Temporal and Spatial Characteristics of CO2 Flux in Plateau Urban Wetlands and Their Influencing Factors Based on Eddy Covariance Technique
Abstract
1. Introduction
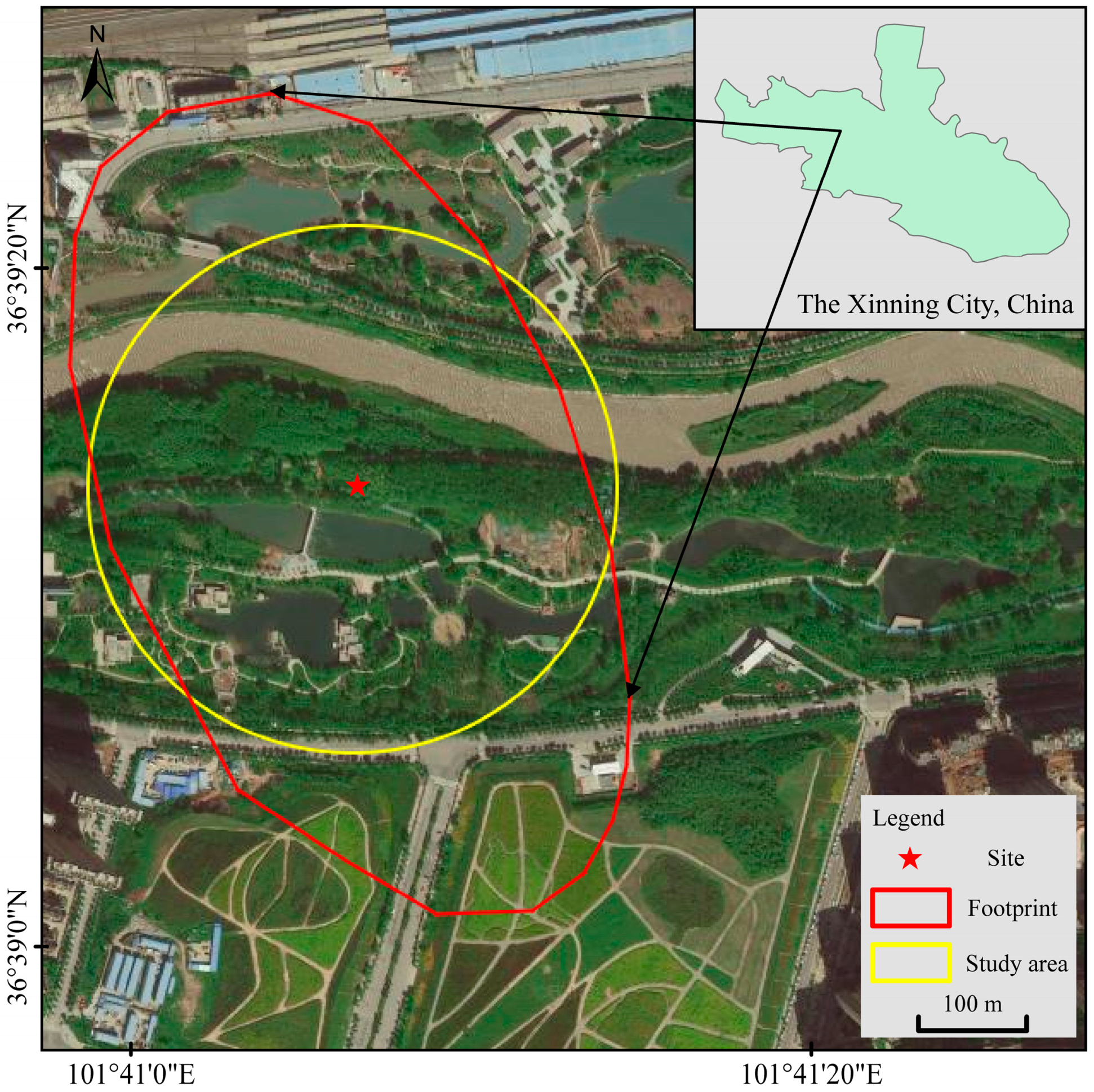
2. Study Sites
2.1. Description of the Area
2.2. Instrument Setting and Field Observation
2.3. Eddy Covariance Technology
3. Materials and Methods
3.1. Data Quality Control
3.2. Data Interpolation
3.3. Split of CO2 Flux Data
3.4. Data Statistical Analysis
4. Results
4.1. Daily Dynamic Change of CO2 Flux
4.2. Monthly Dynamic Change of CO2 Flux
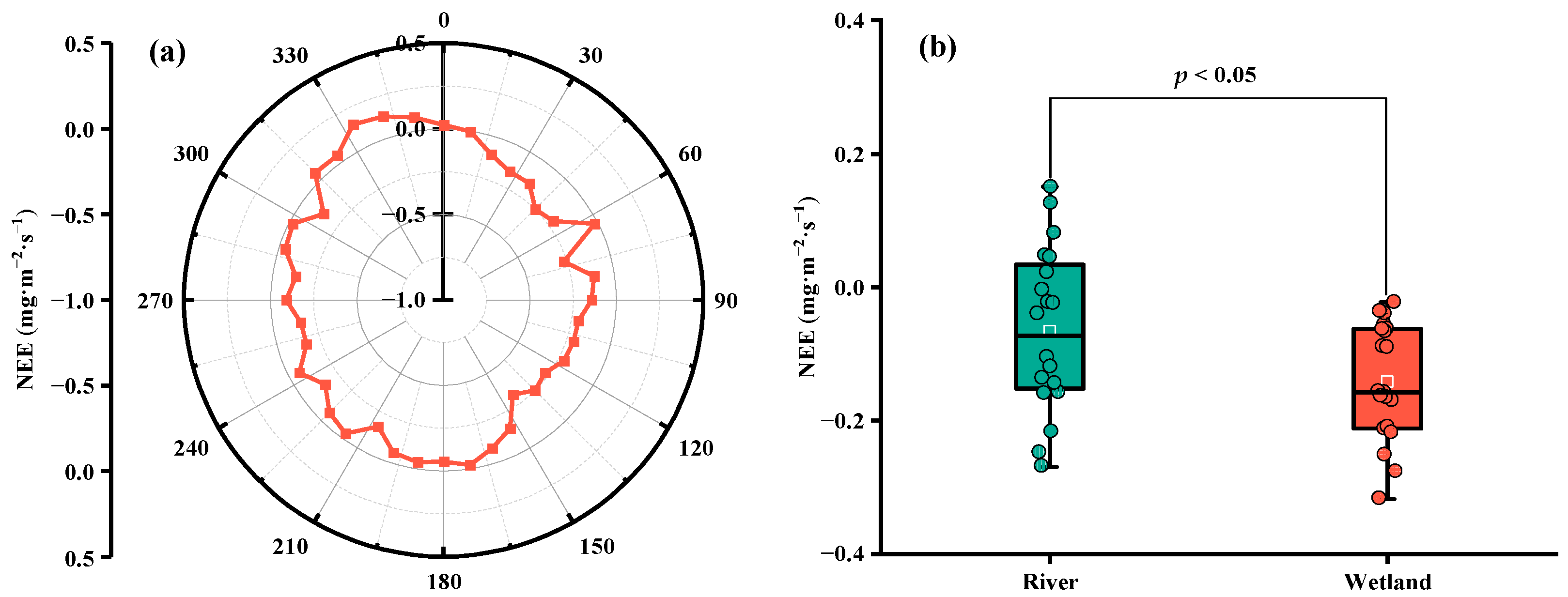
4.3. Spatial Variation of CO2 Flux
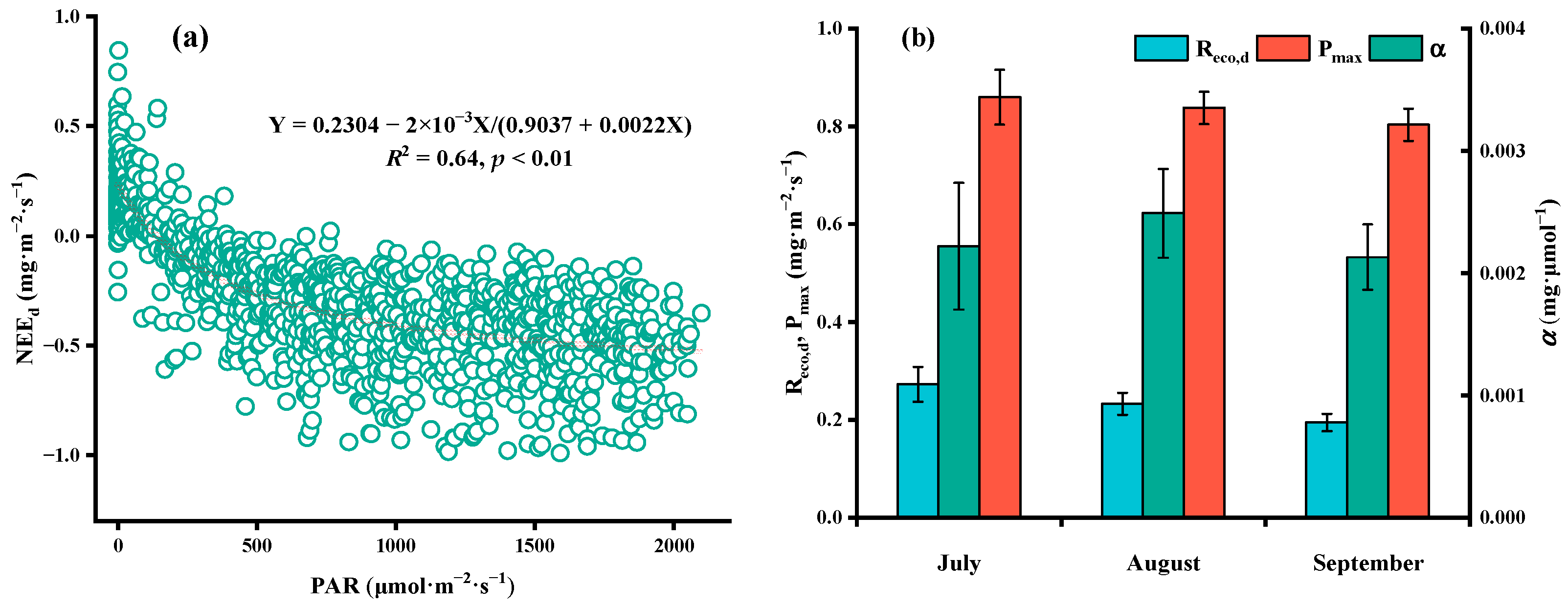
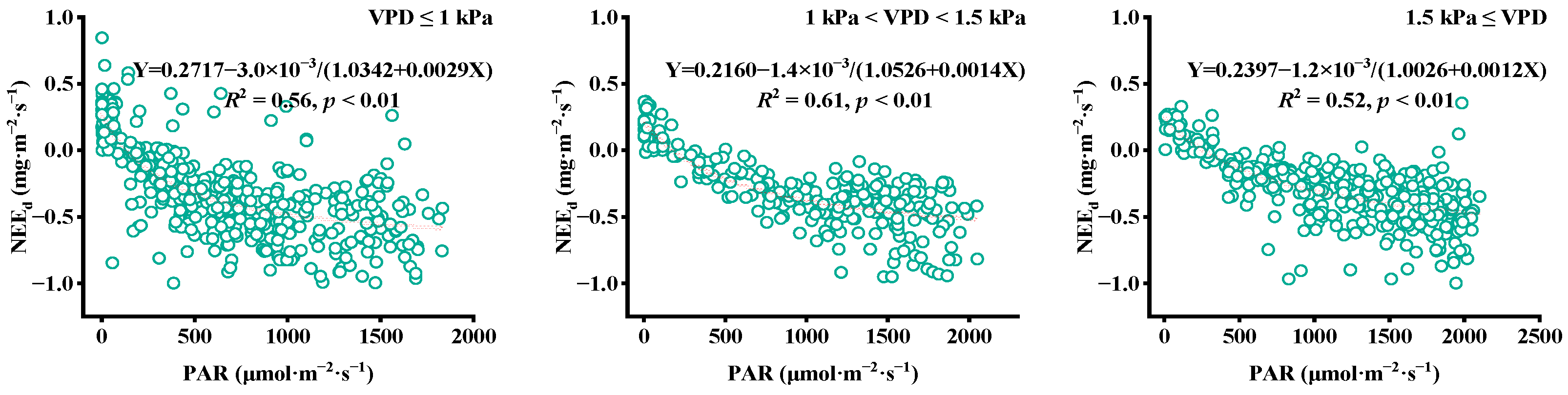
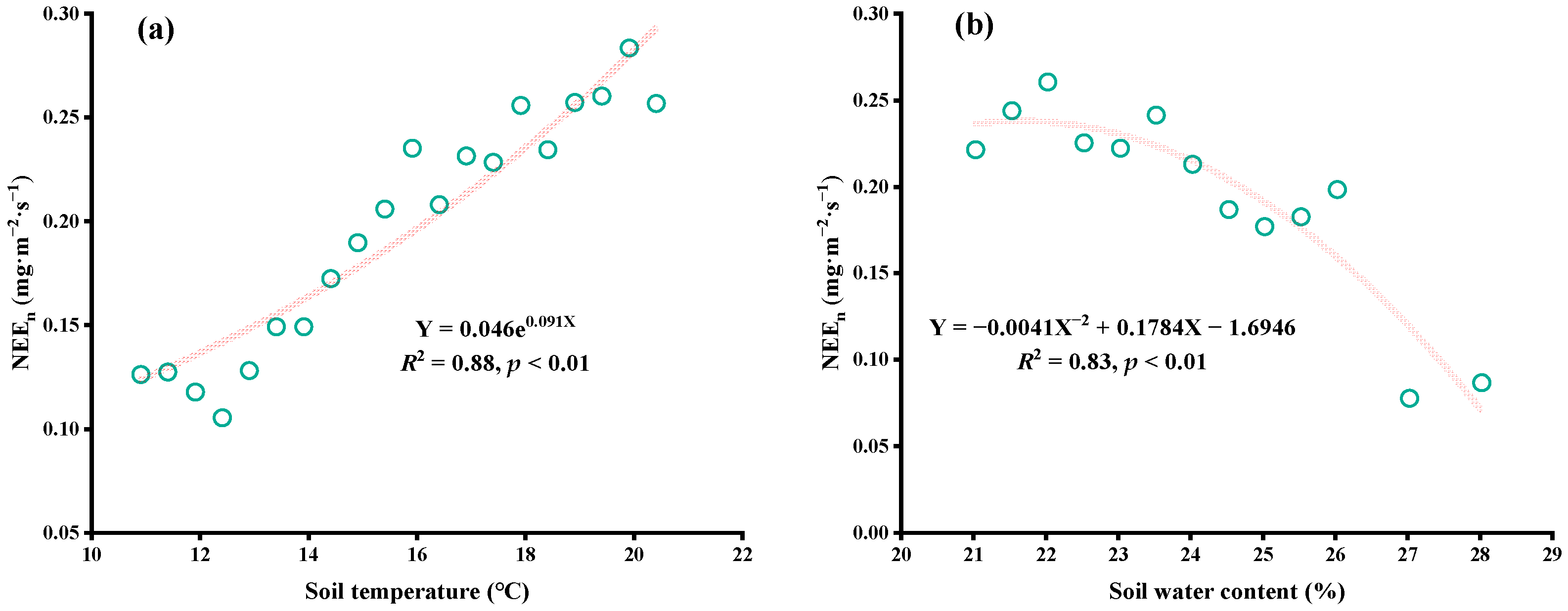
4.4. Factors Affecting CO2 Flux
5. Discussion
5.1. Factors Affecting Daytime CO2 Flux
5.2. Factors Influencing CO2 Flux at Night
5.3. Importance and Particularity of CO2 Fluxes of Plateau Urban Wetlands in Warm Seasons
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Q.; Guo, B.; Zhao, C.; Xing, B. Characteristics of CH4 and CO2 emissions and influence of water and salinity in the Yellow River delta wetland, China. Environ. Pollut. 2018, 239, 289–299. [Google Scholar] [CrossRef]
- Xiao, S.; Wang, Y.; Liu, D.; Yang, Z.; Lei, D.; Zhang, C. Diel and seasonal variation of methane and carbon dioxide fluxes at Site Guojiaba, the Three Gorges Reservoir. J. Environ. Sci. 2013, 25, 2065–2071. [Google Scholar] [CrossRef]
- Yang, D.X.; Mao, X.F.; Wei, X.Y.; Tao, Y.Q.; Zhang, Z.F.; Ma, J.H. Water-air interface greenhouse gas emissions (CO2, CH4, and N2O) emissions were amplified by continuous dams in an urban river in Qinghai–Tibet Plateau, China. Water 2020, 12, 759. [Google Scholar] [CrossRef]
- Mao, X.; Wei, X.; Engel, B.; Wei, X.; Zhang, Z.; Tao, Y.; Wang, W. Network-based perspective on water-air interface GHGs flux on a cascade surface-flow constructed wetland in Qinghai-Tibet Plateau, China. Ecol. Eng. 2020, 151, 105862. [Google Scholar] [CrossRef]
- Mao, X.; Wei, X.; Engel, B.; Wang, W.; Jin, X.; Jin, Y. Biological response to 5 years of operations of cascade rubber dams in a plateau urban river, China. River Res. Appl. 2020. [Google Scholar] [CrossRef]
