Insights into Gastrointestinal Virome: Etiology and Public Exposure
Abstract
1. Introduction
2. Enteric Virome in Infants
3. Viral Etiology
3.1. Adenovirus
3.2. Rotavirus
3.3. Norovirus
3.4. Hepatitis A Virus (HAV)
3.5. Astrovirus
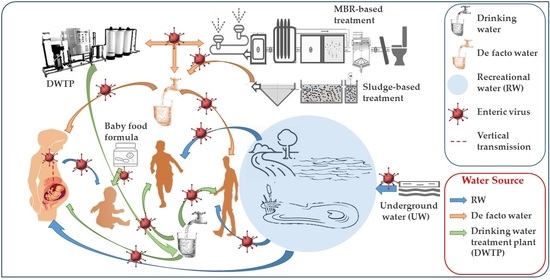
4. Public Exposure to Municipal Wastewater
5. Discharge of Wastewater
6. Reuse of Treated Water
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Carding, S.R.; Davis, N.; Hoyles, L. The Human Intestinal Virome in Health and Disease. Aliment. Pharmacol. Ther. 2017, 46, 800–815. [Google Scholar] [CrossRef]
- Lim, E.S.; Zhou, Y.; Zhao, G.; Bauer, I.K.; Droit, L.; Ndao, I.M.; Warner, B.B.; Tarr, P.I.; Wang, D.; Holtz, L.R. Early Life Dynamics of the Human Gut Virome and Bacterial Microbiome in Infants. Nat. Med. 2015, 21, 1228. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Schubert, A.M.; Zackular, J.P.; Iverson, K.D.; Young, V.B.; Petrosino, J.F. Stabilization of the Murine Gut Microbiome Following Weaning. Gut Microbes 2012, 3, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, R.; Rodgers, R.; Rodriguez, C.; Handley, S.A.; Ndao, I.M.; Tarr, P.I.; Warner, B.B.; Lim, E.S.; Holtz, L.R. Discordant Transmission of Bacteria and Viruses from Mothers to Babies at Birth. Microbiome 2019, 7, 1–13. [Google Scholar] [CrossRef]
- Minot, S.; Bryson, A.; Chehoud, C.; Wu, G.D.; Lewis, J.D.; Bushman, F.D. Rapid Evolution of the Human Gut Virome. Proc. Natl. Acad. Sci. USA 2013, 110, 12450–12455. [Google Scholar] [CrossRef]
- Lawrence, R.M. Transmission of infectious diseases through breast milk and breastfeeding. In Breastfeeding; Elsevier: Amsterdam, The Netherlands, 2022; pp. 393–456. [Google Scholar]
- Khuroo, M.S.; Khuroo, M.S.; Khuroo, N.S. Transmission of Hepatitis E Virus in Developing Countries. Viruses 2016, 8, 253. [Google Scholar] [CrossRef] [PubMed]
- Khuroo, M.S.; Kamili, S.; Khuroo, M.S. Clinical Course and Duration of Viremia in Vertically Transmitted Hepatitis E Virus (HEV) Infection in Babies Born to HEV-Infected Mothers. J. Viral Hepat. 2009, 16, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Grimwood, K.; Lambert, S.B.; Milne, R.J. Rotavirus Infections and Vaccines. Pediatr. Drugs 2010, 12, 235–256. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.K.; Shrestha, J.; Andreassen, A.K.; Strand, T.A.; Dudman, S.; Dembinski, J.L. Genetic Diversity of Astrovirus in Children from a Birth Cohort in Nepal. Front. Microbiol. 2021, 11, 3537. [Google Scholar] [CrossRef]
- Armbrust, S.; Kramer, A.; Olbertz, D.; Zimmermann, K.; Fusch, C. Norovirus Infections in Preterm Infants: Wide Variety of Clinical Courses. BMC Res. Notes 2009, 2, 1–6. [Google Scholar] [CrossRef]
- Elnifro, E.M.; Cooper, R.J.; Dady, I.; Hany, S.; Mughal, Z.M.; Klapper, P.E. Three Nonfatal Cases of Neonatal Adenovirus Infection. J. Clin. Microbiol. 2005, 43, 5814–5815. [Google Scholar] [CrossRef]
- Seitz, S.R.; Leon, J.S.; Schwab, K.J.; Lyon, G.M.; Dowd, M.; McDaniels, M.; Abdulhafid, G.; Fernandez, M.L.; Lindesmith, L.C.; Baric, R.S. Norovirus Infectivity in Humans and Persistence in Water. Appl. Environ. Microbiol. 2011, 77, 6884–6888. [Google Scholar] [CrossRef]
- Ogorzaly, L.; Bertrand, I.; Paris, M.; Maul, A.; Gantzer, C. Occurrence, Survival, and Persistence of Human Adenoviruses and F-Specific RNA Phages in Raw Groundwater. Appl. Environ. Microbiol. 2010, 76, 8019–8025. [Google Scholar] [CrossRef] [PubMed]
- Rzeżutka, A.; Cook, N. Survival of Human Enteric Viruses in the Environment and Food. FEMS Microbiol. Rev. 2004, 28, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Raphael, R.A.; Sattar, S.A.; Springthorpe, V.S. Long-Term Survival of Human Rotavirus in Raw and Treated River Water. Can. J. Microbiol. 1985, 31, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Gorgich, M.; Mata, T.M.; Martins, A.; Caetano, N.S.; Formigo, N. Application of Domestic Greywater for Irrigating Agricultural Products: A Brief Study. Energy Rep. 2020, 6, 811–817. [Google Scholar] [CrossRef]
- Angelakis, A.N.; Asano, T.; Bahri, A.; Jimenez, B.E.; Tchobanoglous, G. Water Reuse: From Ancient to Modern Times and the Future. Front. Environ. Sci. 2018, 6, 26. [Google Scholar] [CrossRef]
- Libutti, A.; Gatta, G.; Gagliardi, A.; Vergine, P.; Pollice, A.; Beneduce, L.; Disciglio, G.; Tarantino, E. Agro-Industrial Wastewater Reuse for Irrigation of a Vegetable Crop Succession under Mediterranean Conditions. Agric. Water Manag. 2018, 196, 1–14. [Google Scholar] [CrossRef]
- Fountoulakis, M.S.; Markakis, N.; Petousi, I.; Manios, T. Single House On-Site Grey Water Treatment Using a Submerged Membrane Bioreactor for Toilet Flushing. Sci. Total Environ. 2016, 551, 706–711. [Google Scholar] [CrossRef]
- Becerra-Castro, C.; Lopes, A.R.; Vaz-Moreira, I.; Silva, E.F.; Manaia, C.M.; Nunes, O.C. Wastewater Reuse in Irrigation: A Microbiological Perspective on Implications in Soil Fertility and Human and Environmental Health. Environ. Int. 2015, 75, 117–135. [Google Scholar] [CrossRef]
- Nour, I.; Hanif, A.; Alanazi, I.O.; Al-Ashkar, I.; Alhetheel, A.; Eifan, S. Novel Insights of Waterborne Human Rotavirus A in Riyadh (Saudi Arabia) Involving G2 Predominance and Emergence of a Thermotolerant Sequence. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bisseux, M.; Colombet, J.; Mirand, A.; Roque-Afonso, A.-M.; Abravanel, F.; Izopet, J.; Archimbaud, C.; Peigue-Lafeuille, H.; Debroas, D.; Bailly, J.-L. Monitoring Human Enteric Viruses in Wastewater and Relevance to Infections Encountered in the Clinical Setting: A One-Year Experiment in Central France, 2014 to 2015. Eurosurveillance 2018, 23, 17–00237. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, A.K.; Sachdeva, P. Coronavirus Disease 2019 (COVID-19): A New Challenge in Untreated Wastewater. Can. J. Civ. Eng. 2020, 47, 1005–1009. [Google Scholar] [CrossRef]
- Tran, H.N.; Le, G.T.; Nguyen, D.T.; Juang, R.-S.; Rinklebe, J.; Bhatnagar, A.; Lima, E.C.; Iqbal, H.M.; Sarmah, A.K.; Chao, H.-P. SARS-CoV-2 Coronavirus in Water and Wastewater: A Critical Review about Presence and Concern. Environ. Res. 2020, 110265. [Google Scholar] [CrossRef] [PubMed]
- Bivins, A.; Greaves, J.; Fischer, R.; Yinda, K.C.; Ahmed, W.; Kitajima, M.; Munster, V.J.; Bibby, K. Persistence of SARS-CoV-2 in Water and Wastewater. Environ. Sci. Technol. Lett. 2020, 7, 937–942. [Google Scholar] [CrossRef]
