Abstract
Nitrate is a widely disseminated water pollutant and has been linked to health disorders, including hypothyroidism. Here, we evaluated the relationship between thyroid function and chronic exposure to nitrates in rural zone families, in addition to the genetic and autoimmune factors. Exposure and effect biomarkers, thyroid hormones, and autoantibodies of tiroperoxidase were measured, as well the presence of two FOXE1 polymorphisms (rs965513, rs1867277). Pearson’s correlation, principal component analysis, Kruskal–Wallis, and chi-squared tests were used for statistical analysis. A total of 102 individuals were analyzed; 45% presented subclinical hypothyroidism, a negative correlation was observed between methemoglobin and the total T3 (r = −0.43, p = 0.001) and free T3 levels (r = −0.34, p = 0.001), as well as between TSH and the free T4 (r = −0.41, p = 0.0001) and total T4 (r = −0.36, p = 0.0001). A total of 15.7% had positive antithyroid ab-TPO, while the polymorphic genotype (AA) represented only 3% (rs965513) and 4% (rs1867277) among subjects with subclinical hypothyroidism. The high frequency of subclinical hypothyroidism in the population under study could be related, mainly, to chronic exposure through the consumption of nitrate-contaminated water.
1. Introduction
Groundwater is the main source of drinking water, as well as a stable and reliable resource that meets the demand for human consumption [1]. Access to water is important to sustain the basic activities of all living beings and is closely related to the development of urban and rural areas. [2]. However, in recent years, the contamination of these has been conditioned by the increase in the levels of nitrogen compounds, specifically nitrate, as a result of leaching, mainly due to anthropogenic activities such as the use of soils for intensive agriculture [3], intensive livestock farming [4], the use of nitrogenated chemical fertilizers [5], the use of animal manure as an organic fertilizer, the production and disposal of urban wastewater [6], the disposal of septic waste and effluents from septic tanks or leaks that also contribute to pollution in settlement environments [7,8]. The latter, in conjunction with geological features [9], rainfall fluctuations [10], interactions with surface water [11], changes in water tables [12], and the presence of biological processes such as denitrification [13], can promote the variation of nitrate concentrations, thus exceeding the permissible limit in water for human consumption established by the WHO, which is 50 mg/L nitrate ion—equivalent to 11.3 mg/L NO3-N (nitrate-nitrogen) [14].
Nitrate is a nitrogenous compound, widely found and disseminated in nature [15] through plants, soil, air, and water [16]. Some of the characteristics of nitrate salts, mainly sodium nitrate and calcium nitrate, indicate that these compounds are colorless, odorless, tasteless, and highly soluble, making them difficult to detect in drinking water [17]. By the action of nitrifying algae and bacteria, nitrite and ammonia are easily converted via oxidation into nitrate, which is the most abundant stable nitrogenated form [18]. Nitrogen is a component of many biomolecules, which is why it can also be obtained via an endogenous pathway that metabolizes nitric oxide via L-arginine-nitric oxide synthase. In the exogenous pathway, nitrogen is obtained via the diet from the consumption of vegetables, green leafy vegetables, processed foods containing nitrogen as an additive, and water contaminated with high nitrate concentrations. Both endogenous and exogenous pathways are found in humans [19]. Once ingested, nitrogen is absorbed via the gastrointestinal mucosa and the proximal duodenum, reaching a bioavailability of over 90% [20]. It is then disseminated around the entire body via systemic circulation, with approximately 25% absorbed via salivary gland uptake, and then transported to the stomach by means of enterosalivary circulation. Finally, 65% of the nitrate ingested is eliminated in urine in the form of nitrate, ammonium, or urea [21]. The exogenous nitrate–nitrite–NO pathway plays an important role due to its precursor action in the formation of nitrite and, in turn, of the genotoxic N-nitroso compounds present in endogenous nitrosation [22]. Moreover, the ingestion of high nitrate concentrations in water used for human consumption has been found to be related to the following: the appearance of methemoglobinemia, [23] alterations in biochemical parameters [24] and metabolic functions [25], the development of cancers, such as bladder [26], colorectal [27], gastric [28], and thyroid [29], and the development of thyroid function disorders [30], such as goiter [31], thyroid hypertrophy [32,33], and subclinical hypothyroidism (SCH) [34].
Further to its well-established carcinogenic action [35], nitrate has been suggested as a thyroid disruptor [36], as it is one of the major inhibitors of the sodium–iodide symporter channel. Acting competitively in the uptake of iodine by the thyroid glands, nitrate alters the hypothalamic–pituitary–thyroid axis, thus conditioning the appearance of hypothyroidism [37]. The presence of nitrates in the thyroid follicle has been associated with changes in the gene expression of sodium–iodide symporter, thyroid peroxidase, thyroglobulin, and the FOXE1 transcription factor, which are key elements in the biosynthesis of thyroid hormones [38].
SCH is characterized by thyroid-stimulating hormone (TSH) levels that exceed the normal parameters for thyroid hormone levels in both total and free T4 tests [39]. On a global level, the main cause of SCH is a low level of iodine ingestion [40], while auto-immune disorders are the second most common cause [41], followed by genetic factors [42] and endocrine disruptors [43]. The global prevalence of SCH is 10% [44], while in Mexico, it is 8% [45].
Some rural areas of the state of Durango are characterized by intensive agricultural practices, including one of the main producing regions of dairy and beef cattle and one of the largest milk basins in Mexico. These practices cause serious deterioration and contamination of the region’s groundwater reserves due to the high nitrate levels, which are mainly attributed to agriculture and livestock farming. Across different rural locations in the municipality of Lerdo, Durango, studies have been conducted to ascertain the extent of the levels of nitrate in water used for human consumption. A high nitrate level in potable water is >11 mg/L NO3-N, as established in the 2019 amendment to the corresponding Official Mexican Standard, NOM-127-SSA-1994 [46]. Moreover, impacts on the health of the region’s inhabitants have been reported, such as high levels of methemoglobin (MetHb), effects on sperm quality, and the presence of Heinz bodies. In our research group, a study was carried out in women living in areas with high levels of nitrates in drinking water, and we found alterations in metabolic parameters and thyroid hormone levels, as well as genotoxic damage and a higher incidence of SCH. In addition, the study participants reported that more than one member of their family suffers from thyroid disorders that were detected in their primary health care centers. Therefore, the objective of this study was to analyze the families of women previously identified with SCH in order to analyze the possible environmental, genetic, and immunological factors that could explain the high incidence of SCH in rural populations of Lerdo, Durango, Mexico.
2. Materials and Methods
2.1. Study Population
A familial, observational, descriptive, and cross-sectional study was conducted on individuals previously identified with thyroid dysfunction and residing in rural areas of the municipality of Lerdo, Durango, Mexico. Lerdo is located in central northern Mexico in the Chihuahuan Desert Region. The average annual temperature is 21 °C, with 200–300 mm of annual rainfall. Mainly, the territory of this municipality is used for agricultural and livestock activities. Due to the low rainfall in the area, people extract water from wells at high depth both for consumption and for the irrigation of crops, so they are exposed to pollutants, among which the presence of nitrates has been determined. The study population comprised families residing in properties of five localities within the municipality— 21 de Marzo, Juan E. García, La Loma, Nazareno, and San Jacinto, which are located between 1200 and 1300 m asl (Figure 1).