- Wang, L.L.; Yang, T.; Gao, C.; Gao, D.; Lu, C.F.; Wang, Y.Q. Diurnal Variation of Net Ecosystem CO2 Exchange of Nanji Wetland Ecosystem in Poyang Lake. J. Ecol. Rural Environ. 2017, 33, 1007–1012. [Google Scholar] [CrossRef]
- Yang, Y.P.; Cao, G.Z.; Hou, P.; Jiang, W.G.; Chen, Y.H.; Li, J. Monitoring and evaluation for climate regulation service of urban wetlands with remote sensing. Geogr. Res. 2013, 32, 73–80. [Google Scholar] [CrossRef]
- Smith, R.M.; Kaushal, S.S.; Beaulieu, J.J.; Pennino, M.J.; Welty, C. Influence of infrastructure on water quality and greenhouse gas dynamics in urban streams. Biogeosciences 2017, 14, 2831–2849. [Google Scholar] [CrossRef]
- Borges, A.V.; Darchambeau, F.; Lambert, T.; Morana, C.; Allen, G.H.; Tambwe, E.; Toengaho Sembaito, A.; Mambo, T.; Nlandu Wabakhangazi, J.; Descy, J.P. Variations in dissolved greenhouse gases (CO2, CH4, N2O) in the Congo River network overwhelmingly driven by fluvial-wetland connectivity. Biogeosciences 2019, 16, 3801–3834. [Google Scholar] [CrossRef]
- Maucieri, C.; Barbera, A.C.; Vymazal, J.; Borin, M. A review on the main affecting factors of greenhouse gases emission in constructed wetlands. Agric. For. Meteorol. 2017, 236, 175–193. [Google Scholar] [CrossRef]
- Nuamah, L.A.; Li, Y.P.; Pu, Y.S.; Nwankwegu, A.S.; Haikuo, Z.; Norgbey, E.; Banahene, P.; Bofah-Buoh, R. Constructed wetlands, status, progress, and challenges. The need for critical operational reassessment for a cleaner productive ecosystem. J. Clean. Prod. 2020, 269, 122340. [Google Scholar] [CrossRef]
- Badiou, P.; Page, B.; Ross, L. A comparison of water quality and greenhouse gas emissions in constructed wetlands and conventional retention basins with and without submerged macrophyte management for storm water regulation. Ecol. Eng. 2019, 127, 292–301. [Google Scholar] [CrossRef]
- Ma, J.J.; Ullah, S.; Niu, A.; Liao, Z.N.; Qin, Q.H.; Xu, S.J.; Lin, C.X. Heavy metal pollution increases CH4 and decreases CO2 emissions due to soil microbial changes in a mangrove wetland: Microcosm experiment and field examination. Chemosphere 2021, 269, 128735. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Sardans, J.; Hou, L.J.; Liu, M.; Xu, C.B.; Peñuelas, J. Climatic temperature controls the geographical patterns of coastal marshes greenhouse gases emissions over China. J. Hydrol. 2020, 590, 125378. [Google Scholar] [CrossRef]
