Onsite Wastewater Treatment Upgrade for Water Reuse in Cooling Towers and Toilets
Abstract
1. Introduction
2. Materials and Methods
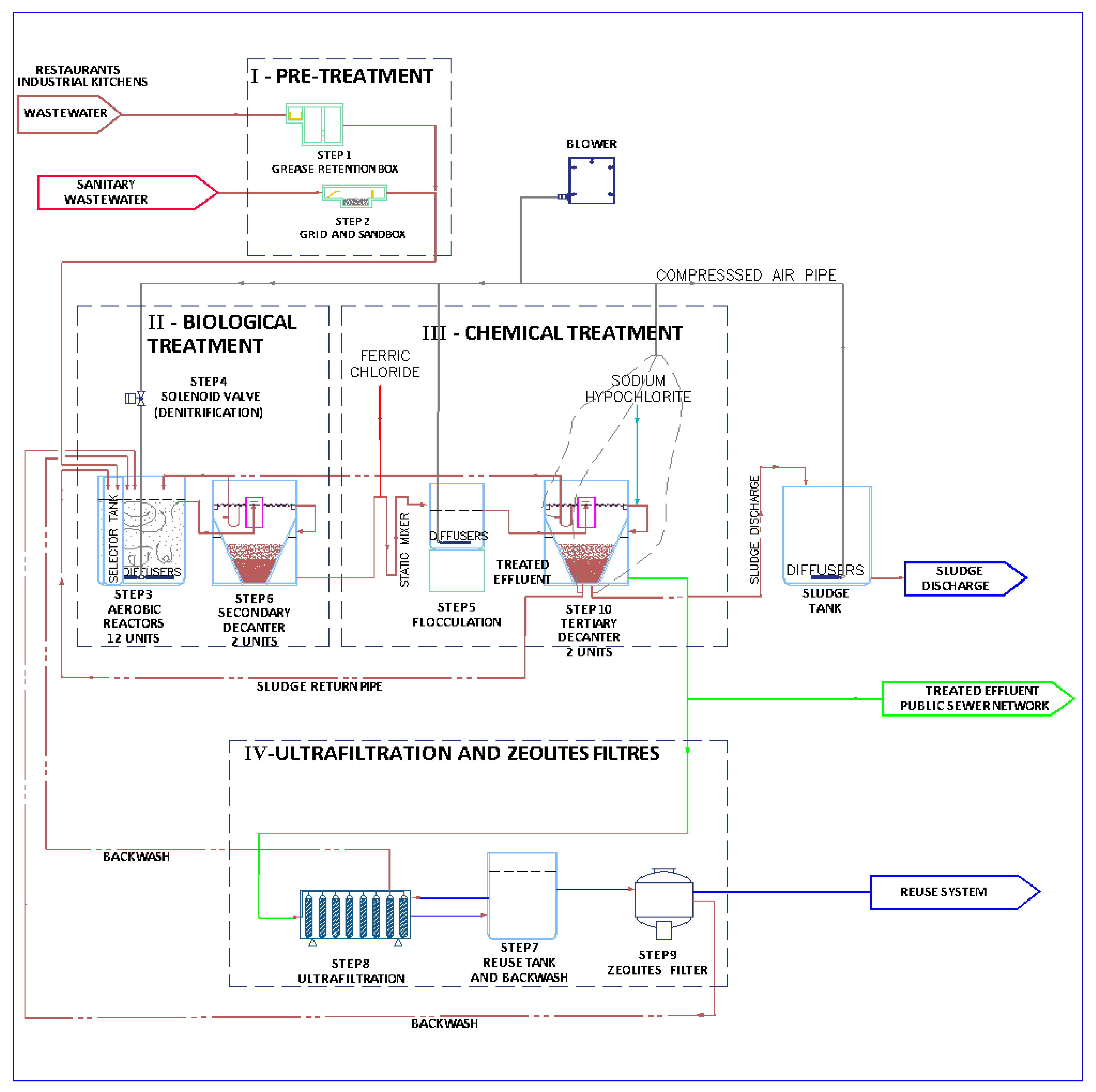
2.1. Implementation of a Wastewater Treatment Plant on a Commercial Scale
2.2. Experimental Procedures–JAR Tests
2.3. Set-Up of the WWTP Operation
2.4. Monitoring of the WWTP Operation
2.5. Analytical Methods
3. Results
3.1. Start of WWTP
3.2. Jar Test Experiments Results
3.3. Set-Up of the WWTP Operation
Adjustments in the WWTP and Implementation of the 10th Stage
3.4. Monitoring of WWTP Operation
4. Discussion
5. Conclusions
- (1)
- The inclusion of a tertiary decanter improved the quality of the water for reuse.
