Modelling Microplastics in the River Thames: Sources, Sinks and Policy Implications
Abstract
1. Introduction
2. Methods
2.1. Measuring Microplastics in Rivers
2.2. Application of INCA Microplastics Model to the River Thames—Model Structure
2.3. Thames Case Study
3. Results
3.1. Driving Climate and Spatial Data
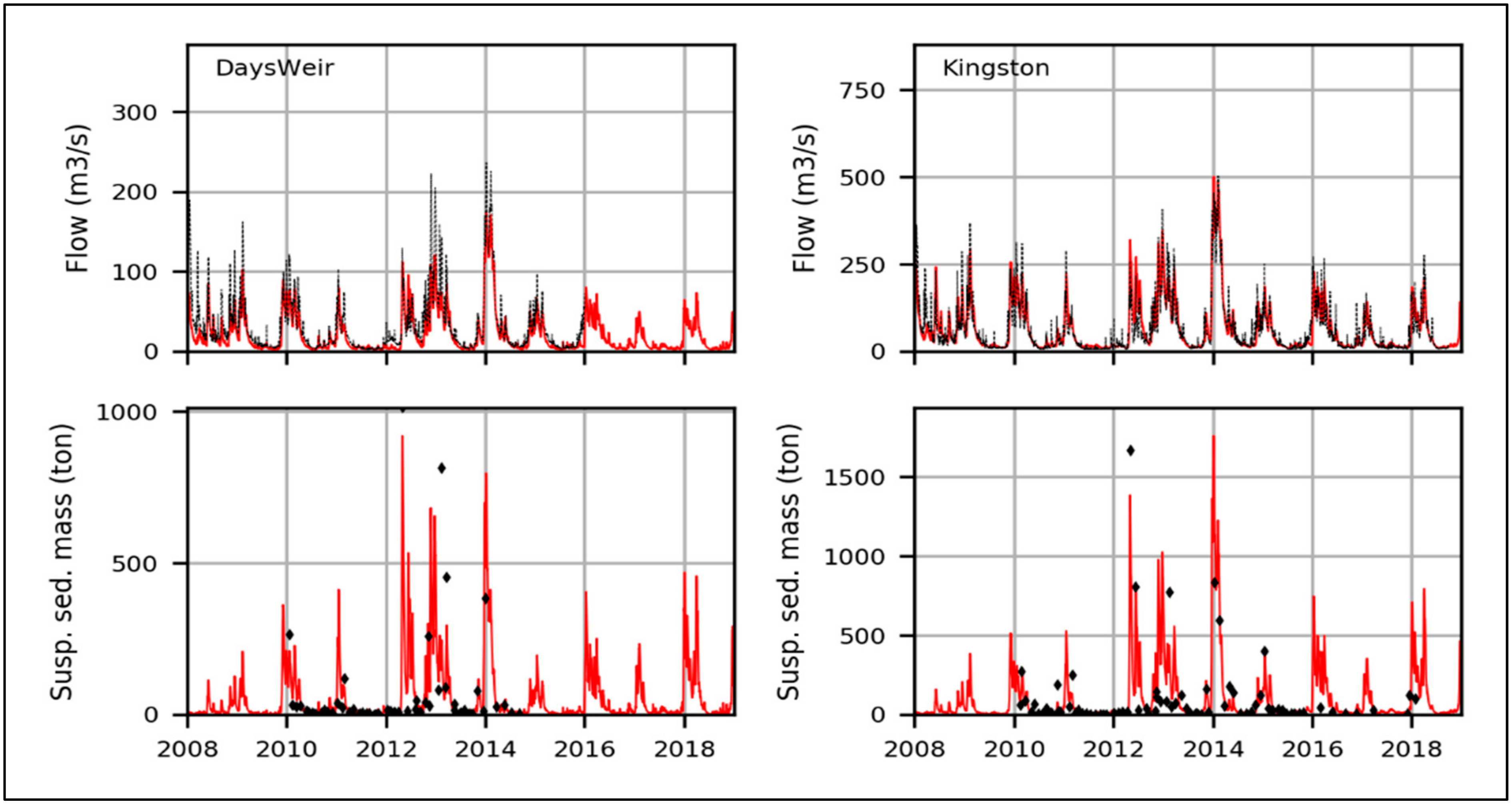
3.2. Model Calibration and Validation
3.3. Model Comparison with MP Measurements for Sediments and Surface Waters
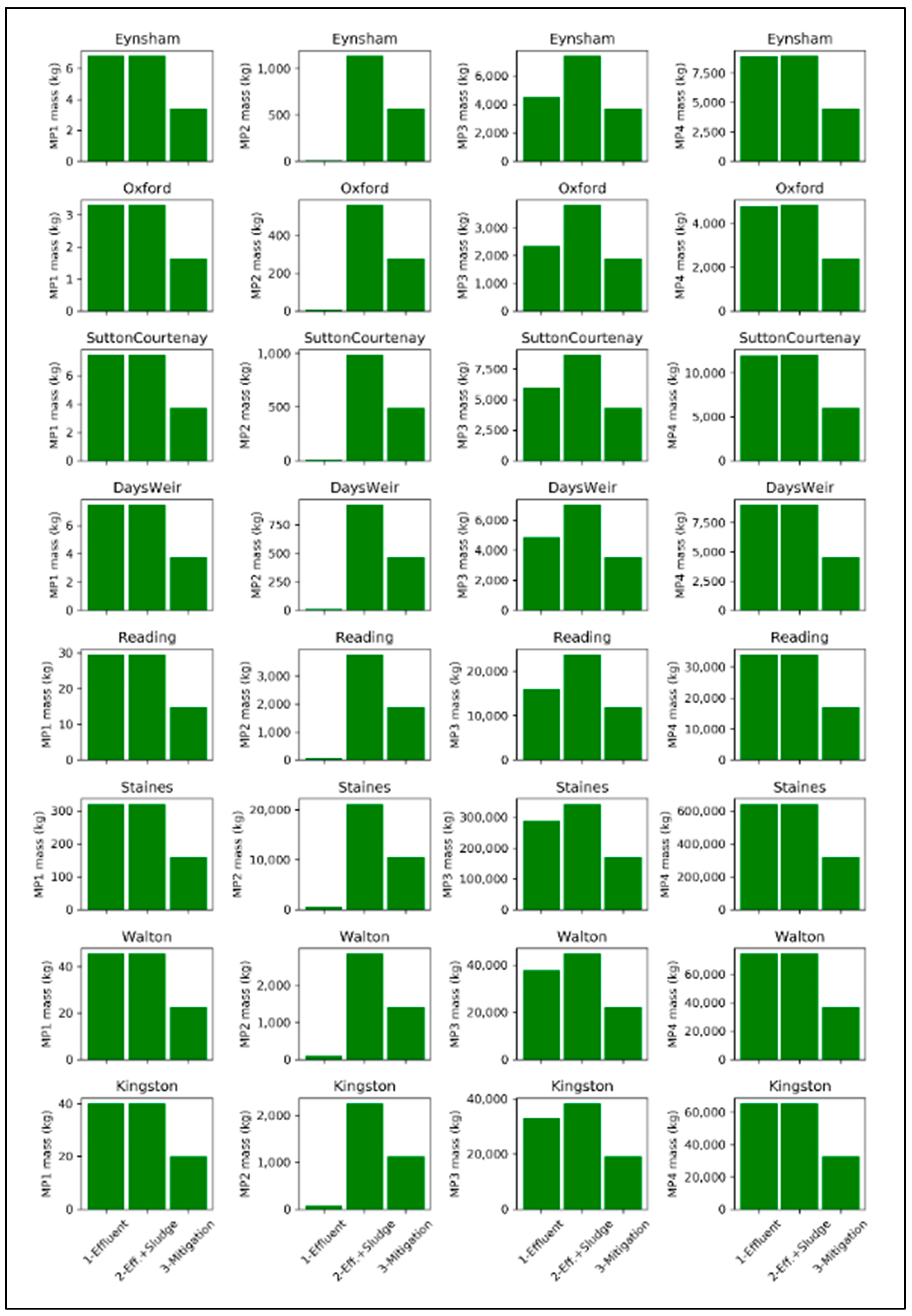
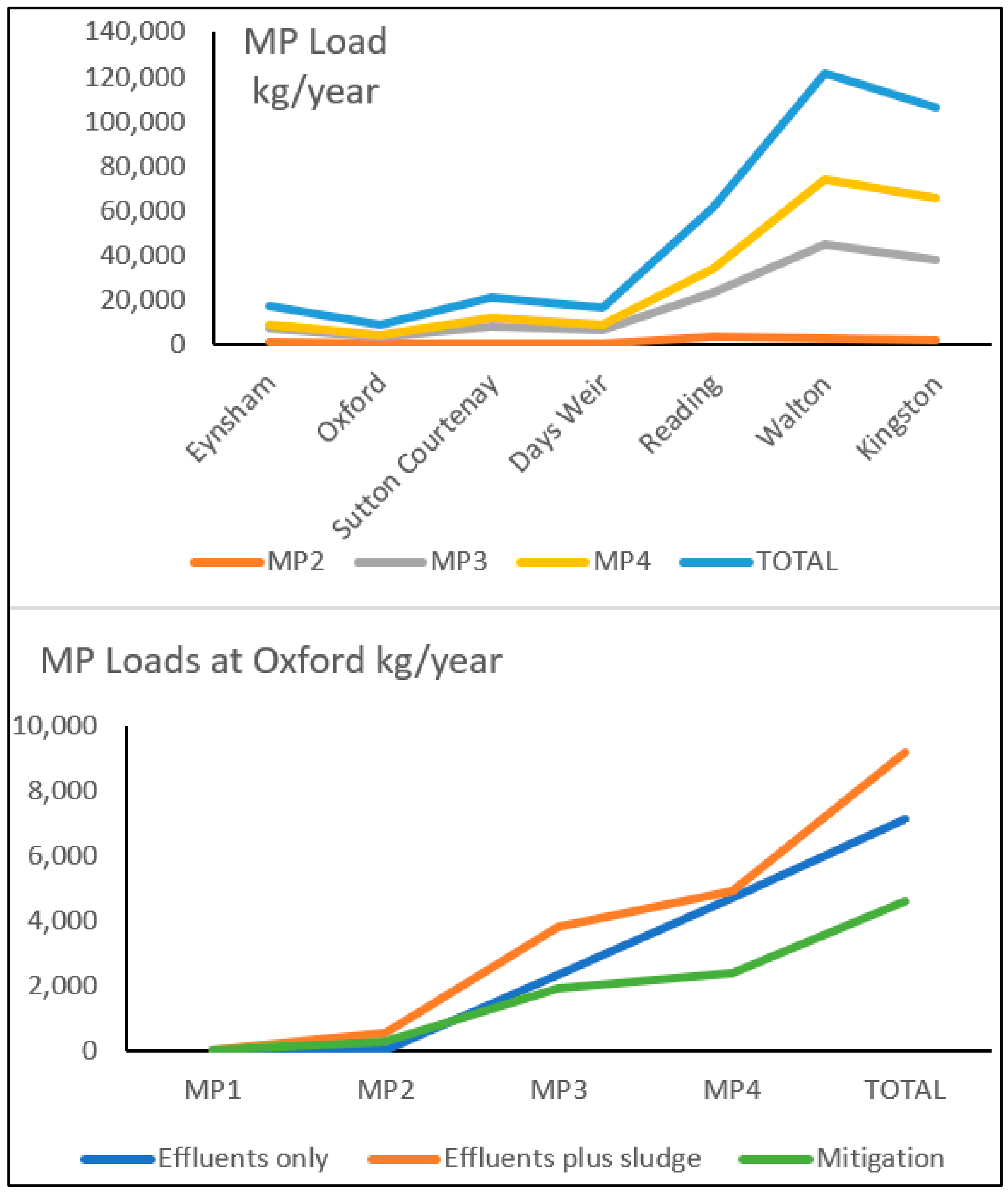
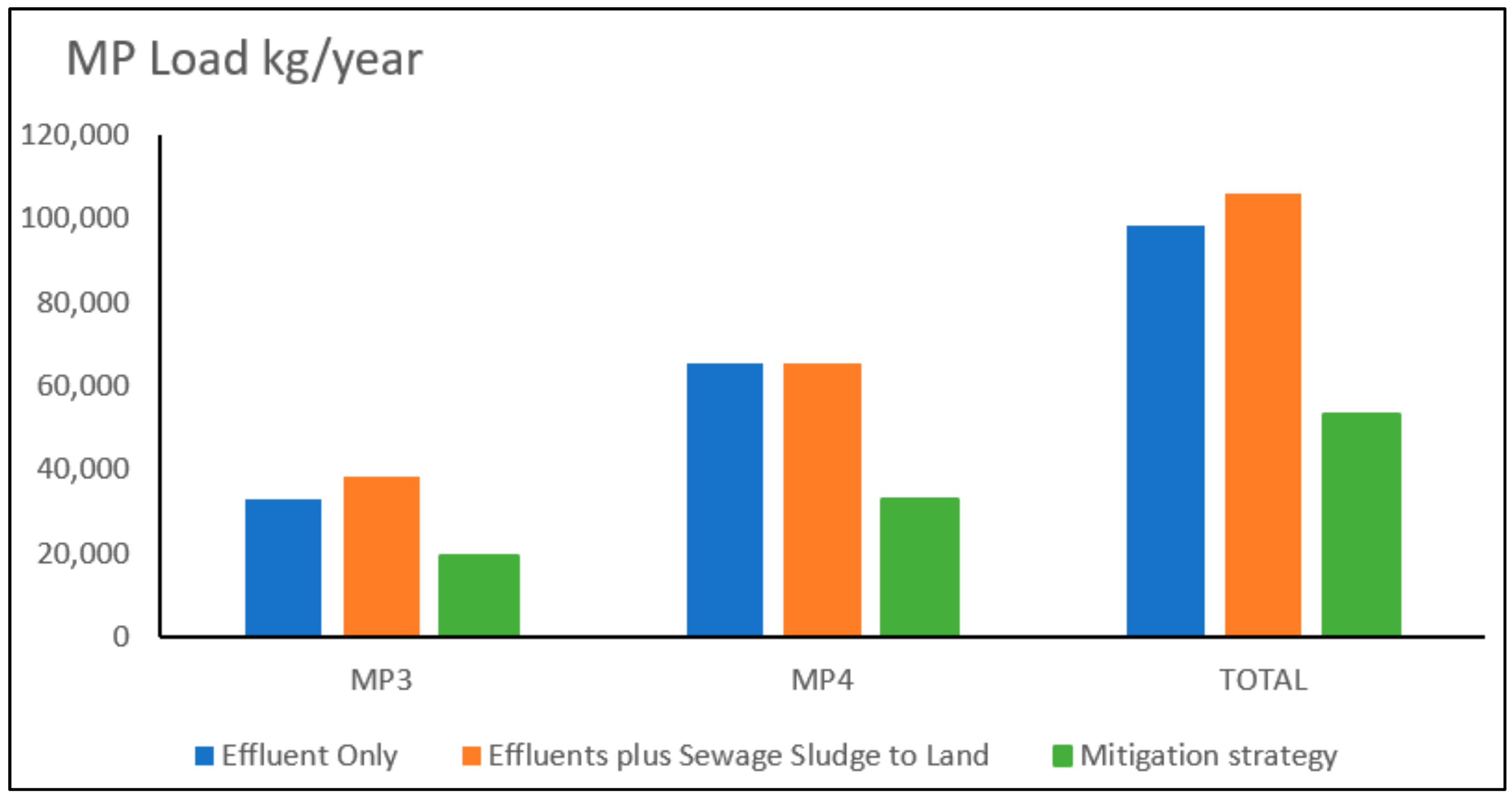
- Scenario 1: Microplastics input from wastewater effluent
- Scenario 2: Microplastics input from wastewater effluent and sludges application in agriculture
- Scenario 3: Mitigation
4. Discussion
4.1. Approaches to Microplastics Mitigation through Policy, Regulation and Management
4.2. Mitigation Approaches at the Source
4.3. Impacts of BREXIT and the UK Leaving the EU
4.4. Legal Controls on Plastic Transport
4.5. Taxation Controls
4.6. Other Diffuse Sources
4.7. Mitigation Approaches along Pathways
4.8. Mitigation Approaches through Standardization of Measurement
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Royal Society. Microplastics in Freshwater and Soil. In An Evidence Synthesis; Publication of the Royal Society of London: London, UK, 2019. [Google Scholar]
- Liu, Y.; Wang, Z.; Wang, S.; Fang, H.; Ye, N.; Wang, D. Ecotoxicological effects on Scenedesmus obliquus and Danio rerio Co-exposed to polystyrene nano-plastic particles and natural acidic organic polymer. Environ. Toxicol. Pharmacol. 2019, 67, 21–28. [Google Scholar] [CrossRef]
- Plastics Europe Plastics—The Facts 2018. In An Analysis of European Plastics Production, Demand and Waste Data; Plastics Europe: Wemmel, Belgium, 2018.
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef]
