Phosphorus Removal from Wastewater: The Potential Use of Biochar and the Key Controlling Factors
Abstract
1. Introduction
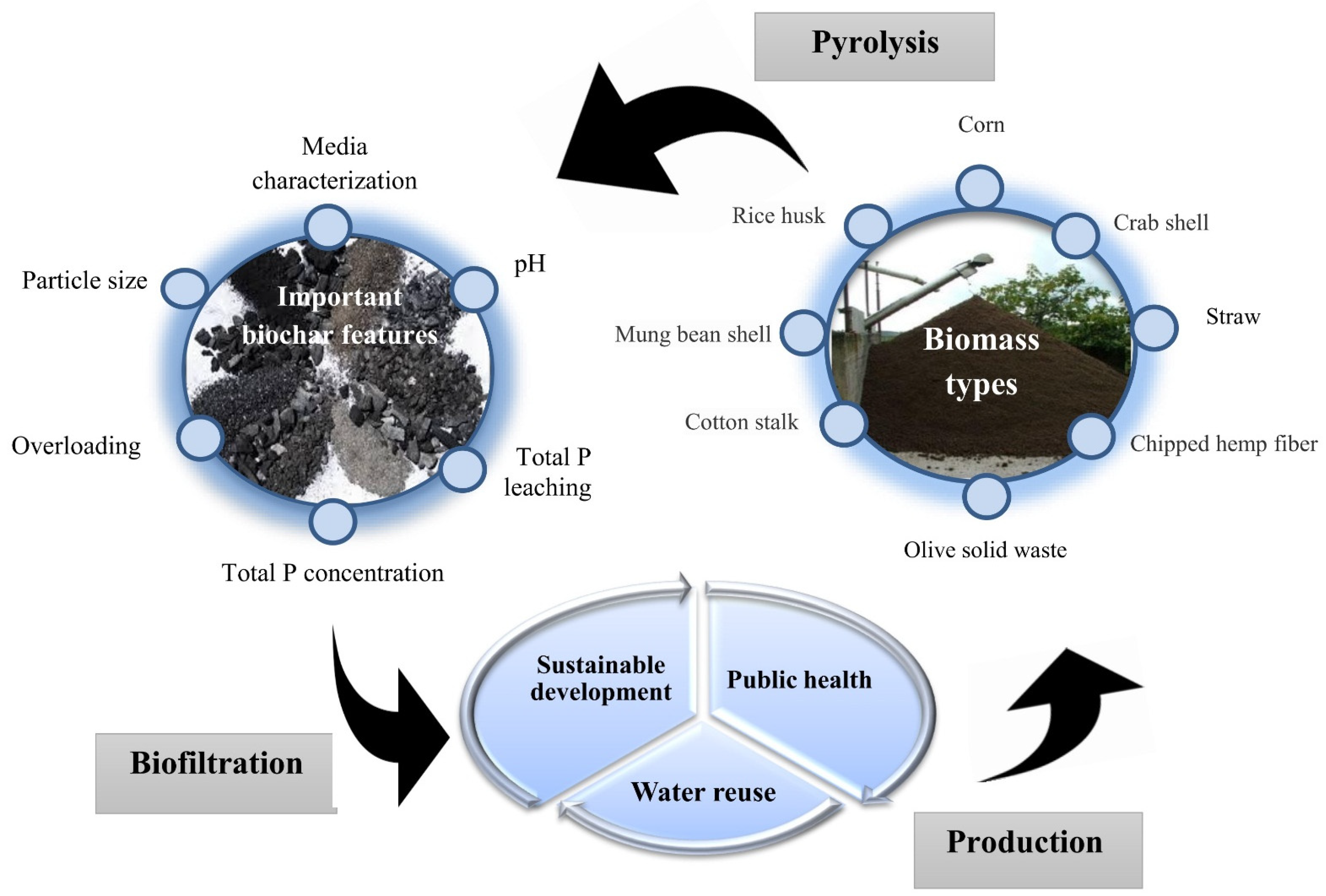
2. The Importance of Biochar Application Compared to Other Methods
3. Other Suggested Adsorbents along with Biochar
3.1. Metal Oxides and Hydroxides
3.2. Carbonates and Hydroxides of Calcium and Magnesium Cations
3.3. Hydrotalcite
3.4. Anion Exchange Resins
3.5. Industrial Wastes
3.6. Organic Wastes
3.7. Hydrochars
4. The Most Important Factors Affecting the Sorption of P by Biochar
4.1. Biochar Surface Area
4.2. Influence of Metal Oxides on the Sorption of P by Biochar
4.3. The Effect of Coexisting Bulk Solution Anions on the Sorption of Phosphorus by Biochar
4.4. Influence of Feedstock Types and Pyrolysis Temperature on Removal of Phosphorus from Wastewater and Aqueous Solutions
4.5. Structural Characteristics
4.6. Electrical Conductivity (EC)
4.7. Metal Composition
4.8. pH
4.9. Zeta Potential
4.10. CEC and AEC
5. P sorption Thermodynamic and Mechanisms in Biochar
6. Conclusions, Perspectives and Future Direction
- Development of biochar modification methods by salts of different metallic cations and determination of effective and efficient methods for removal of P from wastewater.
- Evaluation of the stability of biochar after removal of P and determination of P retention by biochar.
- Determination of P release from biochar surface by different extractors and application of biochar as soil P release fertilizer.
- Evaluation of P removal efficiency by biochar in real or simulated wastewater.
- A study of biochar recovery methods to reduce costs and reuse in wastewater treatment.
- A study of multiple activation/treatment methods of biochar to increase the P sorption capacity.
- An investigation of the relationship between types of metal cations and pyrolysis temperature to better understand the mechanisms of P sorption by biochar.
