Seasonal Variations in Groundwater Quality and Hydrogeochemistry in the Endemic Areas of Chronic Kidney Disease of Unknown Etiology (CKDu) in Sri Lanka
Abstract
:1. Introduction
2. Materials and Methods
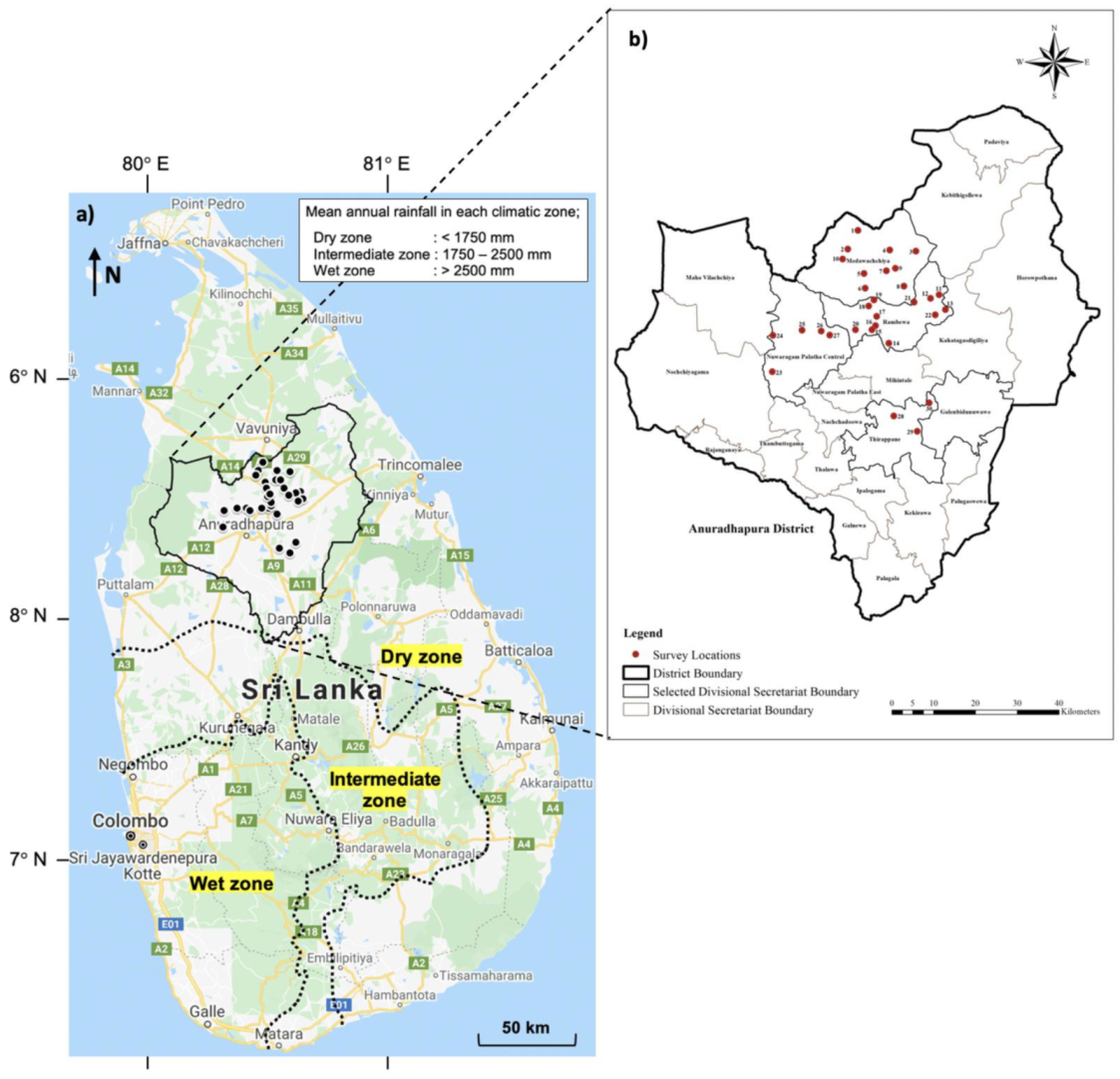
2.1. Study Area
2.2. Sampling and Water Quality Analysis
2.3. Data Analysis
3. Results and Discussion
3.1. Groundwater Quality and Compliance with Drinking Water Standards
3.2. Major Ion Chemistry and Chemical Processes
3.2.1. Correlation Coefficient Matrix
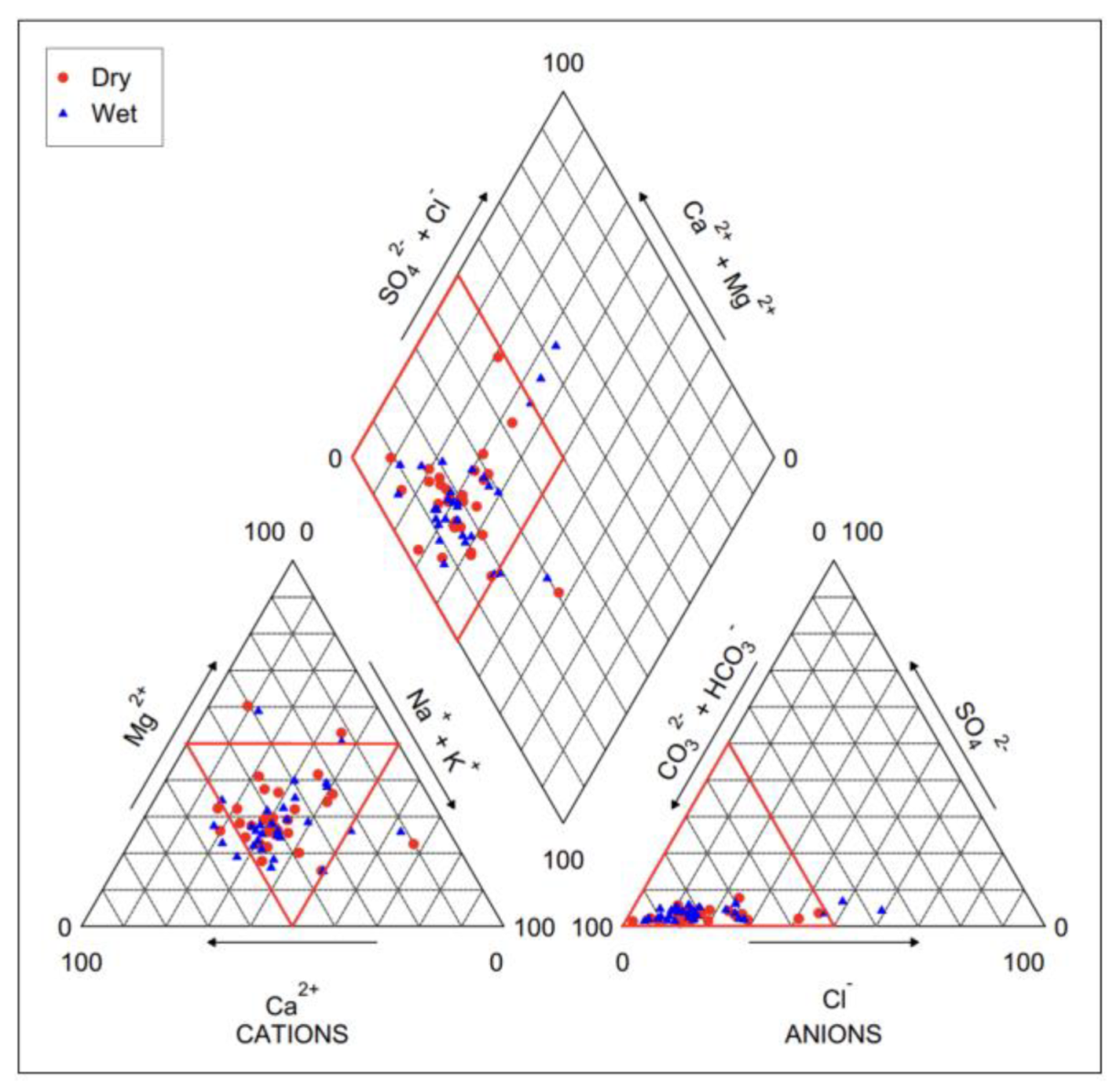
3.2.2. Piper Diagram
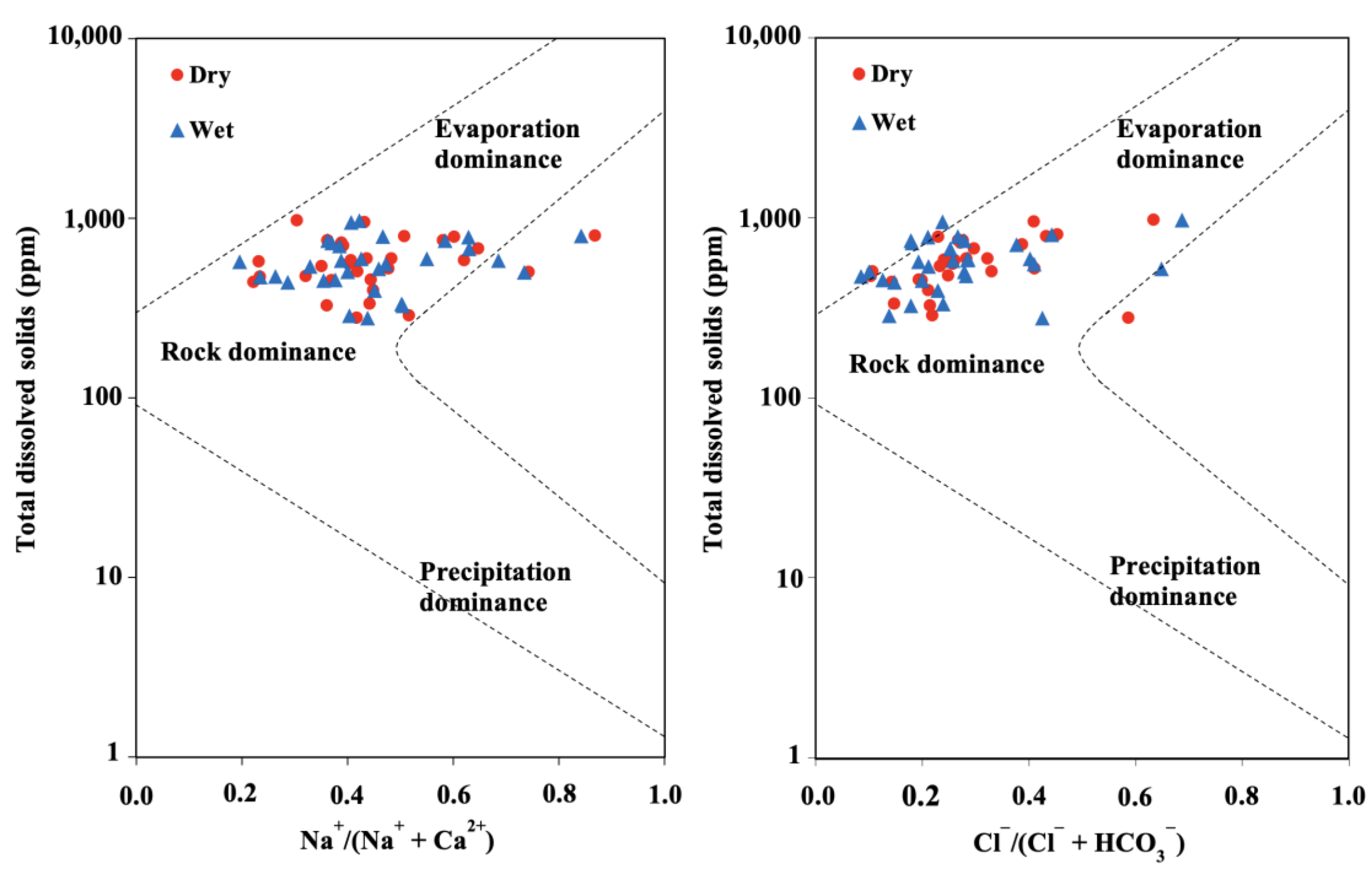
3.2.3. Gibbs Diagram
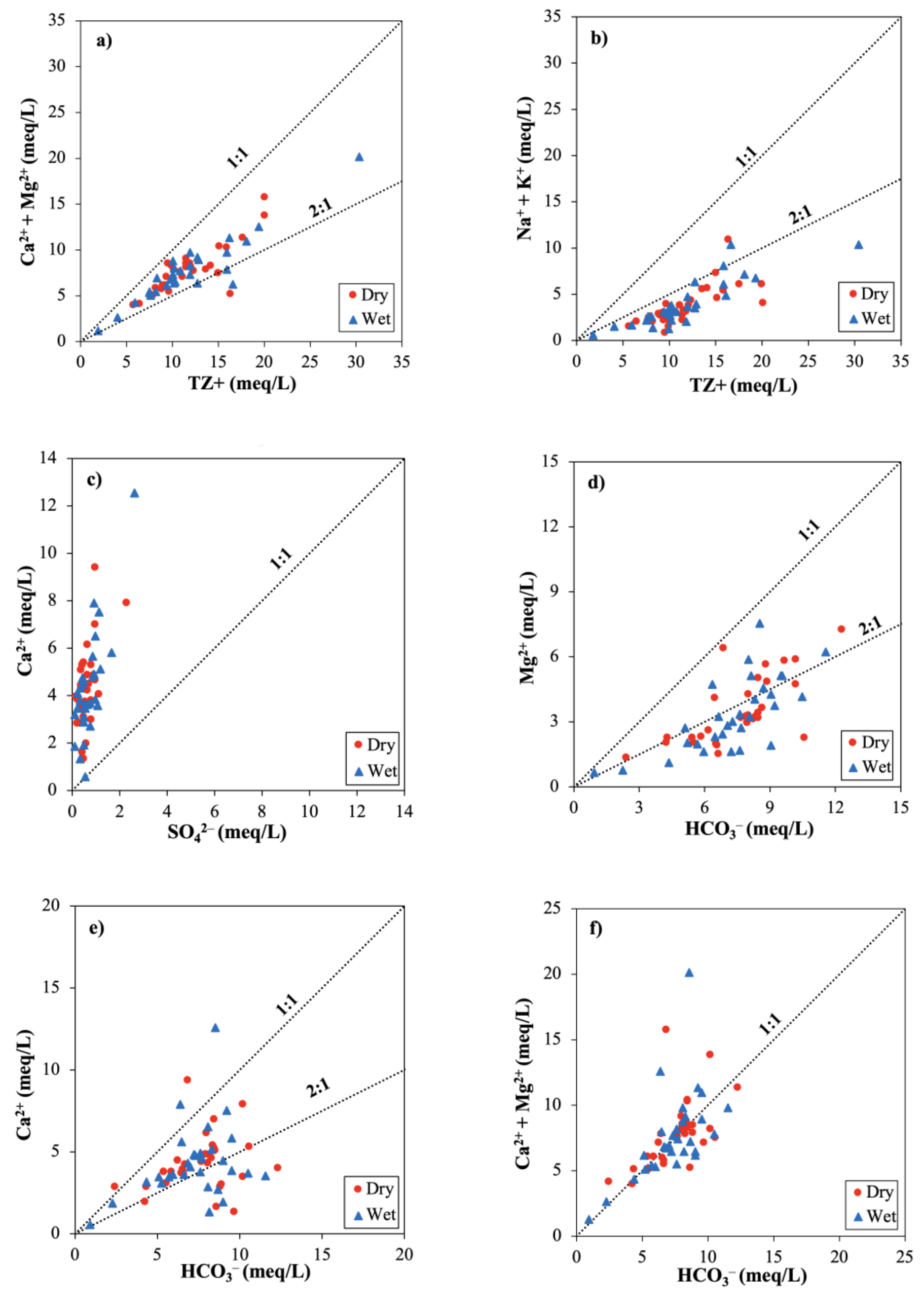
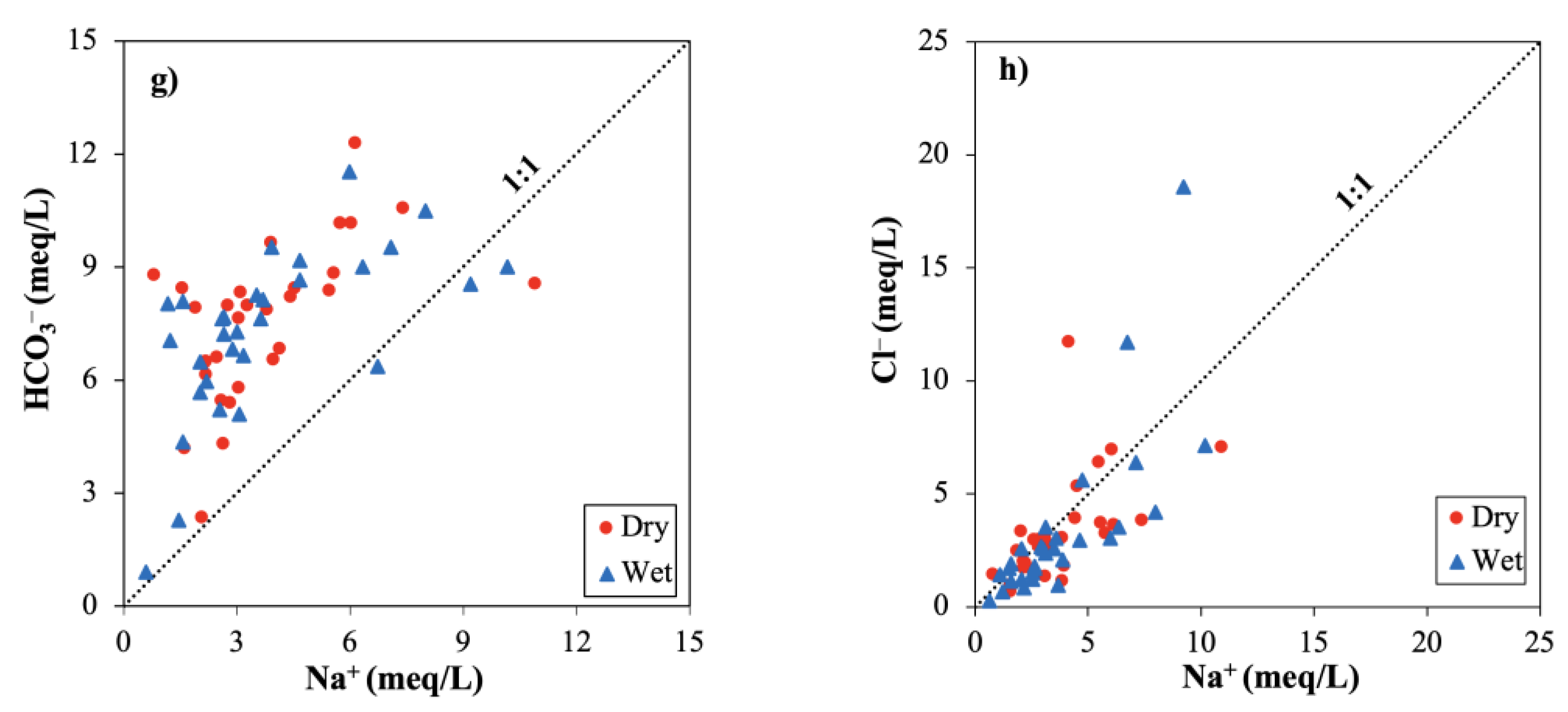
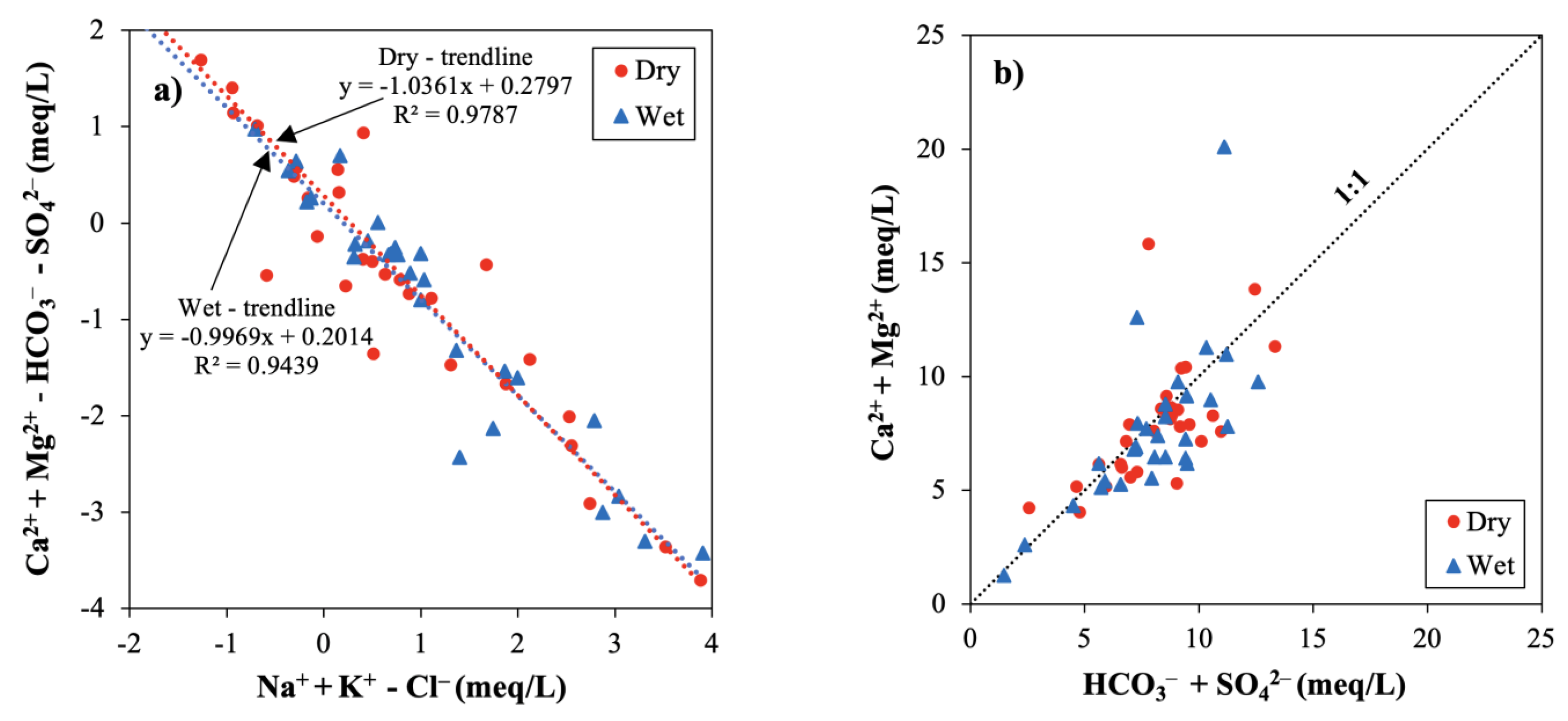
3.2.4. Hydrogeochemical Plots
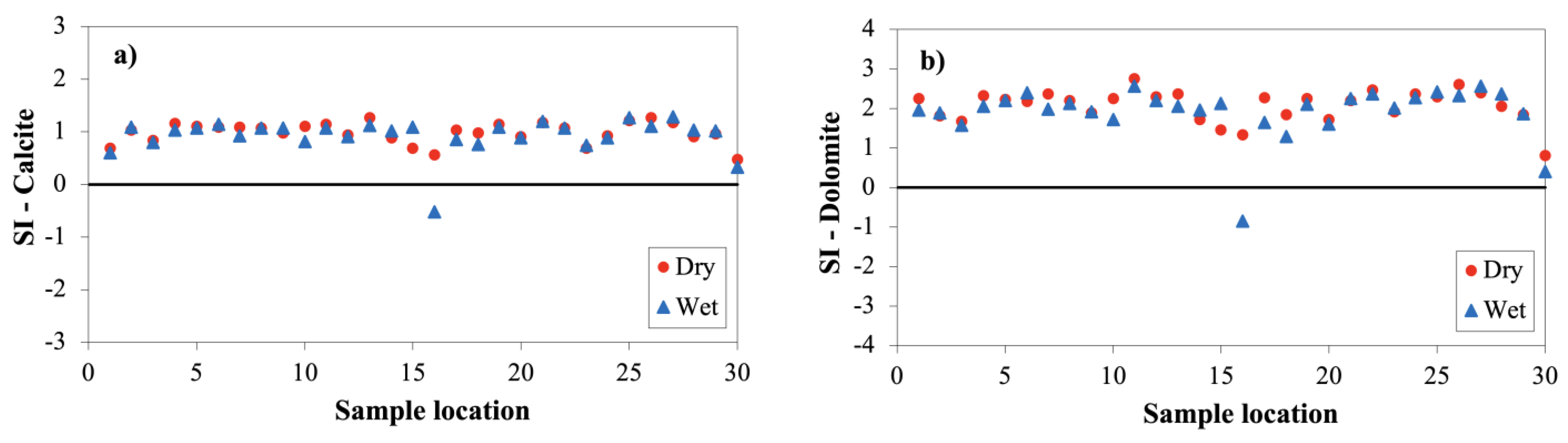
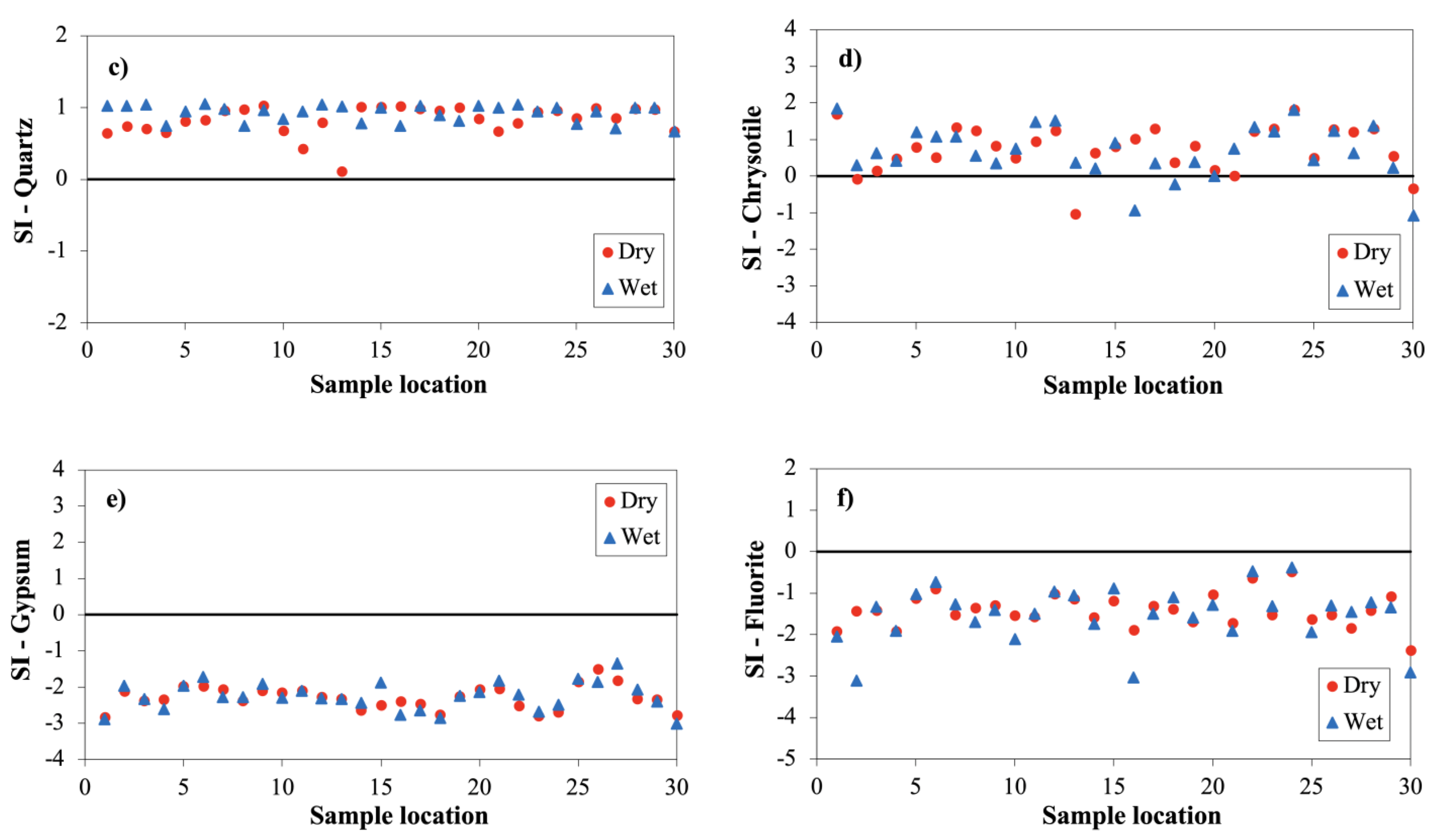
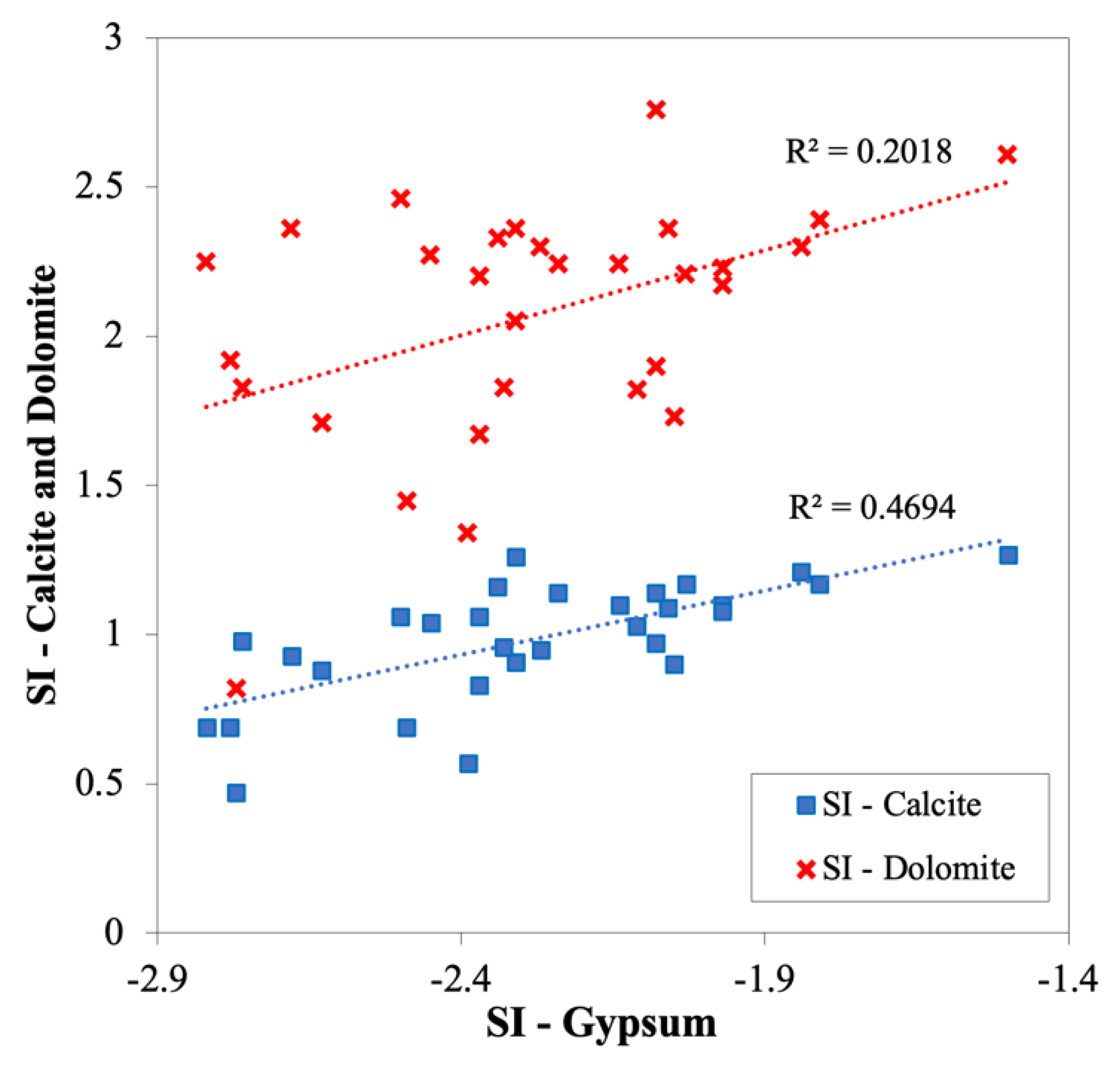
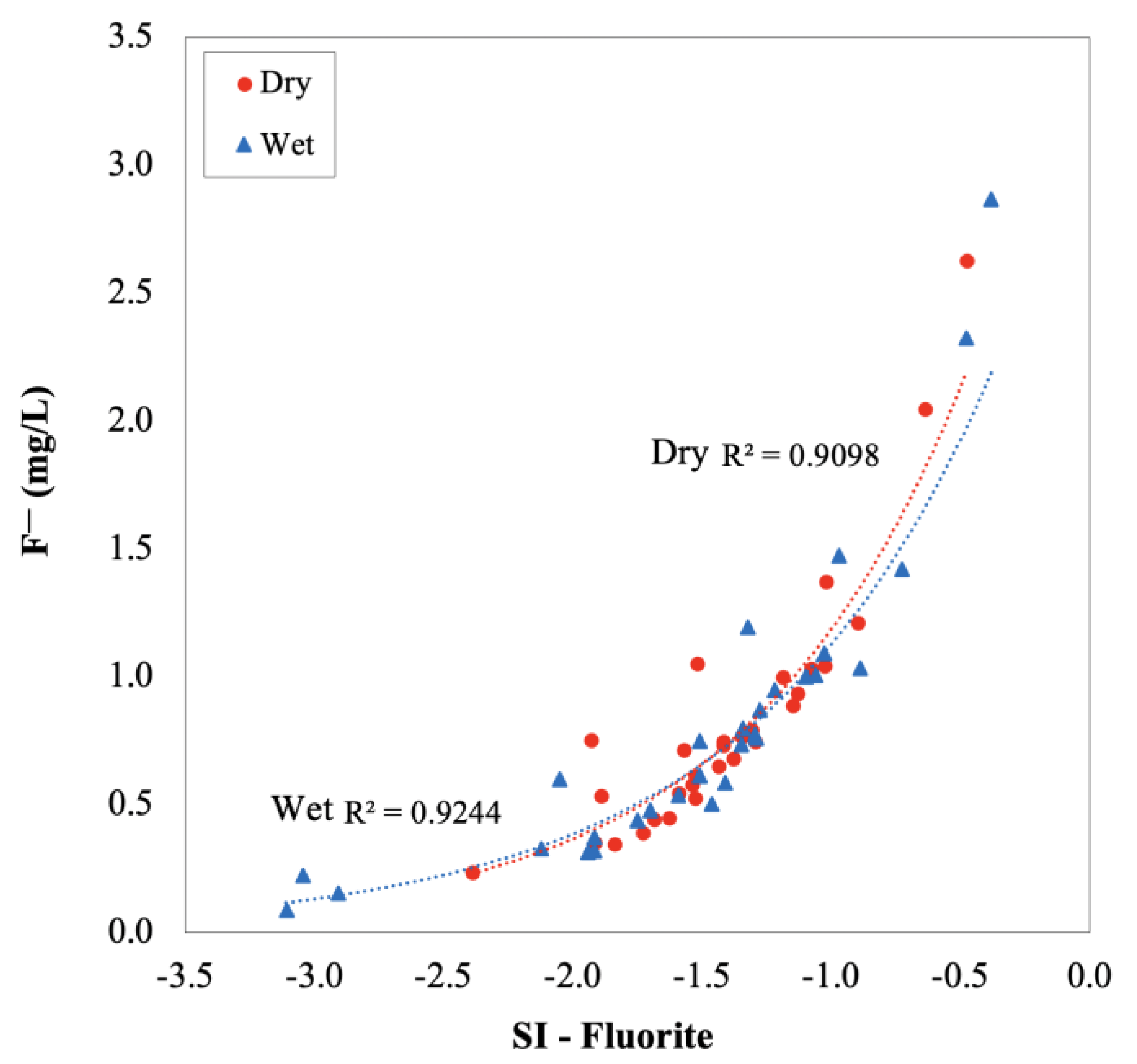