- Allen, S.; Allen, D.; Phoenix, V.R.; Le Roux, G.; Jiménez, P.D.; Simonneau, A.; Binet, S.; Galop, D. Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat. Geosci. 2019, 12, 339–344. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ma, J.; Ji, R.; Pan, K.; Miao, A.-J. Microplastics in aquatic environments: Occurrence, accumulation, and biological effects. Sci. Total Environ. 2020, 703, 134699. [Google Scholar] [CrossRef]
- Wagner, M.; Scherer, C.; Alvarez-Muñoz, D.; Brennholt, N.; Bourrain, X.; Buchinger, S.; Fries, E.; Grosbois, C.; Klasmeier, J.; Marti, T.; et al. Microplastics in freshwater ecosystems: What we know and what we need to know. Environ. Sci. Eur. 2014, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Waldschläger, K.; Lechthaler, S.; Stauch, G.; Schüttrumpf, H. The way of microplastic through the environment—Application of the source-pathway-receptor model (review). Sci. Total Environ. 2020, 713, 136584. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Österlund, H.; Marsalek, J.; Viklander, M. The pollution conveyed by urban runoff: A review of sources. Sci. Total Environ. 2020, 709, 136125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, Y. Effects of microplastics on wastewater and sewage sludge treatment and their removal: A review. Chem. Eng. J. 2020, 382, 122955. [Google Scholar] [CrossRef]
- Wu, P.; Huang, J.; Zheng, Y.; Yang, Y.; Zhang, Y.; He, F.; Chen, H.; Quan, G.; Yan, J.; Li, T.; et al. Environmental occurrences, fate, and impacts of microplastics. Ecotoxicol. Environ. Saf. 2019, 184, 109612. [Google Scholar] [CrossRef] [PubMed]
- Horton, A.A.; Svendsen, C.; Williams, R.J.; Spurgeon, D.J.; Lahive, E. Large microplastic particles in sediments of tributaries of the River Thames, UK—Abundance, sources and methods for effective quantification. Mar. Pollut. Bull. 2017, 114, 218–226. [Google Scholar] [CrossRef]
- Turner, S.; Horton, A.A.; Rose, N.L.; Hall, C. A temporal sediment record of microplastics in an urban lake, London, UK. J. Paleolimnol. 2019, 61, 449–462. [Google Scholar] [CrossRef]
- Brahney, J.; Hallerud, M.; Heim, E.; Hahnenberger, M.; Sukumaran, S. Plastic rain in protected areas of the United States. Science 2020, 368, 1257–1260. [Google Scholar] [CrossRef] [PubMed]
