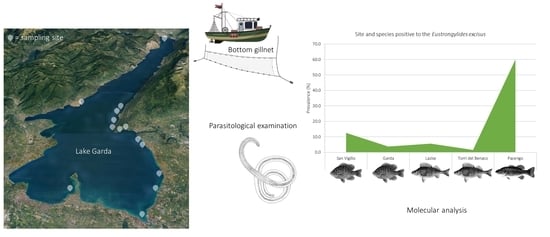
Could Fish Feeding Behaviour and Size Explain Prevalence Differences of the Nematode Eustrongylides excisus among Species? The Case Study of Lake Garda
Abstract
1. Introduction
2. Materials and Methods
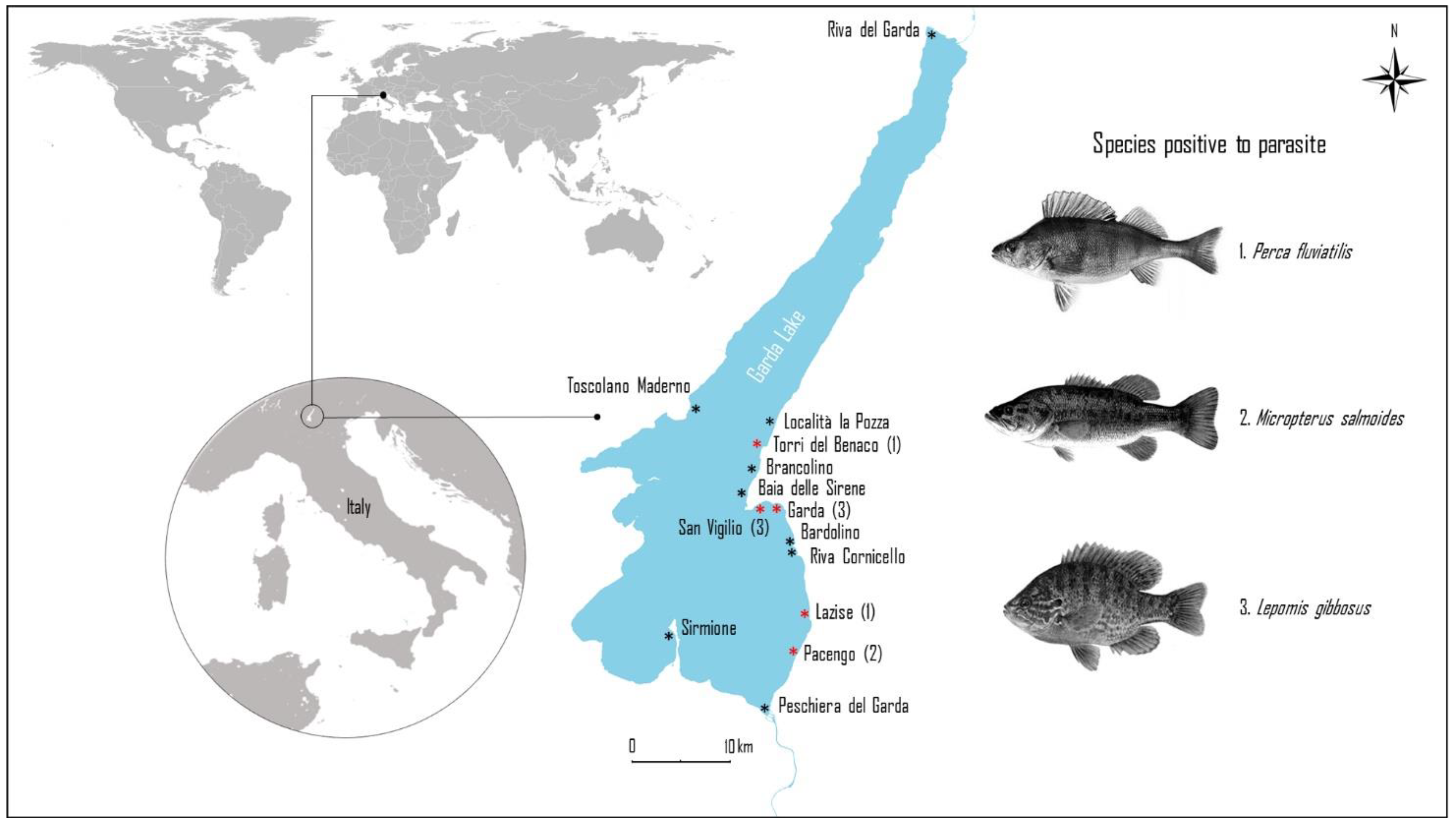
2.1. Study Area
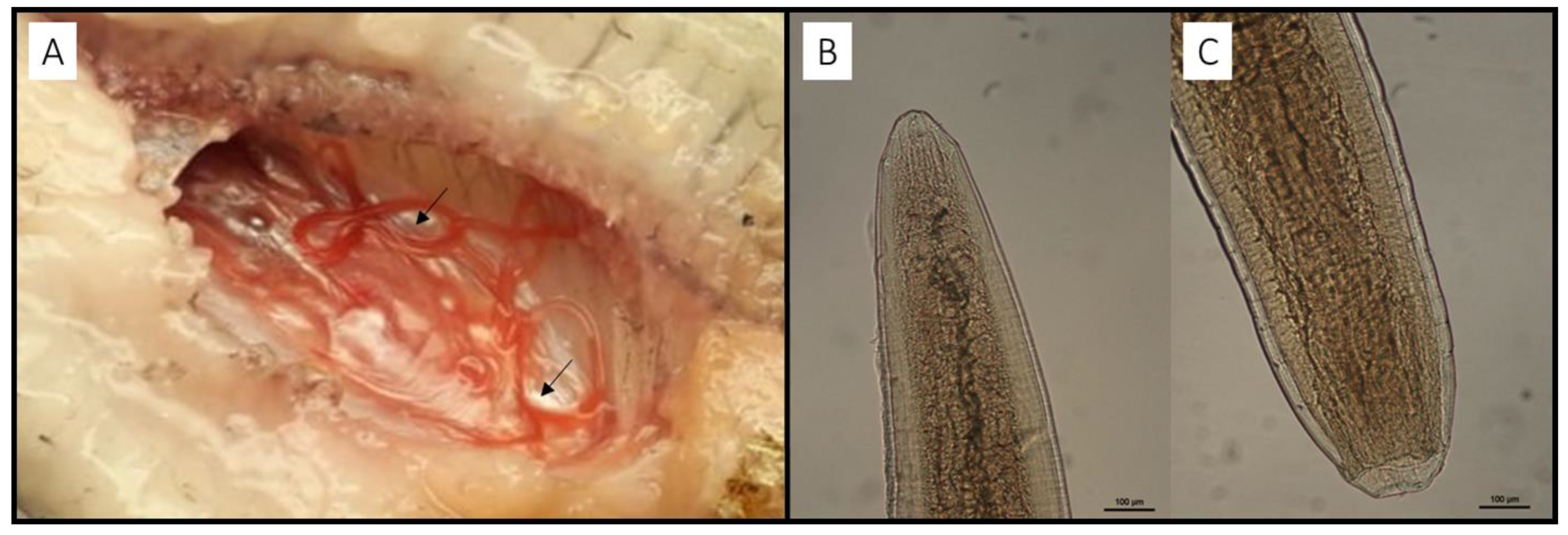
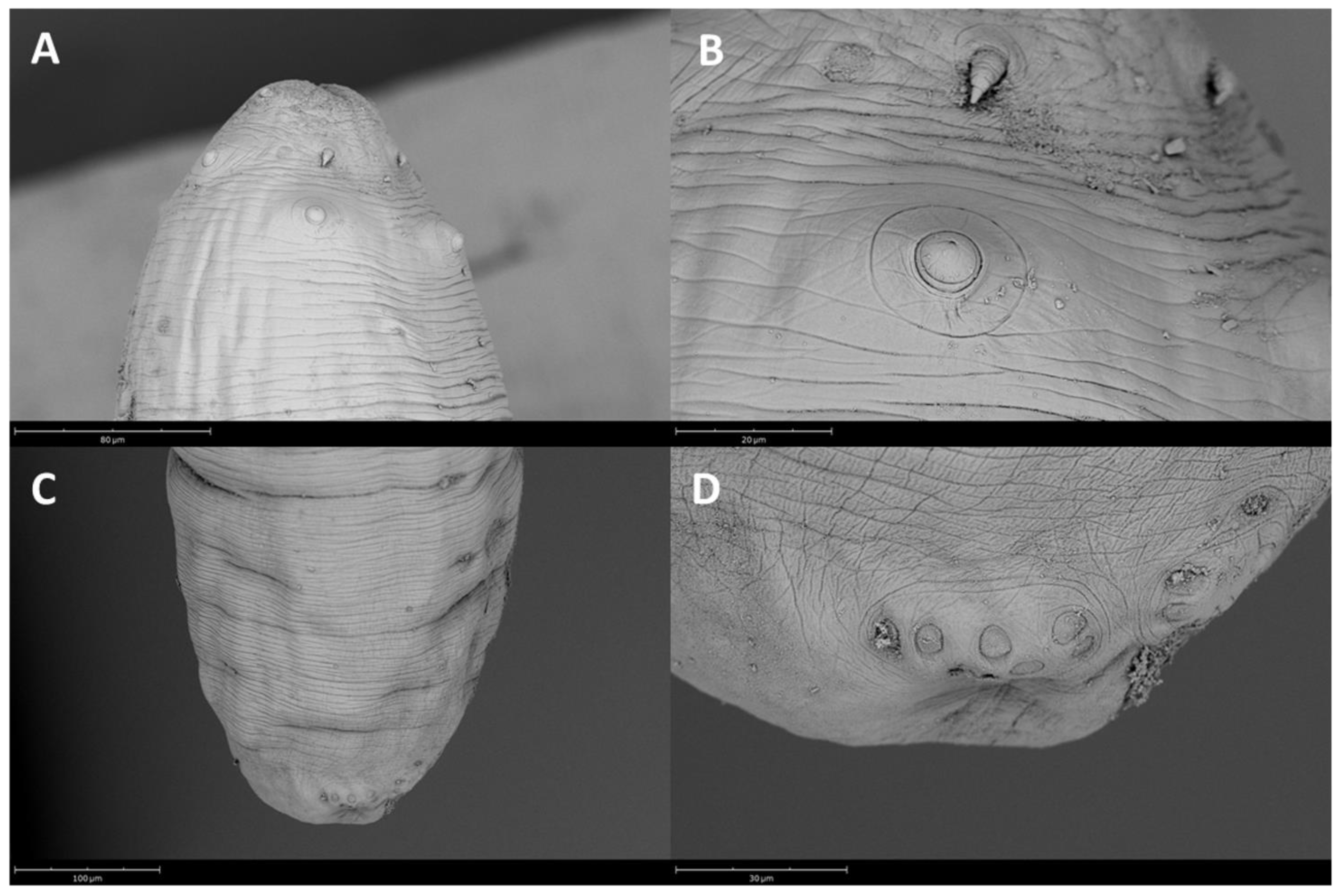
2.2. Parasitological Examination
2.3. Molecular Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pekmezci, G.Z.; Bolukbas, C.S. Morphological and molecular characterization of Eustrongylides excisus larvae (Nematoda: Dioctophymatidae) in Sander lucioperca (L.) from Northern Turkey. Parasitol. Res. 2021, 120, 2269–2274. [Google Scholar] [CrossRef]
- Measures, L.N. Revision of the genus Eustrongylides Jagerskiold, 1909 (Nematoda: Dioctophymatoidea) of piscivorous birds. Can. J. Zool. 2013, 66, 885–895. [Google Scholar] [CrossRef]
- Novakov, N.; Bjelic-Cabrilo, O.; Circovic, M.; Jubojevik, D.; Lujic, J. Eustrongylidosis of European Catfish (Siluris glaris). Bulg. J. Agric. Sci. 2013, 1, 72–76. [Google Scholar]
- Measures, L.N. Epizootiology, pathology, and description of Eustrongylides tubifex (Nematoda: Dioctophymatoidea) in fish. Can. J. Zool. 1988, 66, 2212–2222. [Google Scholar] [CrossRef]
- Measures, L.N. The development of Eustrongylides tubifex (Nematoda: Dioctophymatoidea) in oligochaetes. J. Parasitol. 1988, 74, 294–304. [Google Scholar] [CrossRef]