3.2.5. Saturation Index (SI)
3.3. Possible Implications for CKDu
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Margat, J.; van der Gun, J. Groundwater around the World; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar] [CrossRef]
- Schmoll, O.; Howard, G.; Chilton, G.; Chorus, I. (Eds.) Protecting Groundwater for Health: Managing the Quality of Drinking-Water Sources; World Health Organization: Geneva, Switzerland, 2013; Volumn 12. [Google Scholar] [CrossRef]
- Wada, Y.; van Beek, L.P.H.; van Kempen, C.M.; Reckman, J.W.T.; Vasak, S.; Bierkens, M.F.P. Global depletion of groundwater resources. Geophys. Res. Lett. 2021, 37. [Google Scholar] [CrossRef] [Green Version]
- Elango, L.; Kannan, R. Rock-water interaction and its control on chemical composition of groundwater. Dev. Environ. Sci. 2007, 5, 229–243. [Google Scholar] [CrossRef]
- Maghrebi, M.; Noori, R.; Partani, S.; Araghi, A.; Barati, R.; Farnoush, H.; Haghighi, A.T. Iran’s groundwater hydrochemistry. Earth Space Sci. 2021, 8, e2021EA001793. [Google Scholar] [CrossRef]
- Meenakshi, G.V.K.; Kavita, R.; Malik, A. Groundwater quality in some villages of Haryana, India: Focus on fluoride and fluorosis. J. Hazard. Mater. 2004, 106, 85–97. [Google Scholar] [CrossRef]
- Smith, A.H.; Lingas, E.O.; Rahman, M. Contamination of drinking-water by arsenic in Bangladesh: A public health emergency. Bull. World Health Organ. 2000, 78, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Gifford, F.J.; Gifford, R.M.; Eddleston, M.; Dhaun, N. Endemic nephropathy around the world. Kidney Int. Rep. 2017, 2, 282–292. [Google Scholar] [CrossRef] [Green Version]
- Weaver, V.M.; Fadrowski, J.J.; Jaar, B.G. Global dimensions of chronic kidney disease of unknown etiology (CKDu): A modern era environmental and/or occupational nephropathy? BMC Nephrol. 2015, 16, 145. [Google Scholar] [CrossRef] [Green Version]