- Van Tung, T.; Tran, Q.B.; Thao, N.T.P.; Hieu, T.T.; Le, S.; Tuan, N.Q.; Sonne, C.; Lam, S.S.; Van Le, Q. Recycling of Aquaculture Wastewater and Sediment for Sustainable Corn and Water Spinach Production. Chemosphere 2021, 268, 129329. [Google Scholar] [CrossRef]
- Garcia, X.; Pargament, D. Reusing Wastewater to Cope with Water Scarcity: Economic, Social and Environmental Considerations for Decision-Making. Resour. Conserv. Recycl. 2015, 101, 154–166. [Google Scholar] [CrossRef]
- Jaramillo, M.F.; Restrepo, I. Wastewater Reuse in Agriculture: A Review about Its Limitations and Benefits. Sustainability 2017, 9, 1734. [Google Scholar] [CrossRef]
- Chaudhry, R.M.; Hamilton, K.A.; Haas, C.N.; Nelson, K.L. Drivers of Microbial Risk for Direct Potable Reuse and de Facto Reuse Treatment Schemes: The Impacts of Source Water Quality and Blending. Int. J. Environ. Res. Public Health 2017, 14, 635. [Google Scholar] [CrossRef]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The Placenta Harbors a Unique Microbiome. Sci. Transl. Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef]
- Bergström, A.; Skov, T.H.; Bahl, M.I.; Roager, H.M.; Christensen, L.B.; Ejlerskov, K.T.; Mølgaard, C.; Michaelsen, K.F.; Licht, T.R. Establishment of Intestinal Microbiota during Early Life: A Longitudinal, Explorative Study of a Large Cohort of Danish Infants. Appl. Environ. Microbiol. 2014, 80, 2889. [Google Scholar] [CrossRef]
- Matamoros, S.; Gras-Leguen, C.; Le Vacon, F.; Potel, G.; de La Cochetiere, M.-F. Development of Intestinal Microbiota in Infants and Its Impact on Health. Trends Microbiol. 2013, 21, 167–173. [Google Scholar] [CrossRef]
- Penders, J.; Thijs, C.; Vink, C.; Stelma, F.F.; Snijders, B.; Kummeling, I.; van den Brandt, P.A.; Stobberingh, E.E. Factors Influencing the Composition of the Intestinal Microbiota in Early Infancy. Pediatrics 2006, 118, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.J.; Lynch, D.B.; Murphy, K.; Ulaszewska, M.; Jeffery, I.B.; O’Shea, C.A.; Watkins, C.; Dempsey, E.; Mattivi, F.; Tuohy, K. Evolution of Gut Microbiota Composition from Birth to 24 Weeks in the INFANTMET Cohort. Microbiome 2017, 5, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Palacio, S.D.; Montes, S.A.; Mancabelli, L. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. MMBR 2017, 81. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.M.; Murphy, K.; Stanton, C.; Ross, R.P.; Kober, O.I.; Juge, N.; Avershina, E.; Rudi, K.; Narbad, A.; Jenmalm, M.C. The Composition of the Gut Microbiota throughout Life, with an Emphasis on Early Life. Microb. Ecol. Health Dis. 2015, 26, 26050. [Google Scholar] [CrossRef]
- McCann, A.; Ryan, F.J.; Stockdale, S.R.; Dalmasso, M.; Blake, T.; Ryan, C.A.; Stanton, C.; Mills, S.; Ross, P.R.; Hill, C. Viromes of One Year Old Infants Reveal the Impact of Birth Mode on Microbiome Diversity. PeerJ 2018, 6, e4694. [Google Scholar] [CrossRef]
- Kurokawa, K.; Itoh, T.; Kuwahara, T.; Oshima, K.; Toh, H.; Toyoda, A.; Takami, H.; Morita, H.; Sharma, V.K.; Srivastava, T.P. Comparative Metagenomics Revealed Commonly Enriched Gene Sets in Human Gut Microbiomes. Dna Res. 2007, 14, 169–181. [Google Scholar] [CrossRef]
- Mueller, N.T.; Bakacs, E.; Combellick, J.; Grigoryan, Z.; Dominguez-Bello, M.G. The Infant Microbiome Development: Mom Matters. Trends Mol. Med. 2015, 21, 109–117. [Google Scholar] [CrossRef]
- Goedert, J.J.; Hua, X.; Yu, G.; Shi, J. Diversity and Composition of the Adult Fecal Microbiome Associated with History of Cesarean Birth or Appendectomy: Analysis of the American Gut Project. EBioMedicine 2014, 1, 167–172. [Google Scholar] [CrossRef]
- Zhou, P.; Zhou, Y.; Liu, B.; Jin, Z.; Zhuang, X.; Dai, W.; Yang, Z.; Feng, X.; Zhou, Q.; Liu, Y. Perinatal Antibiotic Exposure Affects the Transmission between Maternal and Neonatal Microbiota and Is Associated with Early-Onset Sepsis. Msphere 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.R.; Lee, H.H.; Spina, C.S.; Collins, J.J. Antibiotic Treatment Expands the Resistance Reservoir and Ecological Network of the Phage Metagenome. Nature 2013, 499, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.K.; Looft, T.; Bayles, D.O.; Humphrey, S.; Levine, U.Y.; Alt, D.; Stanton, T.B. Antibiotics in Feed Induce Prophages in Swine Fecal Microbiomes. MBio 2011, 2. [Google Scholar] [CrossRef] [PubMed]
- Sommer, M.O.; Dantas, G. Antibiotics and the Resistant Microbiome. Curr. Opin. Microbiol. 2011, 14, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.M.; Handley, S.A.; Baldridge, M.T.; Droit, L.; Liu, C.Y.; Keller, B.C.; Kambal, A.; Monaco, C.L.; Zhao, G.; Fleshner, P. Disease-Specific Alterations in the Enteric Virome in Inflammatory Bowel Disease. Cell 2015, 160, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.; Friedberg, I.; Ivanov, I.V.; Davidson, L.A.; Goldsby, J.S.; Dahl, D.B.; Herman, D.; Wang, M.; Donovan, S.M.; Chapkin, R.S. A Metagenomic Study of Diet-Dependent Interaction between Gut Microbiota and Host in Infants Reveals Differences in Immune Response. Genome Biol. 2012, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tremaroli, V.; Bäckhed, F. Functional Interactions between the Gut Microbiota and Host Metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- Kinross, J.M.; Darzi, A.W.; Nicholson, J.K. Gut Microbiome-Host Interactions in Health and Disease. Genome Med. 2011, 3, 1–12. [Google Scholar] [CrossRef]
- Reyes, A.; Blanton, L.V.; Cao, S.; Zhao, G.; Manary, M.; Trehan, I.; Smith, M.I.; Wang, D.; Virgin, H.W.; Rohwer, F. Gut DNA Viromes of Malawian Twins Discordant for Severe Acute Malnutrition. Proc. Natl. Acad. Sci. USA 2015, 112, 11941–11946. [Google Scholar] [CrossRef]
- Breitbart, M.; Haynes, M.; Kelley, S.; Angly, F.; Edwards, R.A.; Felts, B.; Mahaffy, J.M.; Mueller, J.; Nulton, J.; Rayhawk, S. Viral Diversity and Dynamics in an Infant Gut. Res. Microbiol. 2008, 159, 367–373. [Google Scholar] [CrossRef]
- Mukhopadhya, I.; Segal, J.P.; Carding, S.R.; Hart, A.L.; Hold, G.L. The Gut Virome: The ‘Missing Link’between Gut Bacteria and Host Immunity? Ther. Adv. Gastroenterol. 2019, 12, 1756284819836620. [Google Scholar] [CrossRef] [PubMed]
- Baschat, A.A.; Towbin, J.; Bowles, N.E.; Harman, C.R.; Weiner, C.P. Prevalence of Viral DNA in Amniotic Fluid of Low-Risk Pregnancies in the Second Trimester. J. Matern. Fetal Neonatal Med. 2003, 13, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.S.; Wang, D.; Holtz, L.R. The Bacterial Microbiome and Virome Milestones of Infant Development. Trends Microbiol. 2016, 24, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.C.; Fleming, D.W.; Borella, A.J.; Olcott, E.S.; Conrad, R.E.; Baron, R.C. Vertical Transmission of Hepatitis A Resulting in an Outbreak in a Neonatal Intensive Care Unit. J. Infect. Dis. 1993, 167, 567–571. [Google Scholar] [CrossRef]
