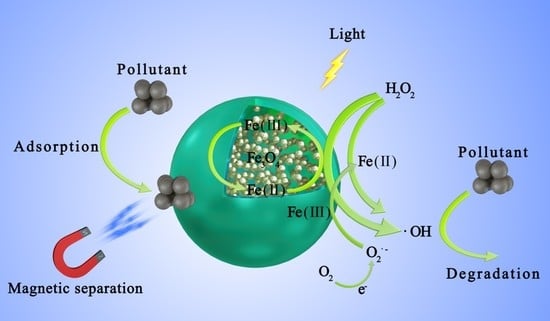
Enhanced Photo–Fenton Removal Efficiency with Core-Shell Magnetic Resin Catalyst for Textile Dyeing Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
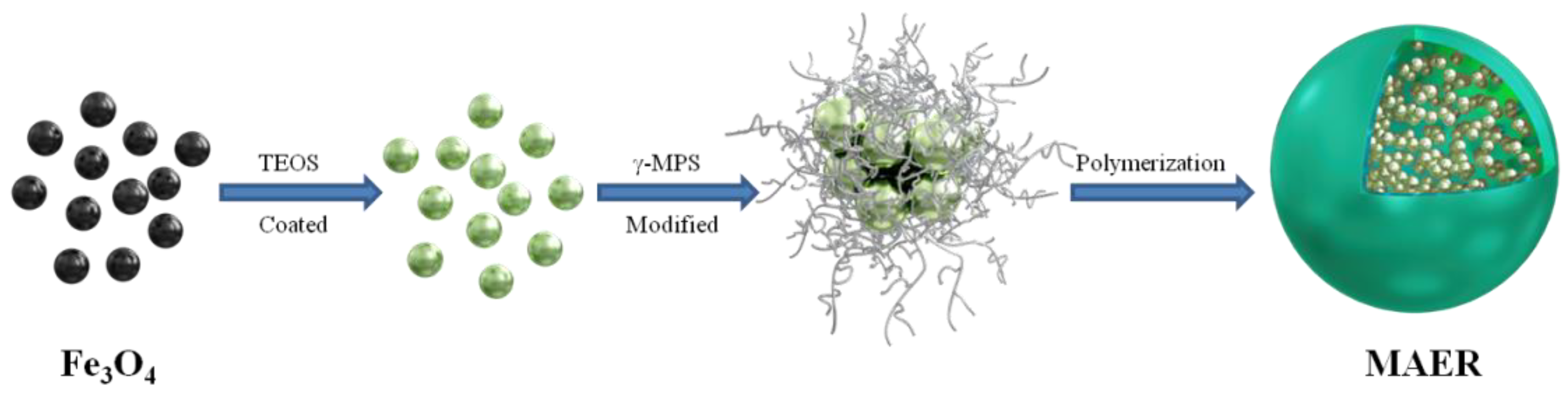
2.2. Preparation of Fe3O4 Nanoparticles
2.3. Preparation of MAER
2.4. Removal Experiments
2.5. Material Characterizations and Chemical Analysis
3. Results and Discussion
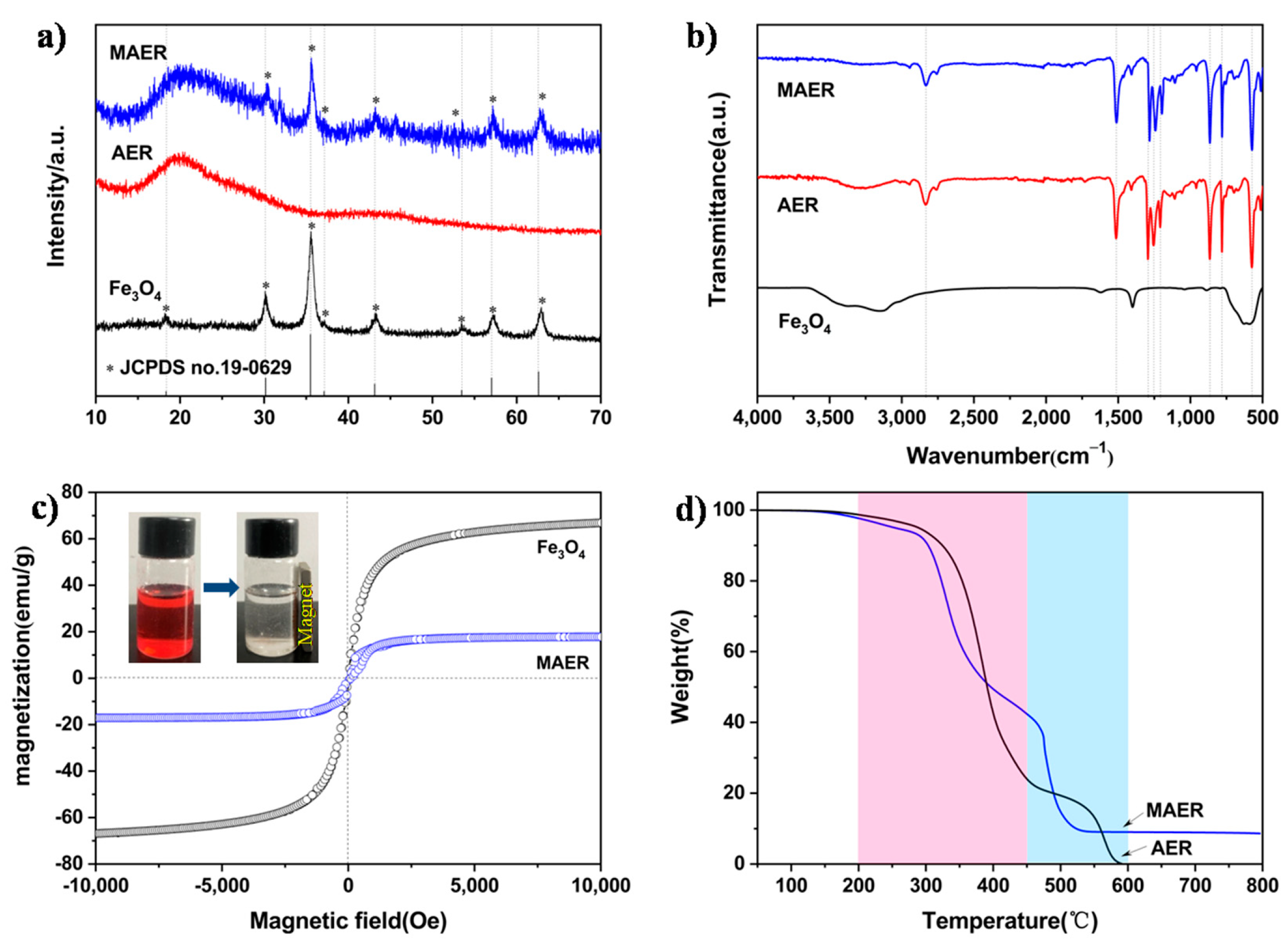
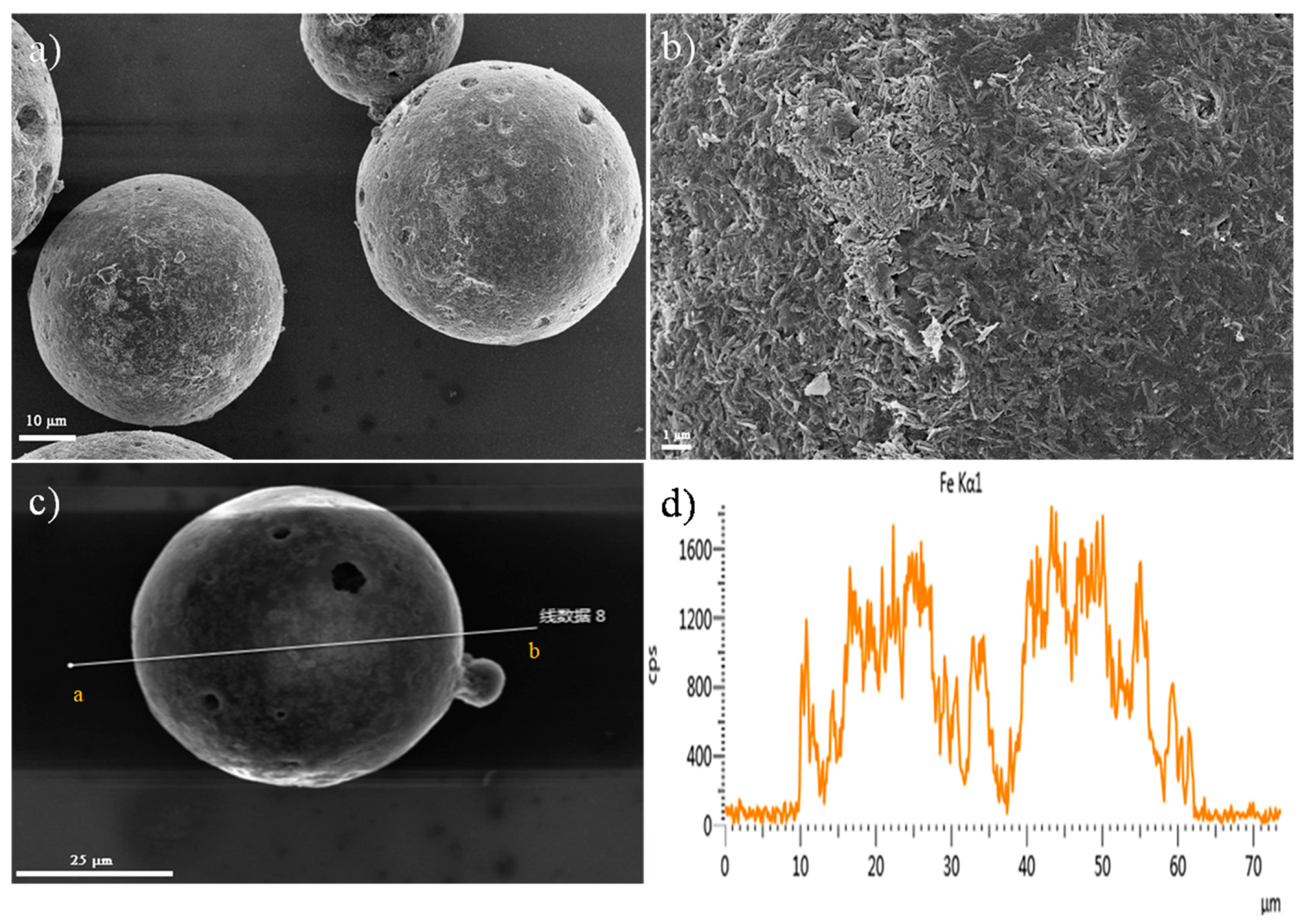
3.1. Characterization of the Prepared Materials
3.2. Adsorption of X-3B
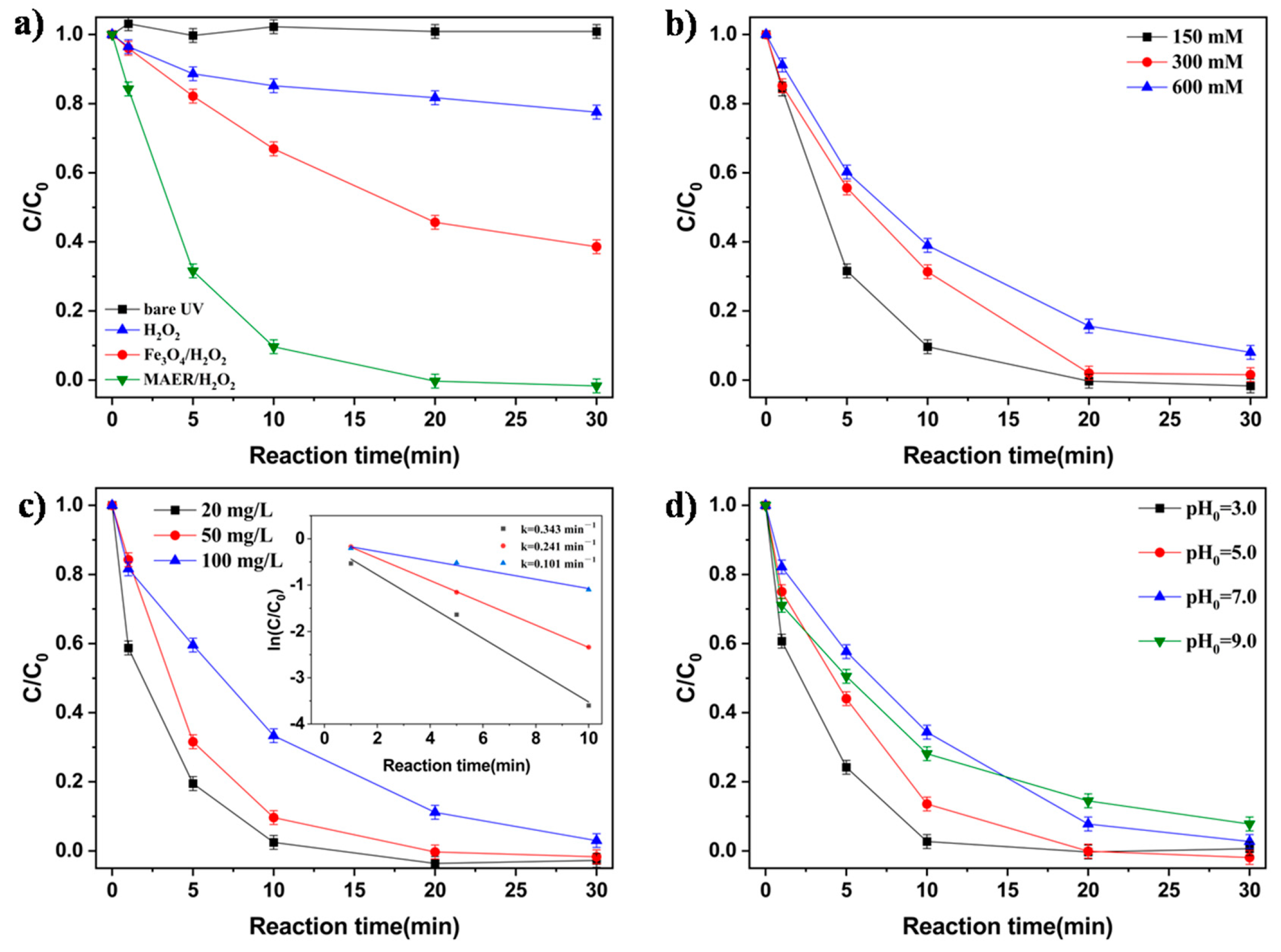
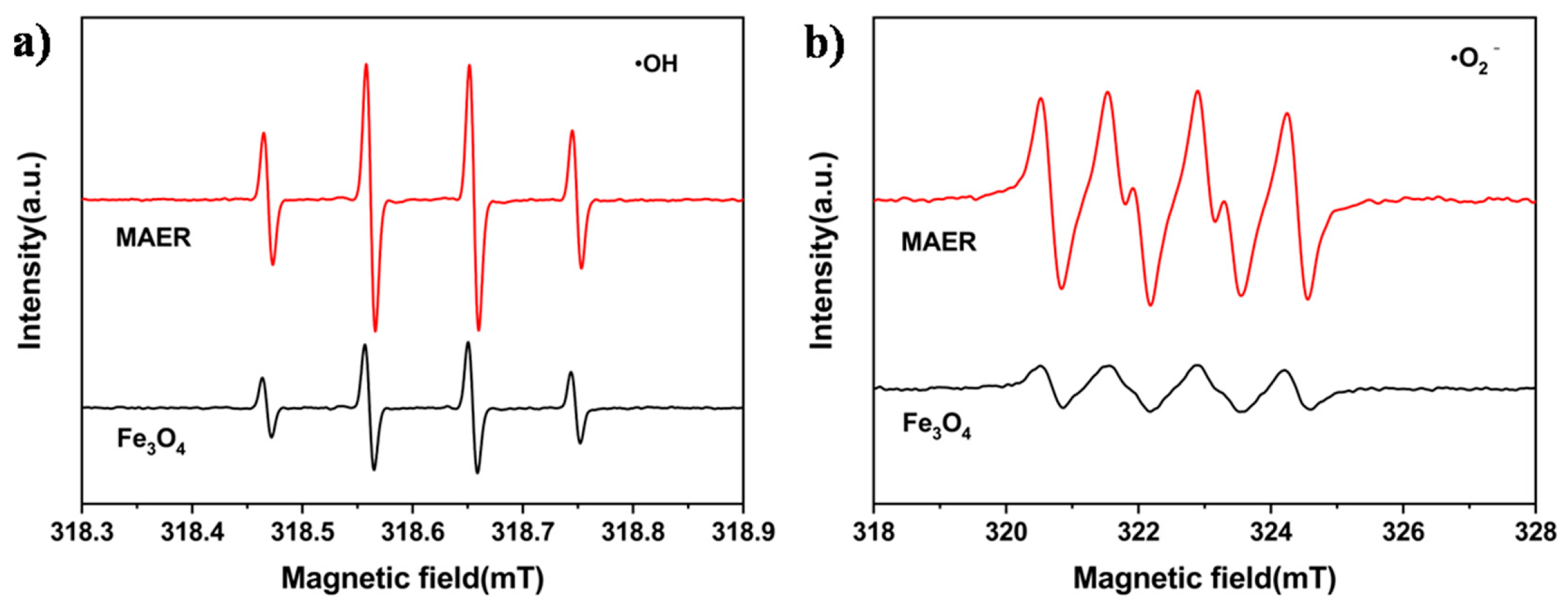
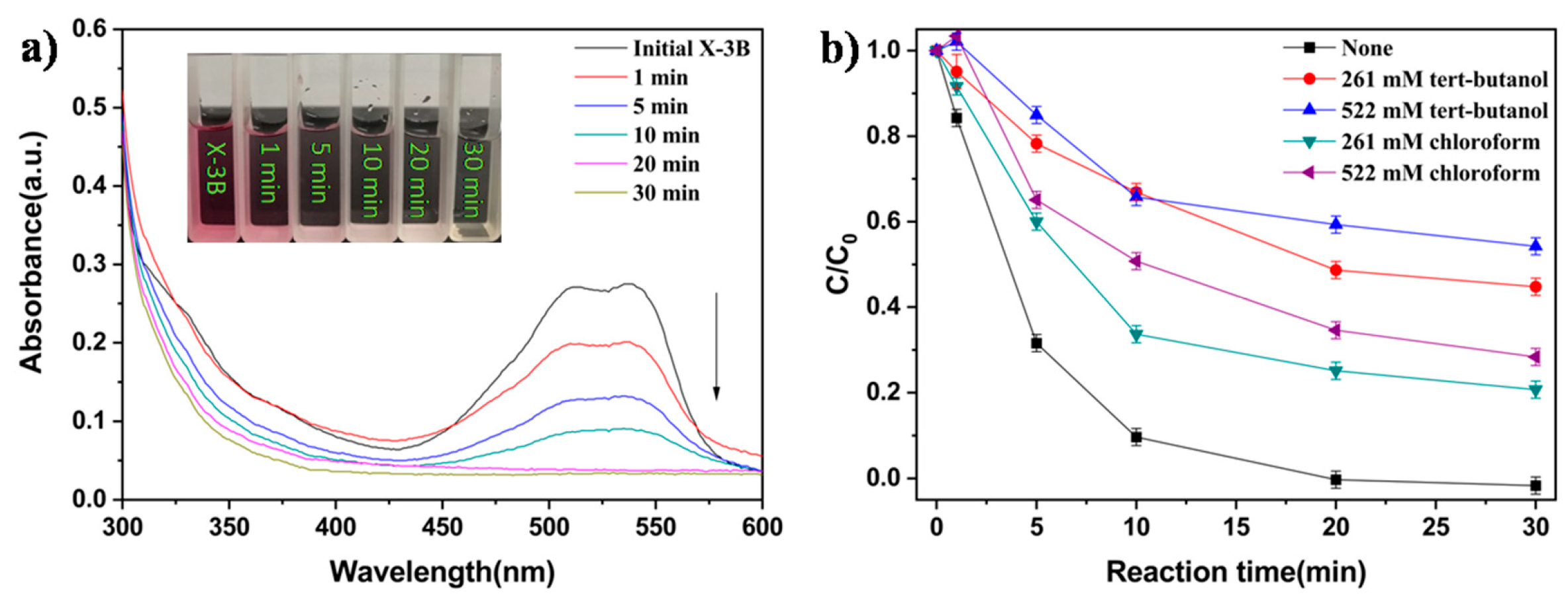
3.3. Removal of X-3B in a UV–Fenton System
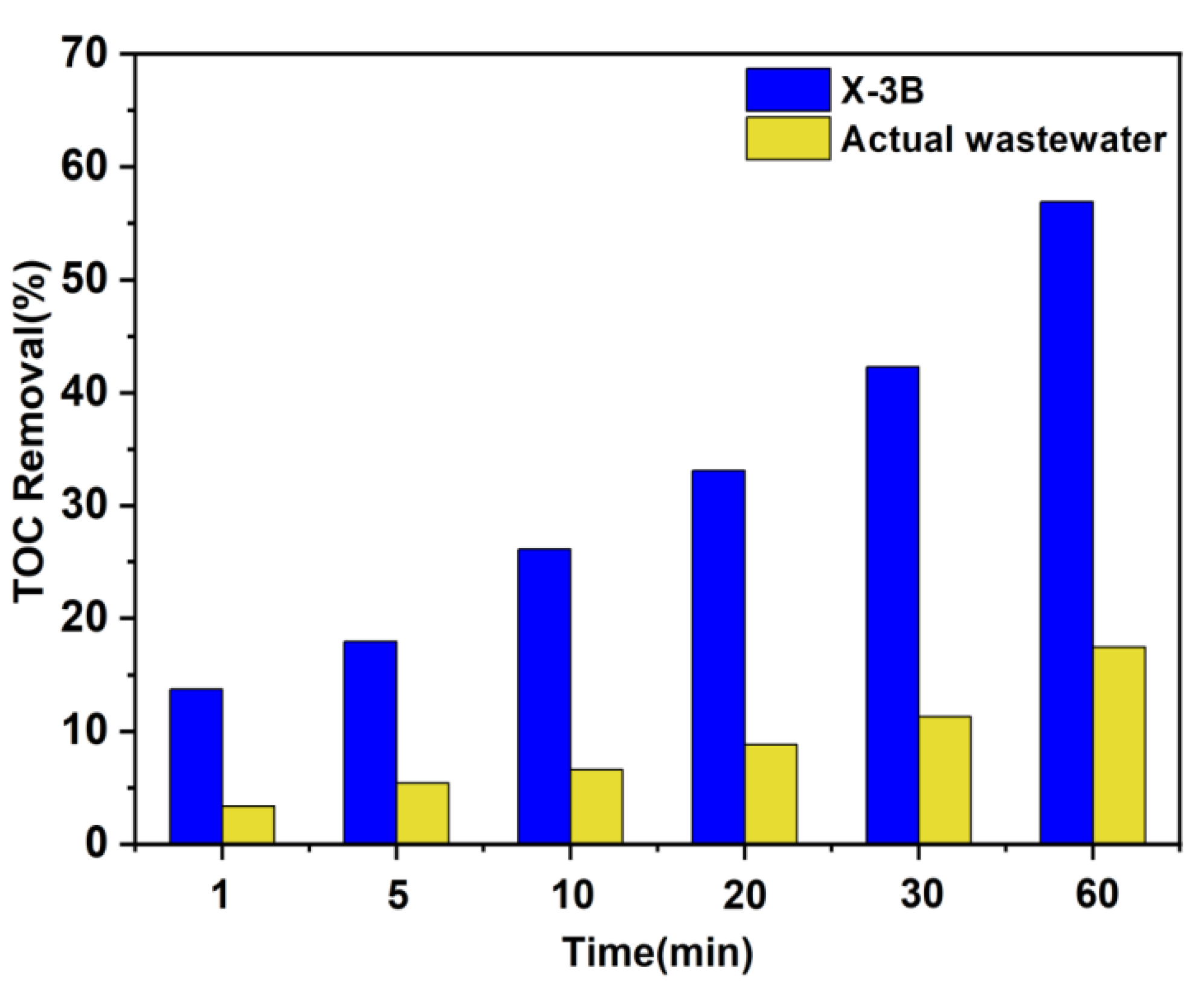
3.4. Practical Application for Removal of Textile Dyeing Wastewater
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, S.; Li, N.; Yuan, D.; Tang, J.; Li, X.; Zhang, C.; Rao, Y. Comparative study of persulfate oxidants promoted photocatalytic fuel cell performance: Simultaneous dye removal and electricity generation. Chemosphere 2019, 234, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.G.; Kumar, S.G.; Reddy, K.M.; Munikrishnappa, C. Photo degradation of Methyl Orange an azo dye by Advanced Fenton Process using zero valent metallic iron: Influence of various reaction parameters and its degradation mechanism. J. Hazard. Mater. 