Comparing GHG Emissions from Drained Oil Palm and Recovering Tropical Peatland Forests in Malaysia
Abstract
1. Introduction
2. Materials and Methods
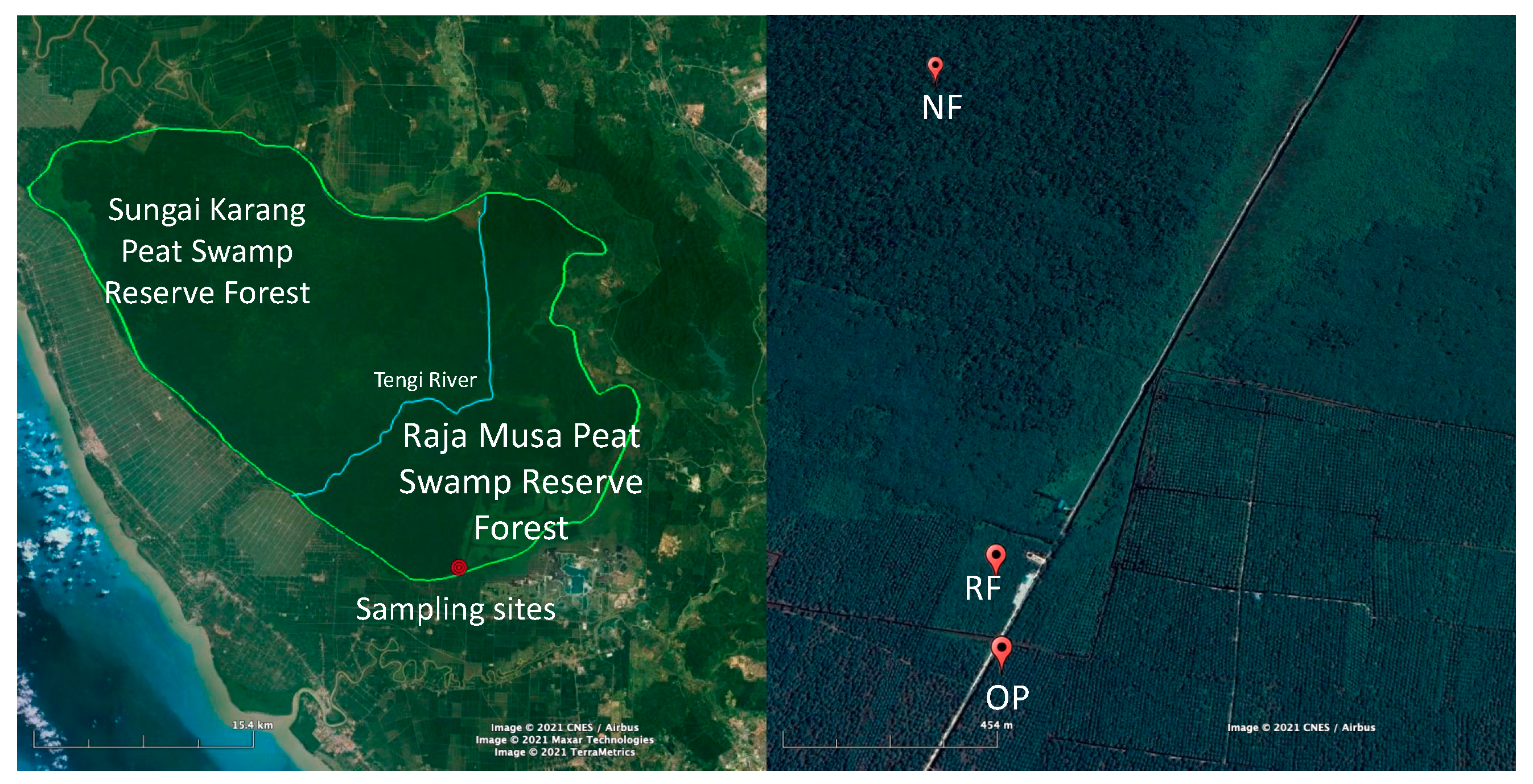
2.1. Study Sites
2.2. Sampling Strategy
2.3. Peat Sampling and Analysis
2.4. Greenhouse Gases Measurement
2.5. Data and Statistical Analysis
3. Results
3.1. Peat Characteristics
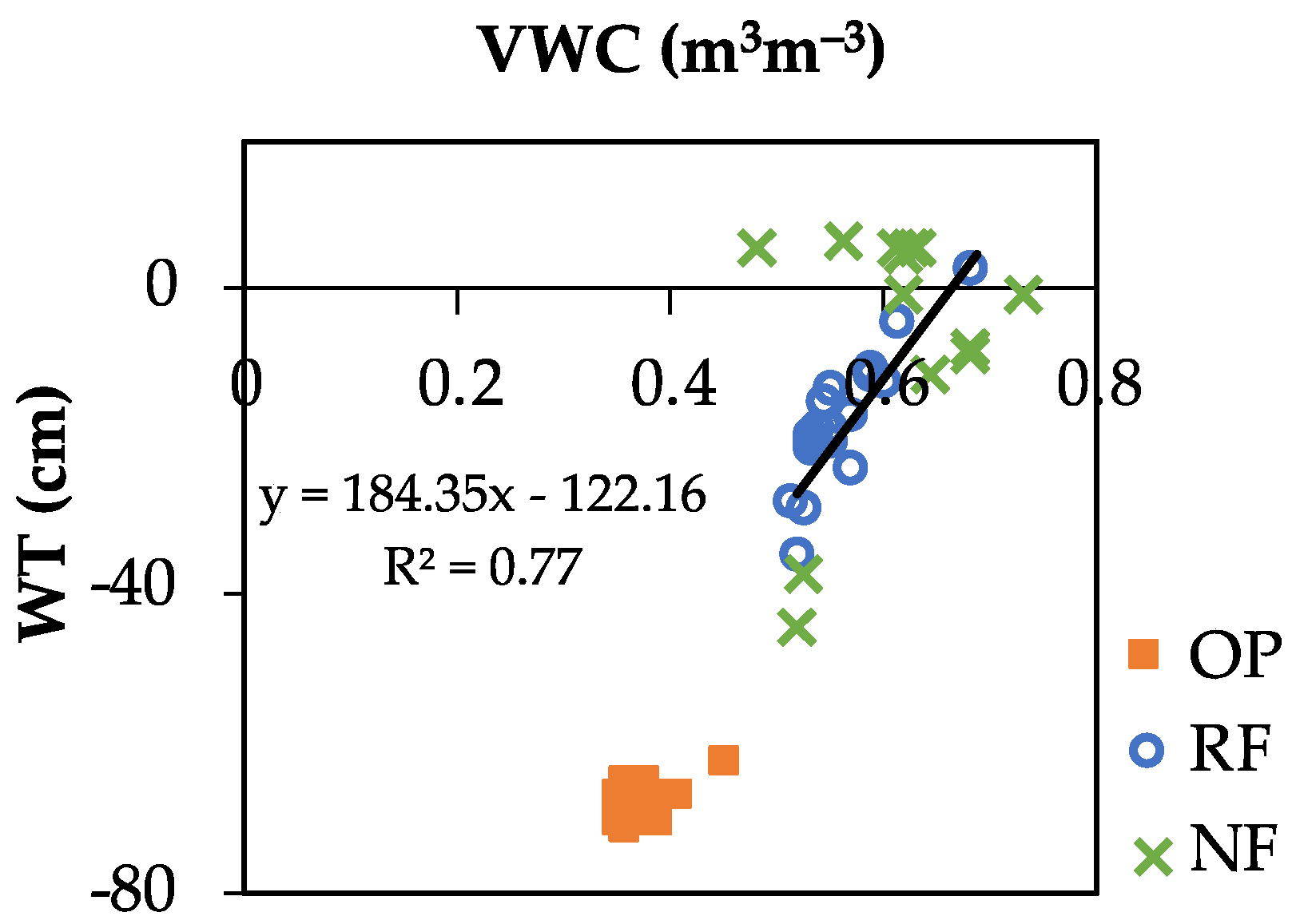
3.2. Variation in Environmental Variables
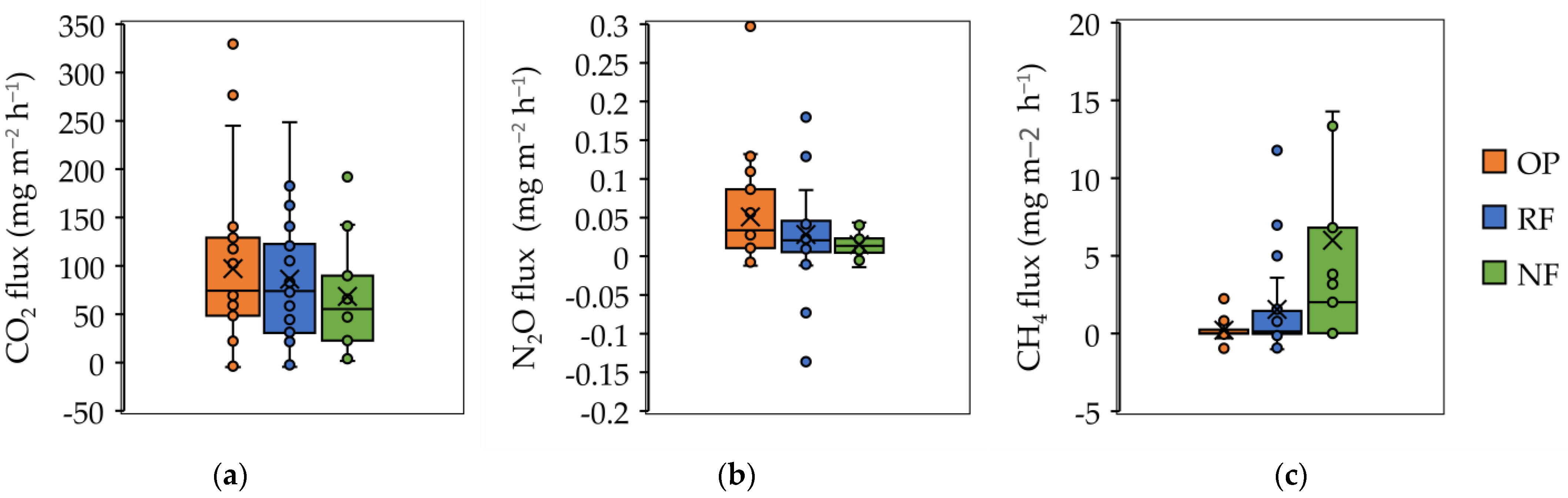
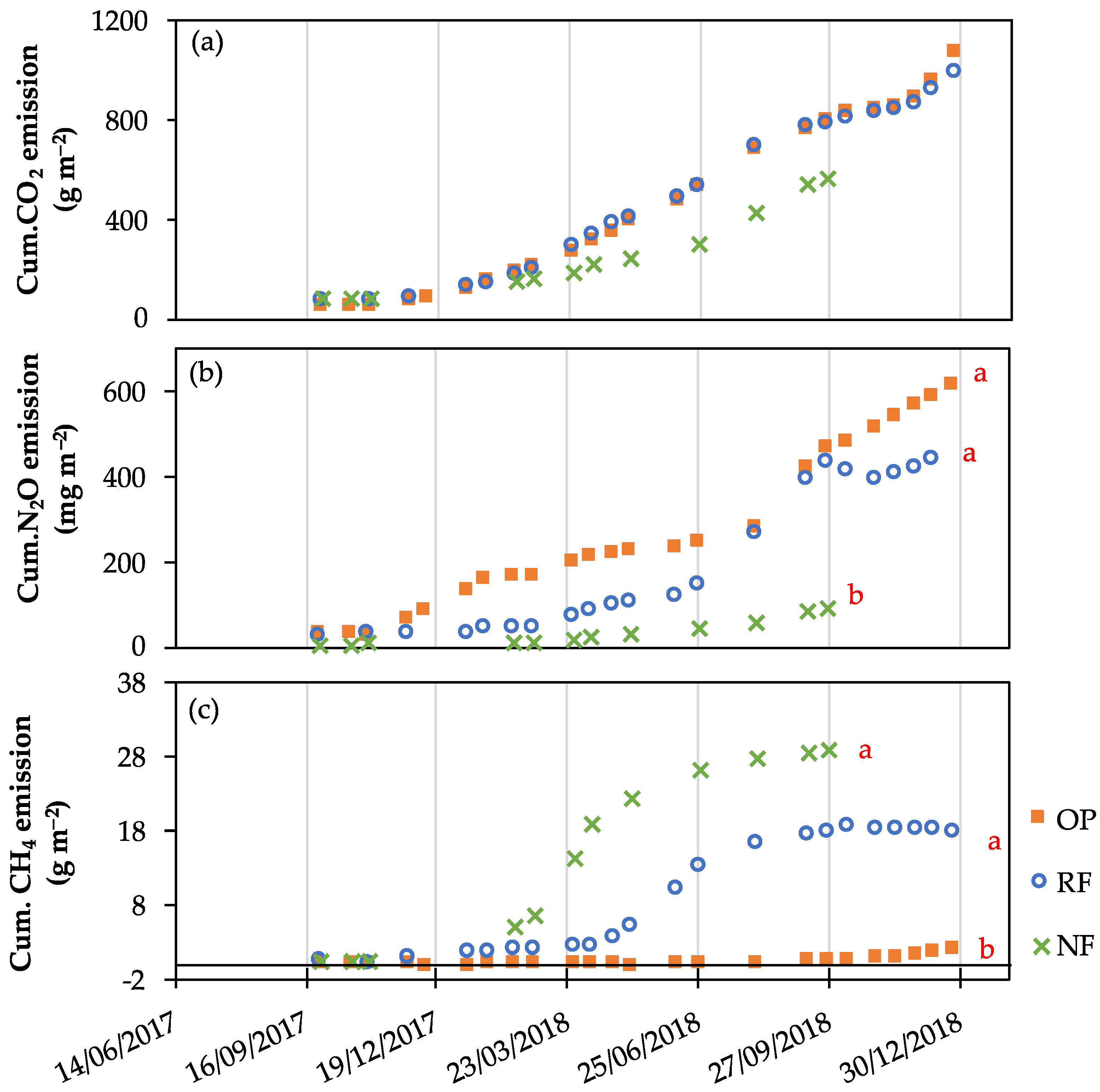
3.3. Variation in GHGs Emission
3.4. Global Warming Potential (GWP)
4. Discussion
4.1. Peat and Environmental Characteristics
4.2. Carbon Dioxide (CO2) Emissions
4.3. Nitrous Oxide (N2O) Emissions
4.4. Methane (CH4) Emissions
4.5. Global Warming Potential (GWP)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. 2013 Supplement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories: Wet-lands; Hiraishi, T., Krug, T., Tanabe, K., Srivastava, N., Baasansuren, J., Fukuda, M., Troxler, T.G., Eds.; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Yu, Z.; Loisel, J.; Brosseau, D.P.; Beilman, D.W.; Hunt, S.J. Global peatland dynamics since the last glacial maximum. Geophys. Res. Lett. 2010, 37, 1–5. [Google Scholar] [CrossRef]
- Loisel, J.; Yu, Z.; Beilman, D.W.; Camill, P.; Alm, J.; Amesbury, M.J.; Anderson, D.; Andersson, S.; Bochicchio, C.; Barber, K.; et al. A database and synthesis of northern peatland soil properties and Holocene carbon and nitrogen accumulation. Holocene 2014, 24, 1028–1042. [Google Scholar] [CrossRef]