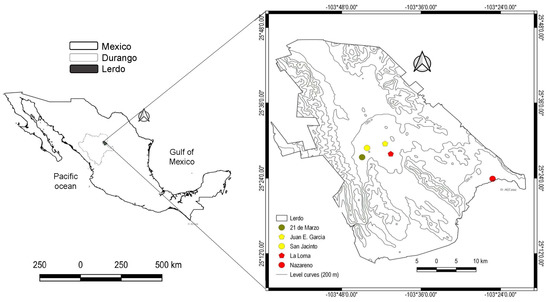
Figure 1.
Geographic location of the study sites in the municipality of Lerdo, Durango, Mexico. The map was created with free and open-source Q−GIS version 3.10.0−A Coruña [47].
The sample comprised 102 subjects who are members of 26 families residing in the abovementioned communities. The inclusion criteria were the following: voluntary participation; signed informed consent; any sex; any age; more than a year of residence in the locations of interest; consumption of water from the municipal network; not receiving medication with either anti-thyroid effects or compounds containing nitrate salts. The following were specified as exclusion criteria: persons with an established diagnosis of congenital, secondary, or tertiary hypothyroidism; persons under treatment with radiation or chemotherapy in the neck region; persons who had any type of resection of the thyroid gland, thyroid cancer, or any diagnosed pathophysiology of the thyroid gland; syndromes or conditions that had any effect on the hypothalamic–pituitary–thyroid axis. All those fulfilling the inclusion criteria were tested for exposure biomarkers (plasma and urinary nitrite), an effect biomarker (percentage of MetHb), and thyroid function (thyroid profile). The genotypes corresponding to the single nucleotide polymorphisms (SNPs) rs965513 and rs1867277 of the FOXE1 gene were also determined. The presence of anti-thyroid peroxidase antibody (TPO-Ab) was determined in the subjects who were found to have SCH. Other variables, such as age, sex, and body mass index, were considered for a better description of the population under study.
2.2. Biological Sample Collection
Study participants were asked to fast for eight hours prior to the collection of a 10 mL peripheral blood sample and a 10 mL sample of the first urine void in the morning, with both samples collected in sterile containers. The blood sample collection was conducted using BD Vacutainer® tubes containing 6 mL of coagulation activator for the recovery of the serum to be used for establishing both the thyroid profile and TPO-Ab levels. Tubes containing 4 mL of ethylenediamine tetraacetic acid (EDTA) were also used to measure plasma nitrite and MetHb levels and for the purification of genomic DNA. Once obtained, the samples were kept at −70 °C until processing.
2.3. Nitrate Exposure via Drinking Water
Although there is a distinction in the literature between low, medium, and high exposure, there is no consensus about the ranges and doses in each area. One strategy is to stratify according to the concentrations found in the specific study to observe the exposure gradient. In the study area, the nitrate levels in the water for human consumption were established by Gandarilla et al. [48], where the study areas were then stratified according to their exposure level. A low level was classified as 4.7 ± 3.3 mg/L (21 de Marzo), a medium level was classified as 32.1 ± 3.7 mg/L (San Jacinto and Juan E. García), and a high level as 56.9 ± 14.7 mg/L (La Loma and Nazareno). The number of subjects/families studied in each nitrate category area was as follows: low, 25/5; medium, 46/12; high, 31/9, respectively.
2.4. Measurement of Methemoglobin Percentage
The percentage of MetHb was established with the spectrophotometry method reported by Sakata et al. [49], using 100 μL of the blood collected in the EDTA tubes and following the manufacturer’s instructions (FAR Diagnostics, Verona, Italy) for measurements conducted via four readings at 630 nm (HACH-DR5000). The first reading (D1) was a hemolyzed preparation, and the second reading (D2) was conducted via the addition of sodium azide. The third (D3) corresponded to a hemolyzed preparation to which potassium ferricyanide was added. Finally, sodium azide was added for the fourth reading (D4). The percentage of MetHb was calculated with the following equation:
Values > 1.5% of the. total MetHb were considered to be higher than the reference values. The reagents used complied with high purity standards (Merck KGaA, Darmstadt, Germany and/or its affiliates).
2.5. Nitrite Measurement
The nitrite concentration was determined, as a stable metabolite, for the plasma and urine samples, which were then deproteinized using absolute ethanol at a 1:7 ratio and centrifuged at 3500 rpm for 15 min. The nitrite levels in the plasma and urine were measured by subjecting 100 μL of supernatant to the Griess colorimetric method, as was reported by Miranda et al. [50], using spectrophotometric quantification (HACH-DR5000) at 540 nm. As a reference, a curve with sodium nitrite and a detection limit between 0.5–50 µmol/mL was performed in triplicate, and each sample was measured by duplicate. All reagents used were high purity (Sigma-Aldrich, Darmstadt, Germany).
2.6. Measurement of Thyroid Function
Thyroid function (TSH), total (tT3) and free T3 (fT3), and total (tT4) and free T4 (fT4) were measured by chemiluminescent immunoassay (IMMULITE®1000 Siemmens™, Gwynedd, UK). The TSH assay had a sensitivity of 0.004 μIU/mL and an upper limit of 75 μIU/mL. The reference ranges for thyroid hormones were TSH, 0.4–4.0 μIU/mL, tT3, 82–179 ng/dL, fT3, 1–40 pg/mL, tT4, 4.5–12.5 μg/dL, and fT4, 0.3–6.0 ng/dL.
2.7. Measurement of TPO-Ab Concentration
For those individuals with TSH levels above the normal range, 100 μL of blood serum was used to conduct a sandwich-type enzyme-linked immunosorbent assay (ELISA) using a commercial TPO-Ab detection kit in accordance with the manufacturer’s instructions (Merck KGaA, Darmstadt, Germany and/or its affiliates). The levels of TPO-Ab+ were determined once values of >35 UI/mL were observed.
2.8. DNA Extraction and Genotypification of FOXE1
Genomic DNA extraction was performed using whole blood following the protocol described by Lahiri and Nurnberger [51], while DNA concentration and purity was established using the NanoDrop 2000 (Thermo Fisher Scientific Inc., Germering, Germany). The SNPs rs965513 and rs1867277 of FOXE1 were genotyped in the StepOne Real-Time PCR (polymerase chain reaction) (Applied Biosystems, Foster City, CA, USA), using hybridization probes (Integrated DNA Technologies rhAmp®, ID Hs.GT.rs965513. G.1 and Hs.GT.rs1867277. G.1) in accordance with the manufacturer’s instructions.
2.9. Statistical Analysis
The data for 16 variables were compiled for 102 individuals, with the database including numerical and categorical variables. Measures of central tendency were analyzed for the description of the data, and Pearson’s correlation for quantitative variables with normal distribution. For non-normal distribution, the variables were standardized by subtracting the average corresponding to the variable from the value of each data point and then dividing by the standard deviation. The multivariate analysis comprised a correlation analysis applied solely for the 11 numerical variables, given that principal component analysis (PCA) requires that the variables used present some level of correlation. For this reason, only the variables with a level of significance greater than or equal to 0.90 were considered. Once the correlations among the variables were identified, a Mahalanobis distance analysis was undertaken to discard the data considered atypical for this set of variables. The PCA was carried out to show, firstly, the distribution of the individuals based on the SCH condition and, secondly, on the nitrate exposure according to the levels detected in the communities in which they live. A heatmap analysis was carried out to group the individuals according to the 11 numerical variables and, at the same time, to show the levels of correlation of each of the variables for each individual. The cluster analysis was undertaken using the Euclidean distance index and the Ward algorithm to construct the groupings, while genotype, subclinical condition, sex, and the NO3-N level to which they were exposed were mapped for the individuals in the heatmap. Moreover, in order to reassign those individuals who had been classified with a non-SCH condition prior to the analysis, a discriminant function analysis was conducted to establish the percentage of correct classifications, which was then statistically corroborated via the Wilkins test. Finally, Kruskal–Wallis and chi-squared tests were performed to determine the differences between the different exposure scenarios, where applicable. All statistical and multivariate analyses were performed with the R software version 4.1.0 [52]; a p-value of <0.05 was considered statistically significant.