- A precise and in-depth study of the mechanisms involved during P removal has yet to be published. Thus, P sorption mechanisms need to be studied to enable the development of more effective methods for producing biochars and optimizing their efficiency for practical application.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elser, J.J.; Bracken, M.E.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef]
- Dodds, W.K.; Smith, V.H. Nitrogen, phosphorus, and eutrophication in streams. Inland Waters 2016, 6, 155–164. [Google Scholar] [CrossRef]
- Gibson, G.; Carlson, R.; Gimpson, J.; Smeltzer, E.; Gerritson, J.; Chapra, S.; Heiskary, S.; Jones, J.; Kennedy, R. Nutrient Criteria Technical Guidance Manual: Lakes and Reservoirs (EPA-822-B-00-001); US Gov. Print. Office: Washington, DC, USA, 2000. [Google Scholar]
- Grover, J.P. Phosphorus-dependent growth kinetics of 11 species of freshwater algae. Limnol. Oceanogr. 1989, 34, 341–348. [Google Scholar] [CrossRef]
- Rybicki, S. Advanced Wastewater Treatment: Phosphorus Removal from Wastewater; Royal Institute of Technology: Stockholm, Sweden, 1997. [Google Scholar]
- Yin, Q.; Zhang, B.; Wang, R.; Zhao, Z. Biochar as an adsorbent for inorganic nitrogen and phosphorus removal from water: A review. Environ. Sci. Pollut. Res. 2017, 24, 26297–26309. [Google Scholar] [CrossRef]
- Wurtsbaugh, W.A.; Paerl, H.W.; Dodds, W.K. Nutrients, eutrophication and harmful algal blooms along the freshwater to marine continuum. Wiley Interdiscip. Rev. Water 2019, 6, 6. [Google Scholar] [CrossRef]
- Codd, G.A.; Morrison, L.F.; Metcalf, J.S. Cyanobacterial toxins: Risk management for health protection. Toxicol. Appl. Pharmacol. 2005, 203, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Codd, G.A. Cyanobacterial toxins, the perception of water quality, and the prioritisation of eutrophication control. Ecol. Eng. 2000, 16, 51–60. [Google Scholar] [CrossRef]
- O’Neil, J.; Davis, T.; Burford, M.; Gobler, C. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Lajayer, B.A.; Najafi, N.; Moghiseh, E.; Mosaferi, M.; Hadian, J. Effects of gamma irradiation on physicochemical and biological characteristics of wastewater effluent and sludge. Int. J. Environ. Sci. Technol. 2020, 17, 1021–1034. [Google Scholar] [CrossRef]
- Sarayu, K.; Sandhya, S. Current Technologies for Biological Treatment of Textile Wastewater–A Review. Appl. Biochem. Biotechnol. 2012, 167, 645–661. [Google Scholar] [CrossRef] [PubMed]
- Nemery, J. Phosphorus and Eutrophication. Encyclopedia of the Environment. 2019. Available online: https://www.encyclopedie-environnement.org/en/water/phosphorus-and-eutrophication/ (accessed on 5 February 2021).
- Fang, C.; Zhang, T.; Li, P.; Jiang, R.-F.; Wang, Y.-C. Application of Magnesium Modified Corn Biochar for Phosphorus Removal and Recovery from Swine Wastewater. Int. J. Environ. Res. Public Heal. 2014, 11, 9217–9237. [Google Scholar] [CrossRef]
- De Quevedo, C.M.G.; Paganini, W.D.S. Detergents as a Source of Phosphorus in Sewage: The Current Situation in Brazil. Water Air Soil Pollut. 2015, 227, 1–12. [Google Scholar] [CrossRef]
- Svenson, A.; Allard, A.-S.; Ek, M. Removal of estrogenicity in Swedish municipal sewage treatment plants. Water Res. 2003, 37, 4433–4443. [Google Scholar] [CrossRef]
- Henze, M.; Comeau, Y. Wastewater characterization. In Biological Wastewater Treatment: Principles Modelling and Design; Loosdrecht, M.C.M., Ekama, G.A., Brdjanovic, D., Eds.; IWA Publishing: London, UK, 2008; pp. 33–52. [Google Scholar]
- Ozengin, N.; Elmaci, A. Performance of duckweed (Lemna minor L.) on different types of wastewater treatment. J. Environ. Biol. 2007, 28, 307–314. [Google Scholar] [PubMed]
- Iloms, E.; Ololade, O.O.; Ogola, H.J.O.; Selvarajan, R. Investigating Industrial Effluent Impact on Municipal Wastewater Treatment Plant in Vaal, South Africa. Int. J. Environ. Res. Public Health 2020, 17, 1096. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.-K.; Abou-Shanab, R.A.I.; Hwang, J.-H.; Timmes, T.C.; Kim, H.-C.; Oh, Y.-K.; Jeon, B.-H. Removal of Nitrogen and Phosphorus from Piggery Wastewater Effluent Using the Green Microalga Scenedesmus obliquus. J. Environ. Eng. 2013, 139, 1198–1205. [Google Scholar] [CrossRef]
- Zhang, L.; Jahng, D. Enhanced anaerobic digestion of piggery wastewater by ammonia stripping: Effects of alkali types. J. Hazard. Mater. 2010, 182, 536–543. [Google Scholar] [CrossRef]
- Banet, T.; Massey, M.S.; Zohar, I.; Litaor, M.I.; Ippolito, J.A. Phosphorus removal from swine wastewater using aluminum-based water treatment residuals. Resour. Conserv. Recycl. X 2020, 6, 100039. [Google Scholar] [CrossRef]
- Ahn, J.-H.; Do, T.H.; Kim, S.D.; Hwang, S. The effect of calcium on the anaerobic digestion treating swine wastewater. Biochem. Eng. J. 2006, 30, 33–38. [Google Scholar] [CrossRef]
- Hansen, K.H.; Angelidaki, I.; Ahring, B.K. Improving thermophilic anaerobic digestion of swine manure. Water Res. 1999, 33, 1805–1810. [Google Scholar] [CrossRef]
- Maruo, M.; Ishimaru, M.; Azumi, Y.; Kawasumi, Y.; Nagafuchi, O.; Obata, H. Comparison of soluble reactive phosphorus and orthophosphate concentrations in river waters. Limnology 2015, 17, 7–12. [Google Scholar] [CrossRef]
- Comber, S.; Gardner, M.; Darmovzalova, J.; Ellor, B. Determination of the forms and stability of phosphorus in wastewater effluent from a variety of treatment processes. J. Environ. Chem. Eng. 2015, 3, 2924–2930. [Google Scholar] [CrossRef]
- Medeiros, J.J.G.; Cid, B.P.; Gómez, E.F. Analytical phosphorus fractionation in sewage sludge and sediment samples. Anal. Bioanal. Chem. 2005, 381, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Nejad, M.H.; Takdastan, A.; Jaafarzadeh, N.; Mogadam, M.A.; Mengelizadeh, N. Removal of Orthophosphate from Municipal Wastewater Using Chemical Precipitation Process in Ahvaz Wastewater Treatment Plant, Iran. Asian J. Chem. 2013, 25, 2565–2568. [Google Scholar] [CrossRef]
- Horne, A.J.; Goldman, C.R. Limnology, 2nd ed.; McGraw-Hill: New York, NY, USA, 1994. [Google Scholar]
- Mueller, D.K.; Helsel, D.R. Nutrients in the Nation’s Waters: Too Much of a Good Thing? Kidd, M.A., Ed.; US Government Printing Office: Washington, DC, USA, 1996. [Google Scholar]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Bolan, N.S. Removal and Recovery of Phosphate From Water Using Sorption. Crit. Rev. Environ. Sci. Technol. 2014, 44, 847–907. [Google Scholar] [CrossRef]
- Anzecc, A. Australian Water Quality Guidelines for Fresh and Marine Waters; Australian and New Zealand Environment and Conservation Council and Agriculture and Resource Management Council of Australia and New Zealand, 2000. Available online: https://www.waterquality.gov.au/sites/default/files/documents/anzecc-armcanz-2000-guidelines-vol1.pdf (accessed on 1 February 2021).
