Modeling and Simulation of Source Separation in Sanitation Systems for Reducing Emissions of Antimicrobial Resistances
Abstract
:1. Introduction
1.1. Significance of the Aquatic Environment for the Dissemination of AMR
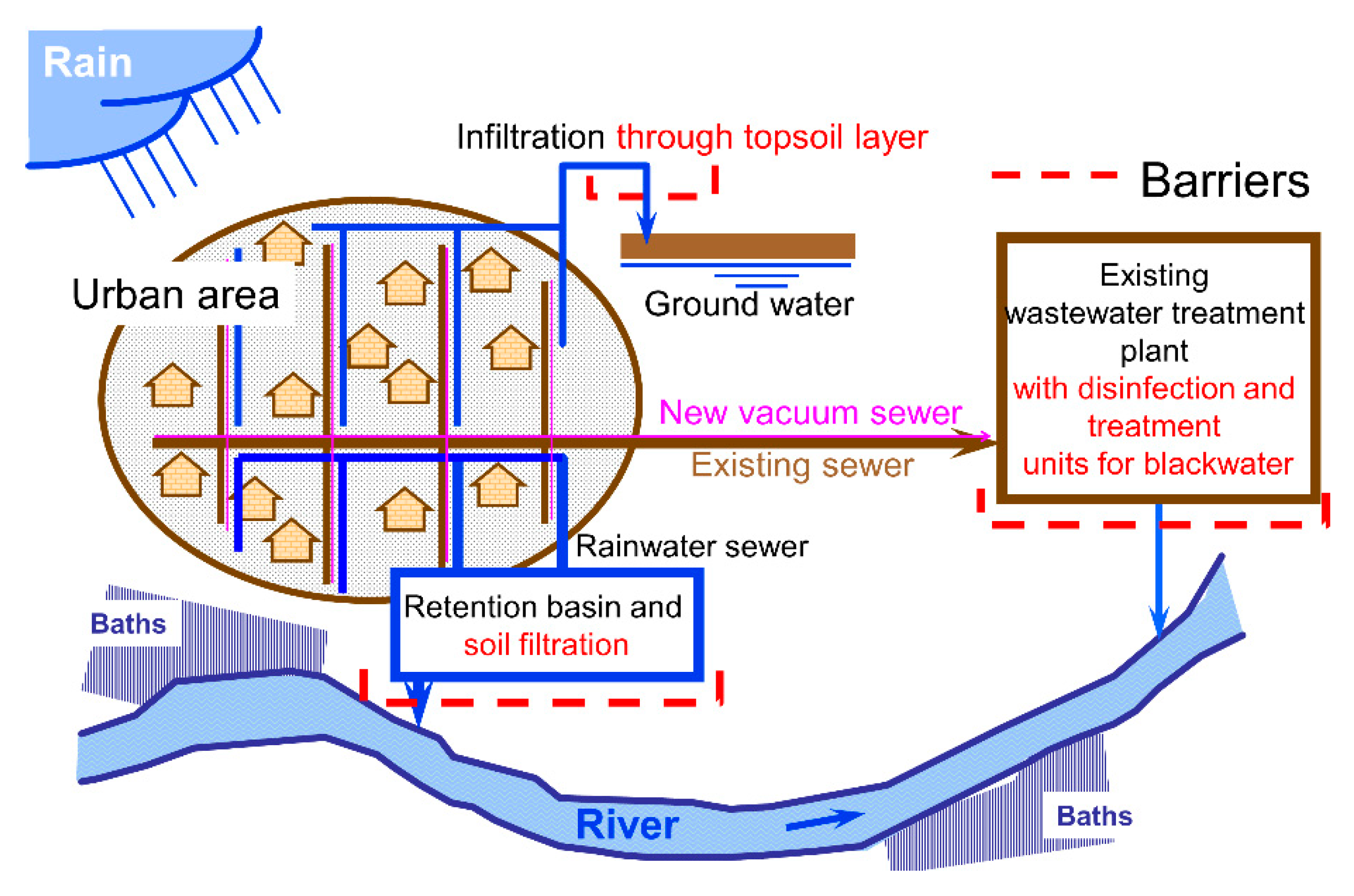
1.2. Input Paths in Urban Drainage
1.3. Reduction of AMR Emissions into the Aquatic Environment
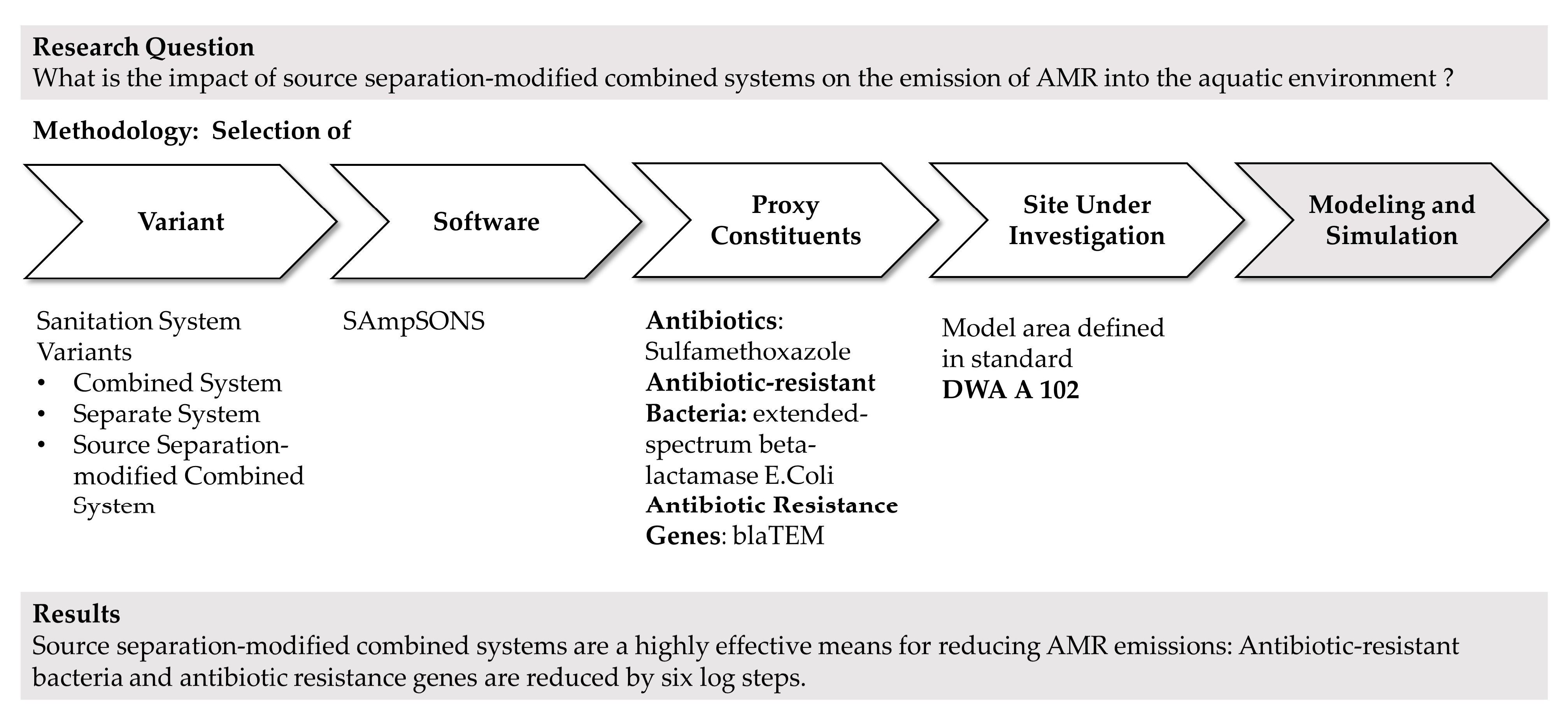
2. Materials and Methods
2.1. Proposed Option
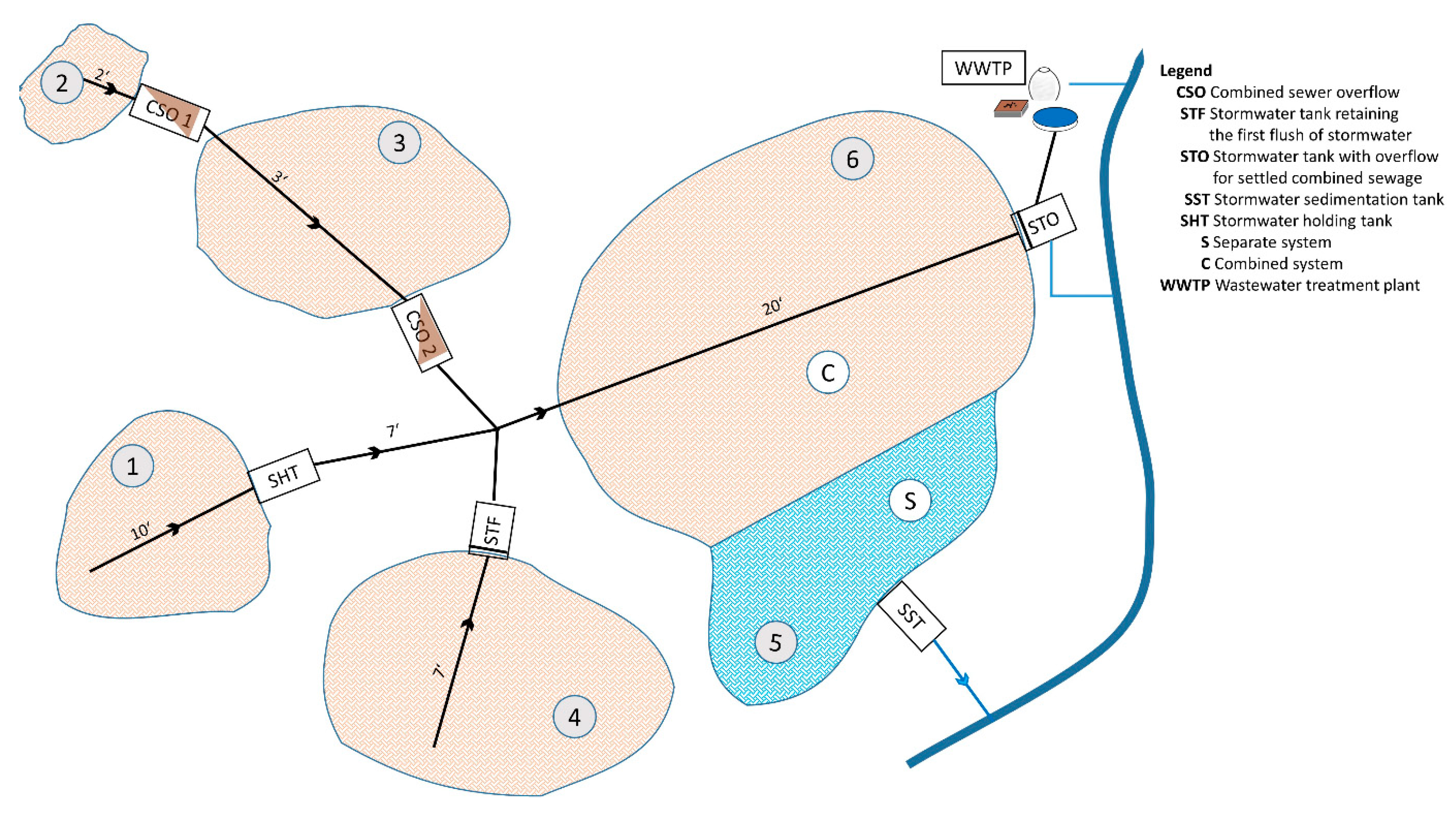
2.2. Sanitation Systems under Comparison
2.2.1. Combined System
2.2.2. Separate System
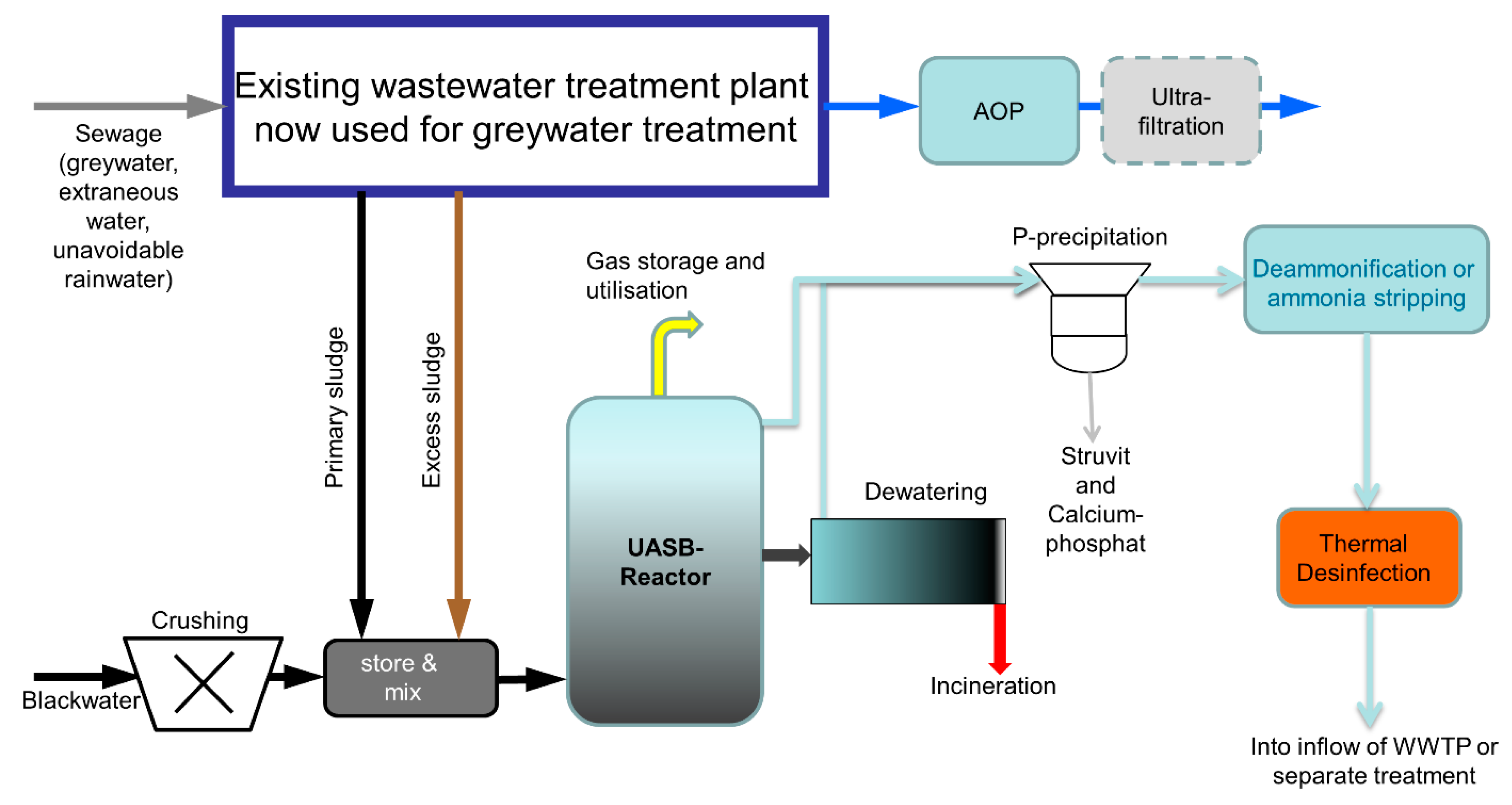
2.2.3. Source-Separation System
2.3. SAmpSONS Simulation Software
3. Results
3.1. Input Data
3.1.1. General Considerations
3.1.2. Selection of Constituents
3.1.3. Material Flows
3.2. Removal Rates of the Treatment Stages
3.2.1. General Considerations
3.2.2. Activated Sludge Process as Aerobic Treatment
| Removal Rates | Fraction into Sludge | Fraction into Effluent | Remark | Literature |
|---|---|---|---|---|
| 1.03 log 10 CFU/100 mL | unclear | rest | [41] | |
| 2.6 logarithm steps | unclear | rest | Median values, high variation | [8] |
| 2.6 logarithm steps | unclear | rest | [39] | |
| 2.44 logarithm steps | unclear | rest | [85] |
3.2.3. Anaerobic Treatment of Blackwater
| Constituent Category | Removal Rates | Fraction into Effluent | Remark |
|---|---|---|---|
| Antibiotics (ABs) | 75% | 25% | Assumption based on [86] |
| Antibiotic-resistant Bacteria (ARB) | 95% | 5% | Assumption based on [91,92,93,94] |
| Antibiotic Resistance Genes (ARGs) | 95% | 5% | Assumption based on [95,96] |
3.2.4. Disinfection of the Liquid Phase
3.2.5. Advanced Oxidative Processes
| Constituent Category | Removal Rates | Fraction into Effluent | Remark | Literature |