- Jauhiainen, J.; Takahashi, H.; Heikkinen, J.E.P.; Martikainen, P.J.; Vasander, H. Carbon fluxes from a tropical peat swamp forest floor. Glob. Chang. Biol. 2005, 11, 1788–1797. [Google Scholar] [CrossRef]
- Couwenberg, J.; Dommain, R.; Joosten, H. Greenhouse gas fluxes from tropical peatlands in South-East Asia. Glob. Chang. Biol. 2010, 16, 1715–1732. [Google Scholar] [CrossRef]
- Jauhiainen, J.; Page, S.E.; Vasander, H. Greenhouse gas dynamics in degraded and restored tropical peatlands. Mires Peat 2016, 17, 1–12. [Google Scholar] [CrossRef]
- Melling, L.; Hatano, R.; Goh, K.J. Soil CO2 Flux from three ecosystems in tropical peatland of Sarawak, Malaysia. Tellus B Chem. Phys. Meteorol. 2005, 57, 1–11. [Google Scholar] [CrossRef]
- Page, S.E. Review of peat surface greenhouse gas emissions from oil palm plantations in Southeast Asia. Indirect Eff. Biofuel Prod. 2011, 15, 1–77. [Google Scholar]
- Susilawati, H.L.; Setyanto, P.; Ariani, M.; Hervani, A.; Inubushi, K. Influence of water depth and soil amelioration on greenhouse gas emissions from peat soil columns. Soil Sci. Plant Nutr. 2016, 62, 57–68. [Google Scholar] [CrossRef][Green Version]
- Page, S.E.; Rieley, J.O.; Banks, C.J. Global and regional importance of the tropical peatland carbon pool. Glob. Chang. Biol. 2011, 17, 798–818. [Google Scholar] [CrossRef]
- Gumbricht, T.; Roman-Cuesta, R.M.; Verchot, L.; Herold, M.; Wittmann, F.; Householder, E.; Herold, N.; Murdiyarso, D. An expert system model for mapping tropical wetlands and peatlands reveals South America as the largest contributor. Glob. Chang. Biol. 2017, 23, 3581–3599. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, J.; Shi, C.; Liew, S.C. Land cover distribution in the peatlands of peninsular Malaysia, Sumatra and Borneo in 2015 with changes since 1990. Glob. Ecol. Conserv. 2016, 6, 67–78. [Google Scholar] [CrossRef]