- Ambaye, T.G.; Vaccari, M.; Van Hullebusch, E.D.; Amrane, A.; Rtimi, S. Mechanisms and adsorption capacities of biochar for the removal of organic and inorganic pollutants from industrial wastewater. Int. J. Environ. Sci. Technol. 2020, 2020, 1–22. [Google Scholar] [CrossRef]
- Hassan, M.; Liu, Y.; Naidu, R.; Parikh, S.J.; Du, J.; Qi, F.; Willett, I.R. Influences of feedstock sources and pyrolysis temperature on the properties of biochar and functionality as adsorbents: A meta-analysis. Sci. Total. Environ. 2020, 744, 140714. [Google Scholar] [CrossRef] [PubMed]
- Chiappero, M.; Norouzi, O.; Hu, M.; Demichelis, F.; Berruti, F.; Di Maria, F.; Mašek, O.; Fiore, S. Review of biochar role as additive in anaerobic digestion processes. Renew. Sustain. Energy Rev. 2020, 131, 110037. [Google Scholar] [CrossRef]
- Dissanayake, P.D.; Choi, S.W.; Igalavithana, A.D.; Yang, X.; Tsang, D.C.; Wang, C.-H.; Kua, H.W.; Lee, K.B.; Ok, Y.S. Sustainable gasification biochar as a high efficiency adsorbent for CO2 capture: A facile method to designer biochar fabrication. Renew. Sustain. Energy Rev. 2020, 124, 109785. [Google Scholar] [CrossRef]
- Patel, S.; Kundu, S.; Halder, P.; Ratnnayake, N.; Marzbali, M.H.; Aktar, S.; Selezneva, E.; Paz-Ferreiro, J.; Surapaneni, A.; De Figueiredo, C.C.; et al. A critical literature review on biosolids to biochar: An alternative biosolids management option. Rev. Environ. Sci. Bio Technol. 2020, 19, 807–841. [Google Scholar] [CrossRef]
- Melia, P.M.; Cundy, A.B.; Sohi, S.P.; Hooda, P.S.; Busquets, R. Trends in the recovery of phosphorus in bioavailable forms from wastewater. Chemosphere 2017, 186, 381–395. [Google Scholar] [CrossRef]
- Inyang, M.; Dickenson, E. The potential role of biochar in the removal of organic and microbial contaminants from potable and reuse water: A review. Chemosphere 2015, 134, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Inyang, M.I.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.; Mosa, A.; Pullammanappallil, P.; Ok, Y.S.; Cao, X. A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit. Rev. Environ. Sci. Technol. 2016, 46, 406–433. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Niazi, N.K.; Hassan, N.E.E.; Bibi, I.; Wang, H.; Tsang, D.C.W.; Ok, Y.S.; Bolan, N.; Rinklebe, J. Wood-based biochar for the removal of potentially toxic elements in water and wastewater: A critical review. Int. Mater. Rev. 2019, 64, 216–247. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Zhang, J.; Liu, J.; Liu, B.; Chen, G. Preparation and application of magnetic biochar in water treatment: A critical review. Sci. Total. Environ. 2020, 711, 134847. [Google Scholar] [CrossRef] [PubMed]
- Chingombe, P.; Saha, B.; Wakeman, R. Surface modification and characterisation of a coal-based activated carbon. Carbon 2005, 43, 3132–3143. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Chen, M. Progress in the preparation and application of modified biochar for improved contaminant removal from water and wastewater. Bioresour. Technol. 2016, 214, 836–851. [Google Scholar] [CrossRef]
- Pan, X.; Gu, Z.; Chen, W.; Li, Q. Preparation of biochar and biochar composites and their application in a Fenton-like process for wastewater decontamination: A review. Sci. Total. Environ. 2021, 754, 142104. [Google Scholar] [CrossRef]
- Matheyarasu, R.; Bolan, N.S.; Naidu, R. Abattoir Wastewater Irrigation Increases the Availability of Nutrients and Influences on Plant Growth and Development. Water Air Soil Pollut. 2016, 227, 253. [Google Scholar] [CrossRef]
- De Rozari, P.; Greenway, M.; El Hanandeh, A. Phosphorus removal from secondary sewage and septage using sand media amended with biochar in constructed wetland mesocosms. Sci. Total. Environ. 2016, 569–570, 123–133. [Google Scholar] [CrossRef]
- Bunce, J.T.; Ndam, E.; Ofiteru, I.D.; Moore, A.; Graham, D.W. A Review of Phosphorus Removal Technologies and Their Applicability to Small-Scale Domestic Wastewater Treatment Systems. Front. Environ. Sci. 2018, 6, 8. [Google Scholar] [CrossRef]
- Yeoman, S.; Stephenson, T.; Lester, J.; Perry, R. The removal of phosphorus during wastewater treatment: A review. Environ. Pollut. 1988, 49, 183–233. [Google Scholar] [CrossRef]
- Metcalfand, E. Wastewater Engineering, Collection, Treatment Disposal; McGraw-Hill: New York, NY, USA, 1979. [Google Scholar]
- Khadhraoui, M.; Watanabe, T.; Kuroda, M. The effect of the physical structure of a porous Ca-based sorbent on its phosphorus removal capacity. Water Res. 2002, 36, 3711–3718. [Google Scholar] [CrossRef]
- Oehmen, A.; Lemos, P.C.; Carvalho, G.; Yuan, Z.; Keller, J.; Blackall, L.L.; Reis, M.A.M. Advances in enhanced biological phosphorus removal: From micro to macro scale. Water Res. 2007, 41, 2271–2300. [Google Scholar] [CrossRef]
- Zou, H.; Wang, Y. Phosphorus removal and recovery from domestic wastewater in a novel process of enhanced biological phosphorus removal coupled with crystallization. Bioresour. Technol. 2016, 211, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kumar, S.; Kwag, J.-H.; Ra, C. Magnesium ammonium phosphate formation, recovery and its application as valuable resources: A review. J. Chem. Technol. Biotechnol. 2012, 88, 181–189. [Google Scholar] [CrossRef]
- Ashekuzzaman, S.; Jiang, J.-Q. Study on the sorption–desorption–regeneration performance of Ca-, Mg- and CaMg-based layered double hydroxides for removing phosphate from water. Chem. Eng. J. 2014, 246, 97–105. [Google Scholar] [CrossRef]
- Jeon, D.J.; Yeom, S.H. Recycling wasted biomaterial, crab shells, as an adsorbent for the removal of high concentration of phosphate. Bioresour. Technol. 2009, 100, 2646–2649. [Google Scholar] [CrossRef]
