Evaluation of Groundwater Suitability for Irrigation and Drinking Purposes in an Agricultural Region of the North China Plain
Abstract
:1. Introduction
2. Materials and Methods
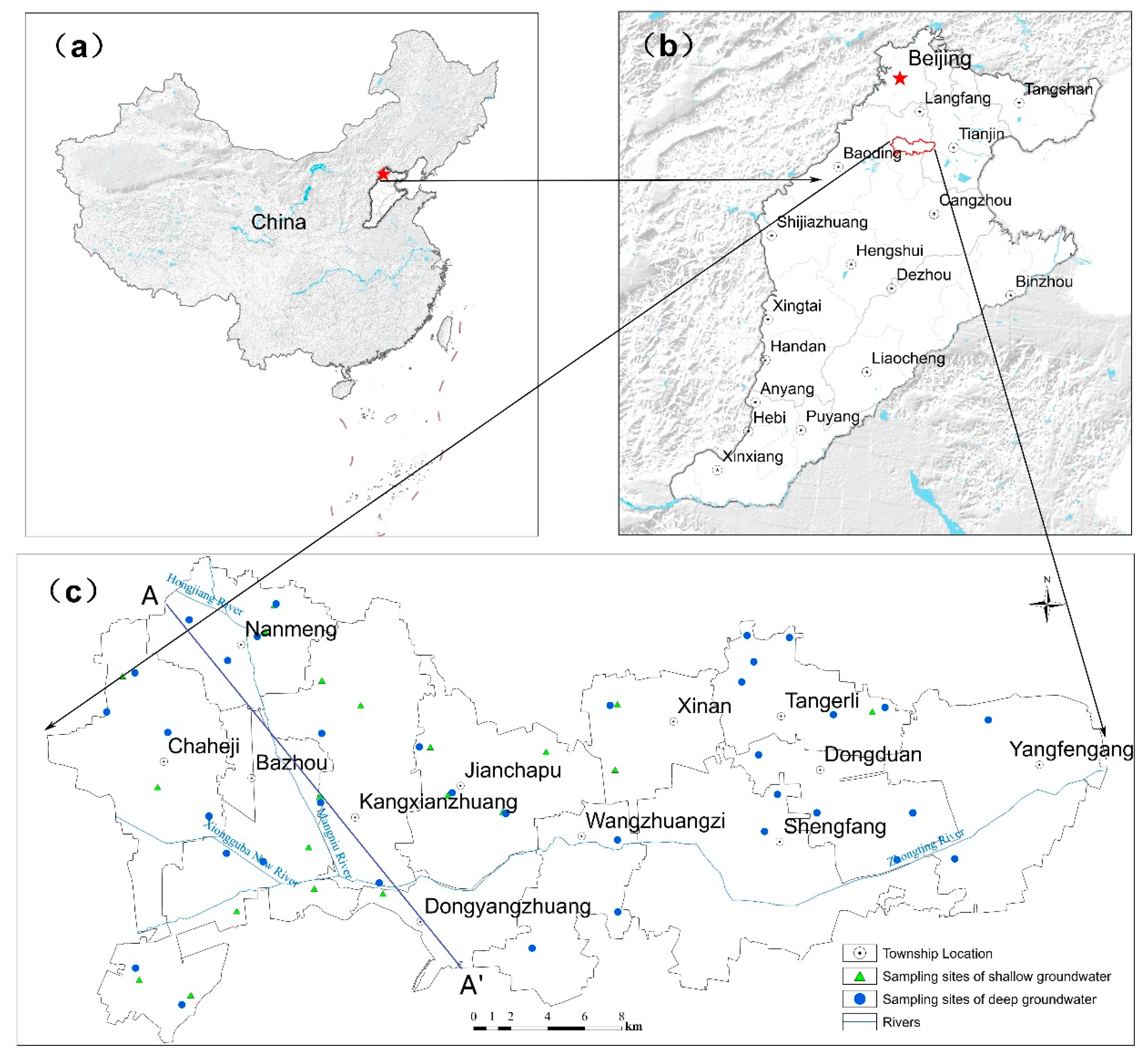
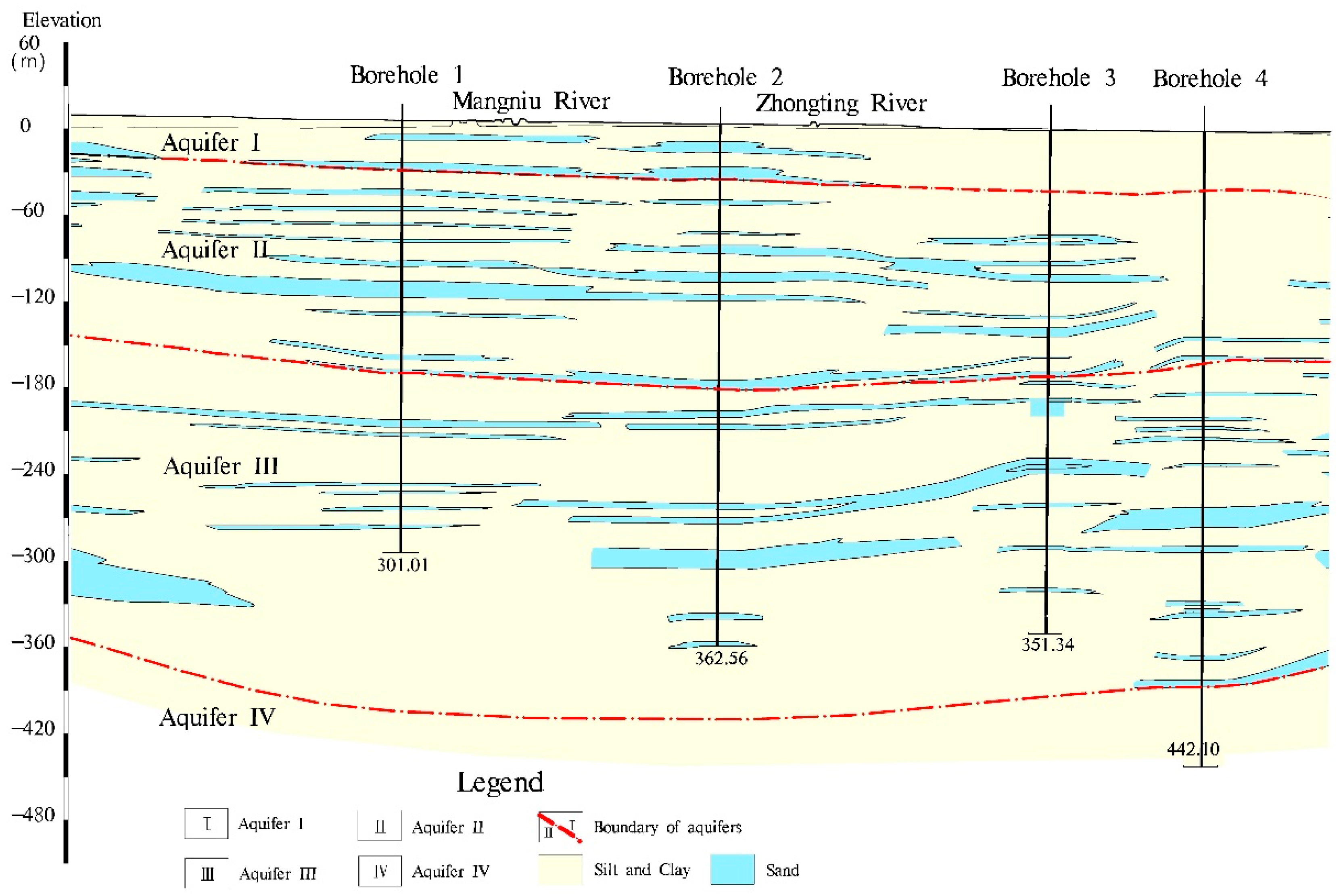
2.1. Study Area
2.2. Methods of Suitability Evaluation for Irrigation Purpose
2.3. Methods of Suitability Evaluation for Drinking
2.4. Water Sampling and Measurement
3. Results and Discussion
3.1. Hydrochemical Characteristics of Main Ions in Groundwater
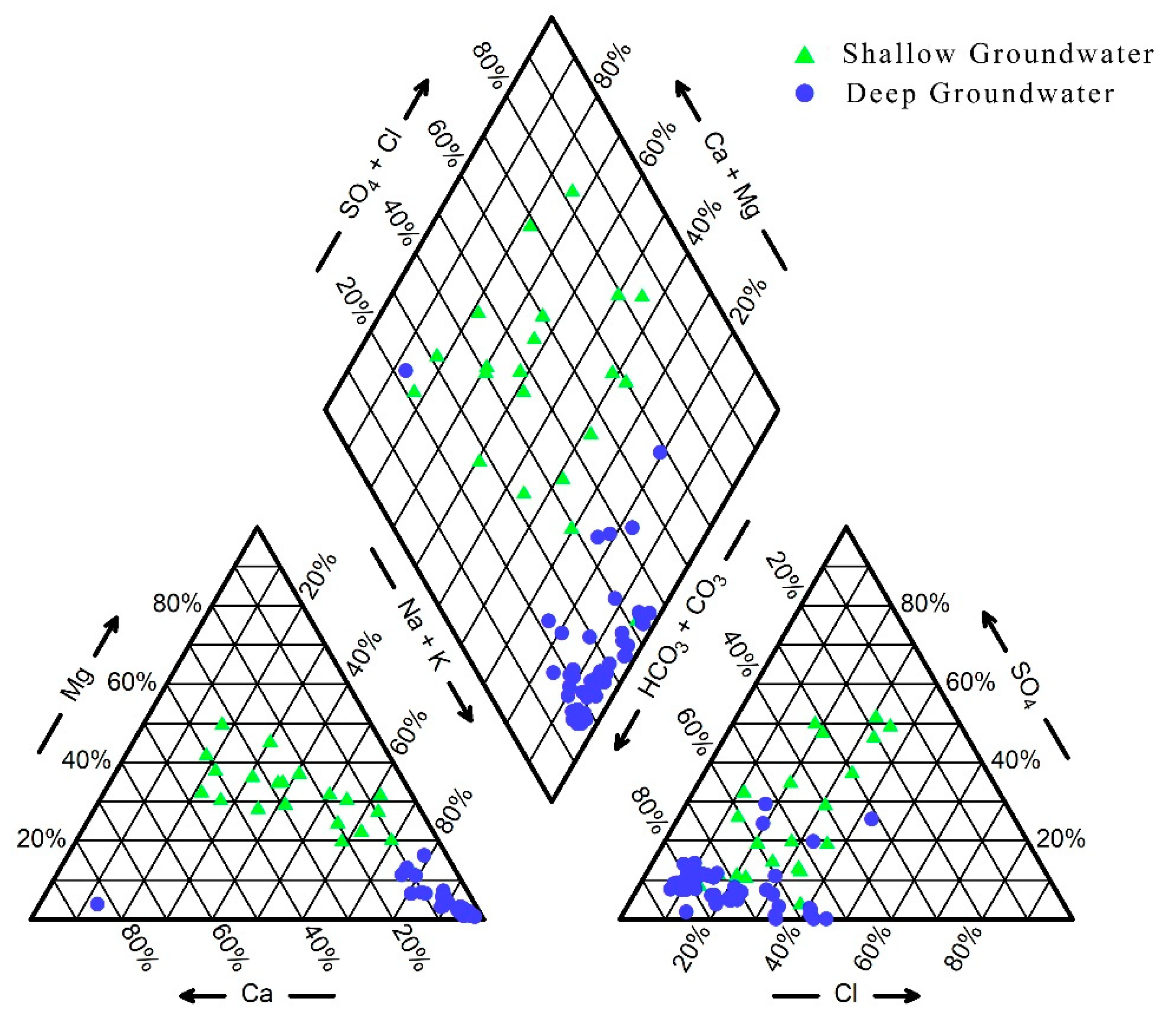
3.2. Hydrochemical Types of Groundwater
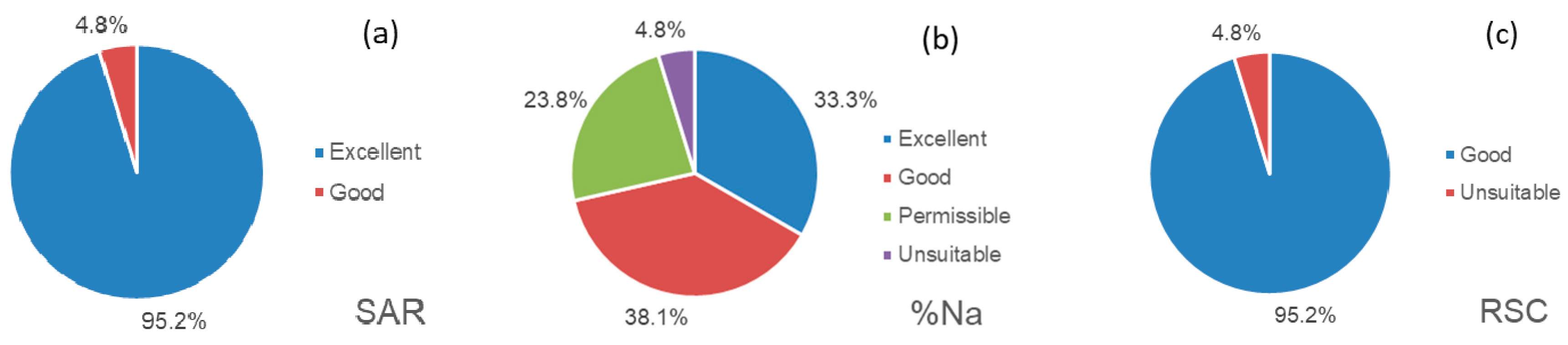
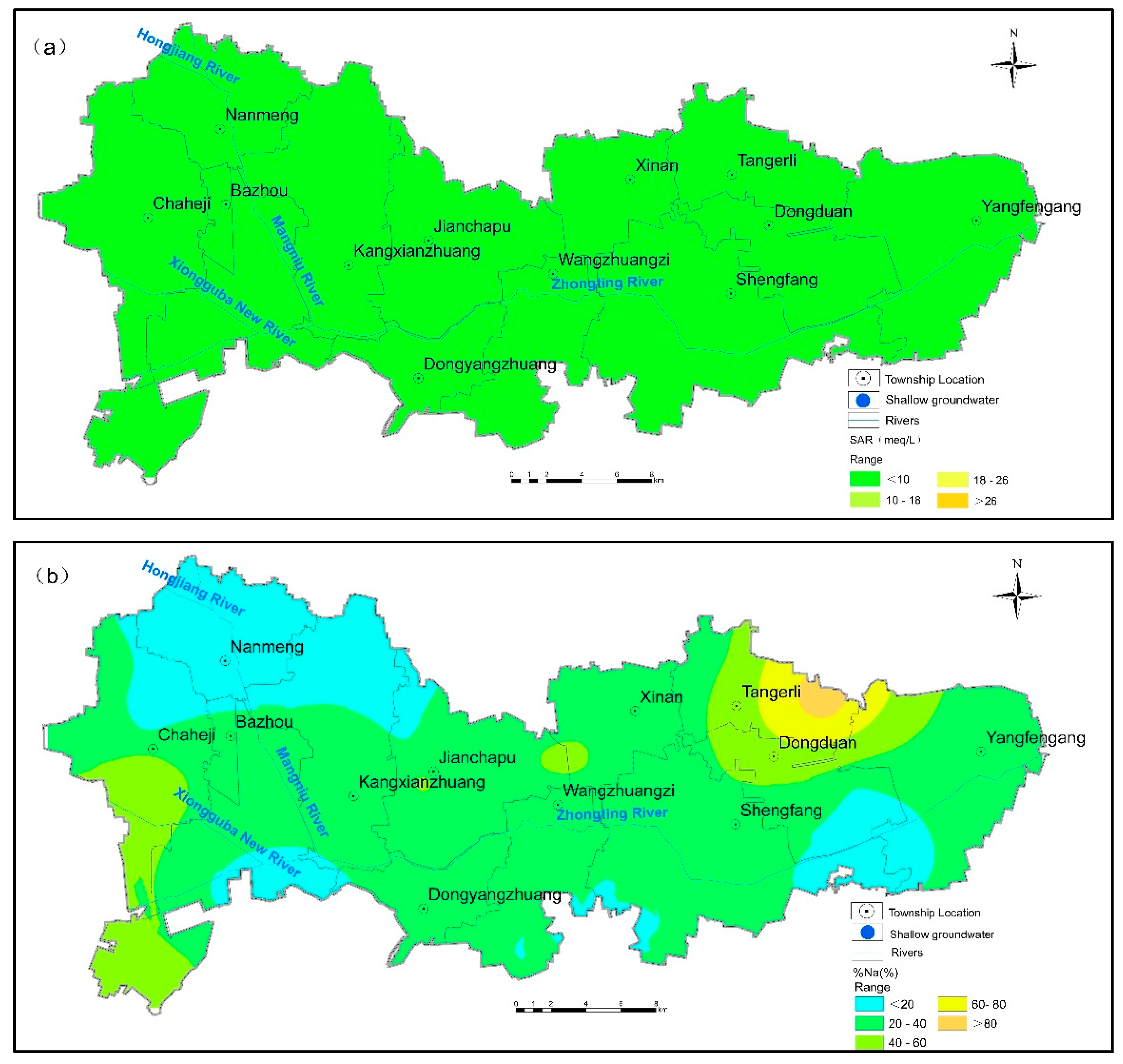
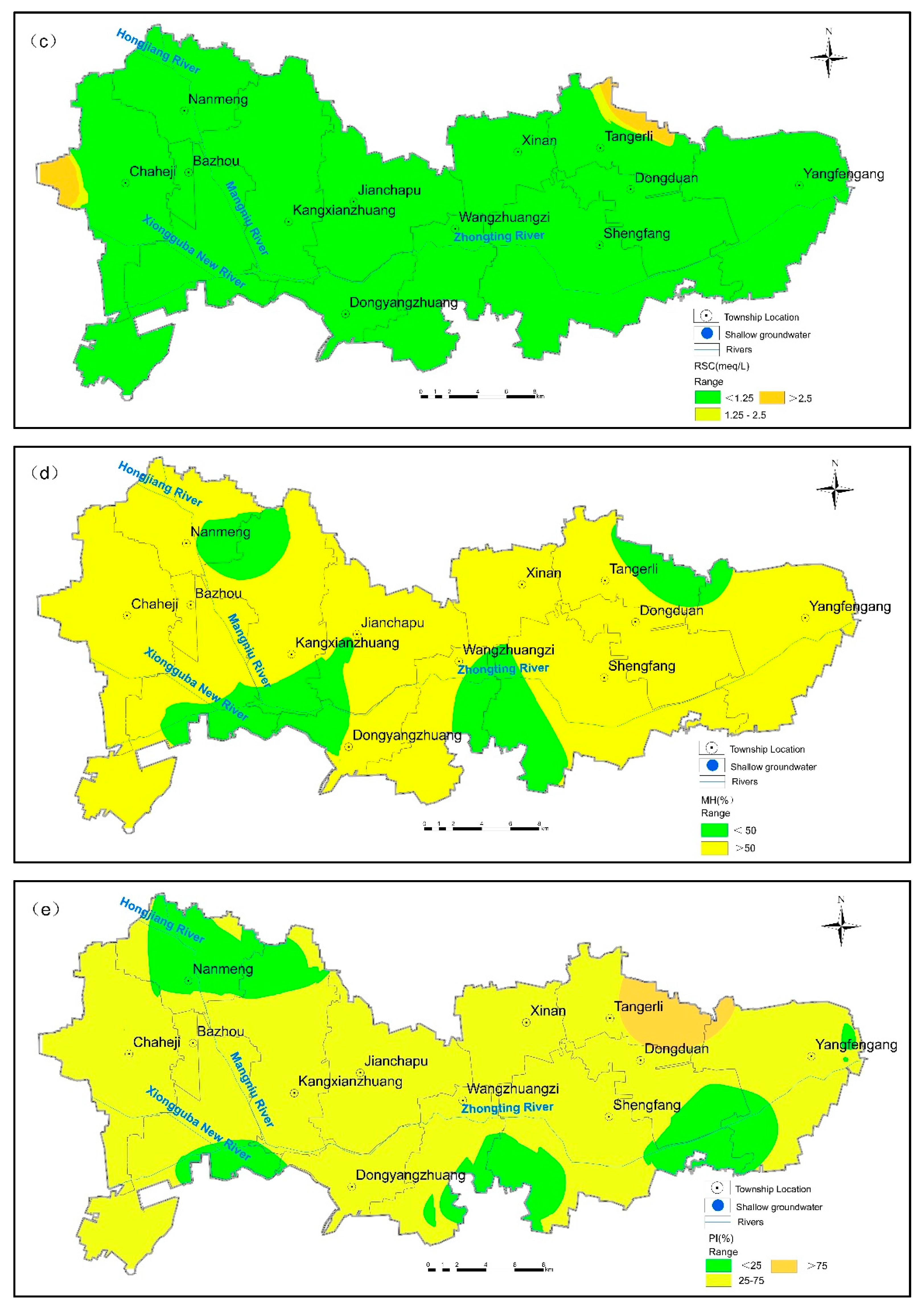
3.3. Suitability Evaluation for Agricultural Irrigation
3.4. Suitability Evaluation for Drinking and Health Risk Assessment
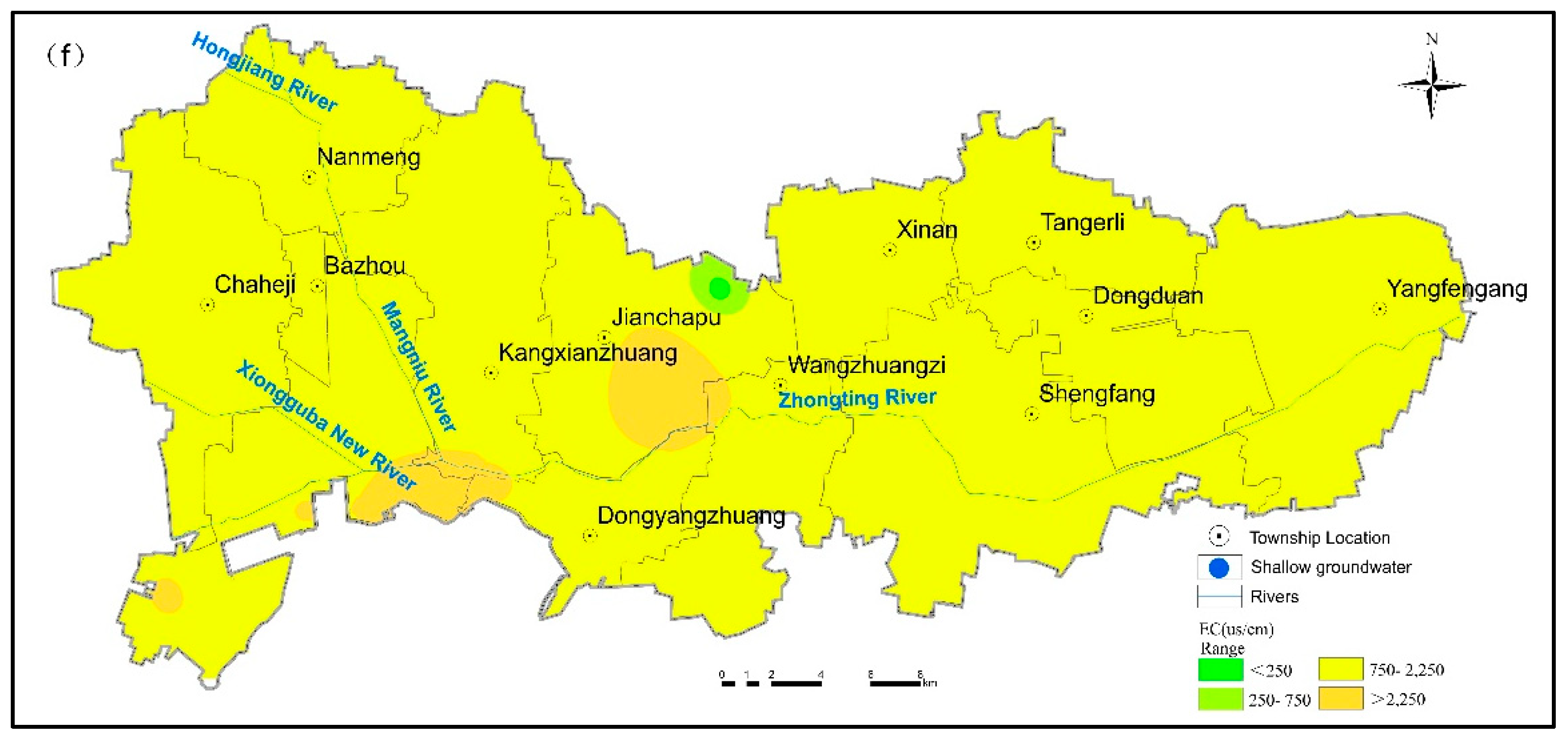
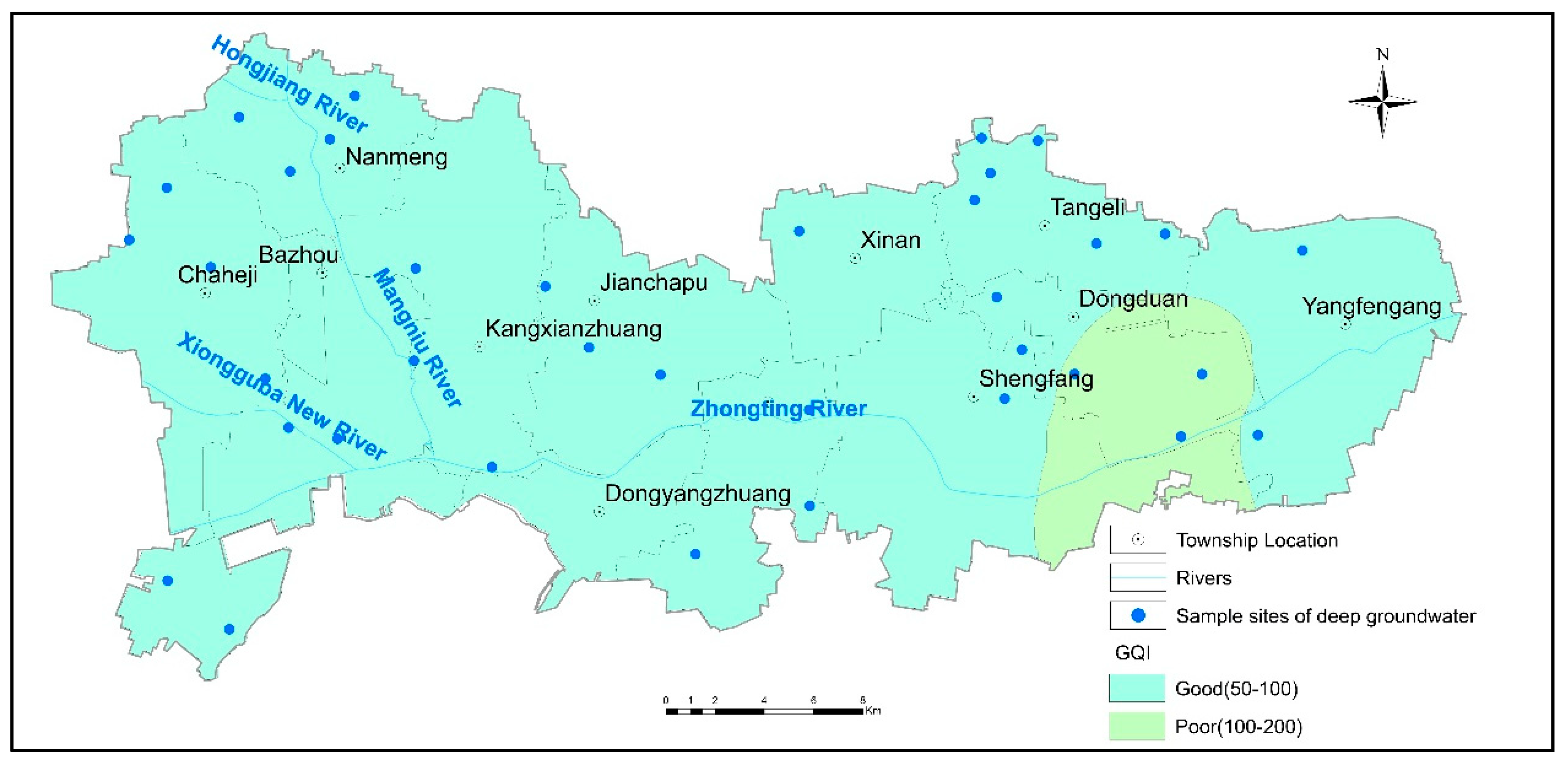
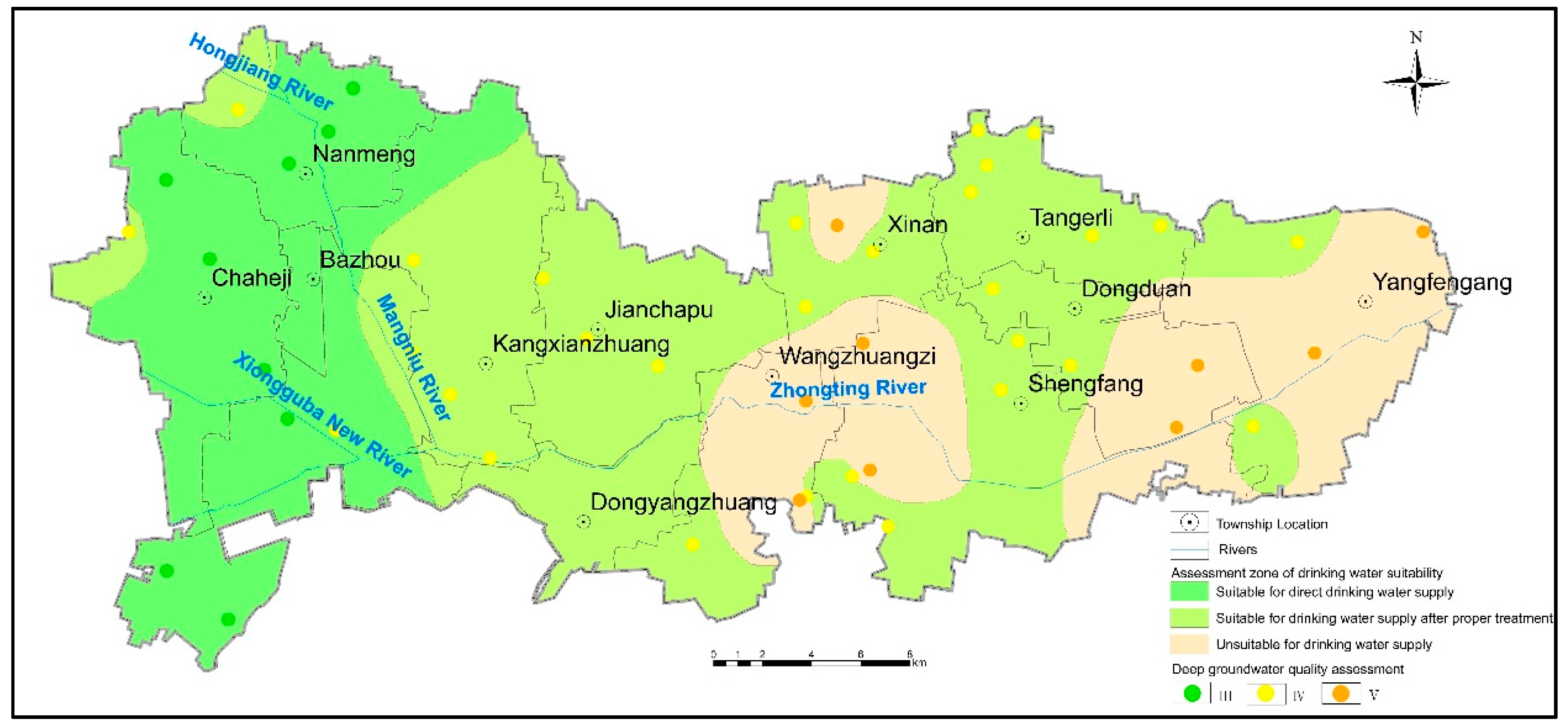
3.4.1. Suitability Evaluation for Drinking
3.4.2. Drinking Water Health Risk Assessment
3.5. Cause of High Fluoride Groundwater Formation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beyene, G.; Aberra, D.; Fufa, F. Evaluation of the suitability of groundwater for drinking and irrigation purposes in Jimma Zone of Oromia, Ethiopia. Groundw. Sustain. Dev. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Karunanidhi, D.; Aravinthasamy, P.; Kumar, D.; Subramani, T.; Roy, P. Sobol sensitivity approach for the appraisal of geomedical health risks associated with oral intake and dermal pathways of groundwater fluoride in a semi-arid region of south India. Ecotoxicol. Environ. Saf. 2020, 194, 1–12. [Google Scholar] [CrossRef]
- Maghrebi, M.; Noori, R.; Partani, S.; Araghi, A.; Haghighi, A. Iran’s Groundwater Hydrochemistry. Earth Space Sci. 2021, 8, 1–17. [Google Scholar] [CrossRef]
- Noori, R.; Maghrebi, M.; Mirchi, A.; Tang, Q.; Bhattarai, R.; Sadegh, M.; Noury, M.; Haghighi, A.; Kløve, B.; Madani, K. Anthropogenic depletion of Iran’s aquifers. Proc. Natl. Acad. Sci. USA 2021, 118, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Medici, G.; Baják, P.; West, L.; Chapman, P.; Banwart, S. DOC and nitrate fluxes from farmland; impact on a dolostone aquifer KCZ. J. Hydrol. 2021, 595, 125658. [Google Scholar] [CrossRef]