|---|---|---|---|---|
| Antibiotics (ABs) | 90% | 10% | [97] | |
| Antibiotic-resistant Bacteria (ARB) | 99.9% | 0.1% | Assumption: treatment of real wastewater for 15 min | [98] |
| Antibiotic Resistance Genes (ARGs) | 99.99% | 0.01% | [98] |
3.3. Specifics of Modeling Using SAmpSONS
3.4. Simulation Results
4. Techno-Economic Challenges
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 23 May 2021).
- Czekalski, N.; von Gunten, U.; Bürgmann, H. Antibiotikaresistenzen im Wasserkreislauf. Aqua Gas 2016, 9, 72–80. [Google Scholar]
- Bush, K.; Courvalin, P.; Dantas, G.; Davies, J.; Eisenstein, B.; Huovinen, P.; Jacoby, G.A.; Kishony, R.; Kreiswirth, B.N.; Kutter, E.; et al. Tackling antibiotic resistance. Nat. Rev. Microbiol. 2011, 9, 894–896. [Google Scholar] [CrossRef]
- Berendonk, T.U.; Manaia, C.M.; Merlin, C.; Fatta-Kassinos, D.; Cytryn, E.; Walsh, F.; Bürgmann, H.; Sørum, H.; Norström, M.; Pons, M.N.; et al. Tackling antibiotic resistance: The environmental framework. Nat. Rev. Microbiol. 2015, 13, 310–317. [Google Scholar] [CrossRef]
- Clatworthy, A.E.; Pierson, E.; Hung, D.T. Targeting virulence: A new paradigm for antimicrobial therapy. Nat. Chem. Biol. 2007, 3, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Buergmann, H.; Czekalski, N.; Bryner, A. Verbreitung Von Antibiotikaresistenzen Im Wasser. Available online: https://www.eawag.ch/fileadmin/Domain1/Beratung/Beratung_Wissenstransfer/Publ_Praxis/Faktenblaetter/fb_antibiotikaresistenzen_juli15.pdf (accessed on 2 July 2021).
- Taylor, J.; Hafner, M.; Yerushalmi, E.; Smith, R.; Bellasio, J.; Vardavas, R.; Bienkowska-Gibbs, T.; Rubin, J. Estimating the Economic Costs of Antimicrobial Resistance: Model and Results; RAND Corporation: Cambridge, UK, 2014. [Google Scholar]
