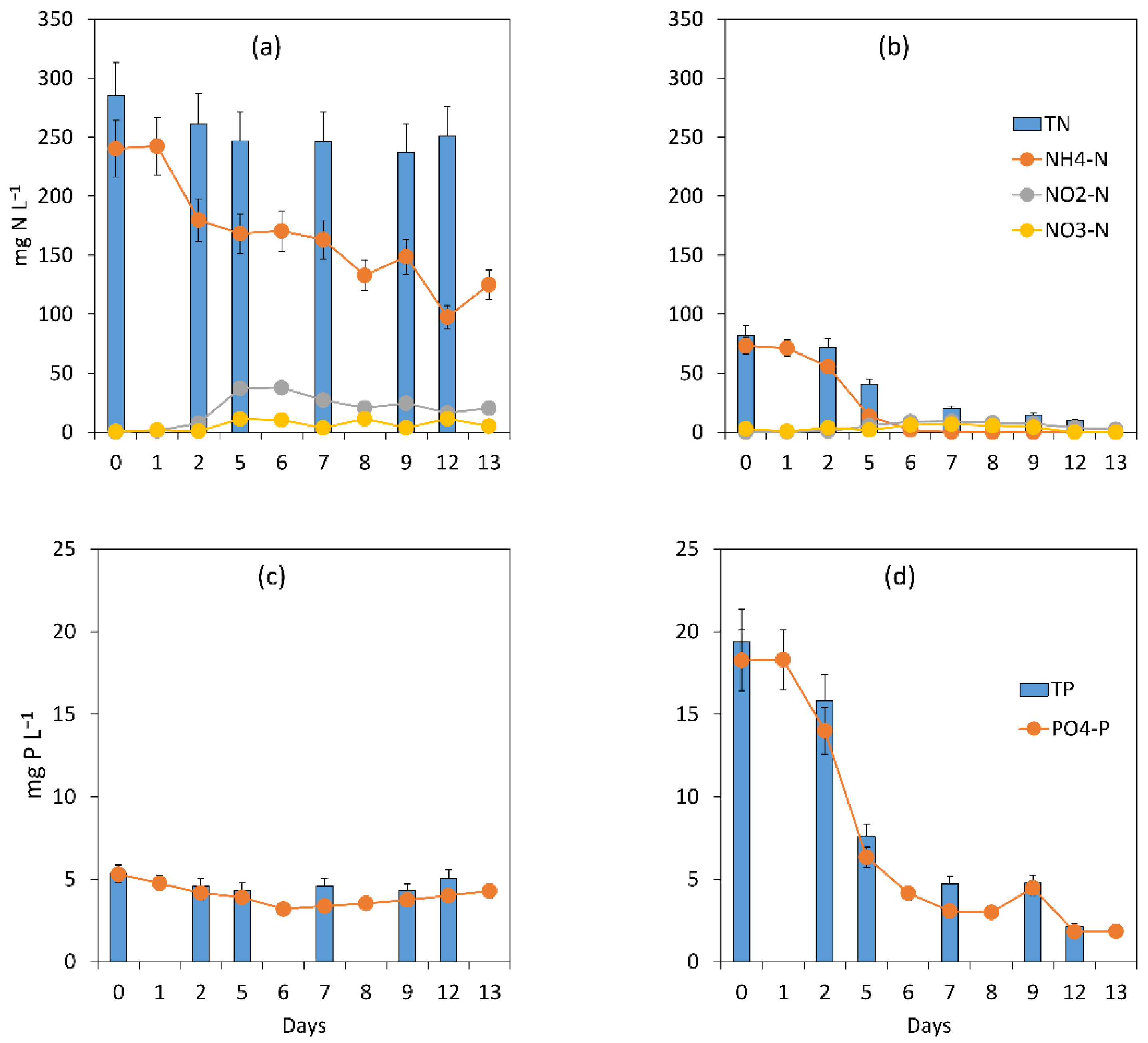
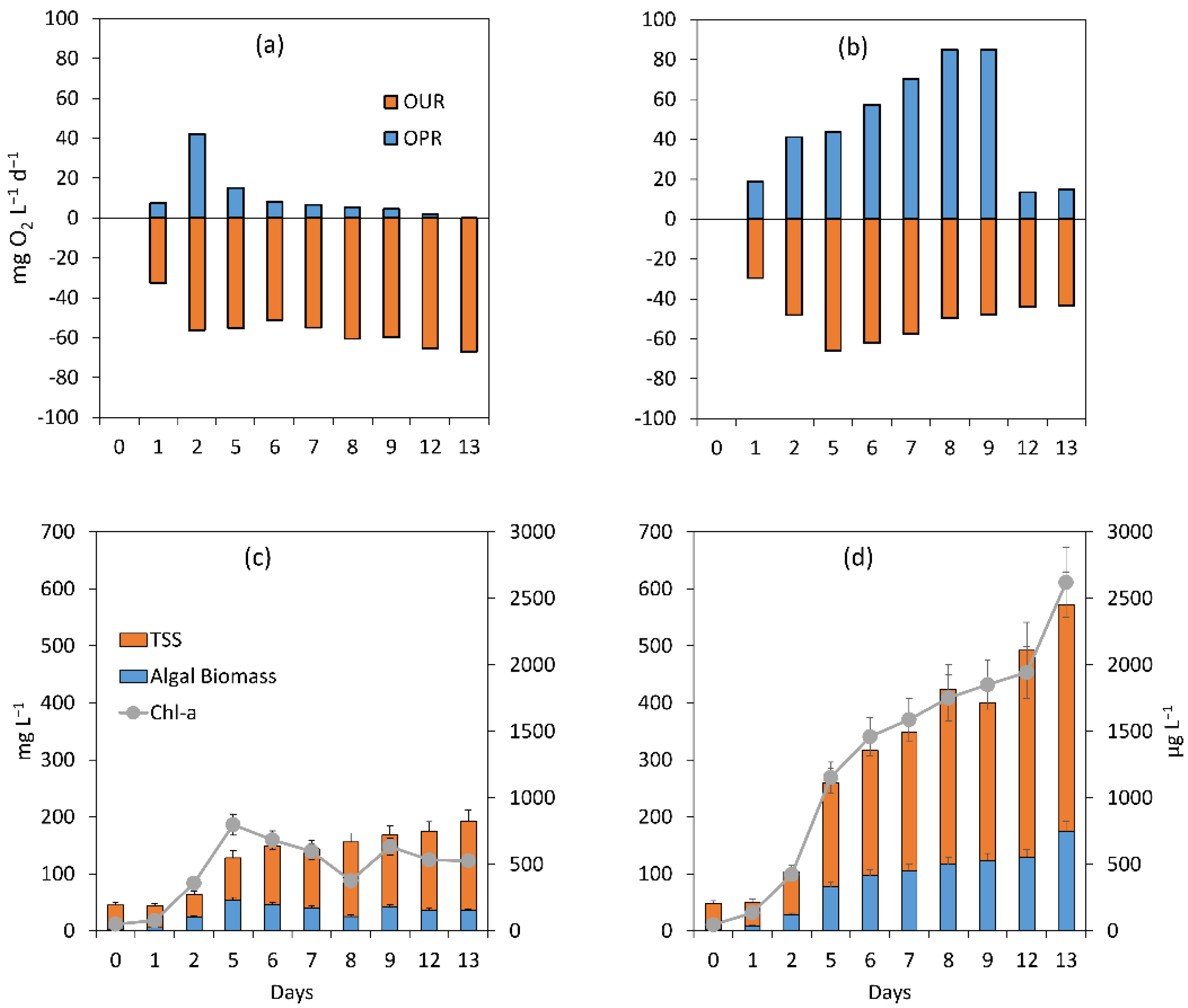
3.2. Evaluation of the Microalgal Bacterial Dynamics under the Impacts of Extreme N:P Ratios
3.2.1. General Assessment of the Main Processes Governing Treatment Performance
3.2.2. Assessment of the Treatment Process Dynamics between Microalgae and Bacteria
3.2.3. Microalgal Bacterial Dynamics Assessment under the Impacts of Extreme N:P Ratios
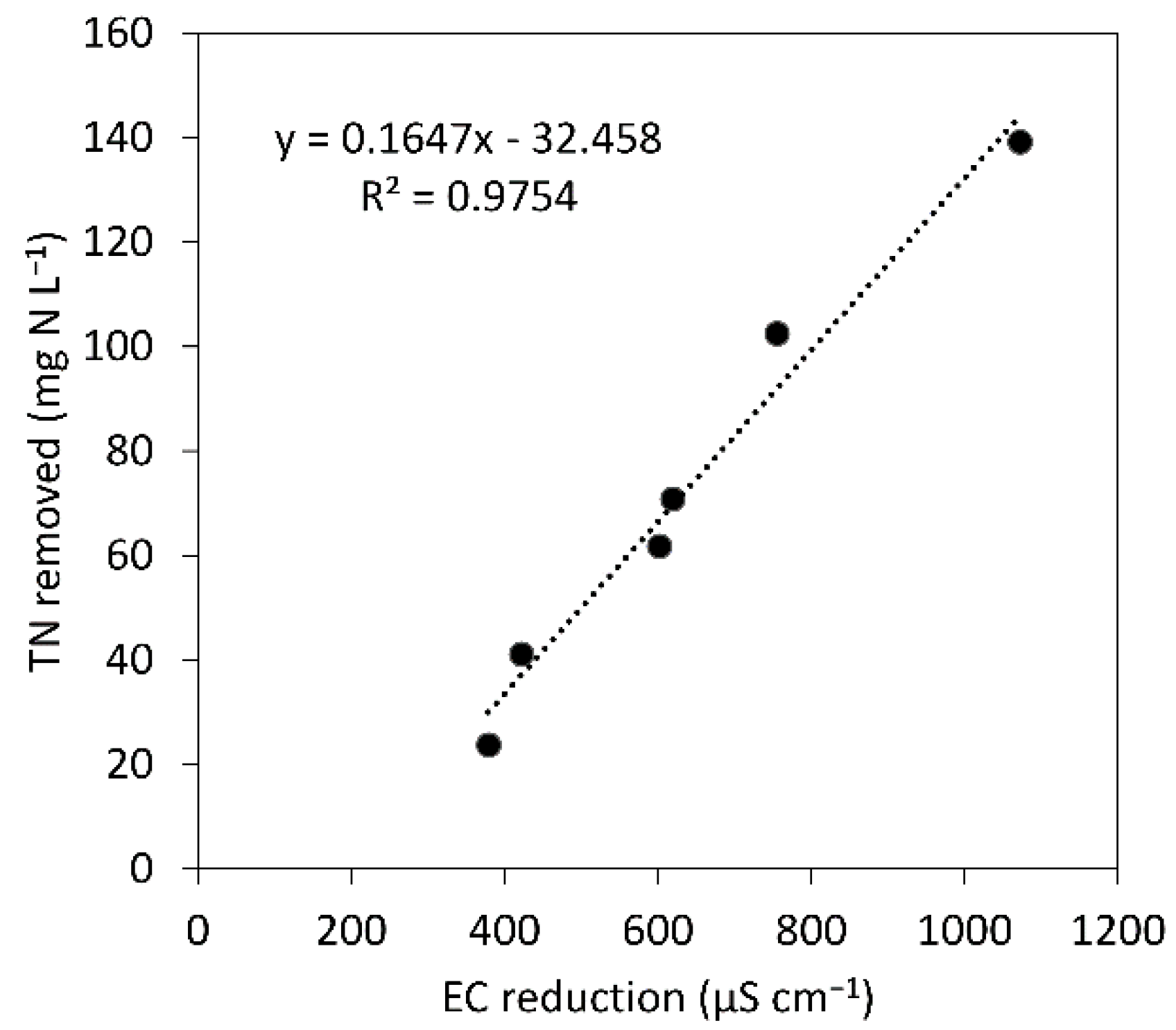
3.3. The Potential Use of Physicochemical Data for Microalgal Bacterial Dynamics Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Chl-a | Chlorophyll-a |
| COD | Chemical Oxygen Demand |
| DO | Dissolved Oxygen |
| EC | Electrical Conductivity |
| LED | Light Emitting Diode |
| N | Nitrogen |
| NH4+-N | Ammonium nitrogen |
| NO2−-N | Nitrite nitrogen |
| NO3−-N | Nitrate nitrogen |
| OPR | Oxygen Production Rate |
| OTR | Oxygen Transfer Rate |
| OUR | Oxygen Uptake Rate |
| ORP | Oxidation-Reduction Potential |
| P | Phosphorus |
| PO43−-P | Orthophosphate |
| TN | Total Nitrogen |
| TP | Total Phosphorus |
| TSS | Total Suspended Solids |
References
- Steinfeld, H.; Wassenaar, T.; Jutzi, S. Livestock Production Systems in Developing Countries: Status, Drivers, Trends. Rev. Sci. Tech. Int. Off. Epizoot. 2006, 25, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Bai, Z.H.; Qin, W.; Xia, L.J.; Oenema, O.; Jiang, R.F.; Ma, L. Environmental, Economic and Social Analysis of Peri-Urban Pig Production. J. Clean. Prod. 2016, 129, 596–607. [Google Scholar] [CrossRef]
- Vu, T.K.V.; Tran, M.T.; Dang, T.T.S. A Survey of Manure Management on Pig Farms in Northern Vietnam. Livest. Sci. 2007, 112, 288–297. [Google Scholar] [CrossRef]
- Baştabak, B.; Koçar, G. A Review of the Biogas Digestate in Agricultural Framework. J. Mater. Cycles Waste Manag. 2020, 22, 1318–1327. [Google Scholar] [CrossRef]
- Aditya, L.; Mahlia, T.M.I.; Nguyen, L.N.; Vu, H.P.; Nghiem, L.D. Microalgae-Bacteria Consortium for Wastewater Treatment and Biomass Production. Sci. Total Environ. 2022, 838, 155871. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Bui, X.-T.; Ngo, H.H.; Nguyen, T.-T.-D.; Nguyen, K.-Q.; Nguyen, H.-H.; Huynh, K.-P.-H.; Némery, J.; Fujioka, T.; Duong, C.H.; et al. Nutrient Recovery and Microalgae Biomass Production from Urine by Membrane Photobioreactor at Low Biomass Retention Times. Sci. Total Environ. 2021, 785, 147423. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for Biodiesel Production and Other Applications: A Review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef] [Green Version]
- Sirajunnisa, A.R.; Surendhiran, D. Algae—A Quintessential and Positive Resource of Bioethanol Production: A Comprehensive Review. Renew. Sustain. Energy Rev. 2016, 66, 248–267. [Google Scholar] [CrossRef]
- Oswald, W.J.; Gotaas, H.B. Photosynthesis in Sewage Treatment. Trans. Am. Soc. Civ. Eng. 1957, 122, 73–97. [Google Scholar] [CrossRef]
