Ecological Efficiency of the Mussel Hyriopsis cumingii (Lea, 1852) on Particulate Organic Matter Filtering, Algal Controlling and Water Quality Regulation
Abstract
1. Introduction
2. Materials and Methods
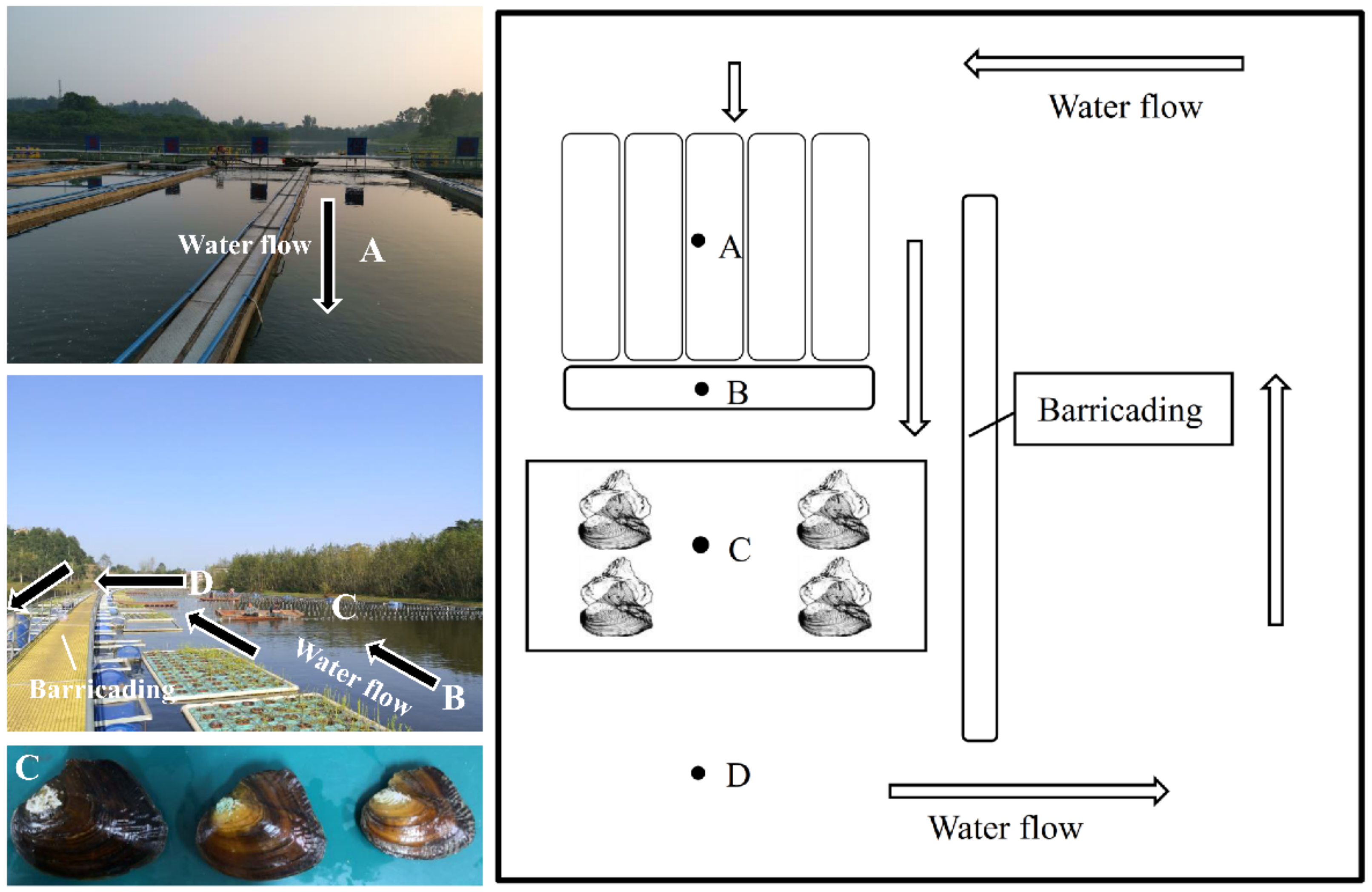
2.1. Shellfish and Experimental Design
2.2. Particulate Organic Matter Detection
2.3. Chlorophyll-a and Phytoplankton Quantitative Detection
2.4. Hydrochemical Parameters Detection of Samples
2.5. Statistical Analysis
3. Results
3.1. Short-Term Effects of H. cumingii on TSS, FA, and POC
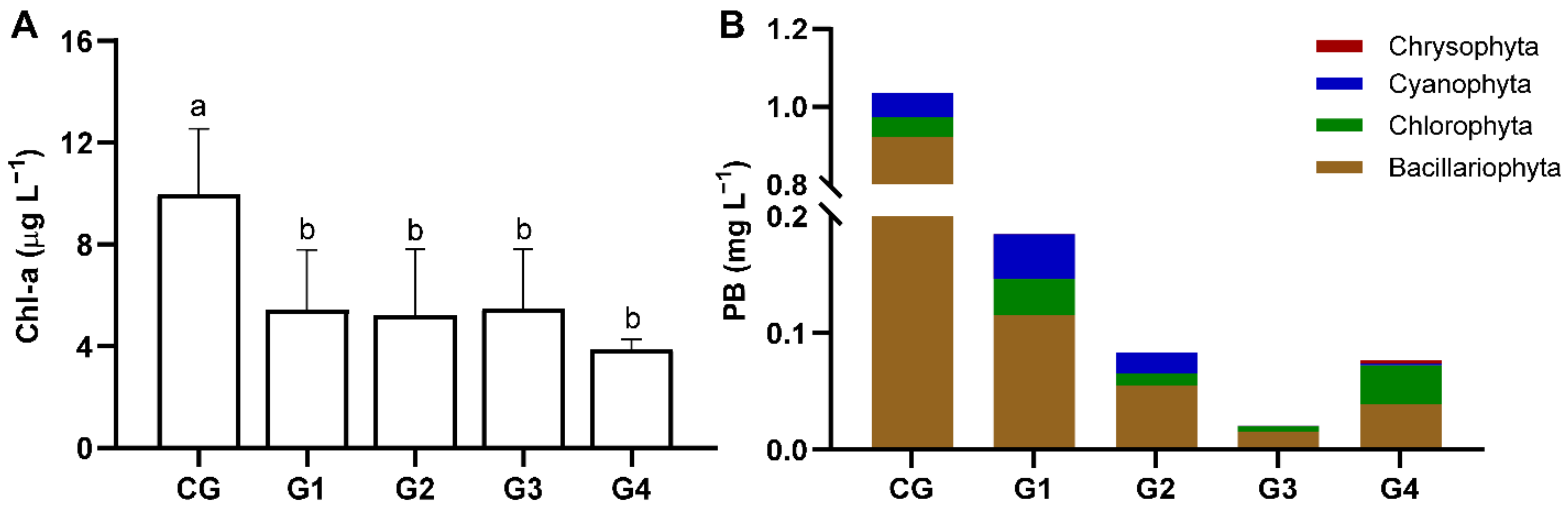
3.2. Filtering Effect of H. cumingii on Phytoplankton
3.3. Effect of H. cumingii on the Water Conditions
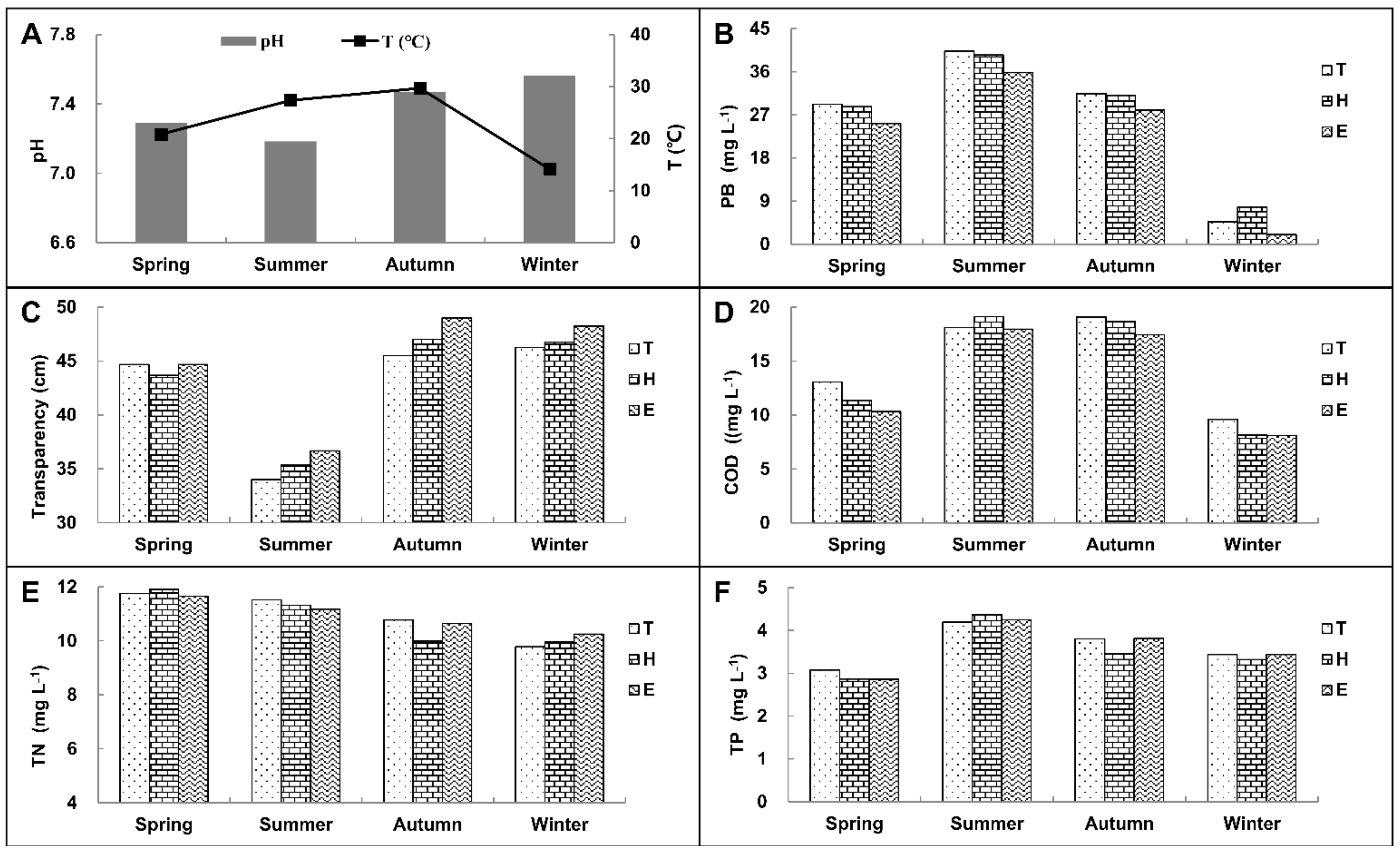
3.4. Annual Survey Results in the Recirculating Aquaculture Pond
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, L.; Wang, W.; Yang, Y.; Yang, C.; Yuan, Z.; Xiong, S.; Diana, J. Environmental impact of aquaculture and countermeasures to aquaculture pollution in China. Environ. Sci. Pollut. Res. 2007, 14, 452–462. [Google Scholar] [CrossRef]
- Horton, M.; Keys, A.; Kirkwood, L.; Mitchell, F.; Kyle, R.; Roberts, D. Sustainable catchment restoration for reintroduction of captive bred freshwater pearl mussels Margaritifera margaritifera. Limnologica 2015, 50, 21–28. [Google Scholar] [CrossRef]
- Kingsford, R.T.; Biggs, H.C.; Pollard, S.R. Strategic adaptive management in freshwater protected areas and their rivers. Biol. Conserv. 2011, 144, 1194–1203. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, F.; Li, S.; Liu, X.; Han, X.; Jiang, K.; Hu, H. China Fishery Statistical Yearbook 2018; China Agriculture Press: Beijing, China, 2018. [Google Scholar]
- Zhang, B.; Cao, C.; Gu, J.; Liu, T. A new environmental protection law, many old problems? Challenges to environmental governance in China. J. Environ. Law 2016, 28, 325–335. [Google Scholar] [CrossRef]
- Wang, L.; He, F.; Sun, J.; Hu, Y.; Huang, T.; Zhang, Y.; Wu, Z. Effects of three biological control approaches and their combination on the restoration of eutrophicated waterbodies. Limnology 2017, 18, 301–313. [Google Scholar] [CrossRef]
