Abstract
Reservoirs are dynamic ecosystems subject to different pressures that influence and compromise their ecological structure. The main objective of this study was to evaluate the potential of using the macroinvertebrate to assess the water quality of four reservoirs (one site in Miranda—M and Pocinho—P; four sites in Aguieira—Ag1 to Ag4; and five sites in Alqueva—Al1 to Al5). The sites were sampled in autumn 2018 (A18), spring and autumn 2019 (S19 and A19) and spring 2020 (S20). In situ physical and chemical parameters were measured and a sample of water and macroinvertebrate were collected for further analyses. Total phosphorus exceeded the allowed concentrations (maximum values recorded: M—0.13 mg/L, P—0.09 mg/L, Ag3—0.22 mg/L and Al5—0.18 mg/L). Total abundance varied between 4 and 3088. Taxonomic richness was always low, between 1 and 12 taxa. The highest Shannon–Wiener value (1.91) was recorded in Ag1_A18 and Al2_A18. Pielou’s evenness varied widely across all reservoirs, from 0.06 to 0.92. Almost all the organisms found were associated with polluted water, according to the index ratings. Organisms tolerant of disturbances (e.g., Chironomidae and Oligochaeta) were associated with sites with the worst water quality, according to the Water Framework Directive (WFD), (M, Ag3, Ag4 and Al5) while organisms with moderate tolerance to disturbances (e.g., Cordullidae and Polycentropodidae) were associated with sites with better water quality (P, Ag1, Ag2 and Al1 to Al4). The macrozoobenthos index (MZB) used proved to be a sensitive tool to Portuguese reservoirs, corroborating most of the results obtained in the remaining analyses, as well as providing a clear ecological potential complementing the analysis carried out by the WFD. Based on this, the macroinvertebrate community appeared to be sensitive and able to characterize the reservoirs’ water quality.
1. Introduction
Over the past few years, a growing concern with the decrease of freshwater quality worldwide has been acknowledged [1,2,3]. Indeed, ensuring the waterbody quality is essential to preserve the ecosystem and conserve the natural structure, function, and dynamics [4] to guarantee the maintenance of the ecological balance [5]. The construction of dams in lotic ecosystems promotes the creation of lentic water bodies upstream (identified as heavily modified and artificial water bodies, such as reservoirs). This latter ecosystem allows the regulation of water levels for flood control, water consumption, irrigation, and hydroelectricity production. However, reservoirs can present low water quality due to changes in the nutrient cycles, promoting the increase of organic matter, nutrient and sediment accumulation, seasonal variations of oxygen and temperature distribution, variable water retention times and, consequently, a decrease of the current velocity and water level fluctuation [6,7,8,9]. Even more, different sedimentological structures may be settled along the reservoir due to the new physical conditions. The variability in loads of dissolved or particulate solids, for example, typically reflects geological variations, use of the adjacent land and precipitation [10]. These interferences change the system morphometry, its physical and chemical characteristics and the functioning and structure of biological communities, namely loss of biodiversity and the loss of ecosystem functions (e.g., less nutrient recycling) [8,11,12], affecting bacterioplankton [13,14], phytoplankton [15,16], zooplankton [17,18], benthic macroinvertebrates [19,20,21,22], fish and macrophytes [10].
The Water Framework Directive 2000/60/EC (WFD) is European legislation affecting water policy and quality [23], which established that each member must protect, improve, and recover all water bodies to attain a good ecological status. According to the WFD approach, the assessment of lentic ecosystems presents a great lack of biological parameters, with phytoplankton being the only parameter historically used. Several authors have already mentioned this scarcity of biological parameters, suggesting other communities (e.g., bacterial, benthic macroinvertebrate and zooplankton) as potential indicators of water quality for lentic ecosystems [14,17,21]. The benthic macroinvertebrate community includes organisms that live or present part of their life cycle on the bottom substrates of a water body [24]. Their distribution is strongly influenced by physical and chemical factors and the type of food available [24,25]. For example, the Chironomidae resist at low concentrations of dissolved oxygen, while the presence of Ephemeroptera, Plecoptera and Trichoptera can be indicative of low anthropic impact environments, being affected by the type of substrates, the heterogeneity of habitats, the current velocity, and the availability of trophic resources [25]. The benthic macroinvertebrate community plays an important role in benthic and pelagic food webs and the cycle of organic matter and energy flow [20]. In lotic ecosystems (e.g., rivers), there is a great taxonomic and functional diversity of benthic macroinvertebrates, which allows the development of metrics for the assessment of the ecological status of these water bodies [26]. In rivers, this community has been considered a good indicator for the assessment of the water quality [20,27]. Furthermore, several authors propose that the benthic macroinvertebrate community can also be a good indicator to assess the water quality in lentic ecosystems [21,28]. However, for reservoirs, the macroinvertebrate community is poorly studied.
The benthic macroinvertebrate community is an important bioindicator used by WFD, namely in river assessment. Furthermore, it is one of the biological communities that the WFD intends to use for the assessment of reservoir water quality, although it is not used yet. In reservoirs, this community tends to be less complex, with reduced diversity and abundance [26]. These previous features tend to reflect the adverse environmental conditions of the reservoirs for these communities [26]. For example, the existence of fluctuation and amplitude of the water level, in the area exposed by lowering, leads to a decrease in diversity and abundance [20,21,27,29,30]. On the other hand, organisms with shorter life cycles and high mobility tend to be able to survive in these conditions, even with high amplitudes of water levels [21]. Thus, the heterogeneity of the types of substrates tends to increase the number of benthic taxa, since a heterogeneous environment affords a greater number of niches [22]. The benthic macroinvertebrate community also tends to vary with depth, generally being more diverse and abundant close to the reservoir margins than in deeper waters [20].
Considering this background, our main objective was to assess whether the benthic macroinvertebrate community can be a good indicator for assessing the ecological potential of heavily modified and artificial water bodies (Miranda, Pocinho, Aguieira, and Alqueva reservoirs). For doing this, the composition (e.g., abundance, diversity, richness and evenness) of benthic macroinvertebrates was analysed to assess the water quality of the target reservoirs. Additionally in each reservoir, specific physical, chemical and biological parameters (phytoplankton Ecological Quality Ratio—EQR) proposed by WFD were quantified and analysed.
2. Materials and Methods
2.1. Study Areas
The present study was conducted in four reservoirs located throughout Portugal: Miranda (M) and Pocinho (P) in the hydrographic basin of the Douro River, Aguieira (Ag) in the hydrographic basin of the Mondego River, and Alqueva (Al) in the hydrographic basin of the Guadiana River (Figure 1). The selection of reservoirs and respective study sites were defined based on previous studies in these areas by our work team [2,31,32,33], with pressures and several other factors already documented (for more detailed information please see [2,31,32,33]).
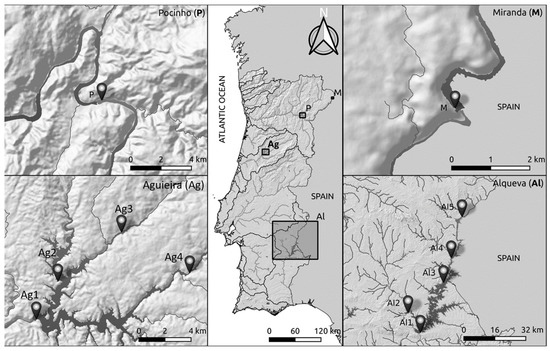
Figure 1.
