Factors Affecting Efficiency of Biosorption of Fe (III) and Zn (II) by Ulva lactuca and Corallina officinalis and Their Activated Carbons
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Algal Biomass
2.2. Preparation of Biosorbent
2.3. Adsorption Procedure
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 67, 303–305. [Google Scholar] [CrossRef] [Green Version]
- Mosleh, Y.Y.; Mofeed, J.; Al-Maghrabi, O.; Fuller, M.P. Residues of heavy metals, PCDDs, PCDFs and DL-PCBs on some medicinal plants collected randomly from the Jeddah, central market. Life Sci. J. 2014, 11, 1–8. [Google Scholar]
- Mosleh, Y.Y.; Mofeed, J.; Al-Maghrabi, O.A.; Mousa, T.; Kadasa, N.M.S. Dietary Intake of Pesticides Based on Vegetable Consumption: A Case Study, Jeddah, Kingd. of Saudi Arabia. Life Sci. J. 2014, 11, 680–688. [Google Scholar]
- Abdel-Gawad, F.K.; Khalil, W.K.B.; Bassem, S.M.; Kumar, V.; Parisi, C.; Inglese, S.; Temraz, T.A.; Nassar, H.F.; Guerriero, G. The duckweed, Lemna minor modulates heavy metal-induced oxidative stress in the Nile tilapia Oreochromis niloticus. Water 2020, 12, 2983. [Google Scholar] [CrossRef]
- Guezgouz, N.; Parisi, C.; Boubsil, S.; Grieco, G.; Hana, S.A.; Guerriero, G. Heavy Metals Assessment in the Medjerda River Basin (Northeastern Algeria): A Preliminary Water Analysis and Toad Skin Biopsy. Proc. Zool. Soc. 2021, 74, 104–113. [Google Scholar] [CrossRef]
- Mosleh, Y.Y.; Mofeed, J. Biochmical biomarkers in algae Scenedesmae obliquus exposed to heavy metals Cd, Cu and Zn. Life Sci. J. 2014, 11, 994–1004. [Google Scholar]
- Guerriero, G.; Bassem, S.M.; Abdel Gawad, F.K. Biological responses of white sea bream (Diplodus sargus, Linnaeus 1758) and sardine (Sardine pilchardus, Walbaum 1792) exposed to heavy metal contaminated water. Emir. J. Food Agric. 2018, 30, 688–694. [Google Scholar] [CrossRef]
- Guerriero, G.; Bassem, S.M.; Khalil, W.K.B.; Temraz, T.A.; Ciarcia, G.; Abdel-Gawad, F.K. Temperature changes and marine fish species (Epinephelus coioides and Sparus aurata): Role of oxidative stress biomarkers in toxicological food studies. Emir. J. Food Agric. 2018, 30, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Mofeed, J. Impacts of ZnO nanoparticles on growth and antioxidant enzymes of the green alga Scenedesmus obliquus. Afr. J. Biol. Sci. 2020, 2, 1–12. [Google Scholar] [CrossRef]
- Tosti, E.; Esposito, M.C. Reprotoxicity of Global Warming in Marine Species. J. Mar. Sci. Res. Dev. 2018, 8, S12-e001. [Google Scholar] [CrossRef]
- Parisi, C.; Guerriero, G. Antioxidative Defense and Fertility Rate in the Assessment of Reprotoxicity Risk Posed by Global Warming. Antioxidants 2019, 8, 622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gentilucci, M.; Moustafa, A.A.; Abdel-Gawad, F.K.; Mansour, S.R.; Coppola, M.R.; Caserta, L.; Inglese, S.; Pambianchi, G.; Guerriero, G. Advances in Egyptian Mediterranean Coast Climate Change Monitoring. Water 2021, 13, 1870. [Google Scholar] [CrossRef]
- Fasulo, S.; Guerriero, G.; Cappello, S.; Colasanti, M.; Schettino, T.; Leonzio, C.; Mancini, G.; Gornati, R. The SYSTEMS BIOLOGY in the study of xenobiotic effects on marine organisms for evaluation of the environmental health status: Biotechnological applications for potential recovery strategies. Rev. Environ. Sci. Biotechnol. 2015, 14, 339–345. [Google Scholar] [CrossRef]
- Piscopo, M.; Trifuoggi, M.; Notariale, R.; Labar, S.; Troisi, J.; Giarra, A.; Rabbito, D.; Puoti, R.; de Benedictis, D.; Brundo, M.V.; et al. Protamine-like proteins’ analysis as an emerging biotechnique for cadmium impact assessment on male mollusk Mytilus galloprovincialis (Lamarck 1819). Acta Biochim. Pol. 2018, 65, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Piscopo, M.; Notariale, R.; Rabbito, D.; Ausió, J.; Olanrewaju, O.S.; Guerriero, G. Mytilus galloprovincialis (Lamarck, 1819) spermatozoa: Hsp70 expression and protamine-like protein property studies. Environ. Sci. Pollut. Res. 2018, 25, 12957–12966. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Pathak, V.V.; Kothari, R.; Kumar, A.; Krishna, S.B.N. Optimization of nutrient stress using C. pyrenoidosa for lipid and biodiesel production in integration with remediation in dairy industry wastewater using response surface methodology. 3 Biotech 2018, 8, 326. [Google Scholar] [CrossRef] [PubMed]
- Hussain, J.; Husain, I.; Arif, M.; Gupta, N. Studies on heavy metal contamination in Godavari river basin. Appl. Water Sci. 2017, 7, 4539–4548. [Google Scholar] [CrossRef]
- Horvath, B.; Gruiz, K. Impact of metalliferous ore mining activity on the environment in Gyongyosorozi, Hungary. Sci. Total Environ. 1996, 184, 215–227. [Google Scholar] [CrossRef]
- Guerriero, G.; Parisi, C.; Abdel-Gawad, F.K.; Hentati, O.; D’Errico, G. Seasonal and pharmaceutical-induced changes in selenoprotein glutathione peroxidase 4 activity in the reproductive dynamics of the soil biosentinel Podarcis sicula (Chordata: Reptilia). Mol. Reprod. Dev. 2019, 1, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, G.; Brundo, M.V.; Labar, S.; Bianchi, A.R.; Trocchia, S.; Rabbito, D.; Palumbo, G.; Abdel-Gawad, F.K.; de Maio, A. Frog (Pelophylax bergeri, Gunther 1986) endocrine disruption assessment: Characterization and role of skin poly(ADP-ribose) polymerases. Environ. Sci. Pollut. Res. 2018, 25, 18303–18313. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Ji, Y.F.; Yang, L.S.; Li, S.J. Effects of mining activity on heavy metals in surface water in lead–zinc deposit area. J. Agro-Environ. Sci. 2007, 26, 103–107. [Google Scholar]
- Scalici, M.; Traversetti, L.; Spani, F.; Malafoglia, V.; Colamartino, M.; Persichini, T.; Cappello, S.; Mancini, G.; Guerriero, G.; Colasanti, M. Shell fluctuating asymmetry in the sea-dwelling benthic bivalve Mytilus galloprovincialis (Lamarck, 1819) as morphological markers to detect environmental chemical contamination. Ecotoxicology 2017, 26, 396–404. [Google Scholar] [CrossRef]
- Redha, A.A. Removal of heavy metals from aqueous media by biosorption. Arab. J. Basic Appl. Sci. 2020, 27, 183–193. [Google Scholar] [CrossRef]
- Balkose, D.; Baltacioglu, H. Adsorption of heavy metal cations from aqueous solutions by wool fibers. J. Chem. Technol. Biotechnol. 1992, 54, 393–397. [Google Scholar] [CrossRef]
- Javadian, H.; Ahmadi, M.; Ghiasvand, M.; Kahrizi, S.; Katal, R. Removal of Cr(VI) by modified brown algae Sargassum bevanom from aqueous solution and industrial wastewater. J. Taiwan Inst. Chem. Eng. 2013, 44, 977–989. [Google Scholar] [CrossRef]
- Prabha, Y.; Soni, S.K.; Sharmita, G. Potential of Algae in Bio-remediation of Wastewater Current Research. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 693–700. [Google Scholar] [CrossRef]
- Jan, A.T.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q.M. Heavy metals and human health: Mechanistic insight into toxicity and counter defense system of antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef] [Green Version]
- Guerriero, G.; Di Finizio, A.; Ciarcia, G. Oxidative Defenses in the Sea Bass, Dicentrarchus labrax. In Oxygen Transport to Tissue XXIV; Dunn, J.F., Swartz, H.M., Eds.; Springer: Boston, MA, USA, 2003; Volume 530, pp. 681–688. [Google Scholar]
- Guerriero, G.; D’Errico, G.; Di Giaimo, R.; Rabbito, D.; Olanrewaju, O.S.; Ciarcia, G. Reactive oxygen species and glutathione antioxidants in the testis of the soil biosentinel Podarcis sicula (Rafinesque 1810). Environ. Sci. Pollut. Res. 2017, 25, 18286–18296. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, W.N.A.S.; Tiandee, S.; Lau, W.J.; Aziz, F.; Ismail, A.F. Potential use of nanofiltration like-forward osmosis membranes for copper ion removal. Chin. J. Chem. Eng. 2020, 28, 420–428. [Google Scholar] [CrossRef]