- Yu, G.R.; Sun, X.M. Principles of Flux Measurement in Terrestrial Ecosystems; Higher Education Press: Beijing, China, 2006; pp. 40–44. [Google Scholar]
- Li, G.D.; Zhang, J.H.; Chen, C.; Tian, H.F.; Zhao, L.P. Research progress on carbon storage and flux in different terrestrial ecosystem in China under globa climate change. Ecol. Environ. Sci. 2013, 22, 873–878. [Google Scholar] [CrossRef]
- Liang, A.L. Research of Space-Borne Remote Sensing for Carbon Dioxide on Validation, Inversion and Application. Ph.D. Thesis, WUHAN University, Wuhan, China, 2018. [Google Scholar]
- Stagakis, S.; Chrysoulakis, N.; Spyridakis, N.; Feigenwinter, C.; Vogt, R. Eddy Covariance measurements and source partitioning of CO2 emissions in an urban environment: Application for Heraklion, Greece. Atmos. Environ. 2019, 201, 278–292. [Google Scholar] [CrossRef]
- Baldocchi, D. Assessing ecosystem carbon balance: Problems and prospects of the eddy covariance technique. Glob. Chang. Biol. 2003, 9, 478–492. [Google Scholar] [CrossRef]
- Chen, S.P.; You, C.H.; Hu, Z.M.; Chen, Z.; Zhang, L.M.; Wang, Q.F. Eddy covariance technique and its applications in flux observations of terrestrial ecosystems. Chin. J. Plant Ecol. 2020, 44, 291–304. [Google Scholar] [CrossRef]
- Richardson, A.D.; Hollinger, D.Y.; Shoemaker, J.K.; Hughes, H.; Savage, K.; Davidson, E.A. Six years of ecosystem-atmosphere greenhouse gas fluxes measured in a sub-boreal forest. Sci. Data 2019, 6, 117. [Google Scholar] [CrossRef]
- Liu, W.T.; Yao, X.L.; Xue, J.Y.; Zhao, Z.H.; Zhang, L.; Wang, X.L.; Cai, Y.J. Seasonal and Spatial Variations in Greenhouse Gases(CH4 and CO2) Emission Along the Riparian Zone of Middle and Lower Reaches of Yangtze River. Resour. Environ. Yangtze Basin 2019, 28, 2718–2726. [Google Scholar] [CrossRef]
- Wei, S.; Han, G.; Jia, X.; Song, W.; Chu, X.; He, W.; Xia, J.; Wu, H. Tidal effects on ecosystem CO2 exchange at multiple timescales in a salt marsh in the Yellow River Delta. Estuarine, Coast. Shelf Sci. 2020, 238, 106727. [Google Scholar] [CrossRef]