Location of the sampling sites in Miranda (M—41°29′24.802″ N, 6°15′55.925″ W), Pocinho (P—41°08′10.884″ N, 7°06′39.074″ W), Aguieira (Ag1—40°20′27.942″ N, 8°11′38.616″ W, Ag2—40°22′01.884″ N, 8°10′28.283″ W, Ag3—40°24′03.488″ N, 8°07′01.150″ W and Ag4—40°22′22.256″ N, 8°03′19.055″ W) and Alqueva (Al1—38°12′07.957″ N, 7°29′19.717″ W, Al2—38°17′35.785″ N, 7°33′41.484″ W, Al3—38°25′58.085″ N, 7°21′03.721″ W, Al4—38°32′49.092″ N, 7°18′13.988″ W and Al5—38°44′15.763″ N, 7°14′15.144″ W).
Miranda reservoir, the first reservoir on the international stretch (bordering Spain) of the Douro River (Figure 1), started operating in 1960. It is the smallest reservoir of this study with an area of 1 km2 [34]. This reservoir tends to present low water level fluctuations (≤1 m) with no trend along the year [35]. According to the 2nd Planning Cycle (2016–2021), Miranda reservoir presented a moderate ecological potential [36]. Pocinho reservoir is the first reservoir on the national stretch of the Douro River (Figure 1), which started operating in 1983 and occupies an area of 8 Km2 [34]. This reservoir also tends to present low water level fluctuations (≤1 m) with no trend along the year [35]. According to the 2nd Planning Cycle (2016–2021), Pocinho reservoir also presented a moderate ecological potential [37]. The Aguieira reservoir is inserted in the Mondego River in the Coimbra district (Figure 1) at the confluence of two secondary rivers, Dão and Criz. This reservoir started operating in 1981 and occupies an area of 20 km2 and was included in the WFD intercalibration exercise [38]. This reservoir presents huge water level fluctuations (≤16 m), often reaching the minimum quota limit in early autumn and the maximum quota limit in early spring [35]. According to the 2nd Planning Cycle (2016–2021), the Aguieira reservoir presented a poor ecological potential [39]. The Alqueva reservoir is located in the Guadiana River, in the Alentejo region (Figure 1). Alqueva is the largest artificial lake in Europe and the most recent reservoir, operating since 2004, with an area of 250 km2 [40]. This reservoir presents high water level fluctuations (≤4 m), reaching the minimum and maximum quota limits at the same time of year as Aguieira [35]. According to the 2nd Planning Cycle (2016–2021), the Alqueva reservoir presented a moderate ecological potential [41].
One site in Miranda (M), one site in Pocinho (P), four sites in Aguieira (Ag1, Ag2, Ag3 and Ag4) and five sites in Alqueva reservoirs (Al1, Al2, Al3, Al4 and Al5) were selected to conduct the current study (Figure 1). These sampling sites were selected based on the dimension of the reservoir, accessibility conditions and types of pressure across reservoirs (e.g., agriculture, industry). Furthermore, this selection considered the existing monitoring stations defined by the national agency responsible for the water monitoring program of each reservoir. Four sampling periods were defined to conduct this study: the end of October in autumn of 2018 (A18), May in spring of 2019 (S19), the end of October in autumn of 2019 (A19) and May in spring of 2020 (S20).
2.2. Sampling Methods and Analyses
In each site, a few meters of the margin (with accessibility on foot), several sub-superficially (<0.50 m depth) physical and chemical parameters were measured in water: pH, conductivity (μS/cm), temperature (°C) and dissolved oxygen (mg/L and %), using a multiparameter probe (Multi 3630 IDS SET F). Additionally, water samples (1 L) were collected and transported to the laboratory at 4 °C and under dark conditions for further analyses.
In the laboratory, the concentration of the following compounds was determined using chromatographic methods. Concentrations of nitrites and nitrates (mg/L) were quantified by liquid chromatography of ions [42]. The total Kjeldahl nitrogen (mg/L) concentration was obtained using the Kjeldahl nitrogen method [43]. The total phosphorus (mg/L) concentration was determined by the application of inductively coupled plasma mass spectrometry [44]. Ammonium (mg/L) concentration was quantified by spectrophotometric and turbidimetric analyses [45], and the content of dissolved organic carbon was determined indirectly through the colour of the water (Coloured Dissolved Organic Carbon (CDOC)) [46]. Turbidity was obtained using a spectrophotometer method using the protocol of Brower et al. [47].
The phytoplankton sampling and analysis were carried out according to the methodology described in [2,48,49]. Only the final values of the Ecological Quality Ratio (EQR) were considered for the interpretation of the results.
Macroinvertebrate community samples were collected using a hand net (0.5 mm of mesh; 0.5 m of length) according to standard procedures [50]. In each site, three drags were performed along one meter of the substrate (essentially sands, gravel, and rocks) and vegetation, resulting in a composite sample. The samples were preserved in formaldehyde 4%. In the laboratory, the samples were screened with tweezers and the organisms were conserved in ethanol 96%. The identification procedure was conducted in a binocular stereoscope and all organisms were identified up to the family taxonomic group, consistent with the taxonomic level used for specific indexes in Europe [51,52]. Oligochaeta was identified up to the subclass using Tachet et al. [53] as a dichotomous identification key.
2.3. Statistical Analysis
According to the WFD [23], the physical and chemical parameters are characterized in two groups, taking into account the reference value: good (green) and moderate (yellow) ecological potential. The biological parameter can be characterized in two forms according to the typology of the reservoir: (i) good or more (green) and moderate or less (yellow) ecological potential for the main course reservoir; and (ii) good or more (green), moderate (yellow), poor (orange) and bad (red) ecological potential for the north reservoirs.
The macroinvertebrate community was characterized regarding abundances, taxonomic richness, the number of families belonging to the orders Ephemeroptera, Plecoptera, Trichoptera (EPT). Shannon–Wiener index was used to estimate the diversity and Pielou’s index was used to estimate the evenness of each sample. The macrozoobenthos index for dam reservoirs (MZB) was applied at all samples, which focuses on the Average Score Per Taxon (AST) and Margalef Diversity Index recorded in the macroinvertebrates community. The results are classified into five groups: excellent (blue), good (green), moderate (yellow) poor (orange) and bad (red), according to [54].
For each hydrographic basin, clustering patterns were achieved by the construction of Complete Linkage dendrograms, based on the Bray-Curtis coefficient, using Primer software v7.0.11. To perceive associations between the environmental variables and the macroinvertebrates communities, a Canonical Correspondence Analysis (CCA) was conducted using CANOCO 4.5® software. Previously, before the latter analysis, environmental variables were standardized, and redundant variables were removed for the analysis.
3. Results and Discussion
3.1. Water Chemistry
Table 1 presents the values of physical, chemical, and biological parameters measured in each site over the sampling period. In general, the four reservoirs tended to present neutral/alkaline pH, with values between 6.7 and 9.7, being the highest values recorded in spring samples. The Aguieira reservoir presented low conductivity values in almost all sites (<100 μS/cm), whereas Miranda and Pocinho reservoirs (>250 μS/cm) and Alqueva reservoir (>500 μS/cm) presented the highest values. The temperature fluctuated considerably throughout the sampling periods, ranging from 9.4 to 33.3 °C. Dissolved oxygen (O2) also fluctuated considerably throughout the sampling periods, showing higher values in the spring samples in all reservoirs. CDOC values were generally low throughout the sampling period, with higher values in P_A18, in Ag3_A18, in all sites of Aguieira in S19, Al2 and Al5 of A18, in all sites of Alqueva in S19 and Al4 and Al5 of S20 (Table 1). Turbidity had generally low values in all sampling periods. The highest value was observed in Ag3_A18. In general, nutrient concentrations were below the detection level (Table 1), which reflects the low concentrations of nutrients recorded. Nitrates (NO3−) and total phosphorus (Ptotal) presented the highest concentrations in the upstream sites (Ag3, Ag4 and Al5). Moreover, nitrate (NO3−) and nitrite (NO2−) concentrations showed seasonality in the Aguieira reservoir with higher values recorded in the spring sampling.