2009, 164, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Guo, J.; Zhou, G.; Wan, X.; Shi, H. Photodegradation of Orange II using waste paper sludge-derived heterogeneous catalyst in the presence of oxalate under ultraviolet light emitting diode irradiation. J. Environ. Sci. 2016, 47, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, X.; Pan, F.; Qin, Y.; Xia, J.; Li, J.; Wu, F. Synthesis of the mesoporous carbon-nano-zero-valent iron composite and activation of sulfite for removal of organic pollutants. Chem. Eng. J. 2018, 353, 542–549. [Google Scholar] [CrossRef]
- Yang, B.; Ying, G.G. Oxidation of benzophenone-3 during water treatment with ferrate (VI). Water Res. 2013, 47, 2458–2466. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, L.; Hu, C.; Wang, X.; Lyu, L.; Sheng, G. Enhanced degradation of organic pollutants over Cu-doped LaAlO3 perovskite through heterogeneous Fenton-like reactions. Chem. Eng. J. 2018, 332, 572–581. [Google Scholar] [CrossRef]
- Dodd, M.C.; Buffle, M.O.; Von Gunten, U. Oxidation of antibacterial molecules by aqueous ozone: Moiety-specific reaction kinetics and application to ozone-based wastewater treatment. Environ. Sci. Technol. 2006, 40, 1969–1977. [Google Scholar] [CrossRef] [PubMed]
- Samy, M.; Ibrahim, M.G.; Alalm, M.G.; Fujii, M.; Ookawara, S.; Ohno, T. Photocatalytic degradation of trimethoprim using S-TiO2 and Ru/WO3/ZrO2 immobilized on reusable fixed plates. J. Water Process Eng. 2020, 33, 101023. [Google Scholar] [CrossRef]
- Michael, I.; Hapeshi, E.; Osorio, V.; Perez, S.; Petrovic, M.; Zapata, A.; Malato, S.; Fatta-Kassinos, D. Solar photocatalytic treatment of trimethoprim in four environmental matrices at a pilot scale: Transformation products and ecotoxicity evaluation. Sci. Total Environ. 