1. Introduction
Downstream migration (DSM) of juvenile fish, an important phenomenon during their life stages, is commonly associated with riverine continuums [
1,
2,
3]. DSM is an efficient way of dispersal of invertebrates and fish in lotic habitats [
2,
4,
5,
6]. However, most of the large and middle-size rivers have been transformed into sequences of lotic and limnetic habitats that are fragmented by dams [
7]. Such transformation created a variety of water reservoirs with novel conditions for DSM, where the emerged ecological barriers and filters modify habitats and influence fish populations and DSM [
8,
9,
10,
11]. Flow regulation appeared to reduce the abundance of larval fish in nursery habitats, altering taxonomic and genetic composition, and disrupting microhabitat relations [
12,
13]. The role of DSM as an efficient way of dispersal of the juvenile fish within the free-flowing rivers substantially changes after river regulation [
14,
15]. This is related not only to the transformation of the flow regime and ecological conditions within newly created water reservoirs, but also to the necessity of overcoming the limnetic–lotic interface, which appears to be caused by damming the river flow [
16,
17,
18,
19].
Although most of the studies regarding the DSM in river systems focus on larval fish downstream drift in the main water flow [
1,
20,
21,
22], growing attention is paid to such aspects of fish behavior as lateral and vertical movements, which control the diel periodicity of DSM and enhance the probability to move into suitable habitats [
2,
3,
23,
24]. In free-flowing (nonregulated) rivers, lateral (cross-flow) movements allow fish to shuttle between the habitat of residence (retention zone) and the migratory habitat (dispersal zone) [
3,
25]. To understand the relative influence of biotic and abiotic factors on the mechanisms controlling DSM, we have to take into account not only the characteristics of migratory habitats, but also residency habitats and the transition zone (interface) between them, where the structure of this zone, topography and flow parameters, are especially important [
25,
26,
27]. The role of the behavior of migrants is most pronounced at the interfaces (bottom, shallow areas near river banks) where the small-scale heterogeneity of the topography and the presence of hydraulic structures provide a template for the coordinated activity of fish [
15,
28,
29]. The whole process of DSM is comprised of a sequence of active and passive components. Short periods of decision making, when migrants enter the drift at dusk and return to the inshore retention zone at dawn, are of particular importance in DSM control [
1,
3,
22,
30].
The modified eco-hydraulic structure of the river at the outlet (water intake) arising from the river regulation radically transforms the interfaces between the residence and migratory habitats. Such a transformation is most pronounced in the lower part of water reservoirs, where the sharp transition from limnetic to lotic habitats occurs. A novel eco-hydraulic pattern may result in juvenile fish that are not adapted to changed conditions being flushed out of retention habitats and entrained in the flow. In turn, this may trigger high mortality rates, poor recruitment, and, over time, significant decline in the population [
5]. Estimates of the mortality rates induced by river damming vary over a wide range (from 0 to more than 90%), with the most frequently observed values at about 10–35% [
31,
32,
33].
Within reservoirs created by damming a river, the following hydrological barriers may be distinguished: (a) a transition zone between lotic and limnetic habitats in the upstream part of the reservoir; (b) an extended gradient zone between the inshore (habitat of residence) and open water areas (migration habitat), which influences the lateral displacement of migrants; (c) a large water body, which slows down and hampers the downstream drift of juvenile fish; (d) a 3-D hydrological structure with a downstream flow through the passage in the dam [
10,
11]. All of these structures may influence the characteristics of DSM. The area associated with the water intake constitutes an eco-hydraulic template where the DSM of fish is under control of the two interacting structures: (1) transformed water flow, which is like a hydraulic 3-D funnel, and (2) a set of adjacent ecological zones (habitats) [
34]. We suppose that different ecological groups of fish respond to these interacting structures in different ways, which results in selective removal/emigration from reservoirs and lakes.
What structures and processes underline selective removal? We hypothesize that this removal results from the interactions between (1) different ecological zones (habitats), which are characterized by specific abiotic and biotic conditions and fish assemblages; (2) a certain flow structure which takes different amounts of water from adjacent ecological zones; (3) behavior and spatial distribution of fish. To test our hypotheses concerning the mechanisms of selective removal of migrating fish, we analyzed data sets on DSM (seasonal and diel patterns) and the spatial distribution of juvenile fish from 13 water reservoirs and lakes in Europe and Asia that were obtained between 1979 and 2004. Depending on the morphology of the water body and the location of the water intake, the composition of the assemblage of migrating fish can be associated with a set of habitats from shallow water littoral and sublittoral zones, and pelagic zones of different depths. Ranging indices of selective removal along the littoral–pelagic gradient, we analyzed the influence of the interaction between hydraulic structures and ecological zones on the removal of migrating fish from limnetic to lotic habitats.