- Firk, J.; Schleiffer, P.; Pinnekamp, J. Einträge von Antibiotikaresistenzen in Gewässer und Bewertung von Möglichkeiten zur Eintragsminderung. In Proceedings of the 53. Essener Tagung Für Wasserwirtschaft, Essen, Germany, 18–20 March 2020; Gesellschaft Zur Förderung Der Siedlungswasserwirtschaft An Der RWTH Aachen E.V.: Aachen, Germany, 2020; pp. 45/1–45/12. [Google Scholar]
- Gullberg, E.; Cao, S.; Berg, O.G.; Ilbäck, C.; Sandegren, L.; Hughes, D.; Andersson, D.I. Selection of resistant bacteria at very low antibiotic concentrations. PLoS Pathog. 2011, 7, e1002158. [Google Scholar] [CrossRef] [Green Version]
- Chow, L.; Waldron, L.; Gillings, M.R. Potential impacts of aquatic pollutants: Sub-clinical antibiotic concentrations induce genome changes and promote antibiotic resistance. Front. Microbiol. 2015, 6, 803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoll, C.; Sidhu, J.P.S.; Tiehm, A.; Toze, S. Prevalence of clinically relevant antibiotic resistance genes in surface water samples collected from Germany and Australia. Environ. Sci. Technol. 2012, 46, 9716–9726. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.F.; Ying, G.G.; Liu, S.; Zhou, L.J.; Zhao, J.L.; Tao, R.; Peng, P.A. Biological degradation and microbial function effect of norfloxacin in a soil under different conditions. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2012, 47, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Pinnekamp, J.; Firk, J.; Keysers, C.; Mousel, D.; Ruppelt, J.; Tondera, K. Elimination von Mikroschadstoffen, Mikroplastik, und antibiotikaresistenten Keimen. In Proceedings of the Bochumer Workshop 8, Bochum, Germany, 6–7 September 2016; Available online: https://docplayer.org/73494442-Elimination-von-mikroschadstoffen-mikroplastik-antibiotikaresistenten-keimen.html (accessed on 25 October 2021).