- Bezzina, J.P.; Robshaw, T.; Dawson, R.; Ogden, M.D. Single metal isotherm study of the ion exchange removal of Cu(II), Fe(II), Pb(II) and Zn(II) from synthetic acetic acid leachate. Chem. Eng. J. 2020, 394, 124862. [Google Scholar] [CrossRef]
- King, J.F.; Szczuka, A.; Zhang, Z.; Mitch, W.A. Efficacy of ozone for removal of pesticides, metals and indicator virus from reverse osmosis concentrates generated during potable reuse of municipal wastewaters. Water Res. 2020, 176, 115744. [Google Scholar] [CrossRef]
- Hassan, A.A.; Al-Isawi, R.; Saleh, Z.A. The Use of Pre-Heated Black Cumin Seeds (Nigella sativa) for Sorption Basic Dyes from Aqueous Solutions. J. Ecol. Eng. 2021, 22, 149–158. [Google Scholar] [CrossRef]
- Arief, V.O.; Trilestarim, K.; Sunarso, J.; Indraswati, N.; Ismadji, S. Recent Progress on biosorption of heavy metals from liquids using low cost biosorbents: Characterization, biosorption parameters and mechanism studies. Clean 2008, 36, 937–962. [Google Scholar] [CrossRef]
- Alalwan, H.A.; Abbas, M.N.; Abudi, Z.N.; Alminshid, A.H. Adsorption of thallium ion (Tl+3) from aqueous solutions by rice husk in a fixed bed column: Experiment and prediction of breakthrough curves. Environ. Technol. Innov. 2018, 12, 1–13. [Google Scholar] [CrossRef]
- Mobark, M.; Mohamed, E.A.; Selim, A.Q.; Sellaoui, L.; Lamine, A.B.; Erto, A.; Bonilia-Petriciolet, A.; Seliem, M.K. Surfactant–modified serpentine for fluoride and Cr(VI) adsorption in single and binary systems: Experimental studies and theoretical modeling. Chem. Eng. J. 2019, 369, 333–343. [Google Scholar] [CrossRef]
- Seliem, M.K.; Mobarak, M.; Selim, A.Q.; Mohamed, E.A.; Halfaya, R.A.; Gomaa, H.K.; Anastopoulos, I.; Giannakoudakis, D.A.; Lima, E.C.; Bonilia-Petriciolet, A.; et al. A novel multifunctional adsorbent of pomegranate peel extract and activated anthracite for Mn(VII) and Cr(VI) uptake from solutions: Experiments and theoretical treatment. J. Mol. Liq. 2020, 311, 113169. [Google Scholar] [CrossRef]
- El Nemr, A.; El Sadaawy, M.M.; Khaled, A.; El Sikaily, A. Adsorption of the anionic dye Direct Red 23 onto new activated carbons developed from Cynara cardunculus: Kinetics, equilibrium and thermodynamics. Blue Biotechnol. J. 2014, 3, 121–142. [Google Scholar]
- Shoaib, A.G.M.; El-Sikaily, A.; ElNemr, A.; Mohamed, A.E.-D.A.; Hassan, A.A. Preparation and characterization of highly surface area activated carbons followed type IV from marine red alga (Pterocladia capillacea) by zinc chloride activation. Biomass Convers. Biorefin. 2020, 1–13. [Google Scholar] [CrossRef]
- Shoaib, A.G.M.; El-Sikaily, A.; ElNemr, A.; Mohamed, A.E.-D.A.; Hassan, A.A. Testing the carbonization condition for high surface area preparation of activated carbon followed type IV from green alga Ulva lactuca. Biomass Convers. Biorefin. 2020, 1–16. [Google Scholar] [CrossRef]
- Babel, S.; Kurmiawan, T.A. Low-cost adsorbents for heavy metalsuptake from contaminated water: A review. J. Hazard. Mater. 2003, B97, 219–243. [Google Scholar] [CrossRef]
- El Nemr, A. Non-Conventional Textile Waste Water Treatment; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2012; 267p. [Google Scholar]
- Kim, K.W.; Lee, H.M.; Kim, B.S.; Hwang, S.H.; Kwac, L.K.; An, K.H.; Kim, B.J. Preparation and thermal properties of polyethylene-based carbonized fibers. Carbon Lett. 2015, 16, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Mosleh, Y.; Mofeed, J.; Nafea, E.; Heham, S. Use of the microalga Scenedesmus obliquus (Turpin) Kutzing to remove some heavy metals from industrial wastewater. Egypt. J. Aquat. Biol. Fish. 2021, 25, 337–354. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Megalea, M.Z. Adsorption of the Heavy Metal Ions onto Bio sorbents: A review. Int. J. Nanomater. Chem. Int. 2018, 4, 27–39. [Google Scholar] [CrossRef]
- Sekhara, K.C.; Kamala, C.T.; Chary, N.S.; Anjaneyulu, Y. Removal of heavy metals using a plant biomass with reference to environmental control. Int. J. Miner. Process. 2003, 68, 37–45. [Google Scholar] [CrossRef]
- Mofeed, J. Biosorption of Heavy Metals from Aqueous Industrial Effluent by Non-living Biomass of Two Marine Green Algae Ulva lactuca and Dunaliella salina as Biosorpents. Catrina Int. J. Environ. Sci. 2017, 16, 43–52. [Google Scholar] [CrossRef]
- Zayadi, N.; Othma, N. Characterization and Optimization of Heavy Metals Biosorption by Fish Scales. Adv. Mater. Res. 2013, 795, 260–265. [Google Scholar]
- Bakar, A.A.; Ali, K.A.B.; Tarmizi, N.A.B.; Ahmad Zia Ul-Saufie, B.; Mohamad Japeri, A.Z.U.; Tammy, N.J.B. Potential of using bladderwort as a biosorbent to remove zinc in wastewater. AIP Conf. Proc. 2016, 1774, 030023. [Google Scholar]
- Mishra, V. Biosorption of zinc ion: A deep comprehension. Appl. Water Sci. 2014, 4, 311–332. [Google Scholar] [CrossRef] [Green Version]
- Apiratikul, R.; Pavasant, P. Batch and column studies of biosorption of heavy metals by Caulerpa lentillifera. Bioresour. Technol. 2008, 99, 2766–2777. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, W.M.; Hassan, A.F.; Azab, Y.A. Biosorption of toxic heavy metals from aqueous solution by Ulva lactuca activated carbon. Egypt. J. Basic Appl. Sci. 2016, 3, 241–249. [Google Scholar] [CrossRef] [Green Version]
- Mofeed, J.; Deyab, M.A.; Mohamed, B.; Moustafa, M.; Negm, S.; El-bilawy, E. Antimicrobial activities of three seaweeds extract against some human viral and bacterial pathogens. Biocell 2022, 46, 247–261. [Google Scholar] [CrossRef]
- Belhadj, S.; Gargouri, M.; Guerriero, G.; Hentati, O. Polysaccharides from the Green Alga Ulva lactuca Improve Antioxidant Balance and Bone Mineral Density in Diabetic Rats. Biomed. Environ. Sci. 2021, 34, 637–640. [Google Scholar]
- Ariano, A.; Musco, N.; Severino, L.; De Maio, A.; Tramice, A.; Tommonaro, G.; Damiano, S.; Genovese, A.; Olanrewaju, O.S.; Bovera, F.; et al. Chemistry of Tropical Eucheumatoids: Potential for Food and Feed Applications. Biomolecules 2021, 11, 804. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, O.S.; Tommonaro, G.; Guerriero, G.; Fogliano, C.; Iodice, C.; Velotto, G.; Tramice, A. New Insight into Marine Biotechnology: Carrageenans Chemical Features and Acetylcholinesterase (AChE) Inhibition Activity of Two Edible Seaweeds of the Genus Kappaphycus. In EMCEI 2019: Recent Advances in Environmental Science from the Euro-Mediterranean and Surrounding Regions, 2nd ed.; Springer: Cham, Switzerland, 2021; pp. 2203–2207. [Google Scholar]
- Olanrewaju, O.S.; De Maio, A.; Lionetti, E.; Bianchi, A.R.; Rabbito, D.; Ariano, A.; Majdoubi, F.-Z.; Guerriero, G. Sea Farms as a Safe and Sustainable Food Source: An Investigation on Use of Seaweeds for Liver Detoxification and Reduced DNA Damage in Lates calcarifer (Bloch, 1790). In EMCEI 2019: Recent Advances in Environmental Science from the Euro-Mediterranean and Surrounding Regions, 2nd ed.; Springer: Cham, Switzerland, 2021; pp. 671–675. [Google Scholar]
- Sari, A.; Tuzen, M. Biosorption of Pb(II) and Cd(II) from aqueous solution using green alga (Ulva lactuca) biomass. J. Hazard. Mater. 2008, 152, 302–308. [Google Scholar] [CrossRef]
- Turner, A.; Lewis, M.S.; Shams, L.; Brown, M.T. Uptake of platinum group elements by the marine macroalga, Ulva lactuca. Mar. Chem. 2007, 105, 271–280. [Google Scholar] [CrossRef]
- Brinza, L.; Dring, M.J.; Gavrilescu, M. Marine micro- and macro-algal species as biosorbents for heavy metals. Environ. Eng. Manag. J. 2007, 6, 237–251. [Google Scholar] [CrossRef]
- Londo, G. The decimal scale for relevés of permanent quadrats. In Sampling Methods and Taxon Analysis in Vegetation Science: Releve Surveys, ‘Vegetationsaufnahmen’, Floristic Analysis of Plant Communities; Handbook of vegetation sciences, pt. 4; Junk: Germany, 1984; pp. 45–49. Available online: https://edepot.wur.nl/459509 (accessed on 2 December 2021).