Our study aimed to reveal the patterns and mechanisms underlying the variability and selectivity of the DSM (emigration) of fish from reservoirs and lakes. We suggest that physical and ecological processes of different scales, among and within habitats, influence the characteristics of fish removal from limnetic to lotic zones. Our focus is given on the eco-hydraulic “mosaic of habitats” typical of the water intake area, which divides limnetic and lotic parts of the lake–riverine systems. Different locations of the water intake sites, both in vertical and horizontal dimensions, along with the varying morphology of the river bed and shorelines, shape a set of eco-hydraulic patterns. The interactions between hydraulic and ecological structures influence from one to several ecological zones (littoral, sublittoral, epipelagic, bathypelagic and bathyal), which comprise different fish assemblages. We analyze the selective (biased) nature of the emigration of fish from limnetic to lotic habitats triggered by the behavioral, ecological and physical processes at the eco-hydraulic interface at the water intake.
2. Eco-Hydraulic Structure of the Area at the Water Intake
The eco-hydraulic structure of the area close to the water intake at the downstream part of water reservoirs (water intake zone) is heterogeneous in terms of both topographical and hydraulic features. Vertical and horizontal gradients of abiotic and biotic factors influence the spatial distribution and DSM of fish, especially the juveniles. Composition of fish assemblages in this area is diverse, and the total concentration of fish is often higher than that at the upstream area of the reservoir, largely due to the drift and the accumulation of migrants [
10]. We suppose that characteristics of fish emigration (removal) from the limnetic habitats of water reservoirs to the downstream lotic part are sensitive to the location of the water intake site and the hydraulic structure of the adjacent water flow. It is difficult to take into account the impact of all potentially important factors influencing the patterns of DSM under the heterogeneous and altering environment of this transition area. We use a robust approach which takes into account an integrated impact of the downstream flow on the 3-D mosaic of ecological zones inhabited by diverse fish populations. This is the first step to analyze the patterns and mechanisms of the selective removal of fish from the limnetic to lotic parts of regulated rivers. This analysis will focus on the effects caused by the differences between the habitats. At the second step, we will aim at revealing the effects caused by the structures and processes within the habitats.
To explore the patterns and mechanisms of the selective removal of fish, we analyzed data sets on the DSM (seasonal and diel patterns) and relative abundance of fish in 13 water reservoirs and lakes in Europe and Asia obtained between 1979 and 2004 (
Table S1). We also studied the vertical distribution of juvenile fish in the head race area of three water reservoirs to assess the relationship between the spatial distribution and DSM patterns (
Table S7). Most of the field studies were carried out during the period between May and November when the intensity of the DSM was the highest for the most abundant fish species. A 3-D hydraulic funnel, which emerges in the area of water intake, entrains water from one to several ecological zones located along horizontal and vertical physical/ecological gradients in the lower part of water reservoirs (
