Evaluation of Galdieria sulphuraria and Chlorella vulgaris for the Bioremediation of Produced Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Sub-Culturing of Algae
2.2. Produced Water
2.3. Experimental Design
- the same salts as the standard media;
- the same amount of salts, as presented in Table 2.
2.4. Analytical Techniques
3. Results
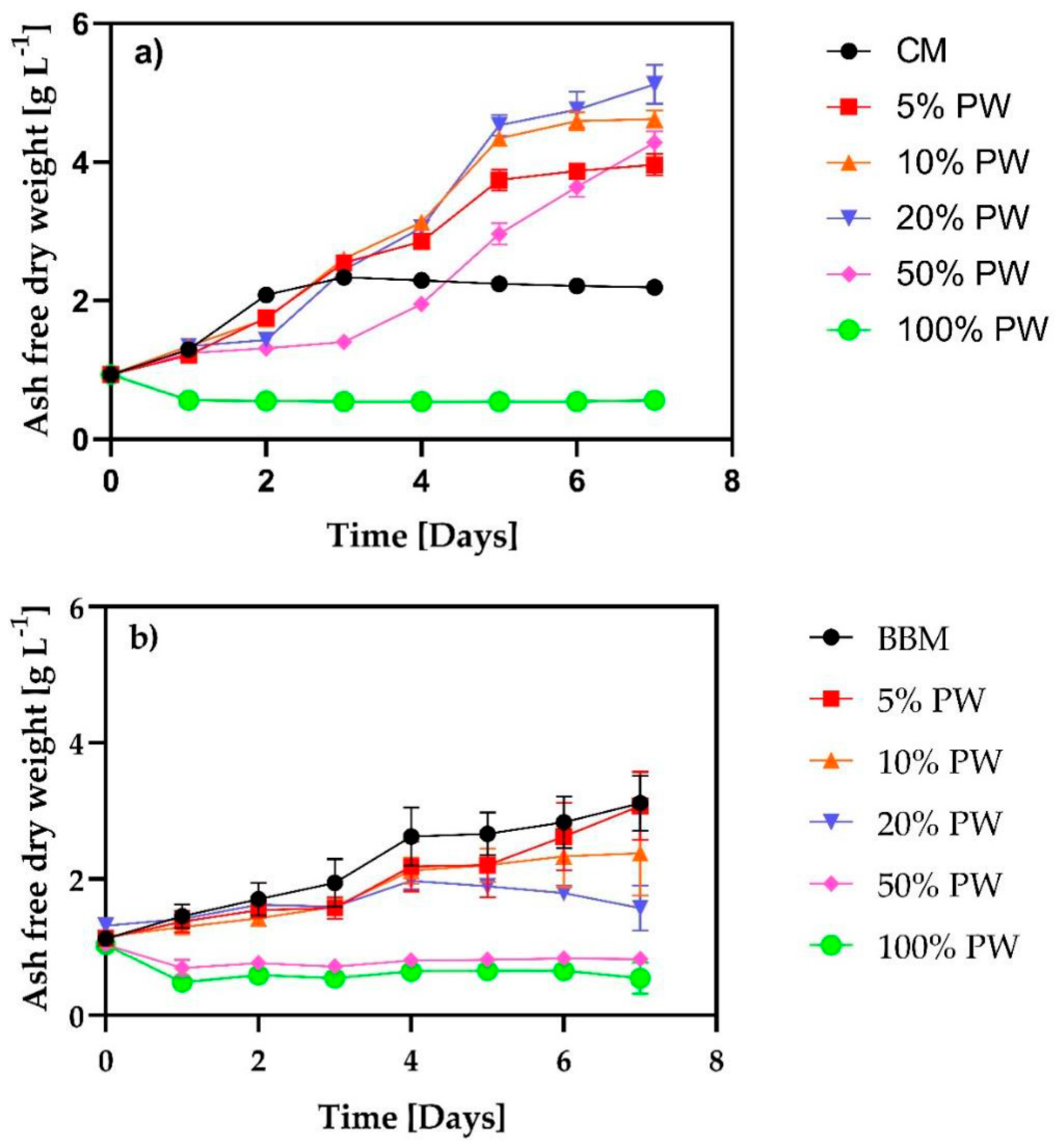
3.1. Algae Biomass Production in PW
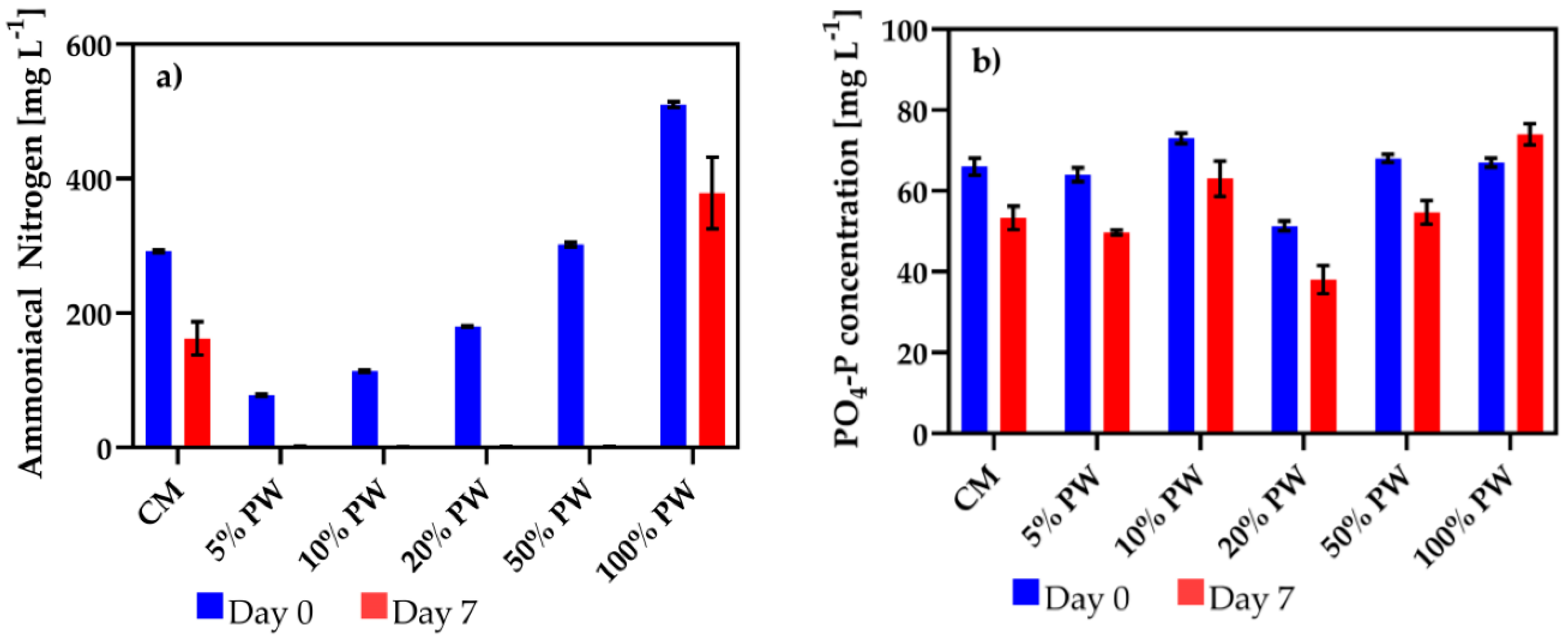