- Ciccarelli, S.; Stolfi, I.; Caramia, G. Management Strategies in the Treatment of Neonatal and Pediatric Gastroenteritis. Infect. Drug Resist. 2013, 6, 133. [Google Scholar]
- Opere, W.M.; John, M.; Ombori, O. Molecular Detection of Human Enteric Adenoviruses in Water Samples Collected from Lake Victoria Waters Along Homa Bay Town, Homa Bay County, Kenya. Food Environ. Virol. 2021, 13, 32–43. [Google Scholar] [CrossRef]
- Dhingra, A.; Hage, E.; Ganzenmueller, T.; Böttcher, S.; Hofmann, J.; Hamprecht, K.; Obermeier, P.; Rath, B.; Hausmann, F.; Dobner, T. Molecular Evolution of Human Adenovirus (HAdV) Species C. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Hashimoto, S.; Gonzalez, G.; Harada, S.; Oosako, H.; Hanaoka, N.; Hinokuma, R.; Fujimoto, T. Recombinant Type Human Mastadenovirus D85 Associated with Epidemic Keratoconjunctivitis since 2015 in Japan. J. Med Virol. 2018, 90, 881–889. [Google Scholar] [CrossRef]
- Robinson, C.M.; Singh, G.; Lee, J.Y.; Dehghan, S.; Rajaiya, J.; Liu, E.B.; Yousuf, M.A.; Betensky, R.A.; Jones, M.S.; Dyer, D.W. Molecular Evolution of Human Adenoviruses. Sci. Rep. 2013, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Seto, D.; Chodosh, J.; Brister, J.R.; Jones, M.S.; Community, A.R. Using the Whole-Genome Sequence to Characterize and Name Human Adenoviruses. J. Virol. 2011, 85, 5701. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, A.J.; Baumgart, D.C. Viral Gastroenteritis in Adults. Recent Pat. Antiinfect. Drug Discov. 2011, 6, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. CDC Acute Respiratory Disease Associated with Adenovirus Serotype 14—Four States, 2006–2007. MMWR. Morb. Mortal. Wkly. Rep. 2007, 56, 1181–1184. [Google Scholar]
- Elmahdy, E.M.; Shaheen, M.N.; Rizk, N.M.; Saad-Hussein, A. Quantitative Detection of Human Adenovirus and Human Rotavirus Group A in Wastewater and El-Rahawy Drainage Canal Influencing River Nile in the North of Giza, Egypt. Food Environ. Virol. 2020, 12, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Farkas, K.; Marshall, M.; Cooper, D.; McDonald, J.E.; Malham, S.K.; Peters, D.E.; Maloney, J.D.; Jones, D.L. Seasonal and Diurnal Surveillance of Treated and Untreated Wastewater for Human Enteric Viruses. Environ. Sci. Pollut. Res. 2018, 25, 33391–33401. [Google Scholar] [CrossRef] [PubMed]
- Fong, T.-T.; Phanikumar, M.S.; Xagoraraki, I.; Rose, J.B. Quantitative Detection of Human Adenoviruses in Wastewater and Combined Sewer Overflows Influencing a Michigan River. Appl. Environ. Microbiol. 2010, 76, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, J.P.; Ahmed, W.; Palmer, A.; Smith, K.; Hodgers, L.; Toze, S. Optimization of Sampling Strategy to Determine Pathogen Removal Efficacy of Activated Sludge Treatment Plant. Environ. Sci. Pollut. Res. 2017, 24, 19001–19010. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Ganesh, A. Water Quality Indicators: Bacteria, Coliphages, Enteric Viruses. Int. J. Environ. Health Res. 2013, 23, 484–506. [Google Scholar] [CrossRef]
- Eischeid, A.C.; Meyer, J.N.; Linden, K.G. UV Disinfection of Adenoviruses: Molecular Indications of DNA Damage Efficiency. Appl. Environ. Microbiol. 2009, 75, 23. [Google Scholar] [CrossRef]
- Brestovitsky, A.; Nebenzahl-Sharon, K.; Kechker, P.; Sharf, R.; Kleinberger, T. The Adenovirus E4orf4 Protein Provides a Novel Mechanism for Inhibition of the DNA Damage Response. PLoS Pathog. 2016, 12, e1005420. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.D.; García-Zapata, M.T.; Anunciação, C.E. Why the Use of Adenoviruses as Water Quality Virologic Marker? Food Environ. Virol. 2011, 3, 138–140. [Google Scholar] [CrossRef]
- Nour, I.; Hanif, A.; Zakri, A.M.; Al-Ashkar, I.; Alhetheel, A.; Eifan, S. Human Adenovirus Molecular Characterization in Various Water Environments and Seasonal Impacts in Riyadh, Saudi Arabia. Int. J. Environ. Res. Public Health 2021, 18, 4773. [Google Scholar] [CrossRef]
- Elmahdy, E.M.; Ahmed, N.I.; Shaheen, M.N.; Mohamed, E.-C.B.; Loutfy, S.A. Molecular Detection of Human Adenovirus in Urban Wastewater in Egypt and among Children Suffering from Acute Gastroenteritis. J. Water Health 2019, 17, 287–294. [Google Scholar] [CrossRef]
- Shaheen, M.N.; Elmahdy, E.M.; Chawla-Sarkar, M. Quantitative PCR-Based Identification of Enteric Viruses Contaminating Fresh Produce and Surface Water Used for Irrigation in Egypt. Environ. Sci. Pollut. Res. 2019, 26, 21619–21628. [Google Scholar] [CrossRef] [PubMed]
- Katayama, H.; Haramoto, E.; Oguma, K.; Yamashita, H.; Tajima, A.; Nakajima, H.; Ohgaki, S. One-Year Monthly Quantitative Survey of Noroviruses, Enteroviruses, and Adenoviruses in Wastewater Collected from Six Plants in Japan. Water Res. 2008, 42, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Sdiri-Loulizi, K.; Hassine, M.; Aouni, Z.; Gharbi-Khelifi, H.; Chouchane, S.; Sakly, N.; Neji-Guédiche, M.; Pothier, P.; Aouni, M.; Ambert-Balay, K. Detection and Molecular Characterization of Enteric Viruses in Environmental Samples in Monastir, Tunisia between January 2003 and April 2007. J. Appl. Microbiol. 2010, 109, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Bortagaray, V.; Lizasoain, A.; Piccini, C.; Gillman, L.; Berois, M.; Pou, S.; del Pilar Díaz, M.; Tort, F.L.; Colina, R.; Victoria, M. Microbial Source Tracking Analysis Using Viral Indicators in Santa Lucía and Uruguay Rivers, Uruguay. Food Environ. Virol. 2019, 11, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Gamazo, P.; Victoria, M.; Schijven, J.F.; Alvareda, E.; Tort, L.F.L.; Ramos, J.; Burutaran, L.; Olivera, M.; Lizasoain, A.; Sapriza, G. Evaluation of Bacterial Contamination as an Indicator of Viral Contamination in a Sedimentary Aquifer in Uruguay. Food Environ. Virol. 2018, 10, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Sibanda, T.; Okoh, A.I. Assessment of the Incidence of Enteric Adenovirus Species and Serotypes in Surface Waters in the Eastern Cape Province of South Africa: Tyume River as a Case Study. Sci. World J. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Osuolale, O.; Okoh, A. Incidence of Human Adenoviruses and Hepatitis A Virus in the Final Effluent of Selected Wastewater Treatment Plants in Eastern Cape Province, South Africa. Virol. J. 2015, 12, 1–8. [Google Scholar] [CrossRef]