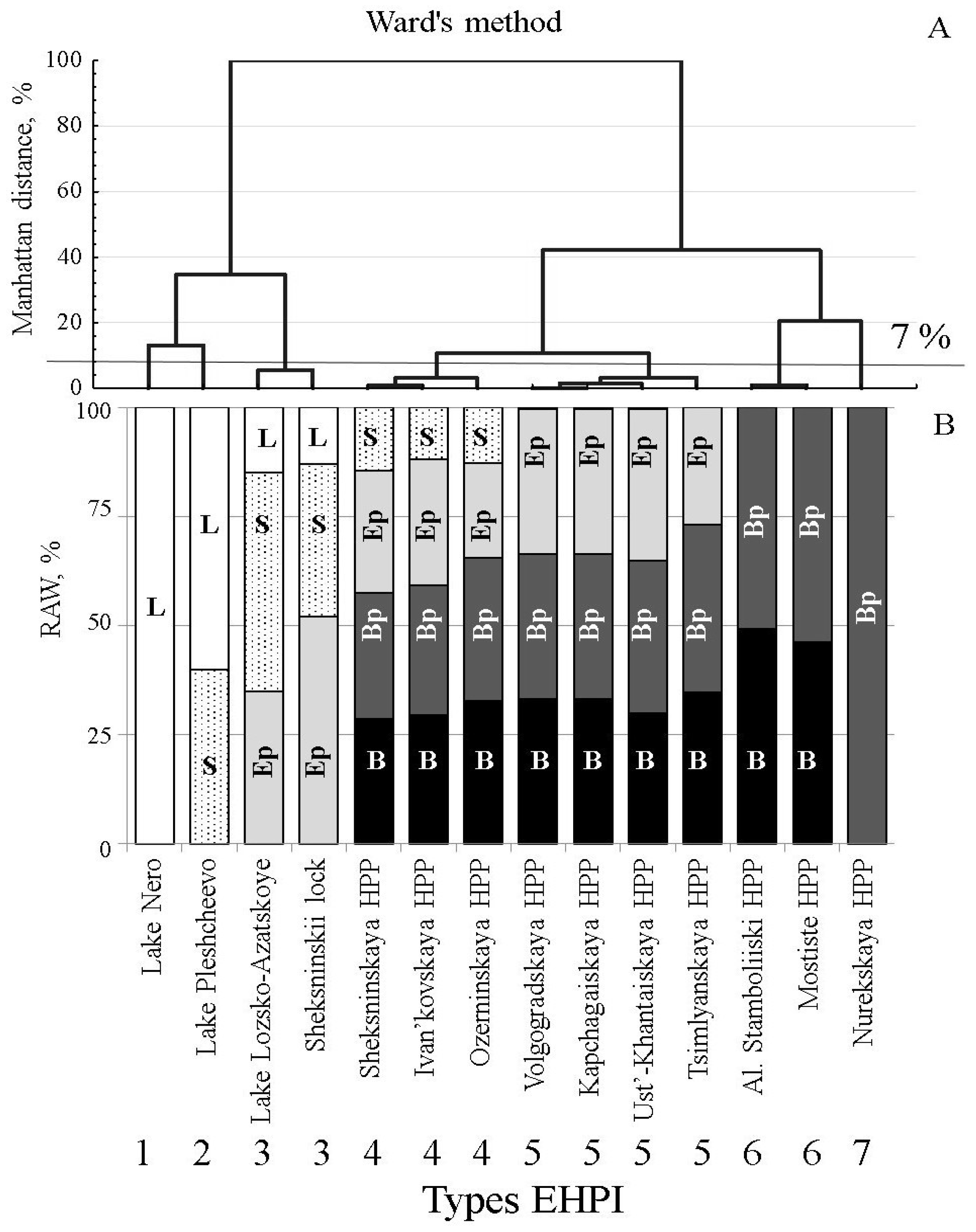
Figures S1 and S2). These ecological zones were roughly grouped into two categories: shallow-water (littoral) and deep-water (pelagic) types of habitats. Cluster analysis (Ward’s-method, Manhattan distance; [
35]) of the set of studied water reservoirs and lakes revealed seven groups of Eco-Hydraulic Patterns of Interaction (EHPI) between water outflow and ecological zones (habitats), which we ranked along the littoral–pelagic gradient (
Figure 1 and
Figure S2; Table S2). To assess the relative amount of water, entrained by downstream flow from adjacent ecological zones, we calculated the parameters of the overlapping between hydraulic and topographical structures at the lower parts of reservoirs where water abstraction takes place (
Tables S2 and S4; Figure S2).
Two water intakes associated with lakes influenced only shallow water ecological zones: Lake Nero—littoral zone (EHPI 1), and Lake Pleshcheevo—littoral and sublittoral zones (EHPI 2). Littoral, sublittoral and epi-pelagic zones were influenced by the water intakes from Lake Lozsko-Azatskoe and the Shipping-lock of the Sheksninskoe Reservoir. Most diverse sets of ecological zones (sublittoral, epi-pelagic, bathi-pelagic and bathyal) were associated with the water intakes of the Sheksninskoe, Ivan’kovskoe and Ozerninskoe water reservoirs. In the reservoirs, where pelagic conditions prevailed in the vicinity of water intakes, the number of ecological zones influenced by the outflow current decreased from three (Volzhskoe, Kapchagaiskoe, Ust’-Khantaiskoe, Tsimlyanskoe reservoirs) to two (Al. Stamboliiski, Mostishte) and to one (Nurekskoe reservoir) (
Figure 1).
Since the composition of fish species within the community considerably differed between the studied reservoirs and lakes, fish species were classified into several ecological groups [
34]. Three groups of the most abundant fish species were typical in all studied water bodies: 1—littoral (shallow water) inhabitants (rudd,
Scardinius erythrophthalmus; crucian carp,
Carassius carassius; goldfish
Carassius auratus; tench,
Tinca tinca; gudgeon,
Gobio gobio; ide,
Leuciscus idus; spiny loach,
Cobitis taenia; pike,
Esox lucius; pumpkinseed,
Lepomis gibbosus; roach,
Rutilus rutilus; bream,
Abramis brama; white bream,
Blicca bjoerkna); 2—pelagic and bathyal inhabitants (common kilka,
Clupeonella cultriventris; black-baked shad,
Alosa kessleri; zander,
Sander lucioperca; Siberian cisco,
Coregonus sardinella; peled,
Coregonus peled; smelt,
Osmerus eperlanus; razorfish,
Pelecus cultratus; ruff,
Gymnocephalus cernuus; burbot,
Lota lota; European catfish,
Silurus glanis); 3—ubiquitous inhabitants (perch,
Perca fluviatilis; bleak,
Alburnus alburnus).
3. Patterns of Eco-Hydraulic Interactions Shape Selective Removal of Fish from Limnetic to Lotic Habitats
Previously, it was found that the characteristics of the downstream migration of cyprinid and percid fishes are strongly influenced by novel ecological structures associated with different parts of water reservoirs [
15]. In comparison with free-flowing rivers, where the intensity and patterns of the DSM of cyprinids and percids are rather similar, a much more intensive emigration of juvenile percids rather than cyprinids was observed from reservoirs having deep-water intakes. We suggested that modified conditions of regulated rivers, especially those at the lower part of water reservoirs (water intake zone), induce a new type of impact of the downstream water flow on fish inhabiting adjacent ecological zones.