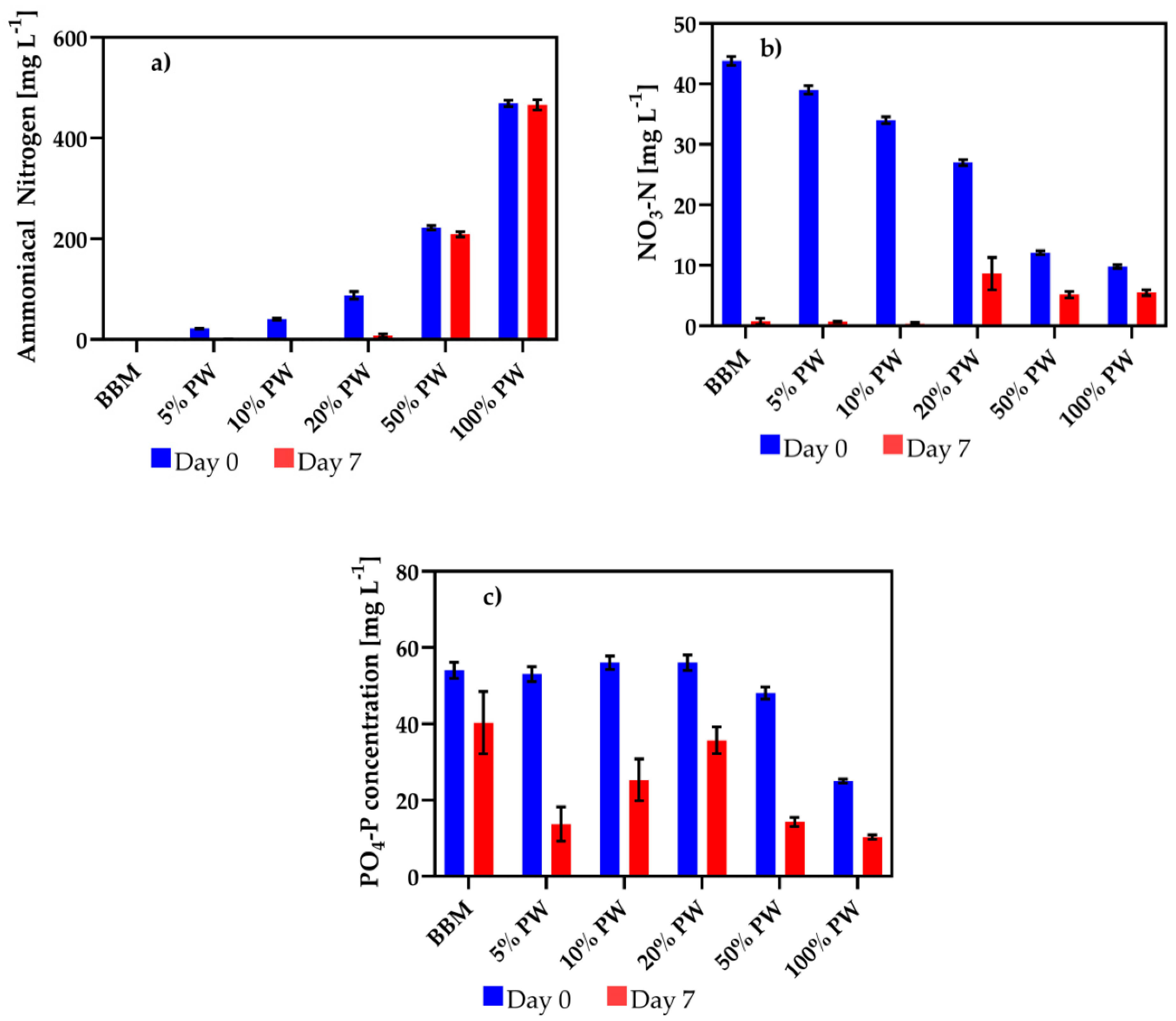
3.2. Nitrogen and Phosphorus Removal by Algae
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yazdan, M.M.S.; Ahad, T.; Jahan, I.; Mazumder, M. Review on the Evaluation of the Impacts of Wastewater Disposal in Hydraulic Fracturing Industry in the United States. Technologies 2020, 8, 67. [Google Scholar] [CrossRef]
- Neff, J.M.; Lee, K.; Deblois, E.M. Produced Water: Overview of Composition, Fates, and Effects. Prod. Water 2011, 3–54. [Google Scholar] [CrossRef]
- Meng, M.; Chen, M.; Sanders, K.T. Evaluating the Feasibility of Using Produced Water from Oil and Natural Gas Production to Address Water Scarcity in California’s Central Valley. Sustainability 2016, 8, 1318. [Google Scholar] [CrossRef]
- Produced Water Treatment Market Size & Share. Industry Report, 2020–2024. Available online: https://www.grandviewresearch.com/industry–analysis/produced–water–treatment–market (accessed on 14 March 2021).