- Noble, A.; Amerasinghe, P.; Manthrithilake, H.; Arasalingam, S. Review of literature on chronic kidney disease of unknown etiology (CKDu) in Sri Lanka. In IWMI Working Paper—158; International Water Management Institute (IWMI): Colombo, Sri Lanka, 2014; 41p. [Google Scholar] [CrossRef]
- Wickramarathna, S.; Balasooriya, S.; Diyabalanage, S.; Chandrajith, R. Tracing environmental aetiological factors of chronic kidney diseases in the dry zone of Sri Lanka—A hydrogeochemical and isotope approach. J. Trace Elements Med. Biol. 2017, 44, 298–306. [Google Scholar] [CrossRef]
- Chandrajith, R.; Nanayakkara, S.; Itai, K.; Aturaliya, T.N.C.; Dissanayake, C.B.; Abeysekera, T.; Harada, K.; Watanabe, T.; Koizumi, A. Chronic kidney diseases of uncertain etiology (CKDue) in Sri Lanka: Geographic distribution and environmental implications. Environ. Geochem. Health 2010, 33, 267–278. [Google Scholar] [CrossRef]
- Imbulana, S.; Oguma, K. Groundwater as a potential cause of Chronic Kidney Disease of unknown etiology (CKDu) in Sri Lanka: A review. J. Water Health 2021, 19, 393–410. [Google Scholar] [CrossRef]
- Rubasinghe, R.; Gunatilake, S.K.; Chandrajith, R. Geochemical characteristics of groundwater in different climatic zones of Sri Lanka. Environ. Earth Sci. 2015, 74, 3067–3076. [Google Scholar] [CrossRef]
- Imbulana, S.; Oguma, K.; Takizawa, S. Evaluation of groundwater quality and reverse osmosis water treatment plants in the endemic areas of Chronic Kidney Disease of Unknown Etiology (CKDu) in Sri Lanka. Sci. Total Environ. 2020, 745, 140716. [Google Scholar] [CrossRef] [PubMed]
- Department of Census and Statistics Sri Lanka. Mid-year Population Estimates by District & Sex 2014–2019. 2019. Available online: http://www.statistics.gov.lk/pophousat/vitalstatistics/midyearpopulation/mid-year%20population%20by%20district.pdf (accessed on 20 October 2021).
