Disinfection of Dental Chair Water Using Aqueous Chlorine Dioxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. ClO2 Measurement
2.3. Oxidation Reduction Potential (ORP) Measurement
2.4. pH Measurement
2.5. Chlorine-like Odor Measurement
2.6. Chlorite Measurement
2.7. Total Bacteria Measurement
2.8. ClO2 Aqueous Solution Application in Dental Clinics
3. Results and Discussion
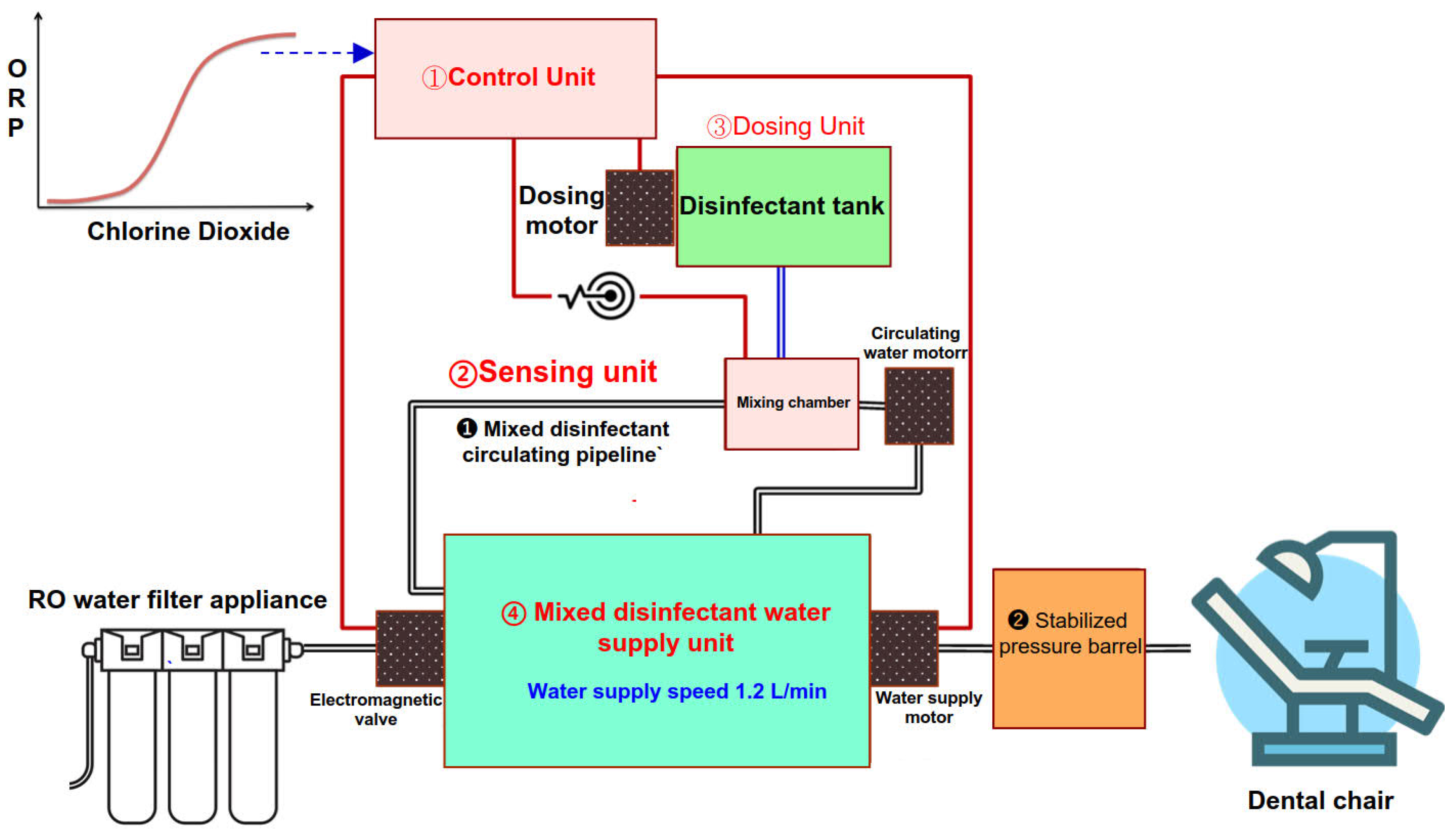
3.1. Water Purification and Disinfectant System
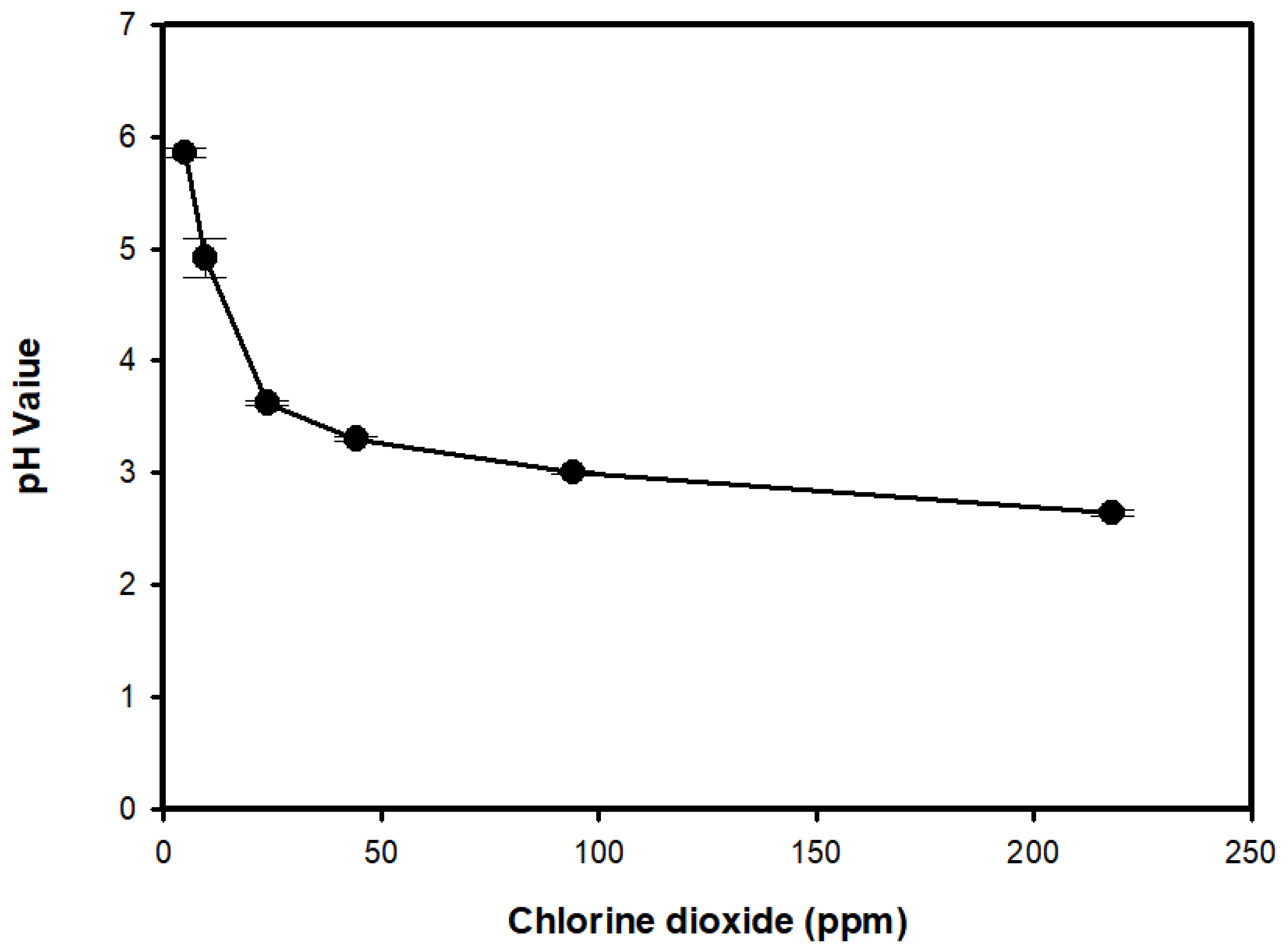
3.2. ClO2 Concentration in ACD and pH
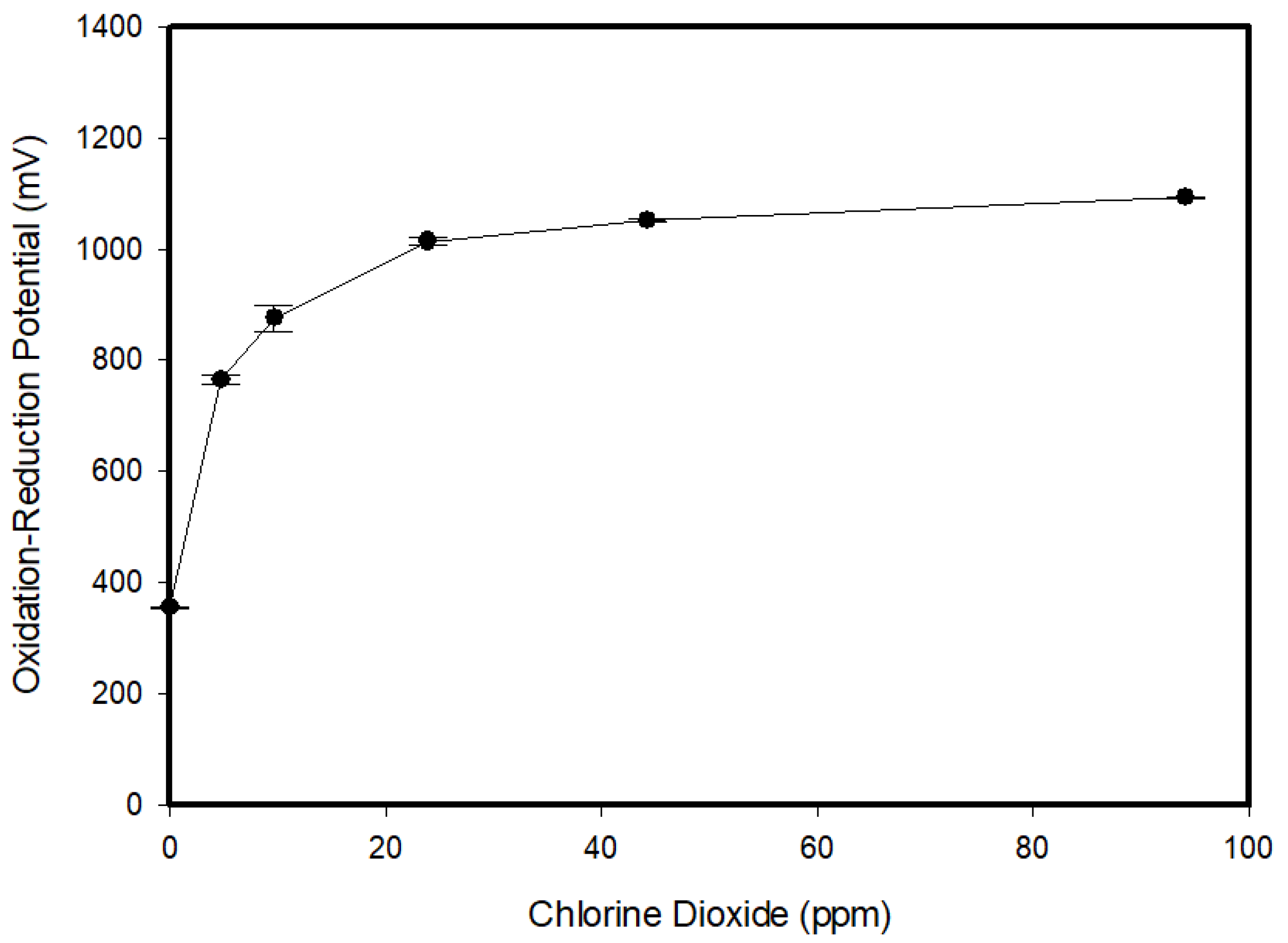
3.3. ClO2 Concentration in ACD and ORP
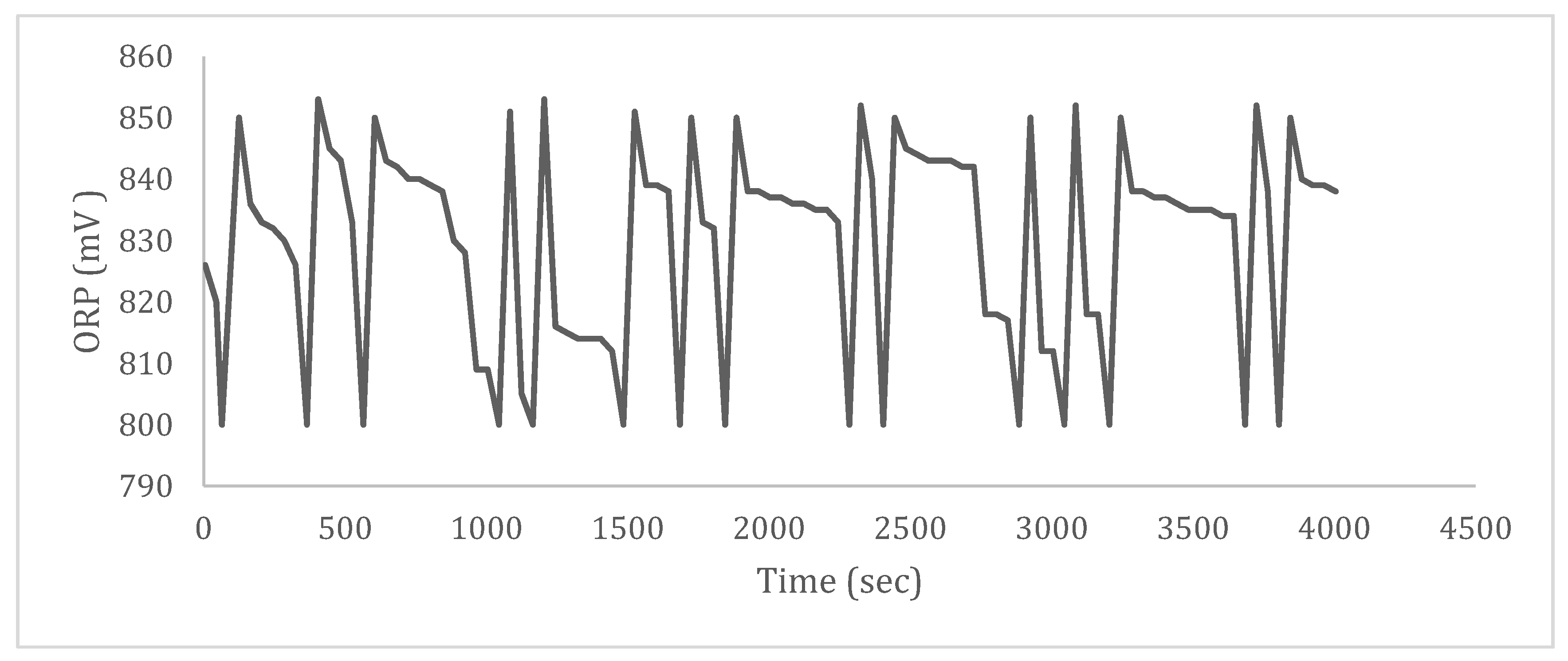
3.4. Dosing Disinfectant between 800 and 900 mV ORP
3.4.1. ORP and Chlorine-like Odor of Water in an Open System
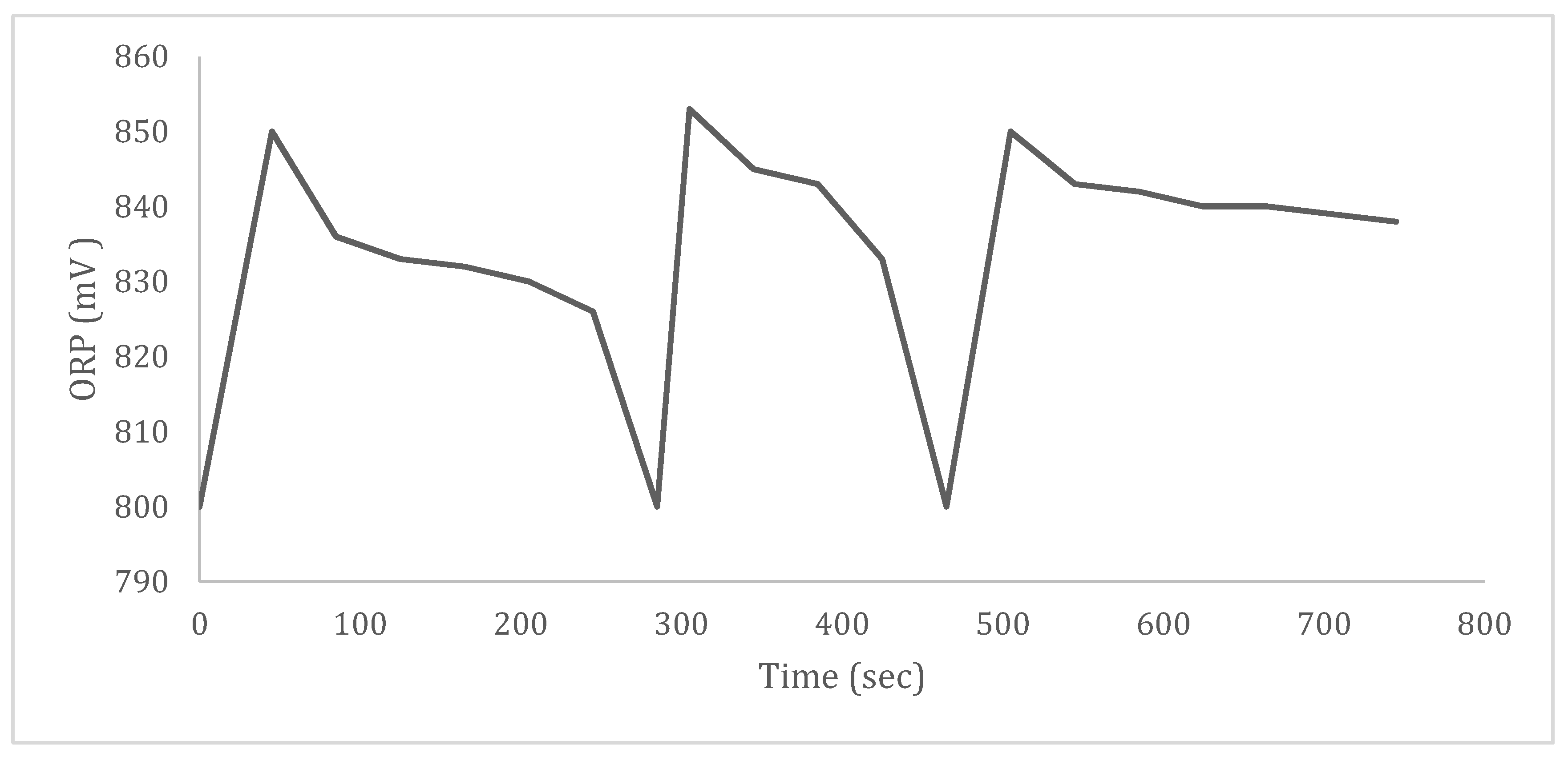
3.4.2. ORP and Chlorine-like Odor of Water in a Closed-Tube System
3.4.3. Automatic Monitoring of Water ORP by Dosing ACD in a Closed-Tube System
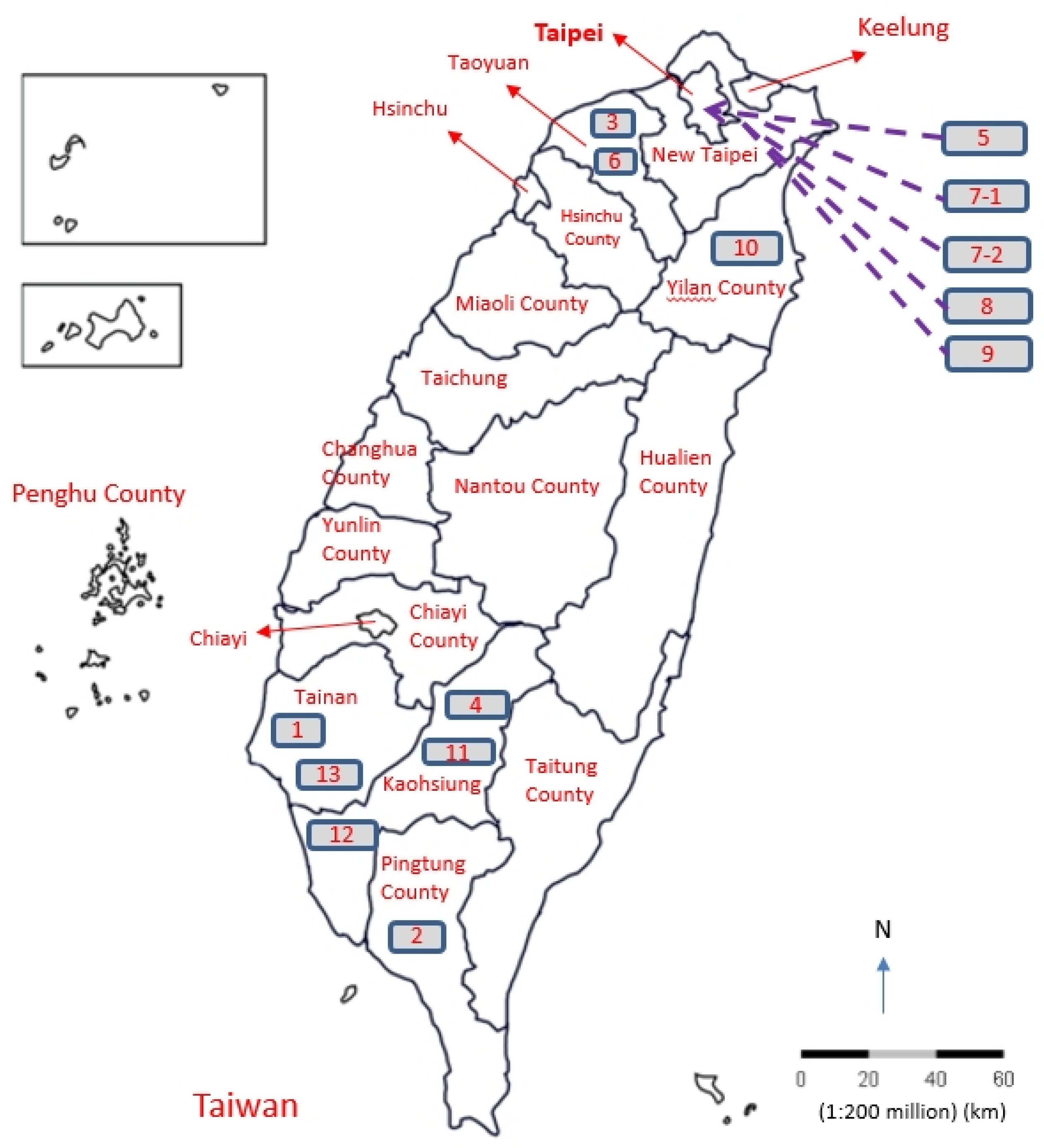
3.5. Clinical Verification
3.5.1. Clinic On-Site Installation
3.5.2. ORP Monitoring of Water System in Clinic
Manual ORP Detection of Water Quality at the Clinic System Outlet
Water Quality in the Dental Clinic