2012, 430, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Peng, Z.; Wang, L.; Yu, Z.; Huang, L.; Sun, L.; Huang, J. Efficient degradation of tetrabromobisphenol A via electrochemical sequential reduction-oxidation: Degradation efficiency, intermediates, and pathway. J. Hazard. Mater. 2018, 343, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Zhang, C.; Tang, S.; Li, X.; Tang, J.; Rao, Y.; Wang, Z.; Zhang, Q. Enhancing CaO2 fenton-like process by Fe(II)-oxalic acid complexation for organic wastewater treatment. Water Res. 2019, 163, 114861. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.S.; Gurol, M.D. Catalytic decomposition of hydrogen peroxide on iron oxide: Kinetics, mechanism, and implications. Environ. Sci. Technol. 1998, 32, 1417–1423. [Google Scholar] [CrossRef]
- Engelmann, M.D.; Bobier, R.T.; Hiatt, T.; Cheng, I.F. Variability of the Fenton reaction characteristics of the EDTA, DTPA, and citrate complexes of iron. Biometals 2003, 16, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Su, H.; Zhu, Y.; Molamahmood, H.V.; Long, M. CaO2 based Fenton-like reaction at neutral pH: Accelerated reduction of ferric species and production of superoxide radicals. Water Res. 2018, 145, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Samy, M.; Ibrahim, M.G.; Alalm, M.G.; Fujii, M. MIL-53(Al)/ZnO coated plates with high photocatalytic activity for extended degradation of trimethoprim via novel photocatalytic reactor. Sep. Purif. Technol. 2020, 249, 117173. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, L.; Yang, Z.; Fan, Y.; Shih, K.; Li, H.; Liu, Y.; Wu, D. Nonradical degradation of microorganic pollutants by magnetic N-doped graphitic carbon: A complement to the unactivated peroxymonosulfate. Chem. Eng. J. 2020, 392, 123724. [Google Scholar] [CrossRef]
