Effect of on-Site Sludge Reduction and Wastewater Treatment Based on Electrochemical-A/O Combined Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrochemical-A/O Combined Sludge Reduction Device
2.2. Sludge Characteristics and Methods of Inoculation and Domestication
2.3. Electrochemical Cell Lysis Experimental Setup
2.4. Experimental Methods
2.4.1. Evaluation Method for Sludge Reduction
2.4.2. Operation Mode of Sludge Reduction Unit
2.4.3. Other Analytical Methods
3. Results
3.1. Sludge Reduction Effect of Two Sets of Processes
3.1.1. Analysis of Sludge Reduction Effect
3.1.2. Analysis of Sludge Reduction Process
3.1.3. Determination of MLVSS and MLSS
3.2. Sewage Treatment Performance of Combined Processes
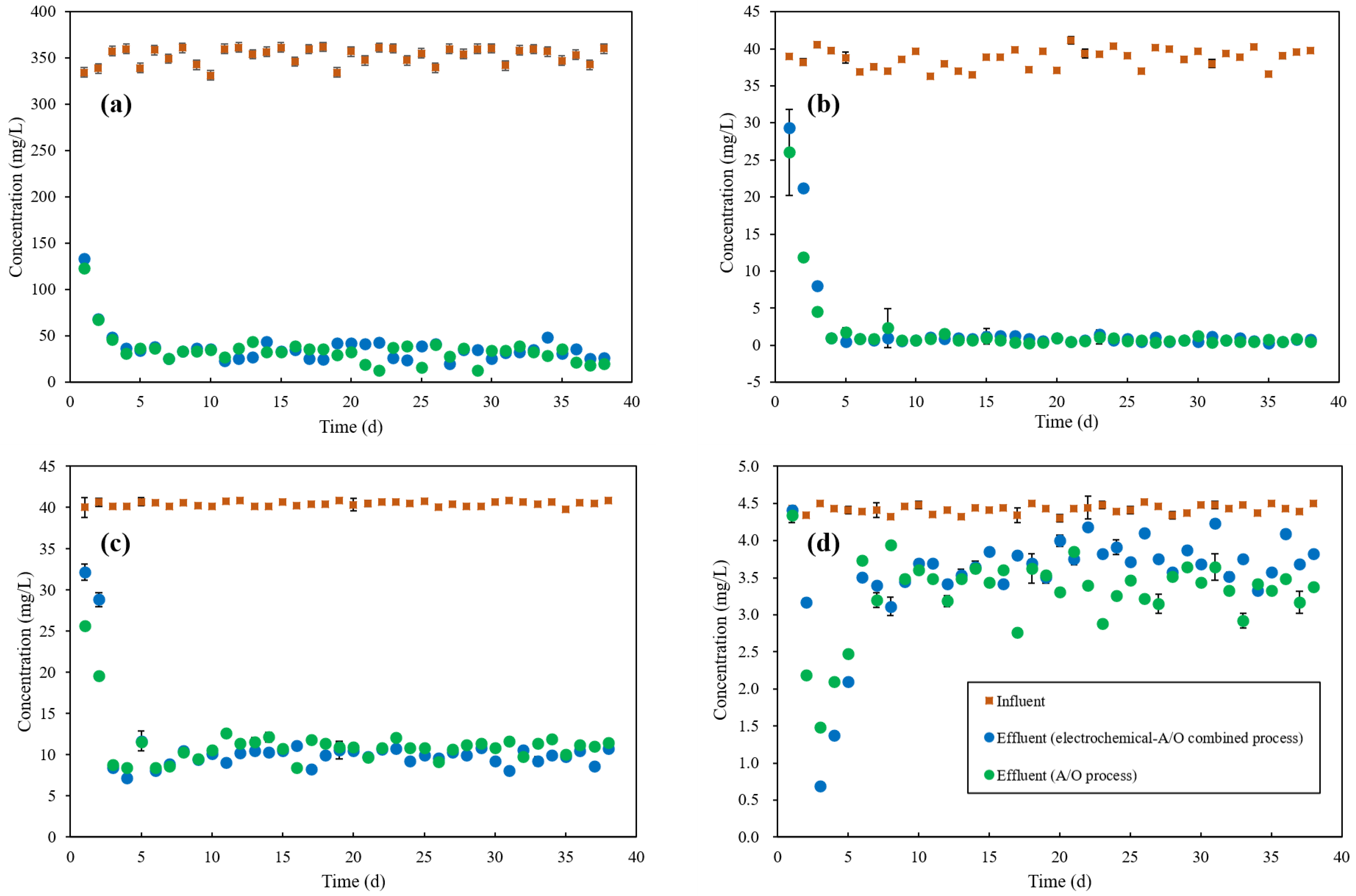
3.2.1. COD Removal
3.2.2. NH4+-N Removal
3.2.3. TN Removal
3.2.4. TP Removal
4. Comparison of Technologies for Reducing Sludge Production
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, L.; Tian, Y.; Zhang, J.; Zuo, W.; Li, H.; Li, A.; Huang, D.; Liu, J.; Liu, Y.; Sun, Z.; et al. Insight into the roles of worm reactor on wastewater treatment; sludge reduction in anaerobic-anoxic-oxic membrane bioreactor (A(2)O-MBR): Performance; mechanism. Chem. Eng. J. 2017, 330, 718–726. [Google Scholar] [CrossRef]
- Zhou, X.; Jin, W.; Wang, L.; Che, L.; Chen, C.; Li, S.; Wang, X.; Tu, R.; Han, S.; Feng, X.; et al. Alum sludge conditioning with ferrous iron/peroxymonosulfate oxidation: Characterization; mechanism. Korean J. Chem. Eng. 2020, 37, 663–669. [Google Scholar] [CrossRef]
- Raheem, A.; Sikarwar, V.S.; He, J.; Dastyar, W.; Dionysiou, D.D.; Wang, W.; Zhao, M. Opportunities; challenges in sustainable treatment; resource reuse of sewage sludge: A review. Chem. Eng. J. 2018, 337, 616–641. [Google Scholar] [CrossRef]
- Erden, G.; Demir, O.; Filibeli, A. Disintegration of biological sludge: Effect of ozone oxidation; ultrasonic treatment on aerobic digestibility. Bioresour. Technol. 2010, 101, 8093–8098. [Google Scholar] [CrossRef] [PubMed]
- Syed-Hassan, S.S.A.; Wang, Y.; Hu, S.; Su, S.; Xiang, J. Thermochemical processing of sewage sludge to energy; fuel: Fundamentals, challenges; considerations. Renew. Sustain. Energy Rev. 2017, 80, 888–913. [Google Scholar] [CrossRef]
- Chan, W.P.; Wang, J. Comprehensive characterisation of sewage sludge for thermochemical conversion processes Based on Singapore survey. Waste Manag. 2016, 54, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Gupta, A.; Novak, J.T.; Goldsmith, C.D. Evolution of nitrogen species in landfill leachates under various stabilization states. Waste Manag. 2017, 69, 225–231. [Google Scholar] [CrossRef]