2.10. Ethical Considerations
This study was approved by the Ethics Committee of the Faculty of Chemical Sciences at the Juarez University of the State of Durango, with a unique assigned registration number of R-2017-123301538X0201-026. The study followed the guidelines, as pertaining to health research, stipulated in articles 13, 14, 17, 21, and 22 of the General Health Law in Mexico.
3. Results
The study population initially comprised 26 females who had been previously diagnosed with SCH and who described other cases in their families. The analysis was carried out on as many members of the subjects’ families as possible in order to explore the possible causes underlying the increased incidence of SCH in these rural populations that have been exposed to an endocrine-disrupting condition, such as nitrates through drinking water. Finally, the sample comprised 102 subjects with an average age of 22 years; 69% were women, 15% had no schooling, and 36% had been educated up to a lower secondary level, while 58% were overweight or presented some degree of obesity, and the majority worked in the home or were students. With regard to water consumption, 85.5% consumed water drawn from the public water system or from private wells, while only 14.5% occasionally consumed purified water from purification machines (see Table 1).

Table 1.
General characteristics of the total study population.
The measurements of MetHb found 79% of subjects to fall outside the reference limit, while the average levels of nitrite in plasma and nitrite in urine were 24.62 µmol/mL and 3.46 µmol/mL, respectively. Regarding the parameters of thyroid function, 45% of subjects presented TSH levels over the reference limits (SCH), 49% had reduced tT3 levels, and 19.6% presented reduced tT4 levels. A total of 15.7% of the SCH cases were positive for the presence of TPO-Ab antibodies (see Table 2).

Table 2.
Determination of biomarkers of interest in the study population.
Regarding the genotypic and allelic distribution of the SNPs, rs965513 and rs1867277 were determined for each exposure scenario to nitrates in water used for human consumption. The SNP rs1867277 was not found in the Hardy–Weinberg equilibrium (chi-square = 0.012), and its genotypic distribution across the exposure scenarios showed a statistically significant difference (chi-square = 0.019) (see Table 3).

Table 3.
Genotypic and allelic frequencies of polymorphisms in the FOXE1 gene observed in the study population, according to the exposure scenarios.
Table 4 shows a summary of the alterations observed in some variables, such as exposure and effect biomarkers for nitrates (measurement of nitrites), thyroid dysfunction (SCH), the genotypic frequency of the SNPs, and the presence of TPO-Ab as an autoimmune factor. Differences were observed in the levels of both nitrates and the exposure (nitrite in urine p = 0.001) and effect (MetHb p = 0.042) biomarkers, while SCH was observed for all levels of chronic exposure (chi-square = 0.817). The SNP rs1867277 only presented in the low exposure scenario, corresponding to 4% (chi-square = 0.043), and, finally, the TPO-Ab percentage was highest in the medium exposure scenario, with 14.2% (see Table 4).

Table 4.
Summary of events by scenario of exposure to nitrates through drinking water.
The statistical analysis compiled the information of 16 variables for the 102 individuals included in the study, while the Pearson correlation analysis only included the continuous quantitative variables. This analysis was performed using the package RcmdrMisc version 2.7.1 [53] in the R software version 4.1.0 [52]. Positive correlations between age and BMI (r = 0.62, p ≤ 0.001), tT3 and fT3 (r = 0.51, p ≤ 0.001), and tT3 and tT4 (r = 0.50, p ≤ 0.001) were found, suggesting a moderate positive relationship. Negative correlations were observed between age and fT3 (r = −0.43, p = 0.001), BMI and fT3 (r = −0.31, p = 0.01), TSH and tT4 (r = −0.36, p = 0.001), TSH and fT4 (r = −0.41, p = 0.001), tT3 and MetHb (r = −0.43, p = 0.001), and fT3 and MetHb (r = −0.34, p = 0.001). All other correlations did not show significant differences (see Figure 2).
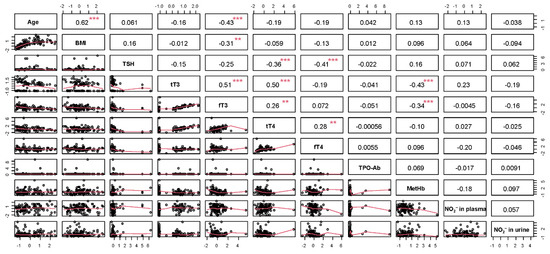
Figure 2.
Pearson correlation analysis (significance level, *** = 0.99; ** = 0.95). BMI = body mass index; TSH = thyroid stimulant hormone; t3T = total T3; fT3 = free T3; t4T = total T4; fT4 = free T4; TPO-Ab = thyroid peroxidase antibody; MetHb = methemoglobin; NO2− = nitrite.
The PCA was performed using the packages ggplot2 version 3.3.5 [54], ggrepel version 0.9.1 [55], factoextra version 1.0.7 [56], FactoMineR version 2.4 [57], paran version 1.5.2 [58], and nFactors version 2.4.1 [59], and revealed that the three first components represent 63.3% of the variability, and the variables that most contributed to the analysis of the first component were tT3, fT3, and tT4T (see Figure 3A), while the main variables in the second component were age and BMI (see Figure 3B). Most of the individuals presenting a SHC condition were identified by increases in the NO2- levels in the blood, BMI, age, TSH level, and MetHb levels, while a greater relationship was found between those individuals with a non-SCH clinical condition and increases in the variables tT3, tT4, fT3, and, mainly, fT4. When the nitrate exposure scenarios were classified, the PCA analysis did not identify a clear condition but did establish that high levels of MetHb are more related to those study locations presenting high levels of nitrate in potable water (see Figure 3).
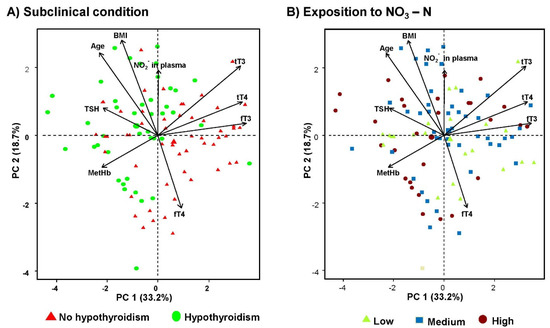
Figure 3.
Principal component analysis (PCA). (A) PCA analysis classifying the condition of subclinical hypothyroidism and (B) PCA analysis classifying nitrate exposure scenarios through drinking water.
Additionally, both multivariate conglomerate and heatmap analyses were undertaken with group subjects according to the numerical variables and, at the same time, the levels of correlation were presented for each of the variables for each individual. The conglomerate analysis was carried out using the Euclidean distance index and the Ward algorithm to construct the groupings, while the heatmap analysis mapped the subject’s genotype and SCH condition, and the level of NO3-N was carried with the package pheatmap version 1.0.12 [60] (see Figure 4).
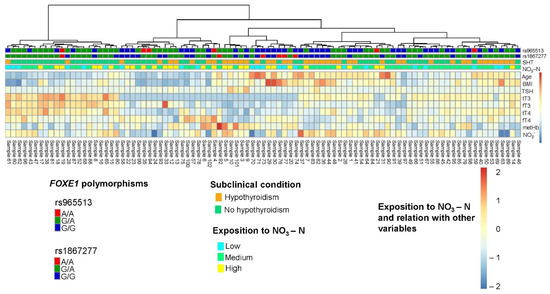
Figure 4.