Table 1.
Physical and chemical parameters (FQ): pH, conductivity (Cond), temperature (Temp), dissolved oxygen (O2) dissolved organic carbon (CDOC), turbidity (Turb), nitrites (NO2−), nitrates (NO3−), total Kjeldahl nitrogen (NKj), total phosphorus (Ptotal) and ammonium (NH4); and the biological parameter (BIO): phytoplankton (Ecological Quality Ratio EQR) for each site and reservoir along the study periods. The highlighted values (in bold) represent the values outside the environmental quality standards. * stands for reference conditions in Miranda, Pocinho and Aguieira reservoirs; ** stands for reference conditions in Alqueva reservoir; (1) [49].
Regarding the threshold values for environmental quality standards according to the WFD, some of the physical and chemical parameters surpassed the thresholds established [23]. The O2 (Table 1), in M_A18, and Ag1 and Ag2 of Aguieira in A19, presented values above the environmental quality standards (O2 of <5 mg/L and 60%) which means that these water bodies, in these periods, are in moderate conditions of environmental quality. Furthermore, Ptotal (Table 1) tends to be the most problematic parameter, exceeding the maximum allowed concentrations according to the environmental quality standard values (≤0.05 mg/L for North reservoir [Miranda, Pocinho and Aguieira] and ≤0.07 mg/L for south reservoir [Alqueva]) in M of A18 and S20, P of A18 and S20, Ag3 of four samplings, Al4_A18 and Al5 of A18, S19 and S20. The values recorded of pH agreed with the environmental quality standards, while the concentration of NO3− was always below the maximum allowed limit.
According to the evaluation of the physical and chemical parameters, the quality of the reservoirs tends to show low water quality, especially in the autumn sampling period. All reservoirs are located in areas exposed to urban and livestock effluent discharges and agricultural runoff with high use of fertilizers that can cause the accumulation of nutrients such as Ptotal and NKj in the reservoir, as observed in Pérez et al. (2010) along the Alqueva reservoir and in the Agência Portuguesa do Ambiente [52] along the Aguieira reservoir. Moreover, all reservoirs were characterized as eutrophic, especially due to high concentrations of Ptotal recorded over the last few years [34,55,56,57], a condition also observed in the present study. Sande-Fouz et al. [58], in an outlet of an agroforestry catchment located in north-western Spain, evaluated the phosphorus concentration along a time scale and observed a slight peak in spring and a high peak in autumn, results that agree with the results of the current study. Moreover, the low DO levels recorded in some sites can be associated with the eutrophic conditions already reported. Data from SNIRH [35] monitoring network show occasionally low O2 in all reservoirs, being 2 mg/L, the lowest concentration observed in Miranda reservoir and 3 mg/L in Pocinho, Aguieira and Alqueva reservoirs. Bordado et al. [34], in the Miranda reservoir, also reported low O2 levels, being 75% of the average values during the 1992–2001 period. On the other hand, Mirás-Avalos et al. [59], in an outlet of a small catchment located in north-western Spain, observed that the highest carbon concentrations were characteristic of the autumn period and seasonality affects the carbon contents and availability. However, in the current study, the highest values were almost always observed in the spring season, except for the Pocinho reservoir where no pattern was observed (Table 1).
Overall, the phytoplankton EQR (Table 1) shows that in the Miranda and Pocinho reservoirs the spring samples presented the worst water quality (moderate or less). The Aguieira reservoir tended to present low water quality, being the Ag3, the most problematic site with the lower EQR values recorded. In addition, it is important to highlight the low quality of the Ag4 site, which in general presented the worst ecological potential. Concerning the Alqueva reservoir, the Al5 site presented a low ecological quality (moderate or less). These reservoirs are classified as eutrophic, and our results corroborate this fact since they present the highest nutrient concentrations (Table 1). The large availability of nutrients favours the overgrowth of phytoplankton [31], namely Cyanobacteria organisms (e.g., Anabaena and Microcystis), that was already associated with poor water quality and recurrently reported in these last two reservoirs [2]. This poor quality can be clearly seen at the Ag3 site where an EQR of −0.50 was obtained. This result was due to a Cyanobacteria bloom, as mentioned in Pinto et al. [2], later identified as a bloom of Microcystis aeruginosa FD4 [33].
3.2. Macroinvertebrate Community
Macroinvertebrate abundance, taxonomic richness, diversity and evenness for each site and sampling period are present in Table 2. In general, no pattern was observed for these four parameters. However, is important to emphasize that Al5_S20 had the highest total abundance (3088 individuals per sampling effort) and Al4_S20 had the lowest value (4 individuals), with both in the Alqueva reservoir. In the Aguieira reservoir, the sampling period with higher total abundances was S19. Overall, Chironomidae (CHI), Oligochaeta (OLI) and Corixidae (CORI), organisms that are mainly found in low-quality water bodies, were the most abundant groups because they tolerate high degrees of pollution [25,60]. Taxonomic richness (Table 2) varied between 1 and 12 taxa, and a higher variation in the number of families was observed in the Alqueva reservoir. Shannon–Wiener diversity was generally low in all sites (Table 2), the highest values being observed in Ag1_A18 (Aguieira) and Al2_A19 (Alqueva) and the lowest value in Al3_S20 when only one taxon was observed. Regarding Pielou’s evenness (Table 2), the values tended to reflect low equitability in almost all sites. Al4_S20 (Alqueva) presented the lowest value (0.06) due to the presence of a dominant taxon, CORI, with 3040 individuals out of 3088 (Table 2). Studies concerning macroinvertebrates in lentic ecosystems (e.g., reservoirs, lakes) are still scarce [22,28,61]. However, other studies have observed low diversity and abundance of macroinvertebrate communities in reservoirs when compared to natural lakes (e.g., [26]). Furthermore, these communities tend to be dominated by groups resistant to organic pollution and pesticides, such as CHI, as mentioned in Palma [4] and Trottier [21].

Table 2.
Abundances of macroinvertebrates (number per sampling effort) in each site of Miranda, Pocinho, Aguieira and Alqueva reservoirs. EP—Ephemeroptera; TR—Trichoptera; DI—Diptera; HE—Heteroptera; MO—Mollusca; AN—Annelida; CR—Crustacea; CO—Coleoptera; OD—Odonata; EPT stands for Ephemeroptera, Plecoptera and Trichoptera.