- Rashid, M.; Khan, M.N.; Jalbani, N. Detection of Human Adenovirus, Rotavirus, and Enterovirus in Tap Water and Their Association with the Overall Quality of Water in Karachi, Pakistan. Food Env. Virol 2021, 13, 44–52. [Google Scholar] [CrossRef]
- Symonds, E.M.; Griffin, D.W.; Breitbart, M. Eukaryotic Viruses in Wastewater Samples from the United States. Appl. Environ. Microbiol. 2009, 75, 1402–1409. [Google Scholar] [CrossRef]
- Staggemeier, R.; Heck, T.M.; Demoliner, M.; Ritzel, R.G.; Röhnelt, N.M.; Girardi, V.; Venker, C.A.; Spilki, F.R. Enteric Viruses and Adenovirus Diversity in Waters from 2016 Olympic Venues. Sci. Total. Environ. 2017, 586, 304–312. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, G.; Pourshaban, M.; Iaconelli, M.; Muscillo, M. Quantitative Real-Time PCR of Enteric Viruses in Influent and Effluent Samples from Wastewater Treatment Plants in Italy. Ann. Ist. Super. Sanita 2010, 46, 266–273. [Google Scholar] [PubMed]
- Iaconelli, M.; Valdazo-González, B.; Equestre, M.; Ciccaglione, A.R.; Marcantonio, C.; Della Libera, S.; La Rosa, G. Molecular Characterization of Human Adenoviruses in Urban Wastewaters Using next Generation and Sanger Sequencing. Water Res. 2017, 121, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Farkas, K.; Cooper, D.M.; McDonald, J.E.; Malham, S.K.; de Rougemont, A.; Jones, D.L. Seasonal and Spatial Dynamics of Enteric Viruses in Wastewater and in Riverine and Estuarine Receiving Waters. Sci. Total. Environ. 2018, 634, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Schijven, J.; Teunis, P.; Suylen, T.; Ketelaars, H.; Hornstra, L.; Rutjes, S. QMRA of Adenovirus in Drinking Water at a Drinking Water Treatment Plant Using UV and Chlorine Dioxide Disinfection. Water Res. 2019, 158, 34–45. [Google Scholar] [CrossRef]
- Li, J.; Lu, X.; Sun, Y.; Lin, C.; Li, F.; Yang, Y.; Liang, Z.; Jia, L.; Chen, L.; Jiang, B. A Swimming Pool-Associated Outbreak of Pharyngoconjunctival Fever Caused by Human Adenovirus Type 4 in Beijing, China. Int. J. Infect. Dis. 2018, 75, 89–91. [Google Scholar] [CrossRef]
- Pang, X.; Qiu, Y.; Gao, T.; Zurawell, R.; Neumann, N.F.; Craik, S.; Lee, B.E. Prevalence, Levels and Seasonal Variations of Human Enteric Viruses in Six Major Rivers in Alberta, Canada. Water Res. 2019, 153, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Sedji, M.I.; Varbanov, M.; Meo, M.; Colin, M.; Mathieu, L.; Bertrand, I. Quantification of Human Adenovirus and Norovirus in River Water in the North-East of France. Environ. Sci. Pollut. Res. 2018, 25, 30497–30507. [Google Scholar] [CrossRef]
- Wang, H.; Sikora, P.; Rutgersson, C.; Lindh, M.; Brodin, T.; Björlenius, B.; Larsson, D.J.; Norder, H. Differential Removal of Human Pathogenic Viruses from Sewage by Conventional and Ozone Treatments. Int. J. Hyg. Environ. Health 2018, 221, 479–488. [Google Scholar] [CrossRef]
- Hellmér, M.; Paxéus, N.; Magnius, L.; Enache, L.; Arnholm, B.; Johansson, A.; Bergström, T.; Norder, H. Detection of Pathogenic Viruses in Sewage Provided Early Warnings of Hepatitis A Virus and Norovirus Outbreaks. Appl. Environ. Microbiol. 2014, 80, 6771–6781. [Google Scholar] [CrossRef]
- McDonald, S.M.; Patton, J.T. Assortment and Packaging of the Segmented Rotavirus Genome. Trends Microbiol. 2011, 19, 136–144. [Google Scholar] [CrossRef]
- Lee, R.M.; Lessler, J.; Lee, R.A.; Rudolph, K.E.; Reich, N.G.; Perl, T.M.; Cummings, D.A. Incubation Periods of Viral Gastroenteritis: A Systematic Review. BMC Infect. Dis. 2013, 13, 1–11. [Google Scholar] [CrossRef]
- Kirkwood, C.D. Genetic and Antigenic Diversity of Human Rotaviruses: Potential Impact on Vaccination Programs. J. Infect. Dis. 2010, 202, S43–S48. [Google Scholar] [CrossRef]
- Giri, S.; Nair, N.P.; Mathew, A.; Manohar, B.; Simon, A.; Singh, T.; Kumar, S.S.; Mathew, M.A.; Babji, S.; Arora, R. Rotavirus Gastroenteritis in Indian Children <5 Years Hospitalized for Diarrhoea, 2012 to 2016. BMC Public Health 2019, 19, 1–10. [Google Scholar]
- Phan, T.; Ide, T.; Komoto, S.; Khamrin, P.; Pham, N.T.K.; Okitsu, S.; Taniguchi, K.; Nishimura, S.; Maneekarn, N.; Hayakawa, S. Genomic Analysis of Group A Rotavirus G12P [8] Including a New Japanese Strain Revealed Evidence for Intergenotypic Recombination in VP7 and VP4 Genes. Infect. Genet. Evol. 2021, 87, 104656. [Google Scholar] [CrossRef] [PubMed]
- Sai, L.; Sun, J.; Shao, L.; Chen, S.; Liu, H.; Ma, L. Epidemiology and Clinical Features of Rotavirus and Norovirus Infection among Children in Ji’nan, China. Virol. J. 2013, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bresee, J.S.; Glass, R.I.; Ivanoff, B.; Gentsch, J.R. Current Status and Future Priorities for Rotavirus Vaccine Development, Evaluation and Implementation in Developing Countries. Vaccine 1999, 17, 2207–2222. [Google Scholar] [CrossRef]
- Alidjinou, E.K.; Sane, F.; Firquet, S.; Lobert, P.-E.; Hober, D. Resistance of Enteric Viruses on Fomites. Intervirology 2018, 61, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Desselberger, U. Rotaviruses. Virus research 2014, 190, 75–96. [Google Scholar] [CrossRef]
- Estes, M.K.; Graham, D.Y.; Smith, E.M.; Gerba, C.P. Rotavirus Stability and Inactivation. J. Gen. Virol. 1979, 43, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Pinon, A.; Vialette, M. Survival of Viruses in Water. Intervirology 2018, 61, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.D.; Birch, C.; Heath, R.; Gust, I. Physicochemical Stability and Inactivation of Human and Simian Rotaviruses. Appl. Environ. Microbiol. 1987, 53, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Bortagaray, V.; Girardi, V.; Pou, S.; Lizasoain, A.; Tort, L.F.L.; Spilki, F.R.; Colina, R.; Victoria, M. Detection, Quantification, and Microbial Risk Assessment of Group A Rotavirus in Rivers from Uruguay. Food Environ. Virol. 2020, 12, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Jain, A. Waterborne viral gastroenteritis: An introduction to common agents. In Water and Health; Springer: Berlin, Germany, 2014; pp. 53–74. [Google Scholar]
- Morozova, O.V.; Sashina, T.A.; Epifanova, N.V.; Kashnikov, A.Y.; Novikova, N.A. Increasing Detection of Rotavirus G2P [4] Strains in Nizhny Novgorod, Russia, between 2016 and 2019. Arch. Virol. 2021, 166, 115–124. [Google Scholar] [CrossRef]