- Xu, H.Y.; Li, B.; Shi, T.N.; Wang, Y.; Komarneni, S. Nanoparticles of magnetite anchored onto few-layer graphene: A highly efficient Fenton-like nanocomposite catalyst. J. Colloid Interf. Sci. 2018, 532, 161–170. [Google Scholar] [CrossRef]
- Hu, X.; Liu, B.; Deng, Y.; Chen, H.; Luo, S.; Sun, C.; Yang, P.; Yang, S. Adsorption and heterogeneous Fenton degradation of 17α-methyltestosterone on nano Fe3O4/MWCNTs in aqueous solution. Appl. Catal. B Environ. 2011, 107, 274–283. [Google Scholar] [CrossRef]
- Gong, Q.; Liu, Y.; Dang, Z. Core-shell structured Fe3O4@GO@MIL-100(Fe) magnetic nanoparticles as heterogeneous photo-Fenton catalyst for 2, 4-dichlorophenol degradation under visible light. J. Hazard. Mater. 2019, 371, 677–686. [Google Scholar] [CrossRef]
- Shuang, C.; Li, P.; Li, A.; Zhou, Q.; Zhang, M.; Zhou, Y. Quaternized magnetic microspheres for the efficient removal of reactive dyes. Water Res. 2012, 46, 4417–4426. [Google Scholar] [CrossRef]
- Lyu, Y.; Kim, J.H.; Gu, X. Developing methodology for service life prediction of PV materials: Quantitative effects of light intensity and wavelength on discoloration of a glass/EVA/PPE laminate. Sol. Energy 2018, 174, 515–526. [Google Scholar] [CrossRef]
- Davarpanah, M.; Ahmadpour, A.; Bastami, T.R. Preparation and characterization of anion exchange resin decorated with magnetite nanoparticles for removal of p-toluic acid from aqueous solution. J. Magn. Magn. Mater. 2015, 375, 177–183. [Google Scholar] [CrossRef]