- Ghahremanzadeh, H.; Noori, R.; Baghvand, A.; Nasrabadi, T. Evaluating the main sources of groundwater pollution in the southern Tehran aquifer using principal component factor analysis. Environ. Geochem. Health 2018, 40, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Bian, J.; Nie, S.; Wang, R.; Wan, H.; Liu, C. Hydrochemical characteristics and quality assessment of groundwater for irrigation use in central and eastern Songnen Plain, Northeast China. Environ. Monit. Assess. 2018, 190, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dev, R.; Bali, M. Evaluation of groundwater quality and its suitability for drinking and agricultural use in district Kangra of Himachal Pradesh, India. J. Saudi. Soc. Agric. Sci. 2019, 18, 462–468. [Google Scholar] [CrossRef]
- Jasmin, I.; Mallikarjuna, P. Evaluation of groundwater suitability for irrigation in the Araniar River Basin, South India—A case study using Gis approach. Irrig Drain. 2015, 64, 600–608. [Google Scholar] [CrossRef]
- Li, P.; Wu, J.; Qian, H. Assessment of groundwater quality for irrigation purposes and identification of hydrogeochemical evolution mechanisms in Pengyang County, China. Environ. Earth Sci. 2013, 69, 2211–2225. [Google Scholar] [CrossRef]
- Gowd, S. Assessment of groundwater quality for drinking and irrigation purposes: A case study of Peddavanka watershed, Anantapur District, Andhra Pradesh, India. Environ. Geol. 2005, 48, 702–712. [Google Scholar] [CrossRef]
- Srinivasamoorthy, K.; Vasanthavigar, M.; Vijayaraghavan, K.; Sarathidasan, R.; Gopinath, S. Hydrochemistry of groundwater in a coastal region of Cuddalore district, Tamilnadu, India: Implication for quality assessment. Arab. J. Geosci. 2013, 6, 441–454. [Google Scholar] [CrossRef]
- Aghazadeh, N.; Mogaddam, A. Assessment of groundwater quality and its suitability for drinking and agricultural uses in the Oshnavieh Area, Northwest of Iran. J. Environ. Prot. 2010, 1, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.; Xiao, Y.; Yin, S.; Hao, Q.; Liu, H.; Hao, Z.; Meng, G.; Pei, Q.; Yan, H. Hydrogeochemical characterization and quality assessment of groundwater in a long-term reclaimed water irrigation area, North China Plain. Water 2018, 10, 1209. [Google Scholar] [CrossRef] [Green Version]
- Anim-Gyampo, M.; Anornu, G.; Appiah-Adjei, E.; Agodzo, S. Quality and health risk assessment of shallow groundwater aquifers within the Atankwidi basin of Ghana. Groundw. Sustain. 2019, 9, 100217. [Google Scholar] [CrossRef]
- Chidambaram, S.; Prasanna, M.; Venkatramanan, S.; Nepolian, M.; Pradeep, K. Groundwater quality assessment for irrigation by adopting new suitability plot and spatial analysis based on fuzzy logic technique. Environ. Res. 2022, 204, 1–15. [Google Scholar]