- Guo, J.; Li, J.; Chen, H.; Bond, P.L.; Yuan, Z. Metagenomic analysis reveals wastewater treatment plants as hotspots of antibiotic resistance genes and mobile genetic elements. Water Res. 2017, 123, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, H.; Blaak, H.; Kemper, M.; van Passel, M.; Hierink, F.; van Leuken, J.; De Roda Husman, A.M.; van der Grinten, E.; Rutgers, M.; Schijven, R.; et al. Bronnen Va Antibioticaresistentie in Het Milieu En Mogelijke Maatregelen; Rijksinstituut voor Volksgezondheid en Milieu RIVM: Bilthoven, The Netherlands, 2017. [CrossRef]
- Graham, D.W.; Bergeron, G.; Bourassa, M.W.; Dickson, J.; Gomes, F.; Howe, A.; Kahn, L.H.; Morley, P.S.; Scott, H.M.; Simjee, S.; et al. Complexities in understanding antimicrobial resistance across domesticated animal, human, and environmental systems. Ann. N. Y. Acad. Sci. 2019, 1441, 17–30. [Google Scholar] [CrossRef]
- Pinnekamp, J.; Firk, J.; Schleiffer, P. Bewertung der urbanen Gewässereinträge und Möglichkeiten der Eintragsminderung. In Proceedings of the Abschlussveranstaltung Des BMBF-Forschungsvorhabens Zu Antibiotikaresistenzen Im Wasserkreislauf (HyReKA), Eggenstein-Leopoldshafen, Germany, 3–4 April 2019. [Google Scholar]
- Mannina, G.; Viviani, G. Separate and combined sewer systems: A long-term modelling approach. Water Sci. Technol. 2009, 60, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Dettmar, J.; Brombach, H. Im Spiegel der Statistik: Abwasserkanalisation und Regenwasserbehandlung in Deutschland. Korresp. Abwasser Abfall 2019, 66, 354–364. [Google Scholar]
- Botturi, A.; Ozbayram, E.G.; Tondera, K.; Gilbert, N.I.; Rouault, P.; Caradot, N.; Gutierrez, O.; Daneshgar, S.; Frison, N.; Akyol, Ç.; et al. Combined sewer overflows: A critical review on best practice and innovative solutions to mitigate impacts on environment and human health. Crit. Rev. Environ. Sci. Technol. 2021, 51, 1585–1618. [Google Scholar] [CrossRef]
- Phillips, P.J.; Chalmers, A.T.; Gray, J.L.; Kolpin, D.W.; Foreman, W.T.; Wall, G.R. Combined sewer overflows: An environmental source of hormones and wastewater micropollutants. Environ. Sci. Technol. 2012, 46, 5336–5343. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, T.G. Mischwasserkanalisation 2021—Quo Vadis? KA Korresp. Abwasser Abfall 2021, 68, 430–439. [Google Scholar]
- Petrie, B. A review of combined sewer overflows as a source of wastewater-derived emerging contaminants in the environment and their management. Environ. Sci. Pollut. Res. 2021, 32095–32110. [Google Scholar] [CrossRef]
- Berger, C.; Falk, C.; Hetzel, F.; Pinnekamp, J.; Ruppelt, J.; Schleiffer, P.; Schmitt, J. Zustand der Kanalisation in Deutschland: Ergebnisse der DWA-Umfrage 2020. KA Korresp. Abwasser Abfall 2020, 67, 939–953. [Google Scholar] [CrossRef]
- Nickel, J.P.; Fuchs, S. Micropollutant emissions from combined sewer overflows. Water Sci. Technol. 2019, 80, 2179–2190. [Google Scholar] [CrossRef] [PubMed]
- Gasperi, J.; Garnaud, S.; Rocher, V.; Moilleron, R. Priority pollutants in wastewater and combined sewer overflow. Sci. Total Environ. 2008, 407, 263–272. [Google Scholar] [CrossRef]
- Gasperi, J.; Cladière, M.; Rocher, V.; Moilleron, R. Combined sewer overflow quality and EU Water Framework Directive. Urban Waters 2009, 1, 124–128. [Google Scholar]
- Weyrauch, P.; Matzinger, A.; Pawlowsky-Reusing, E.; Plume, S.; von Seggern, D.; Heinzmann, B.; Schroeder, K.; Rouault, P. Contribution of combined sewer overflows to trace contaminant loads in urban streams. Water Res. 2010, 44, 4451–4462. [Google Scholar] [CrossRef] [PubMed]
- Dris, R.; Gasperi, J.; Rocher, V.; Saad, M.; Renault, N.; Tassin, B. Microplastic contamination in an urban area: A case study in Greater Paris. Environ. Chem. 2015, 12, 592–599. [Google Scholar] [CrossRef]
- DECHEMA Gesellschaft für Chemische Technik und Biotechnologie. Risikomanagement Von Neuen Schadstoffen Und Krankheitserregern Im Wasserkreislauf Praxishandbuch; DECHEMA Gesellschaft für Chemische Technik und Biotechnologie, Ed.; DECHEMA, Gesellschaft für Chemische Technik und Biotechnologie: Frankfurt am Main, Germany, 2016. [Google Scholar]
- Kay, P.; Hughes, S.R.; Ault, J.R.; Ashcroft, A.E.; Brown, L.E. Widespread, routine occurrence of pharmaceuticals in sewage effluent, combined sewer overflows and receiving waters. Environ. Pollut. 2017, 220, 1447–1455. [Google Scholar] [CrossRef] [Green Version]
- Munro, K.; Martins, C.P.B.; Loewenthal, M.; Comber, S.; Cowan, D.A.; Pereira, L.; Barron, L.P. Evaluation of combined sewer overflow impacts on short-term pharmaceutical and illicit drug occurrence in a heavily urbanised tidal river catchment (London, UK). Sci. Total Environ. 2019, 657, 1099–1111. [Google Scholar] [CrossRef]
- Brunsch, A.F.; Zubieta Florez, P.; Langenhoff, A.A.M.; ter Laak, T.L.; Rijnaarts, H.H.M. Retention soil filters for the treatment of sewage treatment plant effluent and combined sewer overflow. Sci. Total Environ. 2020, 699, 134426. [Google Scholar] [CrossRef]
- Mutzner, L.; Bohren, C.; Mangold, S.; Bloem, S.; Ort, C. Spatial Differences among Micropollutants in Sewer Overflows: A Multisite Analysis Using Passive Samplers. Environ. Sci. Technol. 2020, 54, 6584–6593. [Google Scholar] [CrossRef]