- Bangham, R.V. Parasites of Centrarchidae from southern Florida. Am. Fish. Soc. 1939, 68, 263–268. [Google Scholar] [CrossRef]
- Karmanova, E.M. Dioctophymidea of animals and man and the diseases which they cause. In Fundamentals of Nematodology; Amerind Publishing Co. Academy of Science of the U.S.S.R: New Delhi, India, 1968; p. 383. [Google Scholar]
- Spalding, M.G.; Bancroft, G.T.; Forrester, D.J. Epizootiology of eustrongylidosis in wading birds (Ciconiiformes) in Florida. J. Wildl. Dis. 1993, 29, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Coyner, D.F.; Spalding, M.G.; Forrester, D.J. Epizootiology of Eustrongylides ignotus in Florida: Distribution, density, and natural infections in intermediate hosts. J. Wildl. Dis. 2002, 38, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Menconi, V.; Riina, M.V.; Pastorino, P.; Mugetti, D.; Canola, S.; Pizzul, E.; Bona, M.C.; Dondo, A.; Acutis, P.L.; Prearo, M. First Occurrence of Eustrongylides spp. (Nematoda: Dioctophymatidae) in a Subalpine Lake in Northwest Italy: New Data on Distribution and Host Range. Int. J. Environ. Res. Public Health 2020, 17, 4171. [Google Scholar] [CrossRef] [PubMed]
- Goncharov, S.L.; Soroka, N.M.; Pashkevich, I.Y.; Dubovyi, A.I.; Bondar, A.O. Infection of predatory fish with larvae of Eustrongylides excisus (Nematoda, Dioctophymatidae) in the Delta of the Dnipro River and the Dnipro-Buh Estuary in Southern Ukraine. Vestn. Zool. 2018, 52, 137–144. [Google Scholar] [CrossRef]
