Biomonitoring of Soil Contaminated with Herbicides
Abstract
:1. Introduction
2. Herbicide Classifications
3. Herbicide Fate in Soil and Biological Tools Used in Their Biomonitoring
3.1. Biotests and Bioindicators Used in Soil Monitoring after Herbicide Application
3.2. Bacteria as a Bioindicators of Soil Contaminated with Herbicides
3.3. Biomarkers Used in Soil Monitoring after Herbicide Application
4. Molecular Techniques as a Tool for Herbicide Toxicity Assessment
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, N.; Zhang, Z.; Xue, B.; Ma, J.; Chen, X.; Lu, C. Economic Growth and Pollution Emission in China: Structural Path Analysis. Sustainability 2018, 10, 2569. [Google Scholar] [CrossRef] [Green Version]
- WHO. Health 2020 A European Policy Framework and Strategy for the 21st Century. Available online: https://www.euro.who.int/__data/assets/pdf_file/0011/199532/Health2020-Long.pdf (accessed on 10 May 2021).
- Kaur, K.; Kaur, R. Occupational Pesticide Exposure, Impaired DNA Repair, and Diseases. Indian J. Occup. Environ. Med. 2018, 22, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Wołejko, E.; Jabłońska-Trypuć, A.; Wydro, U.; Butarewicz, A.; Łozowicka, B. Soil Biological Activity as an Indicator of Soil Pollution with Pesticides—A Review. Appl. Soil Ecol. 2020, 147, 103356. [Google Scholar] [CrossRef]
- Granados-Galván, I.A.; Rodríguez-Meza, D.G.; Luna-González, A.; González-Ocampo, H.A. Human Health Risk Assessment of Pesticide Residues in Snappers (Lutjanus) Fish from the Navachiste Lagoon Complex, Mexico. Mar. Pollut. Bull. 2015, 97, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Łozowicka, B.; Wołejko, E.; Kaczyński, P.; Konecki, R.; Iwaniuk, P.; Drągowski, W.; Łozowicki, J.; Tujtebajeva, G.; Wydro, U.; Jablońska-Trypuć, A. Effect of Microorganism on Behaviour of Two Commonly Used Herbicides in Wheat/Soil System. Appl. Soil Ecol. 2021, 162, 103879. [Google Scholar] [CrossRef]
- Popp, J.; Pető, K.; Nagy, J. Pesticide Productivity and Food Security. A Review. Agron. Sustain. Dev. 2013, 33, 243–255. [Google Scholar] [CrossRef]
- Li, Y.-Z.; Wang, C.-Y. 2-Aminobutyric Acid as a Chemical Marker for the Detection of Sulfonylurea Herbicides1. Weed Technol. 2005, 19, 176–182. [Google Scholar] [CrossRef]
- Mesnage, R.; Defarge, N.; Spiroux de Vendômois, J.; Séralini, G.E. Potential Toxic Effects of Glyphosate and Its Commercial Formulations below Regulatory Limits. Food Chem. Toxicol. 2015, 84, 133–153. [Google Scholar] [CrossRef] [Green Version]
- Calderon, M.J.; De Luna, E.; Gomez, J.A.; Hermosin, M.C. Herbicide Monitoring in Soil, Runoff Waters and Sediments in an Olive Orchard. Sci. Total Environ. 2016, 569–570, 416–422. [Google Scholar] [CrossRef]
- Liess, M.; Schäfer, R.B.; Schriever, C.A. The Footprint of Pesticide Stress in Communities--Species Traits Reveal Community Effects of Toxicants. Sci. Total Environ. 2008, 406, 484–490. [Google Scholar] [CrossRef]
- Mulgrew, A.; Williams, P. Biomonitoring of Air Quality Using Plants; Air Hygiene Report; WHO Collab. Centre for Air Quality Management and Air Pollution Control: Berlin, Germany, 2000. [Google Scholar]
- Vischetti, C.; Casucci, C.; De Bernardi, A.; Monaci, E.; Tiano, L.; Marcheggiani, F.; Ciani, M.; Comitini, F.; Marini, E.; Taskin, E.; et al. Sub-Lethal Effects of Pesticides on the DNA of Soil Organisms as Early Ecotoxicological Biomarkers. Front. Microbiol. 2020, 11, 1892. [Google Scholar] [CrossRef] [PubMed]
- Wydro, U.; Wołejko, E.; Łozowicka, B.; Jabłońska-Trypuć, A. Microbial Diversity and P Content Changes after the Application of Sewage Sludge and Glyphosate to Soil. Minerals 2021, 11, 1423. [Google Scholar] [CrossRef]
- Wei, Y.D.; Zheng, H.-G.; Hall, J.C. Role of Auxinic Herbicide-Induced Ethylene on Hypocotyl Elongation and Root/Hypocotyl Radial Expansion. Pest Manag. Sci. 2000, 56, 377–387. [Google Scholar] [CrossRef]
- Van den Brink, P.J.; Blake, N.; Brock, T.C.M.; Maltby, L. Predictive Value of Species Sensitivity Distributions for Effects of Herbicides in Freshwater Ecosystems. Hum. Ecol. Risk Assess. 2006, 12, 645–674. [Google Scholar] [CrossRef]
- Łozowicka, B.; Rutkowska, E.; Jankowska, M.; Hrynko, I.; Kaczynski, P. Toxicological Evaluation of Multi-Class Pesticide Residues in Vegetables and Associated Human Health Risk Study for Adults and Children. Hum. Ecol. Risk Assess. 2016, 22, 1480–1505. [Google Scholar] [CrossRef]
- Rodea-Palomares, I.; González-Pleiter, M.; Martín-Betancor, K.; Rosal, R.; Fernández-Piñas, F. Additivity and Interactions in Ecotoxicity of Pollutant Mixtures: Some Patterns, Conclusions, and Open Questions. Toxics 2015, 3, 342–369. [Google Scholar] [CrossRef] [Green Version]
- Campanale, C.; Massarelli, C.; Losacco, D.; Bisaccia, D.; Triozzi, M.; Uricchio, V.F. The monitoring of pesticides in water matrices and the analytical criticalities: A review. TrAC Trends Anal. Chem. 2021, 144, 116423. [Google Scholar] [CrossRef]
- Wieczerzak, M.; Namieśnik, J.; Kudłak, B. Bioassays as one of the Green Chemistry tools for assessing environmental quality: A review. Environ. Int. 2016, 94, 341–361. [Google Scholar] [CrossRef]
- OECD Environment, Health and Safety Publications. Series on Testing and Assessment. No. 43. Guidance Document on Mammalian Reproductive Toxicity Testing and Assessment. ENV/JM/MONO(2008)16. Available online: https://one.oecd.org/document/ENV/JM/MONO(2008)16/en/pdf (accessed on 24 March 2022).