- Young, S.; Juhl, A.; O’Mullan, G.D. Antibiotic-resistant bacteria in the Hudson River Estuary linked to wet weather sewage contamination. J. Water Health 2013, 11, 297–310. [Google Scholar] [CrossRef]
- Eramo, A.; Reyes, H.D.; Fahrenfeld, N.L. Partitioning of antibiotic resistance genes and fecal indicators varies intra and inter-storm during combined sewer overflows. Front. Microbiol. 2017, 8, 2024. [Google Scholar] [CrossRef]
- Quach-Cu, J.; Herrera-Lynch, B.; Marciniak, C.; Adams, S.; Simmerman, A.; Reinke, R.A. The effect of primary, secondary, and tertiary wastewater treatment processes on antibiotic resistance gene (ARG) concentrations in solid and dissolved wastewater fractions. Water 2018, 10, 37. [Google Scholar] [CrossRef] [Green Version]
- Exner, M.; Schmithausen, R. Verbundvorhaben HyReKA: Hintergrund, Zielsetzung, Methoden. In Proceedings of the Abschlussveranstaltung Des BMBF-Forschungsvorhabens Zu Antibiotikaresistenzen Im Wasserkreislauf (HyReKA), Eggenstein-Leopoldshafen, Germany, 3–4 April 2019. [Google Scholar]
- Jäger, T.; Hembach, N.; Elpers, C.; Wieland, A.; Alexander, J.; Hiller, C.; Krauter, G.; Schwartz, T. Reduction of Antibiotic Resistant Bacteria During Conventional and Advanced Wastewater Treatment, and the Disseminated Loads Released to the Environment. Front. Microbiol. 2018, 9, 2599. [Google Scholar] [CrossRef] [Green Version]
- Hembach, N.; Alexander, J.; Wieland, A.; Hiller, C.X.; Schwartz, T. Untersuchung der Eliminationsleistung verschiedener weiterführender Abwasserreinigungsverfahren zur Keimreduktion. Vom Wasser Das J. 2018, 2, 65–68. [Google Scholar]
- Hiller, C.X.; Hübner, U.; Fajnorova, S.; Schwartz, T.; Drewes, J.E. Antibiotic microbial resistance (AMR) removal efficiencies by conventional and advanced wastewater treatment processes: A review. Sci. Total Environ. 2019, 685, 596–608. [Google Scholar] [CrossRef]
- Mousel, D.; Bastian, D.; Firk, J.; Palmowski, L.; Pinnekamp, J. Removal of pharmaceuticals from wastewater of health care facilities. Sci. Total Environ. 2021, 751, 141310. [Google Scholar] [CrossRef]
- Londong, J. HOSS: Ein Hygiene-orientiertes Siedlungsentwässerungssystem. KA Korresp. Abwasser Abfall 2022. submitted. [Google Scholar]
- Schütze, M.; Wriege-Bechtold, A.; Zinati, T.; Söbke, H.; Wißmann, I.; Schulz, M.; Veser, S.; Londong, J.; Barjenbruch, M.; Alex, J. Simulation and visualization of material flows in sanitation systems for streamlined sustainability assessment. Water Sci. Technol. 2019, 79, 1966–1976. [Google Scholar] [CrossRef]
- Zinati, T.; Wriege-Bechtold, A.; Barjenbruch, M.; Schütze, M.; Schulz, M.; Kraus, M.; Wissmann, I.; Veser, S.; Söbke, H.; Londong, J. SAmpSONS: Softwarewerkzeug zur vergleichenden Vorplanung von Abwasserinfrastrukturen. KA Korresp. Abwasser Abfall. 2021, 68, 1–7. [Google Scholar] [CrossRef]
- Londong, J. Strategien für die Siedlungsentwässerung. KA Korresp. Abwasser Abfall 2000, 47, 1434–1444. [Google Scholar]
- Larsen, T.A.; Udert, K.M.; Lienert, J. Source Separation and Decentralization for Wastewater Management; IWA Publishing: London, UK, 2013; ISBN 9781780401072. [Google Scholar]
- Londong, J. Practical experiences with source separation in Germany. In Source Separation and Decentralization for Wastewater Management; Larsen, T.A., Udert, K.M., Lienert, J., Eds.; IWA Publishing: London, UK, 2013; pp. 423–430. [Google Scholar]
- DWA. Neuartige Sanitärsysteme (NASS); Themenband des DWA-Fachausschusses KA 1 und Seiner 6 Arbeitsgruppen: Hennef, Germany, 2008. [Google Scholar]
- Gao, M.; Zhang, L.; Florentino, A.P.; Liu, Y. Performance of anaerobic treatment of blackwater collected from different toilet flushing systems: Can we achieve both energy recovery and water conservation? J. Hazard. Mater. 2019, 365, 44–52. [Google Scholar] [CrossRef]
- Londong, J.; Wätzel, T.; Giese, T. Combining the production of renewable energy with innovative urban drainage systems—The KREIS Project. In Proceedings of the 13th IWA Specialized Conference on Small Water and Wastewater Systems & 5th IWA Specialized Conference on Resources-Oriented Sanitation, Athens, Greece, 14–16 September 2016. [Google Scholar]
- Veser, S. Intelligent infrastructure systems for urban wastewater management. Research project EvaSENS. Bluefacts. Int. J. Water-Manag. 2012, 2012, 110–114. [Google Scholar]
- Veser, S. Doppel-Inliner-Verfahren Zur Getrennten Erfassung Von Schwarz-Und Grauwasser Im Gebäudebestand. Ph.D. Dissertation, Bauhaus-Universität Weimar, Weimar, Germany, 2015. [Google Scholar]
- Veser, S.; Berndt, M. EVaSENS—Neue Wege der Abwassertrennung im Siedlungsbestand. Wasser Und Abfall 2014, 16, 32–37. [Google Scholar] [CrossRef]
- Hörnlein, S.; Londong, J.; Veser, S.; Berndt, M. Technische Lösungen für Source Separation im Bestand. 2021; in preparation. [Google Scholar]
- Hörnlein, S. A Modelling Approach for Time-Sequential Source Separation of Domestic Wastewaters. 2022; manuscript in preparation. [Google Scholar]
- Gujer, W. Siedlungswasserwirtschaft, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- ifak e.V. SAmpSONS—Visualisation and Sustainability Assessment of Resource-Orientated Sanitation Systems. Available online: https://www.ifak.eu/en/products/sampsons (accessed on 25 October 2021).
