Ecotoxicological Consequences of the Abatement of Contaminants of Emerging Concern by Ozonation—Does Mixture Complexity Matter?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Compounds
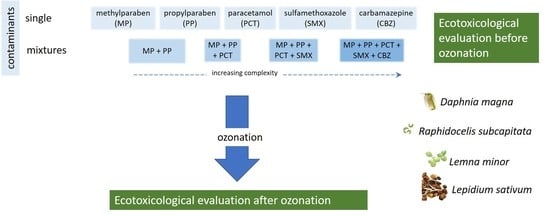
2.2. Experimental Design
2.2.1. Samples Preparation
2.2.2. Ozonation Procedure
2.3. Toxicity Assessment
- Daphnia magna
- Raphidocelis subcapitata
- Lemna minor
- Lepidium sativum
2.4. Chemical Analyses
2.5. Interactive Effects of Mixtures of PPCPs
2.6. Statistical Analyses
3. Results and Discussion
3.1. Detection and Identification of By-Products
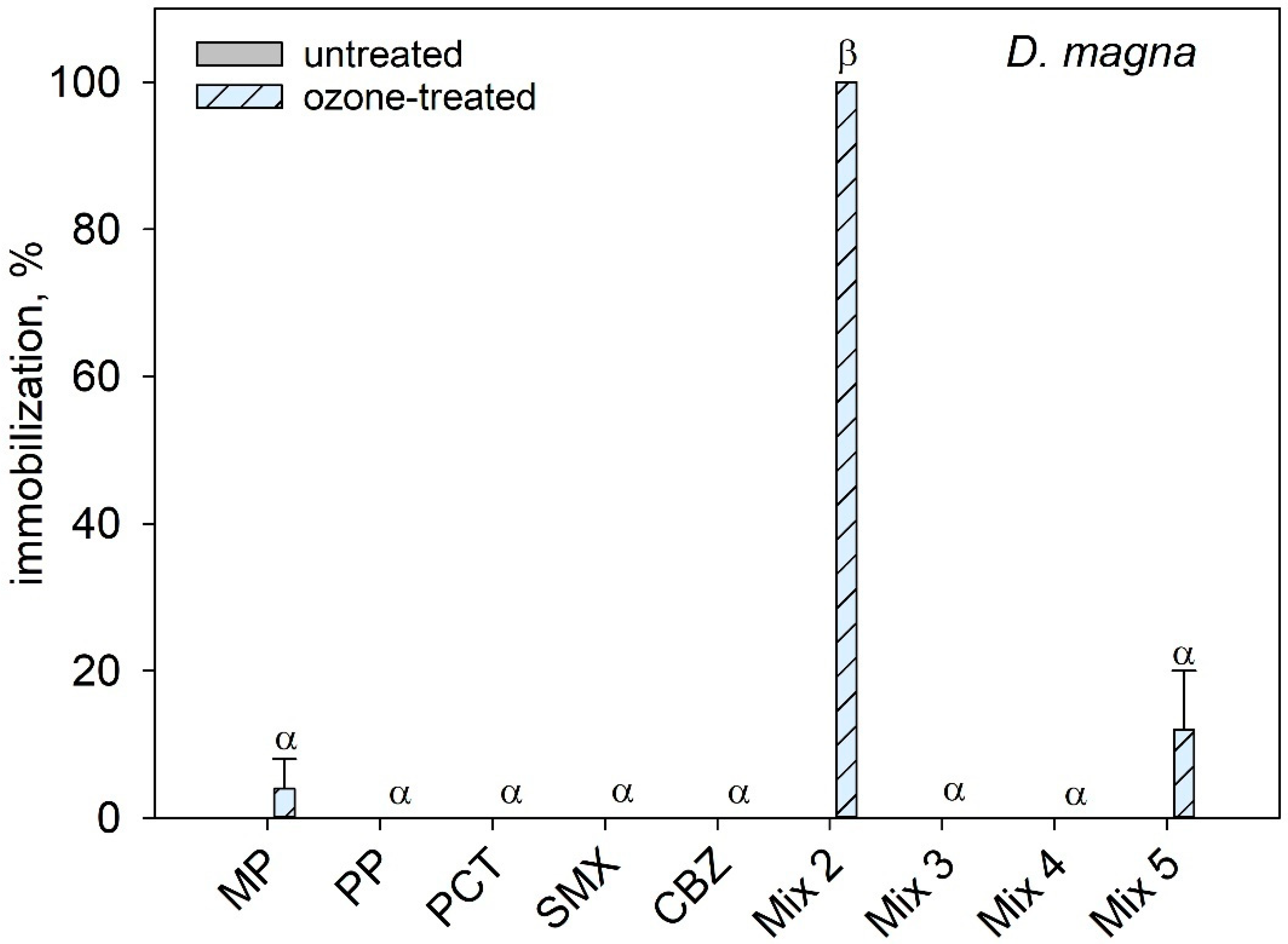
3.2. Toxicity Assessment of PPCPs
3.3. Interactive Effects of Mixtures of PPCPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Balakrishna, K.; Rath, A.; Praveenkumarreddy, Y.; Guruge, K.S.; Subedi, B. A review of the occurrence of pharmaceuticals and personal care products in Indian water bodies. Ecotox. Environ. Saf. 2017, 137, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Keerthanan, S.; Jayasinghe, C.; Biswas, J.K.; Vithanage, M. Pharmaceutical and Personal Care Products (PPCPs) in the environment: Plant uptake, translocation, bioaccumulation, and human health risks. Crit. Rev. Environ. Sci. Technol. 2021, 51, 1221–1258. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef]
- Prada-Vásquez, M.A.; Estrada-Flórez, S.E.; Serna-Galvis, E.A.; Torres-Palma, R.A. Developments in the intensification of photo-Fenton and ozonation-based processes for the removal of contaminants of emerging concern in Ibero-American countries. Sci. Total Environ. 2021, 765, 142699. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.; Costa, R.; Quinta-Ferreira, R.M.; Martins, R.C. Application of ozonation for pharmaceuticals and personal care products removal from water. Sci. Total Environ. 2017, 586, 265–283. [Google Scholar] [CrossRef] [PubMed]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Bourgin, M.; Beck, B.; Boehler, M.; Borowska, E.; Fleiner, J.; Salhi, E.; Teichler, R.; von Gunten, U.; Siegrist, H.; McArdell, C.S. Evaluation of a full-scale wastewater treatment plant upgraded with ozonation and biological post-treatments: Abatement of micropollutants, formation of transformation products and oxidation by-products. Water Res. 2018, 129, 486–498. [Google Scholar] [CrossRef]
- Völker, J.; Stapf, M.; Miehe, U.; Wagner, M. Systematic Review of Toxicity Removal by Advanced Wastewater Treatment Technologies via Ozonation and Activated Carbon. Environ. Sci. Technol. 2019, 53, 7215–7233. [Google Scholar] [CrossRef]