- Li, W.; Wu, C.; Wang, K.; Meng, L.; Lv, L. Nitrogen loss reduction by adding sucrose; beet pulp in sewage sludge composting. Int. Biodeter. Biodegr. 2017, 124, 297–303. [Google Scholar] [CrossRef]
- Han, W.; Mao, Y.; Wei, Y.; Shang, P.; Zhou, X. Bioremediation of aquaculture wastewater with algal-bacterial biofilm combined with the production of selenium rich biofertilizer. Water 2020, 12, 2071. [Google Scholar] [CrossRef]
- Zhou, X.; Jiang, G.; Wang, Q.; Yuan, Z. A review on sludge conditioning by sludge pre-treatment with a focus on advanced oxidation. RSC Adv. 2014, 4, 50644–50652. [Google Scholar] [CrossRef]
- Xiao, K.; Chen, Y.; Jiang, X.; Yang, Q.; Seow, W.Y.; Zhu, W.; Zhou, Y. Variations in physical, chemical; biological properties in relation to sludge dewaterability under Fe (II)-Oxone conditioning. Water Res. 2017, 109, 13–23. [Google Scholar] [CrossRef]
- Wei, Y.S.; Van Houten, R.T.; Borger, A.R.; Eikelboom, D.H.; Fan, Y.B. Minimization of excess sludge production for biological wastewater treatment. Water Res. 2003, 37, 4453–4467. [Google Scholar] [CrossRef]
- Chaplin, B.P. The prospect of electrochemical technologies advancing worldwide water treatment. Acc. Chem. Res. 2019, 52, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Perera, M.K.; Englehardt, J.D.; Dvorak, A.C. Technologies for recovering nutrients from wastewater: A Critical Review. Environ. Eng. Sci. 2019, 36, 511–529. [Google Scholar] [CrossRef]
- Kusakabe, K.; Nishida, H.; Morooka, S.; Kato, Y. Simultaneous electrochemical removal of copper; chemical oxygen-demand using a packed-bed electrode cell. J. Appl. Electrochem. 1986, 16, 121–126. [Google Scholar] [CrossRef]
- Mayen-Mondragon, R.; Ibanez, J.G.; Vasquez-Medrano, R. Simultaneous electrochemical oxidation; reduction of representative organic pollutants. Fresenius Environ. Bull. 2008, 17, 1294–1299. [Google Scholar]
- Llanos, J.; Cotillas, S.; Canizares, P.; Rodrigo, M.A. Effect of bipolar electrode material on the reclamation of urban wastewater by an integrated electrodisinfection/electrocoagulation process. Water Res. 2014, 53, 329–338. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, Q.; Jiang, J.; Wei, L.; Wang, K.; Zhang, Y.; Hou, W.; Yu, H. Electrochemical disinfection; removal of ammonia nitrogen for the reclamation of wastewater treatment plant effluent. Environ. Sci. Pollut. Res. 2017, 24, 5152–5158. [Google Scholar] [CrossRef]
- Fidaleo, M.; Lavecchia, R.; Petrucci, E.; Zuorro, A. Application of a novel definitive screening design to decolorization of an azo dye on boron-doped diamond electrodes. Int. J. Environ. Sci. Technol. 2016, 13, 835–842. [Google Scholar] [CrossRef]