- De Clerck, O.; Coppejans, E. Marina Algae of the Jubail Marine Wildlife Sanctuary, Saudi Arabia; NCWCD: Berthoud, CO, USA, 1996; pp. 199–207. [Google Scholar]
- Brodie, J.; Walker, R.H.; Williamson, C.; Irvine, L.M. Epitypification and redescription of Corallina officinalis L., the type of the genus, and C. elongata Elliset Solander (Corallinales, Rhodophyta). Cryptogam. Algol. 2013, 34, 49–56. [Google Scholar] [CrossRef]
- Ibrahim, W.M.; Salim, E.H.; Azab, Y.A.; Ismail, A.M. Monitoring and removal of cyanobacterial toxins from drinking water by algal-activated carbon. Toxicol. Ind. Health 2015, 32, 1752–1762. [Google Scholar] [CrossRef] [PubMed]
- Pavasant, P.; Apiratikul, R.; Marhaba, T.F. Biosorption of Cu2+, Cd2+, Pb2+ and Zn2+ Using Dried Marine Green Macroalga caulerpa lentillifera. Bioresour. Technol. 2006, 97, 2321–2329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.X.; Shen, Y.C.; Sr, L.I. Epidemiological investigation on mental disorders in 7 areas of China. Chin. J. Psychiatry 1998, 31, 69–77. [Google Scholar]
- Hashim, M.A.; Chu, K.H. Biosorption of cadmium by brown, green, and red seaweeds. Chem. Eng. J. 2004, 97, 249–255. [Google Scholar] [CrossRef]
- Voudrias, E.; Fytianosand, F.; Bozani, E. Sorption description isotherms of dyes from aqueous solutions and waste waters with different sorbent materials, Global Nest. Int. J. 2002, 4, 75–83. [Google Scholar]
- Singh, D.B.; Prasad, G.; Rupainwar, D.C.; Singh, V.N. As (III) removal from aqueous solution by adsorption. Water Air Soil Pollut. 1988, 42, 373–386. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H. Adsorption in solution. J. Phys. Chem. Soc. 1906, 40, 1361–1368. [Google Scholar]
- Sun, L.; Yao, Y.; Wang, L.; Mao, Y.; Huang, Z.; Yao, D.; Lu, W.; Chen, W. Efficient removal of dyes using activated carbon fibers coupled with 8-hydroxyquinoline ferric as a reusable Fentonlike catalyst. Chem. Eng. J. 2014, 240, 413–419. [Google Scholar] [CrossRef]
- Fourest, E.; Roux, J.C. Heavy metal biosorption by fungal mycelial by products: Mechanisms and influence of pH. Appl. Microbiol. Biotechnol. 1992, 37, 399–403. [Google Scholar] [CrossRef]
- Lia, Z.Y.; Guo, S.Y.; Li, L. Study on the process, thermodynamical isotherm and mechanism of Cr(III) uptake by Spirulina platensis. J. Food Eng. 2006, 75, 129–136. [Google Scholar] [CrossRef]
- Shi, D.; Cui, B.; Li, L.; Xu, M.; Zhang, Y.; Peng, X.; Zhang, L.; Song, F.; Ji, L. Removal of calcium and magnesium from lithium concentrated solution by solvent extraction method using D2EHPA. Desalination 2020, 479, 114306. [Google Scholar] [CrossRef]