3.6. Application of the ACD Dental Chair
3.7. Future Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACD | aqueous chlorine dioxide |
| ORP | oxidation-reduction potential |
| RO | reverse osmosis |
| ClO2 | chlorine dioxide |
References
- Spagnolo, A.M.; Sartini, M.; Cristina, M.L. Microbial Contamination of Dental Unit Waterlines and Potential Risk of Infection: A Narrative Review. Pathogens 2020, 9, 651. Available online: https://www.mdpi.com/2076-0817/9/8/651 (accessed on 5 October 2021). [CrossRef] [PubMed]
- Kumar, S.; Atray, D.; Paiwal, D.; Balasubramanyam, G.; Duraiswamy, P.; Kulkarni, S. Dental unit waterlines: Source of contamination and cross-infection. J. Hosp. Infect. 2010, 74, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, J.; Sitkowska, J.; Dutkiewicz, J. Microbial contamination of dental unit waterlines. Ann. Agric. Environ. Med. 2008, 15, 173–179. Available online: https://pubmed.ncbi.nlm.nih.gov/19061251/ (accessed on 5 October 2021). [PubMed]
- Ji, X.Y.; Fei, C.N.; Zhang, Y.; Liu, J.; Liu, H.; Song, J. Three key factors influencing the bacterial contamination of dental unit waterlines: A 6-year survey from 2012 to 2017. Int. Dent. J. 2019, 69, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Reregistration Eligibility Decision (RED) for Chlorine Dioxide and Sodium Chlorite (Case 4023). In US EPA 738-R-06-007; 2006; pp. 3–4. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/red_PC-020503_3-Aug-06.pdf (accessed on 6 October 2021).
- Chlorine Dioxide Gas. In Concise International Chemical Assessment Document 37; World Health Organization: Geneva, Switzerland, 2002; Volume 7, Available online: https://www.who.int/ipcs/publications/cicad/en/cicad37.pdf (accessed on 6 October 2021).
- Kaly-Kullai, K.; Wittmann, M.; Noszticzius, Z.; Rosivall, L. Can chlorine dioxide prevent the spreading of coronavirus or other viral infections? Medical hypotheses. AK J. Physiol. Int. 2020, 107, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Guo, Y.; Yu, P.; Wang, X.; Zhang, X.; Dong, W.; Liu, X.; Guo, C. Chlorine dioxide inhibits the replication of porcine reproductive and respiratory syndrome virus by blocking viral attachment. Infect. Genet. Evol. 2019, 67, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Zoni, R.; Zanelli, R.; Riboldi, E.; Bigliardi, L.; Sansebastiano, G. Investigation on virucidal activity of chlorine dioxide. experimental data on feline calicivirus, HAV and Coxsackie B5. J. Prev. Med. Hyg. 2007, 48, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.W.; Huang, B.S.; Hsu, C.W.; Peng, C.W.; Cheng, M.L.; Kao, J.Y.; Way, T.D.; Yin, H.C.; Wang, S.S. Efficacy and Safety Evaluation of a chlorine dioxide solution. Int. J. of Environ. Res. and Public Health. 2017, 14, 329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Jiao, W.; Du, Y.; Chen, Q.; Su, Z.; Fu, M. Chlorine Dioxide Controls Green Mold Caused by Penicillium digitatum in Citrus Fruits and the Mechanism Involved. J. Agric. Food Chem. 2020, 68, 13897–13905. [Google Scholar] [CrossRef] [PubMed]
- Sanekata, T.; Fukuda, T.; Miura, T.; Morino, H.; Lee, C.; Maeda, K.; Araki, K.; Otake, T.; Kawahata, T.; Shibata, T. Evaluation of the antiviral activity of chlorine dioxide and sodium hypochlorite against feline calicivirus, human influenza virus, measles virus, canine distemper virus, human herpesvirus, human adenovirus, canine adenovirus and canine parvovirus. Biocontrol. Sci. 2010, 15, 45–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubbers, J.R.; Chauhan, S.; Miller, J.K.; Bianchine, J.R. The effects of chronic administration of chlorine dioxide, chlorite and chlorate to normal healthy adult male volunteers. J. Environ. Pathol. Toxicol. Oncol. 1984, 5, 229–238. Available online: https://pubmed.ncbi.nlm.nih.gov/6520728/ (accessed on 9 October 2021). [PubMed]