- Zuorro, A.; Lavecchia, R.; Monaco, M.M.; Iervolino, G.; Vaiano, V. Photocatalytic degradation of Azo dye reactive Violet 5 on Fe-Doped titania catalysts under visible light irradiation. Catalysts 2019, 9, 645. [Google Scholar] [CrossRef]
- Montanaro, D.; Lavecchia, R.; Petrucci, E.; Zuorro, A. UV-assisted electrochemical degradation of coumarin on boron-doped diamond electrodes. Chem. Eng. J. 2017, 323, 512–519. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, Y.; Zhang, J.; Li, N.; Kong, L.; Yu, M.; Zuo, W. Distribution; risk assessment of heavy metals in sewage sludge after ozonation. Environ. Sci. Pollut. Res. 2017, 24, 5118–5125. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, T.L.G.; Elissen, H.H.J.; Temmink, H.; Buisman, C.J.N. Operation of an aquatic worm reactor suitable for sludge reduction at large scale. Water Res. 2011, 45, 4923–4929. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; Wen, W.; Zhang, Y.; Li, R.; Sun, J.; Wang, Y.; Yang, Z.; Liu, J. Enhanced dewaterability of textile dyeing sludge using micro-electrolysis pretreatment. J. Environ. Manag. 2015, 161, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Botte, G.G. Electrochemical treatment of sewage sludge; pathogen inactivation. J. Appl. Electrochem. 2021, 51, 119–130. [Google Scholar] [CrossRef]
- Chaplin, B.P. Critical review of electrochemical advanced oxidation processes for water treatment applications. Environ. Sci. Proc. Impacts 2014, 16, 1182–1203. [Google Scholar] [CrossRef]
- Wu, W.; Huang, Z.; Lim, T. Recent development of mixed metal oxide anodes for electrochemical oxidation of organic pollutants in water. Appl. Catal. A Gen. 2014, 480, 58–78. [Google Scholar] [CrossRef]
- Rajeshwar, K.; Ibanez, J.G.; Swain, G.M. Electrochemistry; the environment. J. Appl. Electrochem. 1994, 24, 1077–1091. [Google Scholar] [CrossRef]
- Loginov, M.; Citeau, M.; Lebovka, N.; Vorobiev, E. Electro-dewatering of drilling sludge with liming; electrode heating. Sep. Purif. Technol. 2013, 104, 89–99. [Google Scholar] [CrossRef]
- Song, L.; Zhu, N.; Yuan, H.; Hong, Y.; Ding, J. Enhancement of waste activated sludge aerobic digestion by electrochemical pre-treatment. Water Res. 2010, 44, 4371–4378. [Google Scholar] [CrossRef] [PubMed]
- Feki, E.; Khoufi, S.; Loukil, S.; Sayadi, S. Improvement of anaerobic digestion of waste-activated sludge by using H2O2 oxidation, electrolysis, electro-oxidation; thermo-alkaline pretreatments. Environ. Sci. Pollut. Res. 2015, 22, 14717–14726. [Google Scholar] [CrossRef]