- Crouau, Y.; Pinelli, E. Comparative Ecotoxicity of Three Polluted Industrial Soils for the Collembola Folsomia Candida. Ecotoxicol. Environ. Saf. 2008, 71, 643–649. [Google Scholar] [CrossRef] [Green Version]
- Ahanger, M.A.; Akram, N.A.; Ashraf, M.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Plant Responses to Environmental Stresses-from Gene to Biotechnology. AoB Plants 2017, 9, plx025. [Google Scholar] [CrossRef] [Green Version]
- HRAC (Herbicide Resistance Action Committee). Global Herbicide Classification Lookup. 2022. Available online: https://hracglobal.com/tools/classification-lookup (accessed on 24 March 2022).
- OECD Environmental Health and Safety Publications Series on Harmonization of Regulatory Oversight in Biotechnology No. 10. Consensus Document on General Information Concerning the Genes and Their Enzymes that Confer Tolerance to Glyphosate Herbicide. ENV/JM/MONO(99)9. Available online: https://www.oecd.org/env/ehs/biotrack/46815618.pdf (accessed on 24 March 2022).
- PPDB (Pesticide Properties DataBase). Available online: http://sitem.herts.ac.uk/aeru/ppdb/ (accessed on 24 March 2022).
- Martínez-Ruiz, E.B.; Martínez-Jerónimo, F. Exposure to the Herbicide 2,4-D Produces Different Toxic Effects in Two Different Phytoplankters: A Green Microalga (Ankistrodesmus falcatus) and a Toxigenic Cyanobacterium (Microcystis aeruginosa). Sci. Total Environ. 2018, 619–620, 1566–1578. [Google Scholar] [CrossRef] [PubMed]
- Bento, C.P.M.; Yang, X.; Gort, G.; Xue, S.; van Dam, R.; Zomer, P.; Mol, H.G.J.; Ritsema, C.J.; Geissen, V. Persistence of Glyphosate and Aminomethylphosphonic Acid in Loess Soil under Different Combinations of Temperature, Soil Moisture and Light/Darkness. Sci. Total Environ. 2016, 572, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Meena, R.; Kumar, S.; Datta, R.; Lal, R.; Vijayakumar, V.; Brtnicky, M.; Sharma, M.; Yadav, G.; Jhariya, M.; Jangir, C.; et al. Impact of Agrochemicals on Soil Microbiota and Management: A Review. Land 2020, 9, 34. [Google Scholar] [CrossRef] [Green Version]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil Quality—A Critical Review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Brussaard, L. Ecosystem services provided by the soil biota. In Soil Ecology and Ecosystem Services; Wall, D.H., Bardgett, R.D., Behan-Pelletier, V., Herrick, J.E., Jones, H., Ritz, K., Six, J., Strong, D.R., van der Putten, W.H., Eds.; Oxford University Press: Oxford, UK, 2012; pp. 45–58. [Google Scholar]
- Łebkowska, M.; Załęska-Radziwiłł, M.; Szyłak-Szydłowski, M.; Kalinowski, R. Toxicity studies on the biodegradation of endosulfan. Eng. Prot. Environ. 2004, 7, 353–365. [Google Scholar]
- Carles, L.; Joly, M.; Bonnemoy, F.; Leremboure, M.; Batisson, I.; Besse–Hoggana, P. Identification of sulfonylurea biodegradation pathways enabled by a novel nicosulfuron–transforming strain Pseudomonas fluorescens SG–1: Toxicity assessment and effect of formulation. J. Hazard. Mater. 2017, 324, 184–193. [Google Scholar] [CrossRef]
- Sarikaya, R.; Yilmaz, M. Investigation of Acute Toxicity and the Effect of 2,4-D (2,4-Dichlorophenoxyacetic Acid) Herbicide on the Behavior of the Common Carp (Cyprinus Carpio L., 1758; Pisces, Cyprinidae). Chemosphere 2003, 52, 195–201. [Google Scholar] [CrossRef]
- Gołombieski, J.I.; Jonas Sutili, F.; Salbego, J.; Seben, D.; Tourem Gressler, L.; Arrudada Cunha, J.; Gressler, L.T.; Zanella, R.; Vaucher, R.d.A.; Marchesan, E.; et al. Imazapyr + imazapic herbicide determines acute toxicity in silver catfish Rhamdia quelen. Ecotox. Environ. Safe 2016, 128, 91–99. [Google Scholar] [CrossRef]
- Geisel, B.G.L.; Schoenau, J.J.; Holm, F.A.; Johnson, E.N. Interactions of ALS inhibiting herbicide residues in three prairie soils. Weed Sci. 2008, 56, 624–627. [Google Scholar] [CrossRef]
- Ros, M. Soil Microbial Activity after Restoration of a Semiarid Soil by Organic Amendments. Soil Biol. Biochem. 2003, 35, 463–469. [Google Scholar] [CrossRef]