- Muñoz, R.; Guieysse, B. Algal–Bacterial Processes for the Treatment of Hazardous Contaminants: A Review. Water Res. 2006, 40, 2799–2815. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Homaidan, A.A.; Ibraheem, I.B.M. Microalgae and Wastewater Treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef] [Green Version]
- Sutherland, D.L.; Howard-Williams, C.; Turnbull, M.H.; Broady, P.A.; Craggs, R.J. Enhancing Microalgal Photosynthesis and Productivity in Wastewater Treatment High Rate Algal Ponds for Biofuel Production. Bioresour. Technol. 2015, 184, 222–229. [Google Scholar] [CrossRef]
- Wang, Y.; Ho, S.-H.; Cheng, C.-L.; Guo, W.-Q.; Nagarajan, D.; Ren, N.-Q.; Lee, D.-J.; Chang, J.-S. Perspectives on the Feasibility of Using Microalgae for Industrial Wastewater Treatment. Bioresour. Technol. 2016, 222, 485–497. [Google Scholar] [CrossRef]
- Rawat, I.; Ranjith Kumar, R.; Mutanda, T.; Bux, F. Dual Role of Microalgae: Phycoremediation of Domestic Wastewater and Biomass Production for Sustainable Biofuels Production. Appl. Energy 2011, 88, 3411–3424. [Google Scholar] [CrossRef]
- Ramanan, R.; Kim, B.-H.; Cho, D.-H.; Oh, H.-M.; Kim, H.-S. Algae–Bacteria Interactions: Evolution, Ecology and Emerging Applications. Biotechnol. Adv. 2016, 34, 14–29. [Google Scholar] [CrossRef] [Green Version]
- Qu, W.; Zhang, C.; Chen, X.; Ho, S.-H. New Concept in Swine Wastewater Treatment: Development of a Self-Sustaining Synergetic Microalgae-Bacteria Symbiosis (ABS) System to Achieve Environmental Sustainability. J. Hazard. Mater. 2021, 418, 126264. [Google Scholar] [CrossRef]
- Van Den Hende, S.; Beelen, V.; Bore, G.; Boon, N.; Vervaeren, H. Up-Scaling Aquaculture Wastewater Treatment by Microalgal Bacterial Flocs: From Lab Reactors to an Outdoor Raceway Pond. Bioresour. Technol. 2014, 159, 342–354. [Google Scholar] [CrossRef]
- Sutherland, D.L.; Turnbull, M.H.; Craggs, R.J. Environmental Drivers That Influence Microalgal Species in Fullscale Wastewater Treatment High Rate Algal Ponds. Water Res. 2017, 124, 504–512. [Google Scholar] [CrossRef]
- García, D.; Posadas, E.; Blanco, S.; Acién, G.; García-Encina, P.; Bolado, S.; Muñoz, R. Evaluation of the Dynamics of Microalgae Population Structure and Process Performance during Piggery Wastewater Treatment in Algal-Bacterial Photobioreactors. Bioresour. Technol. 2018, 248, 120–126. [Google Scholar] [CrossRef] [Green Version]
- Pham, L.A.; Laurent, J.; Bois, P.; Wanko, A. Finding Optimal Algal/Bacterial Inoculation Ratio to Improve Algal Biomass Growth with Wastewater as Nutrient Source. Water SA 2019, 45, 624–631. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Mennerich, A.; Urban, B. Synergistic Cooperation between Wastewater-Born Algae and Activated Sludge for Wastewater Treatment: Influence of Algae and Sludge Inoculation Ratios. Bioresour. Technol. 2012, 105, 67–73. [Google Scholar] [CrossRef]
- Zambrano, J.; Krustok, I.; Nehrenheim, E.; Carlsson, B. A Simple Model for Algae-Bacteria Interaction in Photo-Bioreactors. Algal Res. 2016, 19, 155–161. [Google Scholar] [CrossRef]
- Solimeno, A.; Parker, L.; Lundquist, T.; García, J. Integral Microalgae-Bacteria Model (BIO_ALGAE): Application to Wastewater High Rate Algal Ponds. Sci. Total Environ. 2017, 601–602, 646–657. [Google Scholar] [CrossRef] [Green Version]