- Wang, L.; Ma, L.; Sun, J.; Zhang, Y.; Zhou, Q.; Wu, Z.; He, F. Effects of different aquaculture methods for introduced bivalves (Hyriopsis cumingii) on seston removal and phosphorus balance at the water-sediment interface. J. Freshw. Ecol. 2018, 33, 251–265. [Google Scholar] [CrossRef]
- Jeppesen, E.; Sondergaard, M.; Lauridsen, T.L.; Davidson, T.A.; Liu, Z.; Mazzeo, N.; Meerhoff, M. Biomanipulation as a restoration tool to combat eutrophication: Recent advances and future challenges. Adv. Ecol. Res. 2012, 47, 411–488. [Google Scholar] [CrossRef]
- Vaughn, C.C.; Hakenkamp, C.C. The functional role of burrowing bivalves in freshwater ecosystems. Freshw. Biol. 2001, 46, 1431–1446. [Google Scholar] [CrossRef]
- Enriquez-Ocana, L.F.; Nieves-Soto, M.; Pina-Valdez, P.; Martinez-Cordova, L.R.; Medina-Jasso, M.A. Evaluation of the combined effect of temperature and salinity on the filtration, clearance rate and assimilation efficiency of the mangrove oyster Crassostrea corteziensis. Arch. Biol. Sci. 2012, 64, 479–488. [Google Scholar] [CrossRef]
- Jones HF, E.; Pilditch, C.A.; Bryan, K.R.; Hamilton, D.P. Effects of infaunal bivalve density and flow speed on clearance rates and near-bed hydrodynamics. J. Exp. Mar. Biol. Ecol. 2011, 401, 20–28. [Google Scholar] [CrossRef]
- Tokumon, R.; Cataldo, D.; Boltovskoy, D. Effects of suspended inorganic matter on filtration and grazing rates of the invasive mussel Limnoperna fortunei (Bivalvia: Mytiloidea). J. Molluscan Stud. 2016, 82, 201–204. [Google Scholar] [CrossRef]
- Olden, J.D.; Ray, L.; Mims, M.C.; Horner-Devine, M.C. Filtration rates of the non-native Chinese mystery snail (Bellamya chinensis) and potential impacts on microbial communities. Limnetica 2013, 32, 107–120. [Google Scholar] [CrossRef]