Multivariate cluster and heatmap analysis. BMI = body mass index; TSH = thyroid stimulant hormone; tT3 = total T3; fT3 = free T3; tT4 = total T4; fT4 = free T4; TPO-Ab = thyroid peroxidase antibody; MetHb = methaemoglobin; NO2− = nitrite. The cluster analysis was performed using the Euclidean distance index and Ward’s algorithm to construct the clusters. In the heatmap analysis, the genotype presented by each individual, the SCH condition, and the level of NO3-N to which they were exposed were mapped.
Finally, the discriminant function analysis revealed 81% accuracy in the classification of the subjects. This contrasts with the a priori group, where discriminant function analysis showed that four out of the 95 subjects (numbers 70, 73, 74, and 76) from this group should be classified as likely to develop SCH if the levels for all the numerical variables do not decrease. The discriminant function analysis was statistically corroborated with the Wilkins test.
4. Discussion
The present study is the first to investigate evidence on the possible direct relationship between chronic nitrate exposure via the consumption of contaminated water and alterations in thyroid function in rural areas of Durango, Mexico.
As widely described in the literature, human exposure to nitrates and nitrites occurs endogenously due to the metabolism of NO [61]. It also occurs exogenously in the diet via different concentrations in drinking water and green leafy vegetables [62], food preservatives or flavorings in processed foods [63], and food supplements, such as potassium nitrate and sodium nitrate [64]. It is also present in medications that are mainly used to treat cardiovascular diseases [65]. The fact that the endogenous metabolism produces a lower amount of nitrates and nitrites than that produced by the diet [66] should be noted, as well as the subjects’ null ingestion of food supplements. Therefore, the main source of nitrate exposure in the study population is attributable to the consumption of water contaminated with these compounds.
While few studies have established base levels of nitrates in the organism, Mikiwa et al. [67] found nitrate and nitrite levels of 36.6 ± 18.5 µmol/L and 6.4 ± 2.1 µmol/L, respectively, in the plasma of a sample of ten healthy subjects of Japanese origin. Pimková et al. [68] found nitrite and nitrate levels of 1149 ± 86 nmol/L and 32.78 ± 10.33 µmol/L, respectively, in the plasma of a sample of 23 healthy subjects in the Czech Republic, while Lundberg and Weitzberg [21] reported normal plasma levels of nitrates and nitrites ranging from 20–40 µmol/L and 50–300 nmol/L, respectively. In contrast, the present study obtained blood and urine nitrite levels of 23.95 ± 9 µmol/mL and 4.90 ± 4.90 µmol/mL, respectively, which may be related to the degree of exposure to nitrate in contaminated drinking water.
The physiological level of MetHb ranges from 0–1% [69] to 2% [70]. The lactating population is more prone to developing this condition because of the low level of MetHb reductase activity, while the ease with which fetal hemoglobin is oxidized and its more acidic gastric pH enable the intestinal microbiota to reduce the amount of nitrate ingested into nitrite. High MetHb percentages were observed in this study; 79% of individuals analyzed presented levels over 1.5%, with an average of 2.80 ± 1.88.
Methemoglobinemia is a condition characterized by an abnormally high level (>40%) of MetHb, which is mainly produced by acute exposure to oxidizing compounds, pharmaceuticals, and most chemical agents [71]. However, in this study, the highest MetHb percentage was 12.35%, but the questionnaire information indicates that no prior contact with any medication or chemical agent could explain these high MetHb levels.
Another health aspect of interest to the present study is the state of thyroid function in the presence of chronic nitrate exposure. Nitrate, together with other sodium–iodide symporter inhibitors, is able to alter thyroid function [72], leading to it being suggested as a possible thyroid disruptor in humans [24,36]. In contrast to the findings obtained by Van Maanen et al. [32], Tájtaková et al. [33], Gatseva and Argirova [31], and Ward et al. [29], our results do not provide data on abnormal growth, thyroid nodules, or the presence of any type of thyroid carcinoma. Moreover, while they do not concur with the reduced TSH levels and increased thyroid hormone levels found by the foregoing studies, they do show altered thyroid function after chronic nitrate exposure via potable water, ranging from 4.7 ± 3.3 mg/L to 56.9 ± 14.7 mg/L. The alteration found in the present study corresponds to SCH and occurs due to the increased levels of TSH (up to 45%) and a reduction in tT4 and tT3 (up to 49% and 19.6%, respectively), a finding concurring with that reported by Aschebrook-Kilfoy et al. [34]. Another significant finding of the present study is the 85% urinary nitrite detected, which indicates that the study population exceeds the baseline (1 µmol/mL) and, thus, that high levels of these nitrogenated compounds are excreted by the kidney in the presence of high nitrate concentrations in drinking water. Van Maanen et al. [32] observed an increase in urinary nitrate, which corresponded to the increased nitrate concentration in the drinking water of the study population, thus showing a dose–response relationship.
While previous studies have indicated the relationship between high levels of nitrates in potable water and altered thyroid function, others have shown that the relationship is not completely established, and structural or functional changes in the thyroid gland have not been found [73]. In contrast, the present study identified nitrate concentrations in potable water of up to 56.9 ± 14.7 mg/L, which is considered to be chronic exposure (≥1 year), and further, identified the presence of other conditioning factors for the development of SCH.
The prevalence of SCH found by the present study was 45%, which exceeds the national and global prevalence of 8% and 10%, respectively [45,74], and remains high even under the scenarios of low (40%), medium (45.6%), and high (48.4%) exposure. Hashimoto’s thyroiditis (a form of chronic lymphocytic thyroiditis or chronic autoimmune thyroiditis) is characterized by the autoimmune destruction of the thyroid gland, which leads to epithelial cell apoptosis and diffuse lymphocytic infiltration due to the action of specific B and T cells. Moreover, it also causes follicular destruction [75], which leads to reduced levels of thyroid hormone synthesis. This chronic autoimmune thyroiditis is the main cause of both clinical and subclinical primary hypothyroidism in areas with sufficient or excessive iodine content [74,76]. This is accompanied by the presence of anti-thyroid antibodies, mainly TPO and anti-thyroglobulin antibodies, which are considered biomarkers of thyroid gland damage [77]. In contrast to the 60% established by Bromińska et al. [78] and Malathi et al. [79] and the 50% established by Jayashankar et al. [80], the present study found an SCH level of 15.7% in the presence of TPO-Ab (>35 UI/mL). Therefore, autoimmune disorders would not be the main cause of SCH in the families of the study population. Although the exact etiology of the development of Hashimoto’s thyroiditis remains to be established, the interaction among genetic susceptibility factors, nutritional factors, and environmental triggers may be involved [81].