- Gomes, J.F.; Frasson, D.; Pereira, J.L.; Gonçalves, F.J.M.; Castro, L.M.; Quinta-Ferreira, R.M.; Martins, R.C. Ecotoxicity variation through parabens degradation by single and catalytic ozonation using volcanic rock. Chem. Eng. J. 2019, 360, 30–37. [Google Scholar] [CrossRef]
- Andreozzi, R.; Campanella, L.; Fraysse, B.; Garric, J.; Gonnella, A.; Lo Giudice, R.; Marotta, R.; Pinto, G.; Pollio, A. Effects of advanced oxidation processes (AOPs) on the toxicity of a mixture of pharmaceuticals. Water Sci. Technol. 2004, 50, 23–28. [Google Scholar] [CrossRef]
- Nowak, K.; Ratajczak–Wrona, W.; Górska, M.; Jabłońska, E. Parabens and their effects on the endocrine system. Mol. Cell. Endocrinol. 2018, 474, 238–251. [Google Scholar] [CrossRef] [PubMed]
- OECD, Test No. 202; Daphnia sp., Acute Immobilisation Test. OECD Guidelines for the Testing of Chemicals. Organisation for Economic Co-Operation and Development: Paris, France, 2004; p. 12.
- ASTM E1193-97(2004); Standard Guide for Conducting Daphnia magna Life-Cycle Toxicity Tests. American Society for Testing and Materials: West Conshohocken, PA, USA, 2004.
- OECD, Test No. 201; Freshwater Alga and Cyanobacteria, Growth Inhibition Test. Organisation for Economic Co-Operation and Development: Paris, France, 2011.
- Stein, J.R. Handbook of Phycological Methods: Culture Methods and Growth Measurements; Cambridge University Press: Cambridge, UK, 1973; Volume 1. [Google Scholar]
- OECD, Test N221; Lemna sp. Growth Inhibition Test. OECD Guidelines for the Testing of Chemicals. Organisation for Economic Co-Operation and Development: Paris, France, 2006; p. 22.
- ISO 18763; Soil Quality—Determination of the Toxic Effects of Pollutants on Germination and Early Growth of Higher Plants. International Organization for Standardization: Geneva, Switzerland, 2016.
- Sahu, R.K.; Katiyar, S.; Yadav, A.K.; Kumar, N.; Srivastava, J. Toxicity Assessment of Industrial Effluent by Bioassays. Clean–Soil Air Water 2008, 36, 517–520. [Google Scholar] [CrossRef]
- Trautmann, N.M.; Krasny, M.E. Composting in the Classroom: Scientific Inquiry for High School Students; Kendall/Hunt Publishing Company: Dubuque, IA, USA, 1997. [Google Scholar]
- Kortenkamp, A.; Backhaus, T.; Faust, M. State of the Art Report on Mixture Toxicity—Final Report. Report to the European Commission. 2009. Available online: https://ec.europa.eu/environment/chemicals/effects/pdf/report_mixture_toxicity.pdf (accessed on 26 February 2022).