- Ranasinghe, A.V.; Kumara, G.W.G.P.; Karunarathna, R.H.; De Silva, A.P.; Sachintani, K.G.D.; Gunawardena, J.M.C.N.; Kumari, S.K.C.R.; Sarjana, M.S.F.; Chandraguptha, J.S.; De Silva, M.V.C. The incidence, prevalence and trends of chronic kidney disease and chronic kidney disease of uncertain aetiology (CKDu) in the North Central Province of Sri Lanka: An analysis of 30,566 patients. BMC Nephrol. 2019, 20, 338. [Google Scholar] [CrossRef] [PubMed]
- de Costa, W.A.J.M. Climate change in Sri Lanka: Myth or reality? Evidence from long-term meteorological data. J. Natl. Sci. Found. Sri Lanka 2008, 36, 63. [Google Scholar] [CrossRef] [Green Version]
- Athula, S.; Kanchana, W. Commons vs. Commons: Managing Scarce Water Resources in Dry Zone Village Tank Systems in Sri Lanka; EEPSEA: Los Baños, Philippines, 2011. [Google Scholar] [CrossRef]
- Edirisinghe, E.A.N.V.; Manthrithilake, H.; Pitawala, H.M.T.G.A.; Dharmagunawardhane, H.A.; Wijayawardane, R.L. Geochemical and isotopic evidences from groundwater and surface water for understanding of natural contamination in chronic kidney disease of unknown etiology (CKDu) endemic zones in Sri Lanka. Isot. Environ. Health Stud. 2017, 54, 244–261. [Google Scholar] [CrossRef]
- Maduranga, W.L.S.; Lewangamage, C.S. Development of wind loading maps for Sri Lanka for use with different wind loading codes. Eng. J. Inst. Eng. Sri Lanka 2018, 51, 47. [Google Scholar] [CrossRef] [Green Version]
- Elliott, D.; Schwartz, M.; Scott, G.; Haymes, S.; Heimiller, D.; George, R. Wind Energy Resource Atlas of Sri Lanka and the Maldives; National Renewable Energy Lab.(NREL): Golden, CO, USA, 2003. [CrossRef] [Green Version]
- Gilliland, K.; Simpson, I.; Adderley, W.; Burbidge, C.; Cresswell, A.; Sanderson, D.; Coningham, R.; Manuel, M.; Strickland, K.; Gunawardhana, P.; et al. The dry tank: Development and disuse of water management infrastructure in the Anuradhapura hinterland, Sri Lanka. J. Archaeol. Sci. 2013, 40, 1012–1028. [Google Scholar] [CrossRef] [Green Version]
- Cooray, P.G. The precambrian of Sri Lanka: A historical review. Precambrian Res. 1994, 66, 3–18. [Google Scholar] [CrossRef]
- Irrigation Department Sri Lanka. Soil Map of Sri-Lanka—ESDAC—European Commission. 1988. Available online: https://esdac.jrc.ec.europa.eu/content/soil-map-sri-lanka (accessed on 20 October 2021).
- Kodituwakku, K.A.D.; Weerasinghe, H.A.S.; Wijayawardhana, L.M.J.R. Report on the Availability of Lands for Sugarcane Cultivation in Sri Lanka Sugarcane Research Institute. 2010. Available online: http://sugarres.lk/wp-content/uploads/2020/05/Report-on-Land-Availability-for-Sugarcane-Cultivation.pdf (accessed on 21 November 2021).
- Pathmarajah, S. Use of Groundwater for Agriculture in Sri Lanka. 2002. Available online: https://www.researchgate.net/publication/237022438_Use_of_groundwater_for_agriculture_in_Sri_Lanka (accessed on 20 October 2021).
- Panabokke, C.R.; Ariyaratne, B.R.; Seneviratne, A.; Wijekoon, D.; Molle, F. Characterization and monitoring of the regolith aquifer within four selected cascades (sub-watersheds) of the Malala Oya Basin [Sri Lanka]. In IWMI Working Papers—122; International Water Management Institute (IWMI): Colombo, Sri Lanka, 2007; Available online: http://www.iwmi.cgiar.org/Publications/Working_Papers/working/WOR122.pdf (accessed on 20 October 2021).
- Jackson, P.E. Ion chromatography in environmental analysis. Encycl. Anal. Chem. 2000, 2779–2801. [Google Scholar] [CrossRef]
- Eaton, A.D.; Clesceri, L.S.; Rice, E.W.; Greenberg, A.E.; Franson, M.A.H.; American Public Health Association (Eds.) Standard Methods for the Examination of Water & Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Michalski, R. Applications of ion chromatography for the determination of inorganic cations. Crit. Rev. Anal. Chem. 2009, 39, 230–250. [Google Scholar] [CrossRef]
- Deutsch, W.J. Groundwater Geochemistry: Fundamentals and Applications to Contamination, 1st ed.; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar] [CrossRef]
- Weiner, E.R. Applications of Environmental Aquatic Chemistry—Google Books; Taylor & Francis: Oxfordshire, UK, 2013; Available online: https://www.google.co.jp/books/edition/Applications_of_Environmental_Aquatic_Ch/4ZlqSed6IQwC?hl=en&gbpv=1&dq=calculating+hardness+of+groundwater&pg=PA93&printsec=frontcover (accessed on 19 November 2021).
- R Core Development Team. R: A Language and Environment for Statistical Computing, 3.2.1. 2015. Available online: http://www.r-project.org/ (accessed on 20 October 2021).
- Piper, A.M. A graphic procedure in the geochemical interpretation of water-analyses. Eos Trans. Am. Geophys. Union 1944, 25, 914–928. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mechanisms Controlling World Water Chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef] [PubMed]
- Parkhurst, D.; Appelo, C. Description of Input and Examples for PHREEQC Version 3-A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations. Available online: http://www.hydrochemistry.eu (accessed on 8 May 2021).
- Garrels, R.M.; Mackenzie, F.T. Origin of the Chemical Compositions of Some Springs and Lakes; ACS Publications: Washington, DC, USA, 1967; pp. 222–242. [Google Scholar] [CrossRef]
- Lloyd, J.W.; Heathcote, J.A. Natural Inorganic Hydrochemistry in Relation to Ground Water; Oxford University Press: New York, NY, USA, 1985. [Google Scholar]
- Sri Lanka Standards Institute. Drinking Water Standards: Sri Lanka Standards for Potable Water—SLS 614, 1983; Sri Lanka Standards Institute: Colombo, Sri Lanka, 1947; Available online: https://investsrilanka.com/wp-content/uploads/2020/11/environmental_norms.pdf (accessed on 20 October 2021).