- Fernández-González, M.; Ubeda, J.F.; Cordero-Otero, R.R.; Thanvanthri Gururajan, V.; Briones, A.I. Engineering of an Oenological Saccharomyces Cerevisiae Strain with Pectinolytic Activity and Its Effect on Wine. Int. J. Food Microbiol. 2005, 102, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Siwik-Ziomek, A.; Brzezińska, M.; Lemanowicz, J.; Koper, J.; Szarlip, P. Biological Parameters in Technogenic Soils of a Former Sulphur Mine. Int. Agrophys. 2018, 32, 237–245. [Google Scholar] [CrossRef]
- Carpio, M.J.; García-Delgado, C.; Marín-Benito, J.M.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Soil Microbial Community Changes in a Field Treatment with Chlorotoluron, Flufenacet and Diflufenican and Two Organic Amendments. Agronomy 2020, 10, 1166. [Google Scholar] [CrossRef]
- Tatuśko-Krygier, N.; Jakubus, M. Application of biological methods to assess the toxicity of soils contaminated with heavy metals and the effectiveness of stabilisation processes. Environ. Prot. Eng. 2019, 45, 33–43. [Google Scholar] [CrossRef]
- Wolska, L.; Sagajdakow, A.; Kuczyńska, A.; Namieśnik, J. Application of ecotoxicological studies in integrated environ-mental monitoring: Possibilities and problems. Trends Anal. Chem. 2007, 26, 332–344. [Google Scholar] [CrossRef]
- Sikorski, Ł.; Adomas, B. Biotests in toxicological and ecotoxicological research. Postępy Nauk. Rol. 2010, 4, 121–131. [Google Scholar]
- Sadowski, J.; Kucharski, M.; Sekutowski, T. Ocena zagrożeń upraw następczych przez pozostałości wybranych herbicydów stosowanych w uprawach rzepaku. Prog. Plant Protect. 2007, 47, 246–253. [Google Scholar]
- Sekutowski, T.; Sadowski, J. PHYTOTOXKITTM microbiotest used in detecting herbicide residue in soil. Environ. Prot. Eng. 2009, 35, 105–110. [Google Scholar]
- Iwai, C.B.; Noller, B. Ecotoxicological assessment of diffuse pollution using biomonitoring tool for sustainable land use in Thailand. J. Environ. Sci. 2010, 22, 858–863. [Google Scholar] [CrossRef]
- Valášková, V.; Baldrian, P. Denaturing Gradient Gel Electrophoresis as a Fingerprinting Method for the Analysis of Soil Microbial Communities. Plant Soil Environ. 2009, 55, 413–423. [Google Scholar] [CrossRef] [Green Version]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Dong, D.; Xia, M.; Yang, Y.; Wang, J.; Su, J.; Sun, L.; Yu, H. Oxidative Stress and Antioxidant Capacity: Development and Prospects. New J. Chem. 2020, 44, 11405–11419. [Google Scholar] [CrossRef]
- De la Torre, F.; Salibian, A.; Ferrari, L. Assessment of the pollution impact on biomarkers of effect of a freshwater fish. Chemosphere 2007, 46, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Wu, Y.L.; Chen, Y.G.; Kong, Z.M. Genotoxicity evaluation and a primary risk assessment of organic pollutants in the drinking water sources of Nanjing, China. J. Environ. Sci. 2006, 18, 983–988. [Google Scholar] [CrossRef]
- Claxton, L.D.; Umbuzeiro, G.D.A.; DeMarini, D.M. The Salmonella Mutagenicity Assay: The Stethoscope of Genetic Toxicology for the 21st Century. Environ. Health Perspect. 2010, 118, 1515–1522. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Sharma, K.; Tomar, M.; Malik, V.; Kumar, K.S. Determination of mutagenic potential of imidacloprid in Salmonella Typhimurium–TA 98 and TA 100 following bacterial reverse mutation assay. Int. J. Biotechnol. Bioeng. 2013, 4, 703–710. [Google Scholar]
- Guan, Y.; Wang, X.; Wong, M.; Sun, G.; An, T.; Guo, J.; Zhang, G. Evaluation of Genotoxic and Mutagenic Activity of Organic Extracts from Drinking Water Sources. PLoS ONE 2017, 12, e0170454. [Google Scholar] [CrossRef] [Green Version]
- Gana, J.M.; Ordóñez, R.; Zampini, C.; Hidalgo, M.; Meoni, S.; Isla, M.I. Industrial Effluents and Surface Waters Genotoxicity and Mutagenicity Evaluation of a River of Tucuman, Argentina. J. Hazard. Mater. 2008, 155, 403–406. [Google Scholar] [CrossRef]
- Sforzini, S.; Governa, D.; Boeri, M.; Oliveri, L.; Oldani, A.; Vago, F.; Viarengo, A.; Borrelli, R. Relevance of the Bioavailable Fraction of DDT and Its Metabolites in Freshwater Sediment Toxicity: New Insight into the Mode of Action of These Chemicals on Dictyostelium discoideum. Ecotoxicol. Environ. Saf. 2016, 132, 240–249. [Google Scholar] [CrossRef]