- Ismail, N.S.; Tommerdahl, J.P.; Boehm, A.B.; Luthy, R.G. Escherichia coli reduction by bivalves in an impaired river impacted by agricultural land use. Environ. Sci. Technol. 2016, 50, 11025–11033. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.Y.; Han, X.K.; Liu, X.J.; Li, Q.Q.; Li, J.L. Construction of a high-density genetic map and QTL mapping for pearl quality-related traits in Hyriopsis cumingii. Sci. Rep. 2016, 6, 32608. [Google Scholar] [CrossRef]
- Zhao, Y.; Bai, Z.; Fu, L.; Liu, Y.; Wang, G.; Li, J. Comparison of growth and pearl production in males and females of the freshwater mussel, Hyriopsis cumingii, in China. Aquac. Int. 2013, 21, 1301–1310. [Google Scholar] [CrossRef]
- Sorokin, I.I.; Giovanardi, O.; Pranovi, F.; Sorokin, P.I. Need for restricting bivalve culture in the southern basin of the Lagoon of Venice. Hydrobiologia 1999, 400, 141–148. [Google Scholar] [CrossRef]
- Gallardi, D. Effects of bivalve aquaculture on the environment and their possible mitigation: A review. Fish. Aquac. J. 2014, 5. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, M.; Wu, Z. Can mussels change phytoplankton community structure and enhance prawn production in semi-enclosed prawn ponds? Aquac. Res. 2015, 46, 2559–2564. [Google Scholar] [CrossRef]
- He, H.; Liu, X.; Liu, X.; Yu, J.; Li, K.; Guan, B.; Jeppesen, E.; Liu, Z. Effects of cyanobacterial blooms on submerged macrophytes alleviated by the native Chinese bivalve Hyriopsis cumingii: A mesocosm experiment study. Ecol. Eng. 2014, 71, 363–367. [Google Scholar] [CrossRef]
- Zhao, L.; Walliser, E.O.; Mertz-Kraus, R.; Schoene, B.R. Unionid shells (Hyriopsis cumingii) record manganese cycling at the sediment-water interface in a shallow eutrophic lake in China (Lake Taihu). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 484, 97–108. [Google Scholar] [CrossRef]
- Liu, J.; Yan, H.; Liao, Z.; Zhang, K.; Schmidt, A.R.; Tao, T. Laboratory analysis on the surface runoff pollution reduction performance of permeable pavements. Sci. Total Environ. 2019, 691, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, G.; Wang, R.; Huang, H. Polycyclic aromatic hydrocarbons in the water-SPM-sediment system from the middle reaches of Huai River, China: Distribution, partitioning, origin tracing and ecological risk assessment. Environ. Pollut. 2017, 230, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.Z.; Xu, H.S. The Common Freshwater Phytoplankton Algae atlas of China; Shanghai Science and Technology Press: Shanghai, China, 2010; pp. 1–225. [Google Scholar]