- Murdiyarso, D.; Lilleskov, E.; Kolka, R. Tropical peatlands under siege: The need for evidence-based policies and strategies. Mitig. Adapt. Strat. Glob. Chang. 2019, 24, 493–505. [Google Scholar] [CrossRef]
- Cooper, H.V.; Evers, S.; Aplin, P.; Crout, N.; Dahalan, M.P.B.; Sjogersten, S. Greenhouse gas emissions resulting from conversion of peat swamp forest to oil palm plantation. Nat. Commun. 2020, 11, 407. [Google Scholar] [CrossRef]
- Lam, W.Y.; Kulak, M.; Sim, S.; King, H.; Huijbregts, M.A.J.; Chaplin-Kramer, R. Greenhouse gas footprints of palm oil production in Indonesia over space and time. Sci. Total Environ. 2019, 688, 827–837. [Google Scholar] [CrossRef]
- Hirano, T.; Kusin, K.; Limin, S.; Osaki, M. Carbon dioxide emissions through oxidative peat decomposition on a burnt tropical peatland. Glob. Chang. Biol. 2014, 20, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Jauhiainen, J.; Silvennoinen, H.; Hämäläinen, R.; Kusin, K.; Limin, S.; Raison, R.J.; Vasander, H. Nitrous oxide fluxes from tropical peat with different disturbance history and management. Biogeosciences 2012, 9, 1337–1350. [Google Scholar] [CrossRef]
- Uning, R.; Latif, M.T.; Othman, M.; Juneng, L.; Hanif, N.M.; Nadzir, M.S.M.; Maulud, K.N.A.; Jaafar, W.S.W.M.; Said, N.F.S.; Ahamad, F.; et al. A review of Southeast Asian oil palm and its CO2 fluxes. Sustainability 2020, 12, 5077. [Google Scholar] [CrossRef]
- MPOC—Malaysian Palm Oil Council. Available online: https://mpoc.org.my/ (accessed on 13 October 2020).