- Attermeyer, K.; Flury, S.; Jayakumar, R.; Fiener, P.; Steger, K.; Arya, V.; Wilken, F.; van Geldern, R.; Premke, K. Invasive floating macrophytes reduce greenhouse gas emissions from a small tropical lake. Sci. Rep. 2016, 6, 20424. [Google Scholar] [CrossRef]
- Immerzeel, W.W.; van Beek, L.P.H.; Bierkens, M.F.P. Climate Change Will Affect the Asian Water Towers. Science 2010, 328, 1382–1385. [Google Scholar] [CrossRef]
- Lang, Q.; Niu, Z.G.; Hong, X.Q.; Yang, X.Y. Remote Sensing Monitoring and Change Analysis of Wetlands in the Tibetan Plateau. Geomat. Inf. Sci. Wuhan Univ. 2021, 46, 230–237. [Google Scholar] [CrossRef]
- Meng, W.Q.; He, M.X.; Hu, B.B.; Mo, X.Q.; Li, H.Y.; Liu, B.Q.; Wang, Z.L. Status of wetlands in China: A review of extent, degradation, issues and recommendations for improvement. Ocean Coast. Manag. 2017, 146, 50–59. [Google Scholar] [CrossRef]
- Mao, X.F.; Wei, X.Y.; Chen, Q.; Liu, F.G.; Tao, Y.Q.; Zhang, Z.F. An ECPS framework based assessment on wetland restoration of the Huangshui National Wetland Park in Qinghai Province, China. Geogr. Res. 2019, 38, 760–771. [Google Scholar] [CrossRef]
- Kljun, N.; Calanca, P.; Rotach, M.W.; Schmid, H.P. A simple two-dimensional parameterisation for Flux Footprint Prediction (FFP). Geosci. Model Dev. 2015, 8, 3695–3713. [Google Scholar] [CrossRef]
- Veenendaal, E.; Kolle, O.; Lloyd, J. Seasonal variation in energy fluxes and carbon dioxide exchange for a boad-leaved semi-arid savanna (Mopane Woodland) in Southern Africa. Glob. Chang. Biol. 2003, 10, 318–328. [Google Scholar] [CrossRef]
- McDermitt, D.; Burba, G.; Xu, L.; Anderson, T.; Komissarov, A.; Riensche, B.; Schedlbauer, J.; Starr, G.; Zona, D.; Oechel, W. A new low-power, open-path instrument for measuring methane flux by eddy covariance. Appl. Phys. B Lasers Opt. 2011, 102, 391–405. [Google Scholar] [CrossRef]
- Mahrt, L.; Vickers, D. Relationship of area-averaged carbon dioxide and water vapour fluxes to atmospheric variables. Agric. For. Meteorol. 2002, 112, 195–202. [Google Scholar] [CrossRef]
- Webb, E.K.; Pearman, G.I.; Leuning, R. Correction of flux measurements for density effects due to heat and water-vapor transfer. Q. J. R. Meteorol. Soc. 1980, 106, 85–100. [Google Scholar] [CrossRef]
- Liu, Y.P.; Li, S.S.; Lü, S.H.; Ao, Y.H.; Gao, Y.H. Comparison of Flux Correction Methods for Eddy-Covariance Measurement. Plateau Meteorol. 2013, 32, 1704–1711. [Google Scholar] [CrossRef]
