Use of Stainless-Steel Electrodes on the Electrochemical Oxidation of Naproxen and its Transformation Products in Surface Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Experimental Device
2.3. Characterization of Surface Water
2.4. Degradation of Naproxen and its Oxidation Products
3. Results and Discussion
3.1. Surface Water Characterization
3.2. Degradation of Naproxen
3.3. Degradation of Naproxen Transformation Products (TP)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- DrugBank Online. 2021. Available online: https://go.drugbank.com/drugs/DB00788 (accessed on 2 July 2021).
- Gómez-Oliván, L.M.; Neri-Cruz, N.; Galar-Martínez, M.; Islas-Flores, H.; García-Medina, S. Binary mixtures of diclofenac with paracetamol, ibuprofen, naproxen, and acetylsalicylic acid and these pharmaceuticals in isolated form induce oxidative stress on Hyalella azteca. Environ. Monit. Assess. 2014, 186, 7259–7271. [Google Scholar] [CrossRef] [PubMed]
- Warden, S.J. Prophylactic use of NSAIDs by athletes: A risk/benefit assessment. Physician Sportsmed. 2010, 38, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Serra-Pérez, E.; Álvarez-Torrellas, S.; Águeda, V.I.; Larriba, M.; Ovejero, G.; García, J. Effective removal of naproxen from aqueous solutions by CWAO process using noble metals supported on carbon nanospheres catalysts. Sep. Purif. Technol. 2021, 259, 118084. [Google Scholar] [CrossRef]
- Wojcieszynska, D.; Guzik, U. Naproxen in the environment: Its occurrence, toxicity to nontarget organisms and biodegradation. Appl. Microbiol. Biotechnol. 2020, 104, 1849–1857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebele, A.J.; Abou-Elwafa Abdallah, M.; Harrad, S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Gao, Y.Q.; Gao, N.Y.; Chu, W.H.; Yang, Q.L.; Yin, D.Q. Kinetics and mechanistic investigation into the degradation of naproxen by a UV/chlorine process. RSC Adv. 2017, 7, 33627–33634. [Google Scholar] [CrossRef] [Green Version]