These results would be biased because organisms associated with good water quality (sensitive to disturbances) such as Plecoptera, Trichoptera or Ephemeroptera rarely appear in reservoirs since they are very sensitive to changes in physical and chemical parameters, climatic changes (e.g., precipitation rate), types of substrates, presence of faecal coliforms and heavy metals [62]. In contrast, organisms associated with poor water quality such as CHI and Hirudinea tend to be frequent and sometimes quite dominant [62]. The use of indexes for lakes can be an asset since these ecosystems show many similarities with the reservoirs [28]. Ntislidou et al. [28] developed and applied a new multimetric index (Greek Lake Benthic Macroinvertebrate Index (GLBiI)) to assess the water quality of various lakes based on the WFD approach. This index is based on three ecological quality ratios estimated: (i) total number of taxa; (ii) Simpson’s diversity index and (iii) the percentage of CHI in the profundal zone (max depth of the lake). Unfortunately, this type of approach cannot be applied in our work since the deep zone has not been sampled.
The Bray–Curtis dendrogram of Miranda and Pocinho reservoirs (Figure 2a) revealed that the compositions of benthic macroinvertebrates in the autumn samplings of Pocinho were grouped and were different from the remaining sites. The Miranda reservoir tended to be grouped in a single group except for M_S20 that was more similar to Pocinho, in the same sampling period.
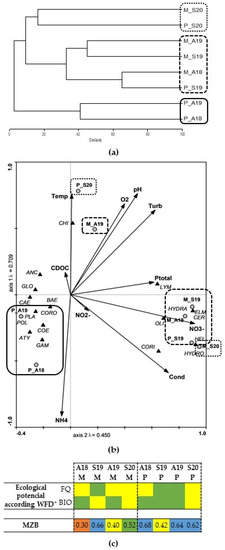
Figure 2.
(a) Miranda and Pocinho complete linkage dendrogram of benthic macroinvertebrates similarity; (b) Canonical Correspondence Analysis (CCA) of the distribution of aquatic invertebrates (see taxa abbreviation in Table 2); (c) ecological potential according to WFD parameters (* see Table 1 for more information) and macrozoobenthos index for dam reservoirs (MZB) through the sampling sites, periods, and environmental variables.
The Miranda and Pocinho CCA (Figure 2b) first axis explains 40.6% of the total variance in the data, while the second axis accounts for 25.8%. The Miranda reservoir tended to be associated with higher nitrate concentrations (NO3−), Coleoptera (ELM, HYDRA, HEL, HYDRO, see taxa abbreviation in Table 2), and Diptera (CER) macroinvertebrate community, except for M_A19 in which no association was recorded with any parameter or taxa. Autumn samples for Pocinho reservoir were associated with low DO concentrations and pH values and organisms of Amphipoda (GAM and CORO), Odonata (COE), Decapoda (ATY), Mollusca (PLA), Ephemeroptera (BAE, CAE) and Trichoptera (POL). Pocinho at_S19 showed a stronger association with NO3− and Coleoptera (HEL and HYDRO) macroinvertebrates, while in S20, temperature (Temp) and Diptera (CHI) organisms were strongly associated.
Additionally, Figure 2c presents the results obtained by the MZB index. According to the classification [54], Miranda presented lower water quality in autumn and good quality in spring. On the other hand, Pocinho presented good quality in all sampling periods, except for S19 where moderate water quality was observed.
Several authors (e.g., [27,60]) have already described that water fluctuations have a strong relationship to the composition of benthic macroinvertebrates. McEwen and Butler [27] reported that benthic macroinvertebrates may be particularly susceptible to water-level changes since they can modify the sediment exposure, temperature regime, wave-induced sediment redistribution and basal productivity. Miranda and Pocinho reservoirs are two small reservoirs that do not show large fluctuations in the volume of water stored, as reported by the SNIRH [35]. Regarding the Miranda reservoir, the water level oscillation is reduced (<1 m) and no major differences in the macroinvertebrate community were recorded throughout the sampling period. This situation can be observed in the CCA (Figure 2b), where almost all Miranda sites tend to be grouped. Moreover, high NO3− concentrations and taxa such as HEL, HYDRO, HYDRA, CER, ELM, OLI and CORI are associated with very polluted waters in which their presence may indicate a high water retention time in the reservoir. However, this contradicts the results obtained by the MZB index. As previously mentioned, this reservoir is characterized by eutrophic conditions [31,32], and according to the results of the WFD evaluation, this reservoir presented a moderate ecological potential in all seasons (Figure 2c). On the other hand, the evaluation of the macroinvertebrates in this reservoir presents contradicting results; however, the MZB index was able to distinguish more sampling sites than the WFD approach itself.
In the case of the Pocinho reservoir, the water level oscillations (<1 m) are also low according to SNIRH [35], a fact verified in the current study, and the sites are grouped seasonally (Figure 2b). Autumn is associated with low values of dissolved oxygen and pH and strongly associated with taxa such as POL, which are organisms related to unpolluted waters, and also with other organisms that are normally present in moderately polluted waters (Figure 2b). Moreover, spring samples are associated with higher temperatures, NO3− and organisms typical of low ecological water quality (e.g., CHI, OLI, CORI and HYDRO). The Pocinho reservoir has been characterized as a eutrophic reservoir [55] due to the highest concentrations of Ptotal recorded (Table 1). Based on the WFD assessment (Figure 2c), and according to other studies, this reservoir has an ecological potential of moderate/good [31,32]. These results by the WFD approach are in agreement with most of the macroinvertebrate results recorded since the sites with high ecological potential (e.g., P_A19) also presented the most sensitive macroinvertebrates to disturbances and better classification according to the MZB (Figure 2c). Therefore, it is possible to observe that the macroinvertebrate community has an important role in the water quality of these two reservoirs.
In the Aguieira reservoir, the Bray–Curtis dendrogram (Figure 3a) shows that Ag3_A18 was very different from the other sites. The remaining sites were grouped into two different groups, in general, according to the sampling season (autumn or spring). The Aguieira CCA (Figure 3b) first axis was responsible for 31.5% of the total variance in the data while the second axis was responsible for 28.8%. In general, this reservoir is grouped by seasons, where spring samplings were associated with high values of DO, pH, CDOC, nitrates and nitrites, while the autumn samplings, except for Ag3_A18, were associated with high values of temperature, conductivity, and some nutrients. Regarding the macroinvertebrate community, the spring samples were associated with a diversity of organisms such as Heteroptera (CORI), Oligochaeta (OLI), Decapoda (ATY), Coleoptera (HYDRO and ELM), Diptera (CHI) and Mollusca (PHY). On the other hand, the autumn samples were associated with Odonata (CORD), Amphipoda (GAM), Mollusca (PLA, LYM), Ephemeroptera (CAE), Hirudinea (GLO), Trichoptera (POL), Diptera (CER) and Coleoptera (DRY). The MBZ index (Figure 3c) shows that the spring samples present lower water quality compared to autumn samples. Moreover, most upstream sites (Ag3 and Ag4) tend to present a lower quality, which corroborates the remaining results obtained.
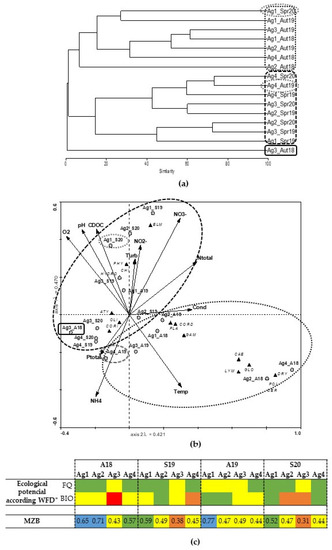
Figure 3.