- Guerin, P.F.; Marapendi, S.; MC Grail, L. Intestinal perforation caused by larval Eustrongylides. Morb. Mort. Week Rep. 1982, 31, 383–389. [Google Scholar]
- Ljubojevic, D.; Novakov, N.; Djordjevic, V.; Radosavljevic, V.; Pelic, M.; Cirkovic, M. Potential parasitic hazards for humans in fish meat. Procedia Food Sci. 2015, 5, 172–175. [Google Scholar] [CrossRef][Green Version]
- Guardone, L.; Ricci, E.; Susini, F.; Polsinelli, E.; Guglielmone, G.; Armani, A. First detection of Eustrongylides excisus (Nematoda: Dioctophymatidae) in big-scale sand smelt (Atherina boyeri) from the lake Massaciuccoli (Northwest Tuscany, Italy): Implications for public health and seafood quality. Food Control 2020, 120, 107517. [Google Scholar] [CrossRef]
- Eiras, J.C.; Pavanelli, G.C.; Takemoto, R.M.; Nawa, Y. An overview of fish-borne nematodiases among returned travelers for recent 25 years–unexpected diseases sometimes far away from the origin. Korean J. Parasitol. 2018, 56, 215. [Google Scholar] [CrossRef]
- Centers for Disease Control. Intestinal perforation caused by larval Eustrongylides—Maryland. MMW 1982, 31, 383–384. [Google Scholar]
- Eberhard, M.L.; Hurwitz, H.; Sun, A.M.; Coletta, D. Intestinal perforation caused by larval Eustrongylides (Nematoda: Dioctophymatoidae) in New Jersey. Am. J. Trop. Med. Hyg. 1989, 40, 648–650. [Google Scholar] [CrossRef]
- Narr, L.L.; O’Donnell, J.G.; Libster, B.; Alessi, P.; Abraham, D. Eustrongylidiasis: A parasitic infection acquired by eating live minnow. Int. J. Osteopath. Med. 1996, 96, 400–402. [Google Scholar] [CrossRef]