- (2)
- From the laboratory scale experiments (jar tests), the zeolite (SFM) and FeCl3 (41.4 mg/L) were chosen for the set-up of the WWTP operation, reducing the time for adjustments in this large full-scale production of quality water for cooling towers.
- (3)
- FeCl3 as a flocculant followed by filtration by zeolites (SFM) resulted in maximum remotion (about 99%) of the biological oxygen demand (BOD5).
- (4)
- The changes in the WWTP operation reduced consistently the TDS including chlorides and ammonia, which are described as corrosion factors in cooling systems.
- (5)
- The concentration of residual organic substrate, N, and P, which are associated with biofouling of cooling systems, was reduced after using the FeCl3 as a flocculant.
- (6)
- The reused water produced by the WWTP presented the reduced capability to form calcium scale (calcium carbonate and calcium phosphate).
- (7)
- The developed treatment demonstrated the feasibility of water reused in air conditioning cooling towers and toilets, saving up to 797 m³/month (27% of consumed water in the mall).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Drinking—Water; World Health Organizations: Geneva, Switzerland, 2019; Available online: https://www.who.int/news-room/fact-sheets/detail/drinking-water (accessed on 18 March 2022).
- UNICEF. Global Framework for Urban Water, Sanitation and Hygiene. Urban Wash. 2019. Available online: https://www.unicef.org/documents/global-framework-urban-water-sanitation-and-hygiene#:~:text=The%20Framework%20is%20based%20on,for%20engagement%20in%20urban%20WASH (accessed on 26 August 2021).
- Capodaglio, A.G. Fit-for-purpose urban wastewater reuse: Analysis of issues and available technologies for sustainable multiple barrier approaches. Crit. Rev. Environ. Sci. Technol. 2021, 51, 1619–1666. [Google Scholar] [CrossRef]
- El-Monaem, E.M.A.; El-Latif, M.M.A.; Eltaweil, A.S.; El-Subruiti, G.M. Cobalt nanoparticles supported on reduced amine-functionalized graphene oxide for catalytic reduction of nitroanilines and organic dyes. Nano 2021, 16, 2150039. [Google Scholar] [CrossRef]
- Hosny, M.; Fawzy, M.; Abdelfatah, A.M.; Fawzy, E.E.; Eltaweil, A.S. Comparative study on the potentialities of two halophytic species in the green synthesis of gold nanoparticles and their anticancer, antioxidant and catalytic efficiencies. Adv. Powder Technol. 2021, 32, 3220–3233. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Hlavínek, P.; Raboni, M. Advances in wastewater nitrogen removal by biological processes: State of the art review. Rev. Ambiente Água 2016, 11, 250–267. [Google Scholar] [CrossRef]
- WHO. The International Statistical Classification of Diseases and Health Related Problems ICD-10, 10th Revision ed; Tabular List; World Health Organizations: Geneva, Switzerland, 2004; Volume 1. [Google Scholar]
- Glibert, P.M. Harmful algae at the complex nexus of eutrophication and climate change. Harmful Algae 2020, 91, 101583. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Omer, A.M.; El-Aqapa, H.G.; Gaber, N.M.; Attia, N.F.; El-Subruiti, G.M.; Mohy-Eldin, M.S.; Abd El-Monaem, E.M. Chitosan based adsorbents for the removal of phosphate and nitrate: A critical review. Carbohydr. Polym. 2021, 274, 118671. [Google Scholar] [CrossRef]
- Liu, T.; Jia, G.; Quan, X. Accelerated start-up and microbial community structures of simultaneous nitrification and denitrification using novel suspended carriers. J. Chem. Technol. Biotechnol. 2018, 93, 577–584. [Google Scholar] [CrossRef]
- Montalvo, S.; Huiliñir, C.; Borja, R.; Sánchez, E.; Herrmann, C. Application of zeolites for biological treatment processes of solid wastes and wastewaters—A review. Bioresour. Technol. 2020, 301, 122808. [Google Scholar] [CrossRef]