- Figueira, M.M.; Volesky, B.; Mathieu, H.J. Instrumental analysis study of iron species biosorption by Sargassum biomass. Environ. Sci. Technol. 1999, 33, 1840–1846. [Google Scholar] [CrossRef]
- Chen, J.P.; Hong, L.A.; Wu, S.N.; Wang, L. Elucidation of interactions between metal ions and Ca alginate-based ion-exchange resin by spectroscopic analysis and modelling simulation. Langmuir 2002, 18, 9413–9421. [Google Scholar] [CrossRef]
- Mofeed, J.; Mosleh, Y.Y. Toxic responses and antioxidative enzymes activity of Scenedesmus obliquus exposed to fenhexamid and atrazine, alone and in mixture. Ecotoxicol. Environ. Saf. 2013, 95, 234–240. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, W.; Han, M. Biosorption of nickel and copper onto treated alga (Undaria pinnatifida): Application of isotherm and kinetic models. J. Hazard. Mater. 2008, 155, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Amini, M.; Younesi, H.; Bahramifar, N.; Lorestani, A.A.Z.; Ghorbani, F.; Daneshi, A.; Sharifzadeh, M. Application of response surface methodology for optimization of lead biosorption in an aqueous solution by Aspergillus niger. J. Hazard. Mater. 2008, 154, 694–702. [Google Scholar] [CrossRef]
- Habtegebrel, M.M.; Khan, M.A. Removal of Zn (II) and Cu (II) Ions from Aqueous Solution by Dried Prosopis juliflora. Mod. Chem. 2018, 6, 6–14. [Google Scholar] [CrossRef]
- Bouzit, L.; Jbari, N.; El Yousfi, F.; Slimani Alaoui, N.; Chaik, A.; Stitou, M. Adsorption of Fe3+ by a living microalgae biomass of Scenedesmus obliquus. Mediterr. J. Chem. 2018, 7, 156–163. [Google Scholar] [CrossRef]
- Malkoc, E. Ni(II) removal from aqueous solutions using cone biomass of Thuja Orient. J. Hazard. Mater. 2006, 137, 899–908. [Google Scholar] [CrossRef]
- Yu, W.W.; Qu, L.; Guo, W.; Peng, X. Experimental Determination of the Extinction Coefficient of CdTe, CdSe, and CdS Nanocrystals. Chem. Mater. 2003, 15, 2854–2860. [Google Scholar] [CrossRef]
- El Sikaily, A.; El Nemr, A.; Khaled, A.; Abdelwahab, O. Removal of toxic chromium from wastewater using green alga Ulva lactuca and its activated Carbon. J. Hazard. Mater. 2007, 148, 216–228. [Google Scholar] [CrossRef]
- Aravindhan, R.; Fathima, N.N.; Rao, J.R.; Nair, B.U. Equilibrium and thermodynamic studies on the removal of basic black dye using calcium alginate beads. Colloids Surf. 2007, 299, 232–238. [Google Scholar] [CrossRef]
- Nuhoglu, Y.; Oguz, E. Removal of coppe r(II) from aqueous solutions by biosorption on the cone biomass of Thuja orientalis. Prosess Biochem. 2003, 38, 1627–1631. [Google Scholar] [CrossRef]