- Zeng, F.; Jin, W.; Zhao, Q. Operation performance of an A/O process combined sewage sludge treatment; phosphorus recovery using human urine. Water Sci. Technol. 2018, 78, 2597–2607. [Google Scholar] [CrossRef]
- Ding, S.; He, J.; Luo, X.; Zheng, Z. Simultaneous nitrogen; carbon removal in a packed A/O reactor: Effect of C/N ratio on microbial community structure. Bioprocess Biosyst. Eng. 2020, 43, 1241–1252. [Google Scholar] [CrossRef]
- Wang, Y.; Du, Z.; Liu, Y.; Wang, H.; Xu, F.; Liu, B.; Zheng, Z. The nitrogen removal; sludge reduction performance of a multi-stage anoxic/oxic (A/O) biofilm reactor. Water Environ. Res. 2020, 92, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Tarn, N.F.; Dai, Y.; Zhang, X.; Li, R.; Zheng, Y.; Wang, L.; Yang, Y. Enhanced performance of pilot-scale hybrid constructed wetlands with A/O reactor in raw domestic sewage treatment. J. Environ. Manag. 2020, 258, 110026. [Google Scholar] [CrossRef]
- Santos, G.O.S.; Doria, A.R.; Vasconcelos, V.M.; Saez, C.; Rodrigo, M.A.; Eguiluz, K.I.B.; Salazar-Banda, G.R. Enhancement of wastewater treatment using novel laser-made Ti/SnO2-Sb anodes with improved electrocatalytic properties. Chemosphere 2020, 259, 127475. [Google Scholar]
- Sun, Y.; Cheng, S.; Li, L.; Yu, Z.; Mao, Z.; Huang, H. Facile sealing treatment with stannous citrate complex to enhance performance of electrodeposited Ti/SnO2-Sb electrode. Chemosphere 2020, 255, 126973. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cheng, S.; Yu, Z.; Li, L.; Li, C.; Yang, J. Elucidating deactivation mechanisms of Pd-doped; un-doped Ti/SnO2-Sb electrodes. J. Alloy Compd. 2020, 834, 155184. [Google Scholar] [CrossRef]
- Polcaro, A.M.; Palmas, S.; Renoldi, F.; Mascia, M. On the performance of Ti/SnO2; Ti/PbO2 anodes in electrochemical degradation of 2-chlorophenol for wastewater treatment. J. Appl. Electrochem. 1999, 29, 147–151. [Google Scholar] [CrossRef]
- Nepa, C. Water and Wastewater Monitoring Methods, 4th ed.; Chinese Environmental Science Publishing House: Beijing, China, 2012. [Google Scholar]
- Zhang, J.; Zhang, J.; Tian, Y.; Li, N.; Kong, L.; Sun, L.; Yu, M.; Zuo, W. Changes of physicochemical properties of sewage sludge during ozonation treatment: Correlation to sludge dewaterability. Chem. Eng. J. 2016, 301, 238–248. [Google Scholar] [CrossRef]
- Nishijima, W.; Mukaidani, T.; Okada, M. DOC removal by multi-stage ozonation-biological treatment. Water Res. 2003, 37, 150–154. [Google Scholar] [CrossRef]
- Qiang, Z.; Wang, L.; Dong, H.; Qu, J. Operation performance of an A/A/O process coupled with excess sludge ozonation; phosphorus recovery: A pilot-scale study. Chem. Eng. J. 2015, 268, 162–169. [Google Scholar] [CrossRef]