- Dunn, C.; Owens, J.; Fears, L.; Nunnerley, L.; Kirby, J.; Armstrong, O.L.; Thomas, P.J.; Aberg, D.; Gilder, W.; Green, D.; et al. An affordable methodology for quantifying waterborne microplastic—An emerging contaminant in inland-waters. J. Limnol. 2019, 79. [Google Scholar] [CrossRef]
- Horton, A.A.; Vijver, M.G.; Lahive, E.; Spurgeon, D.J.; Svendsen, C.; Heutink, R.; van Bodegom, P.M.; Baas, J. Acute toxicity of organic pesticides to Daphnia magna is unchanged by co-exposure to polystyrene microplastics. Ecotoxicol. Environ. Saf. 2018, 166, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Ball, H.; Cross, R.; Grove, E.; Horton, A.; Johnson, A.; Jürgens, M.; Read, D.; Svendsen, C. Sink to River—River to Tap. In A Review of Potential Risks from Nanoparticles and Microplastics; UKWIR: London, UK, 2020. [Google Scholar]
- Horton, A.A.; Cross, R.K.; Read, D.S.; Jürgens, M.D.; Ball, H.L.; Svendsen, C.; Vollertsen, J.; Johnson, A.C. Semi-automated analysis of microplastics in complex wastewater samples. Environ. Pollut. 2021, 268, 115841. [Google Scholar] [CrossRef]
- Whitehead, P.G. Water Quality Modeling; Wiley: Hoboken, NJ, USA, 2016; pp. 1–21. [Google Scholar]
- Mai, L.; Bao, L.-J.; Shi, L.; Wong, C.S.; Zeng, E.Y. A review of methods for measuring microplastics in aquatic environments. Environ. Sci. Pollut. Res. 2018, 25, 11319–11332. [Google Scholar] [CrossRef]
- Nizzetto, L.; Bussi, G.; Futter, M.N.; Butterfield, D.; Whitehead, P.G. A theoretical assessment of microplastic transport in river catchments and their retention by soils and river sediments. Environ. Sci. Process. Impacts 2016, 18, 1050–1059. [Google Scholar] [CrossRef]
- Frias, J.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Nor, N.H.M.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef]
- Alvim, C.B.; Mendoza-Roca, J.; Bes-Piá, A. Wastewater treatment plant as microplastics release source—Quantification and identification techniques. J. Environ. Manag. 2020, 255, 109739. [Google Scholar] [CrossRef]