- Malkoc, E.; Nuhoglu, Y. The Removal of Chromium (VI) From Synthetic Wastewater by Ulothrix zonata. Fresenius Environ. Bull. 2003, 12, 376–381. [Google Scholar]
- Lee, Y.C.; Chang, S.P. The biosorption of heavy metals from aqueous solution by Spirogyra and Cladophora filamentous macroalgae. Bioresour. Technol. 2011, 102, 5297–5304. [Google Scholar] [CrossRef]
- Dorris, K.L.; Yu, B.; Zhang, Y.; Shukla, A.; Shukla, S.S. The removal of heavy metal from aqueous solutions by sawdust adsorption-removal of copper. J. Hazard. Mater. 2000, 80, 33–42. [Google Scholar]
- Abdel-Aty, A.M.; Ammar, N.S.; Abdel-Ghafar, H.H.; Ali, R.K. Biosorption of cadmium and lead from aqueous solution by fresh water alga Anabaena sphaerica biomass. J. Adv. Res. 2013, 4, 367–374. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Kumar, D.; Gaur, J.P. Copper(II) and lead(II) sorption from aqueous solution by non-living Spirogyra neglecta. Bioresour. Technol. 2007, 98, 3622–3629. [Google Scholar] [CrossRef]
- Chang, Y.-C. Microbial Biodegradation of Xenobiotic Compounds; CRC Press: Boca Raton, FL, USA, 2019; p. 248. [Google Scholar]
- Gupta, S.K.; Sriwastav, A.; Ansari, F.A.; Nasr, M.; Nema, A.K. Phycoremediation: An eco-friendly algal technology for bioremediation and bioenergy production. In Phytoremediation Potential of Bioenergy Plants; Bauddh, K., Singh, B., Korstad, J., Eds.; Springer: Singapore, 2017; pp. 431–456. [Google Scholar]
- Aroua, M.K.; Leong, S.P.P.; Teo, L.Y.; Yin, C.Y.; Daud, W.M.A.W. Real time determination of kinetics of adsorption of lead(II) onto palm shell-based activated carbon using ion selective electrode. Bioresour. Technol. 2008, 99, 5786. [Google Scholar] [CrossRef]
- Osinga, T.; Lipinski, W.; Guillot, E.; Olalde, G.; Steinfeld, A. Experimental determination of the extinction coefficient for a packed-bed particulate medium. Exp. Heat Transf. 2006, 19, 69–79. [Google Scholar] [CrossRef]
- Bakatula, E.; Cukrowska, E.; Weiersbye, I.; Mihaly-Cozmuta, L.; Peter, A.; Tutu, H. Biosorption of trace elements from aqueous systems in gold mining sites by the filamentous green algae (Oedogonium sp.). J. Geochem. Explor. 2014, 144, 492–503. [Google Scholar] [CrossRef]
- Chairat, M.; Bremner, J.B. Biosorption of lac dye by the red marine alga Gracilaria tenuistipitata: Biosorption kinetics, isotherms, and thermodynamic parameters. Coloration Technol. 2016, 132, 472–480. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.K.; Rastogi, A.; Nayak, A. Biosorption of nickel onto treated alga (Oedogonium hatei): Application of isotherm and kinetic models. J. Colloid Interface Sci. 2010, 342, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Balarak, D.; Azarpira, H.; Mostafapour, F.K. Thermodynamics of removal of cadmium by adsorption on Barley husk biomass. Pharma Chem. 2016, 8, 243–247. [Google Scholar]