(a) Aguieira complete linkage dendrogram of benthic macroinvertebrates similarity; (b) Canonical Correspondence Analysis (CCA); (c) ecological potential according to WFD parameters (* see Table 1 for more information) and macrozoobenthos index for dam reservoirs (MZB) of the distribution of aquatic invertebrates (see taxa abbreviation in Table 2) through the sampling sites, periods, and environmental variables.
The Aguieira reservoir has frequent cyanobacterial blooms as mentioned by Agência Portuguesa do Ambiente [52] and Vasconcelos et al. [57,63], namely in the Ag3 site, which can alter the macroinvertebrate community present. Furthermore, Cyanobacterial algae blooms can produce toxins able to interfere with the dynamics of this community by eliminating sensitive organisms [11]. Pinto et al. [2] observed cyanobacteria bloom in Ag3_A18, and according to WFD metrics, this site was classified with bad ecological potential. Regarding the macroinvertebrate community, site Ag3_A18 was the most different, presenting the lowest richness and abundance (Table 2 and Figure 3a), which may corroborate the poor water quality observed. Furthermore, Pinto et al. [2] refer that upstream sites (subject to increased pressure from agricultural activities) tend to have worse ecological potential. According to the Aguieira CCA (Figure 3b), Ag3 and Ag4 are closer sites and associated with high resistant organisms (e.g., OLI and CORI) that normally occur in water with poor quality [2,57].
This reservoir showed annually a high-water level oscillation where the stored volume varies up to about 60% with peaks at the end of the dry and rainy season, representing a variation of the quota in the order of 16 m [35]. These oscillations promote the occurrence of drying mechanisms, changes in the substrate composition and availability of the organic matter, leading to great variations in the composition and distribution of benthic organisms [21,28]. The Aguieira dendrogram, CCA and MZB index (Figure 3) showed an evident separation of the sites between seasons. Autumn samplings were associated with taxa linked for a better water classification (e.g., CORD, POL and GAM), while in spring samplings, the taxa recorded were representative of a worse water classification (e.g., CHI and OLI). In spring, this water classification may be associated with the changes in the substrate composition, namely in the margin areas that suffer leachate processes, leading to a loss of habitat for the macroinvertebrate community. The macroinvertebrate community appears to be a potential bioindicator in assessing the water quality of the Aguieira reservoir. Regarding the more polluted sites, they were grouped (Ag3 and Ag4) and the more tolerant organisms (e.g., OLI and CORI) were recorded.
These different assessments may be due to the wide variation in the CHI taxa, as they are in abundance in spring. Kaster and Jacobi [64], in a Wisconsin reservoir, USA, a reservoir with great water level amplitude, observed the same trend in the CHI abundances. The organisms that succeeded to survive the drying and freezing season began to increase their population density during the reservoir filling period, reaching the maximum abundance values at the beginning of June, which is in line with the results obtained in the Aguieira reservoir.
The Alqueva reservoir Bray–Curtis dendrogram (Figure 4a) does not present a clear pattern of sites distribution. However, some trends observed regarding the Al2, Al3 and Al4 at S20 were grouped, and the Al5 (the most distant site of the dam) was also grouped for almost periods (except for A18). In the CCA of Figure 4b, the first axis describes 39% of the total variance in the data, while the second axis explains 14.8%. Most sites were distributed over axis 2; however, their distribution does not follow a clear pattern, since a mixture of the sampling sites and periods was observed (Figure 4a). Only site Al5 presents the four sampling periods close to each other, associated with almost all physical and chemical parameters analysed. Al5 was also associated with Hirudinea (GLO, PIS), Mollusca (ANC, PLA, PHY), Heteroptera (CORI, NEP), Coleoptera (DRY) and Oligochaeta (OLI), essentially organisms associated with very contaminated waters.
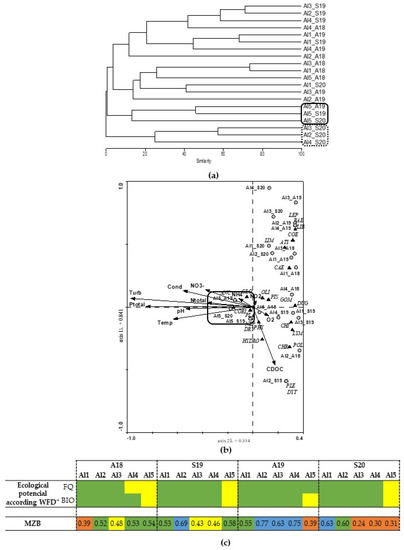
Figure 4.
(a) Alqueva complete linkage dendrogram of benthic macroinvertebrates similarity; (b) Canonical Correspondence Analysis (CCA); (c) ecological potential according to WFD parameters (* see Table 1 for more information) and macrozoobenthos index for dam reservoirs (MZB) of the distribution of aquatic invertebrates (see taxa abbreviation in Table 2) through the sampling sites, periods, and environmental variables.
According to the MZB index (Figure 4c), the sites near the dam (Al1 and Al2) tend to have a better water quality, compared to the other sites, except for Al1_A18 where a poor quality was observed.
The results of the mixing of sampling sites and seasons in the Alqueva reservoir may be due to it being a recently created reservoir (2004) with a large size and very homogeneous conditions. Voshell and Simmons [64] mention that the lack of studies on the initial colonization steps of reservoirs is a limiting factor to perceiving the dynamics of the macroinvertebrates in reservoirs. However, little has been done to understand how biological communities have settled in a new ecosystem. The same authors clarify that the benthic macroinvertebrate community follows a clear ecological succession. The organisms dependent on the terrestrial ecosystem appear first (e.g., Amphipoda), followed by an increase in diversity and the occurrence of dominant species, which are established over the first years. According to our results for the Alqueva reservoir, it appears to be still in an initial phase of ecological succession, with the highest number of taxa (taxa = 25) when compared to the other studied reservoirs, Miranda, Pocinho and Aguieira (oldest reservoirs) (Table 2). The Alqueva reservoir does not present great oscillation in water quotas, having varied only ≈ 2 m, which can facilitate and allow greater colonization. On the other hand, the lack of macrophytes and the homogeneity of the substrate leads to the existence of smaller habitats that can influence organisms to settle in this area. Furthermore, this reservoir has been classified as eutrophic due to high concentrations of Ptotal and concentrations of chlorophyll a, more significantly in the most upstream sites (as Al5) [65]. Indeed, according to Figure 4a,b, the Al5 was the most stable site along the sampling period with the occurrence of organisms with a high tolerance of disturbances (e.g., CORI, PLA, PHY and GLO). Thus, the macroinvertebrate community seems to be able to characterize the upstream site (Al5) with low environmental water quality. However, in the remaining sites, it was not possible to observe an evident evaluation. These sites appeared to be in a similar ecological status, without specific parameters (measured here) able to affect the water body. Furthermore, the macroinvertebrate community observed here was diffuse throughout all locations, not distinguishing differences between sites or seasons. Jorcin et al. [10] also recorded a regular spatial and temporal pattern in the community of benthic organisms in a recent reservoir in Brazil.
4. Conclusions
The current study showed that the benthic macroinvertebrate communities can be a sensitive tool for assessing the ecosystem dynamics of water reservoirs. Fluctuations in the reservoir’s water level concomitantly and the availability of diverse habitats are the factors that seem to most influence these communities. However, the existence of extreme events such as cyanobacteria blooms or high concentrations of nutrients, such as Ptotal, have been shown to also affect the macroinvertebrate community, favouring organisms associated with more polluted areas.