- Lubbers, J.R.; Bianchine, J.R. Effects of the acute rising dose administration of chlorine dioxide, chlorate and chlorite to normal healthy adult male volunteers. J. Environ. Pathol. Toxicol. Oncol. 1984, 5, 215–228. Available online: https://pubmed.ncbi.nlm.nih.gov/6520727/ (accessed on 10 October 2021). [PubMed]
- Lubbers, J.R.; Chauhan, S.; Bianchine, J.R. Controlled clinical evaluations of chlorine dioxide, chlorite and chlorate in man. Fundam. Appl. Toxicol. 1981, 1, 334–338. [Google Scholar] [CrossRef]
- Jang, A.; Szabo, J.; Hosni, A.A.; Coughlin, M.; Bishop, P.L. Measurement of chlorine dioxide penetration in dairy process pipe biofilms during disinfection. Appl. Microbiol. Biotechnol. 2006, 72, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, G.A.; Rand, J.L.; O’leary, K.C.; Rygel, A.C.; Chauret, C.; Andrews, R.C. Disinfectant efficacy of chlorite and chlorine dioxide in drinking water biofilms. Water Res. 2005, 39, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.; Seo, H.S.; Bang, J.; Kim, H.; Beuchat, L.R.; Ryu, J.H. Efficacy of gaseous chlorine dioxide in inactivating Bacillus cereus spores attached to and in a biofilm on stainless steel. Int. J. Food Microbiol. 