- Mwandira, W.; Nakashima, K.; Kawasaki, S.; Arabelo, A.; Banda, K.; Nyambe, I.; Chirwa, M.; Ito, M.; Sato, T.; Igarashi, T.; et al. Biosorption of Pb (II) and Zn (II) from aqueous solution by Oceanobacillus profundus isolated from an abandoned mine. Sci. Rep. 2020, 10, 21189. [Google Scholar] [CrossRef]
- Bano, A.; Hussain, J.; Akbar, A.; Mehmood, K.; Anwar, M.; Hasni, M.S.; Ullah, S.; Sajid, S.; Ali, I. Biosorption of heavy metals by obligate halophilic fungi. Chemosphere 2018, 199, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Aksu, Z. Application of biosorption for the removal of organics pollutants: A review. Process Biochem. 2005, 40, 997–1026. [Google Scholar] [CrossRef]
- Ali, M.H.; Abd Elkarim, M.S.; Haroun, S.A.; Attwa, K.M. Bioremediation of Fe, Zn and Cd ions from aqueous solution using died cells of cyanobacterial mats from extreme habitat, Siwa Oasis, Egypt. Egypt. J. Aquat. Biol. Fish. 2018, 22, 511–522. [Google Scholar] [CrossRef] [Green Version]
- Adekola, F.A.; Hodonou, D.S.S.; Adegoke, H.I. Thermodynamic and kinetic studies of biosorption of iron and manganese from aqueous medium using rice husk ash. Appl. Water Sci. 2016, 6, 319–330. [Google Scholar] [CrossRef] [Green Version]
- Bina, B.; Kermani, M.; Movahedian, H.; Khazaei, Z. Biosorption and Recovery of Copper and Zinc from Aqueous Solutions by Nonliving Biomass of Marine Brown Algae of Sargassum sp. Pak. J. Biol. Sci. 2006, 9, 1525–1530. [Google Scholar] [CrossRef] [Green Version]
- Shaaban, A.M.; Badawy, R.K.; Mansour, H.A.; Mohamed, E.; Abdel-Rahman, M.E.; Yasmin, I.E.; Aboulsoud, Y.I.E. Competitive algal biosorption of Al3+, Fe3+, and Zn2+ and treatment application of some industrial effluents from Borg El-Arab region. Egypt. J. Appl. Phycol. 2017, 29, 3221–3234. [Google Scholar] [CrossRef]
- Chen, F.; Chen, B.; Gan, G.; Zhang, L.; Liang, Y.; Wu, S. Biosorption of Zn+2 and Ni+2, Cu+2 by Four Kinds of Active Microalgae. J. Mater. Sci. Chem. Eng. 2016, 4, 12–19. [Google Scholar] [CrossRef]
- El-Naggar, N.E.A.; Hamouda, R.A.; Mousa, I.E.; Abdel-Hamid, M.S.; Rabei, N.H. Statistical optimization for cadmium removal using Ulva fasciata biomass: Characterization, immobilization and application for almost-complete cadmium removal from aqueous solutions. Sci. Rep. 2018, 8, 12456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esmaeili, A.; Ghasemi, S. Evaluation of the Activated Carbon Prepared of Algae Marine Gracilaria for the Biosorption of Ni (II) from Aqueous Solutions. World Appl. Sci. J. 2009, 6, 515–518. [Google Scholar]