- Santos, L.H.; Araújo, A.N.; Fachini, A.; Pena, A.; Delerue-Matos, C.; Montenegro, M.C. Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. J. Hazard. Mater. 2010, 175, 45–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abreu, E.; Fidelis, M.Z.; Fuziki, M.E.; Malikoski, R.M.; Mastsubara, M.C.; Imada, R.E.; de Tuesta, J.L.D.; Gomes, H.T.; Anziliero, M.D.; Baldykowski, B.; et al. Degradation of emerging contaminants: Effect of thermal treatment on Nb2O5 as photocatalyst. J. Photochem. Photobiol. A Chem. 2021, 419, 113484. [Google Scholar] [CrossRef]
- de Tuesta, J.L.D.; de Almeida, F.V.M.; Oliveira, J.R.P.; Praça, P.; Guerreiro, M.C.; Gomes, H.T. Kinetic insights on wet peroxide oxidation of caffeine using EDTA-functionalized low-cost catalysts prepared from compost generated in municipal solid waste treatment facilities. Environ. Technol. Innov. 2021, 24, 101984. [Google Scholar] [CrossRef]
- Fayyaz, A.; Saravanakumar, K.; Talukdar, K.; Kim, Y.; Yoon b, Y.; Park, C.M. Catalytic oxidation of naproxen in cobalt spinel ferrite decorated Ti3C2Tx MXene activated persulfate system: Mechanisms and pathways. Chem. Eng. J. 2021, 407, 127842. [Google Scholar] [CrossRef]
- Changanaqui, K.; Alarcón, H.; Brillas, E.; Sirés, I. Blue LED light-driven photoelectrocatalytic removal of naproxen from water: Kinetics and primary by-products. J. Electroanal. Chem. 2020, 867, 114192. [Google Scholar] [CrossRef]
- Aguilar, C.M.; Chairez, I.; Rodríguez, J.L.; Tiznado, H.; Santillán, R.; Arrieta, D.; Poznyak, T. Inhibition effect of ethanol in naproxen degradation by catalytic ozonation with NiO. RSC Adv. 2019, 9, 14822–14833. [Google Scholar] [CrossRef] [Green Version]
- Benitez, F.J.; García, J.; Acero, J.L.; Real, F.J.; Roldan, G. Non-catalytic and catalytic wet air oxidation of pharmaceuticals in ultra-pure and natural waters. Process Saf. Environ. Prot. 2011, 89, 334–341. [Google Scholar] [CrossRef]
- Zhao, X.; Qu, J.; Liu, H.; Qiang, Z.; Liu, R.; Hu, C. Photoelectrochemical degradation of anti-inflammatory pharmaceuticals at Bi2/MoO6-boron-doped diamond hybrid electrode under visible light irradiation. Appl. Catal. B Environ. 2009, 91, 539–545. [Google Scholar] [CrossRef]