- Araújo, A.P.D.C.; de Melo, N.F.S.; Junior, A.G.D.O.; Rodrigues, F.P.; Fernandes, T.; Vieira, J.E.D.A.; Rocha, T.L.; Malafaia, G. How much are microplastics harmful to the health of amphibians? A study with pristine polyethylene microplastics and Physalaemus cuvieri. J. Hazard. Mater. 2020, 382, 121066. [Google Scholar] [CrossRef]
- Whitehead, P.G.; Wilson, E.; Butterfield, D.; Seed, K. A semi-distributed integrated flow and nitrogen model for multiple source assessment in catchments (INCA): Part II—application to large river basins in south Wales and eastern England. Sci. Total Environ. 1998, 210–211, 559–583. [Google Scholar] [CrossRef]
- Wade, A.J.; Durand, P.; Beaujouan, V.; Wessel, W.; Raat, K.J.; Whitehead, P.G.; Butterfield, D.; Rankinen, K.; Lepisto, A. A nitrogen model for European catchments: INCA, new model structure and equations. Hydrol. Earth Syst. Sci. 2002, 6, 559–582. [Google Scholar] [CrossRef]
- Wade, A.J.; Whitehead, P.G.; Butterfield, D. The Integrated Catchments model of Phosphorus dynamics (INCA-P), a new approach for multiple source assessment in heterogeneous river systems: Model structure and equations. Hydrol. Earth Syst. Sci. 2002, 6, 583–606. [Google Scholar] [CrossRef]
- Lazar, A.N.; Butterfield, D.; Futter, M.N.; Rankinen, K.; Thouvenot-Korppoo, M.; Jarritt, N.; Lawrence, D.S.; Wade, A.J.; Whitehead, P.G. An assessment of the fine sediment dynamics in an upland river system: INCA-Sed modifications and implications for fisheries. Sci. Total Environ. 2010, 408, 2555–2566. [Google Scholar] [CrossRef]
- Crossman, J.; Whitehead, P.; Futter, M.; Jin, L.; Shahgedanova, M.; Castellazzi, M.; Wade, A. The interactive responses of water quality and hydrology to changes in multiple stressors, and implications for the long-term effective management of phosphorus. Sci. Total Environ. 2013, 454–455, 230–244. [Google Scholar] [CrossRef]
- Jin, L.; Whitehead, P.G.; Futter, M.N.; Lu, Z. Modelling the impacts of climate change on flow and nitrate in the River Thames: Assessing potential adaptation strategies. Hydrol. Res. 2012, 43, 902–916. [Google Scholar] [CrossRef]