- Tan, L.; Xu, J.; Xue, X.; Lou, Z.; Zhu, J.; Baig, S.A.; Xu, X. Multifunctional nanocomposite Fe3O4@SiO2–mPD/SP for selective removal of Pb(Ⅱ) and Cr(Ⅵ) from aqueous solutions. RSC Adv. 2014, 4, 45920–45929. [Google Scholar] [CrossRef]
- Peng, S.; Zhang, W.; He, J.; Yang, X.; Wang, D.; Zeng, G. Enhancement of Fenton oxidation for removing organic matter from hypersaline solution by accelerating ferric system with hydroxylamine hydrochloride and benzoquinone. J. Environ. Sci. 2016, 41, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Al-Anazi, A.; Abdelraheem, W.H.; Scheckel, K.; Nadagouda, M.N.; O’Shea, K.; Dionysiou, D.D. Novel franklinite-like synthetic zinc-ferrite redox nanomaterial: Synthesis, and evaluation for degradation of diclofenac in water. Appl. Catal. B Environ. 2020, 275, 119098. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, J. Fenton-like degradation of 2, 4-dichlorophenol using Fe3O4 magnetic nanoparticles. Appl. Catal. B Environ. 2012, 123, 117–126. [Google Scholar] [CrossRef]
- Du, Y.K.; Yang, P.; Mou, Z.G.; Hua, N.P.; Jiang, L. Thermal decomposition behaviors of PVP coated on platinum nanoparticles. J. Appl. Polym. Sci. 2006, 99, 23–26. [Google Scholar] [CrossRef]
- Babaeivelni, K.; Khodadoust, A.P.; Bogdan, D. Adsorption and removal of arsenic (V) using crystalline manganese (II, III) oxide: Kinetics, equilibrium, effect of pH and ionic strength. J. Environ. Sci. Health Part A 2014, 49, 1462–1473. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Feng, Y.; Li, J.L.; Yang, B.; Ying, G.G. Removal of Sulfadiazine Using 3D Interconnected Petal-Like Magnetic Reduced Graphene Oxide (MrGO) Nanocomposites. Water 2020, 12, 1933. [Google Scholar] [CrossRef]