Despite the low ecological quality reflected in the indexes of macroinvertebrate communities (diversity and evenness), it is possible to observe that sites with better ecological quality are those where species associated with fewer disturbances are found (e.g., Cordullidae and Polycentropodidae). The opposite can be observed for sites with worse quality, where organisms such as Chironomidae and Oligochaeta are found, reflecting once again the ability of these taxonomic groups to quantify the ecological status of lentic ecosystems. The macrozoobenthos index (MZB) for dam reservoirs proved to be useful to apply in Portuguese reservoirs. Furthermore, it was clearly able to distinguish sites and classify these water bodies as excellent or poor, which alerts us to disturbances in the dynamics of these ecosystems. The combination of other biological factors with macroinvertebrates is an advantage in understanding these ecosystems, as they provide valuable ecosystem services that are not taken into account by the current WFD assessment. For these reasons, we encourage that macroinvertebrates would be a reliable and sensitive indicator to assess the quality of heavily modified water bodies such as reservoirs.
Author Contributions
All authors participated in the research and/or article preparation. I.P., S.R. and S.C.A. carried out the conceptualization and field work. I.P. carried out the laboratory work and wrote the original draft and all authors performed the final review. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Funds (through the FCT—Foundation for Science and Technology) and by the European Regional Development Fund (through COMPETE2020 and PT2020) through the research project ReDEFine (POCI-01-0145-FEDER–029368) and the strategic program UIDB/04423/2020 and UIDP/04423/2020. Sara Antunes and Sara Rodrigues were hired through the Regulamento do Emprego Científico e Tecnológico—RJEC from the Portuguese Foundation for Science and Technology (FCT) program (CEECIND/01756/2017 and 2020.00464.CEECIND, respectively).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.-I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.-H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater Biodiversity: Importance, Threats, Status and Conservation Challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Pinto, I.; Rodrigues, S.; Lage, O.M.; Antunes, S.C. Assessment of Water Quality in Aguieira Reservoir: Ecotoxicological Tools in Addition to the Water Framework Directive. Ecotoxicol. Environ. Saf. 2021, 208, 111583. [Google Scholar] [CrossRef] [PubMed]
- Setty, K.E.; Kayser, G.L.; Bowling, M.; Enault, J.; Loret, J.F.; Serra, C.P.; Alonso, J.M.; Mateu, A.P.; Bartram, J. Water Quality, Compliance, and Health Outcomes among Utilities Implementing Water Safety Plans in France and Spain. Int. J. Hyg. Environ. Health 2017, 220, 513–530. [Google Scholar] [CrossRef] [PubMed]
- Palma, P.; Matos, C.; Alvarenga, P.; Köck-Schulmeyer, M.; Simões, I.; Barceló, D.; López de Alda, M.J. Ecological and Ecotoxicological Responses in the Assessment of the Ecological Status of Freshwater Systems: A Case-Study of the Temporary Stream Brejo of Cagarrão (South of Portugal). Sci. Total Environ. 2018, 634, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Naiman, R.J.; Latterell, J.J.; Pettit, N.E.; Olden, J.D. Flow Variability and the Biophysical Vitality of River Systems. Comptes Rendus-Geosci. 2008, 340, 629–643. [Google Scholar] [CrossRef]
- Ackermann, W.; White, G.; Worthington, E.; Ivens, J. Man-Made Lakes: Their Problems and Environmental Effects. In Geophysical Monograph Series; Ackermann, W.C., White, G.F., Worthington, E.B., Ivens, J.L., Eds.; American Geophysical Union: Washington, DC, USA, 1973; ISBN 9781118664117. [Google Scholar]
- Farley, M. Encyclopedia of Lakes and Reservoirs. In Encyclopedia of Earth Sciences Series; Bengtsson, L., Herschy, R.W., Fairbridge, R.W., Eds.; Springer: Dordrecht, The Netherlands, 2012; ISBN 978-1-4020-5616-1. [Google Scholar]
- Simões, N.R.; Nunes, A.H.; Dias, J.D.; Lansac-Tôha, F.A.; Velho, L.F.M.; Bonecker, C.C. Impact of Reservoirs on Zooplankton Diversity and Implications for the Conservation of Natural Aquatic Environments. Hydrobiologia 2015, 758, 3–17. [Google Scholar] [CrossRef]
- Ward, J.V.; Stanford, J.A. The Serial Discontinuity Concept: Extending the Model to Floodplain Rivers. Regul. Rivers Res. Manag. 1995, 10, 159–168. [Google Scholar] [CrossRef]
- Jorcin, A.; Nogueira, M.G.; Belmont, R. Spatial and Temporal Distribution of the Zoobenthos Community during the Filling up Period of Porto Primavera Reservoir (Paraná River, Brazil). Braz. J. Biol. 2009, 69, 19–29. [Google Scholar] [CrossRef]
- do Nascimento Filho, S.L.; De França, E.J.; De Melo, J.; do Nascimento Moura, A. Interactiis coons between Benthic Microalgae, Nutrients and Benthic Macroinvertebrates in Reservoirs from the Semi-Arid Neotropical Region. Fundam. Appl. Limnol. 2019, 192, 237–254. [Google Scholar] [CrossRef]
- Jovem-Azevêdo, D.; Bezerra-Neto, J.F.; Azevêdo, E.L.; Gomes, W.I.A.; Molozzi, J.; Feio, M.J. Dipteran Assemblages as Functional Indicators of Extreme Droughts. J. Arid. Environ. 2019, 164, 12–22. [Google Scholar] [CrossRef]
- de Figueiredo, D.R.; Pereira, M.J.; Moura, A.; Silva, L.; Bárrios, S.; Fonseca, F.; Henriques, I.; Correia, A. Bacterial Community Composition over a Dry Winter in Meso- and Eutrophic Portuguese Water Bodies. FEMS Microbiol. Ecol. 2007, 59, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Llirós, M.; Inceoğlu, Ö.; García-Armisen, T.; Anzil, A.; Leporcq, B.; Pigneur, L.-M.; Viroux, L.; Darchambeau, F.; Descy, J.-P.; Servais, P. Bacterial Community Composition in Three Freshwater Reservoirs of Different Alkalinity and Trophic Status. PLoS ONE 2014, 9, e1161452014. [Google Scholar] [CrossRef] [PubMed]
- Nydick, K.R.; Lafrancois, B.M.; Baron, J.S.; Johnson, B.M. Nitrogen Regulation of Algal Biomass, Productivity, and Composition in Shallow Mountain Lakes, Snowy Range, Wyoming, USA. Can. J. Fish. Aquat. Sci. 2004, 61, 1256–1268. [Google Scholar] [CrossRef]
- Sun, X.; Mwagona, P.C.; Shabani, I.E.; Hou, W.; Li, X.; Zhao, F.; Chen, Q.; Zhao, Y.; Liu, D.; Li, X.; et al. Phytoplankton Functional Groups Response to Environmental Parameters in Muling River Basin of Northeast China. Ann. Limnol.-Int. J. Limnol. 2019, 55, 17. [Google Scholar] [CrossRef]