- Dytczak, M.A.; Londry, K.; Siegrist, H.; Oleszkiewicz, J.A. Extracellular polymers in Partly ozonated return activated sludge: Impact on flocculation; dewaterability. Water Sci. Technol. 2006, 54, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Takdastan, A.; Azimi, A.; Jaafarzadeh, N. Biological excess sludge reduction in municipal wastewater treatment by chlorine. Asian J. Chem. 2010, 22, 1665–1674. [Google Scholar]
- Wang, G.; Jun, S.; Shen, H.; Liang, S.; He, X.; Zhang, M.; Xie, Y.; Li, L.; Hu, Y. Reduction of excess sludge production in sequencing batch reactor through incorporation of chlorine dioxide oxidation. J. Hazard. Mater. 2011, 192, 93–98. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, P.; Yang, J.; Chen, Y. Ultrasonic reduction of excess sludge from the activated sludge system. J. Hazard. Mater. 2007, 145, 515–519. [Google Scholar] [CrossRef]
- Rai, C.; Rao, P. Influence of sludge disintegration by high pressure homogenizer on microbial growth in sewage sludge: An approach for excess sludge reduction. Clean Technol. Environ. Policy 2009, 11, 437–446. [Google Scholar] [CrossRef]
- Camacho, P.; Ginestet, P.; Audic, J.M. Understanding the mechanism of thermal disintegrating treatment in the reduction of sludge production. Water Sci. Technol. 2005, 52, 235–245. [Google Scholar] [CrossRef]
- Low, E.W.; Chase, H.A.; Milner, M.G.; Curtis, T.P. Uncoupling of metabolism to reduce biomass production in the activated sludge process. Water Res. 2000, 34, 3204–3212. [Google Scholar] [CrossRef]
- Basim, Y.; Jaafarzadeh, N.; Farzadkia, M. A novel biological method for sludge volume reduction by aquatic worms. Int. J. Environ. Sci. Dev. 2016, 7, 253–256. [Google Scholar] [CrossRef][Green Version]
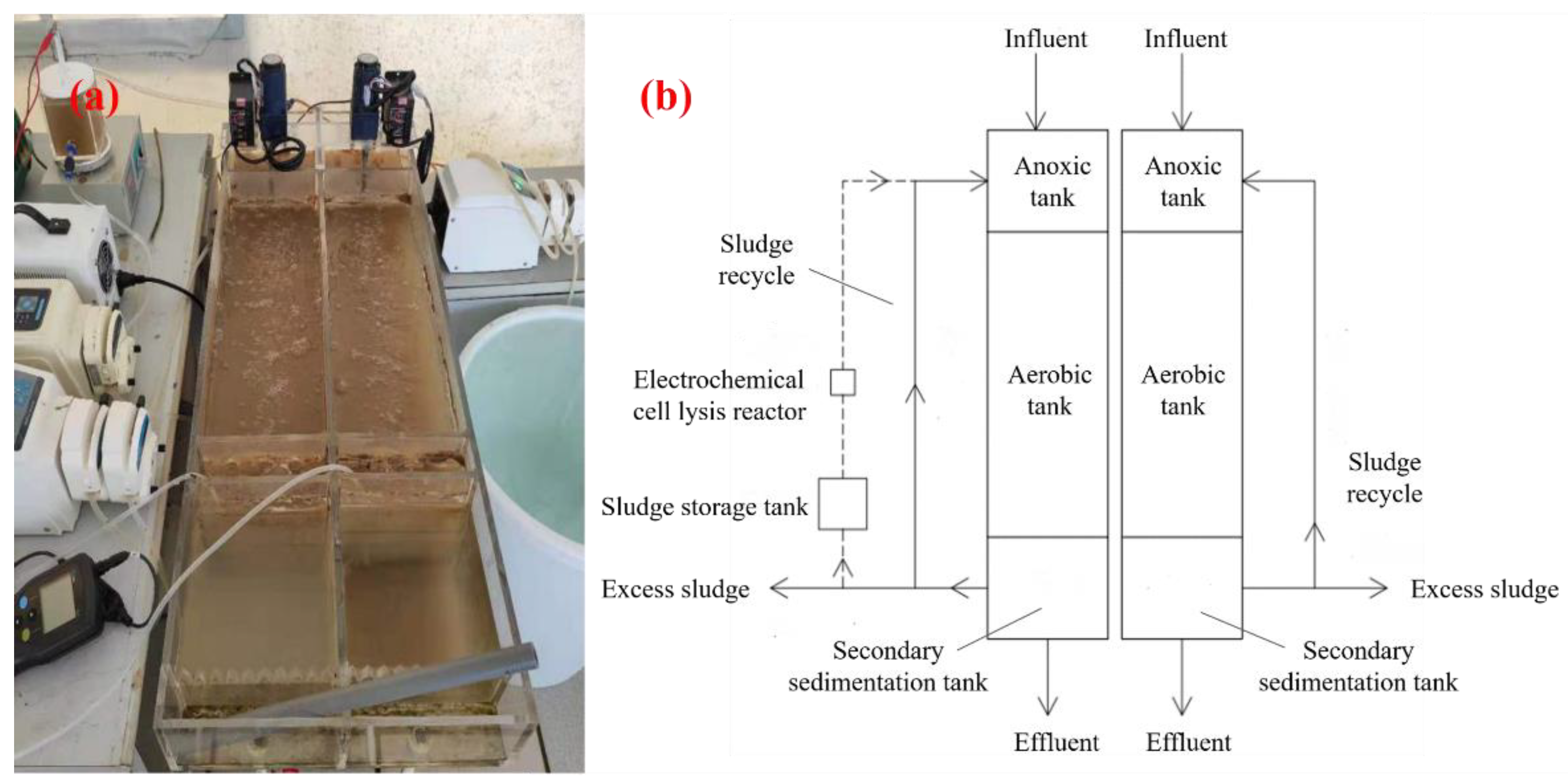

| Operating Parameters | Value |
|---|---|
| Hydraulic retention time (HRT) (h) | 8 |
| Temperature (°C) | 22~25 |
| Dissolved oxygen (mg/L) | 2~3 |
| Mixed liquor suspended solids (MLSS) concentration (mg/L) | 3000 |
| Return sludge ratio (%) | 100 |
| Internal sludge recycle ratio (%) | 400 |
| Sludge retention time (SRT) (d) | 14 |
| Influent flow rate (L/d) | 120 |
| Treatment Technologies | Treatment Conditions | Sludge Reduction Effect | Sludge Reduction Cost | Advantages | Disadvantages | References | |
|---|---|---|---|---|---|---|---|
| Chemical | Ozone | 0.03 g O3/g TSS | 25% | 260.8 $/t TSS | Improved sludge settleability; process applied at full scale | High investment and operating costs; increase in effluent COD | [44] |