- Eberhard, M.L.; Ruiz–Tiben, E. Cutaneous emergence of Eustrongylides in two persons from South Sudan. Am. J. Trop. Med. Hyg. 2014, 90, 315–317. [Google Scholar] [CrossRef]
- Chai, J.Y.; Murrell, K.D.; Lymbery, A.J. Fish-borne parasitic zoonoses: Status and issues. Int. J. Parasitol. 2005, 35, 1233–1254. [Google Scholar] [CrossRef]
- Macpherson, C.N. Human behaviour and the epidemiology of parasitic zoonoses. Int. J. Parasitol. 2005, 35, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Gustinelli, A.; Menconi, V.; Prearo, M.; Caffara, M.; Righetti, M.; Scanzio, T.; Raglio, A.; Fioravanti, M.L. Prevalence of Diphyllobothrium latum (Cestoda: Diphyllobothriidae) plerocercoids in fish species from four Italian lakes and risk for the consumers. Int. J. Food Microbiol. 2016, 235, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Menconi, V.; Manfrin, C.; Pastorino, P.; Mugetti, D.; Cortinovis, L.; Pizzul, E.; Pallavicini, A.; Prearo, M. First Report of Clinostomum complanatum (Trematoda: Digenea) in European Perch (Perca fluviatilis) from an Italian Subalpine Lake: A Risk for Public Health? Int. J. Environ. Res. Public Health 2020, 17, 1389. [Google Scholar] [CrossRef] [PubMed]
- Branciari, R.; Ranucci, D.; Miraglia, D.; Valiani, A.; Veronesi, F.; Urbani, E.; Lo Vaglio, G.; Pascucci, L.; Franceschini, R. Occurrence of parasites of the genus Eustrongylides spp. (Nematoda: Dioctophymatidae) in fish caught in Trasimeno Lake, Italy. Ital. J. Food Saf. 2016, 5, 6130. [Google Scholar] [CrossRef] [PubMed]