- Guida, S.; Potter, C.; Jefferson, B.; Soares, A. Preparation and evaluation of zeolites for ammonium removal from municipal wastewater through ion exchange process. Sci. Rep. 2020, 10, 12426. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yang, Z.; Dai, H.; Lu, X.; Peng, L.; Tan, X.; Shi, L.; Fahim, R. Preparation and application of modified zeolites as adsorbents in wastewater treatment. Water Sci. Technol. 2018, 2017, 621–635. [Google Scholar] [CrossRef]
- Huang, L.; Yan, T.; Mahmoud, A.D.; Li, S.; Zhang, J.; Shia, L.; Zhang, D. Enhanced water purification via redox interfaces created by an atomic layer deposition strategy. Environ. Sci. Nano 2021, 8, 950–959. [Google Scholar] [CrossRef]
- Mahmoud, A.D.; Franke, M.; Stelter, M.; Braeutigam, P. Mechanochemical versus chemical routes for graphitic precursors and their performance in micropollutants removal in water. Powder Technol. 2020, 366, 629–640. [Google Scholar] [CrossRef]
- Hespanhol, I. Um novo paradigma para a gestão de recursos hídricos. Estud. Avançados 2008, 22, 131–158. [Google Scholar] [CrossRef]
- Rupiper, A.M.; Loge, F.J. Identifying and overcoming barriers to onsite non-potable water reuse in California from local stakeholder perspectives. Resour. Conserv. Recycl. X 2019, 4, 100018. [Google Scholar] [CrossRef]
- Metcalf and Eddy Inc.; Tchobanoglous, G.; Stensel, H.D.; Tsuchihashi, R.; Burton, F. Wastewater Engineering: Treatment and Resource Recovery; Mc Graw-Hill Education: New York, NY, USA, 2014. [Google Scholar]
- Asano, T.; Burton, F.L.; Leverenz, H.L.; Tsuchihashi, R.; Tchobanoglous, G. Water Reuse: Issues, Technologies, and Applications; Mc Graw-Hill: New York, NY, USA, 2007. [Google Scholar]
- Trovat, J. Tratamento de Água Para Sistema de Resfriamento, Cursos Online. 2004. Available online: https://www.snatural.com.br/PDF_arquivos/Torre-Caldeira-Tratamento-Agua.pdf (accessed on 19 May 2016).
- Wallin, J.; Knutsson, J.; Karpouzoglou, T. A multi-criteria analysis of Building level graywater reuse for personal hygiene. Resour. Conserv. Recycl. 2021, 12, 200054. [Google Scholar] [CrossRef]
- GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. Available online: https://www.thelancet.com/article/S0140-6736(19)30041-8/fulltext (accessed on 20 February 2020). [CrossRef]
- ANA—Agência Nacional de Águas. Conservação e Reuso da Água em Edificações; FIESP—Federação das Indústrias de São Paulo in association with Ministério do Meio Ambiente: São Paulo, Brazil, 2015. [Google Scholar]
- SES/SIMA–Resolução Conjunta n° 01 SES/SIMA DOE de 14-02-2020, Seção I, 47–48; da Secretaria Estadual da Saúde e Secretaria de Infraestrutura e Meio Ambiente—Disciplina o Reuso Direto de Água Não Potável. Governo do Estado de São Paulo: São Paulo, Brazil, 2020. Available online: http://www.mpsp.mp.br/portal/page/portal/cao_urbanismo_e_meio_ambiente/legislacao/leg_estadual/leg_est_resolucoes/Resol-cjta-SES-SIMA-01-2020_Processo-ssrh-90-2016_reuso-de-agua-nao-potavel_fins_urbano_ETE.pdf (accessed on 26 August 2021).
- ABNT NBR 16783; Uso de Fontes Alternativas de Água não Potável em Edificações. Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 2019.
- Arévolo, J.; Ruiz, L.M.; Parada-Albarracín, J.A.; Gonzáles-Pérez, D.M.; Pérez, J.; Moreno, B.; Gómez, M.A. Wastewater reuse after treatment by MBR. Microfiltration or ultrafiltration? Desalination 2012, 299, 22–27. [Google Scholar] [CrossRef]
- Subtil, E.L.; Hespanhol, I.; Mierzwa, J.C. Biorreatores com Membranas Submersas (BRMs): Alternativa promissora para o tratamento de esgotos sanitários para reuso. Ambiente Água 2013, 8, 129–142. [Google Scholar] [CrossRef][Green Version]
- Von Sperling, M. Princípios do Tratamento Biológico de Águas Residuárias. Lodos Ativados; UFMG: Belo Horizonte, Brazil, 2002. [Google Scholar]
- ABNT NBR 12209; Elaboração de Projetos Hidráulicos-Sanit´Rios de Estações de Tratamento de Esgotos Sanitários. Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 2011.