- Whitehead, P.G.; Crossman, J.; Balana, B.B.; Futter, M.N.; Comber, S.D.W.; Jin, L.; Skuras, D.; Wade, A.J.; Bowes, M.J.; Read, D.S. A cost-effectiveness analysis of water security and water quality: Impacts of climate and land-use change on the River Thames system. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2013, 371, 20120413. [Google Scholar] [CrossRef]
- Whitehead, P.; Leckie, H.; Rankinen, K.; Butterfield, D.; Futter, M.; Bussi, G. An INCA model for pathogens in rivers and catchments: Model structure, sensitivity analysis and application to the River Thames catchment, UK. Sci. Total Environ. 2016, 572, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Bussi, G.; Whitehead, P.G.; Bowes, M.J.; Read, D.S.; Prudhomme, C.; Dadson, S.J. Impacts of climate change, land-use change and phosphorus reduction on phytoplankton in the River Thames (UK). Sci. Total Environ. 2016, 572, 1507–1519. [Google Scholar] [CrossRef]
- Lu, Q.; Futter, M.; Nizzetto, L.; Bussi, G.; Juergens, M.; Whitehead, P. Fate and transport of polychlorinated biphenyls (PCBs) in the River Thames catchment—Insights from a coupled multimedia fate and hydrobiogeochemical transport model. Sci. Total Environ. 2016, 572, 1461–1470. [Google Scholar] [CrossRef]
- Bussi, G.; Dadson, S.J.; Prudhomme, C.; Whitehead, P.G. Modelling the future impacts of climate and land-use change on suspended sediment transport in the River Thames (UK). J. Hydrol. 2016, 542, 357–372. [Google Scholar] [CrossRef]
- Bussi, G.; Janes, V.; Whitehead, P.G.; Dadson, S.J.; Holman, I.P. Dynamic response of land use and river nutrient concentration to long-term climatic changes. Sci. Total Environ. 2017, 590, 818–831. [Google Scholar] [CrossRef]
- Futter, M.N.; Erlandsson, M.A.; Butterfield, D.; Whitehead, P.G.; Oni, S.K.; Wade, A.J. PERSiST: A flexible rainfall-runoff modelling toolkit for use with the INCA family of models. Hydrol. Earth Syst. Sci. 2014, 18, 855–873. [Google Scholar] [CrossRef]