- García-Chicote, J.; Armengol, X.; Rojo, C. Zooplankton Abundance: A Neglected Key Element in the Evaluation of Reservoir Water Quality. Limnologica 2018, 69, 46–54. [Google Scholar] [CrossRef]
- Geraldes, A.M.; Silva-Santos, P.; Pasupuleti, R. Zooplankton Community Structure in a Deep Reservoir: Seasonal Trends and Structuring Variables? In Proceedings of the International Zoological Congress of “Grigore Antipa” Museum; Popa, L.O., Adam, C., Chisamera, G., Iorgu, E., Murariu, D., Popa, O.P., Eds.; “Grigore Antipa” National Museum of Natural History: Bucharest, Romania, 2016. [Google Scholar]
- Hinojosa-Garro, D.; Mason, C.F.; Underwood, G.J.C. Influence of Macrophyte Spatial Architecture on Periphyton and Macroinvertebrate Community Structure in Shallow Water Bodies under Contrasting Land Management. Fundam. Appl. Limnol. 2010, 177, 19–37. [Google Scholar] [CrossRef]
- Magbanua, F.S.; Mendoza, N.Y.B.; Uy, C.J.C.; Matthaei, C.D.; Ong, P.S. Water Physicochemistry and Benthic Macroinvertebrate Communities in a Tropical Reservoir: The Role of Water Level Fluctuations and Water Depth. Limnologica 2015, 55, 13–20. [Google Scholar] [CrossRef]
- Trottier, G.; Embke, H.; Turgeon, K.; Solomon, C.; Nozais, C.; Gregory-Eaves, I. Macroinvertebrate Abundance Is Lower in Temperate Reservoirs with Higher Winter Drawdown. Hydrobiologia 2019, 834, 199–211. [Google Scholar] [CrossRef]
- Zerlin, R.A.; Henry, R. Does Water Level Affect Benthic Macro-Invertebrates of a Marginal Lake in a Tropical River-Reservoir Transition Zone? Braz. J. Biol. 2014, 74, 408–419. [Google Scholar] [CrossRef] [PubMed]
- European Community Commission (ECC). Commission Directive 2000/60/EC of the European Parliament and the Council establishing the framework for community action in the field of water policy. Off. J. Eur. Union 2000, 327, 1–73. [Google Scholar]
- Mugnai, R.; Nessimian, J.L.; BaptistaA, D.F. Manual de Identificação de Macroinvertebrados Aquáticos Do Estado Do Rio de Janeiro; de Janeiro, R., Ed.; Tecnical books Editora: Rio de Janeiro, Brazil, 2010; ISBN 8561368101. [Google Scholar]
- Chagas, F.B.; Rutkoski, C.F.; Bieniek, G.B.; Vargas, G.D.L.P.; Hartmann, P.A.; Hartmann, M.T. Utilização Da Estrutura de Comunidades de Macroinvertebrados Bentônicos Como Indicador de Qualidade Da Água Em Rios No Sul Do Brasil. Rev. Ambiente E Agua 2017, 12, 416–425. [Google Scholar] [CrossRef][Green Version]
- Ferreira, T.; Cortes, R.V.; Morais, M. Qualidade Ecológica e Gestão Integrada de Albufeiras; INAG: Lisboa, Portugal, 2009. [Google Scholar]
- McEwen, D.C.; Butler, M.G. The Effects of Water-Level Manipulation on the Benthic Invertebrates of a Managed Reservoir. Freshw. Biol. 2010, 55, 1086–1101. [Google Scholar] [CrossRef]
- Ntislidou, C.; Lazaridou, M.; Tsiaoussi, V.; Bobori, D.C. A New Multimetric Macroinvertebrate Index for the Ecological Assessment of Mediterranean Lakes. Ecol. Indic. 2018, 93, 1020–1033. [Google Scholar] [CrossRef]
- White, M.S.; Xenopoulos, M.A.; Hogsden, K.; Metcalfe, R.A.; Dillon, P.J. Natural Lake Level Fluctuation and Associated Concordance with Water Quality and Aquatic Communities within Small Lakes of the Laurentian Great Lakes Region. Hydrobiologia 2008, 613, 21–31. [Google Scholar] [CrossRef]
- White, M.S.; Xenopoulos, M.A.; Metcalfe, R.A.; Somers, K.M. Water Level Thresholds of Benthic Macroinvertebrate Richness, Structure, and Function of Boreal Lake Stony Littoral Habitats. Can. J. Fish. Aquat. Sci. 2011, 68, 1695–1704. [Google Scholar] [CrossRef]
- Rodrigues, S.; Pinto, I.; Formigo, N.; Antunes, S.C. Microalgae Growth Inhibition-Based Reservoirs Water Quality Assessment to Identify Ecotoxicological Risks. Water 2021, 13, 2605. [Google Scholar] [CrossRef]
- Rodrigues, S.; Pinto, I.; Martins, F.; Formigo, N.; Antunes, S.C. Can Biochemical Endpoints Improve the Sensitivity of the Biomonitoring Strategy Using Bioassays with Standard Species, for Water Quality Evaluation? Ecotoxicol. Environ. Saf. 2021, 215, 112151. [Google Scholar] [CrossRef]
- Pinto, I.; Calisto, R.; Serra, C.R.; Lage, O.M.; Antunes, S.C. Bacterioplankton Community as a Biological Element for Reservoirs Water Quality Assessment. Water 2021, 13, 2836. [Google Scholar] [CrossRef]
- Bordalo, A.A.; Teixeira, R.; Wiebe, W.J. A Water Quality Index Applied to an International Shared River Basin: The Case of the Douro River. Environ. Manag. 2006, 38, 910–920. [Google Scholar] [CrossRef]
- SNIRH. Sistema Nacional de Informação de Recursos Hídricos SNIRH. Sistema Nacional de Informação de Recursos Hídricos. Available online: https://snirh.apambiente.pt/index.php?idMain= (accessed on 24 September 2019).
- Agência Portuguesa do Ambiente. Plano de Gestão de Região Hidrográfica, Parte 5-Objetivos, Anexo II.1, Região Hidrográfica Do Douro (RH3); Agência Portuguesa do Ambiente: Lisboa, Portugal, 2016. [Google Scholar]
- Agência Portuguesa do Ambiente. Plano de Gestão de Região Hidrográfica, Parte 5-Objetivos, Anexo II.2, Região Hidrográfica Do Douro (RH3); Agência Portuguesa do Ambiente: Lisboa, Portugal, 2016. [Google Scholar]
- Pádua, J.; Bernardo, J.M.; Alves, M.H. Exercício de Intercalibração Em Massas de Água Fortemente Modificadas–Albufeiras, No Âmbito Da Directiva Quadro Da Água. In Proceedings of the 9th Congresso da Água; INAG: Lisboa, Portugal, 2005; pp. 1–14. [Google Scholar]
- Agência Portuguesa do Ambiente. Plano de Gestão de Região Hidrográfica, Parte 5-Objetivos, Anexo II.1, Região Hidrográfica Do Vouga, Mongedo e Lis (RH4); Agência Portuguesa do Ambiente: Lisboa, Portugal, 2016. [Google Scholar]
- Pérez, J.R.; Loureiro, S.; Menezes, S.; Palma, P.; Fernandes, R.M.; Barbosa, I.R.; Soares, A.M.V.M. Assessment of Water Quality in the Alqueva Reservoir (Portugal) Using Bioassays. Environ. Sci. Pollut. Res. 2010, 17, 688–702. [Google Scholar] [CrossRef] [PubMed]
- Agência Portuguesa do Ambiente. Plano de Gestão de Região Hidrográfica, Parte 5-Objetivos, Anexo II.1, Região Hidrográfica Do Guadiana (RH7); Agência Portuguesa do Ambiente: Lisboa, Portugal, 2016. [Google Scholar]
- ISO. NF EN ISO 10304-1 Water Quality—Determination of Dissolved Anions by Liquid Chromatography of Ions—Part 1: Determination of Bromide, Chloride, Fluoride, Nitrate, Nitrite, Phosphate and Sulfate. 2007. Available online: https://www.iso.org/standard/46004.html (accessed on 24 September 2019).