- Falge, E.; Baldocchi, D.; Olson, R.; Anthoni, P.; Aubinet, M.; Bernhofer, C.; Burba, G.; Ceulemans, R.; Clement, R.; Dolman, H. Gap filling strategies for defensible annual sums of net ecosystem exchange. Agric. For. Meteorol. 2001, 107, 43–69. [Google Scholar] [CrossRef]
- Ruimy, A.; Jarvis, P.G.; Baldocchi, D.D.; Saugier, B. CO2 Fluxes over Plant Canopies and Solar Radiation: A Review. Advances in Ecological Research. 1995, 26, 1–68. [Google Scholar] [CrossRef]
- Lloyd, J.; Taylor, J.A. On the Temperature Dependence of Soil Respiration. Funct. Ecol. 1994, 8, 315–323. [Google Scholar] [CrossRef]
- Yang, J.F.; Yang, X.N.; Wang, J.H.; Duan, Y.M.; Qi, X.N.; Zhang, L.S. Characteristics of CO2 Flux in a Mature Apple (Malus demestica) Orchard Ecosystem on the Loess Plate. Environ. Sci. 2018, 39, 2339–2350. [Google Scholar] [CrossRef]
- Hinojo-Hinojo, C.; Castellanos, A.E.; Rodriguez, J.C.; Delgado-Balbuena, J.; Romo-León, J.R.; Celaya-Michel, H.; Huxman, T.E. Carbon and Water Fluxes in an Exotic Buffelgrass Savanna. Rangel. Ecol. Manag. 2016, 69, 334–341. [Google Scholar] [CrossRef]
- Li, Z.H.; Luo, W.J.; Du, H.; Song, T.Q.; Peng, H.J.; Wang, Y.W.; Wang, S.J. CO2 Flux and Its Driving Factors in a Karst Evergreen Deciduous Broadleaf Mixed Forest in Dry Season. Earth Environ. 2020, 48, 525–536. [Google Scholar] [CrossRef]
- Zhao, H.C.; Jia, G.S.; Wang, H.S.; Zhang, A.Z.; Xu, X.Y. Diurnal Variations of the Carbon Fluxes of Semiarid Meadow Steppe and Typical Steppe in China. Clim. Environ. Res. 2020, 25, 172–184. [Google Scholar] [CrossRef]
- Anthoni, P.M.; Law, B.E.; Unsworth, M.H. Carbon and water vapor exchange of an open-canopied ponderosa pine ecosystem. Agric. For. Meteorol. 1999, 95, 151–168. [Google Scholar] [CrossRef]
- Tan, L.P.; Liu, S.X.; Mo, X.G.; Yang, L.H.; Lin, Z.H. Environmental controls over energy, water and carbon fluxes in a plantation in Northern China. Chin. J. Plant Ecol. 2015, 39, 773–784. [Google Scholar] [CrossRef]
- Jarvis, P.; Morison, J. The Control of Transpiration and Photosynthesis by the Stomata; Cambridge University Press: Cambridge, UK, 1981; Volume 8, pp. 247–279. [Google Scholar]