- Whitehead, P.G.; Costigan, P.A.; Bridges, E.M.; Powlson, D.S.; Goss, M.J.; Goulding, K. Modelling nitrate from agriculture into public water supplies. Philos. Trans. R. Soc. B Biol. Sci. 1990, 329, 403–410. [Google Scholar] [CrossRef]
- Nizzetto, L.; Futter, M.; Langaas, S. Are Agricultural Soils Dumps for Microplastics of Urban Origin? Environ. Sci. Technol. 2016, 50, 10777–10779. [Google Scholar] [CrossRef] [PubMed]
- Horton, A.A.; Dixon, S.J. Microplastics: An introduction to environmental transport processes. Wiley Interdiscip. Rev. Water 2018, 5, e1268. [Google Scholar] [CrossRef]
- Gupta, H.V.; Kling, H.; Yilmaz, K.K.; Martinez, G.F. Decomposition of the mean squared error and NSE performance criteria: Implications for improving hydrological modelling. J. Hydrol. 2009, 377, 80–91. [Google Scholar] [CrossRef]
- Smith, L. Plastic Waste. House of Commons Briefing Paper, Number 08515, 31 March 2020. Available online: www.commonslibrary.parliament.uk/research-briefings (accessed on 17 November 2020).
- Verschoor, A.J.; de Valk, E.; Potential Measures against Microplastic Emissions to Water. National Institute for Public Health and Environment, NL. Report 2017-0193. Available online: www.rivm.nl (accessed on 21 July 2020).
- Tournier, V.; Topham, C.M.; Gilles, A.; David, B.; Folgoas, C.; Moya-Leclair, E.; Kamionka, E.; Desrousseaux, M.-L.; Texier, H.; Gavalda, S.; et al. An engineered PET depolymerase to break down and recycle plastic bottles. Nat. Cell Biol. 2020, 580, 216–219. [Google Scholar] [CrossRef]
- Simon, M.; Vianello, A.; Vollertsen, J. Removal of >10 µm Microplastic Particles from Treated Wastewater by a Disc Filter. Water 2019, 11, 1935. [Google Scholar] [CrossRef]
- Talvitie, J.; Mikola, A.; Koistinen, A.; Setälä, O. Solutions to microplastic pollution—Removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Res. 2017, 123, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Eur Eau Microplastics and the Water Sector—Current Knowledge, Challenges and Possible Solutions. Briefing Note, 6 August 2019. Available online: www.eureau.org (accessed on 15 November 2020).