- Subramani, T.; Elango, L.; Damodarasamy, S. Groundwater quality and its suitability for drinking and agricultural use in Chithar River Basin, Tamil Nadu, India. Environ. Geol. 2005, 47, 1099–1110. [Google Scholar] [CrossRef]
- Adimalla, N.; Li, P.; Venkatayogi, S. Hydrogeochemical evaluation of groundwater quality for drinking and irrigation purposes and integrated interpretation with water quality index studies. Environ. Process. 2018, 5, 363–383. [Google Scholar] [CrossRef]
- Adimalla, N.; Vasa, S.; Li, P. Evaluation of groundwater quality, Peddavagu in Central Telangana (PCT), South India: An insight of controlling factors of fluoride enrichment. Model. Earth Syst. Environ. 2018, 4, 841–852. [Google Scholar] [CrossRef]
- Abd El-Aziz, S. Evaluation of groundwater quality for drinking and irrigation purposes in the north-western area of Libya (Aligeelat). Environ. Earth Sci. 2017, 76, 1–7. [Google Scholar] [CrossRef]
- Ehya, F.; Marbouti, Z. Hydrochemistry and contamination of groundwater resources in the Behbahan plain, SW Iran. Environ. Earth Sci. 2016, 75, 1–13. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, D.; Yang, J.; Wei, X.; Qin, J.; Ou, S.; Zhang, Z.; Zou, Y. Probabilistic risk assessment of Chinese residents’ exposure to fluoride in improved drinking water in endemic fluorosis areas. Environ. Pollut. 2017, 222, 118–125. [Google Scholar] [CrossRef]
- Aradpour, S.; Noori, R.; Tang, Q.; Bhattarai, R.; Hooshyaripor, F.; Hosseinzadeh, M.; Haghighi, A.; Klöve, B. Metal contamination assessment in water column and surface sediments of a warm monomictic man-made lake: Sabalan Dam Reservoir, Iran. Hydrol. Res. 2020, 51, 799–814. [Google Scholar] [CrossRef]
- Li, P.; He, X.; Li, Y.; Xiang, G. Occurrence and health implication of fluoride in groundwater of loess aquifer in the Chinese loess plateau: A case study of Tongchuan, Northwest China. Expo. Health 2019, 11, 95–107. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, J. Health risk assessment of fluorine in fertilizers from a fluorine contaminated region based on the oral bioaccessibility determined by Biomimetic Whole Digestion-Plasma in-vitro Method (BWDPM). J. Hazard Mater. 2020, 383, 121124. [Google Scholar] [CrossRef] [PubMed]