- Abbas, M.; Adil, M.; Ehtisham-ul-Haque, S.; Munir, B.; Yameen, M.; Ghaffar, A.; Shar, G.A.; Asif Tahir, M.; Iqbal, M. Vibrio Fischeri Bioluminescence Inhibition Assay for Ecotoxicity Assessment: A Review. Sci. Total Environ. 2018, 626, 1295–1309. [Google Scholar] [CrossRef]
- Jabłońska-Trypuć, A.; Wydro, U.; Serra-Majem, L.; Wołejko, E.; Butarewicz, A. The Analysis of Bifenox and Dichlobenil Toxicity in Selected Microorganisms and Human Cancer Cells. Int. J. Environ. Res. Public Health 2019, 16, 4137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harishankar, M.K.; Sasikala, C.; Ramya, M. Efficiency of the intestinal bacteria in the degradation of the toxic pesticide, chlorpyrifos. Biotech 2013, 3, 137–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medo, J.; Maková, J.; Medová, J.; Lipková, N.; Cinkocki, R.; Omelka, R.; Javoreková, S. Changes in Soil Microbial Community and Activity Caused by Application of Dimethachlor and Linuron. Sci. Rep. 2021, 11, 12786. [Google Scholar] [CrossRef] [PubMed]
- Souza, T.D.S.; Fontanetti, C.S. Morphological Biomarkers in the Rhinocricus Padbergi Midgut Exposed to Contaminated Soil. Ecotoxicol. Environ. Saf. 2011, 74, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Steckling, N.; Gotti, A.; Bose-O’Reilly, S.; Chapizanis, D.; Costopoulou, D.; De Vocht, F.; Garí, M.; Grimalt, J.O.; Heath, E.; Hiscock, R.; et al. Biomarkers of Exposure in Environment-Wide Association Studies—Opportunities to Decode the Exposome Using Human Biomonitoring Data. Environ. Res. 2018, 164, 597–624. [Google Scholar] [CrossRef]
- Lavezzari, G.; Womack, A.W. Industry Perspectives on Biomarker Qualification. Clin. Pharmacol. Ther. 2016, 99, 208–213. [Google Scholar] [CrossRef] [Green Version]
- Poblete-Naredo, I.; Albores, A. Molecular biomarkers to assess health risks due to environmental contaminants exposure. Biomedica 2016, 36, 309–335. [Google Scholar] [CrossRef]
- Lionetto, M.G.; Caricato, R.; Giordano, M.E. Pollution Biomarkers in Environmental and Human Biomonitoring. Open Biomark. J. 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kroon, F.; Streten, C.; Harries, S. A Protocol for Identifying Suitable Biomarkers to Assess Fish Health: A Systematic Review. PLoS ONE 2017, 12, e0174762. [Google Scholar] [CrossRef]
- Traven, L.; Mićović, V.; Vukić Lušić, D.; Smital, T. The Responses of the Hepatosomatic Index (HSI), 7-Ethoxyresorufin-O-Deethylase (EROD) Activity and Glutathione-S-Transferase (GST) Activity in Sea Bass (Dicentrarchus labrax, Linnaeus 1758) Caged at a Polluted Site: Implications for Their Use in Environmental Risk Assessment. Environ. Monit. Assess. 2013, 185, 9009–9018. [Google Scholar] [CrossRef]
- Weichert, F.G.; Axén, C.; Förlin, L.; Inostroza, P.A.; Kammann, U.; Welling, A.; Sturve, J.; Asker, N. A Multi-Biomarker Study on Atlantic Salmon (Salmo salar L.) Affected by the Emerging Red Skin Disease in the Baltic Sea. J. Fish Dis. 2021, 44, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Petersen, I.L.; Hansen, H.C.B.; Ravn, H.W.; Sørensen, J.C.; Sørensen, H. Metabolic Effects in Rapeseed (Brassica napus L.) Seedlings after Root Exposure to Glyphosate. Pestic. Biochem. Physiol. 2007, 89, 220–229. [Google Scholar] [CrossRef]
- Petersen, I.L. Biomarkers in Herbicide Exposed Plants; Department of Basic Sciences and Environment, University of Copenhagen: København, Denmark, 2009. [Google Scholar]
- Nkongolo, K.K.; Narendrula-Kotha, R. Advances in Monitoring Soil Microbial Community Dynamic and Function. J. Appl. Genet. 2020, 61, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Osborn, A.M. Advantages and Limitations of Quantitative PCR (Q-PCR)-Based Approaches in Microbial Ecology: Application of Q-PCR in Microbial Ecology. FEMS Microbiol. Ecol. 2009, 67, 6–20. [Google Scholar] [CrossRef] [Green Version]