- Wágner, D.S.; Valverde-Pérez, B.; Sæbø, M.; Bregua de la Sotilla, M.; Van Wagenen, J.; Smets, B.F.; Plósz, B.G. Towards a Consensus-Based Biokinetic Model for Green Microalgae—The ASM-A. Water Res. 2016, 103, 485–499. [Google Scholar] [CrossRef] [Green Version]
- Solimeno, A.; García, J. Microalgae-Bacteria Models Evolution: From Microalgae Steady-State to Integrated Microalgae-Bacteria Wastewater Treatment Models—A Comparative Review. Sci. Total Environ. 2017, 607–608, 1136–1150. [Google Scholar] [CrossRef]
- Vergara, C.; Muñoz, R.; Campos, J.L.; Seeger, M.; Jeison, D. Influence of Light Intensity on Bacterial Nitrifying Activity in Algal-Bacterial Photobioreactors and Its Implications for Microalgae-Based Wastewater Treatment. Int. Biodeterior. Biodegrad. 2016, 114, 116–121. [Google Scholar] [CrossRef]
- Decostere, B.; Van Hulle, S.W.; Duyck, M.; Maere, T.; Vervaeren, H.; Nopens, I. The Use of a Combined Respirometric–Titrimetric Setup to Assess the Effect of Environmental Conditions on Micro-Algal Growth Rate. J. Chem. Technol. Biotechnol. 2014, 91, 248–256. [Google Scholar] [CrossRef]
- Flores-Salgado, G.; Thalasso, F.; Buitrón, G.; Vital-Jácome, M.; Quijano, G. Kinetic Characterization of Microalgal-Bacterial Systems: Contributions of Microalgae and Heterotrophic Bacteria to the Oxygen Balance in Wastewater Treatment. Biochem. Eng. J. 2021, 165, 107819. [Google Scholar] [CrossRef]
- Zanetti, L.; Frison, N.; Nota, E.; Tomizioli, M.; Bolzonella, D.; Fatone, F. Progress in Real-Time Control Applied to Biological Nitrogen Removal from Wastewater. A Short-Review. Desalination 2012, 286, 1–7. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, Y.; Lv, D.; Wang, M.; Peng, Y.; Li, B. Advanced Nitrogen and Phosphorus Removal in the Pre-Denitrification Anaerobic/Anoxic/Aerobic Nitrification Sequence Batch Reactor (Pre-A2NSBR) Treating Low Carbon/Nitrogen (C/N) Wastewater. Chem. Eng. J. 2016, 302, 296–304. [Google Scholar] [CrossRef]
- Robles, Á.; Capson-Tojo, G.; Gales, A.; Viruela, A.; Sialve, B.; Seco, A.; Steyer, J.-P.; Ferrer, J. Performance of a Membrane-Coupled High-Rate Algal Pond for Urban Wastewater Treatment at Demonstration Scale. Bioresour. Technol. 2020, 301, 122672. [Google Scholar] [CrossRef] [PubMed]
- Foladori, P.; Petrini, S.; Andreottola, G. Evolution of Real Municipal Wastewater Treatment in Photobioreactors and Microalgae-Bacteria Consortia Using Real-Time Parameters. Chem. Eng. J. 2018, 345, 507–516. [Google Scholar] [CrossRef]
- Pham, L.A.; Laurent, J.; Bois, P.; Teshome, T.M.; Wanko, A. Operating a Semi-Continuous Raceway Pond Allows to Link PH and Oxygen Dynamics to the Interaction between Microalgae and Bacteria. Desalin. Water Treat. 2021, 211, 105–116. [Google Scholar] [CrossRef]
- Su, Y.; Mennerich, A.; Urban, B. Municipal Wastewater Treatment and Biomass Accumulation with a Wastewater-Born and Settleable Algal-Bacterial Culture. Water Res. 2011, 45, 3351–3358. [Google Scholar] [CrossRef]