2014, 188, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.P.; Pepich, B.V.; Hautman, D.P.; Munch, D.J.; Salhi, E. Method 326.0 Determination of Inorganic Oxyhalide Disinfection By-Products in Drinking Water Using Ion Chromotography Incorporating the Addition of a Suppressor Acidified Postcolumn Reagent for Trace Bromate Analysis. Desalination 2008, 224, 231–239. Available online: https://www.researchgate.net/publication/222557829_Determination_of_inorganic_oxyhalide_disinfection_by-products_in_bottled_water_by_EPA_Method_3260_for_trace_bromate_analysis (accessed on 13 October 2021).
- Chlorite and Chlorate in Drinking-water. In WHO/SDE/WSH/05.08/86; World Health Organization: Geneva, Switzerland, 2005; Available online: https://www.who.int/water_sanitation_health/dwq/chemicals/chlorateandchlorite0505.pdf (accessed on 14 October 2021).
- Drinking Water Quality Standards. In Agricultural Environmental Protection; Executive Yuan Bulletin: Taipei, Taiwan, 2017; Volume 23, p. 6. Available online: https://gazette.nat.gov.tw/EG_FileManager/eguploadpub/eg023006/ch07/type1/gov60/num29/OEg.pdf (accessed on 15 October 2021).
- Oxidation-Reduction Potential. Fact Sheets. In Environmental Health; 2016. Available online: https://www.health.nsw.gov.au/environment/factsheets/Pages/orp.aspx (accessed on 5 October 2021).
- Kohn, W.G.; Collins, A.S.; Cleveland, J.L.; Harte, J.A.; Eklund, K.J.; Malvitz, D.M.; Centers for Disease Control and Prevention (CDC). Guidelines for infection control in dental health-care settings-2003. MMWR Recomm. Rep. 2003, 52, 1–61. Available online: https://pubmed.ncbi.nlm.nih.gov/14685139/ (accessed on 18 October 2021). [PubMed]
- Dental Unit Waterlines. Oral Health Topics; American Dental Association: Chicago, IL, USA, 2019; Available online: https://www.ada.org/en/member-center/oral-health-topics/dental-unit-waterlines (accessed on 13 October 2021).
- Standards for drinking water quality. GB 5749-2006. In National Standards of the People’s Republic of China; Ministry of Health: Beijing, China, 2006; Available online: http://tradechina.dairyaustralia.com.au/wp-content/uploads/2018/08/GB-5749-2006-Standards-for-Drinking-Water-Quality.pdf (accessed on 15 October 2021).
- Material Compatibility. ClorDiSys. 2014. Available online: https://www.clordisys.com/materialcompatibility.php (accessed on 19 October 2021).