- DWA. Arbeitsblatt DWA-A 102-2/BWK-A 3-2—Grundsätze Zur Bewirtschaftung Und Behandlung Von Regenwetterabflüssen Zur Einleitung in Oberflächengewässer—Teil 2: Emissionsbezogene Bewertungen Und Regelungen; DWA: Hennef, Germany, 2020. [Google Scholar]
- DWA-Arbeitsgruppe ES-2.1. Systembezogene Anforderungen und Grundsätze. Arbeits—Und Merkblattreihe DWA-A/M 102 (BWK-A/M 3) Zusatzdatei: Anwendungsbeispiele; DWA: Hennef, Germany, 2020; Volume 102. [Google Scholar]
- Firk, J.; (RWTH Aachen University, Aachen, Germany). Personal communication, 2021.
- Exner, M.; Schmithausen, R. Synthese Und Abschlussbericht HyReKA. Hygienisch-Medizinische Relevanz Und Kontrolle Antibiotika-Resistenter Krankheitserreger in Klinischen, Landwirtschaftlichen Und Kommunalen Abwässern Und Deren Bedeutung in Rohwässern; Bonn/Karlsruhe/Dresden/Aachen/Bad Elster, Germany. 2020. Available online: https://docplayer.org/199750169-Hyreka-synthese-und-abschlussbericht-bonn-karlsruhe-dresden-aachen-bad-elster-2020.html (accessed on 24 November 2021).
- Rau, W.; Metzger, S. Bestandsaufnahme Der Spurenstoffsituation Von Kläranlagen in Baden-Württemberg; Kompetenzzentrum Spurenstoffe BW: Stuttgart, Germany, 2017; Available online: https://koms-bw.de/cms/content/media/BAn_Bericht-Teil1_veroeffentl_final.pdf (accessed on 24 November 2021).
- Wellbrock, K.; Knobloch, J.K.-W.; Heim, M.; Grottker, M. Spurenstoffe Und Multiresistente Bakterien in Den Entwässerungssystemen Schleswig-Holsteins. Kiel/Lübeck/Hamburg, Germany. 2019. Available online: https://www.th-luebeck.de/fileadmin/media/01_Hochschule/05_Fachbereiche/Bauwesen/Bilder/Projekte/PrioSH/Zusammenfassung_und_Ausblick.pdf (accessed on 24 November 2021).
- Forth, W.; Henschler, D.; Rummel, W. Allgemeine Und Spezielle Pharmakologie Und Toxikologie; 8. Auflage; Urban & Fischer München: Jena, Germany, 2001. [Google Scholar]
- Feldmann, D.F. Modellberechnungen Zum Verhalten Und Verbleib Von Arzneimittelrückständen Im Krankenhausabwasser Und Beurteilungs-Möglichkeiten Ihres Ökotoxikologischen Gefährdungspotentials; Technische Universität Berlin: Berlin, Germany, 2005. [Google Scholar]
- Alexander, J.; Hembach, N.; Schwartz, T. Evaluation of antibiotic resistance dissemination by wastewater treatment plant effluents with different catchment areas in Germany. Sci. Rep. 2020, 10, 8952. [Google Scholar] [CrossRef]
- Alexander, J. Mikrobiologische Charakterisierung Von Anthropogen Beeinflussten Wassersystemen Und Evaluierung Von Abwasserbehandlungsverfahren; Karlsruher Institut für Technologie (KIT): Karlsruhe, Germany, 2017. [Google Scholar]
- Fatta-Kassinos, D.; Meric, S.; Nikolaou, A. Pharmaceutical residues in environmental waters and wastewater: Current state of knowledge and future research. Anal. Bioanal. Chem. 2011, 399, 251–275. [Google Scholar] [CrossRef]
- Gracia-Lor, E.; Sancho, J.V.; Serrano, R.; Hernández, F. Occurrence and removal of pharmaceuticals in wastewater treatment plants at the Spanish Mediterranean area of Valencia. Chemosphere 2012, 87, 453–462. [Google Scholar] [CrossRef] [Green Version]
- Narumiya, M.; Nakada, N.; Yamashita, N.; Tanaka, H. Phase distribution and removal of pharmaceuticals and personal care products during anaerobic sludge digestion. J. Hazard. Mater. 2013, 260, 305–312. [Google Scholar] [CrossRef]
- Koschorreck, J. Arzneimittel in Der Umwelt: Zu Risiken Und Nebenwirkungen Fragen Sie Das Umweltbundesamt; Federal Environment Agency: Dessau, Germany, 2005.
- Hörsing, M.; Ledin, A.; Grabic, R.; Fick, J.; Tysklind, M.; la Cour Jansen, J.; Andersen, H.R. Determination of sorption of seventy-five pharmaceuticals in sewage sludge. Water Res. 2011, 45, 4470–4482. [Google Scholar] [CrossRef] [Green Version]
- Verlicchi, P.; Galletti, A.; Petrovic, M.; Barceló, D.; Al Aukidy, M.; Zambello, E. Removal of selected pharmaceuticals from domestic wastewater in an activated sludge system followed by a horizontal subsurface flow bed—Analysis of their respective contributions. Sci. Total Environ. 2013, 454–455, 411–425. [Google Scholar] [CrossRef]
- Göbel, A.; McArdell, C.S.; Joss, A.; Siegrist, H.; Giger, W. Fate of sulfonamides, macrolides, and trimethoprim in different wastewater treatment technologies. Sci. Total Environ. 2007, 372, 361–371. [Google Scholar] [CrossRef]
- Suárez, S.; Ramil, M.; Omil, F.; Lema, J.M. Removal of pharmaceutically active compounds in nitrifying-denitrifying plants. Water Sci. Technol. 2005, 52, 9–14. [Google Scholar] [CrossRef]
- Radjenović, J.; Petrović, M.; Barceló, D. Fate and distribution of pharmaceuticals in wastewater and sewage sludge of the conventional activated sludge (CAS) and advanced membrane bioreactor (MBR) treatment. Water Res. 2009, 43, 831–841. [Google Scholar] [CrossRef]
- Fanck, B.; Heberer, T. Multi Method for the Detection of Antibiotic Residues in Various Waters Using Solid-Phase Extraction and Liquid Chromatography/Mass Spectrometry. 2005; manuscript. [Google Scholar]
- DWA-Fachausschuss KA-8 Antibiotika und antibiotikaresistente Bakterien und Gene im Wasserkreislauf. KA Korresp. Abwasser Abfall 2018, 65, 545–550. [CrossRef]
- Kistemann, T.; Rind, E.; Rechenburg, A.; Koch, C.; Claßen, T.; Herbst, S.; Wienand, I.; Exner, M. A comparison of efficiencies of microbiological pollution removal in six sewage treatment plants with different treatment systems. Int. J. Hyg. Environ. Health 2008, 211, 534–545. [Google Scholar] [CrossRef]
- Güde, H.; Eckenfels, S.; Palmer, A.; Fitz, S.; Pietruske, J.; McTaggart, K.; Haibel, B.; Setzer, T. Erfassung Und Bewertung Von Eintragswegen Für Belastungen Mit Fäkalkeimen Im Einzugsgebiet Der Seefelder Aach (Bodenseekreis); Langenargen, Germany, 2001. Available online: https://pudi.lubw.de/detailseite/-/publication/45442-Erfassung_und_Bewertung_von_Eintragswegen_f%C3%BCr_Belastungen_mit_F%C3%A4kalkeimen_im_Einzugsgebiet_der_Seefe.pdf (accessed on 5 May 2001).