- Tonks, A.J.; Aplin, P.; Beriro, D.J.; Cooper, H.; Evers, S.; Vane, C.H.; Sjögersten, S. Impacts of conversion of tropical peat swamp forest to oil palm plantation on peat organic chemistry, physical properties and carbon stocks. Geoderma 2016, 289, 36–45. [Google Scholar] [CrossRef]
- Charters, L. Peat swamp forest conservation withstands pervasive land conversion to oil palm plantation in North Selangor, Malaysia. Int. J. Remote Sens. 2019, 40, 7409–7438. [Google Scholar] [CrossRef]
- Wilson, S.; Blain, D.; Couwenberg, J.; Evans, C.D.; Murdiyarso, D.; Page, S.E.; Renou-Wilson, F.; Rieley, J.O.; Strack, M.; Tuittila, E.-S. Greenhouse gas emission factors associated with rewetting of organic soils. Mires Pear 2016, 17, 1–28. [Google Scholar] [CrossRef]
- Günther, A.; Barthelmes, A.; Huth, V.; Joosten, H.; Jurasinski, G.; Koebsch, F.; Couwenberg, J. Prompt rewetting of drained peatlands reduces climate warming despite methane emissions. Nat. Commun. 2020, 11, 1644. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Adelodun, A.A.; Khan, M.F.; Krisnawati, H.; Garcia-Menendez, F. Towards an improved understanding of greenhouse gas emissions and fluxes in tropical peatlands of Southeast Asia. Sustain. Cities Soc. 2020, 53, 101881. [Google Scholar] [CrossRef]
- Murdiyarso, D.; Saragi-Sasmito, M.F.; Rustini, A. Greenhouse gas emissions in restored secondary tropical peat swamp forests. Mitig. Adapt. Strat. Glob. Chang. 2019, 24, 507–520. [Google Scholar] [CrossRef]
- Ritzema, H.; Limin, S.; Kusin, K.; Jauhiainen, J.; Wösten, H. Canal blocking strategies for hydrological restoration of degraded tropical peatlands in Central Kalimantan, Indonesia. Catena 2014, 114, 11–20. [Google Scholar] [CrossRef]
- Nagano, T.; Osawa, K.; Ishida, T.; Sakai, K.; Vijarnsorn, P.; Jongskul, A.; Phetsuk, S.; Waijaroen, S.; Yamanoshita, T.; Norisada, M.; et al. Subsidence and soil CO2 efflux in tropical peatland in Southern Thailand under various water table and management conditions. Mires Peat 2013, 11, 1–20. [Google Scholar]
- Hooijer, A.; Page, S.; Jauhiainen, J.; Lee, W.A.; Lu, X.X.; Idris, A.; Anshari, G. Subsidence and carbon loss in drained tropical peatlands. Biogeosciences 2012, 9, 1053–1071. [Google Scholar] [CrossRef]
- Girkin, N.; Turner, B.; Ostle, N.; Sjogersten, S. Root-derived CO2 flux from a tropical peatland. Wetl. Ecol. Manag. 2018, 26, 985–991. [Google Scholar] [CrossRef]
- Dhandapani, S.; Ritz, K.; Evers, S.; Sjögersten, S. Environmental impacts as affected by different oil palm cropping systems in tropical peatlands. Agric. Ecosyst. Environ. 2019, 276, 8–20. [Google Scholar] [CrossRef]
- Dhandapani, S.; Ritz, K.; Evers, S.; Yule, C.M.; Sjögersten, S. Are secondary forests second-rate? Comparing peatland greenhouse gas emissions, chemical and microbial community properties between primary and secondary forests in Peninsular Malaysia. Sci. Total Environ. 2019, 655, 220–231. [Google Scholar] [CrossRef]
- Deshmukh, C.S.; Julius, D.; Nardi; Evans, C.D.; Susanto, A.P.; Page, S.E.; Gauci, V.; Laurén, A.; Sabiham, S.; Agus, F.; et al. Impact of forest plantation on methane emissions from tropical peatland. Glob. Chang. Biol. 2020, 26, 2477–2495. [Google Scholar] [CrossRef] [PubMed]
- Khasanah, N.; van Noordwijk, M. Subsidence and carbon dioxide emissions in a smallholder peatland mosaic in Sumatra, Indonesia. Mitig. Adapt. Strat. Glob. Chang. 2019, 24, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Matysek, M.; Evers, S.; Samuel, M.K.; Sjogersten, S. High heterotrophic CO2 emissions from a Malaysian oil palm plantations during dry-season. Wetl. Ecol. Manag. 2018, 26, 415–424. [Google Scholar] [CrossRef]
- Husnain, H.; Wigena, I.G.P.; Dariah, A.; Marwanto, S.; Setyanto, P.; Agus, F. CO2 Emissions from tropical drained peat in Sumatra, Indonesia. Mitig. Adapt. Strat. Glob. Chang. 2014, 19, 845–862. [Google Scholar] [CrossRef]
- Hergoualc’h, K.; Hendry, D.T.; Murdiyarso, D.; Verchot, L.V. Total and heterotrophic soil respiration in a swamp forest and Oil palm plantations on peat in Central Kalimantan, Indonesia. Biogeochemistry 2017, 135, 203–220. [Google Scholar] [CrossRef]
- Melling, L.; Hatano, R.; Goh, K.J. Nitrous oxide emissions from three ecosystems in tropical peatland of Sarawak, Malaysia. Soil Sci. Plant Nutr. 2007, 53, 792–805. [Google Scholar] [CrossRef]
- Wakhid, N.; Hirano, T.; Okimoto, Y.; Nurzakiah, S.; Nursyamsi, D. Soil carbon dioxide emissions from a rubber plantation on tropical peat. Sci. Total Environ. 2017, 581–582, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Swails, E.; Jaye, D.; Verchot, L.; Hergoualc’h, K.; Schirrmann, M.; Borchard, N.; Wahyuni, N.; Lawrence, D. Will CO2 emissions from drained tropical peatlands decline over time? Links between soil organic matter quality, nutrients, and C mineralization rates. Ecosystems 2018, 21, 868–885. [Google Scholar] [CrossRef]
- Swails, E.; Hergoualc’h, K.; Verchot, L.; Novita, N.; Lawrence, D. Spatio-temporal variability of peat CH4 and N2O fluxes and their contribution to peat GHG budgets in Indonesian forests and oil palm plantations. Front. Environ. Sci. 2021, 9, 617828. [Google Scholar] [CrossRef]
- Jauhiainen, J.; Hooijer, A.; Page, S.E. Carbon dioxide emissions from an acacia plantation on peatland in Sumatra, Indonesia. Biogeosciences 2012, 9, 617–630. [Google Scholar] [CrossRef]
- Marwanto, S.; Sabiham, S.; Funakawa, S. Importance of CO2 production in subsoil layers of drained tropical peatland under mature oil palm plantation. Soil Tillage Res. 2019, 186, 206–213. [Google Scholar] [CrossRef]
- Ishikura, K.; Hirano, T.; Okimoto, Y.; Hirata, R.; Kiew, F.; Melling, L.; Aeries, E.B.; Lo, K.S.; Musin, K.K.; Waili, J.W.; et al. Soil carbon dioxide emissions due to oxidative peat decomposition in an oil palm plantation on tropical peat. Agric. Ecosyst. Environ. 2018, 254, 202–212. [Google Scholar] [CrossRef]
- Chaddy, A.; Melling, L.; Ishikura, K.; Hatano, R. Soil N2O emissions under different N rates in an oil palm plantation on tropical peatland. Agriculture 2019, 9, 213. [Google Scholar] [CrossRef]
- Melling, L.; Hatano, R.; Kah, J.G. Methane fluxes from three ecosystems in tropical peatland of Sarawak, Malaysia. Soil Biol. Biochem. 2005, 37, 1445–1453. [Google Scholar] [CrossRef]
- Melling, L.; Goh, K.J.; Hatano, R. Short-term effect of urea on CH4 flux under the oil palm (Elaeis guineensis) on tropical peatland in Sarawak, Malaysia. Soil Sci. Plant Nutr. 2006, 52, 788–792. [Google Scholar] [CrossRef]
- Purnomo, H.; Okarda, B.; Dewayani, A.A.; Ali, M.; Achdiawan, R.; Kartodihardjo, H.; Pacheco, P.; Juniwaty, K.S. Reducing forest and land fires through good palm oil value chain governance. For. Policy Econ. 2018, 91, 94–106. [Google Scholar] [CrossRef]
- GEC—A Non-Profit Organisation Established in 1998 to Work on Environmental Issues of Global Importance. Available online: https://www.gec.org.my/ (accessed on 13 October 2021).