- Park, J.B.K.; Craggs, R.J.; Shilton, A.N. Wastewater Treatment High Rate Algal Ponds for Biofuel Production. Bioresour. Technol. 2011, 102, 35–42. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Simões, M.; Pires, J.C.M. The Effect of Light Supply on Microalgal Growth, CO2 Uptake and Nutrient Removal from Wastewater. Energy Convers. Manag. 2014, 85, 530–536. [Google Scholar] [CrossRef] [Green Version]
- Iasimone, F.; Panico, A.; De Felice, V.; Fantasma, F.; Iorizzi, M.; Pirozzi, F. Effect of Light Intensity and Nutrients Supply on Microalgae Cultivated in Urban Wastewater: Biomass Production, Lipids Accumulation and Settleability Characteristics. J. Environ. Manag. 2018, 223, 1078–1085. [Google Scholar] [CrossRef]
- Ferreira, V.S.; Pinto, R.F.; Sant’Anna, C. Low Light Intensity and Nitrogen Starvation Modulate the Chlorophyll Content of Scenedesmus dimorphus. J. Appl. Microbiol. 2016, 120, 661–670. [Google Scholar] [CrossRef] [Green Version]
- Xin, L.; Hong-ying, H.; Ke, G.; Jia, Y. Growth and Nutrient Removal Properties of a Freshwater Microalga Scenedesmus Sp. LX1 under Different Kinds of Nitrogen Sources. Ecol. Eng. 2010, 36, 379–381. [Google Scholar] [CrossRef]
- Yu, B.S.; Sung, Y.J.; Hong, M.E.; Sim, S.J. Improvement of Photoautotrophic Algal Biomass Production after Interrupted CO2 Supply by Urea and KH2PO4 Injection. Energies 2021, 14, 778. [Google Scholar] [CrossRef]
- Lorenzen, C.J. Determination of Chlorophyll and Pheo-Pigments: Spectrophotometric Equations1. Limnol. Oceanogr. 1967, 12, 343–346. [Google Scholar] [CrossRef]
- Park, J.; Craggs, R. Wastewater Treatment and Algal Production in High Rate Algal Ponds with Carbon Dioxide Addition. Water Sci. Technol. 2010, 61, 633–639. [Google Scholar] [CrossRef]
- Garcia-Ochoa, F.; Gomez, E. Bioreactor Scale-up and Oxygen Transfer Rate in Microbial Processes: An Overview. Biotechnol. Adv. 2009, 27, 153–176. [Google Scholar] [CrossRef]
- Pham, L.A.; Laurent, J.; Bois, P.; Wanko, A. Impacts of Operational Conditions on Oxygen Transfer Rate, Mixing Characteristics and Residence Time Distribution in a Pilot Scale High Rate Algal Pond. Water Sci. Technol. 2018, 78, 1782–1791. [Google Scholar] [CrossRef]
- López-Pacheco, I.Y.; Carrillo-Nieves, D.; Salinas-Salazar, C.; Silva-Núñez, A.; Arévalo-Gallegos, A.; Barceló, D.; Afewerki, S.; Iqbal, H.M.N.; Parra-Saldívar, R. Combination of Nejayote and Swine Wastewater as a Medium for Arthrospira maxima and Chlorella vulgaris Production and Wastewater Treatment. Sci. Total Environ. 2019, 676, 356–367. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Pires, J.C.M.; Simões, M. A Review on the Use of Microalgal Consortia for Wastewater Treatment. Algal Res. 2017, 24, 403–415. [Google Scholar] [CrossRef]
- Shi, P.; Shen, H.; Wang, W.; Chen, W.; Xie, P. The Relationship between Light Intensity and Nutrient Uptake Kinetics in Six Freshwater Diatoms. J. Environ. Sci. 2015, 34, 28–36. [Google Scholar] [CrossRef]