- Omar, M.; Shanableh, A.; Al Zaylaie, M. Modification of the swelling characteristics and phosphorus retention of bentonite clay using alum. Soils Found. 2016, 56, 861–868. [Google Scholar] [CrossRef]
- Jiang, C.; Jia, L.; He, Y.; Zhang, B.; Kirumba, G.; Xie, J. Adsorptive removal of phosphorus from aqueous solution using sponge iron and zeolite. J. Colloid Interface Sci. 2013, 402, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Modarresi, S.; Benjamin, M.M. Efficient phosphorus removal from MBR effluent with heated aluminum oxide particles (HAOPs). Water Res. 2019, 159, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Mateus, D.M.; Vaz, M.M.; Pinho, H.J. Fragmented limestone wastes as a constructed wetland substrate for phosphorus removal. Ecol. Eng. 2012, 41, 65–69. [Google Scholar] [CrossRef]
- Li, S.; Cooke, R.A.; Wang, L.; Ma, F.; Bhattarai, R. Characterization of fly ash ceramic pellet for phosphorus removal. J. Environ. Manag. 2017, 189, 67–74. [Google Scholar] [CrossRef]
- Lan, Y.; Zhang, S.; Wang, J.; Smith, R. Phosphorus removal using steel slag. Acta Met. Sin. 2006, 19, 449–454. [Google Scholar] [CrossRef]
- Dai, L.; Tan, F.; Li, H.; Zhu, N.; He, M.; Zhu, Q.; Hu, G.; Wang, L.; Zhao, J. Calcium-rich biochar from the pyrolysis of crab shell for phosphorus removal. J. Environ. Manag. 2017, 198, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Ngo, H.; Guo, W.; Zhang, J.; Liang, S.; Lee, D.; Nguyen, P.; Bui, X. Modification of agricultural waste/by-products for enhanced phosphate removal and recovery: Potential and obstacles. Bioresour. Technol. 2014, 169, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Mehta, C.M.; Khunjar, W.O.; Nguyen, V.; Tait, S.; Batstone, D.J. Technologies to Recover Nutrients from Waste Streams: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 385–427. [Google Scholar] [CrossRef]
- Cui, Q.; Xu, J.; Wang, W.; Tan, L.; Cui, Y.; Wang, T.; Li, G.; She, D.; Zheng, J. Phosphorus recovery by core-shell γ-Al2O3/Fe3O4 biochar composite from aqueous phosphate solutions. Sci. Total. Environ. 2020, 729, 138892. [Google Scholar] [CrossRef]
- Zhou, Y.-F.; Haynes, R.J. Sorption of Heavy Metals by Inorganic and Organic Components of Solid Wastes: Significance to Use of Wastes as Low-Cost Adsorbents and Immobilizing Agents. Crit. Rev. Environ. Sci. Technol. 2010, 40, 909–977. [Google Scholar] [CrossRef]
- Elzinga, E.J.; Sparks, D.L. Phosphate adsorption onto hematite: An in situ ATR-FTIR investigation of the effects of pH and loading level on the mode of phosphate surface complexation. J. Colloid Interface Sci. 2007, 308, 53–70. [Google Scholar] [CrossRef]
- Chitrakar, R.; Tezuka, S.; Sonoda, A.; Sakane, K.; Ooi, K.; Hirotsu, T. Phosphate adsorption on synthetic goethite and akaganeite. J. Colloid Interface Sci. 2006, 298, 602–608. [Google Scholar] [CrossRef]
- Genz, A.; Kornmüller, A.; Jekel, M. Advanced phosphorus removal from membrane filtrates by adsorption on activated aluminium oxide and granulated ferric hydroxide. Water Res. 2004, 38, 3523–3530. [Google Scholar] [CrossRef] [PubMed]
- Makris, K.C.; Harris, W.G.; O’Connor, G.A.; El-Shall, H. Long-term phosphorus effects on evolving physicochemical properties of iron and aluminum hydroxides. J. Colloid Interface Sci. 2005, 287, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Sansalone, J.J.; Ma, J. Parametric Evaluation of Batch Equilibria for Storm-Water Phosphorus Adsorption on Aluminum Oxide Media. J. Environ. Eng. 2009, 135, 737–746. [Google Scholar] [CrossRef]
- Roques, H.; Nugroho-Jeudy, L.; Lebugle, A. Phosphorus removal from wastewater by half-burned dolomite. Water Res. 1991, 25, 959–965. [Google Scholar] [CrossRef]
- Goh, K.-H.; Lim, T.-T.; Dong, Z. Application of layered double hydroxides for removal of oxyanions: A review. Water Res. 2008, 42, 1343–1368. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xu, Z.P.; Qiao, S.Z.; Liu, Q.; Xu, Y.; Qian, G. Enhanced removal of triphosphate by MgCaFe-Cl-LDH: Synergism of precipitation with intercalation and surface uptake. J. Hazard. Mater. 2011, 189, 586–594. [Google Scholar] [CrossRef]
- Koilraj, P.; Kannan, S. Phosphate uptake behavior of ZnAlZr ternary layered double hydroxides through surface precipitation. J. Colloid Interface Sci. 2010, 341, 289–297. [Google Scholar] [CrossRef]
- Chitrakar, R.; Tezuka, S.; Hosokawa, J.; Makita, Y.; Sonoda, A.; Ooi, K.; Hirotsu, T. Uptake properties of phosphate on a novel Zr–modified MgFe–LDH(CO3). J. Colloid Interface Sci. 2010, 349, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Blaney, L.M.; Cinar, S.; Sengupta, A.K. Hybrid anion exchanger for trace phosphate removal from water and wastewater. Water Res. 2007, 41, 1603–1613. [Google Scholar] [CrossRef]
- Johir, M.; George, J.; Vigneswaran, S.; Kandasamy, J.; Grasmick, A. Removal and recovery of nutrients by ion exchange from high rate membrane bio-reactor (MBR) effluent. Desalination 2011, 275, 197–202. [Google Scholar] [CrossRef]
- Awual, R.; Jyo, A.; El-Safty, S.A.; Tamada, M.; Seko, N. A weak-base fibrous anion exchanger effective for rapid phosphate removal from water. J. Hazard. Mater. 2011, 188, 164–171. [Google Scholar] [CrossRef]
- Akhurst, D.J.; Jones, G.B.; Clark, M.W.; McConchie, D. Phosphate Removal from Aqueous Solutions using Neutralised Bauxite Refinery Residues (Bauxsol™). Environ. Chem. 2006, 3, 65–74. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Luan, Z.; Peng, X.; Zhu, C.; Chen, Z.; Zhang, Z.; Fan, J.; Jia, Z. Phosphate removal from aqueous solutions using raw and activated red mud and fly ash. J. Hazard. Mater. 2006, 137, 374–383. [Google Scholar] [CrossRef]