- Jabatan Perhutanan Semenanjung Malaysia—Forestry Department Peninsular Malaysia. Available online: https://www.forestry.gov.my/en/ (accessed on 13 October 2021).
- 14 Day Weather Forecast | World Weather Online. Available online: https://www.worldweatheronline.com/ (accessed on 13 October 2021).
- Local, National, & Global Daily Weather Forecast | AccuWeather. Available online: https://www.accuweather.com/ (accessed on 13 October 2021).
- Yang, M.; Yu, G.; He, N.; Grace, J.; Wang, Q.; Zhou, Y. A method for estimating annual cumulative soil/ecosystem respiration and CH4 flux from sporadic data collected using the chamber method. Atmosphere 2019, 10, 623. [Google Scholar] [CrossRef]
- Hassler, E.; Corre, M.D.; Tjoa, A.; Damris, M.; Utami, S.R.; Veldkamp, E. Soil fertility controls soil-atmosphere carbon dioxide and methane fluxes in a tropical landscape converted from lowland forest to rubber and oil palm plantations. Biogeosciences 2015, 12, 5831–5852. [Google Scholar] [CrossRef]
- Dhandapani, S.; Evers, S.; Ritz, K.; Sjögersten, S. Nutrient and trace element concentrations influence greenhouse gas emissions from Malaysian tropical peatlands. Soil Use Manag. 2021, 37, 138–150. [Google Scholar] [CrossRef]
- Kai, T.; Mukai, M.; Araki, K.S.; Adhikari, D.; Kubo, M. Analysis of chemical and biological soil properties in organically and conventionally fertilized apple orchards. J. Agric. Chem. Environ. 2016, 05, 92–99. [Google Scholar] [CrossRef][Green Version]
- Brogowski, Z.; Kwasowski, W.; Madyniak, R. Calculating particle density, bulk density, and total porosity of soil based on its texture. Soil Sci. Annu. 2014, 65, 139–149. [Google Scholar] [CrossRef]
- Little, K.M.; Metelerkamp, B.; Smith, C.W. A comparison of three methods of soil water content determination. S. Afr. J. Plant Soil 1998, 15, 80–89. [Google Scholar] [CrossRef]
- Donald Nicholas, D.J.; Nason, A. Determination of Nitrate and Nitrite; Academic Press: Cambridge, MA, USA, 1957; Volume 3, pp. 981–984. [Google Scholar] [CrossRef]
- Fichtner, T.; Goersmeyer, N.; Stefan, C. Influence of soil pore system properties on the degradation rates of organic substances during soil aquifer treatment (SAT). Appl. Sci. 2019, 9, 496. [Google Scholar] [CrossRef]
- Levy, P.E.; Gray, A.; Leeson, S.R.; Gaiawyn, J.; Kelly, M.P.C.; Cooper, M.D.A.; Dinsmore, K.J.; Jones, S.K.; Sheppard, L.J. Quantification of uncertainty in trace gas fluxes measured by the static chamber method. Eur. J. Soil Sci. 2011, 62, 811–821. [Google Scholar] [CrossRef]
- Myhre, G. Anthropogenic and natural radiative forcing. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Firdaus, M.S.; Gandaseca, S.; Ahmed, O.H. Effect of drainage and land clearing on selected peat soil physical properties of secondary peat swamp forest. Int. J. Phys. Sci. 2011, 6, 5462–5466. [Google Scholar] [CrossRef]
- Page, S.E.; Wust, R.A.J.; Weiss, D.; Rieley, J.O.; Shotyk, W.; Limin, S.H. A record of late Pleistocene and Holocene carbon accumulation and climate change from an equatorial peat bog (Kalimantan, Indonesia): Implications for past, present and future carbon dynamics. J. Quat. Sci. 2004, 19, 625–635. [Google Scholar] [CrossRef]
- Minkkinen, K.; Laine, J. Effect of forest drainage on the peat bulk density of pine mires in Finland. Can. J. For. Res. 1998, 28, 178–186. [Google Scholar] [CrossRef]
- Laiho, R.; Penttilä, T.; Laine, J. Variation in soil nutrient concentrations and bulk density within peatland forest sites. Silva Fenn. 2004, 38, 29–41. [Google Scholar] [CrossRef]
- Couwenberg, J.; Hooijer, A. Towards robust subsidence-based soil carbon emission factors for peat soils in South-East Asia, with special reference to oil palm plantations. Mires Peat 2013, 12, 1–13. [Google Scholar]
- Moore, S.; Evans, C.D.; Page, S.E.; Garnett, M.H.; Jones, T.G.; Freeman, C.; Hooijer, A.; Wiltshire, A.J.; Limin, S.H.; Gauci, V. Deep instability of deforested tropical peatlands revealed by fluvial organic carbon fluxes. Nature 2013, 493, 660–663. [Google Scholar] [CrossRef]
- Könönen, M.; Jauhiainen, J.; Laiho, R.; Kusin, K.; Vasander, H. Physical and chemical properties of tropical peat Under Stabilised land uses. Mires Peat 2015, 16, 1–13. [Google Scholar]
- Wosten, J.H.M.; Ismail, A.B.; van Wijk, A.L.M. Peat subsidence and its practical implications: A case study in Malaysia. Geoderma 1997, 78, 25–36. [Google Scholar] [CrossRef]
- Anshari, G.Z.; Afifudin, M.; Nuriman, M.; Gusmayanti, E.; Arianie, L.; Susana, R.; Nusantara, R.W.; Sugardjito, J.; Rafiastanto, A. Drainage and land use impacts on changes in selected peat properties and peat degradation in West Kalimantan Province, Indonesia. Biogeosciences 2010, 7, 3403–3419. [Google Scholar] [CrossRef]
- Hoyos-Santillan, J.; Lomax, B.H.; Large, D.; Turner, B.L.; Boom, A.; Lopez, O.R.; Sjögersten, S. Getting to the root of the problem: Litter decomposition and peat formation in lowland neotropical peatlands. Biogeochemistry 2015, 126, 115–129. [Google Scholar] [CrossRef]
- Hoyos-Santillan, J. Quality not quantity: Organic matter composition controls of CO2 and CH4 fluxes in neotropical peat profiles. Soil Biol. Biochem. 2016, 103, 86–96. [Google Scholar] [CrossRef]
- Cooper, H.V. From peat swamp forest to oil palm plantations: The stability of tropical peatland carbon. Geoderma 2019, 34, 109–117. [Google Scholar] [CrossRef]
- Satrio, A.E.; Gandaseca, S.; Ahmed, O.H.; Majid, N.M.A. Effect of logging operation on soil carbon storage of a tropical peat swamp forest. Am. J. Environ. Sci. 2009, 5, 748–752. [Google Scholar] [CrossRef]
- Dariah, A.; Marwanto, S.; Agus, F. Root- and peat-based CO2 emissions from oil palm plantations. Mitig. Adapt. Strat. Glob. Chang. 2014, 19, 831–843. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. Agriculture, forestry and other land use (AFOLU). In Climate Change 2014 Mitigation of Climate Change; Cambridge University Press: Cambridge, UK, 2015; pp. 811–922. [Google Scholar] [CrossRef]
- Carlson, K.M.; Goodman, L.K.; May-Tobin, C.C. Modeling relationships between water table depth and peat soil carbon loss in Southeast Asian plantations. Environ. Res. Lett. 2015, 10, 074006. [Google Scholar] [CrossRef]