- Jain, P.; Sharma, M.; Dureja, P.; Sarma, P.M.; Lal, B. Bioelectrochemical Approaches for Removal of Sulfate, Hydrocarbon and Salinity from Produced Water. Chemosphere 2017, 166, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Agrawal, S.; Nawaz, T.; Pan, S.; Selvaratnam, T. A Review of Algae—Based Produced Water Treatment for Biomass and Biofuel Production. Water 2020, 12, 2351. [Google Scholar] [CrossRef]
- Guerra, K.; Dahm, K.; Dundorf, S. Oil and Gas. Produced Water Management and Beneficial Use in the Western United States; 0704-0188; US Department of the Interior, Bureau of Reclamation: Washington, DC, USA, 2011. Available online: https://www.usbr.gov/research/dwpr/reportpdfs/report157.pdf (accessed on 11 March 2021).
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Produced Water Treatment: Application of Air Gap Membrane Distillation. Desalination 2013, 309, 46–51. [Google Scholar] [CrossRef]
- Das, P.; Abdulquadir, M.; Thaher, M.; Khan, S.; Chaudhary, A.K.; Alghasal, G.; Al–Jabri, H.M.S.j. Microalgal Bioremediation of Petroleum–Derived Low Salinity and Low PH Produced Water. J. Appl. Phycol. 2019, 31, 435–444. [Google Scholar] [CrossRef]
- Ranjbar, S.; Quaranta, J.D.; Tehrani, R.; Van Aken, B. lgae–Based Treatment of Hydraulic Fracturing Produced Water: Metal Removal and Biodiesel Production by the Halophilic Microalgae Dunaliella salina. Miami, FL, USA, 2015. Available online: https://www.researchgate.net/publication/281462885_Algae-Based_Treatment_of_Hydraulic_Fracturing_Produced_Water_Metal_Removal_and_Biodiesel_Production_by_the_Halophilic_Microalgae_Dunaliella_salina (accessed on 11 March 2021).
- Nawaz, T.; Rahman, A.; Pan, S.; Dixon, K.; Petri, B.; Selvaratnam, T. A Review of Landfill Leachate Treatment by Microalgae: Current Status and Future Directions. Processes 2020, 8, 384. [Google Scholar] [CrossRef]
- Hopkins, T.C.; Graham, E.J.S.; Schuler, A.J. Biomass and Lipid Productivity of Dunaliella tertiolecta in a Produced Water—Based Medium over a Range of Salinities. J. Appl. Phycol. 2019, 31, 3349–3358. [Google Scholar] [CrossRef]
- Hopkins, T.C.; Graham, E.J.S.; Schwilling, J.; Ingram, S.; Gómez, S.M.; Schuler, A.J. Effects of Salinity and Nitrogen Source on Growth and Lipid Production for a Wild Algal Polyculture in Produced Water Media. Algal Res. 2019, 38, 101406. [Google Scholar] [CrossRef]
- Graham, E.J.S.; Dean, C.A.; Yoshida, T.M.; Twary, S.N.; Teshima, M.; Alvarez, M.A.; Zidenga, T.; Heikoop, J.M.; Perkins, G.B.; Rahn, T.A.; et al. Oil and Gas Produced Water as a Growth Medium for Microalgae Cultivation: A Review and Feasibility Analysis. Algal Res. 2017, 24, 492–504. [Google Scholar] [CrossRef]
- Johnson, R.J.; Jurawan, I.; Frenzel, M.; Price, A.C. The Identification and Mechanism of a Scenedesmus spp. Causing Bio—Fouling of an Oil Field Produced Water Treatment Plant. Int. Biodeterior. Biodegrad. 2016, 108, 207–213. [Google Scholar] [CrossRef]
- Godfrey, V. Production of Biodiesel from Oleaginous Organisms Using Underutilized Wastewaters. Utah State University: Logan, UT, USA, 2012. Available online: https://digitalcommons.usu.edu/cgi/viewcontent.cgi?article=2330&context=etd (accessed on 11 March 2021).