- Zhou, X.; Zheng, J. Hydrobiology Atlas of Chongqing Section of the Three Gorges Reservoir Area; China Environmental Science Press: Beijing, China, 2005; pp. 1–125. [Google Scholar]
- Yu, X.B.; Hao, K.; Ling, F.; Wang, G.X. Aquatic environmental safety assessment and inhibition mechanism of chemicals for targeting Microcystis aeruginosa. Ecotoxicology 2014, 23, 1638–1647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, J.; Ling, J.; Wang, Y.; Zhang, S. Phytoplankton distribution and their relationship to environmental variables in Sanya Bay, South China Sea. Sci. Mar. 2010, 74, 783–792. [Google Scholar] [CrossRef]
- Wei, Y.; Sun, J.; Zhang, X.; Wang, J.; Huang, K. Picophytoplankton size and biomass around equatorial eastern Indian Ocean. MicrobiologyOpen 2019, 8, e00629. [Google Scholar] [CrossRef]
- Spatharis, S.; Roelke, D.L.; Dimitrakopoulos, P.G.; Kokkoris, G.D. Analyzing the (mis) behavior of Shannon index in eutrophication studies using field and simulated phytoplankton assemblages. Ecol. Indic. 2011, 11, 697–703. [Google Scholar] [CrossRef]
- Wei, F.S.; Qi, W.Q.; Bi, T.; Sun, Z.; Huang, Y. The Standard Methods of Water and Wastewater Monitoring and Analysis of China; China Environmental Science Press: Beijing, China, 2002. [Google Scholar]
- Chen, G.M. Ammonium molybdate spectrophotometric method for determination of total phosphorus in municipal sewage sludge. China Water Wastewater 2006, 22, 85–86. [Google Scholar]
- Meng, X.; Zhu, C.; Chen, X. Alkaline potassium persulfate UV spectrophotometric determination of total nitrogen frequently asked questions and solutions. Environ. Sci. Manag. 2010, 35, 126–128. [Google Scholar]