- Villanueva-Rodríguez, M.; Bello-Mendoza, R.; Hernández-Ramírez, A.; Ruiz-Ruiz, E.J. Degradation of anti-inflammatory drugs in municipal wastewater by heterogeneous photocatalysis and electro-Fenton process. Environ. Technol. 2018, 40, 2436–2445. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Motti, C.A.; Glass, B.D.; Oelgemüller, M. Solar photolysis versus TiO2 mediated solar photocatalysis: A kinetic study of the degradation of naproxen and diclofenac in various water matrices. Environ. Sci. Pollut. Res. 2016, 23, 17437–17448. [Google Scholar] [CrossRef]
- Regmi, C.; Kshetri, Y.K.; Pandey, R.P.; Lee, S.W. Visible-light-driven S and W co-doped dendritic BiVO4 for efficient photocatalytic degradation of naproxen and its mechanistic analysis. Mol. Catal. 2018, 453, 149–160. [Google Scholar] [CrossRef]
- Amini, Z.; Givianrad, M.H.; Saber-Tehrani, M.; Azar, P.A.; Husain, S.W. Synthesis of N doped TiO2/SiO2/Fe3O4 magnetic nanocomposites as a novel purple LED illumination-driven photocatalyst for photocatalytic and photoelectrocatalytic degradation of naproxen: Optimization and different scavenger agents study. J. Environ. Sci. Health A 2019, 54, 1254–1267. [Google Scholar] [CrossRef]
- González, T.; Domínguez, J.R.; Palo, P.; Sánchez-Martín, J. Conductive-diamond electrochemical advanced oxidation of naproxen in aqueous solution: Optimizing the process. J. Chem. Technol. Biotechnol. 2011, 86, 121–127. [Google Scholar] [CrossRef]
- Coria, G.; Sirés, I.; Brillas, E.; Nava, J.L. Influence of the anode material on the degradation of naproxen by Fenton-based electrochemical processes. Chem. Eng. J. 2016, 304, 817–825. [Google Scholar] [CrossRef]
- Chin, C.J.M.; Chen, T.-Y.; Lee, M.; Chang, C.F.; Liu, Y.-T.; Kuo, Y.-T. Effective anodic oxidation of naproxen by platinum nanoparticles coated FTO glass. J. Hazard. Mater. 2014, 277, 110–119. [Google Scholar] [CrossRef]