- Pan, S.; Dixon, K.L.; Nawaz, T.; Rahman, A.; Selvaratnam, T. Evaluation of Galdieria sulphuraria for Nitrogen Removal and Biomass Production from Raw Landfill Leachate. Algal Res. 2021, 54, 102183. [Google Scholar] [CrossRef]
- Das, C.; Naseera, K.; Ram, A.; Meena, R.M.; Ramaiah, N. Bioremediation of Tannery Wastewater by a Salt—Tolerant Strain of Chlorella vulgaris. J. Appl. Phycol. 2017, 29, 235–243. [Google Scholar] [CrossRef]
- Selvaratnam, T.; Pegallapati, A.K.; Montelya, F.; Rodriguez, G.; Nirmalakhandan, N.; Van Voorhies, W.; Lammers, P.J. Evaluation of a Thermo–Tolerant Acidophilic Alga, Galdieria sulphuraria, for Nutrient Removal from Urban Wastewaters. Bioresour. Technol. 2014, 156, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Toplin, J.A.; Norris, T.B.; Lehr, C.R.; McDermott, T.R.; Castenholz, R. Biogeographic and Phylogenetic Diversity of Thermoacidophilic Cyanidiales in Yellowstone National Park, Japan, and New Zealand. Appl. Environ. Microbiol. 2008, 74, 2822–2833. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Kumar, S.; Bafana, A.; Dahoumane, S.; Jeffryes, C. Individual and Combined Effects of Extracellular Polymeric Substances and Whole Cell Components of Chlamydomonas reinhardtii on Silver Nanoparticle Synthesis and Stability. Molecules 2019, 24, 956. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Kumar, S.; Bafana, A.; Lin, J.; Dahoumane, S.A.; Jeffryes, C. A Mechanistic View of the Light—Induced Synthesis of Silver Nanoparticles Using Extracellular Polymeric Substances of Chlamydomonas reinhardtii. Molecules 2019, 24, 3506. [Google Scholar] [CrossRef] [PubMed]
- Badrinarayanan, I. Using Produced Water to Grow Microalgae. In Proceedings of the International Petroleum Environmental Conference, San Antonio, TX, USA, 30 October–1 November 2017; p. 20. [Google Scholar]
- Arriada, A.A.; Abreu, P.C. Nannochloropsis oculata Growth in Produced Water: An Alternative for Massive Microalgae Biomass Production. Braz. J. Pet. Gas 2014, 8, 119–125. [Google Scholar] [CrossRef]
- Calderón–Delgado, I.C.; Mora–Solarte, D.A.; Velasco–Santamaría, Y.M. Physiological and Enzymatic Responses of Chlorella vulgaris Exposed to Produced Water and Its Potential for Bioremediation. Environ. Monit. Assess. 2019, 191, 399. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Unit | Measured Values (Sample Size, n = 5) |
|---|---|---|
| pH | – | 6.71 ± 0.05 |
| Density | kg m−3 | 1075 ± 3 |
| Total Solids (TS) | mg L−1 | 111,876 ± 1119 |
| Total Suspended Solids (TSS) | mg L−1 | 674 ± 246 |
| Total Dissolved Solids (TDS) | mg L−1 | 111,202 ± 1046 |
| Electrical Conductivity (EC) | mS cm−1 | 122.42 ± 0.43 |
| NH3–N | mg L−1 | 452 ± 8 |
| Growth Media. | NO3–N mg L−1 | PO4–P mg L−1 | NO3–Salt | PO4–Salt |
|---|---|---|---|---|
| CM | – | ~66 | – | KH2PO4 |
| BBM | ~42 | ~54 | NaNO3 | K2HPO4, KH2PO4 |
| Experiment | Growth Rate (g L−1 d−1) | |
|---|---|---|
| G. sulphuraria | C. vulgaris | |
| Standard Media | 0.47 ± 0.04 | 0.37 ± 0.17 |
| 5% PW | 0.56 ± 0.05 | 0.37 ± 0.14 |
| 10% PW | 0.68 ± 0.03 | 0.24 ± 0.07 |
| 20% PW | 0.72 ± 0.05 | 0.17 ± 0.04 |