- Dedkov, Y.M.; Elizarova, O.V.; Kelina, S.Y. Dichromate method for the determination of chemical oxygen demand. J. Anal. Chem. 2000, 55, 777–781. [Google Scholar] [CrossRef]
- Yan, L.L.; Zhang, G.F.; Liu, Q.G.; Li, J.L. Optimization of culturing the freshwater pearl mussels, Hyriopsis cumingii with filter feeding Chinese carps (bighead carp and silver carp) by orthogonal array design. Aquaculture 2009, 292, 60–66. [Google Scholar] [CrossRef]
- Canuel, E.A. Relations between river flow, primary production and fatty acid composition of particulate organic matter in San Francisco and Chesapeake Bays: A multivariate approach. Org. Geochem. 2001, 32, 563–583. [Google Scholar] [CrossRef]
- Volkman, J.K.; Revill, A.T.; Holdsworth, D.G.; Fredericks, D. Organic matter sources in an enclosed coastal inlet assessed using lipid biomarkers and stable isotopes. Org. Geochem. 2008, 39, 689–710. [Google Scholar] [CrossRef]
- Bauer, J.E.; Cai, W.-J.; Raymond, P.A.; Bianchi, T.S.; Hopkinson, C.S.; Regnier, P.A.G. The changing carbon cycle of the coastal ocean. Nature 2013, 504, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Porcal, P.; Dillon, P.J.; Molot, L.A. Photochemical production and decomposition of particulate organic carbon in a freshwater stream. Aquat. Sci. 2013, 75, 469–482. [Google Scholar] [CrossRef]
- Gladyshev, M.I.; Sushchik, N.N.; Anishchenko, O.V.; Makhutova, O.N.; Kolmakov, V.I.; Kalachova, G.S.; Kolmakova, A.A.; Dubovskaya, O.P. Efficiency of transfer of essential polyunsaturated fatty acids versus organic carbon from producers to consumers in a eutrophic reservoir. Oecologia 2011, 165, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yin, K.; Wang, L.; Chen, F.; Zhang, D.; Yang, Y. The sources and accumulation rate of sedimentary organic matter in the Pearl River Estuary and adjacent coastal area, Southern China. Estuar. Coast. Shelf Sci. 2009, 85, 190–196. [Google Scholar] [CrossRef]
- Boyer, J.N.; Kelble, C.R.; Ortner, P.B.; Rudnick, D.T. Phytoplankton bloom status: Chlorophyll a biomass as an indicator of water quality condition in the southern estuaries of Florida, USA. Ecol. Indic. 2009, 9, S56–S67. [Google Scholar] [CrossRef]