- Mascher, F.; Karl, F.; Folli, B.; Kittinger, C.; Lipp, M.; Mascher, W.; Pfeifer, B.; Platzer, S.; Schmutz, R.; Schwaiger, M. Die Reiningungsleistung Einer Kommunalen Abwasserreinigungsanlage Im Hinblick Auf Bakterielle Fäkalindikatoren Und Die Auswirkungen Auf Den Vorfluter; Graz, Austria, 2014. Available online: http://docplayer.org/74428142-Endbericht-zum-projekt-a.html (accessed on 25 October 2021).
- Feuerpfeil, I.; López-Pila, J.; Schmidt, R.; Schneider, E.; Szewzyk, R. Antibiotikaresistente Bakterien und Antibiotika in der Umwelt. Bundesgesundheitsblatt Gesundheitsforsch. Gesundh. 1999, 42, 37–50. [Google Scholar] [CrossRef]
- Adler, N.; Balzer, F.; Blondzik, K.; Brauer, F.; Chorus, I.; Ebert, I.; Fiedler, T.; Grummt, T.; Heidemeier, J.; Hein, A. Antibiotika Und Antibiotikaresistenzen in Der Umwelt: Hintergrund, Herausforderungen Und Handlungsoptionen; Umweltbundesamt: Dessau, Germany, 2018.
- Pallares-Vega, R.; Blaak, H.; van der Plaats, R.; de Roda Husman, A.M.; Hernandez Leal, L.; van Loosdrecht, M.C.M.; Weissbrodt, D.G.; Schmitt, H. Determinants of presence and removal of antibiotic resistance genes during WWTP treatment: A cross-sectional study. Water Res. 2019, 161, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Chen, Z.; Gao, J.; Xie, Y.; Li, L.; Qin, S.; Wang, Q.; Mao, D.; Luo, Y. Simultaneous removal of antibiotics and antibiotic resistance genes from pharmaceutical wastewater using the combinations of up-flow anaerobic sludge bed, anoxic-oxic tank, and advanced oxidation technologies. Water Res. 2019, 159, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Carballa, M.; Omil, F.; Ternes, T.; Lema, J.M. Fate of pharmaceutical and personal care products (PPCPs) during anaerobic digestion of sewage sludge. Water Res. 2007, 41, 2139–2150. [Google Scholar] [CrossRef]
- Galler, H.; Zarfel, G.; Feierl, G.; Haas, D.; Leitner, E.; Melkes, A.; Posch, J.; Reinthaler, F.F.; Winter, I. Nachweis Antibiotikaresistenter Escherichia Coli—Stämme Aus Klärschlamm Unter Berücksichtigung Der Verschiedenen Klärschlammbehandlungsverfahren; Abfall-und Ressourcenwirtschaft: Graz, Austria, 2010. [Google Scholar]
- Glaeser, S.; Schauss, T.; Dott, W.; Kämpfer, P. Biogasanlagen—Quelle oder Senke für Antibiotika-resistente Bakterien? BIOspektrum 2016, 22, 656–658. [Google Scholar] [CrossRef]
- Saunders, O.; Harrison, J.; Fortuna, A.M.; Whitefield, E.; Bary, A. Effect of anaerobic digestion and application method on the presence and survivability of E. coli and fecal coliforms in dairy waste applied to soil. Water Air Soil Pollut. 2012, 223, 1055–1063. [Google Scholar] [CrossRef]
- Smith, S.R.; Lang, N.L.; Cheung, K.H.M.; Spanoudaki, K. Factors controlling pathogen destruction during anaerobic digestion of biowastes. Waste Manag. 2005, 25, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Avery, L.M.; Anchang, K.Y.; Tumwesige, V.; Strachan, N.; Goude, P.J. Potential for Pathogen reduction in anaerobic digestion and biogas generation in Sub-Saharan Africa. Biomass Bioenergy 2014, 70, 112–124. [Google Scholar] [CrossRef]
- Espinosa, M.F.; Verbyla, M.E.; Vassalle, L.; Rosa-Machado, A.T.; Zhao, F.; Gaunin, A.; Mota, C.R. Reduction and partitioning of viral and bacterial indicators in a UASB reactor followed by high rate algal ponds treating domestic sewage. Sci. Total Environ. 2021, 760, 144309. [Google Scholar] [CrossRef]
- Moerland, M.J.; Borneman, A.; Chatzopoulos, P.; Fraile, A.G.; van Eekert, M.H.A.; Zeeman, G.; Buisman, C.J.N. Increased (Antibiotic-resistant) pathogen indicator organism removal during (hyper)thermophilic anaerobic digestion of concentrated black water for safe nutrient recovery. Sustainability 2020, 12, 9336. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, Y.; Pruden, A. Effect of temperature on removal of antibiotic resistance genes by anaerobic digestion of activated sludge revealed by metagenomic approach. Appl. Microbiol. Biotechnol. 2015, 99, 7771–7779. [Google Scholar] [CrossRef] [PubMed]