- Xu, J.; Ding, W.; Wu, F.; Mailhot, G.; Zhou, D.; Hanna, K. Rapid catalytic oxidation of arsenite to arsenate in an iron (III)/sulfite system under visible light. Appl. Catal. B Environ. 2016, 186, 56–61. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, S.; Yuan, Y.; Xu, J.; Zhu, Y.; Li, J.; Wu, F. A novel heterogeneous system for sulfate radical generation through sulfite activation on a CoFe2O4 nanocatalyst surface. J. Hazard. Mater. 2017, 324, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.M.; Yang, C.W.; Cheng, X.Z.; Xu, S.C.; Fan, Z.P.; Wang, G.H.; Wang, S.B.; Guan, X.F.; Sun, X.H. Specifically enhancement of heterogeneous Fenton-like degradation activities for ofloxacin with synergetic effects of bimetallic Fe-Cu on ordered mesoporous silicon. Sep. Purif. Technol. 2017, 189, 357–365. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, S.; Zhou, D.; Wu, F. A simple Cr(VI)–S(IV)–O2 system for rapid and simultaneous reduction of Cr (VI) and oxidative degradation of organic pollutants. J. Hazard. Mater. 2016, 307, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Villamena, F.A.; Weavers, L.K. Kinetics and mechanism of ultrasonic activation of persulfate: An in situ EPR spin trapping study. Environ. Sci. Technol. 2017, 51, 3410–3417. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, N.; Chen, S.; Yan, R.; Li, P.; Qu, Y.; Jing, L. Synthesis of nano SnO2-coupled mesoporous molecular sieve titanium phosphate as a recyclable photocatalyst for efficient decomposition of 2, 4-dichlorophenol. Nano Res. 2018, 11, 1612–1624. [Google Scholar] [CrossRef]