- Emenike, C.; Tenebe, I.; Jarvis, P. Fluoride contamination in groundwater sources in Southwestern Nigeria: Assessment using multivariate statistical approach and human health risk. Ecotoxicol. Environ. Saf. 2018, 156, 391–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, Y.; Lei, Y.; Wu, J.; Teng, Y.; Wang, J.; Zhao, X.; Pan, X. Does the groundwater nitrate pollution in China pose a risk to human health? A critical review of published data. Environ. Sci. Pollut. Res. 2016, 24, 3640–3653. [Google Scholar] [CrossRef]
- Jianmin, B.; Yu, W.; Juan, Z. Arsenic and fluorine in groundwater in western Jilin Province, China: Occurrence and health risk assessment. Nat. Hazards 2015, 77, 1903–1914. [Google Scholar] [CrossRef]
- Qin, L.; Pang, X.; Zeng, H.; Liang, Y.; Mo, L.; Wang, D.; Dai, J. Ecological and human health risk of sulfonamides in surface water and groundwater of Huixian karst wetland in Guilin, China. Sci. Total Environ. 2020, 708, 134552. [Google Scholar] [CrossRef]
- Adimalla, N.; Qian, H. Groundwater quality evaluation using water quality index (WQI) for drinking purposes and human health risk (HHR) assessment in an agricultural region of Nanganur, south India. Ecotoxicol. Environ. Saf. 2019, 176, 153–161. [Google Scholar] [CrossRef]
- Aradpour, S.; Noori, R.; Naseh, M.; Hosseinzadeh, M.; Safavi, S.; Ghahraman-Rozegar, F.; Maghrebi, M. Alarming carcinogenic and non-carcinogenic risk of heavy metals in Sabalan dam reservoir, Northwest of Iran. Environ. Pollut. Bioavailab. 2021, 33, 278–291. [Google Scholar] [CrossRef]
- Fang, H.; Lin, Z.; Fu, X. Spatial variation, water quality, and health risk assessment of trace elements in groundwater in Beijing and Shijiazhuang, North China Plain. Environ. Sci. Pollut. Res. 2021, 28, 57046–57059. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Z.; Zhang, Y.; Sun, Z.; Sun, T. Hydrochemical evaluation of groundwater quality and human health risk assessment of nitrate in the largest peninsula of China based on high-density sampling: A case study of Weifang. J. Clean. Prod. 2021, 322, 1–12. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhao, X.; Teng, Y.; Li, X.; Zhang, J.; Wu, J.; Zuo, R. Groundwater nitrate pollution and human health risk assessment by using HHRA model in an agricultural area, NE China. Ecotoxicol. Environ. Saf. 2017, 137, 130–142. [Google Scholar] [CrossRef]
- Adimalla, N.; Li, P. Occurrence, health risks, and geochemical mechanisms of fluoride and nitrate in groundwater of the rockdominant semi-arid region, Telangana State, India. Hum. Ecol. Risk Assess. 2019, 25, 81–103. [Google Scholar] [CrossRef]