- Puerta, Y.T.; Guimarães, P.S.; Martins, S.E.; de Martinez Gaspar Martins, C. Toxicity of methylparaben to green microalgae species and derivation of a predicted no effect concentration (PNEC) in freshwater ecosystems. Ecotox. Environ. Saf. 2020, 188, 109916. [Google Scholar] [CrossRef] [PubMed]
- Madsen, T.; Boyd, H.B.; Nylén, D.; Pedersen, A.R.; Petersen, G.I.; Simonsen, F. Environmental and health assessment of substances in household detergents and cosmetic detergent products. Environ. Proj. 2001, 615, 221. [Google Scholar]
- Yamamoto, H.; Tamura, I.; Hirata, Y.; Kato, J.; Kagota, K.; Katsuki, S.; Yamamoto, A.; Kagami, Y.; Tatarazako, N. Aquatic toxicity and ecological risk assessment of seven parabens: Individual and additive approach. Sci. Total Environ. 2011, 410, 102–111. [Google Scholar] [CrossRef]
- Di Poi, C.; Costil, K.; Bouchart, V.; Halm-Lemeille, M.-P. Toxicity assessment of five emerging pollutants, alone and in binary or ternary mixtures, towards three aquatic organisms. Environ. Sci. Pollut. Res. 2018, 25, 6122–6134. [Google Scholar] [CrossRef]
- Coors, A.; Vollmar, P.; Sacher, F.; Thoma, A. Joint Effects of Pharmaceuticals and Chemicals Regulated under REACH in Wastewater Treatment Plant Effluents–Evaluating Concepts for a Risk Assessment by Means of Experimental Scenarios. 2016. Available online: https://www.bmuv.de/fileadmin/Daten_BMU/Pools/Forschungsdatenbank/fkz_3712_64_419_klaeranlagenablaeufe_bf.pdf (accessed on 26 February 2022).
- Kamaya, Y.; Fukaya, Y.; Suzuki, K. Acute toxicity of benzoic acids to the crustacean Daphnia magna. Chemosphere 2005, 59, 255–261. [Google Scholar] [CrossRef]
- Terasaki, M.; Makino, M.; Tatarazako, N. Acute toxicity of parabens and their chlorinated by-products with Daphnia magna and Vibrio fischeri bioassays. J. Appl. Toxicol. 2009, 29, 242–247. [Google Scholar] [CrossRef]
- Dobbins, L.L.; Usenko, S.; Brain, R.A.; Brooks, B.W. Probabilistic ecological hazard assessment of parabens using Daphnia magna and Pimephales promelas. Environ. Toxicol. Chem. 2009, 28, 2744–2753. [Google Scholar] [CrossRef]
- Lee, J.; Park, N.; Kho, Y.; Lee, K.; Ji, K. Phototoxicity and chronic toxicity of methyl paraben and 1, 2-hexanediol in Daphnia magna. Ecotoxicology 2017, 26, 81–89. [Google Scholar] [CrossRef]
- Nunes, B.; Antunes, S.C.; Santos, J.; Martins, L.; Castro, B.B. Toxic potential of paracetamol to freshwater organisms: A headache to environmental regulators? Ecotox. Environ. Saf. 2014, 107, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Minguez, L.; Pedelucq, J.; Farcy, E.; Ballandonne, C.; Budzinski, H.; Halm-Lemeille, M.-P. Toxicities of 48 pharmaceuticals and their freshwater and marine environmental assessment in northwestern France. Environ. Sci. Pollut. Res. 2016, 23, 4992–5001. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, S.; Petre, J.; Lucaciu, I.; Stoica, C.; Nita-Lazar, M. Risk screening of pharmaceutical compounds in Romanian aquatic environment. Environ. Monit. Assess. 2016, 188, 379. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Choi, K.; Jung, J.; Park, S.; Kim, P.-G.; Park, J. Aquatic toxicity of acetaminophen, carbamazepine, cimetidine, diltiazem and six major sulfonamides, and their potential ecological risks in Korea. Environ. Int. 2007, 33, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Henschel, K.P.; Wenzel, A.; Diedrich, M.; Fliedner, A. Environmental Hazard Assessment of Pharmaceuticals. Regul. Toxicol. Pharm. 1997, 25, 220–225. [Google Scholar] [CrossRef]
- Daniel, D.; Dionísio, R.; de Alkimin, G.D.; Nunes, B. Acute and chronic effects of paracetamol exposure on Daphnia magna: How oxidative effects may modulate responses at distinct levels of organization in a model species. Environ. Sci. Pollut. Res. 2019, 26, 3320–3329. [Google Scholar] [CrossRef]