- Dezfuli, B.S.; Manera, M.; Lorenzoni, M.; Pironi, F.; Shinn, A.P.; Giari, L. Histopathology and the inflammatory response of European perch, Perca fluviatilis muscle infected with Eustrongylides sp. (Nematoda). Parasit. Vectors 2015, 8, 227. [Google Scholar] [CrossRef]
- Ciutti, F.; Beltrami, M.E.; Confortini, I.; Cianfanelli, S.; Cappelletti, C. Non-indigenous invertebrates, fish and macrophytes in Lake Garda (Italy). J. Limnol. 2020, 70, 315–320. [Google Scholar] [CrossRef]
- Salmaso, N.; Mosello, R.; Garibaldi, L.; Decet, F.; Brizzio, M.C.; Cordella, P. Vertical mixing as a determinant of trophic status in deep lakes: A case study from two lakes south of the Alps (Lake Garda and Lake Iseo). J. Limnol. 2003, 62, 33–41. [Google Scholar] [CrossRef]
- Volta, P.; Jeppesen, E.; Sala, P.; Galafassi, S.; Foglini, C.; Puzzi, C.; Winfield, I.J. Fish assemblages in deep Italian subalpine lakes: History and present status with an emphasis on non-native species. Hydrobiologia 2018, 824, 255–270. [Google Scholar] [CrossRef]
- Kottelat, M.; Freyhof, J. Handbook of European freshwater fishes. Publications Kottelat. Copeia 2008, 2008, 725–727. [Google Scholar]
- EC (European Community). Regulation (EC) No. 2075/2005 of the European Parliament and of the Council of 5 December 2005 Laying down Specific Rules on Official Controls for Trichinella in Meat. OJEU. 338, 75–76. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32005R2075 (accessed on 12 December 2021).