- Sumarga, E.; Hein, L.; Hooijer, A.; Vernimmen, R. Hydrological and economic effects of oil palm cultivation in Indonesian Peatlands. Ecol. Soc. 2016, 21, 52. [Google Scholar] [CrossRef]
- Hergoualc’h, K.; Verchot, L.V. Greenhouse gas emission factors for land use and land-use change in Southeast Asian peatlands. Mitig. Adapt. Strat. Glob. Chang. 2014, 19, 789–807. [Google Scholar] [CrossRef]
- Marwanto, S.; Agus, F. Is CO2 flux from oil palm plantations on peatland controlled by soil moisture and/or soil and air temperatures? Mitig. Adapt. Strat. Glob. Chang. 2014, 19, 809–819. [Google Scholar] [CrossRef]
- Manning, F.C.; Kho, L.K.; Hill, T.C.; Cornulier, T.; Teh, Y.A. Carbon emissions from oil palm plantations on peat soil. Front. For. Glob. Chang. 2019, 2, 37. [Google Scholar] [CrossRef]
- Sundari, S.; Hirano, T.; Yamada, H.; Kusin, K.; Limin, S. Effect of groundwater level on soil respiration in tropical peat swamp forests. J. Agric. Meteorol. 2012, 68, 121–134. [Google Scholar] [CrossRef]
- Fenner, N.; Freeman, C. Drought-induced carbon loss in peatlands. Nat. Geosci. 2011, 4, 895–900. [Google Scholar] [CrossRef]
- Yule, C.M.; Gomez, L.N. Leaf litter decomposition in a tropical peat swamp forest in Peninsular Malaysia. Wetl. Ecol. Manag. 2009, 17, 231–241. [Google Scholar] [CrossRef]
- Bader, C.; Müller, M.; Schulin, R.; Leifeld, J. Peat decomposability in managed organic soils in relation to land use, organic matter composition and temperature. Biogeosciences 2018, 15, 703–719. [Google Scholar] [CrossRef]
- Robroek, B.J.M.; Albrecht, R.J.H.; Hamard, S.; Pulgarin, A.; Bragazza, L.; Buttler, A.; Jassey, V.E. Peatland vascular plant functional types affect dissolved organic matter chemistry. Plant Soil 2016, 407, 135–143. [Google Scholar] [CrossRef]
- Wösten, J.H.M.; Clymans, E.; Page, S.E.; Rieley, J.O.; Limin, S.H. Peat–water interrelationships in a tropical peatland ecosystem in Southeast Asia. Catena 2008, 73, 212–224. [Google Scholar] [CrossRef]
- Samaritani, E.; Siegenthaler, A.; Yli-Petäys, M.; Buttler, A.; Christin, P.A.; Mitchell, E.A.D. Seasonal net ecosystem carbon exchange of a regenerating cutaway bog: How long does it take to restore the C-sequestration function? Restor. Ecol. 2011, 19, 480–489. [Google Scholar] [CrossRef]
- Urbanová, Z.; Picek, T.; Hájek, T.; Bufková, I.; Tuittila, E.-S. Vegetation and carbon gas dynamics under a changed hydrological regime in central European peatlands. Plant Ecol. Divers. 2012, 5, 89–103. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, M.P.; Taxak, A.K. Effect of vegetation communities and altitudes on the soil organic carbon stock in Kotli Bhel-1A Catchment, India. CLEAN—Soil, Air, Water 2017, 45, 1600650. [Google Scholar] [CrossRef]
- Bartolucci, N.N.; Anderson, T.R.; Ballantine, K.A. Restoration of retired agricultural land to wetland mitigates greenhouse gas emissions. Restor. Ecol. 2021, 29, e13314. [Google Scholar] [CrossRef]
- Sangok, F.E.; Maie, N.; Melling, L.; Watanabe, A. Evaluation on the decomposability of tropical forest peat soils after conversion to an oil palm plantation. Sci. Total Environ. 2017, 587–588, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Sjögersten, S.; Aplin, P.; Gauci, V.; Peacock, M.; Siegenthaler, A.; Turner, B.L. Temperature response of ex-situ greenhouse gas emissions from tropical peatlands: Interactions between forest type and peat moisture conditions. Geoderma 2018, 324, 47–55. [Google Scholar] [CrossRef]
- Mäkiranta, P.; Laiho, R.; Fritze, H.; Hytönen, J.; Laine, J.; Minkkinen, K. Indirect regulation of heterotrophic peat soil respiration by water level via microbial community structure and temperature sensitivity. Soil Biol. Biochem. 2009, 41, 695–703. [Google Scholar] [CrossRef]
- Kandel, T.P.; Lærke, P.E.; Elsgaard, L. Annual emissions of CO2, CH4 and N2O from a temperate peat bog: Comparison of an undrained and four drained sites under permanent grass and arable crop rotations with cereals and potato. Agric. For. Meteorol. 2018, 256–257, 470–481. [Google Scholar] [CrossRef]
- Ishikura, K.; Hirata, R.; Hirano, T.; Okimoto, Y.; Wong, G.X.; Melling, L.; Aeries, E.B.; Kiew, F.; Lo, K.S.; Musin, K.K.; et al. Carbon dioxide and methane emissions from peat soil in an undrained tropical peat swamp forest. Ecosystems 2019, 22, 1852–1868. [Google Scholar] [CrossRef]
- Toma, Y.; Takakai, F.; Darung, U.; Kuramochi, K.; Limin, S.H.; Dohong, S.; Hatano, R. Nitrous oxide emission derived from soil organic matter decomposition from tropical agricultural peat soil in Central Kalimantan, Indonesia. Soil Sci. Plant Nutr. 2011, 57, 436–451. [Google Scholar] [CrossRef]
- Takakai, F.; Morishita, T.; Hashidoko, Y.; Darung, U.; Kuramochi, K.; Dohong, S.; Limin, S.H.; Hatano, R. Effects of agricultural land-use change and forest fire on N2O emission from tropical peatlands, Central Kalimantan, Indonesia. Soil Sci. Plant Nutr. 2006, 52, 662–674. [Google Scholar] [CrossRef]
- Oktarita, S.; Hergoualc’H, K.; Anwar, S.; Verchot, L.V. Substantial N2O emissions from peat decomposition and N fertilization in an oil palm plantation exacerbated by hotspots. Environ. Res. Lett. 2017, 12, 104007. [Google Scholar] [CrossRef]
- Maljanen, M.; Sigurdsson, B.D.; Guömundsson, J.; Öskarsson, H.; Huttunen, J.T.; Martikainen, P.J. Greenhouse gas balances of managed peatlands in the Nordic countries present knowledge and gaps. Biogeosciences 2010, 7, 2711–2738. [Google Scholar] [CrossRef]
- Audet, J.; Hoffmann, C.C.; Andersen, P.M.; Baattrup-Pedersen, A.; Johansen, J.R.; Larsen, S.E.; Kjaergaard, C.; Elsgaard, L. Nitrous oxide fluxes in undisturbed riparian wetlands located in agricultural catchments: Emission, uptake and controlling factors. Soil Biol. Biochem. 2014, 68, 291–299. [Google Scholar] [CrossRef]
- Davidson, E.A.; Matson, P.A.; Vitousek, P.M.; Riley, R.; Dunkin, K.; García-Méndez, G.; Maass, J.M. Processes regulating soil emissions of NO and N2O in a seasonally dry tropical forest. Ecology 1993, 74, 130–139. [Google Scholar] [CrossRef]
- Linn, D.M.; Doran, J.W. Effect of water-filled pore space on carbon dioxide and nitrous oxide production in tilled and nontilled soils. Soil Sci. Soc. Am. J. 1984, 48, 1267–1272. [Google Scholar] [CrossRef]