- Nunes, B.; Pinto, G.; Martins, L.; Gonçalves, F.; Antunes, S.C. Biochemical and standard toxic effects of acetaminophen on the macrophyte species Lemna minor and Lemna gibba. Environ. Sci. Pollut. Res. 2014, 21, 10815–10822. [Google Scholar] [CrossRef]
- Ferrari, B.; Mons, R.; Vollat, B.; Fraysse, B.; Paxēaus, N.; Giudice, R.L.; Pollio, A.; Garric, J. Environmental risk assessment of six human pharmaceuticals: Are the current environmental risk assessment procedures sufficient for the protection of the aquatic environment? Environ. Toxicol. Chem. Int. J. 2004, 23, 1344–1354. [Google Scholar] [CrossRef]
- Grabarczyk, Ł.; Mulkiewicz, E.; Stolte, S.; Puckowski, A.; Pazda, M.; Stepnowski, P.; Białk-Bielińska, A. Ecotoxicity screening evaluation of selected pharmaceuticals and their transformation products towards various organisms. Environ. Sci. Pollut. Res. 2020, 27, 26103–26114. [Google Scholar] [CrossRef]
- Isidori, M.; Lavorgna, M.; Nardelli, A.; Pascarella, L.; Parrella, A. Toxic and genotoxic evaluation of six antibiotics on non-target organisms. Sci. Total Environ. 2005, 346, 87–98. [Google Scholar] [CrossRef]
- Zhang, Y.; He, D.; Chang, F.; Dang, C.; Fu, J. Combined Effects of Sulfamethoxazole and Erythromycin on a Freshwater Microalga, Raphidocelis subcapitata: Toxicity and Oxidative Stress. Antibiotics 2021, 10, 576. [Google Scholar] [CrossRef] [PubMed]
- Marciocha, D.; Kalka, J.; Turek-Szytow, J.; Wiszniowski, J.; Surmacz-Górska, J. Oxidation of sulfamethoxazole by UVA radiation and modified Fenton reagent: Toxicity and biodegradability of by-products. Water Sci. Technol. 2009, 60, 2555–2562. [Google Scholar] [CrossRef]
- Xiong, Q.; Liu, Y.-S.; Hu, L.-X.; Shi, Z.-Q.; Ying, G.-G. Levofloxacin and sulfamethoxazole induced alterations of biomolecules in Pseudokirchneriella subcapitata. Chemosphere 2020, 253, 126722. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.-P.; Liu, B.-Y.; Yu, H.-J.; Liu, W.-Q.; Yang, Y.-F. Toxic effects of erythromycin, ciprofloxacin and sulfamethoxazole exposure to the antioxidant system in Pseudokirchneriella subcapitata. Environ. Pollut. 2013, 172, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Kim, Y.; Kim, J.; Jeong, D.-H.; Choi, K. Environmental levels of ultraviolet light potentiate the toxicity of sulfonamide antibiotics in Daphnia magna. Ecotoxicology 2008, 17, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Choi, K. Hazard assessment of commonly used agricultural antibiotics on aquatic ecosystems. Ecotoxicology 2008, 17, 526–538. [Google Scholar] [CrossRef] [PubMed]
- Drzymała, J.; Kalka, J. Ecotoxic interactions between pharmaceuticals in mixtures: Diclofenac and sulfamethoxazole. Chemosphere 2020, 259, 127407. [Google Scholar] [CrossRef]
- Białk-Bielińska, A.; Stolte, S.; Arning, J.; Uebers, U.; Böschen, A.; Stepnowski, P.; Matzke, M. Ecotoxicity evaluation of selected sulfonamides. Chemosphere 2011, 85, 928–933. [Google Scholar] [CrossRef]
- Jeong, H.-S.; Ahn, C.-H.; Lee, G.-M.; Kim, S.-J.; Lee, Y.-W. Environmental Risk Assessment of Carbamazepine to Aquatic Organisms. J. Korean Soc. Urban Environ. 2019, 19, 103–109. [Google Scholar] [CrossRef]
- Jos, A.; Repetto, G.; Rios, J.C.; Hazen, M.J.; Molero, M.L.; del Peso, A.; Salguero, M.; Fernández-Freire, P.; Pérez-Martín, J.M.; Cameán, A. Ecotoxicological evaluation of carbamazepine using six different model systems with eighteen endpoints. Toxicol. Vitr. 2003, 17, 525–532. [Google Scholar] [CrossRef]
- Cleuvers, M. Aquatic ecotoxicity of pharmaceuticals including the assessment of combination effects. Toxicol. Lett. 2003, 142, 185–194. [Google Scholar] [CrossRef]
- Tay, K.S.; Rahman, N.A.; Abas, M.R.B. Ozonation of parabens in aqueous solution: Kinetics and mechanism of degradation. Chemosphere 2010, 81, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- El Najjar, N.H.; Touffet, A.; Deborde, M.; Journel, R.; Leitner, N.K.V. Kinetics of paracetamol oxidation by ozone and hydroxyl radicals, formation of transformation products and toxicity. Sep. Purif. Technol. 2014, 136, 137–143. [Google Scholar] [CrossRef]