- EFSA. Scientific Opinion on Risk Assessment of Parasites in Fishery Products. EFSA Panel on Biological Hazards. Available online: https://www.sanipes.gob.pe/archivos/2010-EFSA.pdf (accessed on 10 June 2021).
- Codex Alimentarius Commission. Standard for salted Atlantic Herring and Salted Sprat. Codex Stan, 244–2004, 1–8. 2004. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/all-standards/en/ (accessed on 10 June 2021).
- Moravec, F. Nematodes parasitic in fishes as larvae. In Parasitic Nematodes of Freshwater Fishes of Europe; Moravec, F., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994; pp. 374–421. [Google Scholar]
- Mazzone, A.; Caffara, M.; Gustinelli, A.; Agnetti, F.; Sgariglia, E.; Lo Vaglio, G.; Fioravanti, M.L. Morphological and molecular characterization of larval and adult stages of Eustrongylides excisus (Nematoda: Dioctophymatoidea) with histopathological observations. J. Parasitol. 2019, 105, 882–889. [Google Scholar] [CrossRef]
- Cribb, T.H.; Anderson, G.R.; Adlard, R.D.; Bray, R.A. A DNA-based demonstration of a three-host lifecycle for the Bivesiculidae (Platyhelminthes: Digenea). Int. J. Parasitol. 1998, 28, 1791–1795. [Google Scholar] [CrossRef]
- Gustinelli, A.; Caffara, M.; Florio, D.; Otachi, E.O.; Wathuta, E.M.; Fioravanti, M.L. First description of the adult stage of Clinostomum cutaneum Paperna, 1964 (Digenea: Clinostomidae) from grey herons Ardea cinerea L. and a redescription of the metacercaria from the Nile tilapia Oreochromis niloticus niloticus (L.) in Kenya. Syst. Parasitol. 2010, 76, 39–51. [Google Scholar] [CrossRef]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Klimpel, S.; Kuhn, T.; Münster, J.; Dörge, D.D.; Klapper, R.; Kochmann, J. Parasites of Marine Fish and Cephalopods, 1st ed.; Springer International Publishing, 2019; Chapter 1; pp. 1–10. Available online: https://link.springer.com/book/10.1007/978-3-030-16220-7 (accessed on 12 December 2021).
- Gagliardi, A.; Preatoni, D.; Wauters, L.; Martinoli, A. Selective predators or choosy fishermen? Relation between fish harvest, prey availability and great cormorant (Phalacrocorax carbo sinensis) diet. Ital. J. Zool. 2015, 82, 544–555. [Google Scholar] [CrossRef]
- Atkinson, C.T.; Thomas, N.J.; Hunter, D.B. Parasitic Diseases of Wild Birds. In Eustrongylidosis; Spalding, G.M., Forrester, D.J., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 289–315. [Google Scholar]
- Cole, R. Field Manual of Wildlife Diseases. General Field Procedures and Disease of Birds. In Eustrongyloidosis; Milton, F., Franson, J.C., Eds.; US Geological Survey, 2012; pp. 223–228. Available online: https://pubs.usgs.gov/itr/1999/field_manual_of_wildlife_diseases.pdf (accessed on 12 December 2021).