Alterations in the synthesis and secretion of thyroid hormones may be a consequence of a perturbation in the expression of the genes encoding thyroid transcription factors or the presence of genetic variations [82]. The gene FOXE1 plays an important role in the growth and development of thyroid glands and the proliferation and differentiation of follicular thyroid cells and acts as a regulator of cell function, growth, and differentiation [83]. Two of its polymorphisms, rs965513 [A] and rs1867277 [A], contribute independently to establishing a predisposition for the development of papillary thyroid cancer [84]. Similarly, the presence of the allele of the rs965513 polymorphism impacts thyroid function, reducing the levels of TSH and fT4 and increasing fT3 levels [85], and is associated with the development of hypothyroidism and goiter [86]. Furthermore, the presence of the allele of the rs965513 polymorphism participates in the recruitment of the USF1/2 factors by the FOXE1 promoter, resulting in an alteration in the state of the FOXE1 gene expression [87]. Furthermore, it has been found that genetic variations in FOXE1, including rs965513 and rs1867277, are risk factors associated with increased susceptibility to differentiated thyroid cancer [88]. In terms of the results of the present study, the frequency of the occurrence of the polymorphic allele in the study population was 30% for rs965513 [A] and 37% for rs1867277 [A], which is in line with the global (rs965513 [A] = 20% and rs1867277 [A] = 31%) and national (rs965513 [A] = 26% and rs1867277 [A] = 29%) levels. Considering that only the AA genotype of both polymorphisms could be the cause of thyroid function alterations, the present study found the presence of the genetic variants rs965513 and rs2200733 in only 3 and 4%, respectively, of the SCH cases found, thus reducing the impact of genetic factors in the study population.
Increased BMI is one correlation plausibly connected to the presence of SCH, in that hypothyroidism and SCH provide the conditions for reduced metabolic function, alterations in thermogenesis, and processes involving the metabolism of both glucose and lipids. This translates into weight gain over the long term once the level of thyroid hormone synthesis begins to decrease [89]. Given the foregoing, it is possible that the correlation between age and BMI found in the present study may reveal that the older a subject, the greater their exposure to nitrates and, consequently, the greater the alteration in metabolic processes, thus resulting in weight gain. However, we also consider that the correlation would be affected by other factors, such as nutritional variation, sedentarism, and even the inherent relation with growth.
It should be noted that some studies indicate a relationship between an increased BMI and increased thyroid hormone (fT3) levels [90,91], while others maintain that changes in the serum thyroid hormone (f4T) may cause increases in BMI [92]. The foregoing is the opposite of the negative correlation found in the present study between BMI and fT3 levels. We consider that high nitrate exposure would play an important role in the thyroid profile and requires attention in the study population.
In terms of the relationship observed between age and thyroid profile, the results of the present study provide evidence of a negative and statistically significant correlation. However, the production of hormones regulated by the endocrine system, including the thyroid hormones, decreases due to aging, which causes general morphological and physiological changes [93]. Nevertheless, the importance of the chronic presence of nitrate as an inhibitor of iodine uptake in the thyroid gland cannot be understated.
It is of the utmost importance to highlight that the following environmental characteristics of the rural region in which the present study was carried out are problems with real impacts on human health: intermittent rainfall; soil/aquifer contamination via inorganic nitrate and other compounds; over-fertilization; over-use of land for forage crops; intensive livestock and agricultural practices. These problems are not only present in rural areas of Durango, Mexico, they are also public health and environmental problems in other regions of the world [94].
As discussed previously, the development of SCH may have a multifactorial origin. However, this study suggests that the SCH observed in the population may be due to chronic exposure to high levels of nitrate in water for human consumption, which alters thyroid function.
Some of the limitations of this study include the lack of urinary iodine quantification; even though it is considered a good indicator of the nutritional iodine status in humans, it does not provide direct information on thyroid function. In addition, the distribution of iodized salt in the general population and the absence of congenital hypothyroidism would indicate that the population has an adequate source of iodine intake. It is necessary to designate more robust study types, such as case-controls or cohorts, in order to investigate and specify the factors that condition the presence and development of SCH in the analyzed population.
5. Conclusions
The results of this study suggest that environmental factors have a direct and significant influence on thyroid dysfunction due to chronic exposure to nitrates in drinking water. Therefore, the high frequency of SCH observed in the study population could be due to nitrate contamination of drinking water and not a response to an additive effect and other causal factors. This study analyzed some concentrations of nitrate, within permissible limits, that can be found in drinking water and suggests that exposure to high concentrations of nitrates during critical periods of development can have significant long-term consequences. It is necessary to know the role that nitrate in drinking water plays as an endocrine disruptor. Our findings contribute to the implementation of public health measures that seek to guarantee access to water resources of adequate quality, in addition to raising awareness in the rural population of Durango about the risk of consuming water with a high content of nitrates.
Author Contributions
Conceptualization, E.G.-T., R.P.-M. and E.Y.C.-R.; methodology, E.G.-T., R.P.-M., A.G.-Z. and E.Y.C.-R.; formal analysis, E.G.-T., R.P.-M., A.G.-Z. and E.Y.C.-R.; investigation, E.G.-T., R.P.-M. and E.Y.C.-R.; writing—original draft preparation, E.G.-T., R.P.-M., A.G.-Z. and E.Y.C.-R.; writing—review and editing, E.G.-T., R.P.-M., A.G.-Z. and E.Y.C.-R.; supervision, R.P.-M., A.G.-Z., and E.Y.C.-R.; project administration, R.P.-M. and E.Y.C.-R.; funding acquisition, E.Y.C.-R. All authors have read and agreed to the published version of the manuscript.
Funding