- Bányai, K.; László, B.; Duque, J.; Steele, A.D.; Nelson, E.A.S.; Gentsch, J.R.; Parashar, U.D. Systematic Review of Regional and Temporal Trends in Global Rotavirus Strain Diversity in the Pre Rotavirus Vaccine Era: Insights for Understanding the Impact of Rotavirus Vaccination Programs. Vaccine 2012, 30, A122–A130. [Google Scholar] [CrossRef]
- Rivera, R.; Forney, K.; Castro, M.R.; Rebolledo, P.A.; Mamani, N.; Patzi, M.; Halkyer, P.; Leon, J.S.; Iñiguez, V. Rotavirus Genotype Distribution during the Pre-Vaccine Period in Bolivia: 2007–2008. Int. J. Infect. Dis. 2013, 17, e762–e767. [Google Scholar] [CrossRef]
- Almalki, S.S.R. Molecular Detection of Hepatitis A Virus and Rotavirus in Water Samples Collected from Albaha, Saudi Arabia. Egypt. Acad. J. Biol. Sciences. C Physiol. Mol. Biol. 2018, 10, 59–68. [Google Scholar] [CrossRef]
- Miura, T.; Gima, A.; Akiba, M. Detection of Norovirus and Rotavirus Present in Suspended and Dissolved Forms in Drinking Water Sources. Food Environ. Virol. 2019, 11, 9–19. [Google Scholar] [CrossRef]
- Neveen Magdy, R.; Allayeh, A.K. Genotyping of Rotaviruses in River Nile in Giza, Egypt. Iran. J. Public Health 2020, 49, 173. [Google Scholar]
- Osuolale, O.; Okoh, A. Human Enteric Bacteria and Viruses in Five Wastewater Treatment Plants in the Eastern Cape, South Africa. J. Infect. Public Health 2017, 10, 541–547. [Google Scholar] [CrossRef]
- Yousuf, F.A.; Siddiqui, R.; Khan, N.A. Presence of Rotavirus and Free-Living Amoebae in the Water Supplies of Karachi, Pakistan. Rev. Inst. Med. Trop. São Paulo 2017, 59. [Google Scholar] [CrossRef]
- Naqvi, S.S.; Javed, S.; Naseem, S.; Sadiq, A.; Khan, N.; Sattar, S.; Shah, N.A.; Bostan, N. G3 and G9 Rotavirus Genotypes in Waste Water Circulation from Two Major Metropolitan Cities of Pakistan. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Kitajima, M.; Iker, B.C.; Pepper, I.L.; Gerba, C.P. Relative Abundance and Treatment Reduction of Viruses during Wastewater Treatment Processes—Identification of Potential Viral Indicators. Sci. Total. Environ. 2014, 488, 290–296. [Google Scholar] [CrossRef]
- Fumian, T.M.; Leite, J.P.G.; Rose, T.L.; Prado, T.; Miagostovich, M.P. One Year Environmental Surveillance of Rotavirus Specie A (RVA) Genotypes in Circulation after the Introduction of the Rotarix® Vaccine in Rio de Janeiro, Brazil. Water Res. 2011, 45, 5755–5763. [Google Scholar] [CrossRef]
- Ruggeri, F.M.; Bonomo, P.; Ianiro, G.; Battistone, A.; Delogu, R.; Germinario, C.; Chironna, M.; Triassi, M.; Campagnuolo, R.; Cicala, A. Rotavirus Genotypes in Sewage Treatment Plants and in Children Hospitalized with Acute Diarrhea in Italy in 2010 and 2011. Appl. Environ. Microbiol. 2015, 81, 241–249. [Google Scholar] [CrossRef]
- Lodder, W.J.; Van Den Berg, H.; Rutjes, S.A.; de Roda Husman, A.M. Presence of Enteric Viruses in Source Waters for Drinking Water Production in The Netherlands. Appl. Environ. Microbiol. 2010, 76, 5965–5971. [Google Scholar] [CrossRef]
- Shi, D.; Ma, H.; Miao, J.; Liu, W.; Yang, D.; Qiu, Z.; Shen, Z.; Yin, J.; Yang, Z.; Wang, H. Levels of Human Rotaviruses and Noroviruses GII in Urban Rivers Running through the City Mirror Their Infection Prevalence in Populations. Sci. Total. Environ. 2021, 754, 142203. [Google Scholar] [CrossRef]
- Fonager, J.; Stegger, M.; Rasmussen, L.D.; Poulsen, M.W.; Rønn, J.; Andersen, P.S.; Fischer, T.K. A Universal Primer-Independent next-Generation Sequencing Approach for Investigations of Norovirus Outbreaks and Novel Variants. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Vinjé, J. Advances in Laboratory Methods for Detection and Typing of Norovirus. J. Clin. Microbiol. 2015, 53, 373. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Hall, A.J.; Robinson, A.E.; Verhoef, L.; Premkumar, P.; Parashar, U.D.; Koopmans, M.; Lopman, B.A. Global Prevalence of Norovirus in Cases of Gastroenteritis: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2014, 14, 725–730. [Google Scholar] [CrossRef]
- Lartey, B.L.; Quaye, O.; Damanka, S.A.; Agbemabiese, C.A.; Armachie, J.; Dennis, F.E.; Enweronu-Laryea, C.; Armah, G.E. Understanding Pediatric Norovirus Epidemiology: A Decade of Study among Ghanaian Children. Viruses 2020, 12, 1321. [Google Scholar] [CrossRef]
- Center for Disease Control and Prevention (CDC). Norovirus Worldwide; CDC: Atlanta, GA, USA, 2018. [Google Scholar]
- Mans, J. Norovirus Infections and Disease in Lower-Middle-and Low-Income Countries, 1997–2018. Viruses 2019, 11, 341. [Google Scholar] [CrossRef]
- Mathew, S.; Alansari, K.; Smatti, M.K.; Zaraket, H.; Al Thani, A.A.; Yassine, H.M. Epidemiological, Molecular, and Clinical Features of Norovirus Infections among Pediatric Patients in Qatar. Viruses 2019, 11, 400. [Google Scholar] [CrossRef]
- KY, G.; Knipe DM, H.P.; Cohen, J.L.; Griffin, D.E.; Lamb, R.A.; Martin, M.A.; Racaniello, V.R. Caliciviridae: The Noroviruses. In Fields Virology, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; pp. 582–608. [Google Scholar]
- Chhabra, P.; de Graaf, M.; Parra, G.I.; Chan, M.C.-W.; Green, K.; Martella, V.; Wang, Q.; White, P.A.; Katayama, K.; Vennema, H. Updated Classification of Norovirus Genogroups and Genotypes. J. Gen. Virol. 2019, 100, 1393. [Google Scholar] [CrossRef]
- Parra, G.I.; Squires, R.B.; Karangwa, C.K.; Johnson, J.A.; Lepore, C.; Sosnovtsev, S.V.; Green, K.Y. Static and Evolving Norovirus Genotypes: Implications for Epidemiology and Immunity. PLoS Pathog. 2017, 13, e1006136. [Google Scholar] [CrossRef]
- Caddy, S.L.; De Rougemont, A.; Emmott, E.; El-Attar, L.; Mitchell, J.A.; Hollinshead, M.; Belliot, G.; Brownlie, J.; Le Pendu, J.; Goodfellow, I. Evidence for Human Norovirus Infection of Dogs in the United Kingdom. J. Clin. Microbiol. 2015, 53, 1873. [Google Scholar] [CrossRef]
- Caddy, S.; Breiman, A.; le Pendu, J.; Goodfellow, I. Genogroup IV and VI Canine Noroviruses Interact with Histo-Blood Group Antigens. J. Virol. 2014, 88, 10377. [Google Scholar] [CrossRef]
- Summa, M.; von Bonsdorff, C.-H.; Maunula, L. Evaluation of Four Virus Recovery Methods for Detecting Noroviruses on Fresh Lettuce, Sliced Ham, and Frozen Raspberries. J. Virol. Methods 2012, 183, 154–160. [Google Scholar] [CrossRef]
- Farkas, T. Natural Norovirus Infections in Rhesus Macaques. Emerg. Infect. Dis. 2016, 22, 1272. [Google Scholar] [CrossRef]
- Motomura, K.; Yokoyama, M.; Ode, H.; Nakamura, H.; Mori, H.; Kanda, T.; Oka, T.; Katayama, K.; Noda, M.; Tanaka, T. Divergent Evolution of Norovirus GII/4 by Genome Recombination from May 2006 to February 2009 in Japan. J. Virol. 2010, 84, 8085. [Google Scholar] [CrossRef]