- Huxman, T.E.; Turnipseed, A.A.; Sparks, J.P.; Harley, P.C.; Monson, R.K. Temperature as a control over ecosystem CO2 fluxes in a high-elevation, subalpine forest. Oecologia 2003, 134, 537–546. [Google Scholar] [CrossRef]
- Luo, Q.; Wen, J.; Wang, X.; Tian, H.; Wang, Z.L. Analysis of the Diurnal Characteristics of Water and Heat & CO2 Exchanges at the Alpine Wetland in the Source Region of the Yellow River. Plateau Meteorol. 2017, 36, 667–674. [Google Scholar] [CrossRef]
- Wu, F.T.; Cao, S.K.; Cao, G.C.; Han, G.Z.; Lin, Y.Y.; Cheng, S.Y. Variation of CO2 Flux of Alpine Wetland Ecosystem of Kobresia tibetica Wet Meadow in Lake Qinghai. J. Ecol. Rural Environ. 2018, 34, 124–131. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A.; Luo, Y.Q. On the variability of respiration in terrestrial ecosystems: Moving beyond Q10. Glob. Chang. Biol. 2006, 12, 154–164. [Google Scholar] [CrossRef]
- Zhan, X.Y.; Yu, G.R.; Zheng, Z.M.; Wang, Q.F. Carbon Emission and Spatial Pattern of Soil Respiration of Terrestrial Ecosystems in China: Based on Geostatistic Estimation of Flux Measurement. Prog. Geogr. 2012, 31, 97–108. [Google Scholar] [CrossRef]
- Wang, H.B.; Ma, M.G.; Wang, X.F.; Tan, J.L.; Gen, L.Y.; Yu, W.P.; Jia, S.Z. Carbon flux variation characteristics and its influencing factors in an alpine meadow ecosystem on eastern Qinghai-Tibetan plateau. J. Arid Land Resour. Environ. 2014, 28, 50–56. [Google Scholar] [CrossRef]
- Xing, Q.H.; Han, G.X.; Yu, J.B.; Wu, L.X.; Qiong, Y.L.; Mao, P.L.; Wang, G.M.; Xie, B.H. Net ecosystem CO2 exchange and its controlling factors during the growing season in an inter-tidal salt marsh in the Yellow River Estuary, China. Acta Ecol. Sin. 2014, 34, 4966–4979. [Google Scholar] [CrossRef]
- Peng, F.J.; Ge, J.W.; Li, Y.Y.; Li, J.Q.; Zhou, Y.; Zhang, Z.Q. Characteristics of CO2 Flux and Their Effect Factors in Dajiuhu Peat Wetland of Shennongjia. Ecol. Environ. Sci. 2017, 26, 453–460. [Google Scholar] [CrossRef]
- Špunda, V.; Kalina, J.; Urban, O.; Luis, V.C.; Sibisse, I.; Puértolas, J.; Šprtová, M.; Marek, M.V. Diurnal dynamics of photosynthetic parameters of Norway spruce trees cultivated under ambient and elevated CO2: The reasons of midday depression in CO2 assimilation. Plant Sci. 2005, 168, 1371–1381. [Google Scholar] [CrossRef]