| Chlorine | 0.23 g Cl2/g TSS | 45% | 94.5 $/t TSS | Low investment and operating costs compared to ozonation | Formation of by-products; worsened sludge settleability; increase in effluent COD; only applied at lab scale | [45] | |
| Chloride dioxide | 0.01 g ClO2/g TSS | 36% | 162.6 $/t TSS | Low investment and operating costs compared to ozonation | Formation of by-products; increase in effluent COD | [46] | |
| Physical | Ultrasonic | 25 kHz; 120 kW/kg TSS; 15 min | 91% | 2637.4 $/t TSS | Low investment costs | Erosion of sonotrodes; high operating costs; Worsened sludge settleability | [47] |
| High pressure homogenization | 10,700 kJ/kg TSS | 94% | 253 $/t TSS | Improved sludge settleability | High investment and operating costs; increase in effluent COD; only applied at pilot scale | [48] | |
| Thermal | 90 °C; 45 min | 60% | -- | Improved sludge settleability | High investment and operating costs; increase in effluent COD; only applied at pilot scale | [49] | |
| Electrical | 18 V; 90 min | 37% | 186.2 $/t TSS | Improved sludge settleability; low investment and operating costs compared to other physical technologies | Only applied at lab scale | This study | |
| Others | Un-coupler | para-nitrophenol (pNP)100 mg/L | 25% | 156.2 $/t TSS | Improved sludge settleability | Formation of by-products; increase in effluent COD | [50] |
| Predation | Lumbriculus variegatus | 33% | -- | Low operating costs; improved sludge settleability; process applied at full scale | Large space required for predation reactor; difficult to control the quantities of protozoa and metazoa | [51] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Han, W.; Zhou, X.; Jin, W.; Liu, W.; Gao, S.; Zhao, Z.; Chen, Y.; Jiang, G. Effect of on-Site Sludge Reduction and Wastewater Treatment Based on Electrochemical-A/O Combined Process. Water 2021, 13, 941. https://doi.org/10.3390/w13070941
He Z, Han W, Zhou X, Jin W, Liu W, Gao S, Zhao Z, Chen Y, Jiang G. Effect of on-Site Sludge Reduction and Wastewater Treatment Based on Electrochemical-A/O Combined Process. Water. 2021; 13(7):941. https://doi.org/10.3390/w13070941
Chicago/Turabian StyleHe, Zhongqi, Wei Han, Xu Zhou, Wenbiao Jin, Wentao Liu, Shuhong Gao, Zhicheng Zhao, Yidi Chen, and Guangming Jiang. 2021. "Effect of on-Site Sludge Reduction and Wastewater Treatment Based on Electrochemical-A/O Combined Process" Water 13, no. 7: 941. https://doi.org/10.3390/w13070941
APA StyleHe, Z., Han, W., Zhou, X., Jin, W., Liu, W., Gao, S., Zhao, Z., Chen, Y., & Jiang, G. (2021). Effect of on-Site Sludge Reduction and Wastewater Treatment Based on Electrochemical-A/O Combined Process. Water, 13(7), 941. https://doi.org/10.3390/w13070941