- Öztürk, H.; Barışçı, S.; Turkay, O. Paracetamol degradation and kinetics by advanced oxidation processes (AOPs): Electro-peroxone, ozonation, goethite catalyzed electro-fenton and electro-oxidation. Environ. Eng. Res. 2021, 26, 180332. [Google Scholar] [CrossRef]
- Gonçalves, A.G.; Órfão, J.J.; Pereira, M.F.R. Catalytic ozonation of sulphamethoxazole in the presence of carbon materials: Catalytic performance and reaction pathways. J. Hazard. Mater. 2012, 239, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, J. Degradation of sulfamethoxazole by ozonation combined with ionizing radiation. J. Hazard. Mater. 2021, 407, 124377. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, Z.; Xu, Y.; Tian, J.; Qi, H.; Lin, W.; Cui, F. Oxidation of sulfamethoxazole (SMX) by chlorine, ozone and permanganate—A comparative study. J. Hazard. Mater. 2014, 274, 258–269. [Google Scholar] [CrossRef]
- Rodayan, A.; Roy, R.; Yargeau, V. Oxidation products of sulfamethoxazole in ozonated secondary effluent. J. Hazard. Mater. 2010, 177, 237–243. [Google Scholar] [CrossRef]
- Abellán, M.N.; Gebhardt, W.; Schröder, H.F. Detection and identification of degradation products of sulfamethoxazole by means of LC/MS and −MSn after ozone treatment. Water Sci. Technol. 2008, 58, 1803–1812. [Google Scholar] [CrossRef]
- McDowell, D.C.; Huber, M.M.; Wagner, M.; von Gunten, U.; Ternes, T.A. Ozonation of Carbamazepine in Drinking Water: Identification and Kinetic Study of Major Oxidation Products. Environ. Sci. Technol. 2005, 39, 8014–8022. [Google Scholar] [CrossRef]
- Alharbi, S.K.; Price, W.E.; Kang, J.; Fujioka, T.; Nghiem, L.D. Ozonation of carbamazepine, diclofenac, sulfamethoxazole and trimethoprim and formation of major oxidation products. Desalination Water Treat. 2016, 57, 29340–29351. [Google Scholar] [CrossRef]
- Kråkström, M.; Saeid, S.; Tolvanen, P.; Kumar, N.; Salmi, T.; Kronberg, L.; Eklund, P. Ozonation of carbamazepine and its main transformation products: Product determination and reaction mechanisms. Environ. Sci. Pollut. Res. 2020, 27, 23258–23269. [Google Scholar] [CrossRef]
- Andreozzi, R.; Marotta, R.; Pinto, G.; Pollio, A. Carbamazepine in water: Persistence in the environment, ozonation treatment and preliminary assessment on algal toxicity. Water Res. 2002, 36, 2869–2877. [Google Scholar] [CrossRef]
- Pandey, D.; Mishra, N.; Singh, P. Relative phytotoxicity of hydroquinone on rice (Oryza sativa L.) and associated aquatic weed green musk chara (Chara zeylanica Willd.). Pestic. Biochem. Phys. 2005, 83, 82–96. [Google Scholar]
- Tay, K.S.; Rahman, N.A.; Abas, M.R.B. Kinetic studies of the degradation of parabens in aqueous solution by ozone oxidation. Environ. Chem. Lett. 2010, 8, 331–337. [Google Scholar] [CrossRef]
- Guerra, R. Ecotoxicological and chemical evaluation of phenolic compounds in industrial effluents. Chemosphere 2001, 44, 1737–1747. [Google Scholar] [CrossRef]
- Bährs, H.; Putschew, A.; Steinberg, C.E. Toxicity of hydroquinone to different freshwater phototrophs is influenced by time of exposure and pH. Environ. Sci. Pollut. Res. 2013, 20, 146–154. [Google Scholar] [CrossRef]
- Lee, P.Y.; Chen, C.Y. Toxicity and quantitative structure–activity relationships of benzoic acids to Pseudokirchneriella subcapitata. J. Hazard. Mater. 2009, 165, 156–161. [Google Scholar] [CrossRef]
- Kamaya, Y.; Tsuboi, S.; Takada, T.; Suzuki, K. Growth stimulation and inhibition effects of 4-hydroxybenzoic acid and some related compounds on the freshwater green alga Pseudokirchneriella subcapitata. Arch. Environ. Con. Tox. 2006, 51, 537–541. [Google Scholar] [CrossRef]
- Devillers, J.; Steiman, R.; Seigle-Murandi, F.; Prévot, P.; André, C.; Benoit-Guyod, J.L. Combination of single-species laboratory tests for the assessment of the ecotoxicity of p-Benzoquinone. Toxic. Assess. 1990, 5, 405–416. [Google Scholar] [CrossRef]