- Mattison, K.; Shukla, A.; Cook, A.; Pollari, F.; Friendship, R.; Kelton, D.; Bidawid, S.; Farber, J.M. Human Noroviruses in Swine and Cattle. Emerg. Infect. Dis. 2007, 13, 1184. [Google Scholar] [CrossRef]
- van Der Poel, W.H.; Vinjé, J.; Van der Heide, R.; Herrera, M.-I.; Vivo, A.; Koopmans, M.P. Norwalk-like Calicivirus Genes in Farm Animals. Emerg. Infect. Dis. 2000, 6, 36. [Google Scholar] [CrossRef]
- Jung, K.; Wang, Q.; Kim, Y.; Scheuer, K.; Zhang, Z.; Shen, Q.; Chang, K.-O.; Saif, L.J. The Effects of Simvastatin or Interferon-α on Infectivity of Human Norovirus Using a Gnotobiotic Pig Model for the Study of Antivirals. PLoS ONE 2012, 7, e41619. [Google Scholar] [CrossRef]
- Bok, K.; Parra, G.I.; Mitra, T.; Abente, E.; Shaver, C.K.; Boon, D.; Engle, R.; Yu, C.; Kapikian, A.Z.; Sosnovtsev, S.V. Chimpanzees as an Animal Model for Human Norovirus Infection and Vaccine Development. Proc. Natl. Acad. Sci. USA 2011, 108, 325–330. [Google Scholar] [CrossRef]
- Souza, M.; Azevedo, M.S.P.; Jung, K.; Cheetham, S.; Saif, L.J. Pathogenesis and Immune Responses in Gnotobiotic Calves after Infection with the Genogroup II. 4-HS66 Strain of Human Norovirus. J. Virol. 2008, 82, 1777. [Google Scholar] [CrossRef]
- Wolf, S.; Williamson, W.; Hewitt, J.; Lin, S.; Rivera-Aban, M.; Ball, A.; Scholes, P.; Savill, M.; Greening, G.E. Molecular Detection of Norovirus in Sheep and Pigs in New Zealand Farms. Vet. Microbiol. 2009, 133, 184–189. [Google Scholar] [CrossRef]
- Oliver, S.L.; Brown, D.W.G.; Green, J.; Bridger, J.C. A Chimeric Bovine Enteric Calicivirus: Evidence for Genomic Recombination in Genogroup III of the Norovirus Genus of the Caliciviridae. Virology 2004, 326, 231–239. [Google Scholar] [CrossRef][Green Version]
- Smith, D.B.; McFadden, N.; Blundell, R.J.; Meredith, A.; Simmonds, P. Diversity of Murine Norovirus in Wild-Rodent Populations: Species-Specific Associations Suggest an Ancient Divergence. J. Gen. Virol. 2012, 93, 259–266. [Google Scholar] [CrossRef]
- Karst, S.M.; Wobus, C.E.; Lay, M.; Davidson, J.; Virgin, H.W. STAT1-Dependent Innate Immunity to a Norwalk-like Virus. Science 2003, 299, 1575–1578. [Google Scholar] [CrossRef]
- Ford-Siltz, L.A.; Mullis, L.; Sanad, Y.M.; Tohma, K.; Lepore, C.J.; Azevedo, M.; Parra, G.I. Genomics Analyses of GIV and GVI Noroviruses Reveal the Distinct Clustering of Human and Animal Viruses. Viruses 2019, 11, 204. [Google Scholar] [CrossRef]
- Di Martino, B.; Di Profio, F.; Melegari, I.; Sarchese, V.; Cafiero, M.A.; Robetto, S.; Aste, G.; Lanave, G.; Marsilio, F.; Martella, V. A Novel Feline Norovirus in Diarrheic Cats. Infect. Genet. Evol. 2016, 38, 132–137. [Google Scholar] [CrossRef]
- Martella, V.; Lorusso, E.; Decaro, N.; Elia, G.; Radogna, A.; D’Abramo, M.; Desario, C.; Cavalli, A.; Corrente, M.; Camero, M.; et al. Detection and Molecular Characterization of a Canine Norovirus. Emerg. Infect. Dis. 2008, 14, 1306–1308. [Google Scholar] [CrossRef]
- Wang, Q.-H.; Han, M.G.; Cheetham, S.; Souza, M.; Funk, J.A.; Saif, L.J. Porcine Noroviruses Related to Human Noroviruses. Emerg. Infect. Dis. 2005, 11, 1874. [Google Scholar] [CrossRef]
- Parra, G.I. Emergence of Norovirus Strains: A Tale of Two Genes. Virus Evol. 2019, 5, vez048. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, M.; Wang, H.; Wang, D.; Chen, C.; Jing, Q.; Geng, J.; Li, T.; Zhang, Z.; Yang, Z. An Outbreak of Norovirus-Related Acute Gastroenteritis Associated with Delivery Food in Guangzhou, Southern China. BMC Public Health 2020, 20, 1–7. [Google Scholar] [CrossRef]
- Xue, L.; Cai, W.; Gao, J.; Zhang, L.; Dong, R.; Li, Y.; Wu, H.; Chen, M.; Zhang, J.; Wang, J. The Resurgence of the Norovirus GII. 4 Variant Associated with Sporadic Gastroenteritis in the Post-GII. 17 Period in South China, 2015 to 2017. BMC Infect. Dis. 2019, 19, 1–8. [Google Scholar] [CrossRef]
- Ge, L.; Chen, X.; Liu, J.; Zheng, L.; Chen, C.; Luo, S.; Guo, P.; Kong, J.; Song, Y.; Huo, Y. Genomic and Biological Characterization of a Pandemic Norovirus Variant GII. 4 Sydney 2012. Virus Genes 2020, 1–8. [Google Scholar] [CrossRef]
- Tohma, K.; Lepore, C.J.; Gao, Y.; Ford-Siltz, L.A.; Parra, G.I. Population Genomics of GII. 4 Noroviruses Reveal Complex Diversification and New Antigenic Sites Involved in the Emergence of Pandemic Strains. MBio 2019, 10. [Google Scholar] [CrossRef]
- Robilotti, E.; Deresinski, S.; Pinsky, B.A. Norovirus. Clin. Microbiol. Rev. 2015, 28, 134. [Google Scholar] [CrossRef]
- Bull, R.A.; Eden, J.-S.; Rawlinson, W.D.; White, P.A. Rapid Evolution of Pandemic Noroviruses of the GII. 4 Lineage. PLoS Pathog. 2010, 6, e1000831. [Google Scholar] [CrossRef]
- Bitler, E.J.; Matthews, J.E.; Dickey, B.W.; Eisenberg, J.N.S.; Leon, J.S. Norovirus Outbreaks: A Systematic Review of Commonly Implicated Transmission Routes and Vehicles. Epidemiol. Infect. 2013, 141, 1563–1571. [Google Scholar] [CrossRef]
- Lei, H.; Li, Y.; Xiao, S.; Lin, C.-H.; Norris, S.L.; Wei, D.; Hu, Z.; Ji, S. Routes of Transmission of Influenza A H1N1, SARS CoV, and Norovirus in Air Cabin: Comparative Analyses. Indoor Air 2018, 28, 394–403. [Google Scholar] [CrossRef]
- Xiao, S.; Tang, J.W.; Li, Y. Airborne or Fomite Transmission for Norovirus? A Case Study Revisited. Int. J. Environ. Res. Public Health 2017, 14, 1571. [Google Scholar] [CrossRef]
- de Graaf, M.; van Beek, J.; Koopmans, M.P. Human Norovirus Transmission and Evolution in a Changing World. Nat. Rev. Microbiol. 2016, 14, 421–433. [Google Scholar] [CrossRef]
- Bernstein, D.I.; Atmar, R.L.; Lyon, G.M.; Treanor, J.J.; Chen, W.H.; Jiang, X.; Vinjé, J.; Gregoricus, N.; Frenck, R.W., Jr.; Moe, C.L. Norovirus Vaccine against Experimental Human GII. 4 Virus Illness: A Challenge Study in Healthy Adults. J. Infect. Dis. 2015, 211, 870–878. [Google Scholar] [CrossRef]
- Teunis, P.F.; Moe, C.L.; Liu, P.; Miller, S.E.; Lindesmith, L.; Baric, R.S.; Le Pendu, J.; Calderon, R.L. Norwalk Virus: How Infectious Is It? J. Med Virol. 2008, 80, 1468–1476. [Google Scholar] [CrossRef]
- Dolin, R.; Blacklow, N.R.; DuPont, H.; Formal, S.; Buscho, R.F.; Kasel, J.A.; Chames, R.P.; Hornick, R.; Chanock, R.M. Transmission of Acute Infectious Nonbacterial Gastroenteritis to Volunteers by Oral Administration of Stool Filtrates. J. Infect. Dis. 1971, 123, 307–312. [Google Scholar] [CrossRef]