- Aghapour, S.; Bina, B.; Tarrahi, M.; Amiri, F.; Ebrahimi, A. Distribution and health risk assessment of natural fluoride of drinking groundwater resources of Isfahan, Iran, using GIS. Environ. Monit. Assess. 2018, 190, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Qian, H.; Wu, J.; Chen, J.; Zhang, Y.; Zhang, H. Occurrence and hydrogeochemistry of fluoride in alluvial aquifer of Weihe River, China. Env. Earth Sci. 2014, 71, 3133–3145. [Google Scholar] [CrossRef]
- Narsimha, A.; Sudarshan, V. Assessment of fluoride contamination in groundwater from Basara, Adilabad District, Telangana State, India. Appl. Water. Sci. 2017, 7, 2717–2725. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhou, H.; Qian, K.; Xie, X.; Xue, X.; Yang, Y.; Wang, Y. Fluoride and iodine enrichment in groundwater of North China Plain: Evidences from speciation analysis and geochemical modeling. Sci. Total Environ. 2017, 598, 239–248. [Google Scholar] [CrossRef]
- Fallahzadeh, R.; Miri, M.; Taghavi, M.; Gholizadeh, A.; Anbarani, R. Spatial variation and probabilistic risk assessment of exposure to fluoride in drinking water. Food Chem. Toxicol. 2018, 113, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.; Kumar, V.; Gupta, N.; Kumar, S.; Rezania, S.; Singh, N. Human health risk assessment: Study of a population exposed to fluoride through groundwater of Agra city, India. Regul. Toxicol. Pharmacol. 2019, 106, 68–80. [Google Scholar] [CrossRef]
- Gu, X.; Xiao, Y.; Yin, S.; Pan, X.; Niu, Y.; Shao, J.; Cui, Y.; Zhang, Q.; Hao, Q. Natural and anthropogenic factors affecting the shallow groundwater quality in a typical irrigation area with reclaimed water, North China Plain. Environ. Monit. Assess. 2017, 189, 1–12. [Google Scholar] [CrossRef]
- Liu, H.; Guo, H.; Yang, L.; Wu, L.; Li, F.; Li, S.; Ni, P.; Liang, X. Occurrence and formation of high fluoride groundwater in the Hengshui area of the North China Plain. Environ. Earth Sci. 2015, 74, 2329–2340. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, H.; Wang, Y.; Wang, G.; Wang, H.; Zang, X.; Zhu, J. Groundwater hydrogeochemical characteristics and quality suitability assessment for irrigation and drinking purposes in an agricultural region of the North China plain. Environ. Earth Sci. 2021, 80, 162. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, J. The current situation and countermeasures about the utilization of groundwater resources in Bazhou. Hebei Water Res. 2010, 32, 1–2. (In Chinese) [Google Scholar]