- Bakire, S.; Yang, X.; Ma, G.; Wei, X.; Yu, H.; Chen, J.; Lin, H. Developing predictive models for toxicity of organic chemicals to green algae based on mode of action. Chemosphere 2018, 190, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Arambašić, M.B.; Bjelić, S.; Subakov, G. Acute toxicity of heavy metals (copper, lead, zinc), phenol and sodium on Allium cepa L., Lepidium sativum L. and Daphnia magna St.: Comparative investigations and the practical applications. Water Res. 1995, 29, 497–503. [Google Scholar] [CrossRef]
- Kim, K.T.; Lee, Y.G.; Kim, S.D. Combined toxicity of copper and phenol derivatives to Daphnia magna: Effect of complexation reaction. Environ. Int. 2006, 32, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Aruoja, V.; Sihtmäe, M.; Dubourguier, H.-C.; Kahru, A. Toxicity of 58 substituted anilines and phenols to algae Pseudokirchneriella subcapitata and bacteria Vibrio fischeri: Comparison with published data and QSARs. Chemosphere 2011, 84, 1310–1320. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.; Jesus, F.; Domingues, E.; Gonçalves, F.; Pereira, J.L.; Martins, R.C. Photocatalytic oxidation of pharmaceutical contaminants of emerging concern using sunlight and visible radiation: Mechanism and ecotoxicological evaluation. J. Water Process Eng. 2021, 43, 102204. [Google Scholar] [CrossRef]
- Miazek, K.; Brozek-Pluska, B. Effect of PHRs and PCPs on microalgal growth, metabolism and microalgae-based bioremediation processes: A review. Int. J. Mol. Sci. 2019, 20, 2492. [Google Scholar] [CrossRef]
- del Mar Gómez-Ramos, M.; Mezcua, M.; Agüera, A.; Fernández-Alba, A.R.; Gonzalo, S.; Rodríguez, A.; Rosal, R. Chemical and toxicological evolution of the antibiotic sulfamethoxazole under ozone treatment in water solution. J. Hazard. Mater. 2011, 192, 18–25. [Google Scholar] [CrossRef]
- Guateque-Londoño, J.F.; Serna-Galvis, E.A.; Ávila-Torres, Y.; Torres-Palma, R.A. Degradation of Losartan in Fresh Urine by Sonochemical and Photochemical Advanced Oxidation Processes. Water 2020, 12, 3398. [Google Scholar] [CrossRef]
- Fernandes, E.; Contreras, S.; Medina, F.; Martins, R.C.; Gomes, J. N-doped titanium dioxide for mixture of parabens degradation based on ozone action and toxicity evaluation: Precursor of nitrogen and titanium effect. Process Saf. Environ. 2020, 138, 80–89. [Google Scholar] [CrossRef]
- Punzi, M.; Nilsson, F.; Anbalagan, A.; Svensson, B.-M.; Jönsson, K.; Mattiasson, B.; Jonstrup, M. Combined anaerobic–ozonation process for treatment of textile wastewater: Removal of acute toxicity and mutagenicity. J. Hazard. Mater. 2015, 292, 52–60. [Google Scholar] [CrossRef]




| Treatments | Chemical Composition | Ozonation Conditions (Reaction Time; TOD) |
|---|---|---|
| MP | MP | 10 min; 7.56 mg O3 L−1 |
| PP | PP | 8 min; 10.85 mg O3 L−1 |
| PCT | PCT | 20 min; 16.70 mg O3 L−1 |
| SMX | SMX | 6 min; 6.53 mg O3 L−1 |
| CBZ | CBZ | 1.5 min; 2.10 mg O3 L−1 |
| Mix 2 | MP+PP | 12 min; 12.39 mg O3 L−1 |
| Mix 3 | MP+PP+PCT | 30 min; 14.17 mg O3 L−1 |
| Mix 4 | MP+PP+PCT+SMX | 40 min; 20.85 mg O3 L−1 |
| Mix 5 | MP+PP+PCT+SMX+CBZ | 60 min; 25.09 mg O3 L−1 |
| PPCP | Species | Endpoint | EC50 (mg L−1) | Reference |
|---|---|---|---|---|
| Methylparaben | R. subcapitata | Growth inhibition (72 h) | 92.8 | [21] |
| R. subcapitata | Growth inhibition (72 h) | 91 | [22] | |
| R. subcapitata | Growth inhibition (72 h) | 80 | [23] | |
| R. subcapitata | Growth inhibition (72 h) | 35.25 | [24] | |
| R. subcapitata | Growth inhibition (72 h) | 73.8 | [25] | |
| R. subcapitata | Yield inhibition (72 h) | 18.9 | [25] | |
| D. magna | Immobilization (48 h) | 11.2 | [22] | |
| D. magna | Immobilization (48 h) | 41.1 | [26] | |
| D. magna | Immobilization (48 h) | 62 | [27] | |
| D. magna | Immobilization (48 h) | 24.6 | [28] | |