- Du, Z.; Zhu, Y.; Zhu, L.; Zhang, J.; Li, B.; Wang, J.; Wang, J.; Zhang, C.; Cheng, C. Effects of the Herbicide Mesotrione on Soil Enzyme Activity and Microbial Communities. Ecotoxicol. Environ. Saf. 2018, 164, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Mattana, S.; Chelinho, S.; Sousa, J.P.; Alcañiz, J.M.; Domene, X. Nonylphenol Causes Shifts in Microbial Communities and Nitrogen Mineralization in Soil Microcosms. Ecotoxicol. Environ. Saf. 2019, 181, 395–403. [Google Scholar] [CrossRef]
- Mijangos, I.; Becerril, J.M.; Albizu, I.; Epelde, L.; Garbisu, C. Effects of Glyphosate on Rhizosphere Soil Microbial Communities under Two Different Plant Compositions by Cultivation-Dependent and -Independent Methodologies. Soil Biol. Biochem. 2009, 41, 505–513. [Google Scholar] [CrossRef]
- Tortella, G.R.; Mella-Herrera, R.A.; Sousa, D.Z.; Rubilar, O.; Acuña, J.J.; Briceño, G.; Diez, M.C. Atrazine Dissipation and Its Impact on the Microbial Communities and Community Level Physiological Profiles in a Microcosm Simulating the Biomixture of On-Farm Biopurification System. J. Hazard. Mater. 2013, 260, 459–467. [Google Scholar] [CrossRef] [Green Version]
- Semenov, M.V. Metabarcoding and Metagenomics in Soil Ecology Research: Achievements, Challenges, and Prospects. Biol. Bull. Rev. 2021, 11, 40–53. [Google Scholar] [CrossRef]
- Knight, R.; Vrbanac, A.; Taylor, B.C.; Aksenov, A.; Callewaert, C.; Debelius, J.; Gonzalez, A.; Kosciolek, T.; McCall, L.I.; McDonald, D.; et al. Best practices for analysing microbiomes. Nat. Rev. Microbiol. 2018, 16, 410–422. [Google Scholar] [CrossRef] [Green Version]
- Serbent, M.P.; Dos Anjos Borges, L.G.; Quadros, A.; Marconatto, L.; Tavares, L.B.B.; Giongo, A. Prokaryotic and Microeukaryotic Communities in an Experimental Rice Plantation under Long-Term Use of Pesticides. Environ. Sci. Pollut. Res. Int. 2021, 28, 2328–2341. [Google Scholar] [CrossRef] [PubMed]
- Storck, V.; Nikolaki, S.; Perruchon, C.; Chabanis, C.; Sacchi, A.; Pertile, G.; Baguelin, C.; Karas, P.A.; Spor, A.; Devers-Lamrani, M.; et al. Lab to Field Assessment of the Ecotoxicological Impact of Chlorpyrifos, Isoproturon, or Tebuconazole on the Diversity and Composition of the Soil Bacterial Community. Front. Microbiol. 2018, 9, 1412. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.M.; Hoilett, N.; Lorenz, N.; Dick, R.P.; Liles, M.R.; Ramsier, C.; Kloepper, J.W. Glyphosate Effects on Soil Rhizosphere-Associated Bacterial Communities. Sci. Total Environ. 2016, 543, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Møller, P.; Stopper, H.; Collins, A.R. Measurement of DNA damage with the comet assay in high-prevalence diseases: Current status and future directions. Mutagenesis 2020, 35, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Chapman, P.M. Determining When Contamination Is Pollution—Weight of Evidence Determinations for Sediments and Effluents. Environ. Int. 2007, 33, 492–501. [Google Scholar] [CrossRef]
- Ersson, C.; Møller, P.; Forchhammer, L.; Loft, S.; Azqueta, A.; Godschalk, R.W.L.; van Schooten, F.-J.; Jones, G.D.D.; Higgins, J.A.; Cooke, M.S.; et al. An ECVAG Inter-Laboratory Validation Study of the Comet Assay: Inter-Laboratory and Intra-Laboratory Variations of DNA Strand Breaks and FPG-Sensitive Sites in Human Mononuclear Cells. Mutagenesis 2013, 28, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Saleem, M.; Wang, C. Effects of Biochar on the Earthworm (Eisenia foetida) in Soil Contaminated with and/or without Pesticide Mesotrione. Sci. Total Environ. 2019, 671, 52–58. [Google Scholar] [CrossRef]
- Prado, R.; Díaz, R.; Rioboo, C.; Abalde, J.; Herrero, C.; Cid, A. Use of the comet assay to evaluate pesticide toxicity on non-target microalgae. In Genotoxicity: Evaluation, Testing and Prediction; Kocsis, A., Molnar, H., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2009; pp. 311–319. [Google Scholar]
- Cenkci, S.; Yildiz, M.; Ciğerci, I.H.; Bozdağ, A.; Terzi, H.; Terzi, E.S.A. Evaluation of 2,4-D and Dicamba Genotoxicity in Bean Seedlings Using Comet and RAPD Assays. Ecotoxicol. Environ. Saf. 2010, 73, 1558–1564. [Google Scholar] [CrossRef]