- Sutherland, D.L.; Turnbull, M.H.; Broady, P.A.; Craggs, R.J. Effects of Two Different Nutrient Loads on Microalgal Production, Nutrient Removal and Photosynthetic Efficiency in Pilot-Scale Wastewater High Rate Algal Ponds. Water Res. 2014, 66, 53–62. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, W.; Yen, H.-W.; Ho, S.-H.; Lo, Y.-C.; Cheng, C.-L.; Ren, N.; Chang, J.-S. Cultivation of Chlorella vulgaris JSC-6 with Swine Wastewater for Simultaneous Nutrient/COD Removal and Carbohydrate Production. Bioresour. Technol. 2015, 198, 619–625. [Google Scholar] [CrossRef]
- Alcántara, C.; Domínguez, J.M.; García, D.; Blanco, S.; Pérez, R.; García-Encina, P.A.; Muñoz, R. Evaluation of Wastewater Treatment in a Novel Anoxic–Aerobic Algal–Bacterial Photobioreactor with Biomass Recycling through Carbon and Nitrogen Mass Balances. Bioresour. Technol. 2015, 191, 173–186. [Google Scholar] [CrossRef]
- Arashiro, L.T.; Ferrer, I.; Rousseau, D.P.L.; Van Hulle, S.W.H.; Garfí, M. The Effect of Primary Treatment of Wastewater in High Rate Algal Pond Systems: Biomass and Bioenergy Recovery. Bioresour. Technol. 2019, 280, 27–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lage, S.; Toffolo, A.; Gentili, F.G. Microalgal Growth, Nitrogen Uptake and Storage, and Dissolved Oxygen Production in a Polyculture Based-Open Pond Fed with Municipal Wastewater in Northern Sweden. Chemosphere 2021, 276, 130122. [Google Scholar] [CrossRef] [PubMed]
- Prieto, F.; Barrado, E.; Vega, M.; Deban, L. Measurement of Electrical Conductivity of Wastewater for Fast Determination of Metal Ion Concentration. Russ. J. Appl. Chem. 2001, 74, 1321–1324. [Google Scholar] [CrossRef]
- De Sousa, D.N.R.; Mozeto, A.A.; Carneiro, R.L.; Fadini, P.S. Electrical Conductivity and Emerging Contaminant as Markers of Surface Freshwater Contamination by Wastewater. Sci. Total Environ. 2014, 484, 19–26. [Google Scholar] [CrossRef]
- Žitnik, M.; Šunta, U.; Godič Torkar, K.; Krivograd Klemenčič, A.; Atanasova, N.; Griessler Bulc, T. The Study of Interactions and Removal Efficiency of Escherichia coli in Raw Blackwater Treated by Microalgae Chlorella vulgaris. J. Clean. Prod. 2019, 238, 117865. [Google Scholar] [CrossRef]
- Renuka, N.; Sood, A.; Ratha, S.K.; Prasanna, R.; Ahluwalia, A.S. Nutrient Sequestration, Biomass Production by Microalgae and Phytoremediation of Sewage Water. Int. J. Phytoremediat. 2013, 15, 789–800. [Google Scholar] [CrossRef]
- Ayre, J.M.; Moheimani, N.R.; Borowitzka, M.A. Growth of Microalgae on Undiluted Anaerobic Digestate of Piggery Effluent with High Ammonium Concentrations. Algal Res. 2017, 24, 218–226. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Wu, X.; Zou, G.; Zhou, T.; Liu, Y.; Ruan, R. Cultivation of Chlorella vulgaris in Manure-Free Piggery Wastewater with High-Strength Ammonium for Nutrients Removal and Biomass Production: Effect of Ammonium Concentration, Carbon/Nitrogen Ratio and PH. Bioresour. Technol. 2019, 273, 203–211. [Google Scholar] [CrossRef]
- Munz, G.; Lubello, C.; Oleszkiewicz, J.A. Factors Affecting the Growth Rates of Ammonium and Nitrite Oxidizing Bacteria. Chemosphere 2011, 83, 720–725. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Liu, T. The Difference in Effective Light Penetration May Explain the Superiority in Photosynthetic Efficiency of Attached Cultivation over the Conventional Open Pond for Microalgae. Biotechnol. Biofuels 2015, 8, 49. [Google Scholar] [CrossRef] [Green Version]