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011; p. 340. [Google Scholar] [CrossRef]
- Zhou, X.; Shen, Y.; Zhang, H.; Song, C.; Li, J.; Liu, Y. Hydrochemistry of the natural low pH groundwater in the coastal aquifers near Beihai, China. J. Ocean Univ. China 2015, 14, 475–483. [Google Scholar] [CrossRef]
- Hem, J.D. Study and interpretation of the chemical characteristics of natural water. In US Geological Survey Water-Supply Paper; Department of the Interior, US Geological Survey: Reston, VA, USA, 1985; Volume 2254. [Google Scholar]
- Jayasumana, C.; Gunatilake, S.; Senanayake, P. Glyphosate, hard water and nephrotoxic metals: Are they the culprits behind the epidemic of chronic kidney disease of unknown etiology in Sri Lanka? Int. J. Environ. Res. Public Health 2014, 11, 2125–2147. [Google Scholar] [CrossRef] [Green Version]
- Dharma-Wardana, M.W.C. Chronic kidney disease of unknown etiology and the effect of multiple-ion interactions. Environ. Geochem. Health 2017, 40, 705–719. [Google Scholar] [CrossRef] [Green Version]
- Wasana, H.M.S.; Perera, G.D.R.K.; Gunawardena, P.D.S.; Fernando, P.S.; Bandara, J. WHO water quality standards Vs Synergic effect(s) of fluoride, heavy metals and hardness in drinking water on kidney tissues. Sci. Rep. 2017, 7, srep42516. [Google Scholar] [CrossRef]
- Balasooriya, S.; Munasinghe, H.; Herath, A.T.; Diyabalanage, S.; Ileperuma, O.A.; Manthrithilake, H.; Daniel, C.; Amann, K.; Zwiener, C.; Barth, J.A.C.; et al. Possible links between groundwater geochemistry and chronic kidney disease of unknown etiology (CKDu): An investigation from the Ginnoruwa region in Sri Lanka. Exp. Health 2019, 12, 823–834. [Google Scholar] [CrossRef]
- Moore, K.B.; Ekwurzel, B.; Esser, B.K.; Hudson, G.B.; Moran, J.E. Sources of groundwater nitrate revealed using residence time and isotope methods. Appl. Geochem. 2006, 21, 1016–1029. [Google Scholar] [CrossRef] [Green Version]
- Ghahremanzadeh, H.; Noori, R.; Baghvand, A.; Nasrabadi, T. Evaluating the main sources of groundwater pollution in the southern Tehran aquifer using principal component factor analysis. Environ. Geochem. Health 2017, 40, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Chandrajith, R.; Padmasiri, J.; Dissanayake, C.; Prematilaka, K. Spatial distribution of fluoride in groundwater of Sri Lanka. J. Natl. Sci. Found. Sri Lanka 2012, 40, 303–309. [Google Scholar] [CrossRef] [Green Version]
- Chandrajith, R.; Dissanayake, C.; Ariyarathna, T.; Herath, H.; Padmasiri, J. Dose-dependent Na and Ca in fluoride-rich drinking water—Another major cause of chronic renal failure in tropical arid regions. Sci. Total Environ. 2011, 409, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, C.B. Water quality and dental health in the Dry Zone of Sri Lanka. Geol. Soc. London Spéc. Publ. 1996, 113, 131–140. [Google Scholar] [CrossRef]
- Jha, M.K.; Shekhar, A.; Jenifer, M.A. Assessing groundwater quality for drinking water supply using hybrid fuzzy-GIS-based water quality index. Water Res. 2020, 179, 115867. [Google Scholar] [CrossRef]
- Naseh, M.R.V.; Noori, R.; Berndtsson, R.; Adamowski, J.; Sadatipour, E. Groundwater pollution sources apportionment in the Ghaen Plain, Iran. Int. J. Environ. Res. Public Health 2018, 15, 172. [Google Scholar] [CrossRef] [Green Version]
- Noori, R.; Maghrebi, M.; Mirchi, A.; Tang, Q.; Bhattarai, R.; Sadegh, M.; Noury, M.; Haghighi, A.T.; Kløve, B.; Madani, K. Anthropogenic depletion of Iran’s aquifers. Proc. Natl. Acad. Sci. USA 2021, 118, e2024221118. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.S. Seasonal variation of groundwater quality in a part of Guntur District, Andhra Pradesh, India. Environ. Earth Sci. 2005, 49, 413–429. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, D.; Zhou, P.; Qu, S.; Liao, F.; Wang, G. Hydrochemical characteristics of groundwater and dominant water—Rock interactions in the Delingha. Water 2020, 12, 836. [Google Scholar] [CrossRef] [Green Version]
- Dissanayake, C.B.; Senaratne, A.; Weerasooriya, S.V.R. Geochemistry of well water and cardiovascular diseases in Sri Lanka. Int. J. Environ. Stud. 1982, 19, 195–203. [Google Scholar] [CrossRef]
- Dissanayake, C.B.; Chandrajith, R. The hydrogeological and geochemical characteristics of groundwater of Sri Lanka. In Groundwater of South Asia; Springer: Singapore, 2018; pp. 405–428. [Google Scholar] [CrossRef]
- McDonough, L.K.; Meredith, K.T.; Nikagolla, C.; Banati, R.B. The influence of water–rock interactions on household well water in an area of high prevalence chronic kidney disease of unknown aetiology (CKDu). NPJ Clean Water 2021, 4, 2. [Google Scholar] [CrossRef]
- Walraevens, K.; Bakundukize, C.; Mtoni, Y.E.; Van Camp, M. Understanding the hydrogeochemical evolution of groundwater in Precambrian basement aquifers: A case study of Bugesera region in Burundi. J. Geochem. Explor. 2018, 188, 24–42. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, J.; Feng, J.; Wang, M.; Wu, G. Hydrogeochemical characteristics and the suitability of groundwater in the alluvial-diluvial plain of Southwest Shandong Province, China. Water 2019, 11, 1577. [Google Scholar] [CrossRef] [Green Version]
- Luo, W.; Gao, X.; Zhang, X. Geochemical processes controlling the groundwater chemistry and fluoride contamination in the Yuncheng Basin, China—An area with complex hydrogeochemical conditions. PLoS ONE 2018, 13, e0199082. [Google Scholar] [CrossRef] [PubMed]
- Chenini, I.; Farhat, B.; Ben Mammou, A. Identification of major sources controlling groundwater chemistry from a multilayered aquifer system. Chem. Speciat. Bioavailab. 2010, 22, 183–189. [Google Scholar] [CrossRef]
- Zaidi, F.K.; Nazzal, Y.; Jafri, M.K.; Naeem, M.; Ahmed, I. Reverse ion exchange as a major process controlling the groundwater chemistry in an arid environment: A case study from northwestern Saudi Arabia. Environ. Monit. Assess. 2015, 187, 1–18. [Google Scholar] [CrossRef]
- Kumar, S.K.; Rammohan, V.; Sahayam, J.D.; Jeevanandam, M. Assessment of groundwater quality and hydrogeochemistry of Manimuktha River basin, Tamil Nadu, India. Environ. Monit. Assess. 2008, 159, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wu, X.; Qian, C.; Zhu, G. Hydrogeochemistry and groundwater quality assessment of high fluoride levels in the Yanchi endorheic region, northwest China. Appl. Geochem. 2018, 98, 404–417. [Google Scholar] [CrossRef]
- Srinivasamoorthy, K.; Chidambaram, S.; Prasanna, M.V.; Vasanthavihar, M.; Peter, J.; Anandhan, P. Identification of major sources controlling groundwater chemistry from a hard rock terrain—A case study from Mettur taluk, Salem district, Tamil Nadu, India. J. Earth Syst. Sci. 2008, 117, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Rajmohan, N.; Elango, L. Identification and evolution of hydrogeochemical processes in the groundwater environment in an area of the Palar and Cheyyar River Basins, Southern India. Environ. Geol. 2004, 46, 47–61. [Google Scholar] [CrossRef]
- Schoeller, H. Qualitative evaluation of groundwater resources. In Methods and Techniques of Ground-water Investigation and Development: Water Resources Series No. 33; United Nations: New York, NY, USA, 1967; pp. 44–48. Available online: https://books.google.co.jp/books?id=xbI8mgEACAAJ&printsec=frontcover&source=gbs_ge_summary_r&cad=0#v=onepage&q&f=false (accessed on 20 October 2021).
- Chidambaram, S.; Anandhan, P.; Prasanna, M.V.; Srinivasamoorthy, K.; Vasanthavigar, M. Major ion chemistry and identification of hydrogeochemical processes controlling groundwater in and around Neyveli Lignite Mines, Tamil Nadu, South India. Arab. J. Geosci. 2012, 6, 3451–3467. [Google Scholar] [CrossRef]
- Ansari, A.; Deodhar, A.; Kumar, U.S. Modeling of geochemical processes and multivariate statistical analysis for hydrochemical assessment of spring water of the Outer Himalaya, India. Environ. Earth Sci. 2019, 78, 665. [Google Scholar] [CrossRef]
- Young, S.M.; Pitawala, A.; Ishiga, H. Factors controlling fluoride contents of groundwater in north-central and northwestern Sri Lanka. Environ. Earth Sci. 2010, 63, 1333–1342. [Google Scholar] [CrossRef]
- Saxena, V.; Ahmed, S. Inferring the chemical parameters for the dissolution of fluoride in groundwater. Environ. Geol. 2003, 43, 731–736. [Google Scholar] [CrossRef]
- Song, X.; Kayane, I.; Tanaka, T.; Shimada, J. A study of the groundwater cycle in Sri Lanka using stable isotopes. Hydrol. Process. 1999, 13, 1479–1496. [Google Scholar] [CrossRef]
- Gwenzi, W. Occurrence, behaviour, and human exposure pathways and health risks of toxic geogenic contaminants in serpentinitic ultramafic geological environments (SUGEs): A medical geology perspective. Sci. Total Environ. 2019, 700, 134622. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Asbestos in Drinking-Water: Draft Background Document for Development of WHO Guidelines for Drinking-Water Quality (Version for Public Review). 2020. Available online: https://www.who.int/docs/default-source/wash-documents/wash-chemicals/gdwq-asbestos-background-document-for-public-review.pdf?ua=1 (accessed on 22 May 2021).
- European Commission’s Joint Research Centre—European Soil Data Centre (ESDAC). Geological Map of Sri-Lanka—ESDAC—European Commission. Geological Map of Sri Lanka. 1982. Available online: https://esdac.jrc.ec.europa.eu/content/geological-map-sri-lanka (accessed on 22 October 2021).