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017; 1504p. [Google Scholar]
- Madigan, M.T.; Martinko, J.M.; Bender, K.S.; Buckley, D.H.; Stahl, D.A. Microbiologia de Brock; Artimed: Porto Alegre, Brazil, 2016. [Google Scholar]
- EPA. Guidelines for Water Reuse; Environmental Protection Agency: Washington, DC, USA, 2012. Available online: https://www.epa.gov/sites/production/files/2019-08/documents/2012-guidelines-water-reuse.pdf (accessed on 2 August 2016).
- CONAMA-Resolução 430; Dispõe Sobre Condições e Padrões de Lançamento de Efluentes. Conselho Nacional do Meio Ambiente: Brasília, 2011.
- CONAMA-Resolução 357; Dispõe Sobre a Classificação dos Corpos de Água e Diretrizes Ambientais Para o Seu Enquadramento, Bem Como Estabelece as Condições e Padrões de Lançamento de Efluentes, e dá Outras Providências. Conselho Nacional do Meio Ambiente: Brasília, 2005.
- Abdessemed, D.; Nezzal, G.; Ben Aim, R. Coagulation-adsorption-ultrafiltration for wastewater treatment and Reuse. Desalination 2000, 131, 307–314. [Google Scholar] [CrossRef]
- Abdessemed, D.; Nezzal, G. Tertiary treatment of a secondary effluent by the coupling of coagulation-adsorption-ultrafiltration for reuse. Desalination 2005, 175, 135–141. [Google Scholar] [CrossRef]
- Dymaczewski, Z. (Ed.) A Sewage Treatment Plant Operator’s Guide; PZITS Poznań: Poznań, Poland, 2011; ISBN 978-83-89696-38-X. [Google Scholar]
- Karczmarczyk, A.; Kowalik, W. Combination of Microscopic Tests of the Activated Sludge and Effluent Quality for More Efficient On-Site Treatment. Water 2022, 14, 489. [Google Scholar] [CrossRef]
- Vesilind, P.A.; Morgan, S.M.; Heine, L.G. Introdução à Engenharia Ambiental; Cengage: São Paulo, Brazil, 2018. [Google Scholar]
- SABESP-NTS 181; Dimensionamento do Ramal Predial de Água, Cavalete e Hidrômetro—Primeira Ligação. Companhia de Saneamento Básico do Estado de São Paulo: São Paulo, Brazil, 2017. Available online: http://www2.sabesp.com.br/normas/nts/NTS181.pdf (accessed on 14 April 2018).
- Ghasemi, Z.; Sourinejad, I.; Kazemian, H.; Rohani, S. Application of zeolites in aquaculture industry: A review. Rev. Aquacult. 2018, 10, 75–95. [Google Scholar] [CrossRef]

| Flocculant/Coagulant | Dosage (mg/L) | Before Treatment (µS/cm) | After Jar-Test Treatment (µS/cm) | ZN 0410 (µS/cm) | Coal (µS/cm) | SFM (µS/cm) |
|---|---|---|---|---|---|---|
| Aluminum Sulfate Al2(SO4)3 | 12.5 | 615 | 617 | −10% | 8% | −10% |
| 5.0 | 615 | 618 | −10% | 8% | −12% | |
| 2.5 | 615 | 617 | −8% | 10% | −13% | |
| 1.25 | 615 | 618 | −10% | 8% | −14% | |
| PAC–Aluminum Polychloride | 10.0 | 582 | 601 | −4% | 3% | −3% |
| 2.5 | 582 | 599 | −1% | 15% | −2% | |
| 1.25 | 582 | 600 | 0% | 15% | −2% | |
| Tannin (Tanfloc) | 82.5 | 582 | 601 | −3% | 65% | 45% |
| 68.75 | 582 | 599 | −2% | 65% | 45% | |
| 55.0 | 582 | 602 | −4% | 19% | −6% | |
| 41.25 | 582 | 595 | −3% | 56% | 4% | |
| 27.5 | 582 | 591 | −4% | 36% | −3% | |
| Ferric Chloride (FeCl3) | 276.0 | 615 | 616 | −7% | 10% | −3% |