- Arango, L.; Cuervo, F.M.; González-Sánchez, A.; Buitrón, G. Effect of Microalgae Inoculation on the Start-up of Microalgae-Bacteria Systems Treating Municipal, Piggery and Digestate Wastewaters. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2016, 73, 687–696. [Google Scholar] [CrossRef]
- Park, J.B.K.; Craggs, R.J. Nutrient Removal in Wastewater Treatment High Rate Algal Ponds with Carbon Dioxide Addition. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2011, 63, 1758–1764. [Google Scholar] [CrossRef]
- Casagli, F.; Zuccaro, G.; Bernard, O.; Steyer, J.-P.; Ficara, E. ALBA: A Comprehensive Growth Model to Optimize Algae-Bacteria Wastewater Treatment in Raceway Ponds. Water Res. 2021, 190, 116734. [Google Scholar] [CrossRef]
- Li, L.; Lollar, B.S.; Li, H.; Wortmann, U.G.; Lacrampe-Couloume, G. Ammonium Stability and Nitrogen Isotope Fractionations for NH4+–NH3(Aq)–NH3(Gas) Systems at 20–70 °C and PH of 2–13: Applications to Habitability and Nitrogen Cycling in Low-Temperature Hydrothermal Systems. Geochim. Cosmochim. Acta 2012, 84, 280–296. [Google Scholar] [CrossRef]
- Collos, Y.; Harrison, P.J. Acclimation and Toxicity of High Ammonium Concentrations to Unicellular Algae. Mar. Pollut. Bull. 2014, 80, 8–23. [Google Scholar] [CrossRef]
- Markou, G.; Depraetere, O.; Muylaert, K. Effect of Ammonia on the Photosynthetic Activity of Arthrospira and Chlorella: A Study on Chlorophyll Fluorescence and Electron Transport. Algal Res. 2016, 16, 449–457. [Google Scholar] [CrossRef]
- Rossi, S.; Díez-Montero, R.; Rueda, E.; Castillo Cascino, F.; Parati, K.; García, J.; Ficara, E. Free Ammonia Inhibition in Microalgae and Cyanobacteria Grown in Wastewaters: Photo-Respirometric Evaluation and Modelling. Bioresour. Technol. 2020, 305, 123046. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, Y.; Xu, J.; Shang, C.; Wang, Z.; Xu, J.; Yuan, Z. Luxury Uptake of Phosphorus Changes the Accumulation of Starch and Lipid in Chlorella sp. under Nitrogen Depletion. Bioresour. Technol. 2015, 198, 165–171. [Google Scholar] [CrossRef]
- Xin, L.; Hong-ying, H.; Ke, G.; Ying-xue, S. Effects of Different Nitrogen and Phosphorus Concentrations on the Growth, Nutrient Uptake, and Lipid Accumulation of a Freshwater Microalga Scenedesmus sp. Bioresour. Technol. 2010, 101, 5494–5500. [Google Scholar] [CrossRef]
- Marcilhac, C.; Sialve, B.; Pourcher, A.-M.; Ziebal, C.; Bernet, N.; Béline, F. Control of Nitrogen Behaviour by Phosphate Concentration during Microalgal-Bacterial Cultivation Using Digestate. Bioresour. Technol. 2015, 175, 224–230. [Google Scholar] [CrossRef]
- Chen, M.; Li, J.; Dai, X.; Sun, Y.; Chen, F. Effect of Phosphorus and Temperature on Chlorophyll a Contents and Cell Sizes of Scenedesmus obliquus and Microcystis aeruginosa. Limnology 2011, 12, 187–192. [Google Scholar] [CrossRef]