- Liman, R.; Ciğerci, İ.H.; Öztürk, N.S. Determination of Genotoxic Effects of Imazethapyr Herbicide in Allium Cepa Root Cells by Mitotic Activity, Chromosome Aberration, and Comet Assay. Pestic. Biochem. Physiol. 2015, 118, 38–42. [Google Scholar] [CrossRef]
- Cerdà, A.; Daliakopoulos, I.N.; Terol, E.; Novara, A.; Fatahi, Y.; Moradi, E.; Salvati, L.; Pulido, M. Long-term monitoring of soil bulk density and erosion rates in two Prunus Persica (L) plantations under flood irrigation and glyphosate herbicide treatment in La Ribera district, Spain. J. Environ. Manag. 2021, 282, 111965. [Google Scholar] [CrossRef]
- Jablonowski, N.D.; Linden, A.; Köppchen, S.; Thiele, B.; Hofmann, D.; Mittelstaedt, W.; Pütz, T.; Burauel, P. Long-term persistence of various 14C-labeled pesticides in soils. Environ Pollut. 2012, 168, 29–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
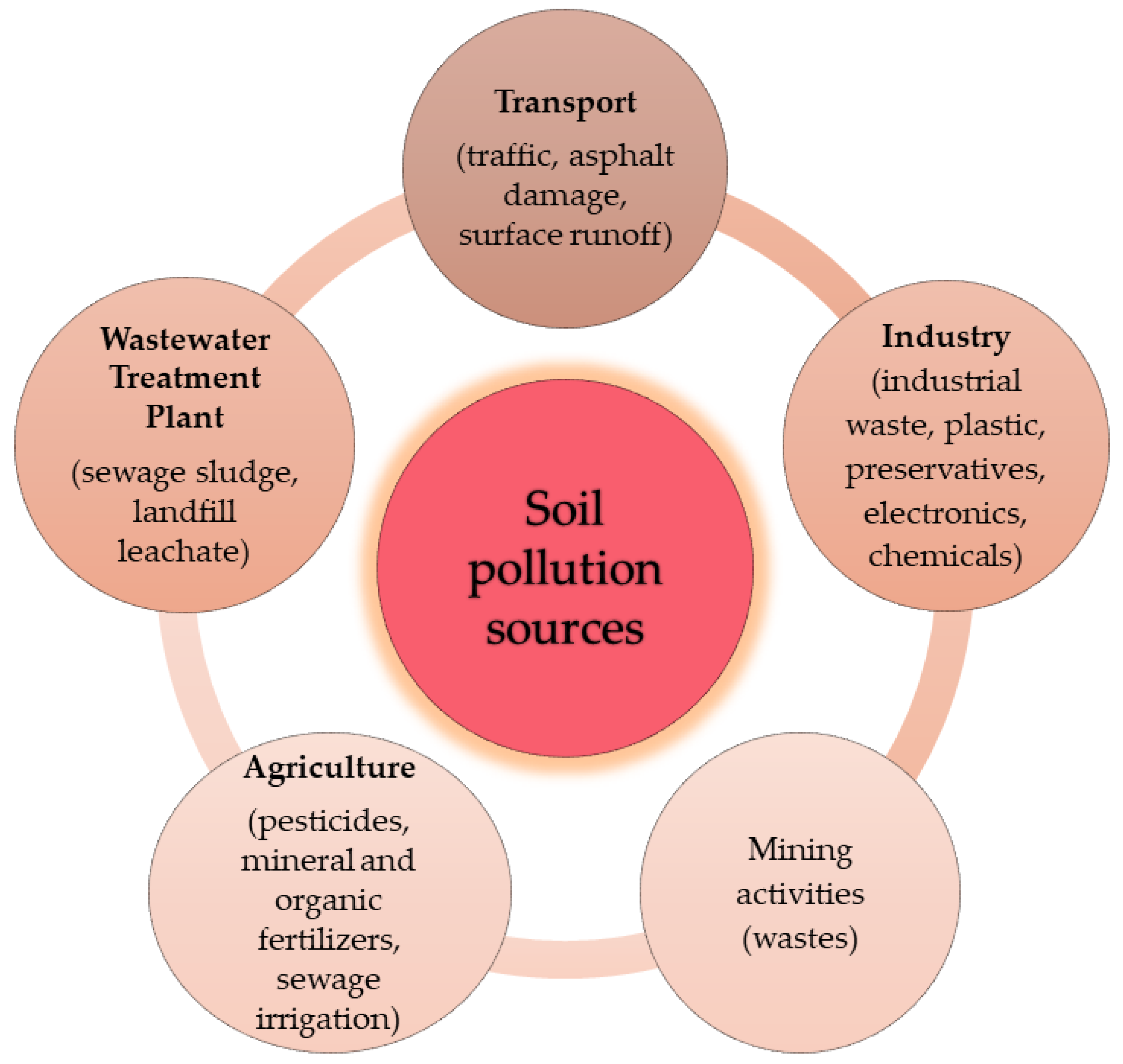

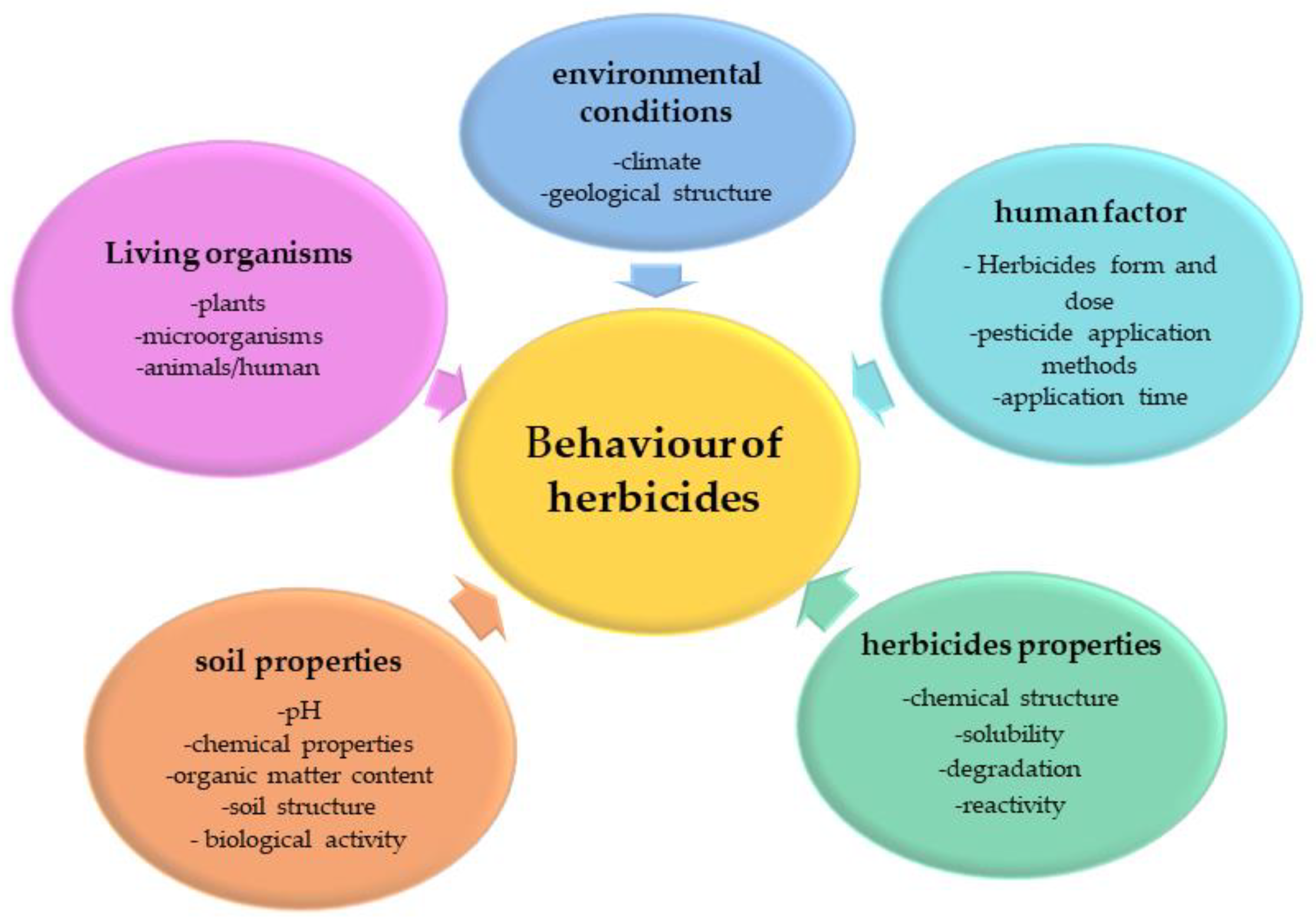
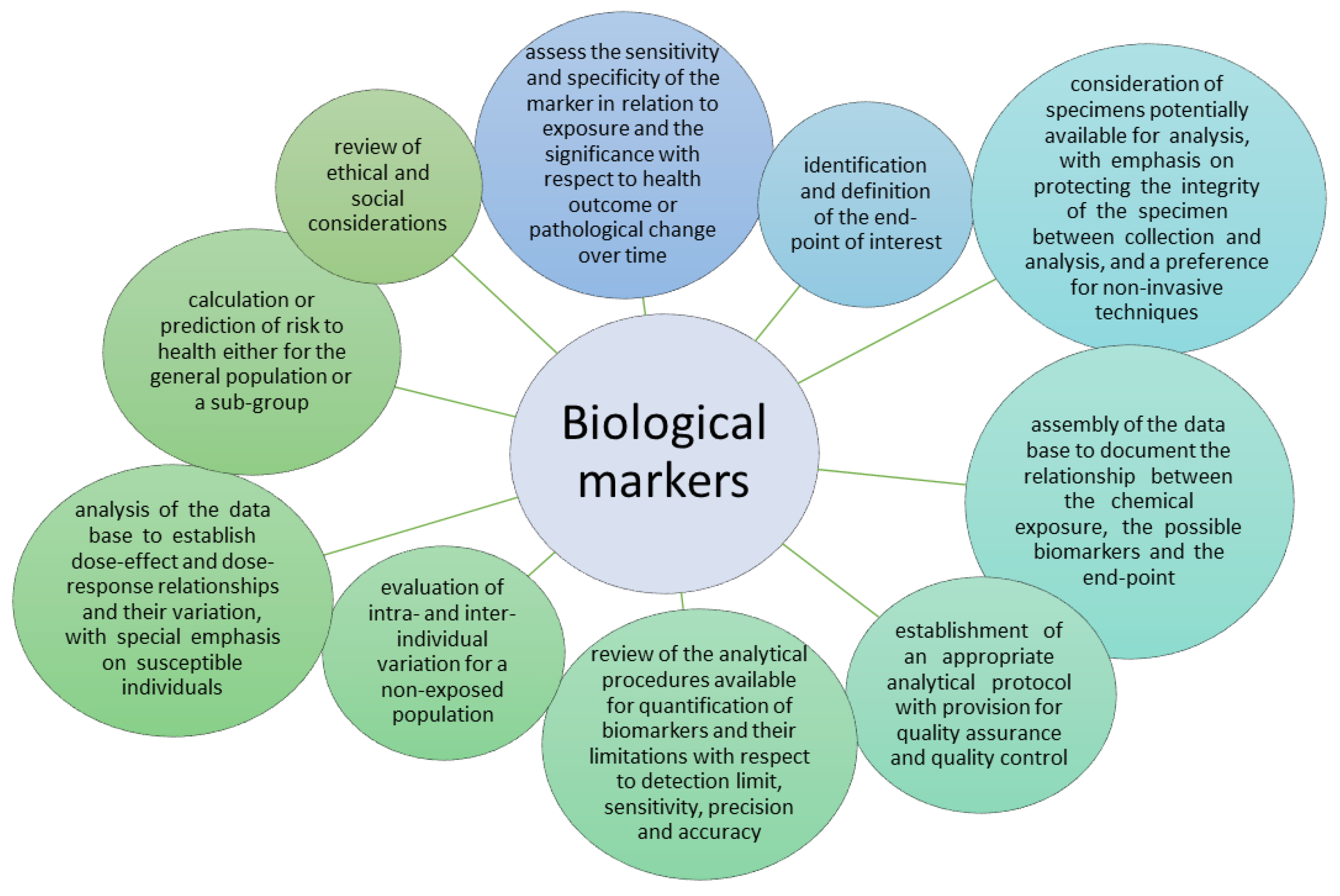
| HRAC Numerical Code | Mechanism of Action | Active Substances | Chemical Family | Environmental Fate [26] | Ecotoxicity | Persistence of Herbicides in Soil (DT50) * |
|---|---|---|---|---|---|---|
| Group 1 | Inhibition of acetyl-CoA carboxylase (ACCase) (Graminicides prevent the production of essential fatty acids in weeds from the grass family.) | Butroxydim, quizalofop-ethyl, quizalofop-P-tefuryl, cycloxydim, fenthiaprop, clethodim, pinoxaden, propaquizafop, tepraloxydim, tralkoxydim | Aryloxyphenoxy-propionates, cyclohexanediones, phenylpyrazoline | Drainflow: Slightly or moderately mobile; Potential for particle-bound transport: Medium | Moderate alert for birds, daphnia, earthworms and fish [26] | from 0.2 to 45 days |
| Group 2 | Inhibition of acetolactate synthase (ALS), also named acetohydroxyacid synthase (AHAS) (prevent the production of some amino acids necessary for protein synthesis) | amidosulfuron, chlorsulfuron, ethametsulfuron-methyl, flazasulfuron, florasulam, foramsulfuron, imazamox, iodosulfuron-methyl-sodium, metosulam, penoxsulam, mesosulfuron-methyl, nicosulfuron, pyroxsulam, propoxycarbazulfuron methyl, flucarbazone-Na, thifensulfuron-methyl, thien-carbazone-methyl, tritosulfuron | Sulfonylurea, imidazolinones, triazolopyrimidine, pyrimidinyl benzoates, sulfonanilides, triazolinones | High leachability and mobile | Moderate alert for birds, earthworms and fish [26] | from 1.85 to 200.2 days |
| Group 3 | Inhibition of microtubule assembly | pendimethalin, benfluralin, butralin, ethalfluralin, pendimethalin, DCPA, trifluralin, propyzamide, dinitramine, oryzalin, isopropalin, butamifos, thiazopyr | Dinitroaniline, phosphoroamidates, pyridines, benzamides, benzoic acid | Potential for particle-bound transport: High Drainflow: Slightly mobile and moderately persistent; Potential for particle-bound transport: Medium | High alert for fish, chronic ecotoxicity [16,26]. Moderate alert for birds, earthworms, daphnia and fish [16,26]. | from 22 to 182.3 days |
| Group 4 | Synthetic auxins (action like indole acetic acid) | 2,4-D, clomeprop, aminopyralid, quinclorac, quinmerac, clopyralid, chlorfenprop, dichlorprop-P, dicamba, fluroxypyr, MCPA, MCPB, mecoprop, picloram, trichlopyr | Phenoxy-carboxylates, pyridine-carboxylates, phenyl carboxylates, quinolone-carboxylates, benzoates | High leachability; Drainflow: Mobile | Moderate alert for birds, earthworms, daphnia and fish [26,27] | from 4.4 to 450 days |
| Group 5 | Inhibition of photosynthesis at PS II (prevent the production of sugar and energy through photosynthesis) | chloridazone, bromacil, brompyrazon, atrazine, desmedipham, hlorth, metamitron, metribuzin, trietazine | Triazine, triazinone, triazolinone, uracil, pyridazinone, phenylcarbamate | Drainflow: Mobile or moderately mobile; Potential for particle-bound transport: High or medium | Moderate alert for birds, daphnia, earthworms, bees and fish [16,26] | from 31 to 75 days |