- Montes, R.H.O.; Lima, A.P.; Cunha, R.R.; Guedes, T.J.; dos Santos, W.T.P.; Nossol, E.; Richter, E.M.; Munoz, R.A.A. Size effects of multi-walled carbon nanotubes on the electrochemical oxidation of propionic acid derivative drugs: Ibuprofen and naproxen. J. Electroanal. Chem. 2016, 775, 342–349. [Google Scholar] [CrossRef]
- Zavala, M.Á.L.; Vega, D.A.; Vega, J.M.Á.; Jerez, O.F.C.; Hernández, R.A.C. Electrochemical oxidation of acetaminophen and its transformation products in surface water: Effect of pH and current density. Heliyon 2020, 6, e03394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anglada, A.; Urtiaga, A.M.; Ortiz, I. Contributions of electrochemical oxidation to waste-water treatment: Fundamentals and review of applications. J. Chem. Technol. Biotechnol. 2009, 84, 1747–1755. [Google Scholar] [CrossRef]
- García-Segura, S.; Ocón, J.D.; Chong, M.N. Electrochemical oxidation remediation of real wastewater effluents—A review. Process SAF Environ. 2018, 113, 48–67. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Keller, J.; Brillas, E.; Radjenovic, J. Removal of organic contaminants from secondary effluent by anodic oxidation with a boron-doped diamond anode as tertiary treatment. J. Hazard. Mater. 2015, 283, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Huitle, C.A.; Panizza, M. Electrochemical oxidation of organic pollutants for wastewater treatment. Curr. Opin. Electrochem. 2018, 11, 62–71. [Google Scholar] [CrossRef]
- Shestakova, M.; Sillanpää, M. Electrode materials used for electrochemical oxidation of organic compounds in wastewater. Rev. Environ. Sci. Biotechnol. 2017, 16, 223–238. [Google Scholar] [CrossRef]
- Albrimi, Y.A.; Eddib, A.; Douch, J.; Berghoute, Y.; Hamdani, M.; Souto, R.M. Electrochemical Behaviour of AISI 316 Austenitic Stainless Steel in Acidic Media Containing Chloride Ions. Int. J. Electrochem. Sci. 2011, 6, 4614–4627. [Google Scholar]
- Iliyasu, I.; Yawas, D.S.; Aku, S.Y. Corrosion Behavior of Austenitic Stainless Steel in Sulphuric Acid at Various Concentrations. Adv. Appl. Sci. Res. 2012, 3, 3909–3915. [Google Scholar]