- Wang, G.; Wang, X.; Wu, L.; Li, X. Contribution and purification mechanism of bio components to pollutants removal in an integrated ecological floating bed. J. Civ. Archit. Environ. Eng. 2015, 4, 136–141. [Google Scholar]
- Xu, H.; Paerl, H.W.; Qin, B.; Zhu, G.; Gao, G. Nitrogen and phosphorus inputs control phytoplankton growth in eutrophic Lake Taihu, China. Limnol. Oceanogr. 2010, 55, 420–432. [Google Scholar] [CrossRef]


| CG | G1 | G2 | G3 | G4 | |
|---|---|---|---|---|---|
| TSS (mg L−1) | 50.33 ± 6.08 a | 46.33 ± 7.81 a | 40.67 ± 2.52 a,b | 39.00 ± 10.26 a,b | 30.67 ± 3.79 b |
| FA (mg L−1) | 16.6 ± 0.04 a | 17.31 ± 0.02 b | 12.38 ± 0.25 c | 12.22 ± 0.19 c | 10.48 ± 0.04 d |
| POC (mg L−1) | 10.72 ± 0.03 a | 4.80 ± 0.13 b | 4.29 ± 0.12 d | 4.41 ± 0.11 c | 4.73 ± 0.23 b |
| Phytoplankton Statistical Analysis | CG | G1 | G2 | G3 | G4 |
|---|---|---|---|---|---|
| Bacillariophyta Biomass (10−3 mg L−1) | 922.20 | 115.66 | 55.04 | 15.15 | 39.30 |
| Navicula simplex Krassk | 386.43 | 17.97 | 10.48 | 5.99 | 1.50 |
| Synedra acus Kützing | 239.65 | 31.95 | 17.97 | 5.99 | 17.97 |
| Synedra ulna Ehrenberg | 103.85 | 10.65 | 3.99 | ||
| Diatoma elongatum Ag. | 83.88 | 2.66 | 4.99 | 1.00 | 3.00 |
| Melosira granulata Ralfs | 67.10 | 51.12 | 20.77 | 10.38 | |
| Navicula cincta Van Heurck | 27.26 | 1.30 | 0.65 | 1.95 | |
| Cocconeis placentula Hust | 4.99 | ||||
| Cyclotella meneghiniana Kütz | 3.99 | 2.00 | |||
| Stauroneis kriegeri Patrick | 3.06 | 0.17 | 0.17 | 0.51 | |
| Gomphonema constrictum Grunow | 2.00 | ||||
| Chlorophyta Biomass (10−3 mg L−1) | 45.63 | 30.49 | 10.28 | 4.94 | 36.43 |
| Gonium sociale | 27.56 | 14.38 | 5.99 | 3.00 | 27.56 |
| Planktosphaeria gelatinosa Smith | 4.49 | 6.66 | 1.65 | ||
| Crucigenia puadrata Morren | 3.99 | 3.20 | 1.20 | ||
| Sceaedesmus cavinaus Chod | 3.20 | 2.66 | 0.85 | 0.80 | |
| Crucigenia tetrapedia Morr | 2.40 | 1.07 | 0.40 | 1.20 | |
| Schroederia spiralis Korsch | 2.00 | ||||
| Selenastrum gracile | 1.20 | 1.33 | 0.60 | ||