- Sidari, F.P.; Stout, J.E.; Van Briesen, J.M.; Bowman, A.M.; Grubb, D.; Neuner, A.; Wagener, M.M.; Yu, V.L. Keeping Legionella out of water systems. J. Am. Water Works Assoc. 2004, 96, 111–119. [Google Scholar] [CrossRef]
- Zhang, Z.; McCann, C.; Stout, J.E.; Piesczynski, S.; Hawks, R.; Vidic, R.; Yu, V.L. Safety and Efficacy of Chlorine Dioxide for Legionella Control in a Hospital Water System. Infect. Control Hospital Epidemiol. 2007, 28, 1009–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| ORP at Stop (mV) | ORP at Stabilization (mV) | Chlorine-Like Odor |
|---|---|---|
| 801 | 811 | 0 |
| 810 | 818 | 0 |
| 820 | 826 | 0 |
| 830 | 837 | 0 |
| 840 | 844 | 0 |
| 850 | 853 | 0 |
| 860 | 863 | 1 |
| 870 | 874 | 1 |
| 880 | 885 | 2 |
| 890 | 893 | 2 |
| 895 | 898 | 3 |
| 896 | 901 | 3 |
| ORP at Dosing Stop (mV) | ORP at Stabilization (mV) | Chlorine-Like Odor |
|---|---|---|
| 801 | 826 | 0 |
| 805 | 838 | 0 |
| 810 | 854 | 0 |
| Sampling Time/Date | Day, ORP (mV) | ||||
|---|---|---|---|---|---|
| 1 6/3 | 2 6/4 | 3 6/5 | 4 6/6 | 5 6/7 | |
| 10:00 | 816 | 832 | 807 | -- | 839 |
| 11:00 | 801 | 820 | 850 | -- | 827 |
| 12:00 | 840 | 806 | 839 | -- | 815 |
| 15:00 | 834 | 847 | 831 | 828 | 807 |
| 16:00 | 819 | 833 | 809 | 812 | 842 |
| 17:00 | 802 | 819 | 846 | 850 | 826 |
| 18:00 | 845 | 803 | 833 | 840 | 809 |
| 19:00 | 836 | 849 | 825 | 823 | 803 |
| 20:00 | 815 | 840 | 804 | 815 | 846 |
| 21:00 | 848 | 825 | 844 | 849 | 830 |
| Sample | Sampling Time | Total Bacterial Colony (CFU/mL) | Chlorite (mg/L) |
|---|---|---|---|
| 1 | 5 June 2019 08:50 | <5 | <0.0004 |
| 2 | 5 June 2019 11:50 | <5 | <0.0004 |
| 3 | 5 June 2019 15:00 | <5 | <0.0004 |
| 4 | 10 June 2019 08:50 | <5 | <0.0004 |
| Clinic Number | Before System Improvement | After System Improvement | ||
|---|---|---|---|---|
| Sampling Time | Total Number of Colonies (CFU/mL) | Sampling Time | Total Number of Colonies (CFU/mL) | |
| 1 | 25 May 2018 | 1400 | 25 July 2018 | <5 |
| 2 | 01 June 2018 | 1300 | 20 June 2018 | <5 |
| 3 | 01 Aug. 2018 | 640 | 08 Aug. 2018 | <5 |
| 4 | 07 Aug. 2018 | 1300 | 27 Aug. 2018 | <5 |
| 5 | 10 Sep. 2018 | 510 | 06 Oct. 2018 | <5 |
| 6 | 25 Jan. 2019 | 200 | 12 Apr. 2019 | <5 |
| 7.1 | 22 July 2019 | 380 | 26 Aug. 2019 | <5 |
| 7.2 | 22 July 2019 | 690 | 21 Aug. 2019 | <5 |
| 8 | 22 July 2019 | 430 | 21 Aug. 2019 | <5 |
| 9 | 22 July 2019 | 980 | 21 Aug. 2019 | <5 |
| 10 | 29 July 2019 | 890 | 05 Aug. 2019 | <5 |
| 11 | 26 Aug. 2019 | 1300 | 02 Sep. 2019 | <5 |
| 12 | 26 Aug. 2019 | 1500 | 02 Sep. 2019 | <5 |
| 13 | 26 Aug. 2019 | 1400 | 02 Sep. 2019 | <5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, L.-L.; Hu, C.-C.; Hsu, C.-W.; Pen, C.-W.; Chen, L.-Y.; Yu, Y.-C.; Carey, J.R.; Yin, H.-C.; Wang, S.-S. Disinfection of Dental Chair Water Using Aqueous Chlorine Dioxide. Water 2021, 13, 3442. https://doi.org/10.3390/w13233442
Wei L-L, Hu C-C, Hsu C-W, Pen C-W, Chen L-Y, Yu Y-C, Carey JR, Yin H-C, Wang S-S. Disinfection of Dental Chair Water Using Aqueous Chlorine Dioxide. Water. 2021; 13(23):3442. https://doi.org/10.3390/w13233442
Chicago/Turabian StyleWei, Li-Lin, Chan-Chih Hu, Chu-Wei Hsu, Chun-Wei Pen, Li-Yu Chen, Yu-Chun Yu, James R. Carey, Hao-Chang Yin, and Shan-Shue Wang. 2021. "Disinfection of Dental Chair Water Using Aqueous Chlorine Dioxide" Water 13, no. 23: 3442. https://doi.org/10.3390/w13233442