- Da Silva Ferreira, V.; Sant’Anna, C. Impact of Culture Conditions on the Chlorophyll Content of Microalgae for Biotechnological Applications. World J. Microbiol. Biotechnol. 2016, 33, 20. [Google Scholar] [CrossRef]
- Muduli, M.; Sonpal, V.; Trivedi, K.; Haldar, S.; Kumar, M.A.; Ray, S. 12—Enhanced Biological Phosphate Removal Process for Wastewater Treatment: A Sustainable Approach. In Wastewater Treatment Reactors; Shah, M.P., Rodriguez-Couto, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 273–287. ISBN 978-0-12-823991-9. [Google Scholar]
- Zhang, X.; Li, J.; Fan, W.-Y.; Sheng, G.-P. Photomineralization of Effluent Organic Phosphorus to Orthophosphate under Simulated Light Illumination. Environ. Sci. Technol. 2019, 53, 4997–5004. [Google Scholar] [CrossRef]
- Camargo, J.A.; Alonso, Á. Ecological and Toxicological Effects of Inorganic Nitrogen Pollution in Aquatic Ecosystems: A Global Assessment. Environ. Int. 2006, 32, 831–849. [Google Scholar] [CrossRef]
- Yang, X.; Xu, M.; Zou, R.; Angelidaki, I.; Zhang, Y. Microbial Protein Production from CO2, H2, and Recycled Nitrogen: Focusing on Ammonia Toxicity and Nitrogen Sources. J. Clean. Prod. 2021, 291, 125921. [Google Scholar] [CrossRef]
- Su, J.-J.; Ding, S.-T.; Chung, H.-C. Establishing a Smart Farm-Scale Piggery Wastewater Treatment System with the Internet of Things (IoT) Applications. Water 2020, 12, 1654. [Google Scholar] [CrossRef]
- Hoffman, J.; Pate, R.C.; Drennen, T.; Quinn, J.C. Techno-Economic Assessment of Open Microalgae Production Systems. Algal Res. 2017, 23, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Ramaswamy, S. Dynamic Process Model and Economic Analysis of Microalgae Cultivation in Open Raceway Ponds. Algal Res. 2017, 26, 330–340. [Google Scholar] [CrossRef]
- Sun, A.; Davis, R.; Starbuck, M.; Ben-Amotz, A.; Pate, R.; Pienkos, P.T. Comparative Cost Analysis of Algal Oil Production for Biofuels. Energy 2011, 36, 5169–5179. [Google Scholar] [CrossRef]
| Parameters | Filtered Wastewater | X5 | X10 |
|---|---|---|---|
| COD (mg COD L−1) | 1173.3 ± 200.6 | 166 ± 31.1 | 93.5 ± 2.1 |
| TN (mg N L−1) | 777.2 ± 103.8 | 167.9 ± 5.8 | 80.3 ± 12.7 |
| NH4+-N (mg N L−1) | 625.3 ± 174.1 | 120.7 ± 39.5 | 67.8 ± 28.1 |
| NO2−-N (mg N L−1) | 0.16 ± 0.03 | 0.18 ± 0.05 | 0.16 ± 0.08 |
| NO3−-N (mg N L−1) | 0.19 ± 0.24 | 5.4 ± 1.2 | 6.9 ± 1.3 |
| TP (mg P L−1) | 35.9 ± 6.0 | 6.9 ± 2.4 | 3.9 ± 1.2 |
| PO43−-P (mg P L−1) | 25.8 ± 5.6 | 3.5 ± 0.3 | 2.7 ± 0.9 |
| TSS (mg L−1) | 369.8 ± 141.6 | 77.0 ± 38.2 | 44.3 ± 31.6 |
| Stage | Name | Light Intensity (μE s−1 m−2) | N:P Ratio | Nutrient Load (Dilution) |
|---|---|---|---|---|
| 1 | HL.X5 | High (12.7 ± 3.1) | 22.1 ± 3.4 | High (x5) |
| HL.X10 | High (12.7 ± 3.1) | 22.1 ± 3.4 | Low (x10) | |
| 2 | LL.X5 | Low (5.9 ± 2.5) | 22.1 ± 3.4 | High (x5) |
| LL.X10 | Low (5.9 ± 2.5) | 22.1 ± 3.4 | Low (x10) | |
| 3 | HL.X10.N | High (12.7 ± 3.1) | High (53.2) | Low (x10) |
| HL.X10.P | High (12.7 ± 3.1) | Low (4.2) | Low (x10) |
| Parameters | HL.X5 | HL.X10 | LL.X5 | LL.X10 | |
|---|---|---|---|---|---|
| COD removal | Rate (mg L−1 d−1) | 6.8 | 4.6 | 4.0 | 3.8 |