- Brillas, E.; Sirés, I.; Oturan, M.A. Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem. Rev. 2009, 109, 6570–6631. [Google Scholar] [CrossRef]
- Diao, Z.H.; Liu, J.J.; Hu, Y.X.; Kong, L.J.; Jiang, D.; Xu, X.R. Comparative study of Rhodamine B degradation by the systems pyrite/H2O2 and pyrite/persulfate: Reactivity, stability, products and mechanism. Sep. Purif. Technol. 2017, 184, 374–383. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical generation by the interaction of transition metals with common oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef] [PubMed]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (⋅OH/⋅O− in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Wilkinson, F.; Helman, W.P.; Ross, A.B. Rate constants for the decay and reactions of the lowest electronically excited singlet state of molecular oxygen in solution: An expanded and revised compilation. J. Phys. Chem. Ref. Data 1995, 24, 663–677. [Google Scholar] [CrossRef]
- Xu, H.Y.; Shi, T.N.; Wu, L.C.; Qi, S.Y. Discoloration of methyl orange in the presence of schorl and H2O2: Kinetics and mechanism. Water Air Soil Pollut. 2013, 224, 1740. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, X.; Lu, S.; Miao, Z.; Xu, M.; Fu, X.; Qiu, Z.; Sui, Q. Degradation of trichloroethylene in aqueous solution by calcium peroxide activated with ferrous ion. J. Hazard. Mater. 2015, 284, 253–260. [Google Scholar] [CrossRef]
- Jin, Y.; Sun, S.P.; Yang, X.; Chen, X.D. Degradation of ibuprofen in water by FeII-NTA complex-activated persulfate with hydroxylamine at neutral pH. Chem. Eng. J. 2018, 337, 152–160. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, X.; Lu, S.; Miao, Z.; Xu, M.; Fu, X.; Qiu, Z.; Sui, Q. Application of calcium peroxide activated with Fe(II)-EDDS complex in trichloroethylene degradation. Chemosphere 2016, 160, 1–6. [Google Scholar] [CrossRef] [PubMed]