- Baral, K.R.; Arthur, E.; Olesen, J.E.; Petersen, S.O. Predicting nitrous oxide emissions from manure properties and soil moisture: An incubation expersiment. Soil Biol. Biochem. 2016, 97, 112–120. [Google Scholar] [CrossRef]
- Elberling, B.; Christiansen, H.H.; Hansen, B.U. Erratum: High nitrous oxide production from thawing permafrost. Nat. Geosci. 2010, 3, 506. [Google Scholar] [CrossRef]
- Syakila, A.; Kroeze, C. The global nitrous oxide budget revisited. Greenh. Gas Meas. Manag. 2011, 1, 17–26. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. B Biol. Sci. 2013, 368. [Google Scholar] [CrossRef]
- Regina, K.; Nykänen, H.; Silvola, J.; Martikainen, P.J. Fluxes of nitrous oxide from boreal peatlands as affected by peatland type, water table level and nitrification capacity. Biogeochemistry 1996, 35, 401–418. [Google Scholar] [CrossRef]
- Ojanen, P.; Minkkinen, K. Rewetting offers rapid climate benefits for tropical and agricultural peatlands but not for forestry-drained peatlands. Glob. Biogeochem. Cycles 2020, 34, 1–16. [Google Scholar] [CrossRef]
- Geng, F.; Li, K.; Liu, X.; Gong, Y.; Yue, P.; Li, Y.; Han, W. Long-term effects of N deposition on N2O emission in an alpine grassland of Central Asia. Catena 2019, 182, 104100. [Google Scholar] [CrossRef]
- Sjögersten, S.; Cheesman, A.W.; Lopez, O.; Turner, B.L. Biogeochemical processes along a nutrient gradient in a tropical ombrotrophic peatland. Biogeochemistry 2011, 104, 147–163. [Google Scholar] [CrossRef]
- Abdalla, M.; Hastings, A.; Truu, J.; Espenberg, M.; Mander, Ü.; Smith, P. Emissions of methane from northern peatlands: A review of management impacts and implications for future management options. Ecol. Evol. 2016, 6, 7080–7102. [Google Scholar] [CrossRef]
- Turetsky, M.R.; Kotowska, A.; Bubier, J.; Dise, N.B.; Crill, P.; Hornibrook, E.R.C.; Minkkinen, K.; Moore, T.R.; Myers-Smith, I.H.; Nykänen, H.; et al. A synthesis of methane emissions from 71 northern, temperate, and subtropical wetlands. Glob. Chang. Biol. 2014, 20, 2183–2197. [Google Scholar] [CrossRef]
- Dommain, R.; Couwenberg, J.; Joosten, H. Development and carbon sequestration of tropical peat domes in South-East Asia: Links to post-glacial sea-level changes and Holocene climate variability. Quat. Sci. Rev. 2011, 30, 999–1010. [Google Scholar] [CrossRef]
- Kurnianto, S.; Warren, M.; Talbot, J.; Kauffman, B.; Murdiyarso, D.; Frolking, S. Carbon accumulation of tropical peatlands over millennia: A modeling approach. Glob. Chang. Biol. 2015, 21, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Girkin, N.T.; Vane, C.H.; Cooper, H.V.; Moss-Hayes, V.; Craigon, J.; Turner, B.L.; Ostle, N.; Sjögersten, S. Spatial variability of organic matter properties determines methane fluxes in a tropical forested peatland. Biogeochemistry 2019, 142, 231–245. [Google Scholar] [CrossRef] [PubMed]
- von Arnold, K.; Weslien, P.; Nilsson, M.; Svensson, B.H.; Klemedtsson, L. Fluxes of CO2, CH4 and N2O from drained coniferous forests on organic soils. For. Ecol. Manag. 2005, 210, 239–254. [Google Scholar] [CrossRef]
- Whalen, S.C. Biogeochemistry of methane exchange between natural wetlands and the atmosphere. Environ. Eng. Sci. 2005, 22, 73–94. [Google Scholar] [CrossRef]
- Leifeld, J.; Menichetti, L. The underappreciated potential of peatlands in global climate change mitigation strategies /704/47/4113 /704/106/47 Article. Nat. Commun. 2018, 9, 1071. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.; Köhler, S.; Glatzel, S.; Jurasinski, G. Methane exchange in a coastal fen in the first year after flooding—A systems shift. PLoS ONE 2015, 10, 1–25. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hemes, K.S.; Chamberlain, S.D.; Eichelmann, E.; Knox, S.H.; Baldocchi, D.D. A biogeochemical compromise: The high methane cost of sequestering carbon in restored wetlands. Geophys. Res. Lett. 2018, 45, 6081–6091. [Google Scholar] [CrossRef]
- Inubushi, K.; Furukawa, Y.; Hadi, A.; Purnomo, E.; Tsuruta, H. Seasonal changes of CO2, CH4 and N2O fluxes in relation to land-use change in tropical peatlands located in coastal area of South Kalimantan. Chemosphere 2003, 52, 603–608. [Google Scholar] [CrossRef]
- Jauhiainen, J.; Limin, S.; Silvennoinen, H.; Vasander, H. Carbon dioxide and methane fluxes in drained tropical peat before and after hydrological restoration. Ecology 2008, 89, 3503–3514. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.G.; Vargas, R.; Bond-Lamberty, B.; Turetsky, M.R. Effects of soil rewetting and thawing on soil gas fluxes: A review of current literature and suggestions for future research. Biogeosciences 2012, 9, 2459–2483. [Google Scholar] [CrossRef]
- Yavitt, J.B.; Fahey, T.J.; Simmons, J.A. Methane and carbon dioxide dynamics in a Northern Hardwood ecosystem. Soil Sci. Soc. Am. J. 1995, 59, 796–804. [Google Scholar] [CrossRef]
- Jackowicz-Korczyński, M.; Christensen, T.R.; Bäckstrand, K.; Crill, P.; Friborg, T.; Mastepanov, M.; Ström, L. Annual cycle of methane emission from a subarctic peatland. J. Geophys. Res. Biogeosci. 2010, 115, G02009. [Google Scholar] [CrossRef]
- Olson, D.M.; Griffis, T.J.; Noormets, A.; Kolka, R.; Chen, J. Interannual, seasonal, and retrospective analysis of the methane and carbon dioxide budgets of a temperate peatland. J. Geophys. Res. Biogeosci. 2013, 118, 226–238. [Google Scholar] [CrossRef]
- Wang, X.; Siciliano, S.; Helgason, B.; Bedard-Haughn, A. Responses of a mountain peatland to increasing temperature: A microcosm study of greenhouse gas emissions and microbial community dynamics. Soil Biol. Biochem. 2017, 110, 22–33. [Google Scholar] [CrossRef]
- Hirano, T.; Jauhiainen, J.; Inoue, T.; Takahashi, H. Controls on the carbon balance of tropical peatlands. Ecosystems 2009, 12, 873–887. [Google Scholar] [CrossRef]
- Lundin, L.; Nilsson, T.; Jordan, S.; Lode, E.; Strömgren, M. Impacts of rewetting on peat, hydrology and water chemical composition over 15 years in two finished peat extraction areas in sweden. Wetl. Ecol. Manag. 2017, 25, 405–419. [Google Scholar] [CrossRef]
- RSPO—Roundtable on Sustainable Palm Oil. Available online: https://www.rspo.org/ (accessed on 13 October 2020).