- Szpyrkowicz, L.; Zilio-Grandi, F.; Kaul, S.N.; Rigoni-Stern, S. Electrochemical treatment of copper cyanide wastewaters using stainless steel electrodes. Water Sci. Technol. 1998, 38, 261–268. [Google Scholar] [CrossRef]
- Abuzaid, N.S.; Al-Hamouz, Z.; Bukhari, A.A.; Essa, M.H. Electrochemical treatment of nitrite using stainless steel electrodes. Water Air Soil Poll. 1999, 109, 429–442. [Google Scholar] [CrossRef]
- Ramachandramoorthy, T.; Rajendran, A.; Padmavathy, S.; Subramanian, B. Electrochemical oxidation of cinnamic acid using stainless steel electrodes. Ionics 2004, 10, 283–287. [Google Scholar] [CrossRef]
- Cañizares, P.; Martínez, F.; Díaz, M.; García-Gómez, J.; Rodrigo, M.A. Electrochemical oxidation of aqueous phenol wastes using active and nonactive electrodes. J. Electrochem. Soc. 2002, 149, D118–D124. [Google Scholar] [CrossRef]
- Wu, Y.; Jin, X.; Liu, Y.; Huang, S.; Wang, F.; Zheng, X.; Wei, D.; Liu, H.; Chen, P. Facile synthesis of solar light-driven Z-scheme Ag2CO3/TNS-001 photocatalyst for the effective degradation of naproxen: Mechanisms and degradation pathways. Sep. Purif. Technol. 2021, 254, 117598. [Google Scholar] [CrossRef]
- Asmar, R.E.; Baalbaki, A.; Khalil, Z.A.; Naim, S.; Bejjani, A. Antoine Ghauch, Iron-based metal organic framework MIL-88-A for the degradation of naproxen in water through persulfate activation. Chem. Eng. J. 2021, 405, 126701. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Zhang, Y.; Zhang, T.; Ye, M. Heterogeneous oxidation of naproxen in the presence of α-MnO2 nanostructures with different morphologies. Appl. Catal. B Environ. 2012, 127, 182–189. [Google Scholar] [CrossRef]
- Sétifi, N.; Debbache, N.; Sehili, T.; Halimi, O. Heterogeneous Fenton-like oxidation of naproxen using synthesized goethite-montmorillonite nanocomposite. J. Photoch. Photobio. A Chem. 2019, 370, 67–74. [Google Scholar] [CrossRef]
- Chen, H.; Lin, T.; Zhang, S.; Xu, H.; Tao, H.; Chen, W. Novel FeII/EDDS/UV/PAA advanced oxidation process: Mechanisms and applications for naproxen degradation at neutral pH and low FeII dosage. Chem. Eng. J. 2021, 417, 127896. [Google Scholar] [CrossRef]