- ISO. NF EN 25663 Water Quality-Determination of Kjeldahl Nitrogen-Method after Mineralization with Selenium. 1984. Available online: https://www.iso.org/standard/11756.html (accessed on 10 November 2021).
- ISO. NF EN ISO 17294-2 Water Quality—Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)—Part 2: Determination of Selected Elements Including Uranium Isotopes. 2016. Available online: https://www.iso.org/standard/62962.html (accessed on 24 September 2019).
- ISO. NF EN ISO 15923-1 Water Quality—Determination of Selected Parameters by Discrete Analysis Systems—Part 1: Ammonium, Nitrate, Nitrite, Chloride, Orthophosphate, Sulfate and Silicate with Photometric Detection. 2013. Available online: https://www.iso.org/standard/55559.html (accessed on 24 September 2019).
- Williamson, C.E.; Morris, D.P.; Pace, M.L.; Olson, O.G. Dissolved Organic Carbon and Nutrients as Regulators of Lake Ecosystems: Resurrection of a More Integrated Paradigm. Limnol. Oceanogr. 1999, 44, 795–803. [Google Scholar] [CrossRef]
- Brower, J.E.; Zar, J.H.; von Ende, C.N. Field and Laboratory Methods for General Ecology, 4th ed.; WCB McGraw-Hill: Boston, MA, USA, 1997. [Google Scholar]
- Instituto da Água. Manual Para a Avaliação Da Qualidade Biológica Da Água Em Lagos e Albufeiras Segundo a Diretiva Quadro Da Água. Protocolo de Amostragem e Análise Para o Fitoplâncton; Ministério do Ambiente do Ordenamento do Território e do Desenvolvimento Regional: Lisboa, Portugal, 2009.
- Agência Portuguesa do Ambiente. Plano de Gestão de Região Hidrográfica-Parte 2-Caracterização e Diagnóstico-Anexos -Região Hidrográfica Do Vouga, Mondego E Lis (Rh4); Agência Portuguesa do Ambiente: Lisboa, Portugal, 2016. [Google Scholar]
- INAG. Manual Para a Avaliação Biológica Da Qualidade Da Água Em Sistemas Fluviais Segundo a Directiva Quadro Da Água Protocolo de Amostragem e Análise Para Os Macroinvertebrados Bentónicos; Instituto daÁgua: Lisbon, Portugal, 2008. [Google Scholar]
- Alba-Tercedor, J.; Sánchez-Ortega, A. A Simple and Quick Method to Evaluate Biological Quality of Renning Freshwater Based on Hellawell (1978). Limnética 1988, 4, 51–56. [Google Scholar]
- Agência Portuguesa do Ambiente. Plano de Ordenamento Da Albufeira Da Aguieira; Agência Portuguesa do Ambiente: Lisboa, Portugal, 2005. [Google Scholar]
- Tachet, H.; Richoux, P.; Bournaud, M.; Usseglio-Polatera, P. Invertébrés d’eau Douce: Systématique, Biologie, Écologie.; CNRS: Paris, France, 2000; ISBN 2-271-05745-0. [Google Scholar]
- Picińska-Fałtynowicz, J.; Błachuta, J. WYTYCZNE METODYCZNE Do Przeprowadzenia Monitoringu i Oceny Potencjału Ekologicznego Zbiorników Zaporowych w Polsce Wersja; Sfinansowana ze środków Narodowego Funduszu Ochrony Środowiska i Gospodarki Wodnej: Wrocław, Poland, 2012. [Google Scholar]
- Cabecinha, E.; Cortes, R.; Alexandre Cabral, J.; Ferreira, T.; Lourenço, M.; Pardal, M. Multi-Scale Approach Using Phytoplankton as a First Step towards the Definition of the Ecological Status of Reservoirs. Ecol. Indic. 2009, 9, 240–255. [Google Scholar] [CrossRef]
- Instituto da Água. Modelação Matemática Da Qualidade Da Água Em Albufeiras Com Planos de Ordenamento-Lll-Albufeira Da Aguieira; Ministério da Agricultura, Mar, Ambiente e Ordenamento do Território. Instituto da Água, I.P.: Lisboa, Portugal, 2011.
- Vasconcelos, V.; Morais, J.; Vale, M. Microcystins and Cyanobacteria Trends in a 14 Year Monitoring of a Temperate Eutrophic Reservoir (Aguieira, Portugal). J. Environ. Monit. 2011, 13, 668–672. [Google Scholar] [CrossRef]
- Fouz, P.S.; Vázquez, E.V.; Avalos, J.M.M. Oscillation of Three Phosphorus Forms and Suspended Solids Content from 1999 to 2007 in a Spanish Agroforestry Catchment under Atlantic Climate. Commun. Soil Sci. Plant Anal. 2012, 43, 288–298. [Google Scholar] [CrossRef]
- Mirás-Avalos, J.M.; Valcárcel Armesto, M.; de Abreu, C.A.; da Silva Dias, R.; Vidal Vázquez, E. Temporal Oscillation and Losses of Three Xarbon Forms in a Microcatchment of NW Spain. Commun. Soil Sci. Plant Anal. 2015, 46, 296–308. [Google Scholar] [CrossRef]
- Martins, F.S.; Formigo, N.; Antunes, S.C. Can Be the Environmental and Biotic Factors Responsible for Macroinvertebrate Communities’ Alterations in Portuguese Alpine Ponds? Limnologica 2020, 83, 125782. [Google Scholar] [CrossRef]
- Pamplin, P.; Almeida, T.; Rocha, O. Composition and Distribution of Benthic Macroinvertebrates in Americana Reservoir (SP, Brazil). Acta Limnol. Bras. 2006, 18, 121–132. [Google Scholar]
- Barbola, I.F.; Moraes, M.F.P.G.; Anazawa, T.M.; Nascimento, E.A.; Sepka, E.R.; Polegatto, C.M.; Milléo, J.; Schühli, G.S. Evaluation of the Aquatic Macroinvertebrate Community as a Tool for Monitoring a Reservoir in the Pitangui River Basin, Paraná, Brazil. Iheringia-Ser. Zool. 2011, 101, 15–23. [Google Scholar] [CrossRef][Green Version]
- Vasconcelos, V.M.; Sivonen, K.; Evans, W.R.; Carmichael, W.W.; Namikoshi, M. Hepatotoxic Microcystin Diversity in Cyanobacterial Blooms Collected in Portuguese Freshwaters. Water Res. 1996, 30, 2377–2384. [Google Scholar] [CrossRef]
- Voshell, J.R.; Simmons, G.M. Colonization and Succession of Benthic Macroinvertebrates in a New Reservoir. Hydrobiologia 1984, 112, 27–39. [Google Scholar] [CrossRef]
- Morais, M.; Serafim, A.; Pinto, P.; Ilhéu, A.; Ruivo, M. Monitoring the water quality in Alqueva reservoir, Guadiana river, southern Portugal. In Reservoir and River Basin Management: Exchange of Experiences from Brazil, Portugal and Germany; Gunkel, G., Sobral, M., Eds.; Universitätsverlag der TU Berlin: Berlin, Germany, 2007; pp. 96–112. ISBN 978-3-7983-2056-7. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).