- Sui, Q.; Zhang, J.; Chen, M.; Tong, J.; Wang, R.; Wei, Y. Distribution of antibiotic resistance genes (ARGs) in anaerobic digestion and land application of swine wastewater. Environ. Pollut. 2016, 213, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Dantas, R.F.; Contreras, S.; Sans, C.; Esplugas, S. Sulfamethoxazole abatement by means of ozonation. J. Hazard. Mater. 2008, 150, 790–794. [Google Scholar] [CrossRef]
- Sousa, J.M.; Macedo, G.; Pedrosa, M.; Becerra-Castro, C.; Castro-Silva, S.; Pereira, M.F.R.; Silva, A.M.T.; Nunes, O.C.; Manaia, C.M. Ozonation and UV254nm radiation for the removal of microorganisms and antibiotic resistance genes from urban wastewater. J. Hazard. Mater. 2017, 323, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Maier, K.; Söbke, H.; Londong, J. Principles of Transition Paths: Purposeful Conversion of Water Infrastructure Systems to Multi Stream Variants. In Proceedings of the Water Efficiency Conference 2015, Exeter, UK, 5–7 August 2015; Memon, F.A., Ed.; WATEF Network/University of Brighton: Exeter, UK, 2015; pp. 359–368. [Google Scholar]

| Choice | Description |
|---|---|
| Site Under Investigation (SUI) | A study area for which a sanitation system is to be assessed must be chosen. The SUI specifies, among other things, the quantity and type of persons or dischargers. |
| Sanitation System | A sanitation system is described by components, each of which is provided by SAmpSONS. |
| Material Flows | The material flows assessed result from the sanitation system chosen or from the respective components of the sanitation system. The material flows are predefined by SAmpSONS. |
| Constituents | SAmpSONS features a set of constituents that are considered for all material flows. Three constituents are freely definable. |
| Removal Rates | For all components, removal rates are to be defined with respect to each constituent. The predefined standard values may also be used unchanged. |
| Subarea 1 | Subarea 2 | Subarea 3 | Subarea 4 | Subarea 5 | Subarea 6 | ||
|---|---|---|---|---|---|---|---|
| Population | P | 3200 | 970 | 600 | 1900 | 1100 | 8200 |
| Wastewater | L/s | 4.8 | 1.1 | 0.9 | 2.9 | 1.5 | 12.4 |
| m3/d | 414.72 | 95.04 | 77.76 | 250.56 | 129.60 | 1071.36 | |
| Stormwater and extraneous water | L/s | 4.8 | 1.1 | 0.9 | 2.9 | 1.5 | 12.4 |
| m3/d | 414.72 | 95.04 | 77.76 | 250.56 | 129.60 | 1071.36 |
| Constituent Category | Removal Rates | Fraction into Effluent | Remark |
|---|---|---|---|
| Antibiotics (ABs) | 0% | 100% | no data found in literature |
| Antibiotic-resistant Bacteria (ARB) | 99.999% | 0.001 | no data found in literature |
| Antibiotic Resistance Genes (ARGs) | 99.999% | 0.001 | no data found in literature |
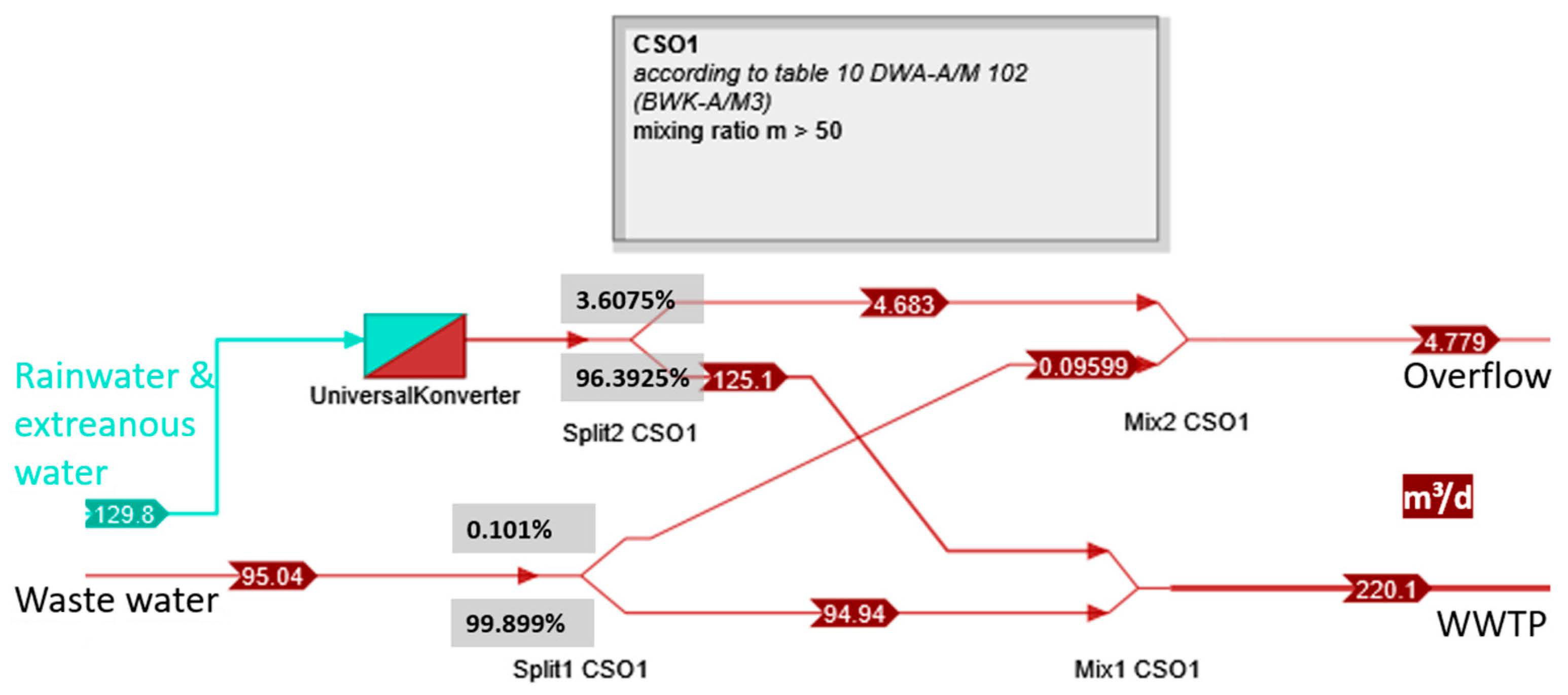
| Structure | Ve,MWÜ | Ve,MWÜ | m | Fraction Wastewater | Fraction Stormwater |
|---|---|---|---|---|---|
| m3/a | m3/d | m3/d | m3/d | ||
| CSO1 | 1744 | 4.7781 | 50 | 0.0956 | 4.6825 |
| AB | ARB | ARG | |
|---|---|---|---|
| Sulfamethoxazole [kg/a] | ESBL-E.Coli [CFU/d] | blaTEM [Cell Equivalent/d] | |
| Load Influent | 0.5475 | 9.5145 × 1014 | 6.1431 × 1014 |
| Emission combined system | 0.2494 | 1.4112 × 1013 | 9.1118 × 1012 |
| Emission separate system | 0.2464 | 4.7572 × 1012 | 3.0715 × 1012 |
| Emission source-separation-modified combined system | 0.0612 | 9.0764 × 104 | 5.8602 × 104 |
| Reduction combined vs. separate system | 1.2% | 66.3% | 66.3% |
| Reduction combined vs. source-separation-modified system | 75.5% | >99.999999% | >99.999999% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Londong, J.; Barth, M.; Söbke, H. Modeling and Simulation of Source Separation in Sanitation Systems for Reducing Emissions of Antimicrobial Resistances. Water 2021, 13, 3342. https://doi.org/10.3390/w13233342
Londong J, Barth M, Söbke H. Modeling and Simulation of Source Separation in Sanitation Systems for Reducing Emissions of Antimicrobial Resistances. Water. 2021; 13(23):3342. https://doi.org/10.3390/w13233342
Chicago/Turabian StyleLondong, Jörg, Marcus Barth, and Heinrich Söbke. 2021. "Modeling and Simulation of Source Separation in Sanitation Systems for Reducing Emissions of Antimicrobial Resistances" Water 13, no. 23: 3342. https://doi.org/10.3390/w13233342