- Cheng, J.; Yin, W.; Chang, Z.; Lundholm, N.; Jiang, Z. Biosorption capacity and kinetics of cadmium (II) on live and dead Chlorella vulgaris. J. Appl. Phycol. 2017, 29, 211–221. [Google Scholar] [CrossRef]
- Li, Y.; Xia, B.; Zhao, Q.; Liu, F.; Du, Q.; Wang, D.; Li, D.; Wang, Z.; Zhang, P.; Xia, Y. Removal of copper ions from aqueous solution by calcium alginate immobilized kaolin. J. Environ. Sci. 2011, 23, 404–411. [Google Scholar] [CrossRef]
- Zaib, M.; Makshoof Athar, M.; Saeed, A.; Farooq, U.; Salman, M.; Nouman Makshoof, M. Equilibrium, kinetic and thermodynamic biosorption studies of Hg(II) on red algal biomass of Porphyridium cruentum. Green Chem. Lett. Rev. 2016, 9, 179–189. [Google Scholar] [CrossRef] [Green Version]
- Su-Hsia, L.; Ruey-Shin, J. Adsorption of phenol and its derivatives from water using synthetic resins and low-cost natural adsorbents: A review. J. Environ. Manag. 2009, 90, 1336–1349. [Google Scholar]
- Arecoa, M.M.; Hanelab, S.; Duranb, J.; dos Santos, A.M. Biosorption of Cu(II), Zn(II), Cd(II) and Pb(II) by dead biomasses of green alga Ulva lactuca and the development of a sustainable matrix for adsorption implementation. J. Hazard. Mater. 2012, 213, 123–132. [Google Scholar] [CrossRef]
- Kumar, Y.P.; King, P.; Prasad, V.S.R.K. Adsorption of zinc from aqueous solution using marine green algae—Ulva fasciata sp. Chem. Eng. J. 2007, 129, 161–166. [Google Scholar] [CrossRef]
- Anilkumar, B.; Chitti, B.N.; Kavitha, G. Biosorption of Zinc on to Gracilaria Corticata (Red Algae) Powder and Optimization using Central Composite Design. J. Appl. Sci. Eng. Methodol. 2016, 2, 412–425. [Google Scholar]
- Benaisa, S.B.; Arhoun, B.; El Mail, R.; Rodriguez-Maroto, J.M. Potential of brown algae biomass as new biosorbent of Iron: Kinetic, equilibrium and thermodynamic study. J. Mater. Environ. Sci. 2018, 9, 2131–2141. [Google Scholar]
- Liu, Y.; Cao, Q.; Luo, F.; Chen, J. Biosorption of Cd2+, Cu2+, Ni2+ and Zn2+ ions from aqueous solutions by pretreated biomass of brown algae. J. Hazard. Mater. 2009, 163, 931–938. [Google Scholar] [CrossRef] [PubMed]
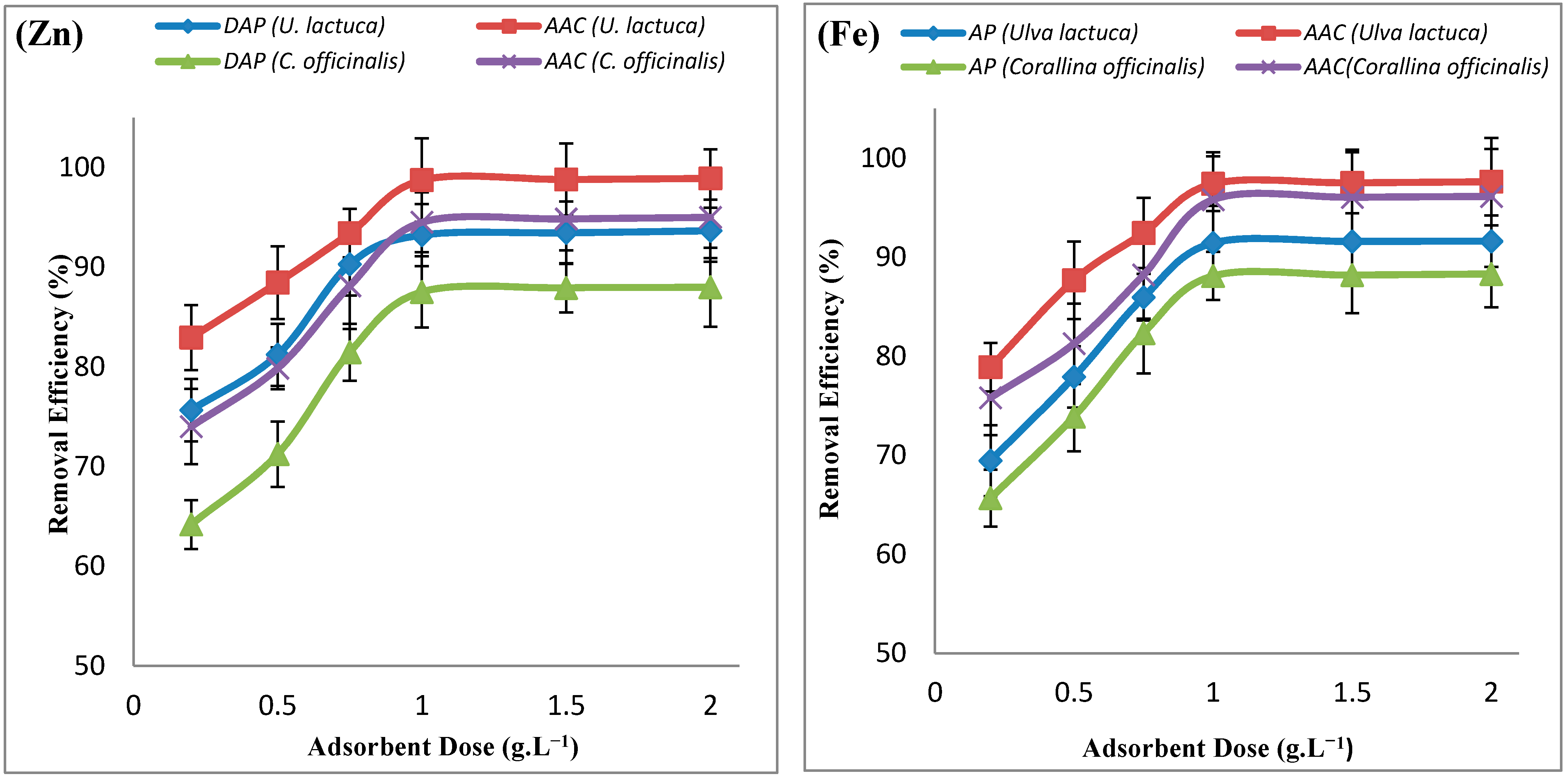
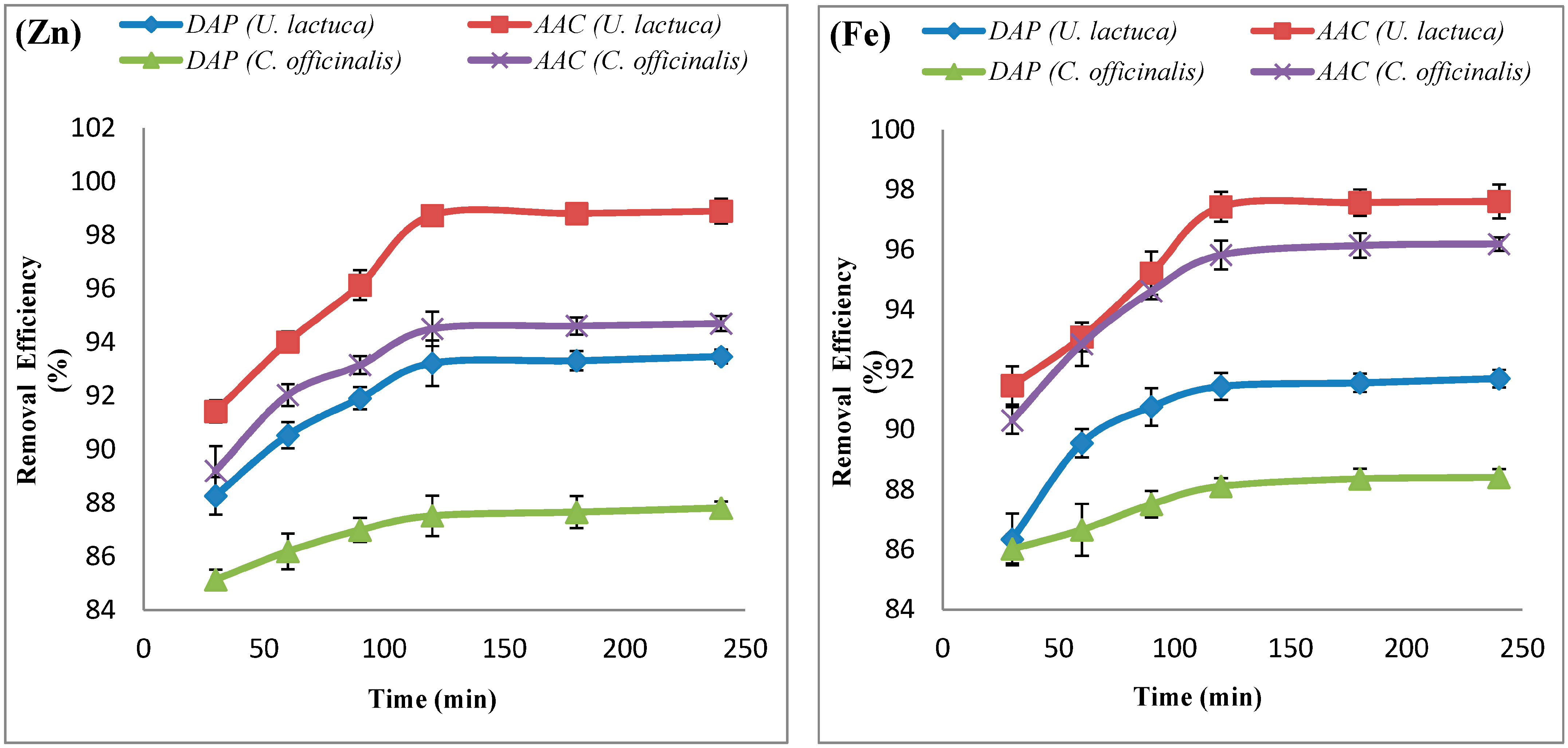

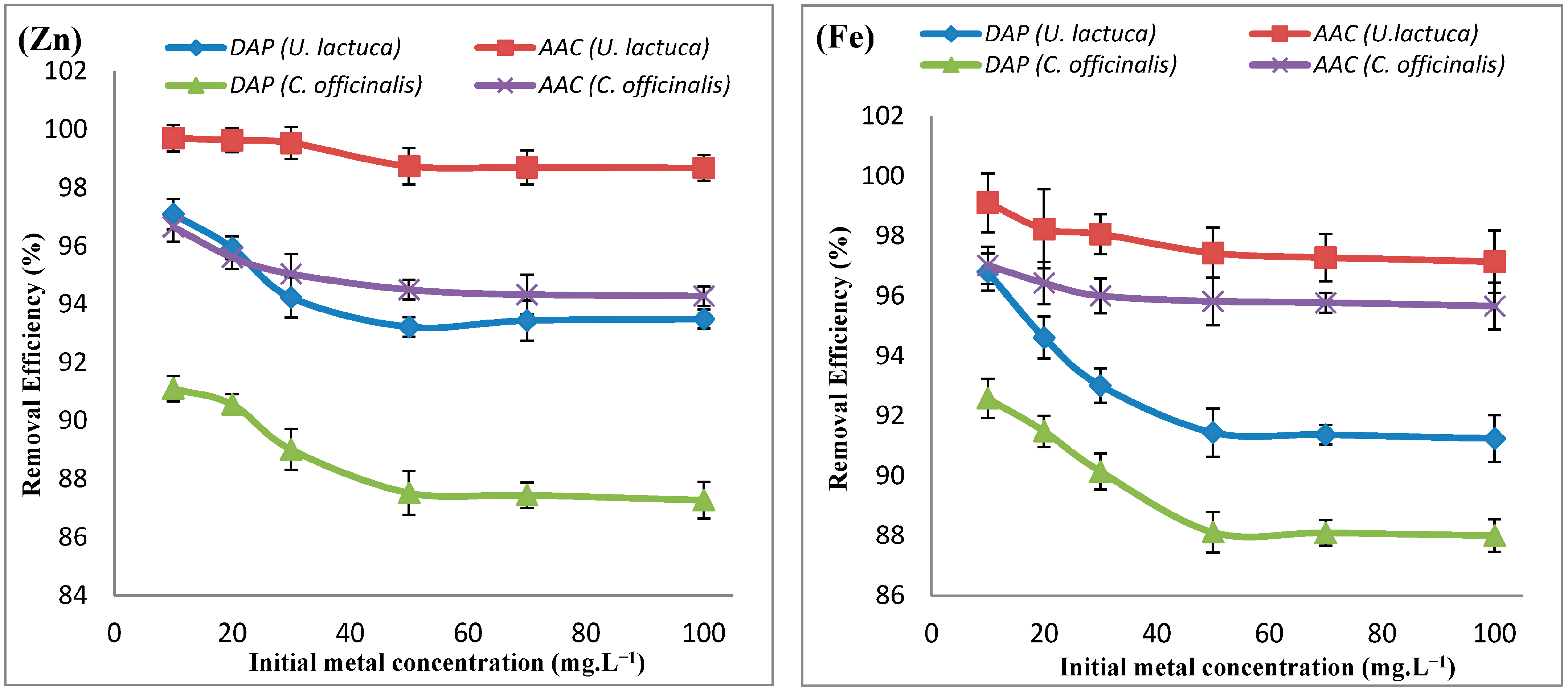
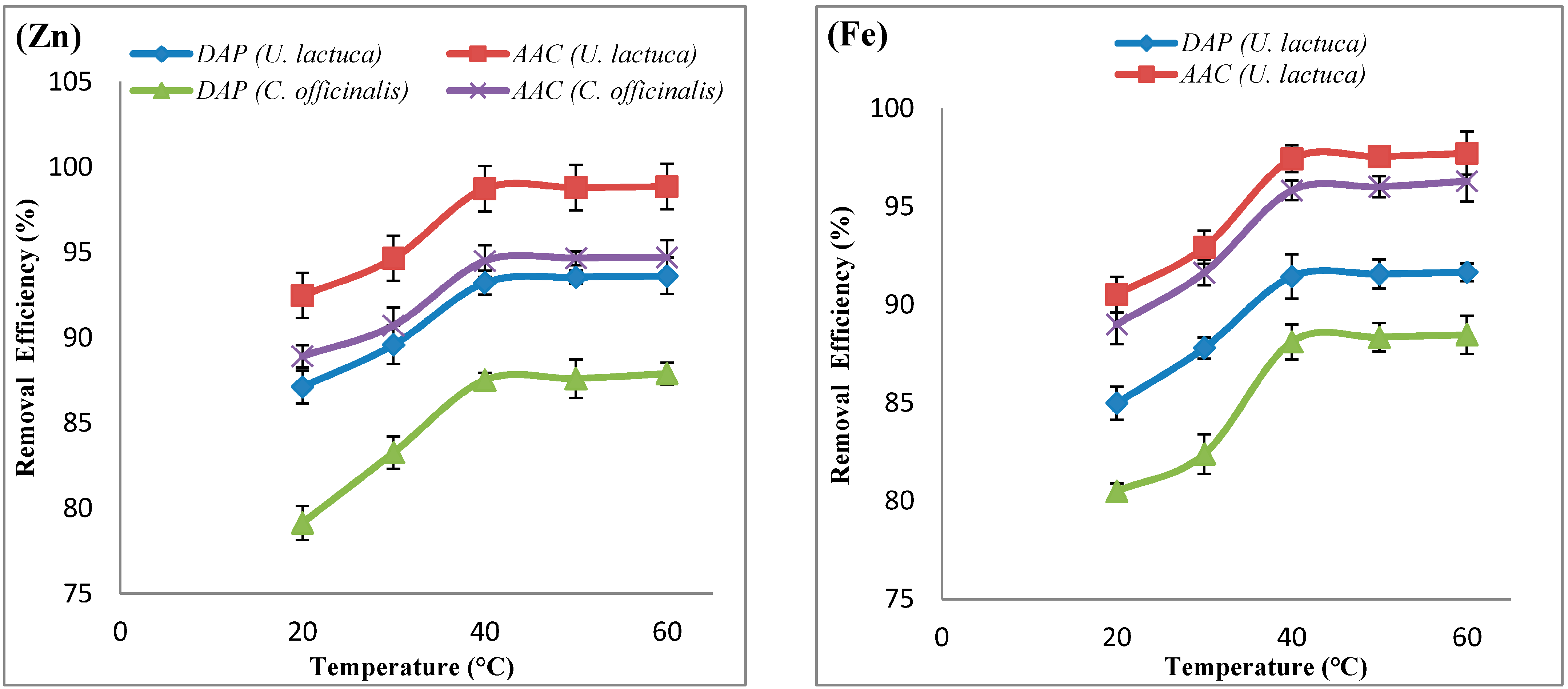
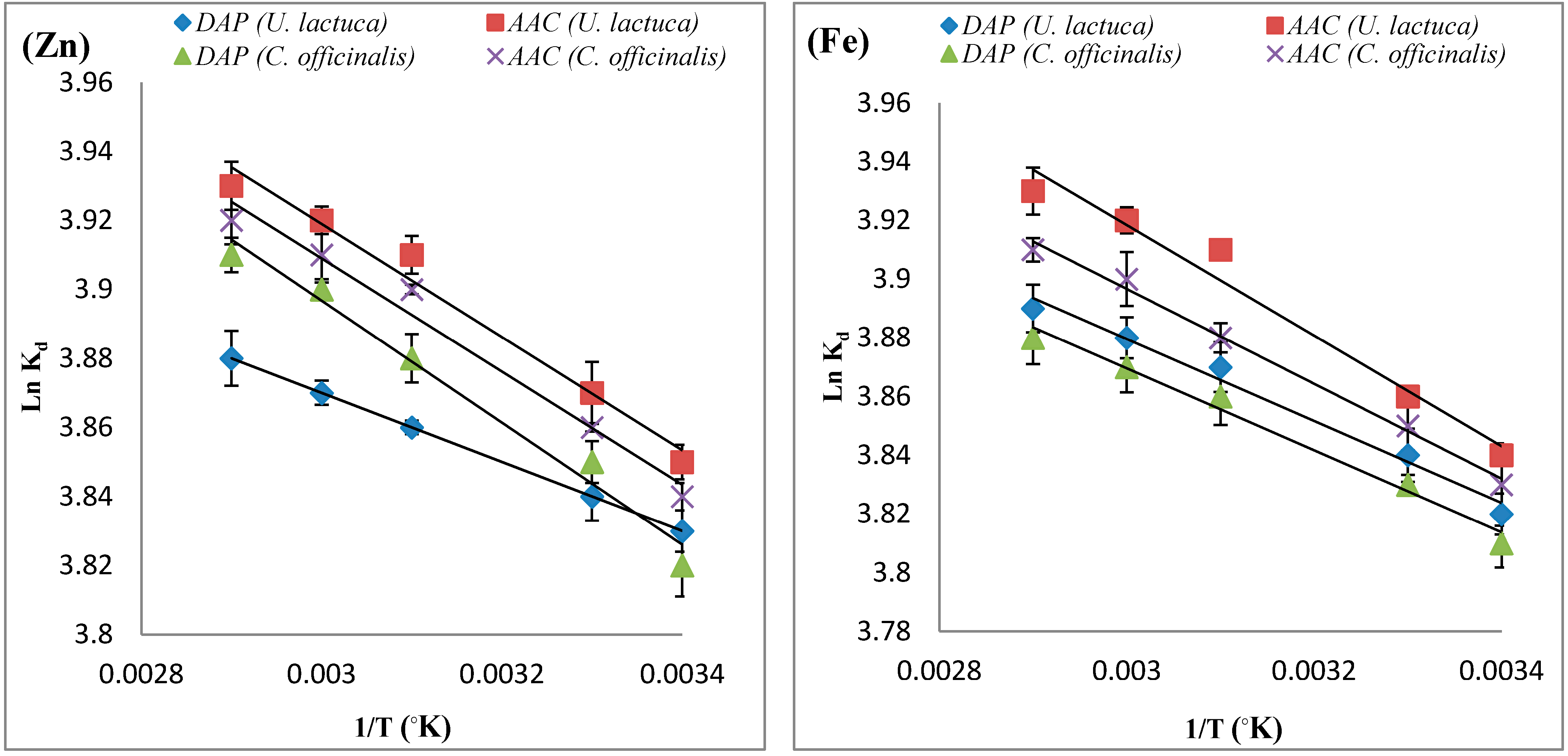
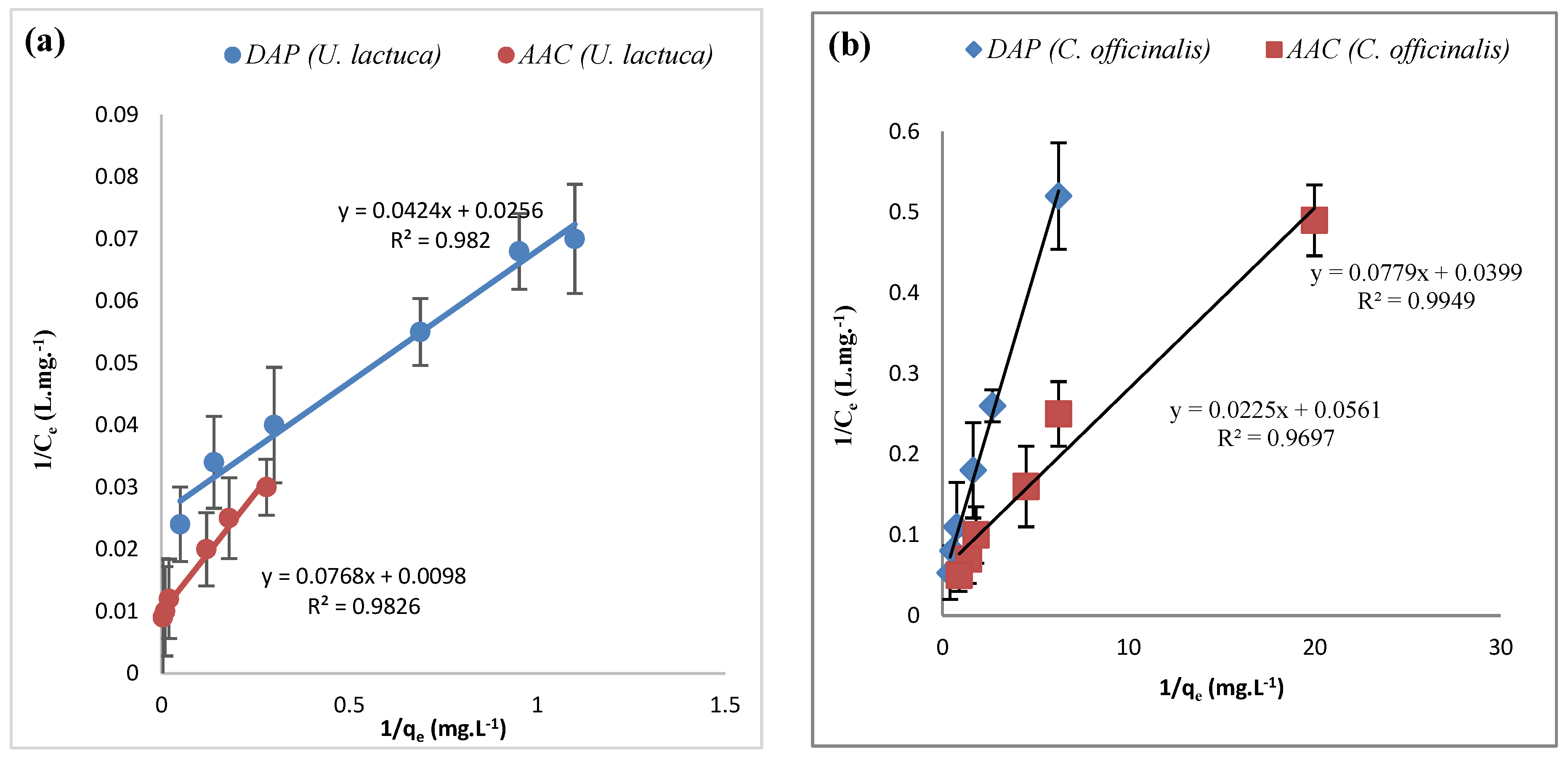
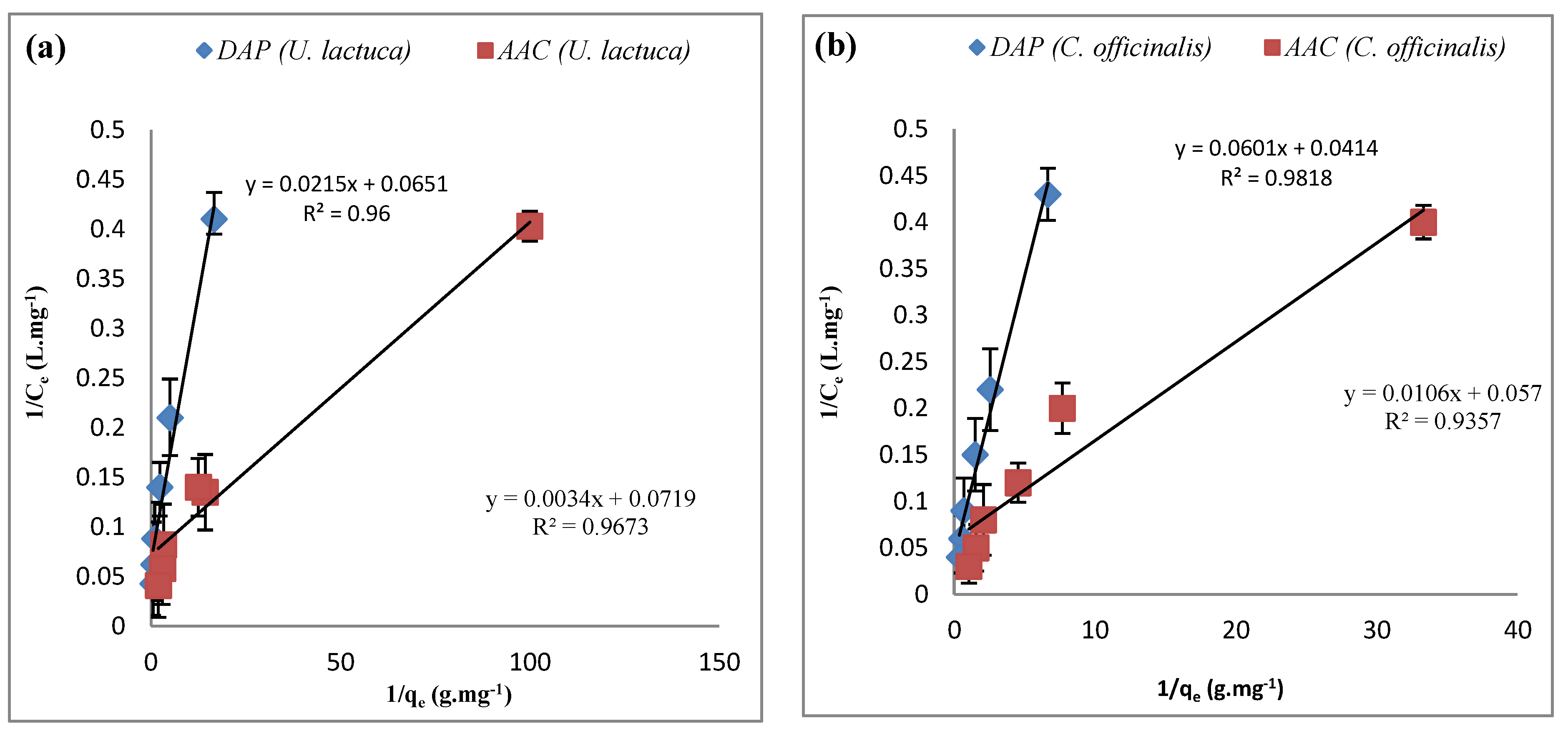
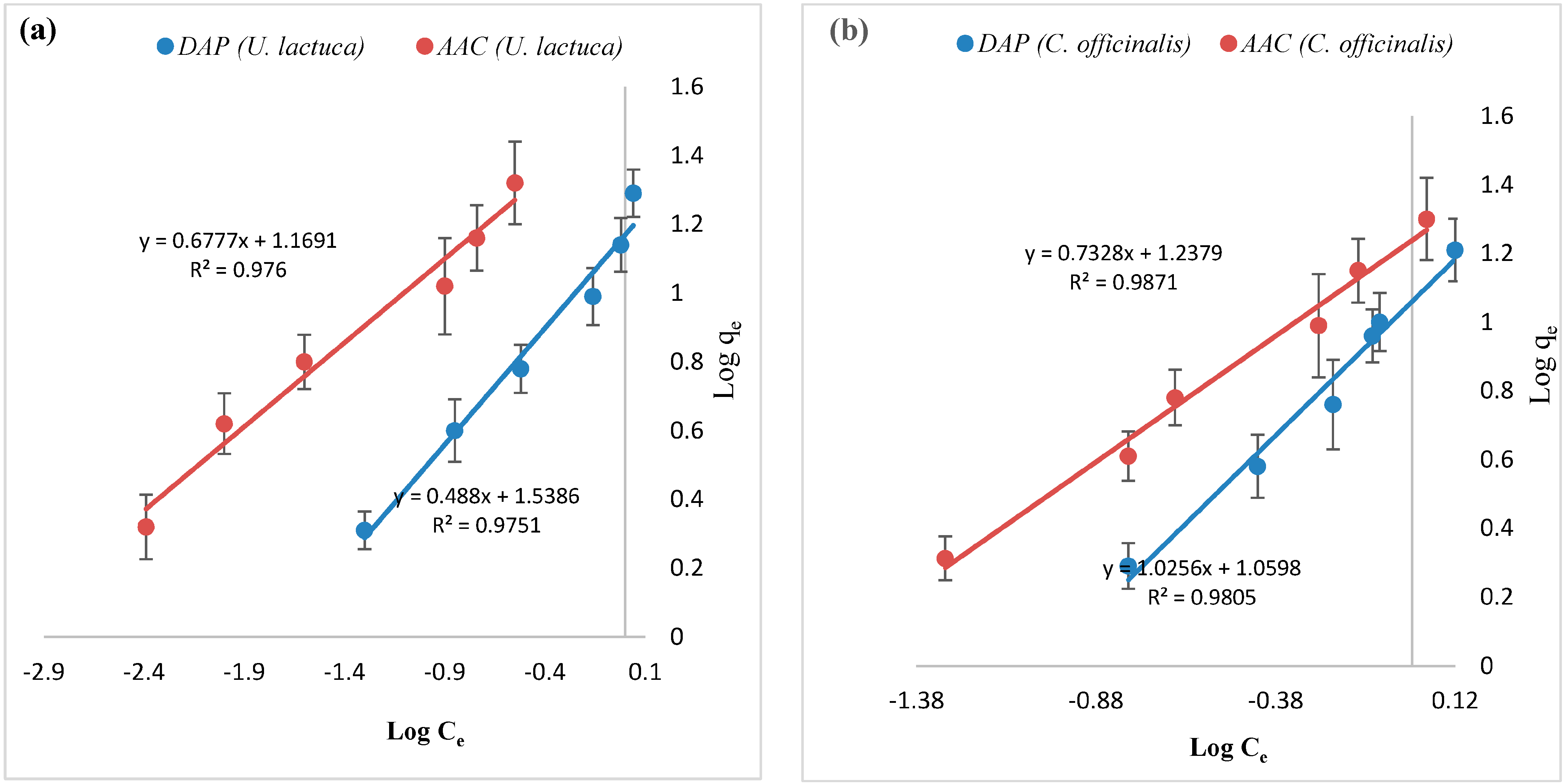