- Wang, S.; Ang, H.; Tadé, M. Novel applications of red mud as coagulant, adsorbent and catalyst for environmentally benign processes. Chemosphere 2008, 72, 1621–1635. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Vilar, V.J.; Botelho, C.M.; Boaventura, R.A. A review of the use of red mud as adsorbent for the removal of toxic pollutants from water and wastewater. Environ. Technol. 2011, 32, 231–249. [Google Scholar] [CrossRef]
- Ugurlu, A. Phosphorus removal by fly ash. Environ. Int. 1998, 24, 911–918. [Google Scholar] [CrossRef]
- Lu, S.; Bai, S.; Zhu, L.; Shan, H. Removal mechanism of phosphate from aqueous solution by fly ash. J. Hazard. Mater. 2009, 161, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Li, X.; Liu, J. Adsorptive removal of phosphate from aqueous solutions using iron oxide tailings. Water Res. 2004, 38, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Barca, C.; Scanu, D.; Podda, N.; Miche, H.; Poizat, L.; Hennebert, P. Phosphorus removal from wastewater by carbonated bauxite residue under aerobic and anoxic conditions. J. Water Process. Eng. 2021, 39, 101757. [Google Scholar] [CrossRef]
- Barca, C.; Gérente, C.; Meyer, D.; Chazarenc, F.; Andrès, Y. Phosphate removal from synthetic and real wastewater using steel slags produced in Europe. Water Res. 2012, 46, 2376–2384. [Google Scholar] [CrossRef]
- Barca, C.; Troesch, S.; Meyer, D.; Drissen, P.; Andrès, Y.; Chazarenc, F. Steel Slag Filters to Upgrade Phosphorus Removal in Constructed Wetlands: Two Years of Field Experiments. Environ. Sci. Technol. 2012, 47, 549–556. [Google Scholar] [CrossRef]
- Marshall, W.E.; Wartelle, L.H. An anion exchange resin from soybean hulls. J. Chem. Technol. Biotechnol. 2004, 79, 1286–1292. [Google Scholar] [CrossRef]
- Van Vinh, N.; Zafar, M.; Behera, S.K.; Park, H.S. Arsenic(III) removal from aqueous solution by raw and zinc-loaded pine cone biochar: Equilibrium, kinetics, and thermodynamics studies. Int. J. Environ. Sci. Technol. 2014, 12, 1283–1294. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction: Practical Design and Theory; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Spataru, A.; Jain, R.; Chung, J.W.; Gerner, G.; Krebs, R.; Lens, P.N.L. Enhanced adsorption of orthophosphate and copper onto hydrochar derived from sewage sludge by KOH activation. RSC Adv. 2016, 6, 101827–101834. [Google Scholar] [CrossRef]
- Chen, T.; Zhou, Z.; Han, R.; Meng, R.; Wang, H.; Lu, W. Adsorption of cadmium by biochar derived from municipal sewage sludge: Impact factors and adsorption mechanism. Chemosphere 2015, 134, 286–293. [Google Scholar] [CrossRef]
- Takaya, C.; Fletcher, L.; Singh, S.; Anyikude, K.; Ross, A. Phosphate and ammonium sorption capacity of biochar and hydrochar from different wastes. Chemosphere 2016, 145, 518–527. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Li, Z.; Feng, R.; Zhang, Y. Characterization of corncob-derived biochar and pyrolysis kinetics in comparison with corn stalk and sawdust. Bioresour. Technol. 2014, 170, 76–82. [Google Scholar] [CrossRef]
- Jung, K.-W.; Hwang, M.-J.; Ahn, K.-H.; Ok, Y.S. Kinetic study on phosphate removal from aqueous solution by biochar derived from peanut shell as renewable adsorptive media. Int. J. Environ. Sci. Technol. 2015, 12, 3363–3372. [Google Scholar] [CrossRef]
- Jung, K.-W.; Kim, K.; Jeong, T.-U.; Ahn, K.-H. Influence of pyrolysis temperature on characteristics and phosphate adsorption capability of biochar derived from waste-marine macroalgae (Undaria pinnatifida roots). Bioresour. Technol. 2016, 200, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guo, H.; Shen, F.; Yang, G.; Zhang, Y.; Zeng, Y.; Wang, L.; Xiao, H.; Deng, S. Biochar produced from oak sawdust by Lanthanum (La)-involved pyrolysis for adsorption of ammonium (NH4+), nitrate (NO3−), and phosphate (PO43−). Chemosphere 2015, 119, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Gao, B.; Inyang, M.; Zimmerman, A.R.; Cao, X.; Pullammanappallil, P.; Yang, L. Removal of phosphate from aqueous solution by biochar derived from anaerobically digested sugar beet tailings. J. Hazard. Mater. 2011, 190, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gao, B.; Yao, Y.; Xue, Y.; Inyang, M. Synthesis of porous MgO-biochar nanocomposites for removal of phosphate and nitrate from aqueous solutions. Chem. Eng. J. 2012, 210, 26–32. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Fang, J.; Creamer, A.E.; Ullman, J.L. Self-assembly of needle-like layered double hydroxide (LDH) nanocrystals on hydrochar: Characterization and phosphate removal ability. RSC Adv. 2014, 4, 28171–28175. [Google Scholar] [CrossRef]
- Jung, K.-W.; Ahn, K.-H. Fabrication of porosity-enhanced MgO/biochar for removal of phosphate from aqueous solution: Application of a novel combined electrochemical modification method. Bioresour. Technol. 2016, 200, 1029–1032. [Google Scholar] [CrossRef]
- Yoon, S.-Y.; Lee, C.-G.; Park, J.-A.; Kim, J.-H.; Kim, S.-B.; Lee, S.-H.; Choi, J.-W. Kinetic, equilibrium and thermodynamic studies for phosphate adsorption to magnetic iron oxide nanoparticles. Chem. Eng. J. 2014, 236, 341–347. [Google Scholar] [CrossRef]
- Zach-Maor, A.; Semiat, R.; Shemer, H. Synthesis, performance, and modeling of immobilized nano-sized magnetite layer for phosphate removal. J. Colloid Interface Sci. 2011, 357, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Sarkhot, D.; Ghezzehei, T.A.; Berhe, A.A. Effectiveness of Biochar for Sorption of Ammonium and Phosphate from Dairy Effluent. J. Environ. Qual. 2013, 42, 1545–1554. [Google Scholar] [CrossRef]