| D. magna | Immobilization (48 h) | 34 | [23] | |
| D. magna | Immobilization (48 h) | 41.23 | [24] | |
| D. magna | Immobilization (48 h) | 36.73 | [29] | |
| L. minor | Yield inhibition, as frond number (7 d) | 22.0 | [25] | |
| L. minor | Growth inhibition, as frond number (7 d) | 27.2 | [25] | |
| Propylparaben | R. subcapitata | Growth inhibition (72 h) | 15 | [22] |
| R. subcapitata | Growth inhibition (72 h) | 36 | [23] | |
| D. magna | Immobilization (48 h) | 15.4 | [22] | |
| D. magna | Immobilization (48 h) | 23 | [27] | |
| D. magna | Immobilization (48 h) | 12.3 | [28] | |
| D. magna | Immobilization (48 h) | 2 | [23] | |
| Paracetamol | R. subcapitata | Growth inhibition (72 h) | 317.4 | [30] |
| R. subcapitata | Growth inhibition (72 h) | >100 | [31] | |
| D. magna | Immobilization (48 h) | 34.99 | [31] | |
| D. magna | Immobilization (48 h) | 4.7 | [30] | |
| D. magna | Immobilization (48 h) | 11.02 | [32] | |
| D. magna | Immobilization (48 h) | 30.1 | [33] | |
| D. magna | Immobilization (48 h) | 50 | [34] | |
| D. magna | Immobilization (48 h) | 2.99 | [35] | |
| L. minor | Yield, as frond number (7 d) | 429.9 | [30] | |
| L. minor | Yield, as frond number (7 d) | 446.6 | [36] | |
| Sulfamethoxazole | R. subcapitata | Growth inhibition (96 h) | 0.146 | [37] |
| R. subcapitata | Growth inhibition (72 h) | 4.36 | [38] | |
| R. subcapitata | Growth inhibition (72 h) | 0.52 | [39] | |
| R. subcapitata | Growth inhibition (96 h) | 0.49 | [40] | |
| R. subcapitata | Growth inhibition (72 h) | 5.4 | [41] | |
| R. subcapitata | Growth inhibition (96 h) | 4.74 | [42] | |
| R. subcapitata | Growth inhibition (96 h) | 4.4 | [43] | |
| R. subcapitata | Growth inhibition (72 h) | 1.12 | [31] | |
| D. magna | Immobilization (48 h) | 98.01 | [31] | |
| D. magna | Immobilization (48 h) | 189.2 | [33] | |
| D. magna | Immobilization (48 h) | 42.74 | [38] | |
| D. magna | Immobilization (48 h) | 43.97 | [38] | |
| D. magna | Immobilization (48 h) | 205.2 | [44] | |
| D. magna | Immobilization (48 h) | 123.1 | [45] | |
| L. minor | Growth inhibition, as frond area (7 d) | 3.07 | [38] | |
| L. minor | Yield inhibition, as frond number (7 d) | 1.48 | [25] | |
| L. minor | Growth inhibition, as frond number (7 d) | 5.02 | [25] | |
| L. minor | Growth inhibition, as frond number (7 d) | 12.56 | [46] | |
| L. minor | Growth inhibition, as frond area (7 d) | 0.21 | [47] | |
| Carbamazepine | R. subcapitata | Growth rate inhibition (72 h) | >100 | [24] |
| R. subcapitata | Growth rate inhibition (72 h) | >100 | [38] | |
| R. subcapitata | Growth rate inhibition (72 h) | >100 | [31] | |
| R. subcapitata | Growth rate inhibition (72 h) | 46.63 | [48] | |
| D. magna | Immobilization (48 h) | 57.56 | [48] | |
| D. magna | Immobilization (48 h) | >100 | [31] | |
| D. magna | Immobilization (48 h) | >100 | [24] | |
| D. magna | Immobilization (48 h) | >100 | [38] | |
| D. magna | Immobilization (48 h) | 97.8 | [49] | |
| D. magna | Immobilization (48 h) | 21.87 | [32] | |
| L. minor | Growth inhibition, as frond area (7 d) | 25.5 | [50] | |
| L. minor | Growth rate, as frond area (7d) | 50.17 | [38] |
| PPCP | Ozonation By-Product | Reference |
|---|---|---|
| Methylparaben (MP) | 1-Hydroxy-methyl paraben; Monohydroxy-methyl paraben; Dihydroxy-methyl paraben; Trihydroxy-methyl paraben | [51] |
| Hydroquinone (HQ) | [51]; present study | |
| Propylparaben (PP) | Monohydroxy-propyl paraben; 1-Hydroxy-propylparaben; Dihydroxy-propyl paraben; Trihydroxy-propyl paraben | [51] |
| 4-Hydroxybenzoic acid (4-HBA) | [51]; present study | |
| Mixture of parabens, including MP and PP | 4-Hydroxybenzoic acid (4-HBA) | [9]; present study |
| 2,4-Dihydroxybenzoic acid (2,4-diHBA) | [9]; present study | |
| 3,4-Dihydroxybenzoic acid (3,4-diHBA) | [9]; present study | |
| 3,4-dimethoxybenzoic acid (3,4-diMeBA) | [9]; present study | |