| Adsorbate | Adsorbent | ΔH° (KJ.mol−1) | ΔS° (KJ.mol−1) | ΔG° (KJ.mol−1) | ||||
|---|---|---|---|---|---|---|---|---|
| T1 (20 °C) | T2 (30 °C) | T3 (40 °C) | T4 (50 °C) | T5 (60 °C) | ||||
| Zn | DAP U. lactuca | 0.83 | 0.0340 | −9.13 | −9.47 | −9.81 | −10.15 | −10.49 |
| ACC U. lactuca | 1.37 | 0.0372 | −9.47 | −9.84 | −10.21 | −10.58 | −10.95 | |
| DAP C. officinalis | 1.72 | 0.0384 | −9.41 | −9.79 | −10.17 | −10.55 | −10.93 | |
| ACC C. officinalis | 1.36 | 0.0360 | −9.35 | −9.54 | −9.9 | −10.26 | −10.62 | |
| Fe | DAP U. lactuca | 1.15 | 0.0357 | −9.31 | −9.66 | −10.07 | −10.38 | −10.73 |
| ACC U. lactuca | 1.57 | 0.0372 | −9.32 | −9.70 | −9.95 | −10.44 | −10.81 | |
| DAP C. officinalis | 1.16 | 0.0355 | −9.24 | −9.59 | −9.74 | −10.30 | −10.66 | |
| ACC C. officinalis | 1.34 | 0.0350 | −9.03 | −9.38 | −10.07 | −10.09 | −10.44 | |
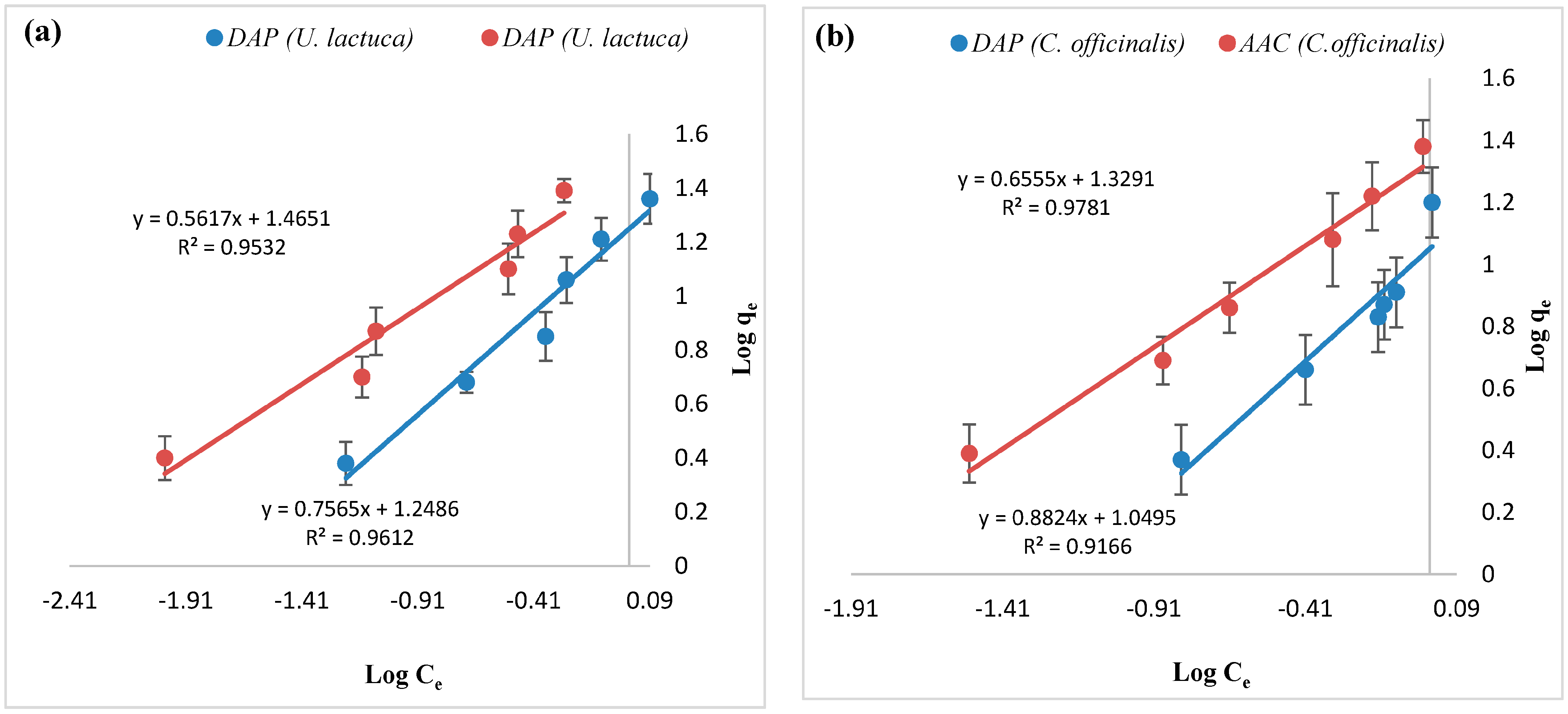
| Metal Ions | Biosorbent | Langmuir Constants | Freundlich Constants | ||||
|---|---|---|---|---|---|---|---|
| qmax (mg.g−1) | b (l.mg−1) | R2 | Kf (mg.g−1) | 1/n | R2 | ||
| Zn+2 | DAP U. lactuca | 23.5 | 1.65 | 0.982 | 14.6 | 0.677 | 0.976 |
| AAC U. lactuca | 13.0 | 7.83 | 0.982 | 34.5 | 0.488 | 0.975 | |
| DAP C. officinalis | 12.8 | 1.95 | 0.987 | 11.4 | 1.02 | 0.980 | |
| AAC C. officinalis | 44.4 | 0.401 | 0.981 | 13.4 | 0.732 | 0.963 | |
| Fe+3 | DAP U. lactuca | 46.5 | 0.330 | 0.981 | 17.7 | 0.756 | 0.961 |
| AAC U. lactuca | 294 | 0.047 | 0.994 | 29.2 | 0.561 | 0.953 | |
| DAP C. officinalis | 16.6 | 1.45 | 0.979 | 11.2 | 0.882 | 0.916 | |
| AAC C. officinalis | 94.3 | 0.185 | 0.998 | 21.3 | 0.655 | 0.978 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ameen, M.M.; Moustafa, A.A.; Mofeed, J.; Hasnaoui, M.; Olanrewaju, O.S.; Lazzaro, U.; Guerriero, G. Factors Affecting Efficiency of Biosorption of Fe (III) and Zn (II) by Ulva lactuca and Corallina officinalis and Their Activated Carbons. Water 2021, 13, 3421. https://doi.org/10.3390/w13233421
Ameen MM, Moustafa AA, Mofeed J, Hasnaoui M, Olanrewaju OS, Lazzaro U, Guerriero G. Factors Affecting Efficiency of Biosorption of Fe (III) and Zn (II) by Ulva lactuca and Corallina officinalis and Their Activated Carbons. Water. 2021; 13(23):3421. https://doi.org/10.3390/w13233421
Chicago/Turabian StyleAmeen, Mahy M., Abdelraouf A. Moustafa, Jelan Mofeed, Mustapha Hasnaoui, Oladokun Sulaiman Olanrewaju, Umberto Lazzaro, and Giulia Guerriero. 2021. "Factors Affecting Efficiency of Biosorption of Fe (III) and Zn (II) by Ulva lactuca and Corallina officinalis and Their Activated Carbons" Water 13, no. 23: 3421. https://doi.org/10.3390/w13233421