- Figueiredo, J.; Pereira, M.; Freitas, M.; Órfão, J. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Arias, C.; Del Bubba, M.; Brix, H. Phosphorus removal by sands for use as media in subsurface flow constructed reed beds. Water Res. 2001, 35, 1159–1168. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, D.; Zhu, L. Transitional Adsorption and Partition of Nonpolar and Polar Aromatic Contaminants by Biochars of Pine Needles with Different Pyrolytic Temperatures. Environ. Sci. Technol. 2008, 42, 5137–5143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gao, B.; Yao, Y.; Inyang, M. Phosphate removal ability of biochar/MgAl-LDH ultra-fine composites prepared by liquid-phase deposition. Chemosphere 2013, 92, 1042–1047. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Chen, J.; Yang, L. Engineered Biochar Reclaiming Phosphate from Aqueous Solutions: Mechanisms and Potential Application as a Slow-Release Fertilizer. Environ. Sci. Technol. 2013, 47, 8700–8708. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, Y.; Wang, H.; Lu, W.; Zhou, Z.; Zhang, Y.; Ren, L. Influence of pyrolysis temperature on characteristics and heavy metal adsorptive performance of biochar derived from municipal sewage sludge. Bioresour. Technol. 2014, 164, 47–54. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, C.; Gray, E.M.; Boyd, S.E.; Yang, H.; Zhang, D. Roles of biochar in improving phosphorus availability in soils: A phosphate adsorbent and a source of available phosphorus. Geoderma 2016, 276, 1–6. [Google Scholar] [CrossRef]
- Shepherd, J.G.; Joseph, S.; Sohi, S.P.; Heal, K.V. Biochar and enhanced phosphate capture: Mapping mechanisms to functional properties. Chemosphere 2017, 179, 57–74. [Google Scholar] [CrossRef]
- Dugdug, A.A.; Chang, S.X.; Ok, Y.S.; Rajapaksha, A.U.; Anyia, A. Phosphorus sorption capacity of biochars varies with biochar type and salinity level. Environ. Sci. Pollut. Res. 2018, 25, 25799–25812. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Chu, B.; Amano, Y.; Machida, M. Removal and recovery of phosphate from water by Mg-laden biochar: Batch and column studies. Colloids Surfaces A: Physicochem. Eng. Asp. 2018, 558, 429–437. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, D.; Li, Y.; Pan, Q.; Wang, J.; Xue, L.; Howard, A. Phosphorus and Nitrogen Adsorption Capacities of Biochars Derived from Feedstocks at Different Pyrolysis Temperatures. Water 2019, 11, 1559. [Google Scholar] [CrossRef]
- Wei, S.; Zhu, M.; Fan, X.; Song, J.; Peng, P.; Li, K.; Jia, W.; Song, H. Influence of pyrolysis temperature and feedstock on carbon fractions of biochar produced from pyrolysis of rice straw, pine wood, pig manure and sewage sludge. Chemosphere 2019, 218, 624–631. [Google Scholar] [CrossRef]
- Melia, P.M.; Busquets, R.; Hooda, P.S.; Cundy, A.B.; Sohi, S.P. Driving forces and barriers in the removal of phosphorus from water using crop residue, wood and sewage sludge derived biochars. Sci. Total. Environ. 2019, 675, 623–631. [Google Scholar] [CrossRef]
- Liao, T.; Li, T.; Su, X.; Yu, X.; Song, H.; Zhu, Y.; Zhang, Y. La(OH)3-modified magnetic pineapple biochar as novel adsorbents for efficient phosphate removal. Bioresour. Technol. 2018, 263, 207–213. [Google Scholar] [CrossRef]
- Zhu, Z.; Huang, C.; Zhu, Y.; Wei, W.; Qin, H. A hierarchical porous adsorbent of nano-α-Fe2O3/Fe3O4 on bamboo biochar (HPA-Fe/C-B) for the removal of phosphate from water. J. Water Process. Eng. 2018, 25, 96–104. [Google Scholar] [CrossRef]
- Wan, S.; Wang, S.; Li, Y.; Gao, B. Functionalizing biochar with Mg–Al and Mg–Fe layered double hydroxides for removal of phosphate from aqueous solutions. J. Ind. Eng. Chem. 2017, 47, 246–253. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, S.; Sun, Y.; Tsang, D.C.; Cheng, K.; Ok, Y.S. Assembling biochar with various layered double hydroxides for enhancement of phosphorus recovery. J. Hazard. Mater. 2019, 365, 665–673. [Google Scholar] [CrossRef]
- Jiang, Y.-H.; Li, A.-Y.; Deng, H.; Ye, C.-H.; Wu, Y.-Q.; Linmu, Y.-D.; Hang, H.-L. Characteristics of nitrogen and phosphorus adsorption by Mg-loaded biochar from different feedstocks. Bioresour. Technol. 2019, 276, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yan, L.; Yu, H.; Yan, T.; Li, X. Adsorption of phosphate from aqueous solution by vegetable biochar/layered double oxides: Fast removal and mechanistic studies. Bioresour. Technol. 2019, 284, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Antunes, E.; Jacob, M.V.; Brodie, G.; Schneider, P.A. Isotherms, kinetics and mechanism analysis of phosphorus recovery from aqueous solution by calcium-rich biochar produced from biosolids via microwave pyrolysis. J. Environ. Chem. Eng. 2018, 6, 395–403. [Google Scholar] [CrossRef]
- Jack, J.; Huggins, T.M.; Huang, Y.; Fang, Y.; Ren, Z.J. Production of magnetic biochar from waste-derived fungal biomass for phosphorus removal and recovery. J. Clean. Prod. 2019, 224, 100–106. [Google Scholar] [CrossRef]
- Haddad, K.; Jellali, S.; Jeguirim, M.; Trabelsi, A.B.H.; Limousy, L. Investigations on phosphorus recovery from aqueous solutions by biochars derived from magnesium-pretreated cypress sawdust. J. Environ. Manag. 2018, 216, 305–314. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, T.; Li, P.; Jiang, R.; Wu, S.; Nie, H.; Wang, Y. Phosphorus recovery from biogas fermentation liquid by Ca–Mg loaded biochar. J. Environ. Sci. 2015, 29, 106–114. [Google Scholar] [CrossRef]
- Liu, F.; Zuo, J.; Chi, T.; Wang, P.; Yang, B. Removing phosphorus from aqueous solutions by using iron-modified corn straw biochar. Front. Environ. Sci. Eng. 2015, 9, 1066–1075. [Google Scholar] [CrossRef]
- Kong, L.; Han, M.; Shih, K.; Su, M.; Diao, Z.; Long, J.; Chen, D.; Hou, L.; Peng, Y. Nano-rod Ca-decorated sludge derived carbon for removal of phosphorus. Environ. Pollut. 2018, 233, 698–705. [Google Scholar] [CrossRef]