- López-Cázares, M.I.; Isaacs-Páez, E.D.; Ascacio-Valdés, J.; Aguilar-González, C.N.; Rangel-Mendez, J.R.; Chazaro-Ruiz, L.F. Electro-assisted naproxen adsorption followed by its electrodegradation and simultaneous electroreactivation of the activated carbon electrode. Sep. Purif. Technol. 2021, 258, 118030. [Google Scholar] [CrossRef]
- Sirés, I.; Brillas, E. Remediation of water pollution caused by pharmaceutical residues based on electrochemical separation and degradation technologies: A review. Environ. Int. 2012, 40, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Sirés, I.; Arias, C.; Cabot, P.L.; Centellas, F.; Rodríguez, R.M.; Garrido, J.A.; Brillas, E. Paracetamol mineralization by advanced electrochemical oxidation processes for wastewater treatment. Environ. Chem. 2004, 1, 26–28. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater, 20th ed.; APHA: Washington, DC, USA; WWA: Washington, DC, USA; WEF: Washington, DC, USA, 2005. [Google Scholar]
- Comninellis, C. Electrocatalysis in the electrochemical conversion/combustion of organic pollutants for wastewater treatment. Electrochim. Acta 1994, 39, 1857–1862. [Google Scholar] [CrossRef]
- Panizza, M.; Cerisola, G. Direct and mediated anodic oxidation of organic pollutants. Chem. Rev. 2009, 109, 6541–6569. [Google Scholar] [CrossRef] [PubMed]
- Boxall, C.; Kelsall, G.H. Hypochlorite electrogeneration. II. Thermodynamics and kinetic model of the anode reaction layer. Inst. Chem. Eng. Symp. Ser. 1992, 127, 59–70. [Google Scholar]
- Fajardo, A.S.; Seca, H.F.; Martins, R.C.; Corceiro, V.N.; Freitas, I.F.; Quinta-Ferreira, M.E.; Quinta-Ferreira, R.M. Electrochemical oxidation of phenolic wastewaters using a batch-stirred reactor with NaCl electrolyte and Ti/RuO2 anodes. J. Electroanal. Chem. 2017, 785, 180–189. [Google Scholar] [CrossRef]