| 50% PW | 0.72 ± 0.05 | 0.00 ± 0.00 |
| 100% PW | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Sum of Squares | Degree of Freedom | Mean Square | F | p-value | α = 0.05 | |
|---|---|---|---|---|---|---|
| G. sulphuraria experiments | ||||||
| Biomass Density (g L−1) | ||||||
| Between Groups | 80.93 | 5 | 16.09 | 618.35 | 1.57 × 10−24 | p < 0.05, there is a significant difference |
| Within Groups | 0.63 | 24 | 0.03 | |||
| Total | 47.71 | 29 | ||||
| Growth Rate (g L−1 d−1) | ||||||
| Between Groups | 3.51 | 5 | 0.70 | 544.86 | 1.80 × 10−21 | p < 0.05, there is a significant difference |
| Within Groups | 0.03 | 21 | 0.00 | |||
| Total | 2.28 | 26 | ||||
| NH4–N Removal (%) | ||||||
| Between Groups | 16,990.40 | 5 | 3398.08 | 110.69 | 1.30× 10−9 | p < 0.05, there is a significant difference |
| Within Groups | 368.38 | 12 | 30.70 | |||
| Total | 17,358.78 | 17 | ||||
| P Removal (%) | ||||||
| Between Groups | 2597.68 | 5 | 519.54 | 27.33 | 3.65 × 10−6 | p < 0.05, there is a significant difference |
| Within Groups | 228.15 | 12 | 19.01 | |||
| Total | 2825.83 | 17 | ||||
| C. vulgaris experiments | ||||||
| Biomass Density (g L−1) | ||||||
| Between Groups | 37.01 | 5 | 7.40 | 45.92 | 3.63 × 10−13 | p < 0.05, there is a significant difference |
| Within Groups | 4.84 | 30 | 0.16 | |||
| Total | 20.33 | 35 | ||||
| Growth Rate (g L−1 d−1) | ||||||
| Between Groups | 0.68 | 5 | 0.14 | 35.33 | 1.06 × 10−11 | p < 0.05, there is a significant difference |
| Within Groups | 0.12 | 30 | 0.00 | |||
| Total | 0.39 | 35 | ||||
| NH4–N Removal (%) | ||||||
| Between Groups | 31,685.05 | 4 | 7921.26 | 1292.35 | 1.61 × 10−13 | p < 0.05, there is a significant difference |
| Within Groups | 61.29 | 10 | 6.13 | |||
| Total | 38,549.13 | 14 | ||||
| NO3–N Removal (%) | ||||||
| Between Groups | 8884.93 | 5 | 1776.99 | 74.65 | 1.29 × 10−8 | p < 0.05, there is a significant difference |
| Within Groups | 285.65 | 12 | 23.80 | |||
| Total | 9170.58 | 17 | ||||
| P Removal (%) | ||||||
| Between Groups | 5466.69 | 5 | 1093.34 | 14.78 | 8.99 × 10−5 | p < 0.05, there is a significant difference |
| Within Groups | 887.49 | 12 | 73.96 | |||
| Total | 6354.18 | 17 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, A.; Pan, S.; Houston, C.; Selvaratnam, T. Evaluation of Galdieria sulphuraria and Chlorella vulgaris for the Bioremediation of Produced Water. Water 2021, 13, 1183. https://doi.org/10.3390/w13091183
Rahman A, Pan S, Houston C, Selvaratnam T. Evaluation of Galdieria sulphuraria and Chlorella vulgaris for the Bioremediation of Produced Water. Water. 2021; 13(9):1183. https://doi.org/10.3390/w13091183
Chicago/Turabian StyleRahman, Ashiqur, Shanglei Pan, Cymone Houston, and Thinesh Selvaratnam. 2021. "Evaluation of Galdieria sulphuraria and Chlorella vulgaris for the Bioremediation of Produced Water" Water 13, no. 9: 1183. https://doi.org/10.3390/w13091183
APA StyleRahman, A., Pan, S., Houston, C., & Selvaratnam, T. (2021). Evaluation of Galdieria sulphuraria and Chlorella vulgaris for the Bioremediation of Produced Water. Water, 13(9), 1183. https://doi.org/10.3390/w13091183