- Cui, X.; Dai, X.; Khan, K.Y.; Li, T.; Yang, X.; Zhenli China (host institution) Hangzhou 310058 Zhejiang University College of Environmental and Resource Sciences Ministry of Education Key Laboratory of Environmental Remediation and Ecological Health. Removal of phosphate from aqueous solution using magnesium-alginate/chitosan modified biochar microspheres derived from Thalia dealbata. Bioresour. Technol. 2016, 218, 1123–1132. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, B.; Gao, Q.; Gao, Y.; Liu, S. Adsorption of phosphorus by different biochars. Spectrosc. Lett. 2017, 50, 73–80. [Google Scholar] [CrossRef]
- Lou, K.; Rajapaksha, A.U.; Ok, Y.S.; Chang, S.X. Pyrolysis temperature and steam activation effects on sorption of phosphate on pine sawdust biochars in aqueous solutions. Chem. Speciat. Bioavailab. 2016, 28, 42–50. [Google Scholar] [CrossRef]
- Li, J.-H.; Lv, G.-H.; Bai, W.-B.; Liu, Q.; Zhang, Y.-C.; Song, J.-Q. Modification and use of biochar from wheat straw (Triticum aestivum L.) for nitrate and phosphate removal from water. Desalination Water Treat. 2014, 57, 1–13. [Google Scholar] [CrossRef]
- Wang, S.; Kong, L.; Long, J.; Su, M.; Diao, Z.; Chang, X.; Chen, D.; Song, G.; Shih, K. Adsorption of phosphorus by calcium-flour biochar: Isotherm, kinetic and transformation studies. Chemosphere 2018, 195, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Veni, D.K.; Kannan, P.; Edison, T.N.J.I.; Senthilkumar, A. Biochar from green waste for phosphate removal with subsequent disposal. Waste Manag. 2017, 68, 752–759. [Google Scholar] [CrossRef]
- Kasak, K.; Truu, J.; Ostonen, I.; Sarjas, J.; Oopkaup, K.; Paiste, P.; Kõiv-Vainik, M.; Mander, Ü.; Truu, M. Biochar enhances plant growth and nutrient removal in horizontal subsurface flow constructed wetlands. Sci. Total. Environ. 2018, 639, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Břendová, K.; Lhotka, M.; Krulikovská, T.; Tlustoš, P.; Száková, J.; Punčochář, M. Biochar physicochemical parameters as a result of feedstock material and pyrolysis temperature: Predictable for the fate of biochar in soil? Environ. Geochem. Heal. 2017, 39, 1381–1395. [Google Scholar] [CrossRef]
- Amonette, J.E.; Joseph, S. Characteristics of biochar: Microchemical properties. In Biochar for environmental management; Lehmann, J., Joseph, S., Eds.; Routledge: Boca Raton, FL, USA, 2009; pp. 65–84. [Google Scholar]
- Jun, M.; Altor, A.E.; Craft, C.B. Effects of Increased Salinity and Inundation on Inorganic Nitrogen Exchange and Phosphorus Sorption by Tidal Freshwater Floodplain Forest Soils, Georgia (USA). Chesap. Sci. 2013, 36, 508–518. [Google Scholar] [CrossRef]
- Hollister, C.C.; Bisogni, J.J.; Lehmann, J. Ammonium, Nitrate, and Phosphate Sorption to and Solute Leaching from Biochars Prepared from Corn Stover (Zea mays L.) and Oak Wood (Quercus spp.). J. Environ. Qual. 2013, 42, 137–144. [Google Scholar] [CrossRef]
- Mukherjee, A.; Zimmerman, A.R. Organic carbon and nutrient release from a range of laboratory-produced biochars and biochar–soil mixtures. Geoderma 2013, 193–194, 122–130. [Google Scholar] [CrossRef]
- Lǚ, J.; Liu, H.; Liu, R.; Zhao, X.; Sun, L.; Qu, J. Adsorptive removal of phosphate by a nanostructured Fe–Al–Mn trimetal oxide adsorbent. Powder Technol. 2013, 233, 146–154. [Google Scholar] [CrossRef]
- Jiang, T.-Y.; Jiang, J.; Xu, R.-K.; Li, Z. Adsorption of Pb(II) on variable charge soils amended with rice-straw derived biochar. Chemosphere 2012, 89, 249–256. [Google Scholar] [CrossRef]
- Banik, C.; Lawrinenko, M.; Bakshi, S.; Laird, D.A. Impact of Pyrolysis Temperature and Feedstock on Surface Charge and Functional Group Chemistry of Biochars. J. Environ. Qual. 2018, 47, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.T.; Lehmann, J. Black carbon decomposition under varying water regimes. Org. Geochem. 2009, 40, 846–853. [Google Scholar] [CrossRef]
- Yin, Q.; Zhang, B.; Wang, R.; Zhao, Z. Phosphate and ammonium adsorption of sesame straw biochars produced at different pyrolysis temperatures. Environ. Sci. Pollut. Res. 2017, 25, 4320–4329. [Google Scholar] [CrossRef]
- Tan, I.; Ahmad, A.; Hameed, B. Adsorption of basic dye on high-surface-area activated carbon prepared from coconut husk: Equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater. 2008, 154, 337–346. [Google Scholar] [CrossRef]
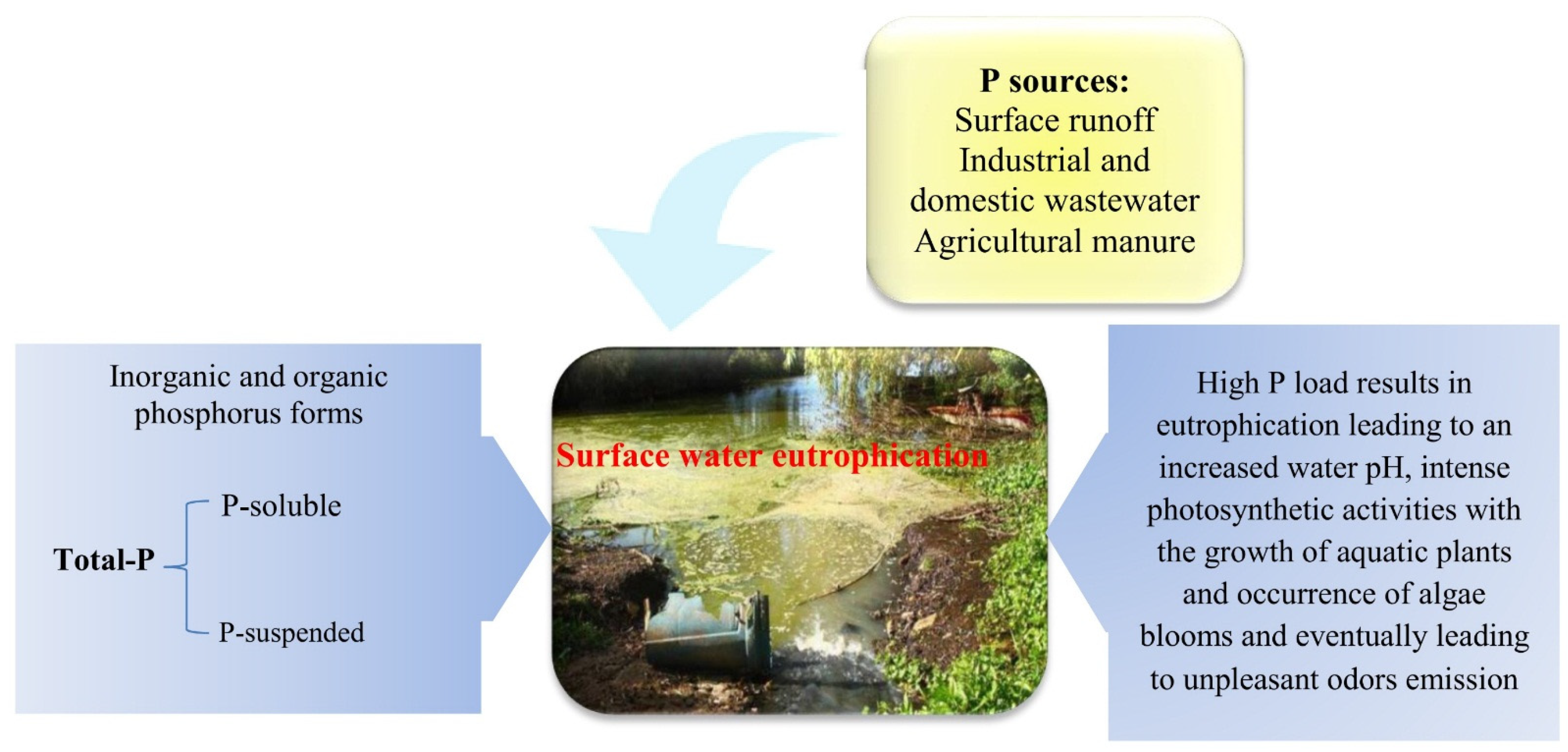


| Wastewater Type | pH Range | P Forms (mg/L) | References |
|---|---|---|---|
| Swine wastewater | 7.8 | 84 (P-PO43−) | [14] |
| Sewage wastewater | 6.3–7.3 | Approximately 4–8 (organic and inorganic P) | [15,16] |
| Domestic/Municipal wastewater | 7–8 | 4–15 (P-PO43−) (P-soluble: 11 and P-suspended: 4) | [17] |
| Municipal/Industrial Wastewater | 4.4–11.1 | 1.5–3.5 (Total P) | [18,19] |
| Piggery wastewater | 6.64 and 8.1 | 4.2 (P-PO43−) | [20,21] |
| Swine wastewater | 6.37–7.62 | 24.1 mg/kg (as orthophosphate) | [22,23,24] |
| Methods | Description | References |
|---|---|---|
| Biological | Biological P removal (i.e., Enhanced Biological Phosphorus Removal (EPBR)) with activated sludge systems is based on the biochemical process coupled to the luxury P-uptake, which relies on phosphorus-accumulating organisms (PAO). | [49,50] |
| Chemical | The most common chemical P removal approaches involve dosing metal salts (i.e., aluminum or iron salts) to either pre-treated wastewater, activated sludge reactors or at the outlet of secondary clarifiers | [49] |
| Physical | Use of reactive media filters which rely on P-sorbing properties of some materials such as natural products (e.g., apatite, bauxite or limestone), industrial waste products (e.g., fly-ash, ochre or steel slag) or man-made products (e.g., FiltraliteTM). | [51] |