- Udeshani, W.; Dissanayake, H.; Gunatilake, S.; Chandrajith, R. Assessment of groundwater quality using water quality index (WQI): A case study of a hard rock terrain in Sri Lanka. Groundw. Sustain. Dev. 2020, 11, 100421. [Google Scholar] [CrossRef]
- Nikagolla, C.; Meredith, K.T.; Dawes, L.A.; Banati, R.B.; Millar, G.J. Using water quality and isotope studies to inform research in chronic kidney disease of unknown aetiology endemic areas in Sri Lanka. Sci. Total Environ. 2020, 745, 140896. [Google Scholar] [CrossRef]
| Water Quality Parameter | Dry Season | Wet Season | p Values from t-Test b | MAL c | % Samples Didn’t Meet MAL c | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | SD a | Min | Max | Mean | SD a | Dry | Wet | |||
| Temperature | 27 | 33 | 30 | 1 | 26 | 34 | 29 | 2 | 0.001 * | – | – | – |
| pH | 6.1 | 7.2 | 6.6 | 0.3 | 6.1 | 7.8 | 7.3 | 0.3 | 2.761 × 10−12 ** | 6.5–8.5 | 33 | 3 |
| Total Alkalinity (mg/L as CaCO3) | 119 | 614 | 379 | 104 | 46 | 578 | 364 | 112 | 0.602 | 200 | 90 | 93 |
| Electrical Conductivity (µS/cm) | 555 | 1941 | 1157 | 364 | 195 | 2820 | 958 | 389 | 0.238 | – | – | – |
| Total Dissolved Solids (ppm) | 278 | 971 | 578 | 182 | 97 | 1410 | 510 | 256 | 0.238 | 500 | 67 | 40 |
| Total Hardness (mg/L as CaCO3) | 202 | 791 | 394 | 130 | 62 | 1005 | 382 | 168 | 0.766 | 250 | 97 | 93 |
| Sodium/Na+ (mg/L) | 19 | 250 | 86 | 48 | 14 | 234 | 87 | 57 | 0.901 | 200 | 3 | 7 |
| Calcium/Ca2+ (mg/L) | 27 | 189 | 87 | 35 | 12 | 252 | 87 | 45 | 0.980 | 100 | 27 | 23 |
| Magnesium/Mg2+ (mg/L) | 17 | 89 | 43 | 19 | 8 | 92 | 40 | 21 | 0.586 | 30 | 70 | 67 |
| Potassium/K+ (mg/L) | 0.44 | 18.71 | 1.77 | 1.18 | 0.34 | 44.60 | 2.66 | 2.36 | 0.146 | – | – | – |
| Bicarbonate/HCO3− (mg/L) | 145 | 749 | 462 | 127 | 56 | 705 | 444 | 136 | 0.602 | – | – | – |
| Chloride/Cl− (mg/L) | 26 | 418 | 106 | 60 | 10 | 660 | 100 | 84 | 0.943 | 250 | 7 | 10 |
| Sulfate/SO42− (mg/L) | 7 | 110 | 29 | 19 | 6 | 125 | 34 | 24 | 0.392 | 250 | 0 | 0 |
| Fluoride/F− (mg/L) | 0.24 | 2.63 | 0.72 | 0.28 | 0.09 | 2.87 | 0.69 | 0.36 | 0.941 | 11.5 d | 27 | 30 |
| Nitrate/NO3− (mg/L) | 0.00 | 19.23 | 1.96 | 2.14 | 0.00 | 1.50 | 0.35 | 0.21 | 0.003 * | 50 | 0 | 0 |
| (a) | |||||||||
| Parameter | TDS | Na+ | K+ | Ca2+ | Mg2+ | HCO3− | Cl− | SO42− | F− |
| TDS | 1.000 | ||||||||
| Na+ | 0.636 | 1.000 | |||||||
| K+ | −0.023 | −0.076 | 1.000 | ||||||
| Ca2+ | 0.608 | 0.041 | 0.143 | 1.000 | |||||
| Mg2+ | 0.608 | 0.337 | −0.174 | 0.249 | 1.000 | ||||
| HCO3− | 0.623 | 0.559 | 0.011 | 0.239 | 0.690 | 1.000 | |||
| Cl− | 0.803 | 0.579 | −0.041 | 0.623 | 0.473 | 0.230 | 1.000 | ||
| SO42− | 0.556 | 0.327 | 0.067 | 0.574 | 0.472 | 0.391 | 0.484 | 1.000 | |
| F− | −0.115 | 0.104 | −0.129 | −0.382 | 0.244 | 0.282 | −0.180 | −0.152 | 1.000 |
| (b) | |||||||||
| Parameter | TDS | Na+ | K+ | Ca2+ | Mg2+ | HCO3− | Cl− | SO42− | F− |
| TDS | 1.000 | ||||||||
| Na+ | 0.670 | 1.000 | |||||||
| K+ | 0.488 | 0.219 | 1.000 | ||||||
| Ca2+ | 0.797 | 0.399 | 0.567 | 1.000 | |||||
| Mg2+ | 0.595 | 0.621 | 0.205 | 0.440 | 1.000 | ||||
| HCO3− | 0.394 | 0.617 | 0.122 | 0.331 | 0.715 | 1.000 | |||
| Cl− | 0.877 | 0.755 | 0.482 | 0.780 | 0.603 | 0.277 | 1.000 | ||
| SO42− | 0.734 | 0.554 | 0.557 | 0.782 | 0.661 | 0.437 | 0.753 | 1.000 | |
| F− | 0.071 | 0.303 | −0.267 | −0.132 | 0.435 | 0.409 | 0.059 | 0.031 | 1.000 |
| Mineral | SI-Dry Season | SI-Wet Season | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | SD a | Min | Max | Mean | SD a | ||
| Calcite | (CaCO3) | 0.47 | 1.27 | 0.98 | 0.20 | −0.53 | 1.29 | 0.93 | 0.34 |
| Aragonite | (CaCO3) | 0.33 | 1.13 | 0.84 | 0.20 | −0.67 | 1.15 | 0.79 | 0.34 |
| Dolomite | (CaMg(CO3)2) | 0.82 | 2.76 | 2.07 | 0.41 | −0.84 | 2.56 | 1.92 | 0.67 |
| Quartz | (SiO2) | 0.11 | 1.03 | 0.83 | 0.20 | 0.66 | 1.05 | 0.92 | 0.12 |
| Chalcedony | (SiO2) | −0.30 | 0.62 | 0.41 | 0.20 | 0.24 | 0.63 | 0.51 | 0.12 |
| Chrysotile | (Mg3Si2O5(OH)4) | −1.04 | 1.81 | 0.75 | 0.63 | −1.09 | 1.84 | 0.67 | 0.70 |
| Sepiolite | (Mg2Si3O7·5OH:3H2O) | −1.62 | 1.68 | 0.79 | 0.67 | −0.66 | 1.88 | 0.93 | 0.61 |
| Gypsum | (CaSO4·2H2O) | −2.82 | −1.50 | −2.28 | 0.32 | −3.00 | −1.35 | −2.25 | 0.39 |
| Anhydrite | (CaSO4) | −3.01 | −1.69 | −2.48 | 0.32 | −3.21 | −1.54 | −2.45 | 0.39 |
| Halite | (NaCl) | −7.62 | −5.82 | −6.75 | 0.45 | −8.41 | −5.52 | −6.84 | 0.61 |
| Fluorite | (CaF2) | −2.39 | −0.48 | −1.42 | 0.40 | −3.11 | −0.38 | −1.52 | 0.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imbulana, S.; Oguma, K.; Takizawa, S. Seasonal Variations in Groundwater Quality and Hydrogeochemistry in the Endemic Areas of Chronic Kidney Disease of Unknown Etiology (CKDu) in Sri Lanka. Water 2021, 13, 3356. https://doi.org/10.3390/w13233356
Imbulana S, Oguma K, Takizawa S. Seasonal Variations in Groundwater Quality and Hydrogeochemistry in the Endemic Areas of Chronic Kidney Disease of Unknown Etiology (CKDu) in Sri Lanka. Water. 2021; 13(23):3356. https://doi.org/10.3390/w13233356
Chicago/Turabian StyleImbulana, Sachithra, Kumiko Oguma, and Satoshi Takizawa. 2021. "Seasonal Variations in Groundwater Quality and Hydrogeochemistry in the Endemic Areas of Chronic Kidney Disease of Unknown Etiology (CKDu) in Sri Lanka" Water 13, no. 23: 3356. https://doi.org/10.3390/w13233356