The present study was funded by the Consejo de Ciencia y Tecnologia del Estado de Durango (COCyTED, or the Council for Science and Technology of the State of Durango), under project number 14889, sub-fund number 12 0515. It also received funding from the Consejo Nacional de Ciencia y Tecnología (CONACYT, or the National Council for Science and Technology, México, www.conacyt.gob.mx, accessed on 2 December 2021) and the Sistema Nacional de Investigadores (SNI, or the National System of Researchers), as awarded to R.P.-M. and A.G.-Z. and E.G.-T. (CVU-599255) received a doctoral scholarship from the CONACYT, under scholarship registry number 429715, grant number 326228.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors thank Leslie Madhai Morales Madrigal, Gerardo Ávila Butrón, Lourdes Froto Madariaga, Verenice Hernández Sifuentes, and Juan Manuel Llanas Wong for their technical assistance in the sampling, the measurement of the biochemical parameters, and genotypification.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Carrard, N.; Foster, T.; Willetts, J. Groundwater as a source of drinking water in Southeast Asia and the Pacific: A multi-country review of current reliance and resource concerns. Water 2019, 11, 1605. [Google Scholar] [CrossRef]
- Velis, M.; Conti, K.I.; Biermann, F. Groundwater and human development: Synergies and trade-offs within the context of the sustainable development goals. Sustain. Sci. 2017, 12, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Serio, F.; Miglietta, P.P.; Lamastra, L.; Ficocelli, S.; Intini, F.; De Leo, F.; De Donno, A. Groundwater nitrate contamination and agricultural land use: A grey water footprint perspective in Southern Apulia region (Italy). Sci. Total Environ. 2018, 645, 1425–1431. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Kim, K.; Powell, M.A. Managing groundwater nitrate contamination from livestock farms: Implication for nitrate management guidelines. Curr. Pollut. Rep. 2016, 2, 178–187. [Google Scholar] [CrossRef]
- Yu, G.; Wang, J.; Liu, L.; Li, Y.; Zhang, Y.; Wang, S. The analysis of groundwater nitrate pollution and health risk assessment in rural areas of Yantai, China. BMC Public Health 2020, 20, 437. [Google Scholar] [CrossRef] [PubMed]
- Shalev, N.; Burg, A.; Gavrieli, I.; Lazar, B. Nitrate contamination sources in aquifers underlying cultivated fields in an arid region—The Arava Valley, Israel. Appl. Geochem. 2015, 63, 322–332. [Google Scholar] [CrossRef]
- Wang, D.; Fu, Q.; Xu, Q.; Liu, Y.; Hao Ngo, H.; Yang, Q.; Zeng, G.; Li, X.; Ni, B.J. Free nitrous acid-based nitrifying sludge treatment in a two-sludge system enhances nutrient removal from low-carbon wastewater. Bioresour. Technol. 2017, 244, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Mester, T.; Balla, D.; Karancsi, G.; Bessenyei, É.; Szabó, G. Effects of nitrogen loading from domestic wastewater on groundwater quality. Water SA 2019, 45, 349–358. [Google Scholar] [CrossRef]
- Wongsanit, J.; Teartisup, P.; Kerdsueb, P.; Tharnpoophasiam, P.; Worakhunpiset, S. Contamination of nitrate in groundwater and its potential human health: A case study of lower Mae Klong river basin, Thailand. Environ. Sci. Pollut. Res. Int. 2015, 22, 11504–11512. [Google Scholar] [CrossRef]
- Kawagoshi, Y.; Suenaga, Y.; Chi, N.L.; Hama, T.; Ito, H.; Duc, L.V. Understanding nitrate contamination based on the relationship between changes in groundwater levels and changes in water quality with precipitation fluctuations. Sci. Total Environ. 2019, 657, 146–153. [Google Scholar] [CrossRef]
- Lasagna, M.; De Luca, D.A.; Franchino, E. Nitrate contamination of groundwater in the western Po Plain (Italy): The effects of groundwater and surface water interactions. Environ. Earth Sci. 2016, 75, 240. [Google Scholar] [CrossRef]
- Rhymes, J.; Jones, L.; Wallace, H.; Jones, T.G.; Dunn, C.; Fenner, N. Small changes in water levels and groundwater nutrients alter nitrogen and carbon processing in dune slack soils. Soil Biol. Biochem. 2016, 99, 28–35. [Google Scholar] [CrossRef]
- Lasagna, M.; De Luca, D.A.; Franchino, E. The role of physical and biological processes in aquifers and their importance on groundwater vulnerability to nitrate pollution. Environ. Earth Sci. 2016, 75, 961. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality: First Addendum to the Fourth Edition; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Takai, K. The nitrogen cycle: A large, fast, and mystifying cycle. Microbes Environ. 2019, 34, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Gassara, F.; Kouassi, A.P.; Brar, S.K.; Belkacemi, K. Green alternatives to nitrates and nitrites in meat-based products. A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2133–2148. [Google Scholar] [CrossRef] [PubMed]
- Almasi, A.; Shokri, R.; Momenzadeh, R.; Rezaei, S.; Jamshidi, A.; Yazdizadeh, R. Distribution of groundwater nitrate in Dehloran, Iran: A case study using GIS. J. Adv. Environ. Health Res. 2016, 4, 155–160. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, H. Assessment of sources and transformation of nitrate in the alluvial-pluvial fan region of north China using a multi-isotope approach. J. Environ. Sci. 2020, 89, 9–22. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Carlström, M.; Weitzberg, E. Metabolic effects of dietary nitrate in health and disease. Cell Metab. 2018, 28, 9–22. [Google Scholar] [CrossRef]
- Harper, C.; Keith, S.; Todd, G.D.; Williams, M.; Wohlers, D.; Diamond, G.L.; Citra, M.J. Toxicological Profile for Nitrate and Nitrite; ATSDR: Atlanta, GA, USA, 2017. [Google Scholar]
- Lundberg, J.O.; Weitzberg, E. Nitric oxide formation from inorganic nitrate. In Nitric Oxide: Biology and Pathobiology, 3rd ed.; Ignarro, L.J., Freeman, B.A., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 157–171. [Google Scholar] [CrossRef]
- Schullehner, J.; Hansen, B.; Thygesen, M.; Pedersen, C.B.; Sigsgaard, T. Nitrate in drinking water and colorectal cancer risk: A nationwide population-based cohort study. Int. J. Cancer 2018, 143, 73–79. [Google Scholar] [CrossRef]
- Fossen, S. Methemoglobinemia: Infants at risk. Curr. Probl. Pediatr. Adolesc. Health Care 2019, 49, 57–67. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Ghasemi, A.; Kabir, A.; Azizi, F.; Hadaegh, F. Is dietary nitrate/nitrite exposure a risk factor for development of thyroid abnormality? A systematic review and meta-analysis. Nitric Oxide 2015, 47, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Maurer, P.; Manfredini, V.; Noal, R.; Nunes, M.; Manica da Cruz, I.B.; da Costa, J. Association between Nitrite/Nitrate and metabolic risk in blacks. Arch. Med. 2015, 8, 1–4. [Google Scholar]
- Espejo-Herrera, N.; Gràcia-Lavedan, E.; Boldo, E.; Aragonés, N.; Pérez-Gómez, B.; Pollán, M.; Molina, A.J.; Fernández, T.; Martín, V.; La Vecchia, C.; et al. Colorectal cancer risk and nitrate exposure through drinking water and diet. Int. J. Cancer 2016, 139, 334–346. [Google Scholar] [CrossRef]
- Jones, R.R.; Weyer, P.J.; DellaValle, C.T.; Inoue-Choi, M.; Anderson, K.E.; Cantor, K.P.; Krasner, S.; Robien, K.; Freeman, L.E.; Silverman, D.T.; et al. Nitrate from drinking water and diet and bladder cancer among postmenopausal women in Iowa. Environ. Health Perspect. 2016, 124, 1751–1758. [Google Scholar] [CrossRef]
- Song, P.; Wu, L.; Guan, W. Dietary nitrates, nitrites, and nitrosamines intake and the risk of gastric cancer: A meta-analysis. Nutrients 2015, 7, 9872–9895. [Google Scholar] [CrossRef]
- Ward, M.H.; Kilfoy, B.A.; Weyer, P.J.; Anderson, K.E.; Folsom, A.R.; Cerhan, J.R. Nitrate intake and the risk of thyroid cancer and thyroid disease. Epidemiology 2010, 21, 389–395. [Google Scholar] [CrossRef]
- Tariqi, A.; Naughton, C. Water, Health, and Environmental Justice in California: Geospatial Analysis of Nitrate Contamination and Thyroid Cancer. Environ. Eng. Sci. 2021, 38, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Gatseva, P.D.; Argirova, M.D. Iodine status and goitre prevalence in nitrate-exposed schoolchildren living in rural Bulgaria. Public Health 2008, 122, 458–461. [Google Scholar] [CrossRef]