| Samples | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|
| k1 (h−1) | Qe (mg/g) | R2 | k2 (g/mg/h) | Qe (mg/g) | R2 | |
| Fe3O4 | 0.13 | 96.7 | 0.936 | 1.10 × 10−3 | 121.5 | 0.953 |
| AER | 1.06 | 25.0 | 0.772 | 4.92 × 10−2 | 27.3 | 0.788 |
| MAER | 0.53 | 182.6 | 0.983 | 3.11 × 10−3 | 208.7 | 0.989 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, J.; Yang, B.; Feng, Y.; Chen, Y.; Wang, L.-G.; You, W.-D.; Ying, G.-G. Enhanced Photo–Fenton Removal Efficiency with Core-Shell Magnetic Resin Catalyst for Textile Dyeing Wastewater Treatment. Water 2021, 13, 968. https://doi.org/10.3390/w13070968
Zhong J, Yang B, Feng Y, Chen Y, Wang L-G, You W-D, Ying G-G. Enhanced Photo–Fenton Removal Efficiency with Core-Shell Magnetic Resin Catalyst for Textile Dyeing Wastewater Treatment. Water. 2021; 13(7):968. https://doi.org/10.3390/w13070968
Chicago/Turabian StyleZhong, Jie, Bin Yang, Yong Feng, Yang Chen, Li-Gao Wang, Wen-Dan You, and Guang-Guo Ying. 2021. "Enhanced Photo–Fenton Removal Efficiency with Core-Shell Magnetic Resin Catalyst for Textile Dyeing Wastewater Treatment" Water 13, no. 7: 968. https://doi.org/10.3390/w13070968
APA StyleZhong, J., Yang, B., Feng, Y., Chen, Y., Wang, L.-G., You, W.-D., & Ying, G.-G. (2021). Enhanced Photo–Fenton Removal Efficiency with Core-Shell Magnetic Resin Catalyst for Textile Dyeing Wastewater Treatment. Water, 13(7), 968. https://doi.org/10.3390/w13070968