- Stegmaier, T.; Oellingrath, E.; Himmel, M.; Fraas, S. Differences in Epidemic Spread Patterns of Norovirus and Influenza Seasons of Germany: An Application of Optical Flow Analysis in Epidemiology. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Richards, G.P.; Watson, M.A.; Meade, G.K.; Hovan, G.L.; Kingsley, D.H. Resilience of Norovirus GII. 4 to Freezing and Thawing: Implications for Virus Infectivity. Food Environ. Virol. 2012, 4, 192–197. [Google Scholar] [CrossRef]
- Kauppinen, A.; Miettinen, I.T. Persistence of Norovirus GII Genome in Drinking Water and Wastewater at Different Temperatures. Pathogens 2017, 6, 48. [Google Scholar] [CrossRef]
- Bozkurt, H.; D’Souza, D.H.; Davidson, P.M. Thermal Inactivation Kinetics of Human Norovirus Surrogates and Hepatitis A Virus in Turkey Deli Meat. Appl. Environ. Microbiol. 2015, 81, 4850. [Google Scholar] [CrossRef]
- Cromeans, T.; Park, G.W.; Costantini, V.; Lee, D.; Wang, Q.; Farkas, T.; Lee, A.; Vinjé, J. Comprehensive Comparison of Cultivable Norovirus Surrogates in Response to Different Inactivation and Disinfection Treatments. Appl. Environ. Microbiol. 2014, 80, 5743. [Google Scholar] [CrossRef]
- Robin, M.; Chassaing, M.; Loutreul, J.; de Rougemont, A.; Belliot, G.; Majou, D.; Gantzer, C.; Boudaud, N. Effect of Natural Ageing and Heat Treatments on GII. 4 Norovirus Binding to Histo-Blood Group Antigens. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Wu, H.M.; Fornek, M.; Schwab, K.J.; Chapin, A.R.; Gibson, K.; Schwab, E.; Spencer, C.; Henning, K. A Norovirus Outbreak at a Long-Term-Care Facility: The Role of Environmental Surface Contamination. Infect. Control. Hosp. Epidemiol. 2005, 26, 802–810. [Google Scholar] [CrossRef]
- Redwan, N.A.; Bagatadah, R.A. Health Risk Assessment Associated with Norovirus Incidence in Raw Wastewater in Jeddah, Saudi Arabia. Austr. J. Basic Appl. Sci. 2012, 6, 43–48. [Google Scholar]
- Shaheen, M.N.; Elmahdy, E.M. Environmental Monitoring of Astrovirus and Norovirus in the Rosetta Branch of the River Nile and the El-Rahawy Drain, Egypt. Water Supply 2019, 19, 1381–1387. [Google Scholar] [CrossRef]
- Shaheen, M.N.F.; Abd El-Daim, S.E.; Ahmed, N.I.; Elmahdy, E.M. Molecular Detection of Three Gastroenteritis Viruses in an Urban Sewage Treatment Plant and River Water in Egypt. Egypt. J. Aquat. Biol. Fish. 2018, 22, 615–627. [Google Scholar] [CrossRef][Green Version]
- Nakamura, K.; Iwai, M.; Zhang, J.; Obara, M.; Horimoto, E.; Hasegawa, S.; Kurata, T.; Takizawa, T. Detection of a Novel Recombinant Norovirus from Sewage Water in Toyama Prefecture, Japan. Jpn. J. Infect. Dis. 2009, 62, 394–398. [Google Scholar]
- Kitajima, M.; Haramoto, E.; Phanuwan, C.; Katayama, H.; Ohgaki, S. Detection of Genogroup IV Norovirus in Wastewater and River Water in Japan. Lett. Appl. Microbiol. 2009, 49, 655–658. [Google Scholar] [CrossRef]
- Mabasa, V.V.; Meno, K.D.; Taylor, M.B.; Mans, J. Environmental Surveillance for Noroviruses in Selected South African Wastewaters 2015–2016: Emergence of the Novel GII. 17. Food Environ. Virol. 2018, 10, 16–28. [Google Scholar] [CrossRef]
- Mans, J.; Netshikweta, R.; Magwalivha, M.; Van Zyl, W.B.; Taylor, M.B. Diverse Norovirus Genotypes Identified in Sewage-Polluted River Water in South Africa. Epidemiol. Infect. 2013, 141, 303–313. [Google Scholar] [CrossRef]
- Bivins, A.; Lowry, S.; Wankhede, S.; Hajare, R.; Murphy, H.M.; Borchardt, M.; Labhasetwar, P.; Brown, J. Microbial Water Quality Improvement Associated with Transitioning from Intermittent to Continuous Water Supply in Nagpur, India. Water Res. 2021, 117301. [Google Scholar] [CrossRef]
- Jahne, M.A.; Brinkman, N.E.; Keely, S.P.; Zimmerman, B.D.; Wheaton, E.A.; Garland, J.L. Droplet Digital PCR Quantification of Norovirus and Adenovirus in Decentralized Wastewater and Graywater Collections: Implications for Onsite Reuse. Water Res. 2020, 169, 115213. [Google Scholar] [CrossRef]
- Rosiles-González, G.; Ávila-Torres, G.; Moreno-Valenzuela, O.A.; Cháidez-Quiroz, C.; Hernández-Flores, C.I.; AcostA–González, G.; Brown, J.K.; Betancourt, W.Q.; Gerba, C.P.; Hernández-Zepeda, C. Norovirus and Human Adenovirus Occurrence and Diversity in Recreational Water in a Karst Aquifer in the Yucatan Peninsula, Mexico. J. Appl. Microbiol. 2019, 127, 1255–1269. [Google Scholar] [CrossRef]
- Myrmel, M.; Lange, H.; Rimstad, E. A 1-Year Quantitative Survey of Noro-, Adeno-, Human Boca-, and Hepatitis E Viruses in Raw and Secondarily Treated Sewage from Two Plants in Norway. Food Environ. Virol. 2015, 7, 213–223. [Google Scholar] [CrossRef]
- Fumian, T.M.; Fioretti, J.M.; Lun, J.H.; Dos Santos, I.A.; White, P.A.; Miagostovich, M.P. Detection of Norovirus Epidemic Genotypes in Raw Sewage Using next Generation Sequencing. Environ. Int. 2019, 123, 282–291. [Google Scholar] [CrossRef]
- Bonadonna, L.; Briancesco, R.; Suffredini, E.; Coccia, A.; Della Libera, S.; Carducci, A.; Verani, M.; Federigi, I.; Iaconelli, M.; Ferraro, G.B. Enteric Viruses, Somatic Coliphages and Vibrio Species in Marine Bathing and Non-Bathing Waters in Italy. Mar. Pollut. Bull. 2019, 149, 110570. [Google Scholar] [CrossRef]
- Lodder, W.J.; de Roda Husman, A.M. Presence of Noroviruses and Other Enteric Viruses in Sewage and Surface Waters in The Netherlands. Appl. Environ. Microbiol. 2005, 71, 1453–1461. [Google Scholar] [CrossRef]
- Shang, X.; Fu, X.; Zhang, P.; Sheng, M.; Song, J.; He, F.; Qiu, Y.; Wu, H.; Lu, Q.; Feng, Y. An Outbreak of Norovirus-Associated Acute Gastroenteritis Associated with Contaminated Barrelled Water in Many Schools in Zhejiang, China. PLoS ONE 2017, 12, e0171307. [Google Scholar] [CrossRef]
- Zhou, X.; Kong, D.-G.; Li, J.; Pang, B.-B.; Zhao, Y.; Zhou, J.-B.; Zhang, T.; Xu, J.-Q.; Kobayashi, N.; Wang, Y.-H. An Outbreak of Gastroenteritis Associated with GII. 17 Norovirus-Contaminated Secondary Water Supply System in Wuhan, China, 2017. Food Environ. Virol. 2019, 11, 126–137. [Google Scholar] [CrossRef]
- McKnight, K.L.; Lemon, S.M. Hepatitis A Virus Genome Organization and Replication Strategy. Cold Spring Harb. Perspect. Med. 2018, 8, a033480. [Google Scholar] [CrossRef]
- Blight, K.J.; Grakoui, A.; Hanson, H.L.; Rice, C.M. The molecular biology of hepatitis C virus. In Hepatitis Viruses; Springer: Berlin, Germany, 2002; pp. 81–108. [Google Scholar]
- Gosert, R.; Cassinotti, P.; Siegl, G.; Weitz, M. Identification of Hepatitis A Virus Non-Structural Protein 2B and Its Release by the Major Virus Protease 3C. J. Gen. Virol. 1996, 77, 247–255. [Google Scholar] [CrossRef]
- Vaughan, G.; Xia, G.; Forbi, J.C.; Purdy, M.A.; Rossi, L.M.G.; Spradling, P.R.; Khudyakov, Y.E. Genetic Relatedness among Hepatitis A Virus Strains Associated with Food-Borne Outbreaks. PLoS ONE 2013, 8, e74546. [Google Scholar] [CrossRef]