- Chen, D.L.; Xu, B.Q.; Yao, T.D.; Guo, Z.T.; Cui, P.; Chen, F.H.; Zhang, R.H.; Zhang, X.Z.; Zhang, Y.L.; Fan, J. Assessment of past, present and future environmental changes on the Tibetan Plateau. Chin. Sci. Bull. 2015, 60, 3025–3035. [Google Scholar] [CrossRef]
- Li, Y.; Kang, X.M.; Hao, Y.B.; Ding, K.; Wang, Y.F.; Cui, X.Y.; Mei, X.R. Carbon, water and heat fluxes of a reed (Phragmites australis) wetland in the Yellow River Delta, China. Acta Ecol. Sin. 2014, 34, 4400–4411. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, X.H.; Wang, S.Y.; Shang, L.Y.; Zhang, Y. Analysis of the near-surface micrometeorology and CO2 flux over the Maqu alpine meadow in summer. J. Glaciol. Geocryol. 2019, 41, 54–63. [Google Scholar] [CrossRef]
- D′Acunha, B.; Morillas, L.; Black, T.A.; Christen, A.; Johnson, M.S. Net Ecosystem Carbon Balance of a Peat Bog Undergoing Restoration: Integrating CO2 and CH4 Fluxes From Eddy Covariance and Aquatic Evasion With DOC Drainage Fluxes. J. Geophys. Res. Biogeosci. 2019, 124, 884–901. [Google Scholar] [CrossRef]
- Li, X.L.; Jia, Q.Y.; Liu, J.M. Seasonal variations in heat and carbon dioxide fluxes observed over a reed wetland in northeast China. Atmos. Environ. 2016, 127, 6–13. [Google Scholar] [CrossRef]
- Griffis, T.J.; Roman, D.T.; Wood, J.D.; Deventer, J.; Fachin, L.; Rengifo, J.; Del Castillo, D.; Lilleskov, E.; Kolka, R.; Chimner, R.A. Hydrometeorological sensitivities of net ecosystem carbon dioxide and methane exchange of an Amazonian palm swamp peatland. Agric. For. Meteorol. 2020, 295, 108167. [Google Scholar] [CrossRef]
- Zhang, L.W.; Xia, X.H.; Liu, S.D.; Zhang, S.B.; Li, S.L.; Wang, J.F.; Wang, G.Q.; Gao, H.; Zhang, Z.R.; Wang, Q.R. Significant methane ebullition from alpine permafrost rivers on the East Qinghai-Tibet Plateau. Nat. Geosci. 2020, 13, 349–354. [Google Scholar] [CrossRef]
- Marescaux, A.; Thieu, V.; Garnier, J. Carbon dioxide, methane and nitrous oxide emissions from the human-impacted Seine watershed in France. Sci. Total Environ. 2018, 643, 247–259. [Google Scholar] [CrossRef]
- Yao, T.D. A comprehensive study of Water-Ecosystem-Human activities reveals unbalancing Asian Water Tower and accompanying potential risks. Chin. Sci. Bull. 2019, 64, 2761–2762. [Google Scholar] [CrossRef]