- Zhang, G.; Yan, X.; Tian, Y.; Yan, M.; Wang, W. Response characteristic and mechanism of shallow groundwater level on the successive years with less rainfall in the agricultural areas of the central Hebei piedmont plain. J. Earth Sci. Environ. 2015, 37, 68–74, (In Chinese with English abstract). [Google Scholar]
- Yan, L.; Qin, Y.; Yang, H.; Zhang, J.; Le, Z. Characteristics of Bazhou ground temperature change from 1964–2015 and its relationship with temperature and precipitation. Mod. Agricult. Sci. Technol. 2018, 3, 223–229. (In Chinese) [Google Scholar]
- Zhang, Z.; Shi, D.; Fuhong, R.; Yin, Z.; Sun, J.; Zhang, C. Evolution of quaternary groundwater system in North China Plain. Sci. China 1997, 40, 276–283. [Google Scholar] [CrossRef]
- Xing, L.; Guo, H.; Zhan, Y. Groundwater hydrochemical characteristics and processes along flow paths in the North China Plain. J. Asian Earth Sci. 2013, 70–71, 250–264. [Google Scholar] [CrossRef]
- Najafzadeh, M.; Homaei, F.; Mohamadi, S. Reliability evaluation of groundwater quality index using data-driven models. Environ. Sci. Pollut. Res. 2021. [Google Scholar] [CrossRef]
- Raju, N. Hydrogeochemical parameters for assessment of groundwater quality in the upper Gunjanaeru River basin, Cuddapah District, Andhra Pradesh, South India. Environ. Geol. 2007, 52, 1067–1074. [Google Scholar] [CrossRef]
- Abboud, I. Geochemistry and quality of groundwater of the Yarmouk basin aquifer, north Jordan. Environ. Geochem. Health 2018, 40, 1405–1435. [Google Scholar] [CrossRef]
- USSL Salinity Laboratory. Diagnosis and Improvement of Saline and Alkaline Soils; US Department of Agriculture Handbook; No. 60; US Department of Agriculture: Washington, DC, USA, 1954.
- Wilcox, L. Classification and Use of Irrigation Water, 4th ed.; USDA, Circular: Washington, DC, USA, 1955; p. 969.
- WHO Guidelines for Drinking Water Quality: Fourth Edition Incorporating the First Addendum; World Health Organization: Geneva, Switzerland, 2017.
- WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2011.
- U.S. EPA. Risk Assessment Guidance for Superfund Volume I—Human Health Evaluation Manual; Office of Emergency and Remedial Response: Washington, DC, USA, 1989.
- U.S. EPA. Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual; Office of Superfund Remediation and Technology Innovation, U.S. Environmental Protection Agency: Washington, DC, USA, 2004.