- Carneiro, J.F.; Aquino, J.M.; Silva, A.J.; Barreiro, J.C.; Cass, Q.B.; Rocha-Filho, R.C. The effect of the supporting electrolyte on the electrooxidation of enrofloxacin using a flow cell with a BDD anode: Kinetics and follow-up of oxidation intermediates and antimicrobial activity. Chemosphere 2018, 206, 674–681. [Google Scholar] [CrossRef] [PubMed]
- García-Espinoza, J.D.; Mijaylova-Nacheva, P.; Avilés-Flores, M. Electrochemical carbamazepine degradation: Effect of the generated active chlorine, transformation pathways and toxicity. Chemosphere 2018, 192, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Baloul, Y.; Aubry, O.; Rabat, H.; Colas, C.; Maunit, B.; Hong, D. Degradation of Paracetamol in Aqueous Solution by Non-Thermal Plasma. In Proceedings of the 15th High Pressure Low Temperature Plasma Chemistry Symposium (HAKONE 15), Brno, Czech Republic, 11–16 September 2016. [Google Scholar]
- Sirés, I.; Garrido, J.A.; Rodríguez, R.M.; Cabot, P.L.; Centellas, F.; Arias, C.; Brillas, E. Electrochemical degradation of paracetamol from water by catalytic action of Fe2+, Cu2+, and UVA light on electrogenerated hydrogen peroxide. J. Electrochem. Soc. 2006, 153, 1–9. [Google Scholar] [CrossRef]
- De Tuesta, J.L.D.; Quintanilla, A.; Casas, A.; Morales-Torres, S.; Faria, J.L.; Silva, A.M.T.; Gomes, H.T. The pH effect on the kinetics of 4-nitrophenol removal by CWPO with doped carbon black catalysts. Catal. Today 2020, 356, 216–225. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Tang, Y.; Wu, Y.; Feng, L.; Zhang, L. Degradation of naproxen in chlorination and UV/chlorine processes: Kinetics and degradation products. Environ. Sci. Pollut. Res. 2019, 26, 34301–34310. [Google Scholar] [CrossRef] [PubMed]
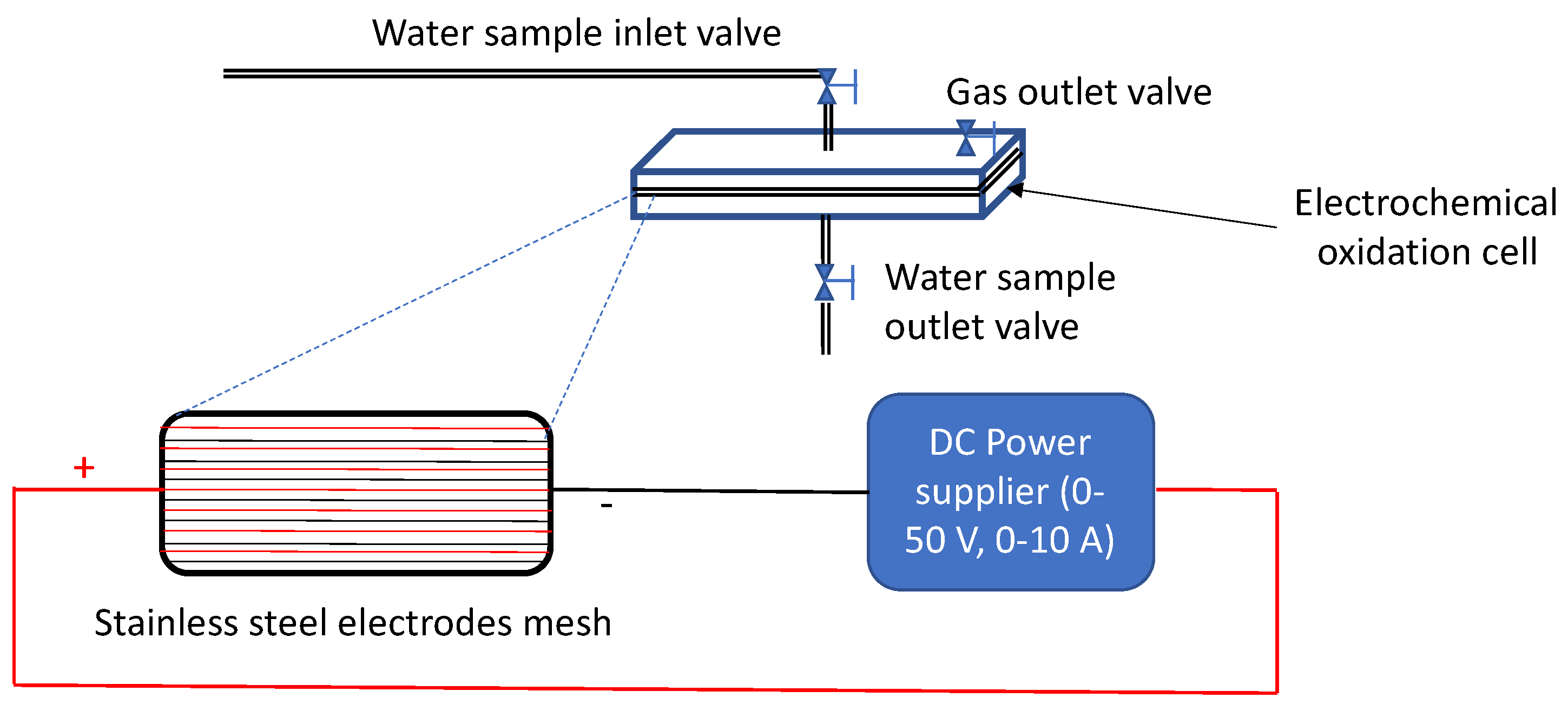
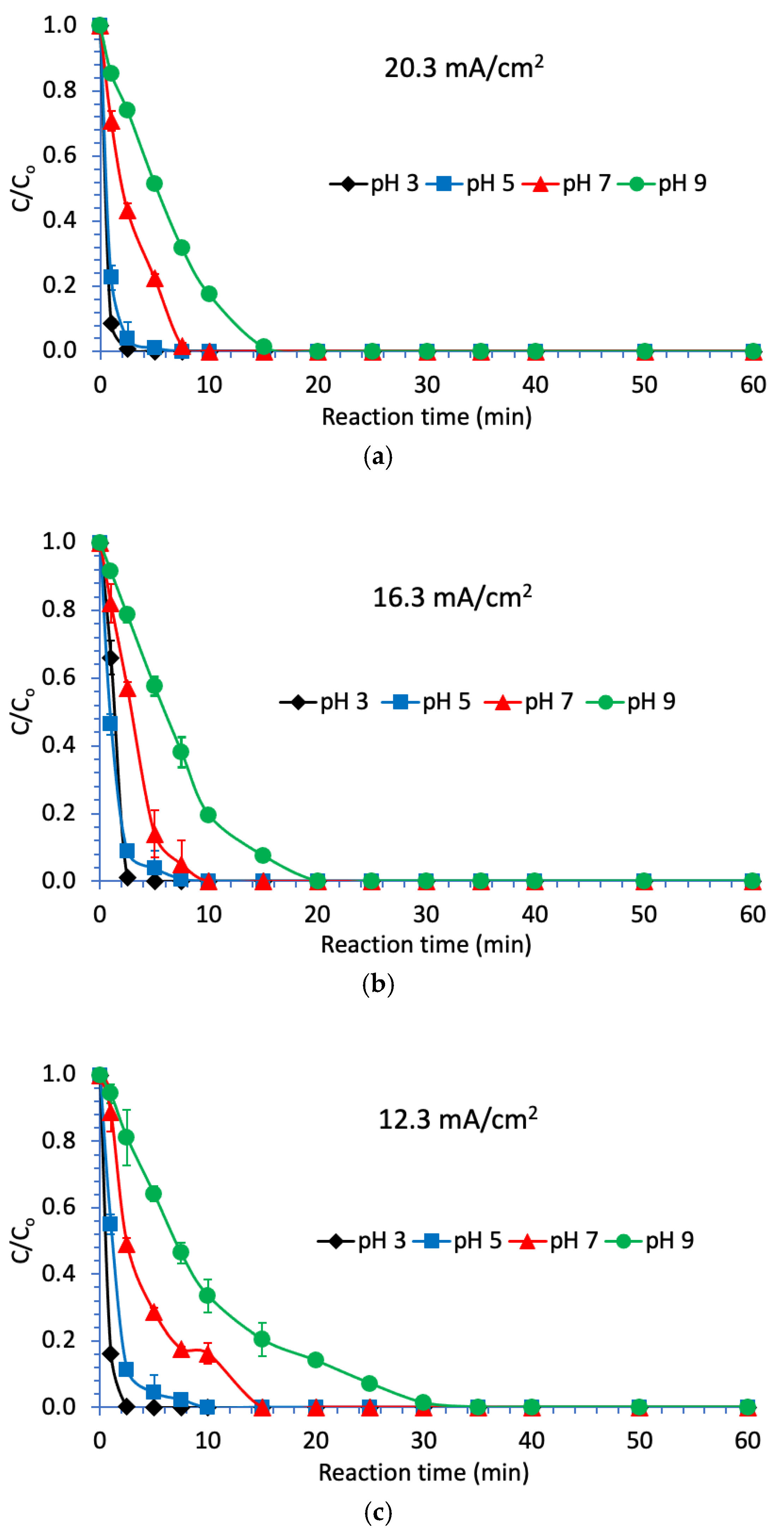
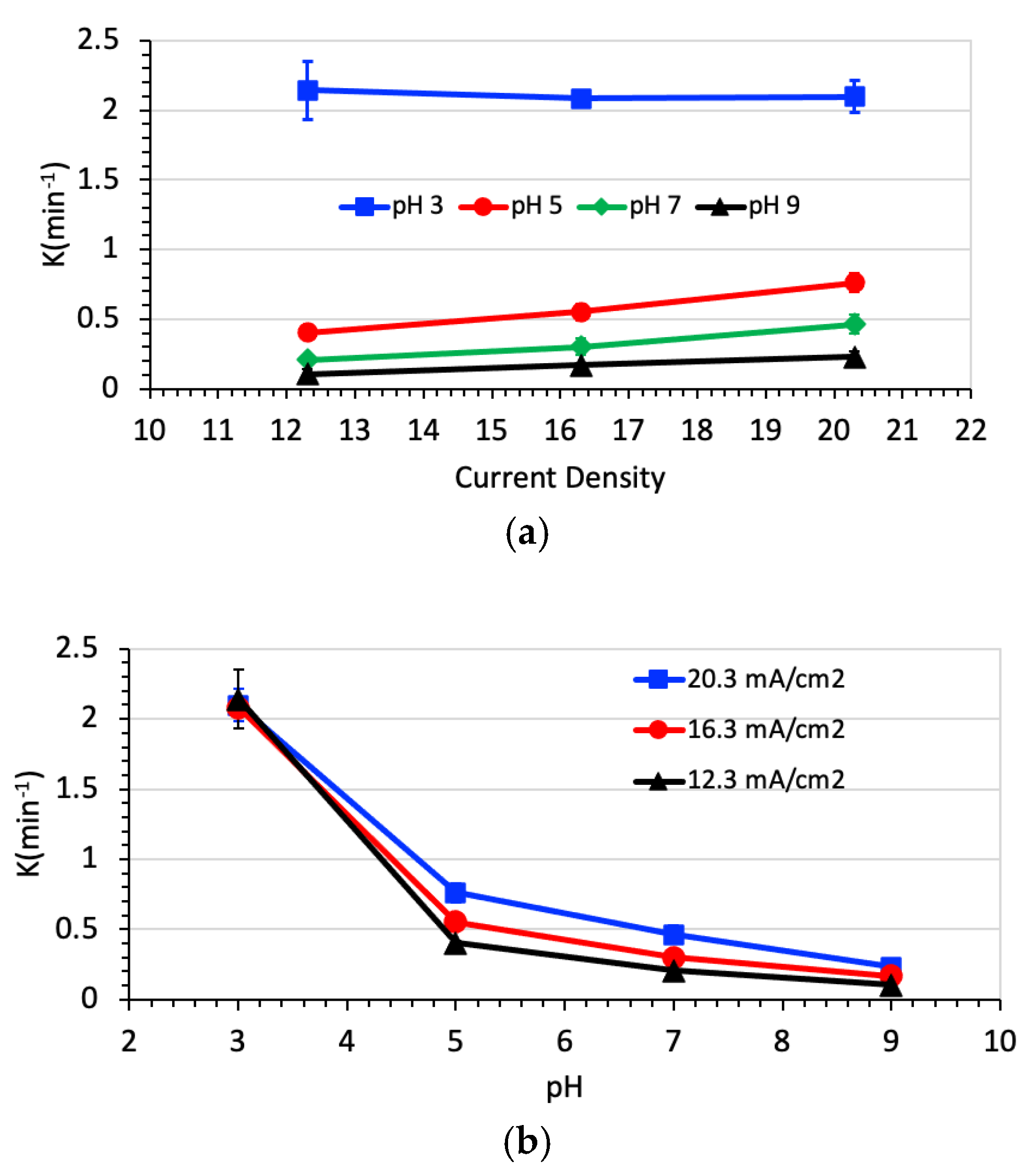
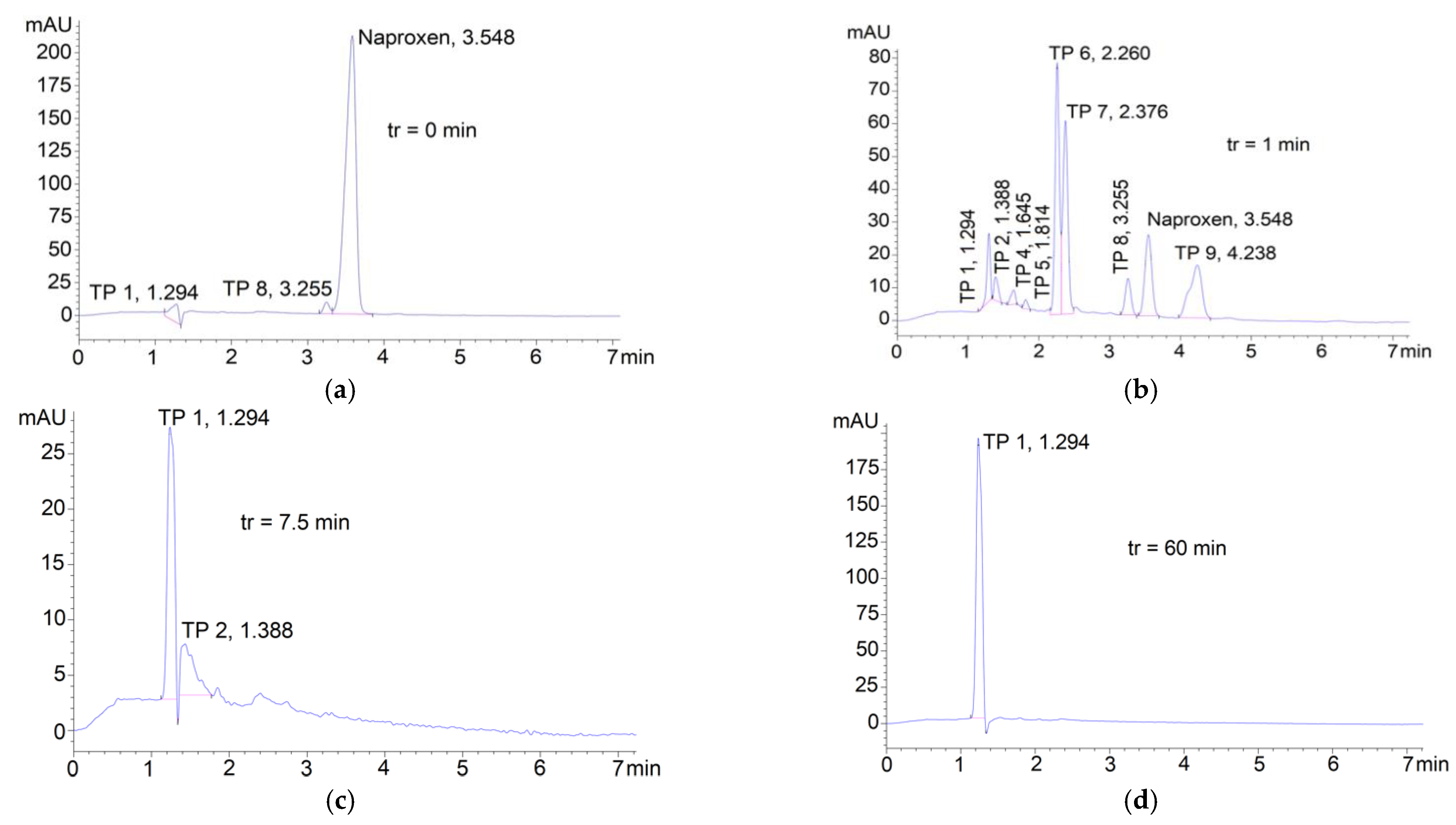
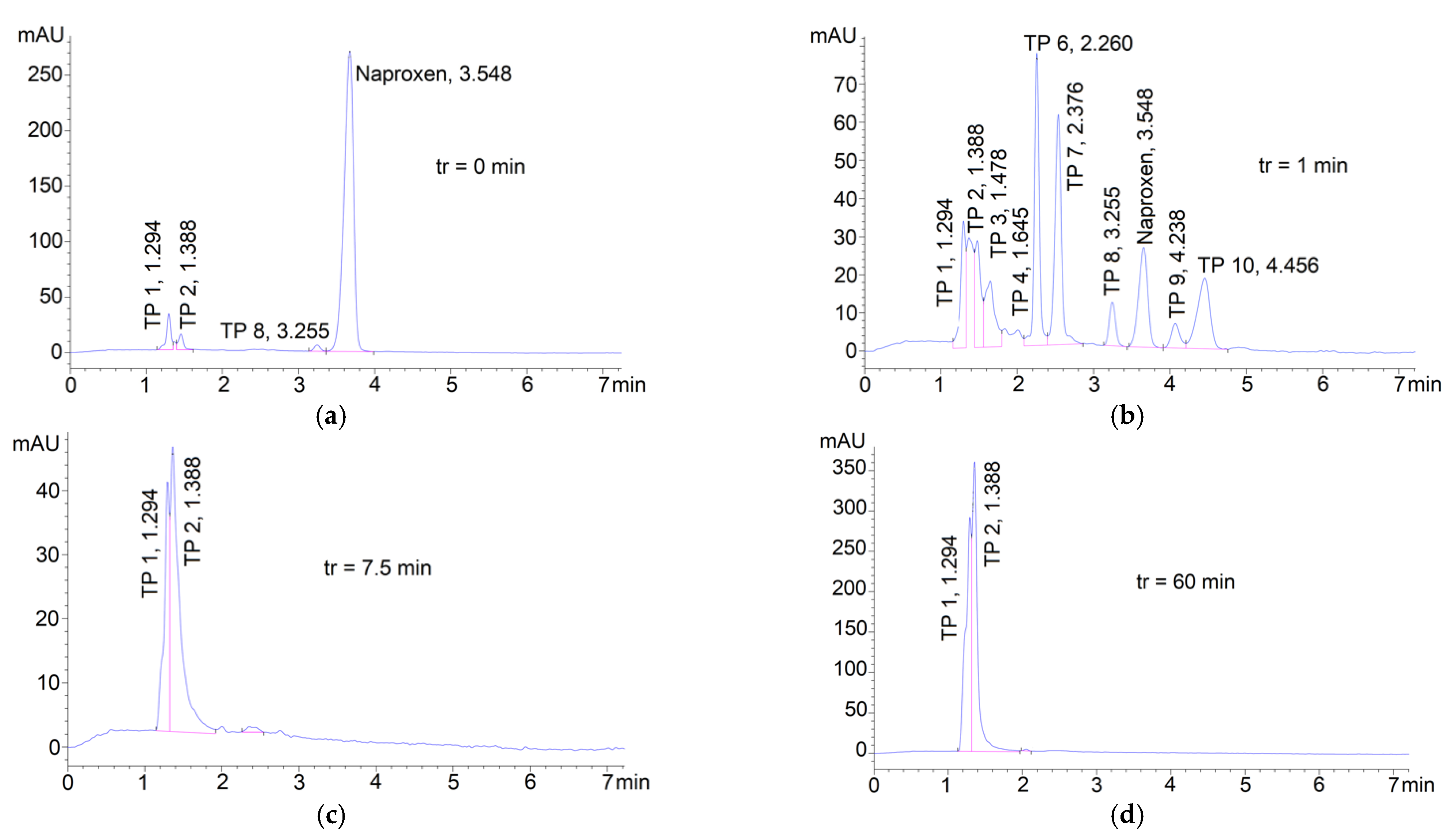
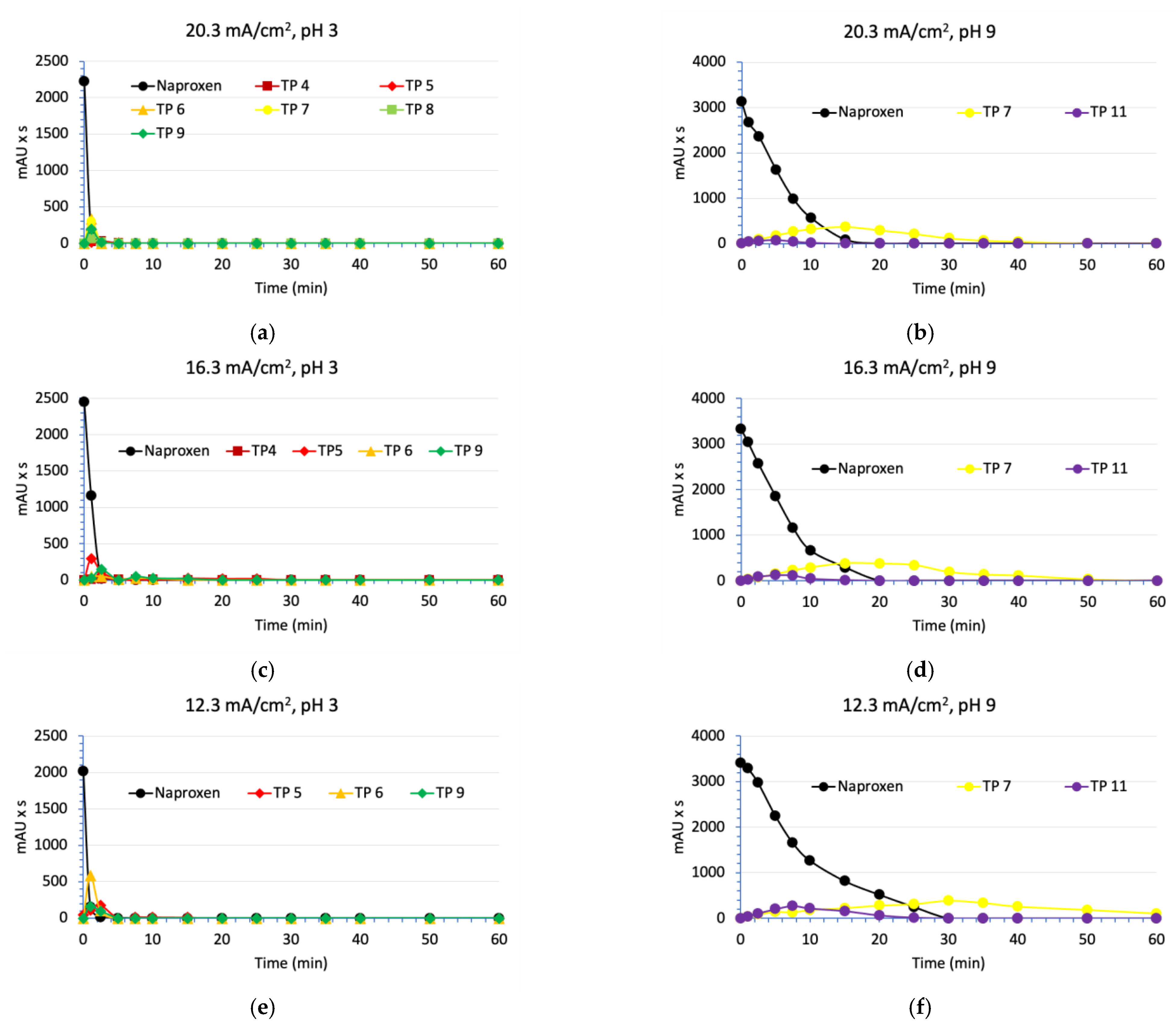
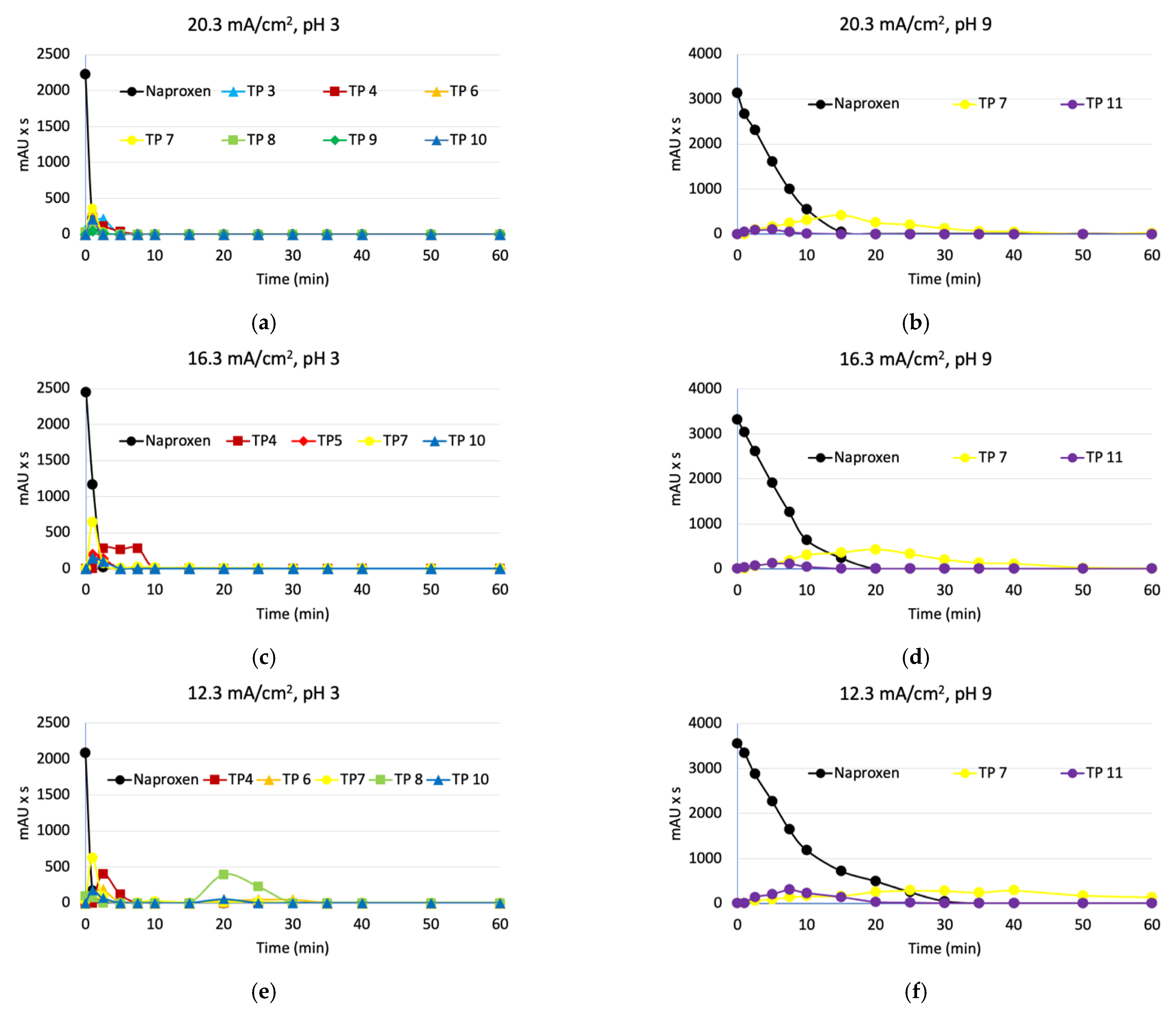
| Parameter | RSW 1 | RSW 1 + NAP 2 Solution | |||
|---|---|---|---|---|---|
| pH 3 | pH 5 | pH 7 | pH 9 | ||
| COD (mg/L) | 20.80 ± 0.96 | 26.20 ± 0.71 | 26.20 ± 0.71 | 26.20 ± 0.71 | 26.20 ± 0.71 |