| p-Benzoquinone (BQ) | [9]; present study | |
| Paracetamol | Hydroquinone (HQ) | [52,53]; present study |
| Maleic acid (MA) | [53] | |
| Oxalic acid (OA) | [53]; present study | |
| p-Benzoquinone (BQ) | [53] | |
| Sulfamethoxazole | p-Benzoquinone (BQ) | [54,55] |
| Oxamic acid; pyruvic acid | [54,55] | |
| 3-amino-5-methylisoxazole (AMI) | [54,56]; present study | |
| Maleic acid | [54,55] | |
| Oxalic acid (OA) | [54,55] | |
| 4-aminobenzene sulfonamide; N-(3-phenylpropyl)-acetamide, 2-methyl-benzoxazole | [57] | |
| p-nitrophenol | [58] | |
| Phenol | [57] | |
| Dehydroxylated-sulfamethoxazole | [56] | |
| Nitro-sulfamethoxazole | [56,58] | |
| Carbamazepine | 1-(2-benzaldehyde)-4-hydro-(1H,3H)-quinazoline-2-one (BQM) | [59,60,61] |
| Anthranilic acid; glyoxal acid; glyoxylic acid | [62] | |
| Oxalic acid (OA) | [62] | |
| 1-(2-benzoic acid)-(1H,3H)-quinazoline-2,4-dione (BaQD) | [59,60] | |
| 1-(2-benzaldehyde)-(1H,3H)-quinazoline-2,4-dione (BQD) | [59,60,61] | |
| BaQM | [60] |
| Ozonation By-Product | Species | Endpoint | EC50 (mg L−1) | Reference |
|---|---|---|---|---|
| Hydroquinone (HQ) (by-product of MP, PCT) | D. magna | Immobilization, 48 h | 0.150 | [65] |
| R. subcapitata | Growth rate, 48 h | 8.92 | [66] | |
| R. subcapitata | Growth rate, 96–120 h | 10.8 | [66] | |
| 4-Hydroxybenzoic acid (4-HBA) (by-product of PP) | D. magna | Immobilization, 48 h | 1690 | [26] |
| R. subcapitata | Yield, 48 h | 270.7 | [67] | |
| R. subcapitata | Growth rate, 48 h | 355.0 | [67] | |
| R. subcapitata | Growth rate, 72 h | 1367 | [68] | |
| R. subcapitata | Growth rate, 96 h | 1602 | [68] | |
| 2,4-Dihydroxybenzoic acid (2,4-diHBA) (by-product of parabens mixture) | D. magna | Immobilization, 48 h | 120 | [26] |
| R. subcapitata | Yield, 48 h | 36.21 | [67] | |
| R. subcapitata | Growth rate, 48 h | 80.14 | [67] | |
| 3,4-Dihydroxybenzoic acid (3,4-diHBA) (by-product of parabens mixture) | D. magna | Immobilization, 48 h | 370 | [26] |
| R. subcapitata | Yield, 48h | 267.1 | [67] | |
| R. subcapitata | Growth rate, 48 h | 726.3 | [67] | |
| p-Benzoquinone (BQ) (by-product of parabens mixture, PCT, SMX) | D. magna | Immobilization, 24 h | 0.124 | [69] |
| Oxalic acid (OA) (by-product of SMX, CBZ) | R. subcapitata | Growth inhibition, 72 h | 4073 | [70] |
| Phenol (by-product of SMX) | L. sativum | Root length, 48 h | 81.2 | [71] |
| D. magna | Mortality, 24 h | 9.6 | [71] | |
| D. magna | Mortality, 48 h | 11.64 | [72] | |
| R. subcapitata | Yield, 72 h | 197 | [73] |
| R. subcapitata | D. magna | |
|---|---|---|
| MP | 0.013 | 0.028 |
| PP | 0.039 | 0.076 |
| PCT | 0.003 | 0.045 |
| SMX | 0.351 | 0.008 |
| CBZ | 0.021 | 0.017 |
| Mix 2 | 0.053 | 0.104 |
| Mix 3 | 0.056 | 0.149 |
| Mix 4 | 0.407 | 0.157 |
| Mix 5 | 0.428 | 0.174 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jesus, F.; Bernardo, C.; Martins, R.C.; Gomes, J.; Pereira, J.L. Ecotoxicological Consequences of the Abatement of Contaminants of Emerging Concern by Ozonation—Does Mixture Complexity Matter? Water 2022, 14, 1801. https://doi.org/10.3390/w14111801
Jesus F, Bernardo C, Martins RC, Gomes J, Pereira JL. Ecotoxicological Consequences of the Abatement of Contaminants of Emerging Concern by Ozonation—Does Mixture Complexity Matter? Water. 2022; 14(11):1801. https://doi.org/10.3390/w14111801
Chicago/Turabian StyleJesus, Fátima, Carla Bernardo, Rui C. Martins, João Gomes, and Joana Luísa Pereira. 2022. "Ecotoxicological Consequences of the Abatement of Contaminants of Emerging Concern by Ozonation—Does Mixture Complexity Matter?" Water 14, no. 11: 1801. https://doi.org/10.3390/w14111801
APA StyleJesus, F., Bernardo, C., Martins, R. C., Gomes, J., & Pereira, J. L. (2022). Ecotoxicological Consequences of the Abatement of Contaminants of Emerging Concern by Ozonation—Does Mixture Complexity Matter? Water, 14(11), 1801. https://doi.org/10.3390/w14111801