- Toussaint, B.; Raffael, B.; Angers-Loustau, A.; Gilliland, D.; Kestens, V.; Petrillo, M.; Rio-Echevarria, I.M.; Eede, G.V.D. Review of micro- and nanoplastic contamination in the food chain. Food Addit. Contam. Part A 2019, 36, 639–673. [Google Scholar] [CrossRef] [PubMed]
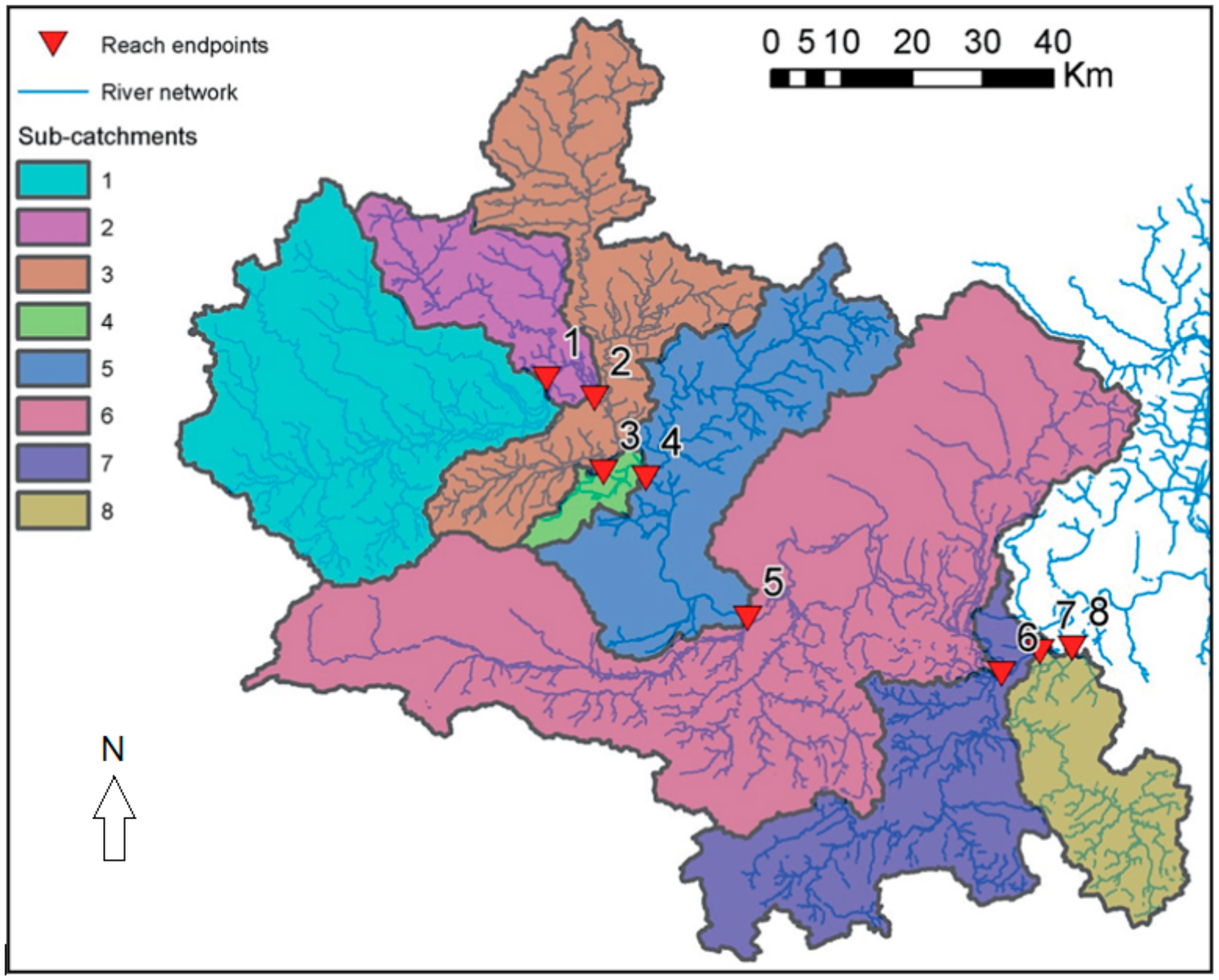

| Reach | Downstream Section | Catchment Area (km2) | Reach Length (m) | Effluent Flow (m3/s) | Mean Slope | % Arable | % Pasture | % Forest | % Urban |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Eynsham | 1609 | 54,100 | 0.50 | 0.0005 | 74.3 | 16.5 | 2.8 | 6.4 |
| 2 | Oxford | 526 | 12,420 | 0.14 | 0.000418 | 60.2 | 16.3 | 5 | 18.5 |
| 3 | Sutton Courtenay | 1288 | 18,960 | 0.70 | 0.000374 | 72.4 | 15.3 | 2.2 | 10 |
| 4 | Days Weir | 58 | 9320 | 0.06 | 0.000128 | 78.8 | 0 | 2.8 | 18.3 |
| 5 | Reading | 1154 | 35,150 | 0.43 | 0.000273 | 72.8 | 10.3 | 8.2 | 8.6 |
| 6 | Staines | 3632 | 70,410 | 3.85 | 0.000406 | 43.9 | 12.2 | 15.1 | 28.7 |
| 7 | Walton | 1102 | 9540 | 0.81 | 0.0001 | 38.8 | 13.1 | 25.3 | 22.7 |
| 8 | Kingston | 589 | 7740 | 0.96 | 0.0003 | 30.5 | 15.4 | 17.7 | 36.3 |
| Class | MP1 | MP2 | MP3 | MP4 | Total |
|---|---|---|---|---|---|
| Eynsham | 7 | 12 | 4514 | 8927 | 13,460 |
| Oxford | 3 | 6 | 2335 | 4795 | 7139 |
| Sutton Courtenay | 7 | 14 | 5913 | 11,989 | 17,923 |
| Days Weir | 7 | 13 | 4848 | 8992 | 13,860 |
| Reading | 30 | 51 | 16,114 | 33,886 | 50,081 |
| Staines | 320 | 616 | 288,816 | 640,622 | 930,374 |
| Walton | 46 | 87 | 37,827 | 74,177 | 112,137 |
| Kingston | 40 | 77 | 33,056 | 65,405 | 98,578 |
| Class | MP1 | MP2 | MP3 | MP4 | Total |
|---|---|---|---|---|---|
| Eynsham | 7 | 1138 | 7394 | 8970 | 17,509 |
| Oxford | 3 | 559 | 3809 | 4817 | 9188 |
| Sutton Courtenay | 7 | 984 | 8630 | 12,030 | 21,651 |
| Days Weir | 7 | 926 | 7013 | 9023 | 16,969 |
| Reading | 30 | 3764 | 23,790 | 34,010 | 61,594 |