| Ankistrodesmus angustus B.S. Korš | 0.40 | 0.27 | 0.20 | 0.20 | 0.20 |
| Cosmarium subtumidum Nordst | 0.40 | ||||
| Sceaedesmus oblipuus Kütz | 0.80 | ||||
| Sceaedesmus dimorphus Kütz | 0.93 | 0.55 | |||
| Pediastrum tetras Ralfs | 1.60 | ||||
| Pediastrum duplex Mey | 1.60 | ||||
| Cyanophyta Biomass (10−3 mg L−1) | 64.44 | 38.30 | 17.77 | 0.53 | 1.64 |
| Nostoc communes Vauch | 43.94 | 13.31 | 15.98 | ||
| Chroococcus minor Näg | 16.11 | 3.15 | 0.59 | 0.15 | 1.63 |
| Phormidium tenue Gom | 4.39 | 21.83 | 1.20 | ||
| Merismopedia punctata Meyen | 0.38 | 0.01 | |||
| Species number | 22 | 17 | 15 | 11 | 16 |
| Total Biomass (10−3 mg L−1) | 1032.27 | 184.45 | 83.09 | 20.62 | 77.09 |
| Phytoplankton Density | 12.00 | 5.53 | 3.06 | 1.72 | 2.01 |
| Diversity index H’ | 3.61 | 3.23 | 3.35 | 1.49 | 2.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Yang, Q.; Zhao, Z.; Tang, X.; Xiong, B.; Su, S.; Wu, Z.; Yao, W. Ecological Efficiency of the Mussel Hyriopsis cumingii (Lea, 1852) on Particulate Organic Matter Filtering, Algal Controlling and Water Quality Regulation. Water 2021, 13, 297. https://doi.org/10.3390/w13030297
Yu X, Yang Q, Zhao Z, Tang X, Xiong B, Su S, Wu Z, Yao W. Ecological Efficiency of the Mussel Hyriopsis cumingii (Lea, 1852) on Particulate Organic Matter Filtering, Algal Controlling and Water Quality Regulation. Water. 2021; 13(3):297. https://doi.org/10.3390/w13030297
Chicago/Turabian StyleYu, Xiaobo, Qinglin Yang, Zhe Zhao, Xiaoqi Tang, Bo Xiong, Shengqi Su, Zhengli Wu, and Weizhi Yao. 2021. "Ecological Efficiency of the Mussel Hyriopsis cumingii (Lea, 1852) on Particulate Organic Matter Filtering, Algal Controlling and Water Quality Regulation" Water 13, no. 3: 297. https://doi.org/10.3390/w13030297
APA StyleYu, X., Yang, Q., Zhao, Z., Tang, X., Xiong, B., Su, S., Wu, Z., & Yao, W. (2021). Ecological Efficiency of the Mussel Hyriopsis cumingii (Lea, 1852) on Particulate Organic Matter Filtering, Algal Controlling and Water Quality Regulation. Water, 13(3), 297. https://doi.org/10.3390/w13030297