The emigration of fish from the limnetic to lotic zone is an important factor affecting the fish communities of water reservoirs. The emigration is characterized by the number of fish (concentration) entrained by the water flow, and the taxonomic/ecological composition of the pool of migrants. The most common technique for the investigation of the drift of the young stages of fish is the deployment of stationary drift nets [
2,
20]. Comparing catches of the drift nets, which gives us information on abundance and composition of migrants, with catches obtained by trawling in the habitats of residence (retention zone—littoral, sublittoral), the selective removal of fish from the habitat of residence into migratory habitat (transit flow) can be assessed in the free-flowing river. This selectivity is mainly related to the motivation for drifting and other aspects of migratory behavior [
38] which are species-specific [
3,
21,
39]. In the free-flowing river, fish, which are ready to migrate, may leave the habitat of residence and enter the flow at almost any location along the river. In the regulated river, the most intensive transition of fish from the retention zone to the downstream flow is located at the lower part of the reservoirs, where water intakes (outlets) entrain fish from different ecological zones associated not only with littoral habitats, but also with pelagic ones, including the epi- and bathy-pelagic.
Using data on the intensity of drift from the studied reservoirs (lakes) and the relative abundance of fish in these water bodies, we assessed the tendency to leave these habitats by means of an index of selective removal (SR-index) for the littoral, pelagic and ubiquitous groups of fish. By analogy with the index of migration intensity [
15,
34], we defined the SR-index as the difference between the relative abundance (rank) of a specific fish species (or group of species) in the pool of migrants and the rank of this species in the pool of residents. On the resulting scale, the SR-index varied between 0 and 1, showing the relative investment of a specific species (group) into the pool of migrants relative to other species (groups) in the same reservoir.
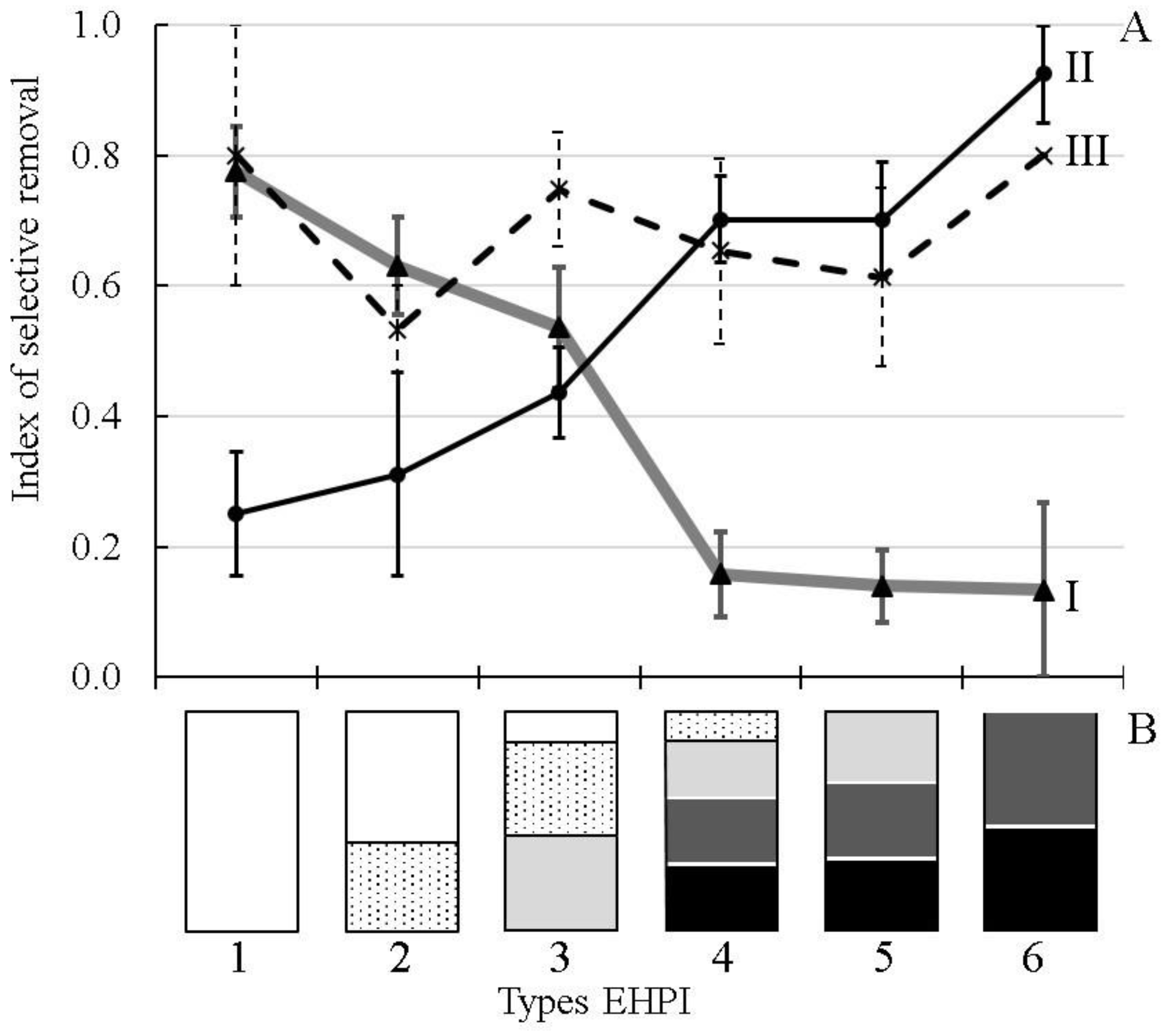
Selective removal of different ecological groups of fishes primarily depended on the interaction between hydraulic and ecological structures (
Figure 2). The highest values of the SR-index for the littoral inhabitants were observed in the water bodies, where the outflow entrained water mainly from the littoral, sublittoral and epi-pelagic zones; the SR-index dropped sharply in the reservoirs, where the outflow influenced largely the pelagic and bathyal zones. A reversed pattern was obtained for the pelagic inhabitants. The third group, ubiquitous fish, displayed a rather high tendency to migrate from the water bodies with all types of Eco-Hydraulic Patterns of Interaction (EHPI) between water flow and ecological zones (