- van Maanen, J.M.S.; van Dijk, A.; Mulder, K.; de Baets, M.H.; Menheere, P.C.A.; van der Heide, D.; Kleinjans, J.C.S. Consumption of drinking water with high nitrate levels causes hypertrophy of the thyroid. Toxicol. Lett. 1994, 72, 365–374. [Google Scholar] [CrossRef]
- Tajtáková, M.; Semanová, Z.; Tomková, Z.; Szökeová, E.; Majoroš, J.; Rádiková, Ž.; Langer, P. Increased thyroid volume and frequency of thyroid disorders signs in schoolchildren from nitrate polluted area. Chemosphere 2006, 62, 559–564. [Google Scholar] [CrossRef]
- Aschebrook-Kilfoy, B.; Heltshe, S.L.; Nuckols, J.R.; Sabra, M.M.; Shuldiner, A.R.; Mitchell, B.D.; Airola, M.; Holford, T.R.; Zhang, Y.; Ward, M.H. Modeled nitrate levels in well water supplies and prevalence of abnormal thyroid conditions among the old order Amish in Pennsylvania. Environ. Health 2012, 11, 6. [Google Scholar] [CrossRef]
- Cantwell, M.; Elliott, C. Nitrates, nitrites and nitrosamines from processed meat intake and colorectal cancer risk. J. Clin. Nutr. Diet. 2017, 3, 27–30. [Google Scholar] [CrossRef]
- Poulsen, R.; Cedergreen, N.; Hayes, T.; Hansen, M. Nitrate: An environmental endocrine disruptor? A review of evidence and research needs. Environ. Sci. Technol. 2018, 52, 3869–3887. [Google Scholar] [CrossRef]
- Yilmaz, B.; Terekeci, H.; Sandal, S.; Kelestimur, F. Endocrine disrupting chemicals: Exposure, effects on human health, mechanism of action, models for testing and strategies for prevention. Rev. Endocr. Metab. Disord. 2019, 21, 127–147. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, M.; Nicola, J.P.; Nazar, M.; Peyret, V.; Lucero, A.M.; Pellizas, C.G.; Masini-Repiso, A.M. Nitric oxide-repressed forkhead factor FoxE1 expression is involved in the inhibition of TSH-induced thyroid peroxidase levels. Mol. Cell. Endocrinol. 2016, 420, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Hennessey, J.V.; Espaillat, R. Subclinical hypothyroidism: A historical view and shifting prevalence. Int. J. Clin. Pract. 2015, 69, 771–782. [Google Scholar] [CrossRef]
- Zimmermann, M.B. Iodine deficiency. In The Thyroid and its Diseases. A Comprehensive Guide for the Clinician; Luster, M., Duntas, L., Wartofsky, L., Eds.; Springer: Cham, Switzerland, 2019; pp. 101–107. [Google Scholar] [CrossRef]
- Ragusa, F.; Fallahi, P.; Elia, G.; Gonnella, D.; Paparo, S.R.; Giusti, C.; Churilov, L.P.; Ferrari, S.M.; Antonelli, A. Hashimotos’ thyroiditis: Epidemiology, pathogenesis, clinic and therapy. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101367. [Google Scholar] [CrossRef] [PubMed]
- Persani, L.; Rurale, G.; de Filippis, T.; Galazzi, E.; Muzza, M.; Fugazzola, L. Genetics and management of congenital hypothyroidism. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 387–396. [Google Scholar] [CrossRef]
- Oliveir, K.J.; Chiamolera, M.I.; Giannocco, G.; Pazos-Moura, C.C.; Ortiga-Carvalho, T.M. Thyroid function disruptors: From nature to chemicals. J. Mol. Endocrinol. 2019, 62, R1–R19. [Google Scholar] [CrossRef]
- Biondi, B.; Cappola, A.R.; Cooper, D.S. Subclinical hypothyroidism. A review. JAMA 2019, 322, 153. [Google Scholar] [CrossRef]
- Bruneel, F.; Raschilas, F.; Bédos, J.P.; Régnier, B.; Wolff, M. Diagnóstico y tratamiento de hipotiroidismo primario y subclínico en el adulto. EMC-Anest.-Reanim. 2016, 28, 1–13. [Google Scholar] [CrossRef]
- Secretaria de Salud. Proyecto de Norma Oficial Mexicana PROY-NOM-127-SSA1-2017. Agua para uso y consumo humano. Límites permisibles de la calidad del agua. Diario Oficial de la Federación, 6 December 2019. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5581179&fecha=06/12/2019 (accessed on 30 July 2020).
- QGIS.org. QGIS Geographic Information System; QGIS Association: 2022. Available online: http://www.qgis.org (accessed on 1 November 2021).
- Gandarilla-Esparza, D.D.; Calleros-Rincón, E.Y.; Macias, H.M.; González-Delgado, M.F.; Vargas, G.G.; Sustaita, J.D.; González-Zamora, A.; Ríos-Sánchez, E.; Pérez-Morales, R. FOXE1 polymorphisms and chronic exposure to nitrates in drinking water cause metabolic dysfunction, thyroid abnormalities, and genotoxic damage in women. Genet. Mol. Biol. 2021, 44, e20210020. [Google Scholar] [CrossRef]
- Sakata, M.; Yoshida, A.; Haga, M. Methemoglobin in blood as determined by double-wavelength spectrophotometry. Clin. Chem. 1982, 28, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef]
- Lahiri, D.K.; Nurnberger, J.I., Jr. A rapid non-enzymatic method for the preparation of HMW DNA from blood for RFLP studies. Nucleic Acids Res. 1991, 19, 5444. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 2 November 2021).
- Fox, J. RcmdrMisc: R Commander Miscellaneous Functions, R package version 2.7.1. R Foundation for Statistical Computing: Vienna, Austria. 2020. Available online: https://CRAN.R-project.org/package=RcmdrMisc (accessed on 2 November 2021).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: New York, NY, USA, 2016; pp. 1–260. [Google Scholar]
- Slowikowski, K. ggrepel: Automatically Position Non-Overlapping Text Labels With ‘ggplot2’, R package version 0.9.1. R Foundation for Statistical Computing: Vienna, Austria. 2021. Available online: https://CRAN.R-project.org/package=ggrepel (accessed on 2 November 2021).
- Kassambara, A.; Mundt, F. factoextra: Extract and Visualize the Results of Multivariate Data Analyses, R package version 1.0.7; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://CRAN.R-project.org/package=factoextra (accessed on 2 November 2021).
- Le, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Dinno, A. paran: Horn’s Test of principal components/Factors, R package version 1.5.2. R Foundation for Statistical Computing: Vienna, Austria. 2018. Available online: https://CRAN.R-project.org/package=paran (accessed on 2 November 2021).
- Raiche, G.; Magis, D. nFactors: Parallel Analysis and Other non Graphical Solutions to the Cattell Scree Test, R package version 2.4.1; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://CRAN.R-project.org/package=nFactors (accessed on 2 November 2021).
- Kolde, R. Pheatmap: Pretty Heatmaps, R package version 1.0.12; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://CRAN.R-project.org/package=pheatmap (accessed on 2 November 2021).
- Lundberg, J.; Weitzberg, E.; Gladwin, M. The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Jonvik, K.L.; Nyakayiru, J.; Pincers, P.J.; Senden, J.M.; van Loon, L.J.; Verdijk, L.B. Nitrate-rich vegetables increase plasma nitrate and nitrite concentrations and lower blood pressure in healthy adults. J. Nutr. 2016, 146, 986–993. [Google Scholar] [CrossRef]
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Younes, M. Re-evaluation of potassium nitrite (E 249) and sodium nitrite (E 250) as food additives. EFSA J. 2017, 15, 4786. [Google Scholar] [CrossRef]
- McDonagh, S.T.J.; Wylie, L.J.; Webster, J.M.A.; Vanhatalo, A.; Jones, A.M. Influence of dietary nitrate food forms on nitrate metabolism and blood pressure in healthy normotensive adults. Nitric Oxide 2018, 72, 66–74. [Google Scholar] [CrossRef]
- Lee, P.M.; Gerriets, V. Nitrates. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK545149/ (accessed on 2 November 2021).