- Guma, S.A. The food and feeding habits of young perch, Perca fluviatilis, in Windermere. Freshw. Biol. 1978, 8, 177–187. [Google Scholar] [CrossRef]
- Persson, L. Asymmetries in competitive and predatory interactions in fish populations. In Size-Structured Populations-Ecology and Evolution; Ebenman, B., Persson, L., Eds.; Springer: Berlin, Germany, 1988; pp. 203–218. [Google Scholar]
- Berezina, N.A.; Strelnikova, A.P. Relationships between the Food Spectrum of Perch Fry (Perca fluviatilis L.) and the Structure of Zoobenthos in Experimental Mesocosms. Biol. Bull. Russ. Acad. Sci. 2001, 28, 311–318. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Report of the Eighth Session of the joint FAO/WHO Codex Alimentarius Commission: Recommended International Standard for Quick Frozen Filet of Cod and Haddock. 1971. Available online: https://www.fao.org/3/c0531e/C0531E00.htm (accessed on 10 June 2021).
- European Regulation (EC). No 853/2004 of the European Parliament and of the Council of 29 April 2004 Laying Down Specific Hygiene Rules for Food of Animal Origin. Available online: http://data.europa.eu/eli/reg/2004/853/oj (accessed on 10 June 2021).
- Legeslative Decree, 2013. Decreto 17/07/2013; GU Serie Generale n.187 del 10–08–2013. pp. 31–32. Available online: https://www.sopranciodue.it/en/business-to-do-safety/fire-protection-laws (accessed on 10 June 2021).
| Sampling Site | Species | Weight (g) | Total Length (cm) | N | Male | Female | ND |
|---|---|---|---|---|---|---|---|
| Peschiera del Garda | Alosa fallax | 57.1 ± 13.0 | 19.5 ± 1.9 | 20 | 45 | 55 | — |
| Lazise | Coregonus lavaretus | 120.2 ± 28.0 | 23.9 ± 2.1 | 6 | 50 | 50 | — |
| Pacengo | 91.8 ± 80.4 | 20.1 ± 7.1 | 5 | 50 | 50 | — | |
| Peschiera del Garda | 99.8 ± 9.1 | 23.0 ± 0.6 | 5 | 60 | 40 | — | |
| Riva del Garda | 321.5 ± 53.8 | 31.8 ± 3.2 | 5 | 0 | 100 | — | |
| Sirmione | 115.21 ± 20.5 | 23.4 ± 1.0 | 10 | 50 | 50 | — | |
| Toscolano Maderno | 277.2 ± 50.5 | 30.1 ± 1.9 | 11 | 45.5 | 54.5 | — | |
| Bardolino | Lepomis gibbosus | 50.6 ± 41.9 b | 12.2 ± 3.0 b | 30 | 26.7 | 50.0 | 23.3 |
| Garda | 26.0 ± 12.4 b | 11.0 ± 1.4 b | 27 | 34.6 | 65.4 | — | |
| San Vigilio | 86.4 ± 23.2 b | 16.5 ± 2.0 a | 24 | 54.2 | 45.8 | — | |
| Toscolano Maderno | 62.3 ± 38.9 b | 13.29 ± 5.8 b | 48 | 37.5 | 62.5 | — | |
| Pacengo | Micropterus salmoides | 93.0 ± 48.8 a | 33.5±29.1 a | 10 | 40 | 60 | — |
| Torri del Benaco | 1168.7 ± 571.0 b | 40.3 ± 3.2 b | 10 | 66.7 | 33.3 | — | |
| Baia delle Sirene | Perca fluviatilis | 120.4 ± 33.4 b | 20.2 ± 1.8 b | 5 | 0 | 100 | — |
| Brancolino | 120.5 ± 27.3 b | 21.0 ± 1.3 b | 14 | 21.4 | 78.6 | — | |