| pentanochlor, chlorotoluron, buturon, isoproturon, linuron, isouron, propanil | Ureas, amides | Drainflow: Moderately or slightly mobile; Potential for particle-bound transport: Medium | Moderate alert for birds, daphnia, earthworms and fish [16,26] | from 0.4 to 57.6 days | ||
| Group 6 | bentazone, bromofenoxim, bromoxynil-octanoate, pyridate | Nitriles, benzothiadiazinone, phenyl-pyridazine | Drainflow: Mobile or non-mobile; Potential for particle-bound transport: Low or medium | Moderate alert for birds and fish High alert: High fish and daphnia, chronic ecotoxicity [26] | from 2.2 to 53 days | |
| Group 9 | Inhibition of 5-enolpyruvyl shikimate-3-phosphate synthase (EPSPS) (prevent the biosynthesis of some amino acids) | glyphosate | Glycine | Drainflow: Slightly mobile; Potential for particle-bound transport: Medium | Moderate alert for birds, earthworms, bees, daphnia and fish [26] | from 1.5 to 53.5 days [28] |
| Group 10 | Inhibition of glutamine synthetase | glufosinate-ammonium, bialaphos/bilanafos | Phosphinic acids | No records | Low alert for bees, acute contact and oral ecotoxicity [26] | ~7.4 days |
| Group 12 | Inhibition of phytoene desaturase (PDS) | beflubutamid, flurochloridone, fluridone, diflufenican, picolinafen | N-Phenyl heterocycles, diphenyl heterocycles, phenyl ethers | Slightly mobile and moderately persistent; Potential for particle-bound transport: Medium and High | Moderate alert for daphnia and earthworms Moderate and high alert for fish [26] | from 6.1 to 94.5 days |
| Group 13 | Inhibition of 1-deoxy-d-xylose-5-phosphate synthase (DOXP synthase) | clomazone, bixlozone | Isoxazolidinone | Drainflow: Moderately mobile | Moderate alert for birds, earthworms, bees, daphnia and fish [12] | from 22.6 to 153 days |
| Group 14 | Inhibition of protoporphyrinogen (PPO) | Acifluorfen oxyfluorfen, nitrofen, flumipropyn bifenox, tiafenacil, oxadiazon carfentrazone-ethyl, oxadiargyl, pyraflufen-ethyl | Diphenyl ether, N-phenyl-oxadiazolones, N-phenyl-imides, N-phenyl-triazolinones, phenylpyrazoles | Drainflow: Slightly mobile and moderately persistent; Potential for particle-bound transport: Medium | High alert: High fish and daphnia, chronic ecotoxicity [26] Moderate alert for birds, daphnia, earthworms and fish [26] | from 0.32 to 502 days |
| Group 15 | Inhibition of very-long-chain fatty acid synthesis (VLCFAs), inhibition of cell division | acetochlor, dimetachlor, dimethenamid-P, benfuresate, flufenacet, metazachlor, anilofos, metolachlor-S, butylate, indanofan, napropamide, pethoxamid, cafenstrole | Azolyl-carboxamides, benzofurans, isoxazolines, oxiranes, thiocarbamates, α-chloroacetamides, α-oxyacetamides, α-thioacetamides | Drainflow: Mobile or moderately mobile and moderately persistent; Potential for particle-bound transport: Medium | High alert for birds, chronic ecotoxicity. Moderate alert for birds, earthworms, daphnia and fish [26]. | from 2.0 to 90 days |
| Group 18 | Inhibition of dihydropteroate (DHP) synthase | asulam | Carbamate | Drainflow: Mobile | Moderate alert for birds, earthworms, daphnia and fish [26]. | ~3.2 days |
| Group 22 | Photosystem I-electron diversion (prevent the production of sugar and energy through photosynthesis; they destroy cell membranes) | diquat, paraquat, morfamquat, cyperquat | Bipyridylium | Very persistent; Potential for particle-bound transport: High | High alert; acute and chronic ecotoxicity for birds [16,26] | from 0 to 3000 days |
| Group 23 | Inhibition of mitosis/microtubule organization | chlorpropham, propham, carbetamide, barban | Carbamate | Drainflow: Moderately mobile | Moderate alert for birds, earthworms, daphnia and fish [26] | from 5.0 to 13.1 days |
| Group 27 | Inhibition of 4-hydroxyphenyl-pyruvate-dioxygenase (4-HPPD) | isoxaflutole, topramezone, pyrasulfotole sulcotrione, tembotrione, mesotrione, tefuryltrione | Triketones, isoxazoles, pyrazoles | Drainflow: Mobile and moderately mobile | High alert for fish, chronic ecotoxicity. Moderate alert for birds, earthworms, bees, daphnia and fish [26]. | from 0.9 to 218 days |