| Staines | 320 | 21,131 | 342,260 | 641,543 | 1,005,254 |
| Walton | 46 | 2850 | 44,684 | 74,288 | 121,868 |
| Kingston | 40 | 2252 | 38,330 | 65,491 | 106,113 |
| Class | MP1 | MP2 | MP3 | MP4 | Total |
|---|---|---|---|---|---|
| Eynsham | 3 | 569 | 3697 | 4485 | 8754 |
| Oxford | 2 | 280 | 1904 | 2409 | 4594 |
| Sutton Courtenay | 4 | 492 | 4315 | 6015 | 10,826 |
| Days Weir | 4 | 463 | 3507 | 4512 | 8485 |
| Reading | 15 | 1882 | 11,895 | 17,005 | 30,797 |
| Staines | 160 | 10,565 | 171,130 | 320,771 | 502,627 |
| Walton | 23 | 1425 | 22,342 | 37,144 | 60,934 |
| Kingston | 20 | 1126 | 19,165 | 32,745 | 53,056 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whitehead, P.G.; Bussi, G.; Hughes, J.M.R.; Castro-Castellon, A.T.; Norling, M.D.; Jeffers, E.S.; Rampley, C.P.N.; Read, D.S.; Horton, A.A. Modelling Microplastics in the River Thames: Sources, Sinks and Policy Implications. Water 2021, 13, 861. https://doi.org/10.3390/w13060861
Whitehead PG, Bussi G, Hughes JMR, Castro-Castellon AT, Norling MD, Jeffers ES, Rampley CPN, Read DS, Horton AA. Modelling Microplastics in the River Thames: Sources, Sinks and Policy Implications. Water. 2021; 13(6):861. https://doi.org/10.3390/w13060861
Chicago/Turabian StyleWhitehead, Paul G., Gianbattista Bussi, Jocelyne M. R. Hughes, Ana T. Castro-Castellon, Magnus D. Norling, Elizabeth S. Jeffers, Cordelia P. N. Rampley, Daniel S. Read, and Alice A. Horton. 2021. "Modelling Microplastics in the River Thames: Sources, Sinks and Policy Implications" Water 13, no. 6: 861. https://doi.org/10.3390/w13060861
APA StyleWhitehead, P. G., Bussi, G., Hughes, J. M. R., Castro-Castellon, A. T., Norling, M. D., Jeffers, E. S., Rampley, C. P. N., Read, D. S., & Horton, A. A. (2021). Modelling Microplastics in the River Thames: Sources, Sinks and Policy Implications. Water, 13(6), 861. https://doi.org/10.3390/w13060861