| Garda | 99.36 ± 20.6 b | 20.41 ± 1.5 b | 11 | 18.2 | 81.8 | — | |
| Localita la Pozza | 125.3 ± 31.6 b | 20.8 ± 1.9 b | 4 | 25 | 65 | — | |
| Lazise | 94.7±24.6 a | 18.7 ± 1.9 a | 30 | 3.3 | 96.7 | — | |
| Riva Cornicello | 111.5±26.8 b | 20.6 ± 1.6 b | 11 | 0 | 100 | — | |
| San Vigilio | 122.4 ± 42.4 b | 21.0 ± 2.0 b | 12 | 16.7 | 83.3 | — | |
| Torri del Benaco | 98.6 ± 32.6 b | 20.9 ± 2.0 b | 65 | 16.9 | 83.1 | — | |
| Toscolano Maderno | 100.6 ± 33.8 b | 20 5 ± 2.1 b | 60 | 45 | 65 | — |
| Sampling Site | Fish Species | N | Positive | P (%; 95% CI) | N of larvae | MI | MA |
|---|---|---|---|---|---|---|---|
| Lazise | Perca fluviatilis | 36 | 2 | 5.55 (0.99–18.14) | 2AV | 1 | 0.05 |
| Torri del Benaco | Perca fluviatilis | 65 | 1 | 1.54 (0.08–8.21) | 1AV | 1 | 0.015 |
| Pacengo | Micropterus salmoides | 10 | 6 | 60 (31.27–83.18) | 3AV;2AD;1PV;2VC | 1.33 | 0.8 |
| Garda | Lepomis gibbosus | 27 | 1 | 3.7 (0.19–18.28) | 1AV | 1 | 0.03 |
| San Vigilio | L. gibbosus | 24 | 3 | 12.5 (4.3–31) | 1AN; 2PD | 1 | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menconi, V.; Tedesco, P.; Pastorino, P.; Confortini, I.; Esposito, G.; Tomasoni, M.; Mugetti, D.; Gustinelli, A.; Dondo, A.; Pizzul, E.; et al. Could Fish Feeding Behaviour and Size Explain Prevalence Differences of the Nematode Eustrongylides excisus among Species? The Case Study of Lake Garda. Water 2021, 13, 3581. https://doi.org/10.3390/w13243581
Menconi V, Tedesco P, Pastorino P, Confortini I, Esposito G, Tomasoni M, Mugetti D, Gustinelli A, Dondo A, Pizzul E, et al. Could Fish Feeding Behaviour and Size Explain Prevalence Differences of the Nematode Eustrongylides excisus among Species? The Case Study of Lake Garda. Water. 2021; 13(24):3581. https://doi.org/10.3390/w13243581
Chicago/Turabian StyleMenconi, Vasco, Perla Tedesco, Paolo Pastorino, Ivano Confortini, Giuseppe Esposito, Mattia Tomasoni, Davide Mugetti, Andrea Gustinelli, Alessandro Dondo, Elisabetta Pizzul, and et al. 2021. "Could Fish Feeding Behaviour and Size Explain Prevalence Differences of the Nematode Eustrongylides excisus among Species? The Case Study of Lake Garda" Water 13, no. 24: 3581. https://doi.org/10.3390/w13243581
APA StyleMenconi, V., Tedesco, P., Pastorino, P., Confortini, I., Esposito, G., Tomasoni, M., Mugetti, D., Gustinelli, A., Dondo, A., Pizzul, E., Fioravanti, M. L., & Prearo, M. (2021). Could Fish Feeding Behaviour and Size Explain Prevalence Differences of the Nematode Eustrongylides excisus among Species? The Case Study of Lake Garda. Water, 13(24), 3581. https://doi.org/10.3390/w13243581