| Sorbent | Amount of PO4-P sorbed (mg·g−1) | Sorption Mechanisms | References |
|---|---|---|---|
| La(OH)3-modified magnetic pineapple biochar Pineapple biochar (the ratio between lanthanum and magnetic biochar is 10 mmol/g in the suspension) | 101.2 | Precipitation, electrostatic interaction, ligand exchange, inner-sphere complexation | [121] |
| 3.7 | Not reported | ||
| Bamboo biochar modified with α-Fe2O3/Fe2O4 | 2.8 | Not reported | [122] |
| Bamboo modified with varying amount of Mg-Al and Mg-Fe (3:1) layered double hydroxide | 32 | Interlayer anion exchange, surface sorption, precipitation | [123] |
| Mg laden bamboo biochar synthesized at 400, 500 and 600 °C | 370 | Ligand exchange, electrostatic attraction | [117] |
| A novel combined electrochemical MgO biochar (prepared from brown marine macroalgae) Brown marine macroalgae (unmodified biochar) | 600 | Detailed mechanisms not reported | [104] |
| 5 | Not reported | ||
| Iron modification waste activated sludge-based biochar | 111 | Ligand exchange | [124] |
| Mg-loaded biochar from different feedstock (Tarto straw, Corn straw, Cassava straw, Chinese fir straw, Banana straw, Camelia oleifera shell) | 31.2 | Surface electrostatic, Mg2+ precipitation, complexation with hydroxyl functional groups | [125] |
| Vegetable biochar/Mg-Al layered double oxide | 132.8 | Electrostatic attraction, surface complexation, anion exchange | [126] |
| Calcium doped biochar produced from biosolids | 147 | Precipitation | [127] |
| Biochar from waste-derived fungal biomass (Magnetic biochar) | 23.9 | P-OH bonding | [128] |
| Biochar derived from magnesium-pretreated cypress sawdust (pyrolysis temperature 400 °C) Biochar derived from magnesium-pre-treated cypress sawdust (pyrolysis temperature 600 °C) | 19.2 | Sorption on active site, precipitation with Mg ions | [129] |
| 33.8 | |||
| Nano Ca-Mg loaded biochar | 326.6 | Not reported | [130] |
| Iron modified corn straw biochar | --- | Precipitation, Fe-O-P-P-C bonds | [131] |
| Nano-rod Ca-decorated sludge derived carbon | 116.8 | Precipitation as hydroxyl apatite | [132] |
| Wood and sewage sludge derived biochar pyrolyzed at various temperature | 0.7–1.2 | Not reported | [120] |
| Magnesium alginate/chitosan modified biochar microspheres from Thalia dealbata | 46.5 | Precipitation with minerals, ligand exchange | [133] |
| Sesame straw biochar produced at different pyrolysis temperature (300, 500 and 700 °C) | 3.45–34.2 | Not reported | [6] |
| Maize-Straw biochar (unmodified) | 8.8 | Not reported | [134] |
| Pine biochar (unmodified) | 13.9 | ||
| Pine sawdust biochar | 2 | Repulsion forces between biochar surface and phosphate ion cause of the low adsorption | [135] |
| Wheat straw biochar with HCl activated and coated with FeCl3·6H2O | 16.6 | Not reported | [136] |
| Calcium Flour biochar | 314.2 | Reaction of Ca(OH)2 and PO4 forming the hydroxylapatite | [137] |
| Cotton stalk solid waste | 50 | Precipitation with Ca, Mg ions | [138] |
| Biochar derived from digested sugar beet tailing | 25 | Main sorption sites: Colloidal and nano sized MgO (particles) on the biochar surface | [101] |
| Biochar/MgAl-LDH fine composite | 350 | Surface sorption, interlayer exchange | [111] |
| Unmodified biochar (different feedstock) | 0–30 | Precipitation with Mg and Ca ions | [96] |
| Biochar derived from Peanut shell | 3.8 | Not reported | [98] |
| The Main Description | Percentage | Feedstock Biochar/Solution Type/Treatment pH Range | References |
|---|---|---|---|
| Calcium-rich biochars for P removal from wastewater | ≈80 | Calcium-rich biochars (pyrolysis of crab shell)/30 mL KH2PO4 solution (80 mg P/L) and biogas effluent of swine manure wastewater (11.59 mg P/L)/11.25–14 | [63] |
| Lightweight expanded clay aggregates (LECA) along with biochar and plant increased P removal from wastewater compared to (LECA + plants) | 22.5 | LECA + with biochar + plant/wastewater in horizontal subsurface flow constructed wetlands (water quality 12.4-17.5 mg/L P-PO4)/6.7–7.1 | [139] |
| The Mg/biochar is more effective than biochar in P removal | 90 | Magnesium (Mg) Modified Corn Biochar/swine Wastewater (84 mg/L PO43−-P)/7.8 | [14] |
| Core-shell γ-Al2O3/Fe3O4 biochar for P removal | 91 | Core-shell γ-Al2O3/Fe3O4 biochar/aqueous phosphate solutions (10–500 mg/L NaH2PO4)/3–11 | [66] |
| Peanut shell-derived biochar is as an alternative and renewable sorptive media for phosphate removal | 61 | Peanut shell-derived biochar/aqueous phosphate solution (5.0 mg/L using KH2PO4)/7 | [98] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nobaharan, K.; Bagheri Novair, S.; Asgari Lajayer, B.; van Hullebusch, E.D. Phosphorus Removal from Wastewater: The Potential Use of Biochar and the Key Controlling Factors. Water 2021, 13, 517. https://doi.org/10.3390/w13040517
Nobaharan K, Bagheri Novair S, Asgari Lajayer B, van Hullebusch ED. Phosphorus Removal from Wastewater: The Potential Use of Biochar and the Key Controlling Factors. Water. 2021; 13(4):517. https://doi.org/10.3390/w13040517
Chicago/Turabian StyleNobaharan, Khatereh, Sepideh Bagheri Novair, Behnam Asgari Lajayer, and Eric D. van Hullebusch. 2021. "Phosphorus Removal from Wastewater: The Potential Use of Biochar and the Key Controlling Factors" Water 13, no. 4: 517. https://doi.org/10.3390/w13040517
APA StyleNobaharan, K., Bagheri Novair, S., Asgari Lajayer, B., & van Hullebusch, E. D. (2021). Phosphorus Removal from Wastewater: The Potential Use of Biochar and the Key Controlling Factors. Water, 13(4), 517. https://doi.org/10.3390/w13040517