| TOC (mg/L) | 5.07 ± 0.03 | 14.20 ± 0.47 | 14.20 ± 0.47 | 14.20 ± 0.47 | 14.20 ± 0.47 |
| Chlorides (mg/L) | 4.20 ± 0.37 | 322.10 ± 1.42 | 203.17 ± 1.07 | 84.23 ± 0.62 | 4.20 ± 0.37 |
| pH | 8.13 ± 0.16 | 3.0 ± 0.01 | 5.0 ± 0.24 | 7.0 ± 0.23 | 9.0 ± 0.06 |
| EC (µs/cm) | 412.00 ± 14.23 | 985 ± 50.21 | 545 ± 9.29 | 490 ± 23.29 | 440 ± 10.12 |
| Turbidity (NTU) | 1.24 ± 0.67 | 1.85 ± 0.86 | 1.11 ± 0.36 | 1.97 ± 0.53 | 2.11 ± 1.12 |
| TS (mg/L) | 322.40 ± 9.24 | - | - | - | - |
| TSS (mg/L) | 61.30 ± 6.82 | - | - | - | - |
| TDS (mg/L) | 261.10 ± 5.27 | - | - | - | - |
| pH | DC Current Density (mA/cm2) | ||
|---|---|---|---|
| 12.3 | 16.3 | 20.3 | |
| 3 | 2.5 | 5.0 | 5.0 |
| 5 | 10.0 | 7.5 | 5.0 |
| 7 | 15.0 | 10.0 | 7.5 |
| 9 | 35.0 | 20.0 | 15.0 |
| DC Density (mA/cm2) | 20.3 | 16.3 | 12.3 | |||
|---|---|---|---|---|---|---|
| pH | TE | SL | TE | SL | TE | SL |
| 3 | 2.5 | 7.5 | 5 | 10 | 5 | 30 |
| 5 2 | 15 | 30 | 15 | 30 | 20 | 40 |
| 7 2 | 25 | 30 | 30 | 30 | 35 | 40 |
| 9 | 45 | 50 | 50 | 50 | 60 | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López Zavala, M.Á.; Anglés Vega, D. Use of Stainless-Steel Electrodes on the Electrochemical Oxidation of Naproxen and its Transformation Products in Surface Water. Water 2021, 13, 3604. https://doi.org/10.3390/w13243604
López Zavala MÁ, Anglés Vega D. Use of Stainless-Steel Electrodes on the Electrochemical Oxidation of Naproxen and its Transformation Products in Surface Water. Water. 2021; 13(24):3604. https://doi.org/10.3390/w13243604
Chicago/Turabian StyleLópez Zavala, Miguel Ángel, and Diego Anglés Vega. 2021. "Use of Stainless-Steel Electrodes on the Electrochemical Oxidation of Naproxen and its Transformation Products in Surface Water" Water 13, no. 24: 3604. https://doi.org/10.3390/w13243604
APA StyleLópez Zavala, M. Á., & Anglés Vega, D. (2021). Use of Stainless-Steel Electrodes on the Electrochemical Oxidation of Naproxen and its Transformation Products in Surface Water. Water, 13(24), 3604. https://doi.org/10.3390/w13243604