- Moretti, C.; Zhuge, Z.; Zhang, G.; Haworth, S.M.C.; Paulo, L.L.; Guimarães, D.D.; Lundberg, J.O. The obligatory role of host microbiota in bioactivation of dietary nitrate. Free Radic. Biol. Med. 2019, 145, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Mikiwa, K.; Tadashi, I.; Kayoko, K.; Atsushi, N.; Masahito, H.; Hiroshi, H. Plasma nitrate/nitrite concentration in healthy population and patients with diabetes mellitus-relationships with gender, aging and diabetic complications. Bull. Osaka Med. Coll. 2002, 48, 1–6. [Google Scholar]
- Pimková, K.; Chrastinová, L.; Suttnar, J.; Štikarová, J.; Kotlín, R.; Čermák, J.; Dyr, J.E. Plasma levels of aminothiols, nitrite, nitrate, and malondialdehyde in myelodysplastic syndromes in the context of clinical outcomes and as a consequence of iron overload. Oxid. Med. Cell. Longev. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Queirós, C.; Salvador, P.; Ventura, A.; Lopes, D. Methemoglobinemia after paracetamol ingestion: A case report. Acta Med. Port. 2017, 30, 753–756. [Google Scholar] [CrossRef] [PubMed]
- Gómez, I.; Franyuti, G.; Hornelas, A. Metahemoglobinemia severa secundaria a sobredosis de dapsona, reporte de caso. Med. Crít. (Col. Mex. Med. Crít.) 2017, 31, 285–287. [Google Scholar]
- Alanazi, M.Q. Drugs may be induced methemoglobinemia. J. Hematol. Thrombo. Dis. 2017, 6, 5–9. [Google Scholar] [CrossRef]
- Horton, M.K.; Blount, B.C.; Valentin-Blasini, L.; Wapner, R.; Whyatt, R.; Gennings, C.; Factor-Litvak, P. CO-occurring exposure to perchlorate, nitrate and thiocyanate alters thyroid function in healthy pregnant women. Environ. Res. 2015, 143, 1–9. [Google Scholar] [CrossRef]
- Hunault, C.C.; Lambers, A.C.; Mensinga, T.T.; van Isselt, J.W.; Koppeschaar, H.P.F.; Meulenbelt, J. Effects of sub-chronic nitrate exposure on the thyroidal function in humans. Toxicol. Lett. 2007, 175, 64–70. [Google Scholar] [CrossRef]
- Duntas, B. Subclinical hypothyroidism. In Thyroid Disorders; Basic Science and Clinical Practice; Springer: Berlin/Heidelberg, Germany, 2019; pp. 203–224. [Google Scholar] [CrossRef]
- Liontiris, M.I.; Mazokopakis, E.E. A concise review of Hashimoto thyroiditis (HT) and the importance of iodine, selenium, vitamin D and gluten on the autoimmunity and dietary management of HT patients. Points that need more investigation. Hell. J. Nucl. Med. 2017, 20, 51–56. [Google Scholar]
- Zimmermann, M.B.; Boelaert, K. Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol. 2015, 3, 286–295. [Google Scholar] [CrossRef]
- Radetti, G. Clinical aspects of Hashimoto’s thyroiditis. Endocr. Dev. 2014, 26, 158–170. [Google Scholar] [CrossRef]
- Bromińska, B.; Bromiński, G.; Owecki, M.; Michalak, M.; Czarnywojtek, A.; Waśko, R.; Ruchała, M. Anti-thyroidal peroxidase antibodies are associated with thyrotropin levels in hypothyroid patients and in euthyroid individuals. Ann. Agric. Environ. Med. 2017, 24, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Malathi, M.; Sudeep, K.; Jeena, E.J. A hospital-based study of anti-TPO titer in patients with thyroid disease. Muller J. Med. Sci. Res. 2013, 4, 74. [Google Scholar] [CrossRef]
- Jayashankar, C.A.; Avinash, S.; Shashidharan, B.; Sarathi, V.; Shruthi, K.R.; Nikethan, D.; Harshavardhan, J. The prevalence of anti-thyroid peroxidase antibodies in subclinical and clinical hypothyroid patients. Int. J. Res. Med. Sci. 2015, 3, 3564. [Google Scholar] [CrossRef]
- Hu, S.; Rayman, M.P. Multiple nutritional factors and the risk of Hashimoto’s thyroiditis. Thyroid 2017, 27, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.P.; López-Márquez, A.; Santisteban, P. Thyroid transcription factors in development, differentiation and disease. Nat. Rev. Endocrinol. 2015, 11, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Zhang, Y.Q. Exploration of the association between FOXE1 gene polymorphism and differentiated thyroid cancer: A meta-analysis. BMC Med. Genet. 2018, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nikitski, A.V.; Rogounovitch, T.I.; Bychkov, A.; Takahashi, M.; Yoshiura, K.; Mitsutake, N.; Saenko, V.A. Genotype analyses in the Japanese and Belarusian populations reveal independent effects of rs965513 and rs1867277 but do not support the role of FOXE1 polyalanine tract length in conferring risk for Papillary Thyroid Carcinoma. Thyroid 2017, 27, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsson, J.; Sulem, P.; Gudbjartsson, D.F.; Jonasson, J.G.; Sigurdsson, A.; Bergthorsson, J.T.; Stefansson, K. Common variants on 9q22.33 and 14q13.3 predispose to thyroid cancer in European populations. Nat. Genet. 2009, 41, 460–464. [Google Scholar] [CrossRef]
- Denny, J.C.; Crawford, D.C.; Ritchie, M.D.; Bielinski, S.J.; Basford, M.A.; Bradford, Y.; Chai, H.S.; Bastarache, L.; Zuvich, R.; Peissig, P.; et al. Variants near FOXE1 are associated with hypothyroidism and other thyroid conditions: Using electronic medical records for genome- and phenome-wide studies. Am. J. Hum. Genet. 2011, 89, 529–542. [Google Scholar] [CrossRef]
- Landa, I.; Ruiz-Llorente, S.; Montero-Conde, C.; Inglada-Pérez, L.; Schiavi, F.; Leskelä, S.; Robledo, M. The variant rs1867277 in FOXE1 gene confers thyroid cancer susceptibility through the recruitment of USF1/USF2 transcription factors. PLoS Genet. 2009, 5, e1000637. [Google Scholar] [CrossRef]
- Geng, P.; Ou, J.; Li, J.; Liao, Y.; Wang, N.; Xie, G.; Liang, H. TITF1 and TITF2 loci variants indicate significant associations with thyroid cancer. Endocrine 2015, 50, 598–607. [Google Scholar] [CrossRef]
- Sanyal, D.; Raychaudhuri, M. Hypothyroidism and obesity: An intriguing link. Indian J. Endocrinol. Metab. 2016, 20, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.N.; Richmond, R.; Davies, N.; Sayers, A.; Stevenson, K.; Woltersdorf, W.; Dayan, C.M. Paradoxical relationship between body mass index and thyroid hormone levels: A study using mendelian randomization. J. Clin. Endocrinol. Metab. 2016, 101, 730–738. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, R.; Huang, F.; Zhang, S.; Lv, Y.; Liu, Q. Thyroid function, body mass index, and metabolic risk markers in euthyroid adults: A cohort study. BMC Endocr. Disord. 2019, 19, 58. [Google Scholar] [CrossRef] [PubMed]
- Abdi, H.; Kazemian, E.; Gharibzadeh, S.; Amouzegar, A.; Mehran, L.; Tohidi, M.; Rashvandi, Z.; Azizi, F. Association between thyroid function and body mass index: A 10-year follow-up. Ann. Nutr. Metab. 2017, 70, 338–345. [Google Scholar] [CrossRef]
- Barbesino, G. Thyroid function changes in the elderly and their relationship to cardiovascular health: A mini-review. Gerontology 2019, 65, 1–8. [Google Scholar] [CrossRef]
- Shukla, S.; Saxena, A. Global status of nitrate contamination in groundwater: Its occurrence, health impacts, and mitigation measures. In Handbook of Environmental Materials Management; Hussain, C., Ed.; Springer: Cham, Switzerland, 2018; pp. 869–888. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).