| 207.0 | 616 | 568 | −8% | 12% | −6% | |
| 138.0 | 615 | 559 | −9% | 20% | −5% | |
| 69.0 | 615 | 549 | −11% | 13% | −8% | |
| 41.4 | 615 | 539 | −12% | 12% | −14% | |
| 13.8 | 615 | 537 | −13% | 10% | −13% |
| Limits Determined by Agencies | Analysis Date | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters (Max) | Unit | ANA | EPA | Manu-Factor | 1 December 2015 | 25 July 2016 | 8 November 2016 | 7 February 2017 | 2 February 2018 |
| TDS | mg/L | 500 | 500 | 2800 | 594 | 472 | 500 | 567 | 130 |
| pH | 6.8–7.2 | 6.0–9.0 | 6.5–8.5 | 7.79 | 6.91 | 7.01 | 6.75 | 6.79 | |
| Chlorides | mg/L | 500 | 500 | 200 | 128 | 50.0 | 159 | 11.5 | 107.0 |
| Nitrites | mg/L | - | - | - | 14.6 | 0.040 | <0.002 | <0.002 | <0.002 |
| Nitrates | mg/L | - | - | - | 23.5 | 15.5 | <0.02 | <0.02 | 1.80 |
| Hardness | mg/L CaCO3 | 650 | 650 | 400 | 120.0 | <0.5 | 92 | 31.0 | 136.0 |
| Alkalinity | mg/L CaCO3 | 350 | 350 | 400 | 35.7 | 98.7 | 144.9 | 42.0 | 86.1 |
| BOD5 | mg/L | - | 25 | - | <2 | <2 | <2 | <2 | 5.0 |
| COD | mg/L | 75 | 75 | - | <50 | <50 | <50 | 84 | <50 |
| TSS | mg/L | 100 | 100 | 20 | 8 | 10 | 167 | 33 | 30 |
| Turbidity | UNT | - | 50 | 20 | 2.03 | 6.60 | 0.55 | 8.1 | 1.3 |
| Ammonia Nitrog. | mg/L | - | 1.0 | - | 2.25 | 2.87 | 13.7 | 1.24 | <0.1 |
| Total Phosphorus | mg/L | - | 4.0 | - | 19.65 | 5.86 | 6.92 | 5.4 | 1.57 |
| Silica | mg/L SiO2 | 50 | 50 | 150 | - | 31.0 | 17.5 | 34.5 | 11.3 |
| Aluminum | mg/L | 0.1 | 0.1 | 0.1 | <0.148 | <0.148 | <0.148 | <0.148 | 0.41 |
| Iron | mg/L | 0.5 | 0.5 | 5.0 | 0.021 | 0.58 | 0.116 | 1.06 | 0.288 |
| Calcium | mg/L | 50 | 50 | - | - | 0.01 | 19.5 | 1.10 | 35.3 |
| Magnesium | mg/L | 30 | 0.5 | - | - | <0.02 | 60.9 | 25.1 | 84.6 |
| Manganese | mg/L | 0.5 | 0.5 | - | 0.059 | 0.497 | 0.299 | 1.55 | 0.110 |
| Bicarbonates | mg/L | 24 | 24 | - | <0.5 | <0.5 | 2.6 | <0.5 | <0.5 |
| Sulfates | mg/L | 200 | 200 | 300 | 22 | 19 | 15 | 32 | 7 |
| Bacteria (Col/mL) | CFU/100mL | - | - | 1 × 10³ | 2.3 × 10⁵ | 1.3 × 10³ | <1 | <20 | <2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papp, L.A.; Rodrigues, F.A.; Júdice, W.A.d.S.; Araújo, W.L. Onsite Wastewater Treatment Upgrade for Water Reuse in Cooling Towers and Toilets. Water 2022, 14, 1612. https://doi.org/10.3390/w14101612
Papp LA, Rodrigues FA, Júdice WAdS, Araújo WL. Onsite Wastewater Treatment Upgrade for Water Reuse in Cooling Towers and Toilets. Water. 2022; 14(10):1612. https://doi.org/10.3390/w14101612
Chicago/Turabian StylePapp, Luiz Antonio, Flávio Aparecido Rodrigues, Wagner Alves de Souza Júdice, and Welington Luiz Araújo. 2022. "Onsite Wastewater Treatment Upgrade for Water Reuse in Cooling Towers and Toilets" Water 14, no. 10: 1612. https://doi.org/10.3390/w14101612
APA StylePapp, L. A., Rodrigues, F. A., Júdice, W. A. d. S., & Araújo, W. L. (2022). Onsite Wastewater Treatment Upgrade for Water Reuse in Cooling Towers and Toilets. Water, 14(10), 1612. https://doi.org/10.3390/w14101612