| Efficiency (%) | 46.8 | 65.2 | 36.1 | 51.6 | |
| TN removal | Rate (mg L−1 d−1) | 12.2 | 6.0 | 9.5 | 3.4 |
| Efficiency (%) | 92.2 | 87.8 | 75.5 | 61.3 | |
| NH4+-N removal | Rate (mg L−1 d−1) | 11.4 | 7.3 | 13.2 | 6.8 |
| Efficiency (%) | 100 | 100 | 100 | 99.2 | |
| TP removal | Rate (mg L−1 d−1) | 0.52 | 0.30 | 0.33 | 0.17 |
| Efficiency (%) | 78.7 | 81.2 | 82.4 | 71.8 | |
| PO43−-P removal | Rate (mg L−1 d−1) | 0.23 | 0.25 | 0.22 | 0.10 |
| Efficiency (%) | 79.9 | 97.4 | 86.1 | 65.1 |
| Experiments | DO (mg L−1) | pH | EC (µS cm−1) | TSS Production Rate (mg L−1 d−1) | Microalgal Production Rate (mg L−1 d−1) |
|---|---|---|---|---|---|
| HL.X5 | 5.2 ± 3.3 | 9.1 ± 0.3 | 1725.8 ± 395.1 | 20.6 | 14.9 |
| HL.X10 | 6.2 ± 2.6 | 9.0 ± 0.4 | 996.4 ± 215.5 | 19.8 | 9.2 |
| LL.X5 | 5.3 ± 2.7 | 8.9 ± 0.9 | 1390.0 ± 275.9 | 18.3 | 7.9 |
| LL.X10 | 4.2 ± 3.8 | 8.6 ± 0.7 | 836.2 ± 137.2 | 15.5 | 7.1 |
| Parameters | HL.X10.N | L.X10.P | |
|---|---|---|---|
| COD removal | Rate (mg L−1 d−1) | 0 (4.2 1) | 0.5 (6.7 2) |
| Efficiency (%) | 0 (23.6 1) | 9.6 (64.4 2) | |
| TN removal | Rate (mg L−1 d−1) | 2.6 | 2.6 |
| Efficiency (%) | 11.9 | 88.0 | |
| NH4+-N removal | Rate (mg L−1 d−1) | 8.9 | 9.2 |
| Efficiency (%) | 48.0 | 100 | |
| TP removal | Rate (mg L−1 d−1) | 0.02 | 1.33 |
| Efficiency (%) | 6.0 | 89.1 | |
| PO43−-P removal | Rate (mg L−1 d−1) | 0.08 | 1.26 |
| Efficiency (%) | 19.3 | 89.9 | |
| TSS production rate (mg L−1 d−1) | 11.3 | 40.3 | |
| Microalgal production rate (mg L−1 d−1) | 2.4 | 13.2 | |
| DO (mg L−1) | 1.8 ± 1.9 | 5.3 ± 3.2 | |
| pH | 6.3 ± 0.8 | 9.5 ± 0.8 | |
| EC (µS cm−1) | 2923.0 ± 97.8 | 782.2 ± 204.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, A.L.; Luu, K.D.; Duong, T.T.; Dinh, T.M.T.; Nguyen, S.Q.; Nguyen, T.K.; Duong, H.C.; Le, Q.P.T.; Le, T.P. Evaluation of Microalgal Bacterial Dynamics in Pig-Farming Biogas Digestate under Impacts of Light Intensity and Nutrient Using Physicochemical Parameters. Water 2022, 14, 2275. https://doi.org/10.3390/w14142275
Pham AL, Luu KD, Duong TT, Dinh TMT, Nguyen SQ, Nguyen TK, Duong HC, Le QPT, Le TP. Evaluation of Microalgal Bacterial Dynamics in Pig-Farming Biogas Digestate under Impacts of Light Intensity and Nutrient Using Physicochemical Parameters. Water. 2022; 14(14):2275. https://doi.org/10.3390/w14142275
Chicago/Turabian StylePham, Anh Le, Khanh Duy Luu, Thuy Thi Duong, Thanh Mai Thi Dinh, Son Quoc Nguyen, Toan Khanh Nguyen, Hung Cong Duong, Quynh Phuong Thi Le, and Thu Phuong Le. 2022. "Evaluation of Microalgal Bacterial Dynamics in Pig-Farming Biogas Digestate under Impacts of Light Intensity and Nutrient Using Physicochemical Parameters" Water 14, no. 14: 2275. https://doi.org/10.3390/w14142275
APA StylePham, A. L., Luu, K. D., Duong, T. T., Dinh, T. M. T., Nguyen, S. Q., Nguyen, T. K., Duong, H. C., Le, Q. P. T., & Le, T. P. (2022). Evaluation of Microalgal Bacterial Dynamics in Pig-Farming Biogas Digestate under Impacts of Light Intensity and Nutrient Using Physicochemical Parameters. Water, 14(14), 2275. https://doi.org/10.3390/w14142275