Figure 2). The obtained results demonstrate the trends (probabilities) for fishes from different ecological groups to be removed from the water bodies having different EHPI. To test this interaction more rigorously and to assess not only the qualitative trends, but also the number (concentration) of fish removed from different types of water intakes, we studied the DSM parameters and the SR-index in the same reservoir with two types of water intakes.
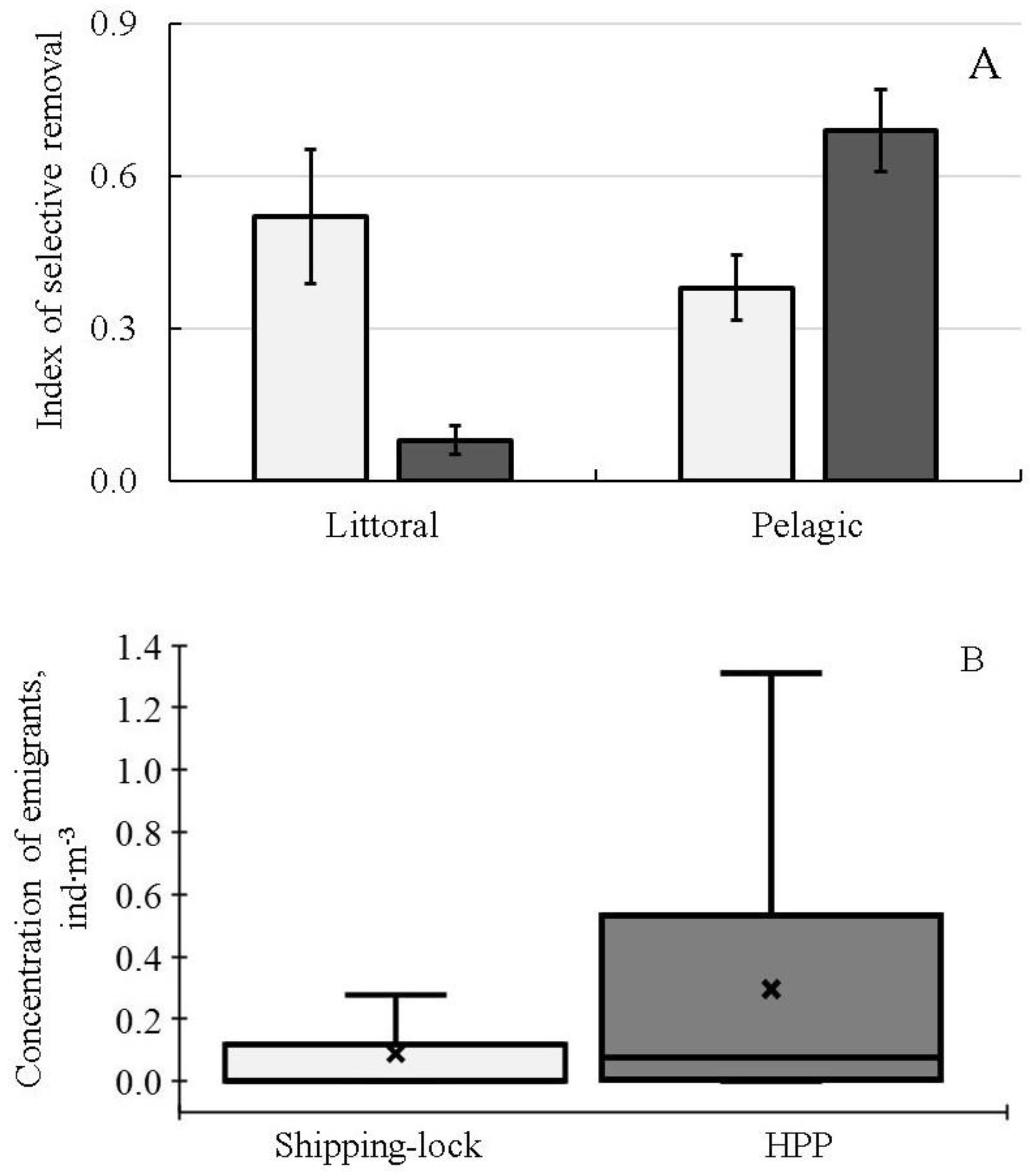
The most detailed studies of seasonal and diel dynamics of DSM and spatial (both vertical and horizontal) distribution of fish abundance were carried out at the lower part (head race) of the Sheksninskoe Reservoir. This area was influenced by the two types of water intakes: (1) The Shipping-lock, located close to the shore where the water is predominantly removed from the shallow water habitats. (2) The deep water intake of the Sheksninskaya hydro-power plant (HPP), which takes water predominantly from the pelagic habitats. Using integrated (i.e., averaged) data on the intensity of fish drift through the two types of water intakes for the period from May to November, we calculated the SR-indices for the littoral and pelagic groups of fish (
Figure 3A). Littoral inhabitants were rudd, tench, gudgeon, ide, spiny loach, pike, roach, bream and white bream, whereas the pelagic ones were zander, Siberian cisco, smelt, razorfish, ruff and burbot. The aim of this analysis was the qualitative assessment of the impact of two types of EHPI on the selective removal of fish inhabiting different ecological zones of the Sheksninskoe Reservoir. We also calculated the average concentration of fish, which were removed from the reservoir through the Shipping-lock and deep-water intake during the same period (
Figure 3B).
The obtained results showed that the two water intakes with different types of EHPI located in the same reservoir that are not far from each other had different impacts on the selective removal of fish from two main ecological groups, namely the littoral and pelagic (
Figure 3A). However, in interpreting these results, we cannot assess the total amount of fish from the different ecological groups that are removed from the ecological zones influenced by outflows through the deep-water and Shipping-lock intakes. Comparison of the data on the tendency to emigrate (
Figure 3A) and the concentrations of fish removed through the two water intakes (
Figure 3B) suggests that the probability to be entrained by the downstream flow is the highest for the pelagic inhabitants influenced by the water intake of the HPP.
Comparisons of the indices of selective removal of fish belonging to the different ecological groups showed that pelagic fishes were significantly more vulnerable to be entrained by the outflow, especially through the HPP water intake, than littoral fishes. Concentration of emigrants through the HPP was three times higher than that of emigrants through the Shipping-lock. These results support our suggestion concerning the higher tendency of pelagic inhabitants to emigrate from the reservoir.
Within the pelagic ecological group, zander is one of the most vulnerable fish for the water flow induced by a deep-water intake of HPPs [
28,
40,
41]. There were several case studies which showed intensive emigration of the juvenile zander from the reservoirs having deep-water intakes. Selective removal from pelagic habitats along with low abundance of available prey (crustaceans such as amphipods and mysids, and shoaling fish juveniles) might be the main reason that induced the enhanced emigration of juvenile zander from water reservoirs [
41,
42]. Eventually, it led to a reduction in the zander populations in water reservoirs [
37,
42]. We suggest that the unsuccessful introduction of zander into the reservoirs with relatively small littoral habitats and a low abundance of pelagic prey fish were mainly related to the intensive selective removal of this species from pelagic habitats [
28,
34,
42]. Intensive emigration of juvenile zander (0+, 1+) during the mid-summer–early autumn period resulted in complete elimination in 3 years of the population introduced into the Al. Stamboliiski reservoir [
42].
4. Ecological and Behavioral Mechanisms of Selective Removal of Fish
More intensive removal of fish from the pelagic habitats than from shallow water (littoral) was related not only to different volumes of water entrained by the outflow from these zones, but also to the structure of the habitats and the behavior of fish. We suggested that a within-habitat physical heterogeneity, which substantially differed between the littoral and pelagic zones, modified not only the characteristics of the transient flow, but also the behavior of fish [
28,
29,
43,
44]. It is expected that these differences might produce both habitat-specific and species-specific variations in the intensity of fish removal by the outflow. To test this hypothesis, two field experiments were carried out at the water intake zones of the Sheksninskoe Reservoir and Lake Pleshcheevo. For both cases, we compared the values of the mean fish concentration obtained near the water intake limnetic zone (C
inhab) with the concentration of migrants through the water intake (C
migr). The Emigration Index, EI = C
migr/C
inhab, was determined for the larvae of the four most abundant species living in the two water bodies. Two species, roach and bream, represented the littoral ecological group, whereas the other two, perch and bleak, represented the ubiquitous (
Table S6).
The EI was much higher for all inhabitants of the Sheksninskoe Reservoir than Lake Pleshcheevo (
Figure 4). The obtained results suggest that the highly heterogeneous sublittoral and littoral zones of the lake, through which fish migrated before reaching the outflow, provide a more efficient retention structure for juvenile fish compared to the more homogeneous pelagic area of the Sheksninskoe Reservoir. The higher retention ability of the cyprinids (roach, bleak, bream) compared to percids (perch) is especially pronounced when juvenile fish emigrate through the heterogeneous shallow water habitats. This is probably related to the morphological and behavioral adaptations of larval cyprinids, which allow them to reside within structured habitats more efficiently than percids do [
29,
41].
Eco-behavioral mechanisms triggering either migration or retention of fish in a certain habitat are based not only on the predisposal of fish for dispersal [
20,
45], but also are substantially influenced by the structure of the residence habitat [
28,
29]. Comparing littoral and pelagic habitats, we suggest that the latter retains fish less efficiently due to the lack of stationary landmarks and shelters, which hamper flushing from the habitat by a transient flow. At the water intake zone of an HPP with the deep-water abstraction, pelagic inhabitants may avoid or minimize emigration provided that they move to the layer with the lowest flow velocity. To test this suggestion, we studied diel changes in the vertical distribution of fish in the limnetic part of the reservoir that is not far from the dam and DSM through the Sheksninskaya HPP and Shipping-lock (
Table S7).
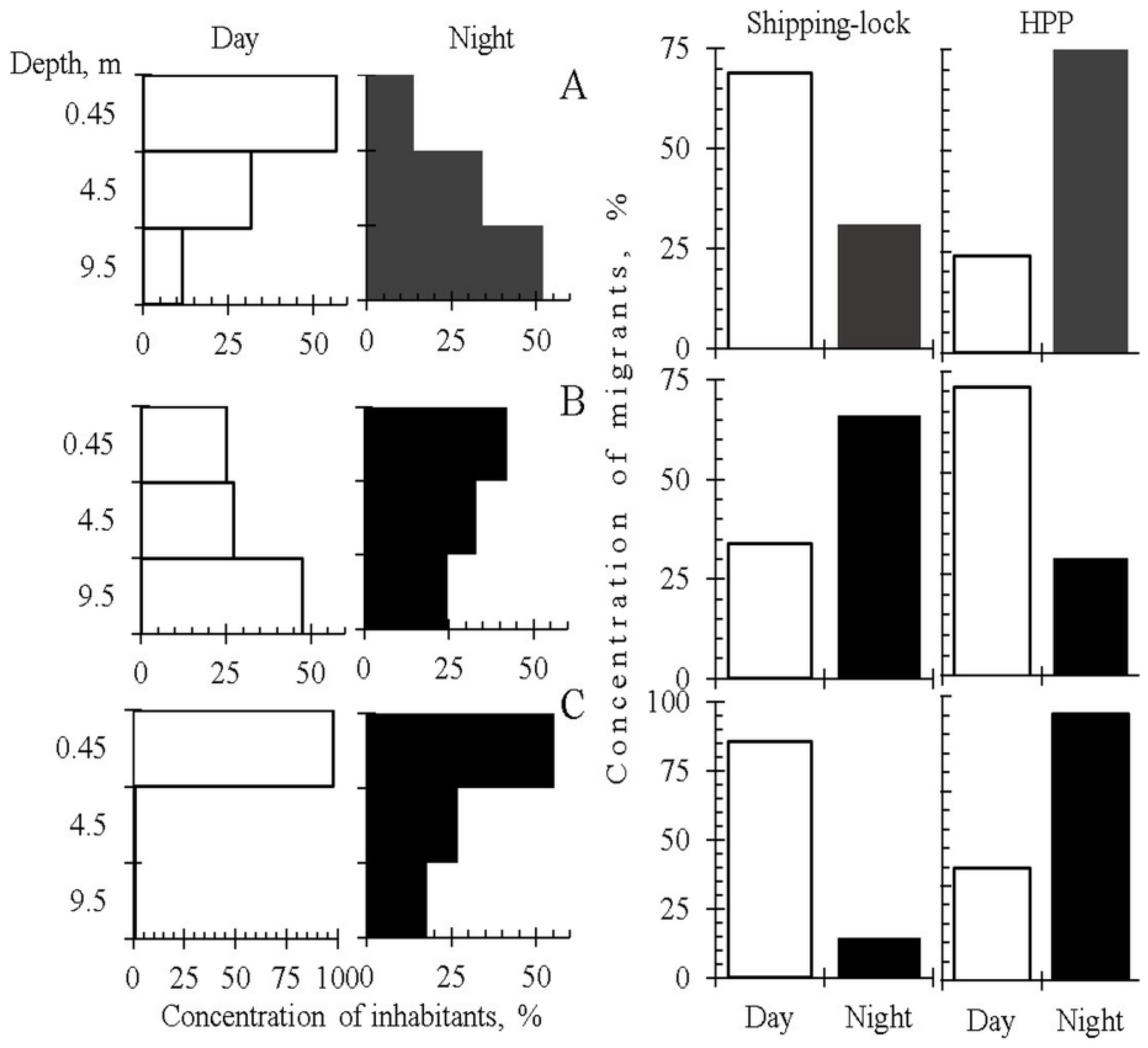
The deep-water intake of the Sheksninskaya HPP abstracts water from the whole water column (bathy-pelagic, bathyal and epi-pelagic zones) and less from the sublittoral zone (
Figure 1). The outflow through the HPP intake impacts mostly on the deepest, bathyal and bathy-pelagic, zones. The water intake of the Shipping-lock, located at the same dam closer to the shore, influences the shallow-water zones (epi-pelagic, sublittoral and littoral) (
Figure 1). Both water intakes supply water from the area located 500–1500 m upstream of the dam. To assess the diel changes in the vertical distribution of juvenile fish, we sampled this area using horizontal trawling at three different depths: 0.45, 4.5 and 9.5 m. To assess the intensity of DSM for three fish species of the pelagic (zander, smelt) and ubiquitous (bleak) ecological groups, we simultaneously sampled outflows through the HPP and Shipping-lock (
Table S7). We found a clear-cut relation between the patterns of the vertical distribution of fish concentration and the intensity of the DSM (
Figure 5). Deepening of the zander and bleak in the night, despite the difference in the patterns of vertical distribution between the two species, caused a concomitant increase in the DSM through the deep-water intake of the HPP. However, smelt displayed an opposite pattern—deepening and the highest intensity of the DSM through the HPP were observed during the daytime (
Figure 5). DSM through the Shipping-lock was the highest during the day for zander and bleak, and at night for smelt. We do not have detailed suggestions concerning the interaction between the vertical distribution and DSM in these species. The importance of the tendency to descend into the deeper layers as a factor enhancing the DSM through the HPP should, however, be emphasized.
The results show that the selective removal of fish through the water intakes at the dam (HPP and Shipping-lock) is a result of the interaction between the EHPI type and species-specific behavior (primarily diel changes of vertical distribution). These results (
Figure 5) support the hypothesis that pelagic inhabitants, especially juvenile zander (
Figure 3), suffer the highest removal rate through the HPP.
5. Conclusions
Anthropogenic regulation deeply influences the hydraulic, morphological and ecological characteristics of the rivers. All these characteristics are important, as they modify the spatial distribution and migrations of fish [
15,
18,
19,
25]. Damming of rivers causes the most serious ecological barriers and filters, which strongly influence both upstream and downstream fish migration [
9,
15]. We found that contrary to free-flowing rivers, where downstream migrants “shuttle” between the shallow-water (littoral) zone and the main flow, at the water intake zone close to the dam, downstream flows of different types entrain fish from different habitats, such as pelagic and littoral. Depending on the pattern of interactions between the water flow and ecological zones, which in turn depends on the location of the water intake and topography of the adjacent area, the outflow through the dam selectively removes fish from the shallow-water and pelagic habitats. We found that the deep-water HPP flow removes much more fish from the pelagic habitats than the littoral ones, while the Shipping-lock intake removes both littoral and pelagic inhabitants. However, the concentration of all migrants through the water intake of the HPP is much higher than that through the Shipping-lock. These effects resulted not only from the higher amount of water going through the HPP intake, but also from the physical structure of the habitats. Different habitats provide different retaining capacities for fish from flashing out. Inhabitants of the deep pelagic layers have the lowest chances to stay within the resident habitat.
The DSM of juvenile fish through the water intakes of different types is a potentially powerful factor, which influences the abundance and composition of fish populations inhabiting man-made reservoirs and natural lakes. Our results and published papers [
1,
3,
15,
21,
22] on the variability of DSM suggest that the management of populations of commercially valuable and/or endangered fish species should take into account traditionally important aspects related to foraging, reproduction and fisheries. Selective removal of fish from the lacustrine populations, especially during the periods of their dispersal/migrations within water bodies, might be also important. Removal of migrants through the water intakes is under control of hydraulic and ecological impacts at different spatio-temporal scales: the whole-lake [
10], between-habitats and within-habitat scales. The pelagic zone of many reservoirs has impoverished fish populations with low abundance. Intensive (selective) removal of fish from this area through the water intake of the limnetic–lotic interface could be one of the factors triggering this effect. For example, in the reservoirs of the Volga and Kama rivers, a strong negative relationship between the fish biomass in the pelagic zone and the intensity of water exchange was observed [
46].
Depending on the qualitative and quantitative impacts of the selective removal of fish on the whole communities or populations of certain species, the main ecological and hydraulic factors should be taken into account to mitigate the negative effects of DSM from water reservoirs. To develop concrete measures to minimize threats, we have to (1) determine a target species or group of fishes; (2) assess the major periods of migratory activity of fishes on both diel and seasonal scales; (3) estimate the rate of transportation of migrants to the lower part of a reservoir—this process is strongly influenced by the topographical complexity of the reservoir and the rate of water exchange [
10,
15]; (4) estimate the eco-hydraulic pattern of interaction (EHPI) between the water flow and ecological zones within the water intake area; (5) assess within-habitat complexity (density of landmarks and shelters), which may hamper the removal of fish from habitats of residence by the outflow. The results of our study reveal the importance of these factors in terms of controlling the DSM in regulated rivers and natural lake–river systems.