- Lemon, S.M.; Ott, J.J.; Van Damme, P.; Shouval, D. Type A Viral Hepatitis: A Summary and Update on the Molecular Virology, Epidemiology, Pathogenesis and Prevention. J. Hepatol. 2018, 68, 167–184. [Google Scholar] [CrossRef]
- Drexler, J.F.; Corman, V.M.; Lukashev, A.N.; van den Brand, J.M.; Gmyl, A.P.; Bruenink, S.; Rasche, A.; Seggewiβ, N.; Feng, H.; Leijten, L.M. Evolutionary Origins of Hepatitis A Virus in Small Mammals. Proc. Natl. Acad. Sci. USA 2015, 112, 15190–15195. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, H.; Cao, J.; Zhou, W.; Yi, Y.; Jia, Z.; Bi, S. Genetic Diversity of Hepatitis A Virus in China: VP3-VP1-2A Genes and Evidence of Quasispecies Distribution in the Isolates. PLoS ONE 2013, 8, e74752. [Google Scholar] [CrossRef]
- Bruni, R.; Taffon, S.; Equestre, M.; Cella, E.; Presti, A.L.; Costantino, A.; Chionne, P.; Madonna, E.; Golkocheva-Markova, E.; Bankova, D. Hepatitis a Virus Genotypes and Strains from an Endemic Area of Europe, Bulgaria 2012–2014. BMC Infect. Dis. 2017, 17, 1–8. [Google Scholar] [CrossRef]
- Yilmaz, H.; Karakullukcu, A.; Turan, N.; Cizmecigil, U.Y.; Yilmaz, A.; Ozkul, A.A.; Aydin, O.; Gunduz, A.; Mete, M.; Zeyrek, F.Y. Genotypes of Hepatitis a Virus in Turkey: First Report and Clinical Profile of Children Infected with Sub-Genotypes IA and IIIA. BMC Infect. Dis. 2017, 17, 1–8. [Google Scholar] [CrossRef]
- D’Andrea, L.; Pérez-Rodríguez, F.J.; De Castellarnau, M.; Manzanares, S.; Lite, J.; Guix, S.; Bosch, A.; Pintó, R.M. Hepatitis A Virus Genotype Distribution during a Decade of Universal Vaccination of Preadolescents. Int. J. Mol. Sci. 2015, 16, 6842–6854. [Google Scholar] [CrossRef]
- Roque-Afonso, A.M.; Desbois, D.; Dussaix, E. Hepatitis A Virus: Serology and Molecular Diagnostics. Future Virol. 2010, 5, 233–242. [Google Scholar] [CrossRef]
- Robertson, B.H.; Jansen, R.W.; Khanna, B.; Totsuka, A.; Nainan, O.V.; Siegl, G.; Widell, A.; Margolis, H.S.; Isomura, S.; Ito, K. Genetic Relatedness of Hepatitis A Virus Strains Recovered from Different Geographical Regions. J. Gen. Virol. 1992, 73, 1365–1377. [Google Scholar] [CrossRef]
- de Oliveira Carneiro, I.; Sander, A.-L.; Silva, N.; Moreira-Soto, A.; Normann, A.; Flehmig, B.; Lukashev, A.N.; Dotzauer, A.; Wieseke, N.; Franke, C.R. A Novel Marsupial Hepatitis A Virus Corroborates Complex Evolutionary Patterns Shaping the Genus Hepatovirus. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- La Rosa, G.; Mancini, P.; Bonanno Ferraro, G.; Iaconelli, M.; Veneri, C.; Paradiso, R.; De Medici, D.; Vicenza, T.; Proroga, Y.T.R.; Di Maro, O. Hepatitis A Virus Strains Circulating in the Campania Region (2015–2018) Assessed through Bivalve Biomonitoring and Environmental Surveillance. Viruses 2021, 13, 16. [Google Scholar] [CrossRef]
- Walker, C.M. Adaptive Immune Responses in Hepatitis A Virus and Hepatitis E Virus Infections. Cold Spring Harb. Perspect. Med. 2019, 9, a033472. [Google Scholar] [CrossRef]
- World Health Organization. Hepatitis A; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Benjamin, M.; Agnihotry, S.; Srivastava, A.; Bolia, R.; Yachha, S.K.; Aggarwal, R. Relationship of Severity of Hepatitis a with Polymorphisms in Hepatitis a Virus Cellular Receptor 1 (HAVCR1) Gene. Ann. Hepatol. 2018, 17, 561–568. [Google Scholar] [CrossRef]
- Matheny, S.C.; Kingery, J.E. Hepatitis A. Am. Fam. Physician 2012, 86, 1027–1034. [Google Scholar]
- Jeong, S.-H.; Lee, H.-S. Hepatitis A: Clinical Manifestations and Management. Intervirology 2010, 53, 15–19. [Google Scholar] [CrossRef]
- Nelson, N.P.; Weng, M.K.; Hofmeister, M.G.; Moore, K.L.; Doshani, M.; Kamili, S.; Koneru, A.; Haber, P.; Hagan, L.; Romero, J.R. Prevention of Hepatitis A Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices; Center for Disease Control and Prevention: Atlanta, GA, USA, 2020. [Google Scholar]
- Augustine, S.A.J.; Eason, T.N.; Simmons, K.J.; Griffin, S.M.; Curioso, C.L.; Ramudit, M.K.D.; Sams, E.A.; Oshima, K.H.; Dufour, A.; Wade, T.J. Rapid Salivary IgG Antibody Screening for Hepatitis A. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef]
- Nainan, O.V.; Xia, G.; Vaughan, G.; Margolis, H.S. Diagnosis of Hepatitis A Virus Infection: A Molecular Approach. Clin. Microbiol. Rev. 2006, 19, 63. [Google Scholar] [CrossRef]
- Fujiwara, K.; Yokosuka, O.; Fukai, K.; Imazeki, F.; Saisho, H.; Omata, M. Analysis of Full-Length Hepatitis A Virus Genome in Sera from Patients with Fulminant and Self-Limited Acute Type A Hepatitis. J. Hepatol. 2001, 35, 112–119. [Google Scholar] [CrossRef]
- Behzadi, M.A.; LeyvA–Grado, V.H.; Namayandeh, M.; Ziyaeyan, A.; Feyznezhad, R.; Dorzaban, H.; Jamalidoust, M.; Ziyaeyan, M. Seroprevalence of Viral Hepatitis A, B, C, D and E Viruses in the Hormozgan Province Southern Iran. BMC Infect. Dis. 2019, 19, 1–12. [Google Scholar] [CrossRef]
- Barrett, C.E. Impact of Public Health Interventions on Drinking Water–Associated Outbreaks of Hepatitis A—United States, 1971–2017. MMWR. Morb. Mortal. Wkly. Rep. 2019, 68. [Google Scholar] [CrossRef]
- Sánchez, G.; Bosch, A. Survival of Enteric Viruses in the Environment and Food. Viruses Foods 2016, 367–392. [Google Scholar]
- Biziagos, E.; Passagot, J.; Crance, J.-M.; Deloince, R. Long-Term Survival of Hepatitis A Virus and Poliovirus Type 1 in Mineral Water. Appl. Environ. Microbiol. 1988, 54, 2705. [Google Scholar] [CrossRef]
- Sewlikar, S.; D’Souza, D.H. Survival of Hepatitis A Virus and Aichi Virus in Cranberry-Based Juices at Refrigeration (4 °C). Food Microbiol. 2017, 62, 251–255. [Google Scholar] [CrossRef]
- Scholz, E.; Heinricy, U.; Flehmig, B. Acid Stability of Hepatitis A Virus. J. Gen. Virol. 1989, 70, 2481–2485. [Google Scholar] [CrossRef]
- Agrawal, A.; Singh, S.; Kolhapure, S.; Hoet, B.; Arankalle, V.; Mitra, M. Increasing Burden of Hepatitis A in Adolescents and Adults and the Need for Long-Term Protection: A Review from the Indian Subcontinent. Infect. Dis. Ther. 2019, 8, 483–497. [Google Scholar] [CrossRef]
- Sánchez, G.; Bosch, A.; Pintó, R.M. Genome Variability and Capsid Structural Constraints of Hepatitis A Virus. J. Virol. 2003, 77, 452. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, W.; Sánchez, G. Hepatitis A Infections from Food. J. Appl. Microbiol. 2020, 129, 1120–1132. [Google Scholar] [CrossRef]
- Scavia, G.; Alfonsi, V.; Taffon, S.; Escher, M.; Bruni, R.; De Medici, D.; Di Pasquale, S.; