Efficient Removal of Antimony(III) in Aqueous Phase by Nano-Fe3O4 Modified High-Iron Red Mud: Study on Its Performance and Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of HRM@nFe3O4
2.3. Characterization of HRM@nFe3O4
2.4. Batch Experiments
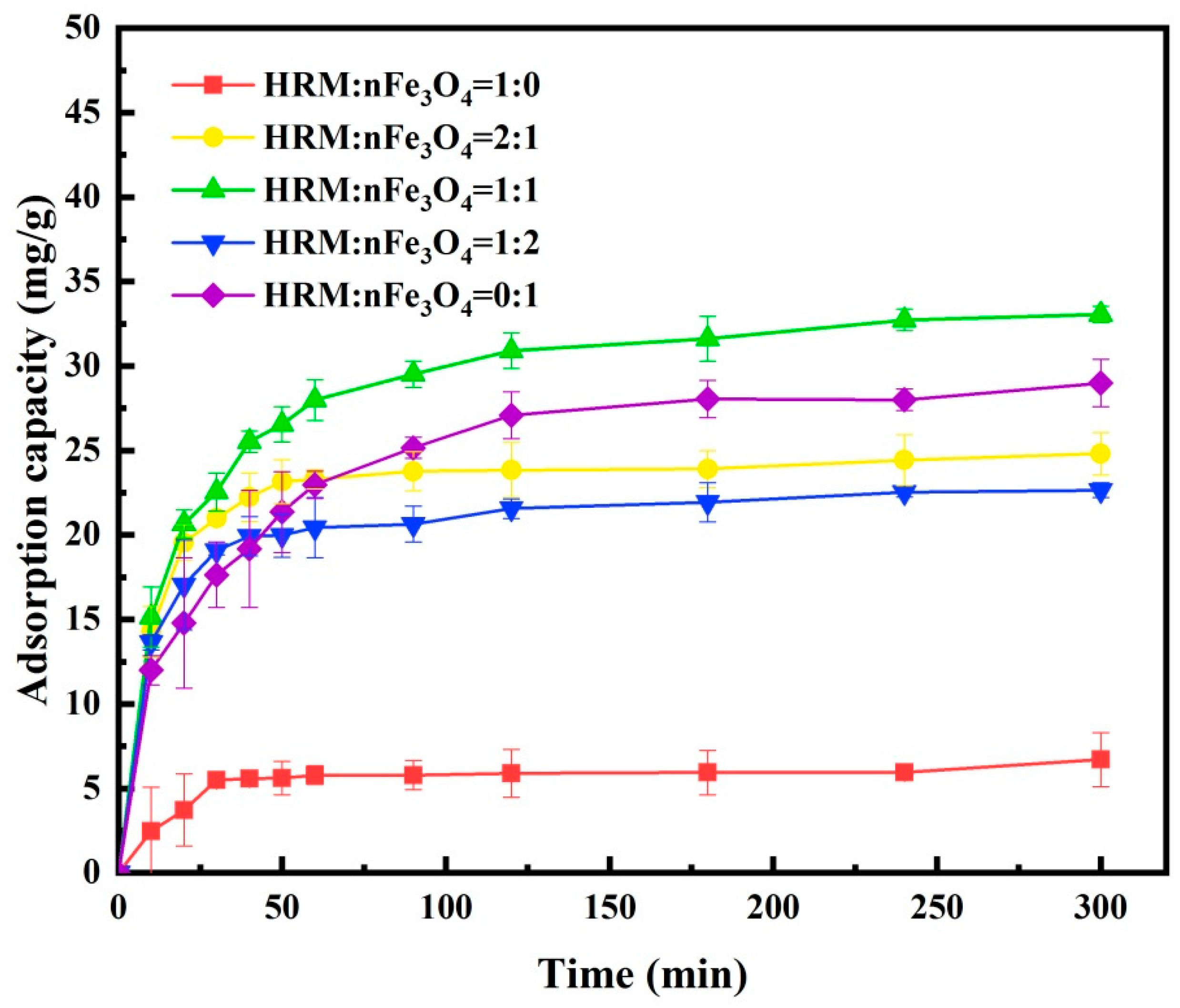
2.4.1. Effect of nFe3O4/HRM Mass Ratio
2.4.2. Adsorption Isotherm and Kinetics
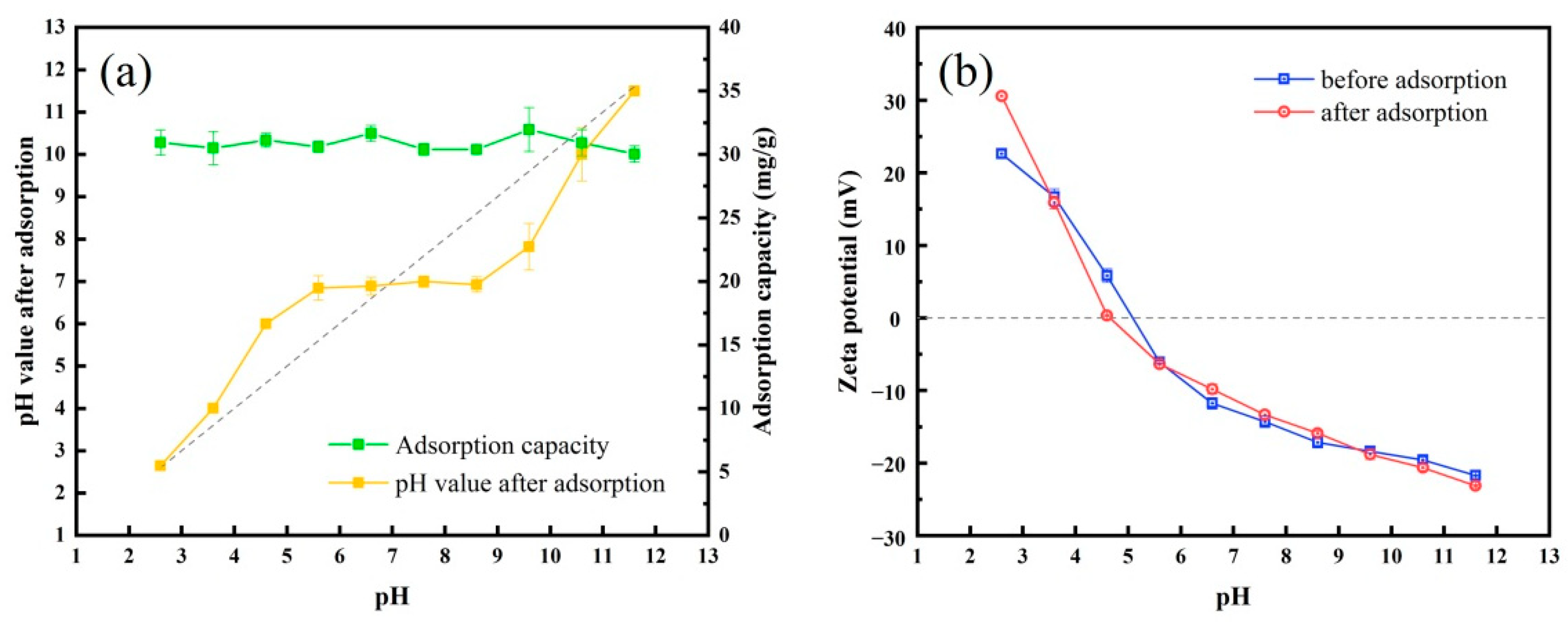
2.4.3. The Influence of Solution PH and Coexisting Ions
2.4.4. Adsorption and Desorption Tests
2.5. Analytical Methods
3. Results and Discussion
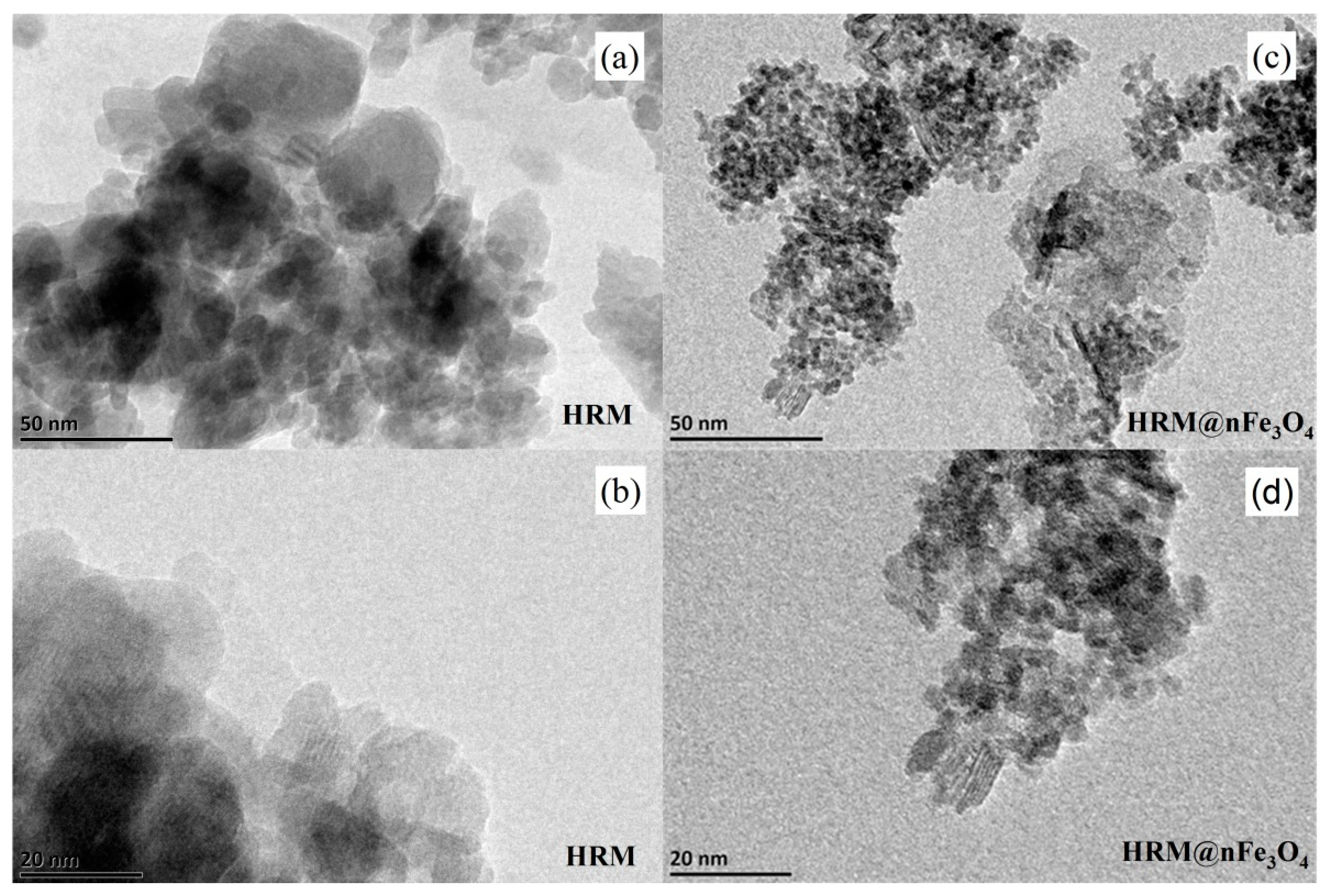
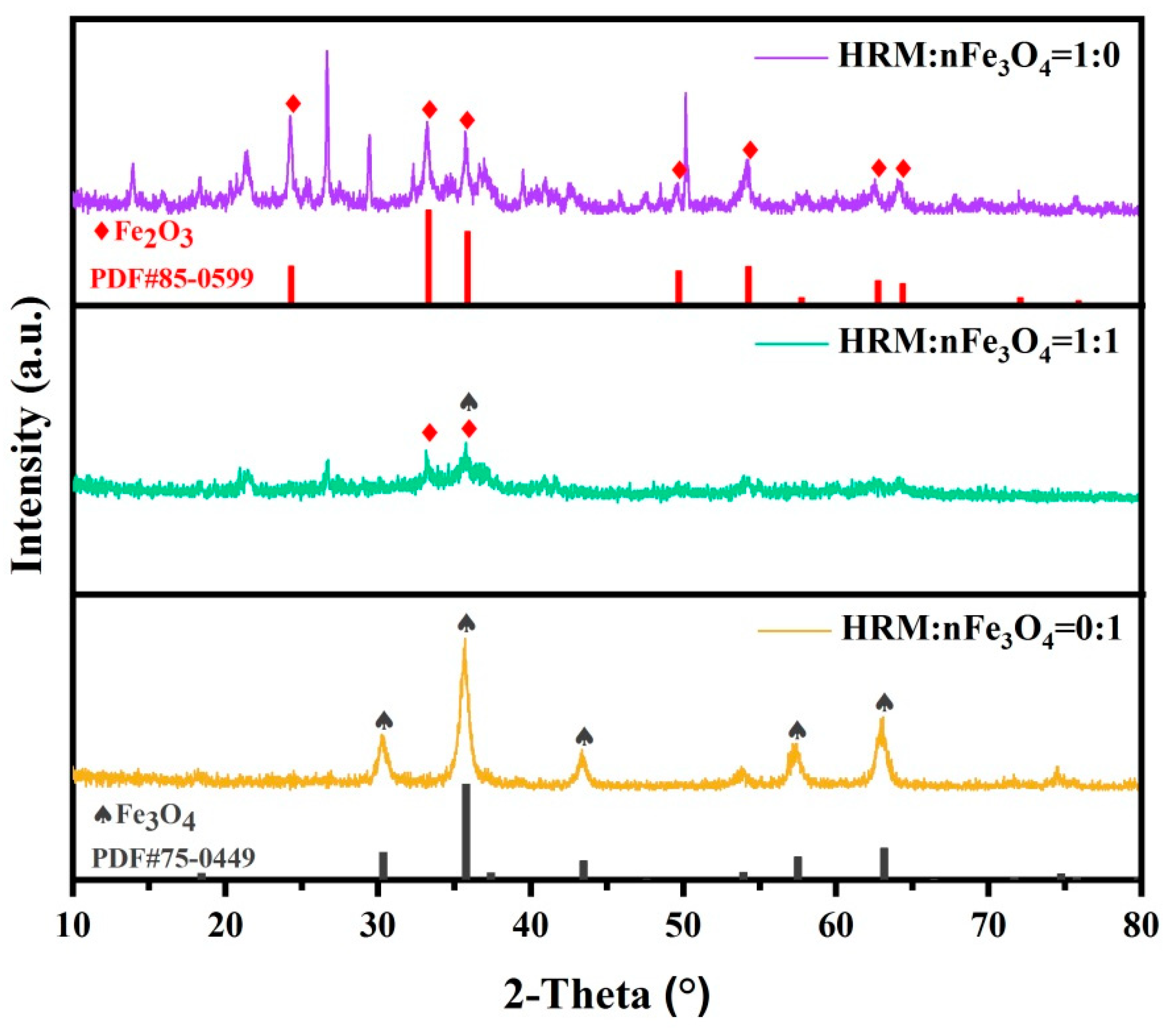
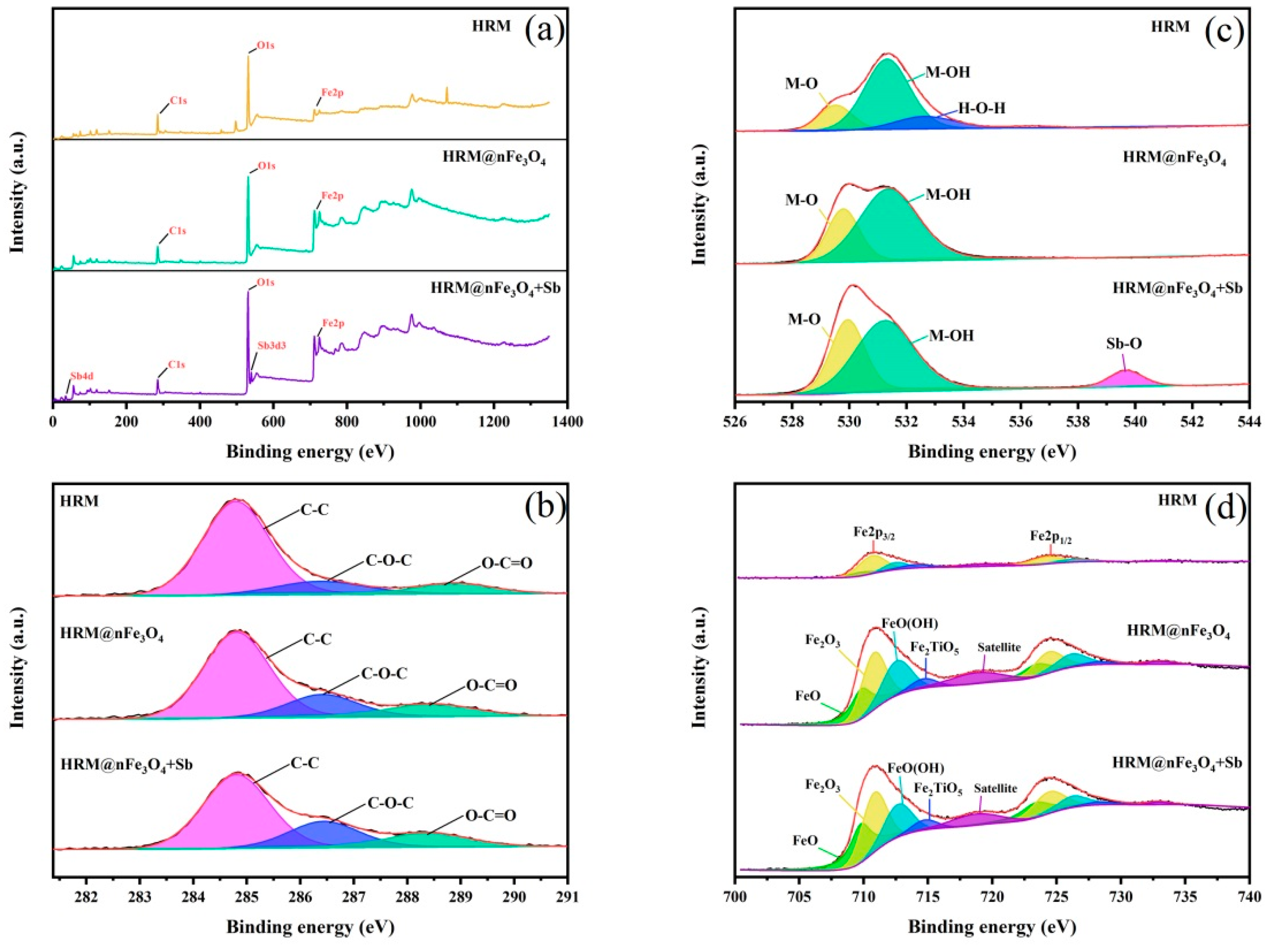
3.1. Structure, Morphology and Surface Properties of HRM@nFe3O4
3.2. Effect of Mass Ratio of nFe3O4/HRM on Sb(III) Adsorption
3.3. Adsorption Behavior
3.3.1. Effect of Solution PH
3.3.2. Influences of Coexisting Ions on Sb(III) Adsorption
3.3.3. Regeneration and Reusability of HRM@ nFe3O4
3.4. Mechanism of Sb(III) Removal by HRM@nFe3O4
3.4.1. Adsorption Kinetics
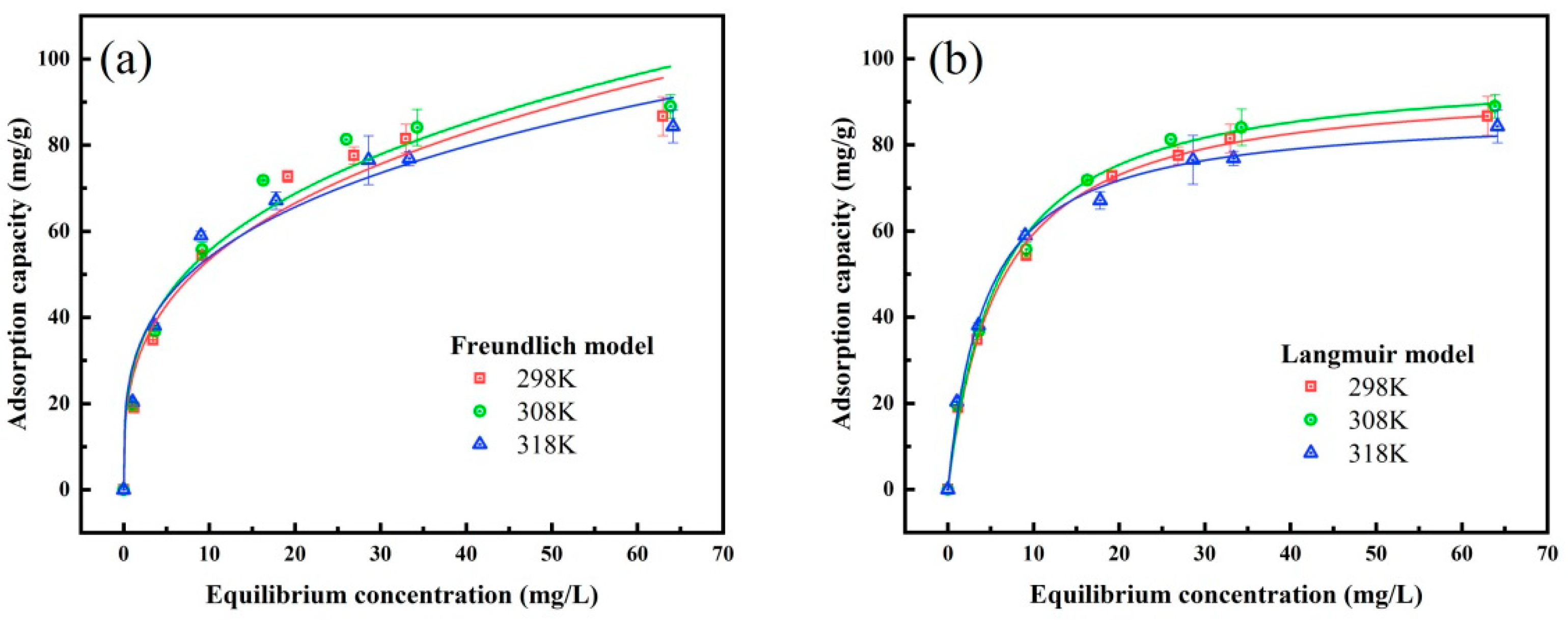
3.4.2. Adsorption Isotherms
3.4.3. Influence of Functional Groups
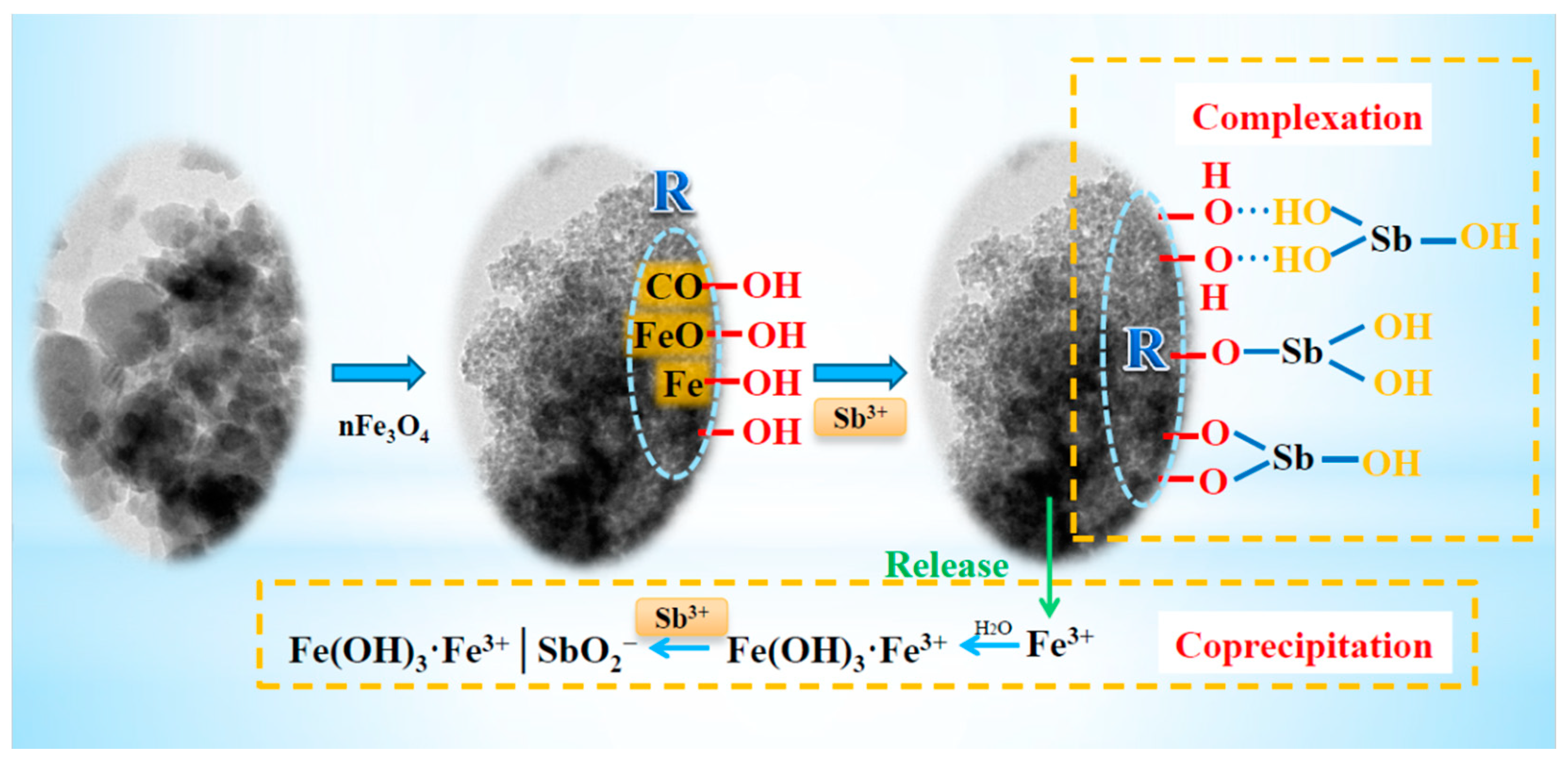
3.4.4. Adsorption Mechanism Reconstruction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, R.; Lei, L.; Su, J.; Zhang, R.; Zhu, Y.; Chen, W.; Wang, L.; Wang, R.; Dai, J.; Lin, Z.; et al. Toxicity of different forms of antimony to rice plant: Effects on root exudates, cell wall components, endogenous hormones and antioxidant system. Sci. Total Environ. 2020, 711, 134589. [Google Scholar] [CrossRef]
- Shan, J.; He, M.; Lin, C.; Ouyang, W.; Liu, X. Simultaneous electrochemical determination of Sb(III) and Sb(V) in Water samples: Deposition potential differences and Sb(III) photooxidation characteristics. Sens. Actuators B Chem. 2020, 305, 127454. [Google Scholar] [CrossRef]
- Yang, X.; Shi, Z.; Yuan, M.; Liu, L. Adsorption of Trivalent Antimony from Aqueous Solution Using Graphene Oxide: Kinetic and Thermodynamic Studies. J. Chem. Eng. Data 2015, 60, 806–813. [Google Scholar] [CrossRef]
- Luo, J.; Luo, X.; Crittenden, J.C.; Qu, J.; Bai, Y.; Peng, Y.; Li, J. Removal of Antimonite (Sb(III)) and Antimonate (Sb(V)) from Aqueous Solution Using Carbon Nanofibers That Are Decorated with Zirconium Oxide (ZrO2). Environ. Sci. Technol. 2015, 49, 11115–11124. [Google Scholar] [CrossRef] [PubMed]
- Filella, M.; Belzile, N.; Chen, Y.-W. Antimony in the environment: A review focused on natural waters: II. Relevant solution chemistry. Earth-Sci. Rev. 2002, 59, 265–285. [Google Scholar] [CrossRef]
- Winship, K. Toxicity of antimony and its compounds. Advers. Drug React. Acute Poisoning Rev. 1987, 6, 67–90. [Google Scholar]
- U.S. Environmental Protection Agency. Drinking Water Standards and Health Advisories; Office of Water: Washington, DC, USA, 2009.
- Guo, W.; Fu, Z.; Zhang, Z.; Wang, H.; Liu, S.; Feng, W.; Zhao, X.; Giesy, J.P. Synthesis of Fe3O4 magnetic nanoparticles coated with cationic surfactants and their applications in Sb(V) removal from water. Sci. Total Environ. 2020, 710, 136302. [Google Scholar] [CrossRef]
- Guo, X.; Wu, Z.; He, M. Removal of antimony(V) and antimony(III) from drinking water by coagulation–flocculation–sedimentation (CFS). Water Res. 2009, 43, 4327–4335. [Google Scholar] [CrossRef]
- Wu, Z.; He, M.; Guo, X.; Zhou, R. Removal of antimony (III) and antimony (V) from drinking water by ferric chloride coagulation: Competing ion effect and the mechanism analysis. Sep. Purif. Technol. 2010, 76, 184–190. [Google Scholar] [CrossRef]
- Wu, F.; Sun, F.; Wu, S.; Yan, Y.; Xing, B. Removal of antimony(III) from aqueous solution by freshwater cyanobacteria Microcystis biomass. Chem. Eng. J. 2012, 183, 172–179. [Google Scholar] [CrossRef]
- Naiya, T.K.; Bhattacharya, A.K.; Das, S.K. Adsorption of Cd (II) and Pb (II) from aqueous solutions on activated alumina. J. Colloid Interface Sci. 2009, 333, 14–26. [Google Scholar] [CrossRef]
- Wang, X.; He, M.; Lin, C.; Gao, Y.; Zheng, L. Antimony(III) oxidation and antimony(V) adsorption reactions on synthetic manganite. Geochemistry 2012, 72, 41–47. [Google Scholar] [CrossRef]
- Bergmann, M.H.; Koparal, A.S. Electrochemical antimony removal from accumulator acid: Results from removal trials in laboratory cells. J. Hazard. Mater. 2011, 196, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Xiao, E.; Dong, Y.; Tang, S.; Krumins, V.; Ning, Z.; Sun, M.; Zhao, Y.; Wu, S.; Xiao, T. Profiling microbial community in a watershed heavily contaminated by an active antimony (Sb) mine in Southwest China. Sci. Total Environ. 2016, 550, 297–308. [Google Scholar] [CrossRef]
- Wei, D.; Li, B.; Luo, L.; Zheng, Y.; Huang, L.; Zhang, J.; Yang, Y.; Huang, H. Simultaneous adsorption and oxidation of antimonite onto nano zero-valent iron sludge-based biochar: Indispensable role of reactive oxygen species and redox-active moieties. J. Hazard. Mater. 2020, 391, 122057. [Google Scholar] [CrossRef]
- Ungureanu, G.; Santos, S.; Boaventura, R.; Botelho, C. Arsenic and antimony in water and wastewater: Overview of removal techniques with special reference to latest advances in adsorption. J. Environ. Manag. 2015, 151, 326–342. [Google Scholar] [CrossRef]
- Bray, A.W.; Stewart, D.I.; Courtney, R.; Rout, S.P.; Humphreys, P.N.; Mayes, W.M.; Burke, I.T. Sustained bauxite residue rehabilitation with gypsum and organic matter 16 years after initial treatment. Environ. Sci. Technol. 2018, 52, 152–161. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Liu, X.; Luo, L.; Zhou, Y.; Wei, J.; Chen, A.; Tang, L.; Wu, H.; Deng, Y.; Zhang, F.; et al. Remediation of Cu, Pb, Zn and Cd-contaminated agricultural soil using a combined red mud and compost amendment. Int. Biodeterior. Biodegrad. 2017, 118, 73–81. [Google Scholar] [CrossRef]
- Li, G.; Liu, M.; Rao, M.; Jiang, T.; Zhuang, J.; Zhang, Y. Stepwise extraction of valuable components from red mud based on reductive roasting with sodium salts. J. Hazard. Mater. 2014, 280, 774–780. [Google Scholar] [CrossRef]
- Lyu, F.; Gao, J.; Sun, N.; Liu, R.; Sun, X.; Cao, X.; Wang, L.; Sun, W. Utilisation of propyl gallate as a novel selective collector for diaspore flotation. Miner. Eng. 2019, 131, 66–72. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, K.; Liu, Y.; Lyu, G.; Li, X.; Chen, X. A Review of Comprehensive Utilization of High-Iron Red Mud of China. Light Met. 2020, 65–71. [Google Scholar] [CrossRef]
- Power, G.; Gräfe, M.; Klauber, C. Bauxite residue issues: I. Current management, disposal and storage practices. Hydrometallurgy 2011, 108, 33–45. [Google Scholar] [CrossRef]
- Ke, W.; Zhang, X.; Zhu, F.; Wu, H.; Zhang, Y.; Shi, Y.; Hartley, W.; Xue, S. Appropriate human intervention stimulates the development of microbial communities and soil formation at a long-term weathered bauxite residue disposal area. J. Hazard. Mater. 2021, 405, 124689. [Google Scholar] [CrossRef]
- Khairul, M.; Zanganeh, J.; Moghtaderi, B. The composition, recycling and utilisation of Bayer red mud. Resour. Conserv. Recycl. 2019, 141, 483–498. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Zhou, Y.; Zhang, J.; Mao, Q.; Yang, Y.; Huang, H.; Liu, Z.; Peng, Q.; Luo, L. Effects of red mud based passivator on the transformation of Cd fraction in acidic Cd-polluted paddy soil and Cd absorption in rice. Sci. Total Environ. 2018, 640-641, 736–745. [Google Scholar] [CrossRef]
- Hua, Y.; Heal, K.V.; Friesl-Hanl, W. The use of red mud as an immobiliser for metal/metalloid-contaminated soil: A review. J. Hazard. Mater. 2017, 325, 17–30. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Hu, G.; Lyu, F.; Yue, T.; Tang, H.; Han, H.; Yang, Y.; Liu, R.; Sun, W. Application of Red Mud in Wastewater Treatment. Minerals 2019, 9, 281. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Liu, L.; Luo, L.; Liu, Y.; Wei, J.; Zhang, J.; Yang, Y.; Chen, A.; Mao, Q.; Zhou, Y. Response of soil microbial communities to red mud-based stabilizer remediation of cadmium-contaminated farmland. Environ. Sci. Pollut. Res. 2018, 25, 11661–11669. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Huang, L.; Xue, S.-G.; Huang, Y.-Y.; Hartley, W.; Cui, M.-Q.; Wong, M.-H. Arsenic sorption by red mud-modified biochar produced from rice straw. Environ. Sci. Pollut. Res. 2017, 24, 18168–18178. [Google Scholar] [CrossRef]
- Yoon, K.; Cho, D.W.; Tsang, Y.F.; Tsang, D.C.; Kwon, E.E.; Song, H. Synthesis of functionalised biochar using red mud, lignin, and carbon dioxide as raw materials. Chem. Eng. J. 2019, 361, 1597–1604. [Google Scholar] [CrossRef]
- Li, Y.; Huang, H.; Xu, Z.; Ma, H.; Guo, Y. Mechanism study on manganese(II) removal from acid mine wastewater using red mud and its application to a lab-scale column. J. Clean. Prod. 2020, 253, 119955. [Google Scholar] [CrossRef]
- Chon, C.-M.; Cho, D.-W.; Nam, I.-H.; Kim, J.-G.; Song, H. Fabrication of Fe/Mn oxide composite adsorbents for adsorptive removal of zinc and phosphate. J. Soils Sediments 2018, 18, 946–956. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Siddiqui, M.; Mubarak, N.; Baloch, H.A.; Abdullah, E.; Mazari, S.A.; Griffin, G.; Srinivasan, M.; Tanksale, A. Iron Oxide Nanomaterials for the Removal of Heavy Metals and Dyes from Wastewater. Nanoscale Mater. Water Purif. 2019, 447–472. [Google Scholar] [CrossRef]
- Hu, Y.; Peng, X.; Ai, Z.; Jia, F.; Zhang, L. Liquid Nitrogen Activation of Zero-Valent Iron and Its Enhanced Cr(VI) Removal Performance. Environ. Sci. Technol. 2019, 53, 8333–8341. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, Y.; Chai, L.; Lei, H.; Yang, W.; Hou, L.; Yuan, T.; Jin, L.; Tang, C.J.; Luo, J. Highly-dispersed Fe2O3 @Celectrode materials for Pb2+ removal by capacitive deionization. Carbon 2019, 153, 12–20. [Google Scholar] [CrossRef]
- Qi, Z.; Joshi, T.P.; Liu, R.; Liu, H.; Qu, J. Synthesis of Ce(III)-doped Fe3O4 magnetic particles for efficient removal of antimony from aqueous solution. J. Hazard. Mater. 2017, 329, 193–204. [Google Scholar] [CrossRef]
- Qi, P.; Luo, R.; Pichler, T.; Zeng, J.; Wang, Y.; Fan, Y.; Sui, K. Development of a magnetic core-shell Fe3O4@TA@UiO-66 microsphere for removal of arsenic(III) and antimony(III) from aqueous solution. J. Hazard. Mater. 2019, 378, 120721. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Liu, G.; Wang, Y.; Shi, B.; Han, K.; Zhuang, Y.; Kong, Y. Simultaneous cationic Cu (II)‒anionic Sb (III) removal by NH2-Fe3O4-NTA core-shell magnetic nanoparticle sorbents synthesized via a facile one-pot approach. J. Hazard. Mater. 2019, 362, 246–257. [Google Scholar] [CrossRef]
- Yu, G.; Fu, F. Exploration of different adsorption performance and mechanisms of core-shell Fe3O4@Ce-Zr oxide composites for Cr(VI) and Sb(III). J. Colloid Interface Sci. 2020, 576, 10–20. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, R.J.; Ren, B.Z.; Hou, B.; Hursthouse, A. Preparation of a novel Fe3O4/HCO composite adsorbent and the mechanism for the removal of antimony (III) from aqueous solution. Sci. Rep. 2019, 9, 13021. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Wang, Z.; He, C.; Lyu, W.; Yan, W.; Yang, L. Enhanced antimonate (Sb(V)) removal from aqueous solution by La-doped magnetic biochars. Chem. Eng. J. 2018, 354, 623–632. [Google Scholar] [CrossRef]
- Liu, S.; Feng, H.; Tang, L.; Dong, H.; Wang, J.; Yu, J.; Feng, C.; Liu, Y.; Luo, T.; Ni, T. Removal of Sb(III) by sulfidated nanoscale zerovalent iron: The mechanism and impact of environmental conditions. Sci. Total Environ. 2020, 736, 139629. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, C.; Zheng, T.; Ma, J.; Zhang, G.; Ren, G.; Wang, L.; Liu, Y. Efficient oxidation and sorption of arsenite using a novel titanium(IV)-manganese(IV) binary oxide sorbent. J. Hazard. Mater. 2018, 353, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhou, J.; Lou, Z.; Zhou, X.; Liu, Y.; Li, Y.; Baig, S.A.; Xu, X. Removal of Sb(V) from aqueous solutions using Fe-Mn binary oxides: The influence of iron oxides forms and the role of manganese oxides. Chem. Eng. J. 2018, 354, 577–588. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting Physisorption Data for Gas Solid Systems with Special Reference to the Determination of Surface-Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Zhang, G.; Wu, Q.; Wang, D. Facile fabrication of nanostructured cerium-manganese binary oxide for enhanced arsenite removal from water. Chem. Eng. J. 2018, 334, 1518–1526. [Google Scholar] [CrossRef]
- Zeng, J.; Qi, P.; Shi, J.; Pichler, T.; Wang, F.; Wang, Y.; Sui, K. Chitosan functionalized iron nanosheet for enhanced removal of As(III) and Sb(III): Synergistic effect and mechanism. Chem. Eng. J. 2020, 382, 122999. [Google Scholar] [CrossRef]
- You, D.; Min, X.; Liu, L.; Ren, Z.; Xiao, X.; Pavlostathis, S.G.; Luo, J.; Luo, X. New insight on the adsorption capacity of metallogels for antimonite and antimonate removal: From experimental to theoretical study. J. Hazard. Mater. 2018, 346, 218–225. [Google Scholar] [CrossRef]
- Yan, L.; Song, J.; Chan, T.; Jing, C. Insights into antimony adsorption on {001} TiO2: XAFS and DFT study. Environ. Sci. Technol. 2017, S1, 6335–6341. [Google Scholar] [CrossRef]
- Guo, X.; Wu, Z.; He, M.; Meng, X.; Jin, X.; Qiu, N.; Zhang, J. Adsorption of antimony onto iron oxyhydroxides: Adsorption behavior and surface structure. J. Hazard. Mater. 2014, 276, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.; Hayat, T.; Alsaedi, A.; Chen, C. Screening of Zirconium-Based Metal–Organic Frameworks for Efficient Simultaneous Removal of Antimonite (Sb(III)) and Antimonate (Sb(V)) from Aqueous Solution. ACS Sustain. Chem. Eng. 2017, 5, 11496–11503. [Google Scholar] [CrossRef]
- Zhao, T.; Tang, Z.; Zhao, X.; Zhang, H.; Wang, J.; Wu, F.; Giesy, J.P.; Shi, J. Efficient removal of both antimonite (Sb(iii)) and antimonate (Sb(v)) from environmental water using titanate nanotubes and nanoparticles. Environ. Sci. Nano 2019, 6, 834–850. [Google Scholar] [CrossRef]
- Iqbal, M.; Saeed, A.; Edyvean, R.G. Bioremoval of antimony(III) from contaminated water using several plant wastes: Optimization of batch and dynamic flow conditions for sorption by green bean husk (Vigna radiata). Chem. Eng. J. 2013, 225, 192–201. [Google Scholar] [CrossRef]
- Pena, M.; Meng, X.; Korfiatis, G.P.; Jing, C. Adsorption Mechanism of Arsenic on Nanocrystalline Titanium Dioxide. Environ. Sci. Technol. 2006, 40, 1257–1262. [Google Scholar] [CrossRef]
- Han, L.; Sun, H.; Ro, K.S.; Sun, K.; Libra, J.A.; Xing, B. Removal of antimony (III) and cadmium (II) from aqueous solution using animal manure-derived hydrochars and pyrochars. Bioresour. Technol. 2017, 234, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Buschnann, J.; Sigg, L. Antimony(III) Binding to Humic Substances: Influence of pH and Type of Humic Acid. Environ. Sci. Technol. 2004, 38, 4535–4541. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Y.; Wang, X.; Li, B.; Zhou, Y.; Liu, D.; Liu, D.; Liu, S. Efficient extraction of antimony(III) by titanate nanosheets: Study on adsorption behavior and mechanism. Ecotoxicol. Environ. Saf. 2021, 207, 111271. [Google Scholar] [CrossRef] [PubMed]
- Thammawong, C.; Opaprakasit, P.; Tangboriboonrat, P.; Sreearunothai, P. Prussian blue-coated magnetic nanoparticles for removal of cesium from contaminated environment. J. Nanopart. Res. 2013, 15, 1–10. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, P.; Liu, F.; Li, F.; An, X.; Liu, J.; Wang, Z.; Shen, C.; Sand, W. Electroactive Modified Carbon Nanotube Filter for Simultaneous Detoxification and Sequestration of Sb(III). Environ. Sci. Technol. 2019, 53, 1527–1535. [Google Scholar] [CrossRef]
- Zhao, X.; Dou, X.; Mohan, D.; Pittman, C.U.; Ok, Y.S.; Jin, X. Antimonate and antimonite adsorption by a polyvinyl alcohol-stabilized granular adsorbent containing nanoscale zero-valent iron. Chem. Eng. J. 2014, 247, 250–257. [Google Scholar] [CrossRef]
- Mishra, S.; Dwivedi, J.; Kumar, A.; Sankararamakrishnan, N. Removal of antimonite (Sb(iii)) and antimonate (Sb(v)) using zerovalent iron decorated functionalized carbon nanotubes. RSC Adv. 2016, 6, 95865–95878. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, F.; Qi, Z.; Shen, C.; Li, F.; Ma, C.; Huang, M.; Wang, Z.; Li, J. Simultaneous oxidation and sorption of highly toxic Sb(III) using a dual-functional electroactive filter. Environ. Pollut. 2019, 251, 72–80. [Google Scholar] [CrossRef] [PubMed]
- De Miguel, E.; Izquierdo, M.; Gómez, A.; Mingot, J.; Barrio-Parra, F. Risk assessment from exposure to arsenic, antimony, and selenium in urban gardens (Madrid, Spain). Environ. Toxicol. Chem. 2017, 36, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, N.; Tan, L.; Zhang, R.; Zhao, Q.; Wang, H. Removal of aqueous As(III) Sb(III) by potassium ferrate (K2FeO4): The function of oxidation and flocculation. Sci. Total Environ. 2020, 726, 138541. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.A.; Sarı, A.; Tuzen, M. Effective adsorption of antimony(III) from aqueous solutions by polyamide-graphene composite as a novel adsorbent. Chem. Eng. J. 2017, 307, 230–238. [Google Scholar] [CrossRef]
- Lan, B.; Wang, Y.; Wang, X.; Zhou, X.; Kang, Y.; Li, L. Aqueous arsenic (As) and antimony (Sb) removal by potassium ferrate. Chem. Eng. J. 2016, 292, 389–397. [Google Scholar] [CrossRef]
- Tu, Y.; Ren, L.-F.; Lin, Y.; Shao, J.; He, Y.; Gao, X.; Shen, Z. Adsorption of antimonite and antimonate from aqueous solution using modified polyacrylonitrile with an ultrahigh percentage of amidoxime groups. J. Hazard. Mater. 2020, 388, 121997. [Google Scholar] [CrossRef]
- He, X.; Min, X.; Luo, X. Efficient Removal of Antimony (III, V) from Contaminated Water by Amino Modification of a Zirconium Metal–Organic Framework with Mechanism Study. J. Chem. Eng. Data 2017, 62, 1519–1529. [Google Scholar] [CrossRef]
- Zhou, Z.; Dai, C.; Zhou, X.; Zhao, J.; Zhang, Y. The Removal of Antimony by Novel NZVI-Zeolite: The Role of Iron Transformation. Water Air Soil Pollut. 2015, 226, 76. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, C.; Alves, M.E.; Ata-Ul-Karim, S.T.; Zhou, D.M.; He, J.Z.; Cui, P.X.; Wang, Y.J. The oxidation and sorption mechanism of Sb on δ-MnO2. Chem. Eng. J. 2018, 342, 429–437. [Google Scholar] [CrossRef]
- Jia, X.; Zhou, J.; Liu, J.; Liu, P.; Yu, L.; Wen, B.; Feng, Y. The antimony sorption and transport mechanisms in removal experiment by Mn-coated biochar. Sci. Total Environ. 2020, 724, 138158. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, G.; Liu, C.; Li, J.; Zheng, T.; Ma, J.; Wang, L.; Jiang, J.; Zhai, X. Enhanced removal of arsenite and arsenate by a multifunctional Fe-Ti-Mn composite oxide: Photooxidation, oxidation and adsorption. Water Res. 2018, 147, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Qiu, S.; Zhang, X.; Nie, G. Nanoconfined hydrous titanium oxides with excellent acid stability for selective and efficient removal of As(V) from acidic wastewater. Chem. Eng. J. 2020, 400, 125907. [Google Scholar] [CrossRef]
- Ge, X.; Liu, J.; Song, X.; Wang, G.; Zhang, H.; Zhang, Y.; Zhao, H. Hierarchical iron containing γ-MnO2 hollow microspheres: A facile one-step synthesis and effective removal of As(III) via oxidation and adsorption. Chem. Eng. J. 2016, 301, 139–148. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.N.; Sun, Y.; Pan, X.; Zhang, D.; Tsang, Y.F. Differences in Sb(V) and As(V) adsorption onto a poorly crystalline phyllomanganate (δ-MnO2): Adsorption kinetics, isotherms, and mechanisms. Process Saf. Environ. Prot. 2018, 113, 40–47. [Google Scholar] [CrossRef]
- Herranz, T.; Rojas, S.; Ojeda, M.; Pérez-Alonso, F.J.; Terreros, P.; Pirota, K.; Fierro, J.L.G. Synthesis, Structural Features, and Reactivity of Fe-Mn Mixed Oxides Prepared by Mi-croemulsion. Chem. Mater. 2006, 18, 2364–2375. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition met-als, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Esfandiari, N.; Kashefi, M.; Mirjalili, M.; Afsharnezhad, S. Role of silica mid-layer in thermal and chemical stability of hierarchical Fe3O4-SiO2-TiO2 nanoparticles for improvement of lead adsorption: Kinetics, thermodynamic and deep XPS investigation. Mater. Sci. Eng. B 2020, 262, 114690. [Google Scholar] [CrossRef]
- Guo, S.; Wang, S.; Wu, N.; Liu, J.; Ni, Y.; Liu, W. Facile synthesis of porous Fe2TiO5 microparticulates serving as anode material with enhanced electrochemical performances. RSC Adv. 2015, 5, 103767–103775. [Google Scholar] [CrossRef]
- Liu, G.; Debnath, S.; Paul, K.W.; Han, W.; Hausner, D.B.; Hosein, H.A.; Michel, F.M.; Parise, J.B.; Sparks, D.L.; Strongin, D.R. Characterization and Surface Reactivity of Ferrihydrite Nanoparticles Assembled in Ferri-tin. Langmuir 2006, 22, 9313–9321. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, S.; Cho, D.W.; Du, Q.; Song, J.; Tsang, D.C. Porous biochar composite assembled with ternary needle-like iron-manganese-sulphur hy-brids for high-efficiency lead removal. Bioresour. Technol. 2019, 272, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhao, Y.; Ma, S.; Zhu, B.; Zhang, J.; Zheng, C. Mercury Removal by Magnetic Biochar Derived from Simultaneous Activation and Magnetiza-tion of Sawdust. Environ. Sci. Technol. 2016, 50, 12040–12047. [Google Scholar] [CrossRef] [PubMed]
- Decarlo, E.H.; Zeltlln, H.; Fernando, Q. Simultaneous Separation of Trace Levels of Germanium, Antimony, Arsenic, and Sele-nium from an Acid Matrix by Adsorbing Colloid Flotation. Anal. Chem. 1981, 53, 1104–11077. [Google Scholar] [CrossRef]


| Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||||
|---|---|---|---|---|---|---|---|
| Sb | C0 (mg·L−1) | qe (mg·g−1) | k1 (min−1) | R2 | qe (mg·g−1) | k2 [mg·(g·min)−1] | R2 |
| 5 | 22.0170 | 0.0214 | 0.9838 | 25.4863 | 0.0004 | 0.9930 | |
| 10 | 31.0136 | 0.1013 | 0.9473 | 33.1181 | 0.0022 | 0.9909 | |
| 20 | 60.8113 | 0.0175 | 0.9688 | 72.2033 | 0.0001 | 0.9831 | |
| Langmuir Model | Freundlich Model | ||||||
|---|---|---|---|---|---|---|---|
| Sb | T (K) | kL (L·mg−1) | qm (mg·g−1) | R2 | kF (L·mg−1) | n | R2 |
| 298 | 0.1661 | 95.0291 | 0.9967 | 25.7512 | 3.1579 | 0.9598 | |
| 308 | 0.1665 | 98.0278 | 0.9948 | 27.2642 | 3.2412 | 0.9557 | |
| 318 | 0.2249 | 87.7352 | 0.9946 | 28.2786 | 3.5599 | 0.9652 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Luo, L.; Luo, S.; Peng, K.; Zhou, Y.; Mao, Q.; Yang, J.; Yang, Y. Efficient Removal of Antimony(III) in Aqueous Phase by Nano-Fe3O4 Modified High-Iron Red Mud: Study on Its Performance and Mechanism. Water 2021, 13, 809. https://doi.org/10.3390/w13060809
Peng Y, Luo L, Luo S, Peng K, Zhou Y, Mao Q, Yang J, Yang Y. Efficient Removal of Antimony(III) in Aqueous Phase by Nano-Fe3O4 Modified High-Iron Red Mud: Study on Its Performance and Mechanism. Water. 2021; 13(6):809. https://doi.org/10.3390/w13060809
Chicago/Turabian StylePeng, Yizhe, Lin Luo, Shuang Luo, Kejian Peng, Yaoyu Zhou, Qiming Mao, Jian Yang, and Yuan Yang. 2021. "Efficient Removal of Antimony(III) in Aqueous Phase by Nano-Fe3O4 Modified High-Iron Red Mud: Study on Its Performance and Mechanism" Water 13, no. 6: 809. https://doi.org/10.3390/w13060809