- Li, C.; Gao, X.; Wang, Y. Hydrogeochemistry of high-fluoride groundwater at Yuncheng Basin, northern China. Sci. Total Environ. 2015, 508, 155–165. [Google Scholar] [CrossRef]
- Alaya, M.; Saidi, S.; Zemni, T.; Zargouni, F. Suitability assessment of deep groundwater for drinking and irrigation use in the Djeffara aquifers (Northern Gabes, south-eastern Tunisia). Environ. Earth Sci. 2014, 71, 3387–3421. [Google Scholar] [CrossRef] [Green Version]
- Narsimha, A.; Sudarshan, V. Contamination of fluoride in groundwater and its effect on human health: A case study in hard rock aquifers of Siddipet, Telangana State, India. Appl. Water Sci. 2017, 7, 2501–2512. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Li, P.; Qian, H. Hydrochemical characterization of drinking groundwater with special reference to fluoride in an arid area of China and the control of aquifer leakage on its concentrations. Environ. Earth Sci. 2015, 73, 8575–8588. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Xie, X.; Depaolo, D. Effects of water-sediment interaction and irrigation practices on iodine enrichment in shallow groundwater. J. Hydrol. 2016, 543, 293–304. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, Y.; Asghari, F.B.; Zuccarello, P.; Conti, G.O.; Ejlali, A.; Mohammadi, A.A.; Ferrante, M. Spatial Distribution Variation and Probabilistic Risk Assessment of Exposure to Fluoride in Ground Water Supplies: A Case Study in an Endemic Fluorosis Region of Northwest Iran. Int. J. Environ. Res. Public Health 2019, 16, 564. [Google Scholar]
- Rao, N.; Sunitha, B.; Sun, L. Mechanisms controlling groundwater chemistry and assessment of potential health risk: A case study from South India. Chemie. Der Erde–Geochem. 2019, 80, 125568. [Google Scholar]
- Gao, Z.; Han, C.; Xu, Y.; Zhao, Z.; Luo, Z.; Liu, J. Assessment of the water quality of groundwater in Bohai Rim and the controlling factors—a case study of northern Shandong Peninsula, north China. Environ. Pollut. 2021, 285, 117482. [Google Scholar] [CrossRef] [PubMed]
- Narsimha, A.; Rajitha, S. Spatial distribution and seasonal variation in fluoride enrichment in groundwater and its associated human health risk assessment in Telangana State, South India. Hum. Ecol. Risk Assess. Int. J. 2018, 24, 2119–2132. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Z.; Cheng, G.; Li, W.; Li, T.; Jiao, J. Groundwater-derived land subsidence in the North China Plain. Environ Earth Sci. 2015, 74, 1415–1427. [Google Scholar] [CrossRef]
- Han, Y.; Zhai, Y.; Guo, M.; Cao, X.; Lu, H. Hydrochemical and Isotopic Characterization of the impact of water diversion on water in drainage channels, groundwater, and Lake Ulansuhai in China. Water 2021, 13, 3033. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Fei, Y.; Liu, J.; Wei, W. Estimation of water released from aquitard compaction indicated by fluorine in Cangzhou. J. Jilin Univ. 2011, 41, 298–302, (In Chinese with English Abstract). [Google Scholar]
- Zhang, R. Study of fluorine increase mechanism in deep groundwater in the Eastern Hebei Plain. Hydrogeol. Engin. Geol. 1991, 18, 56–58, (In Chinese with English Abstract). [Google Scholar]
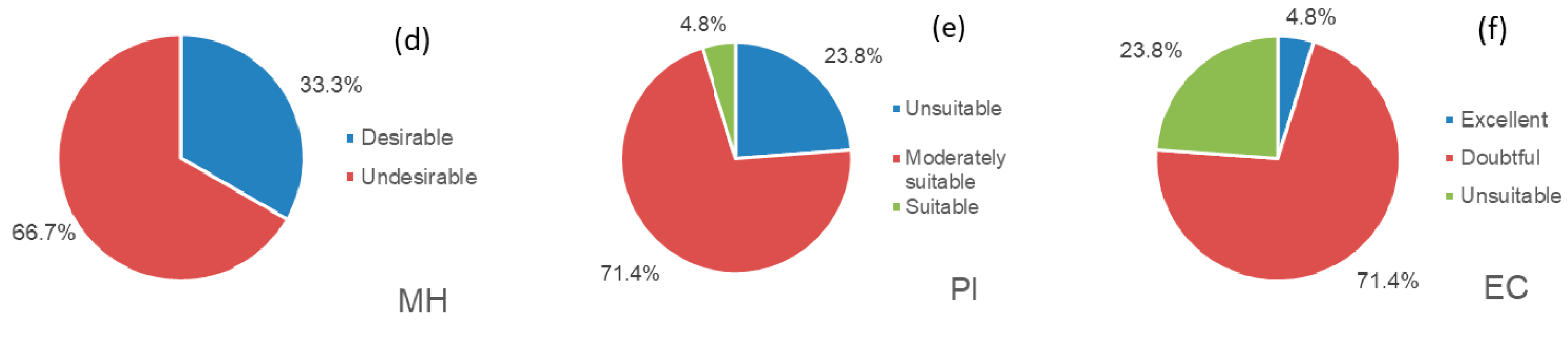
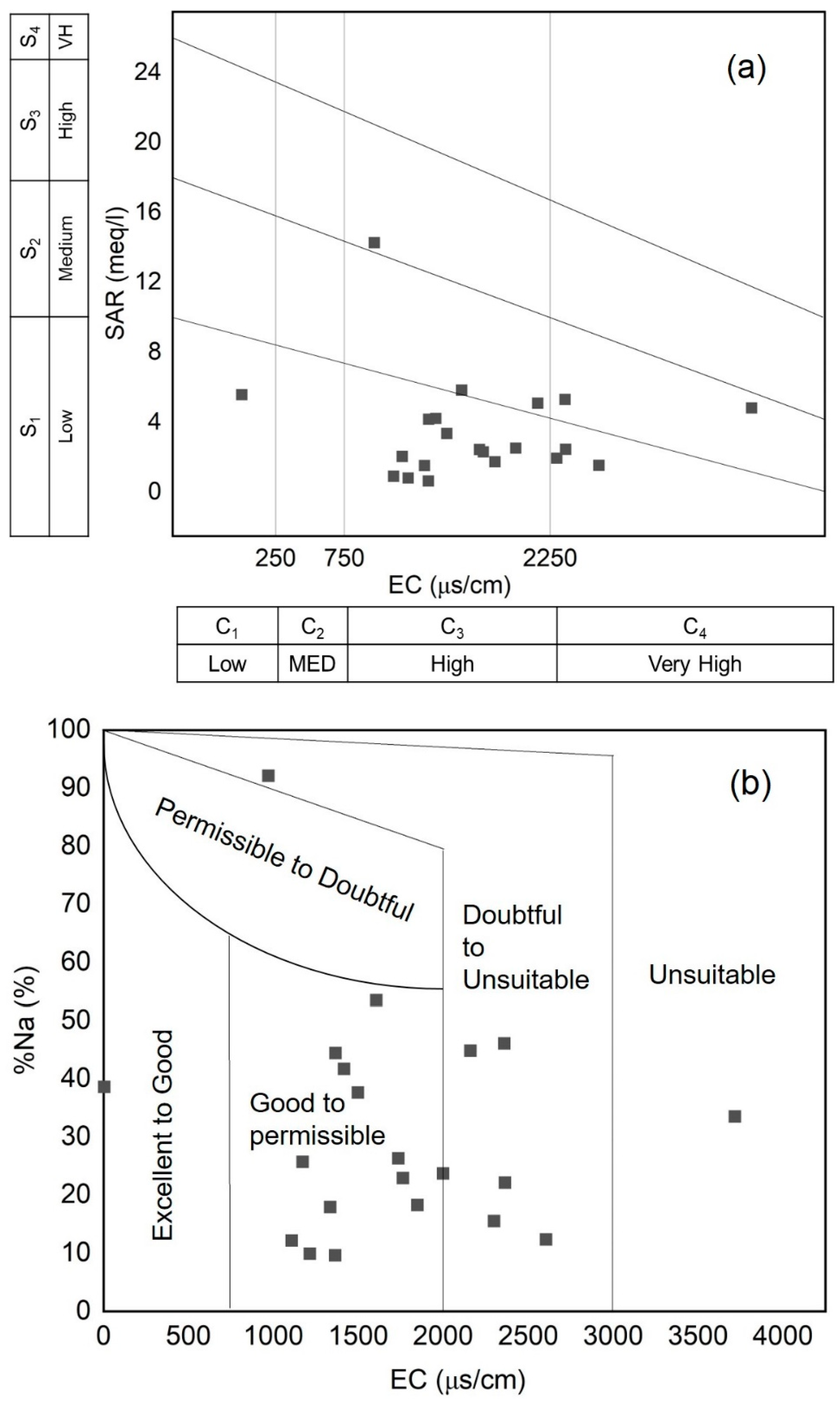
| Index Range Classification | Index Range Classification | Index Range Classification | Standard Formulas | References |
|---|---|---|---|---|
| SAR (meq/L) | <10 | Excellent | Richards (1954) | |
| 10–18 | Good | |||
| 18–26 | Doubtful | |||
| >26 | Unsuitable | |||
| %Na (%) | <20 | Excellent | Wilcox (1955) | |
| 20–40 | Good | |||
| 40–60 | Permissible | |||
| 60–80 | Doubtful | |||
| >80 | Unsuitable | |||
| RSC (meq/L) | <1.25 | Good | Richards (1954) | |
| 1.25–2.5 | Doubtful | |||
| >2.5 | Unsuitable | |||
| MH (%) | <50 | Desirable | Szaboles and Darab (1964) | |
| >50 | Undesirable | |||
| PI (%) | <25 | Unsuitable | Doneen (1964) | |
| 25–75 | Moderately suitable | |||
| >75 | Suitable | |||
| EC (us/cm) | <250 | Excellent | Measured by instruments | Wilcon (1955) |
| 250–750 | Good | |||
| 750–2250 | Doubtful | |||
| >2250 | Unsuitable |
| Parameters | WHO Standards (S) | Weight (wi) | Relative Weight (Wi) |
|---|---|---|---|
| Cl− | 250 (mg/L) | 3 | 0.083 |
| SO42− | 200 (mg/L) | 4 | 0.112 |
| HCO3− | 150 (mg/L) | 3 | 0.083 |
| pH | 6.5–9.2 (mg/L) | 4 | 0.112 |
| TDS | 500 (mg/L) | 5 | 0.139 |
| EC | 500 (us/cm) | 5 | 0.139 |
| Na+ | 200 (mg/L) | 2 | 0.055 |
| K+ | 10 (mg/L) | 2 | 0.055 |
| Mg2+ | 30 (mg/L) | 2 | 0.055 |