- Zhu, L.P.; Ju, J.T.; Qiao, B.J.; Yang, R.M.; Liu, C.; Han, B.P. Recent lake changes of the Asia Water Tower and their climate response: Progress, problems and prospects. Chin. Sci. Bull. 2019, 64, 2796–2806. [Google Scholar] [CrossRef]
- Davidson, T.A.; Audet, J.; Jeppesen, E.; Landkildehus, F.; Lauridsen, T.L.; Søndergaard, M.; Syväranta, J. Synergy between nutrients and warming enhances methane ebullition from experimental lakes. Nat. Clim. Chang. 2018, 8, 156–160. [Google Scholar] [CrossRef]

| Month | GPP Flux mg·m−2·s−1 | Reco Flux mg·m−2·s−1 | NEE Flux mg·m−2·s−1 | CO2 Budget g·m−2·month−1 |
|---|---|---|---|---|
| 7 | 0.30 ± 0.39 a | 0.26 ± 0.06 a | −0.04 ± 0.36 a | −102.05 |
| 8 | 0.25 ± 0.36 a | 0.21 ± 0.06 b | −0.05 ± 0.34 a | −120.53 |
| 9 | 0.22 ± 0.41 b | 0.17 ± 0.29 c | −0.06 ± 0.29 a | −142.82 |
| Time | PAR | Tsoil | Tair | VPD | SWC |
|---|---|---|---|---|---|
| Daytime | −0.446 ** | 0.140 | 0.098 ** | 0.186 ** | −0.064 |
| Night | −0.032 | 0.213 ** | 0.097 | 0.114 | −0.231 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Mao, X.; Zhang, Z.; Tang, W.; Cao, G.; Zhou, H.; Ma, J.; Yin, X. Temporal and Spatial Characteristics of CO2 Flux in Plateau Urban Wetlands and Their Influencing Factors Based on Eddy Covariance Technique. Water 2021, 13, 1176. https://doi.org/10.3390/w13091176
Wu Y, Mao X, Zhang Z, Tang W, Cao G, Zhou H, Ma J, Yin X. Temporal and Spatial Characteristics of CO2 Flux in Plateau Urban Wetlands and Their Influencing Factors Based on Eddy Covariance Technique. Water. 2021; 13(9):1176. https://doi.org/10.3390/w13091176
Chicago/Turabian StyleWu, Yi, Xufeng Mao, Zhifa Zhang, Wenjia Tang, Guangchao Cao, Huakun Zhou, Jianhai Ma, and Xinan Yin. 2021. "Temporal and Spatial Characteristics of CO2 Flux in Plateau Urban Wetlands and Their Influencing Factors Based on Eddy Covariance Technique" Water 13, no. 9: 1176. https://doi.org/10.3390/w13091176
APA StyleWu, Y., Mao, X., Zhang, Z., Tang, W., Cao, G., Zhou, H., Ma, J., & Yin, X. (2021). Temporal and Spatial Characteristics of CO2 Flux in Plateau Urban Wetlands and Their Influencing Factors Based on Eddy Covariance Technique. Water, 13(9), 1176. https://doi.org/10.3390/w13091176