| Properties | OP | RF | NF |
|---|---|---|---|
| Bulk density (gcm−3) | 0.21 ± 0.00 | 0.18 ± 0.02 | 0.17 ± 0.00 |
| Particle density (gcm−3) | 1.53 ± 0.007 | 1.43 ± 0.010 | 1.43 ± 0.005 |
| WFPS (%) | 74.4651 | 72.4581 | 77.6919 |
| Ph | 3.17 ± 0.12 | 3.33 ± 0.15 | 3.50 ± 0.26 |
| WT (cm) | −68.94 ± 244 | −18.38 ± 8.46 | 4.92 ± 17.37 |
| Soil surface temp. (°C) | 27.55 ± 04 | 27.51 ± 0.01 | 26.02 ± 0.95 |
| Air temp. (°C) | 26.86 ± 3.98 | 26.81 ± 4.14 | 26.09 ± 2.94 |
| Total C (%) | 14.36 ± 22.05 | 17.821 ± 28.28 | 16.678 ± 27.10 |
| Total N (%) | 1.53 ± 1.65 | 0.40 ± 0.44 | 0.19 ± 0.03 |
| C/N ratio | 16.46 ± 19.54 | 25.06 ± 26.19 | 76.37 ± 121.71 |
| NO3− -N (mg N Kg−1) | 3.00 ± 2.66 | 1.00 ± 0.01 | 0.00 ± 0.00 |
| NH4+-N (mg N Kg−1) | 21.67 ± 2.08 | 66.67 ± 28.31 | 18.33 ± 23.97 |
| Sites | Annualized Emission of CO2, N2O and CH4 (g m−2 yr−1) [Mean Value (mg m−2 h−1) ± stv] | ||
|---|---|---|---|
| CO2 | N2O | CH4 | |
| OP | 726.99 [97.05 ± 84.42] | 0.42 [0.05 ± 0.06] | 1.09 [0.21 ± 0.59] |
| RF | 680.06 [86.23 ± 66.26] | 0.29 [0.03 ± 0.06] | 12.19 [1.55 ± 3.04] |
| NF | 481.93 [68.33 ± 59.31] | 0.08 [0.01 ± 0.02] | 24.57 [5.99 ± 9.85] |
| Sites | 20-Years GWP (g CO2 eq.m−2 yr−1) [% of Total GWP] | |||
|---|---|---|---|---|
| CO2 | N2O | CH4 | Total | |
| Drained, uncleared forest (Pen. Malaysia) [14] | 5632 ± 13.15 [54] | 5562 ± 21.47 [53] | 52 ± 0.23 [0.5] | 10,486 ± 22.88 |
| 6 months OP (Pen. Malaysia) [14] | 8137 ± 9.54 [32] | 18,242 ± 78.03 [71] | 268 ± 1.20 [1] | 25,853 ± 75.43 |
| OP (this study) | 726.98 [78] | 112.91 [12] | 93.62 [10] | 933.52 |
| 10–15 years old OP (Pen. Malaysia) [14] | 5441 ± 13.25 [56] | 3434 ±15.78 [35] | 767 ± 3.43 [8] | 9736 ± 21.68 |
| Matured OP (Kal. Indonesia) [40] | 3040 ± 8.5 [96] | 130 ± 1.0 [4] | 0.0 ± 0.0 [0] | 3170 ± 8.6 |
| RF (this study) | 680.06 [38] | 80.15 [4] | 1048.69 [58] | 1808.91 |
| 30-yo secondary forest (Kal, Indonesia) [40] | 520 ± 4.4 [51] | 340 ± 0.8 [33] | 160 ± 0.5 [16] | 1020 ± 4.5 |
| NF (this study) | 481.93 [18] | 21.10 [0.8] | 2113.39 [81] | 2616.42 |
| Primary forest (Kal, Indonesia) [14] | −50 ± 3.9 [−51] | 40 ± 0.2 [42] | 100 ± 0.2 [109] | 90 ± 3.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azizan, S.N.F.; Goto, Y.; Doi, T.; Kamardan, M.I.F.; Hara, H.; McTaggart, I.; Kai, T.; Noborio, K. Comparing GHG Emissions from Drained Oil Palm and Recovering Tropical Peatland Forests in Malaysia. Water 2021, 13, 3372. https://doi.org/10.3390/w13233372
Azizan SNF, Goto Y, Doi T, Kamardan MIF, Hara H, McTaggart I, Kai T, Noborio K. Comparing GHG Emissions from Drained Oil Palm and Recovering Tropical Peatland Forests in Malaysia. Water. 2021; 13(23):3372. https://doi.org/10.3390/w13233372
Chicago/Turabian StyleAzizan, Siti Noor Fitriah, Yuji Goto, Toshihiro Doi, Muhammad Imran Firdaus Kamardan, Hirofumi Hara, Iain McTaggart, Takamitsu Kai, and Kosuke Noborio. 2021. "Comparing GHG Emissions from Drained Oil Palm and Recovering Tropical Peatland Forests in Malaysia" Water 13, no. 23: 3372. https://doi.org/10.3390/w13233372
APA StyleAzizan, S. N. F., Goto, Y., Doi, T., Kamardan, M. I. F., Hara, H., McTaggart, I., Kai, T., & Noborio, K. (2021). Comparing GHG Emissions from Drained Oil Palm and Recovering Tropical Peatland Forests in Malaysia. Water, 13(23), 3372. https://doi.org/10.3390/w13233372