| Ca2+ | 75 (mg/L) | 2 | 0.055 |
| TH | 100 (mg/L) | 4 | 0.112 |
| Total weight | 36 | 1 |
| GQI | Type of Water |
|---|---|
| <50 | Excellent |
| 50–100 | Good |
| 100–200 | Poor |
| 200–300 | Very poor |
| >300 | Water unsuitable for drinking |
| Parameter | Shallow Groundwater | Deep Groundwater | Standard Values | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | SD | CV | Min | Max | Mean | SD | CV | GB | WG | |
| pH | 7.31 | 8.50 | 7.85 | 0.34 | 0.04 | 8.20 | 9.15 | 8.60 | 0.17 | 0.02 | 6.5–8.5 | 6.5–8.5 |
| EC (µs/cm) | 3.56 | 3717.10 | 1709.77 | 730.56 | 0.43 | 1.59 | 1556.0 | 698.95 | 269.50 | 0.39 | 1500 | |
| Total hardness (mg/L) | 18.73 | 1482.85 | 673.82 | 351.95 | 0.52 | 10.12 | 82.96 | 24.81 | 14.67 | 0.59 | 450 | 500 |
| Total alkalinity (mg/L) | 268.76 | 712.1 | 494.2 | 120.83 | 0.24 | 185.19 | 418.35 | 286.67 | 51.08 | 0.18 | ||
| CODMn (mg/L) | 0.66 | 5.58 | 1.74 | 1.09 | 0.63 | 0.37 | 1.66 | 0.67 | 0.24 | 0.36 | 3 | |
| Soluble silica (mg/L) | 3.60 | 21.20 | 13.24 | 4.69 | 0.35 | 9.80 | 15.90 | 12.67 | 1.13 | 0.09 | ||
| Carbon dioxide (mg/L) | 0.00 | 85.40 | 38.64 | 27.52 | 0.71 | 0.00 | 2.20 | 0.06 | 0.35 | 6.08 | ||
| K+ (mg/L) | 0.30 | 31.40 | 2.49 | 6.51 | 2.61 | 0.20 | 29.10 | 1.22 | 4.59 | 3.76 | 12 | |
| Na+ (mg/L) | 44.80 | 569.70 | 240.88 | 134.48 | 0.56 | 40.58 | 314.60 | 160.48 | 49.62 | 0.31 | 200 | 200 |
| Ca2+ (mg/L) | 4.70 | 331.20 | 122.91 | 81.78 | 0.67 | 2.90 | 327.10 | 14.44 | 51.49 | 3.57 | 200 | |
| Mg2+ (mg/L) | 1.70 | 175.60 | 89.14 | 40.69 | 0.46 | 0.70 | 9.60 | 2.62 | 2.07 | 0.79 | 150 | |
| Cl− (mg/L) | 55.30 | 550.50 | 220.30 | 132.42 | 0.60 | 13.80 | 231.10 | 53.83 | 48.62 | 0.90 | 250 | 250 |
| SO42− (mg/L) | 16.00 | 1037.36 | 344.71 | 307.02 | 0.89 | 0.35 | 50.07 | 26.43 | 12.09 | 0.46 | 250 | 250 |
| CO32− (mg/L) | 0.00 | 12.00 | 1.57 | 3.16 | 2.01 | 0.00 | 31.20 | 7.59 | 5.48 | 0.72 | ||
| HCO3− (mg/L) | 315.50 | 868.30 | 599.39 | 147.50 | 0.25 | 213.60 | 491.80 | 334.09 | 62.24 | 0.19 | 500 | |
| F− (mg/L) | 0.35 | 2.15 | 1.02 | 0.50 | 0.49 | 0.29 | 4.21 | 2.23 | 1.20 | 0.54 | 1 | 1.5 |
| I− (mg/L) | 0.00 | 0.25 | 0.05 | 0.08 | 1.48 | 0.00 | 0.36 | 0.05 | 0.08 | 1.52 | 0.08 | |
| NH4+ (mg/L) | 0.04 | 0.40 | 0.12 | 0.07 | 0.60 | 0.00 | 0.16 | 0.08 | 0.05 | 0.56 | 0.5 | |
| NO3− (mg/L) | 0.00 | 126.80 | 18.16 | 31.83 | 1.75 | 0.00 | 7.63 | 0.62 | 1.67 | 2.69 | 20 | 50 |
| NO2− (mg/L) | 0.00 | 0.36 | 0.05 | 0.08 | 1.68 | 0.00 | 0.10 | 0.01 | 0.02 | 2.64 | 1 | 3 |
| PO43− (mg/L) | 0.02 | 0.57 | 0.08 | 0.12 | 1.50 | 0.02 | 0.46 | 0.20 | 0.10 | 0.51 | 30 | |
| Fe (mg/L) | 0.01 | 0.07 | 0.02 | 0.02 | 0.91 | 0.01 | 0.10 | 0.02 | 0.02 | 0.87 | 0.3 | |
| Mn (mg/L) | 0.00 | 0.60 | 0.08 | 0.14 | 1.87 | 0.00 | 0.04 | 0.01 | 0.01 | 1.39 | 0.1 | 0.4 |
| Statistics | F− | I− | ||||
|---|---|---|---|---|---|---|
| HQc | HQd | HQ | HQc | HQd | HQ | |
| Children | ||||||
| Min | 0.22 | 0.00037 | 0.22 | 0.000031 | 0.018 | 0.018 |
| Max | 3.1 | 0.0054 | 3.1 | 0.0056 | 3.3 | 3.3 |
| Mean | 1.7 | 0.0029 | 1.7 | 0.00084 | 0.49 | 0.49 |
| Male | ||||||
| Min | 0.15 | 0.00028 | 0.15 | 0.000023 | 0.013 | 0.013 |
| Max | 2.2 | 0.004 | 2.2 | 0.0042 | 2.3 | 2.3 |
| Mean | 1.2 | 0.0021 | 1.2 | 0.00062 | 0.35 | 0.35 |
| Female | ||||||
| Min | 0.17 | 0.00028 | 0.17 | 0.000023 | 0.014 | 0.014 |
| Max | 2.4 | 0.0041 | 2.4 | 0.0042 | 2.5 | 2.5 |
| Mean | 1.3 | 0.0022 | 1.3 | 0.00063 | 0.37 | 0.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Li, M.; Wang, L.; Wang, Y.; Zang, X.; Zhao, X.; Wang, H.; Zhu, J. Evaluation of Groundwater Suitability for Irrigation and Drinking Purposes in an Agricultural Region of the North China Plain. Water 2021, 13, 3426. https://doi.org/10.3390/w13233426
Guo H, Li M, Wang L, Wang Y, Zang X, Zhao X, Wang H, Zhu J. Evaluation of Groundwater Suitability for Irrigation and Drinking Purposes in an Agricultural Region of the North China Plain. Water. 2021; 13(23):3426. https://doi.org/10.3390/w13233426
Chicago/Turabian StyleGuo, Haipeng, Muzi Li, Lu Wang, Yunlong Wang, Xisheng Zang, Xiaobing Zhao, Haigang Wang, and Juyan Zhu. 2021. "Evaluation of Groundwater Suitability for Irrigation and Drinking Purposes in an Agricultural Region of the North China Plain" Water 13, no. 23: 3426. https://doi.org/10.3390/w13233426
APA StyleGuo, H., Li, M., Wang, L., Wang, Y., Zang, X., Zhao, X., Wang, H., & Zhu, J. (2021). Evaluation of Groundwater Suitability for Irrigation and Drinking Purposes in an Agricultural Region of the North China Plain. Water, 13(23), 3426. https://doi.org/10.3390/w13233426