| Group 29 | Inhibition of cell wall (cellulose) synthesis | dichlobenil, isoxaben, hlorthiamide, flupoxam, indaziflam, triaziflam | Nitriles, benzamides, triazolocarboxamide, alkylazines | Drainflow: Moderately mobile; Potential for particle-bound transport: Medium | Moderate alert for birds, earthworms, daphnia and fish [26] | from 25 to 150 days |
| Group 34 | Inhibition of lycopene cyclase | amitrole | Triazole | Drainflow: Moderately mobile | Moderate alert for birds, earthworms, daphnia and fish [26] | ~7.4 days |
| Group Ø | The mode of action of herbicides in group Z is unknown. | quinoclamine, bensulide, iron sulphate, diphenamid, perfluidone, flamprop-m | Acetamides, phosphorodithioate, inorganic compound, trifluoromethane sulfonanilides, arylaminopropionic acid | Drainflow: Slightly mobile or very mobile | High alert for bees, daphnia and fish [26] | from 22 to 1000 days |
| Method | Assays/indicators | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Biological activity measurement | Soil enzyme activity determination (dehydrogenases, catalase, β-glucosidase, etc.) | effective tool to characterize the microbial status of soil, easy and fast tests | Sensitive to environmental changes | [4] |
| Soil respiration analysis | ||||
| Soil microbial biomass/number analysis | ||||
| Biological diversity, community structure and functional gene abundance determination | Fingerprinting methods (DGGE, T-RFLP, etc.) | Easy and fast methods (fingerprint methods), precise methods, independent culture method, highly sensitive | PCR-based methods require DNA sample with high quality and purity, PCR reaction optimization; specialist equipment required (HTS), requires the use of bioinformatics and statistical tools for data analysis | [13,47] |
| Real-time PCR | ||||
| High-throughput sequencing (NGS, TGS) | ||||
| Cell viability | MTT assay | A large range of applications (bacteria, yeast, animal/human cells), rapid and simple test procedure; highly sensitive (ATP assay) | low sensitivity, possible chemical interferences (MTT assay); specialist equipment required (ATP assay) | [20,48] |
| ATP bioluminescence assay | ||||
| Genotoxicity/mutagenicity analysis | Comet assay | Fast, easy, requiring a small amount of test substance, small amount of sample may be used, high sensitivity, can be applied in different samples (plant, animal/human) | may be too sensitive, reaching saturation at a relatively low level of damage, requires careful calibration, specialist equipment required | [13,20] |
| Oxidative stress parameter measurement | ROS analysis | Applied in many different biological models (plant, bacteria, yeast, animal/human cells), easy to conduct, inexpensive, simple, replicable | low sensitivity; the need to maintain a low temperature at each stage of the analysis, high consumption of reagents, limitations related to spectroscopic methods | [49] |
| Activity of enzymes (catalases, superoxide dismutase, glutathione peroxidase) | ||||
| Non-enzymatic oxidant production (glutathione) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wołejko, E.; Wydro, U.; Odziejewicz, J.I.; Koronkiewicz, A.; Jabłońska-Trypuć, A. Biomonitoring of Soil Contaminated with Herbicides. Water 2022, 14, 1534. https://doi.org/10.3390/w14101534
Wołejko E, Wydro U, Odziejewicz JI, Koronkiewicz A, Jabłońska-Trypuć A. Biomonitoring of Soil Contaminated with Herbicides. Water. 2022; 14(10):1534. https://doi.org/10.3390/w14101534
Chicago/Turabian StyleWołejko, Elżbieta, Urszula Wydro, Joanna Irena Odziejewicz, Agata Koronkiewicz, and Agata Jabłońska-Trypuć. 2022. "Biomonitoring of Soil Contaminated with Herbicides" Water 14, no. 10: 1534. https://doi.org/10.3390/w14101534
APA StyleWołejko, E., Wydro, U., Odziejewicz, J. I., Koronkiewicz, A., & Jabłońska-Trypuć, A. (2022). Biomonitoring of Soil Contaminated with Herbicides. Water, 14(10), 1534. https://doi.org/10.3390/w14101534









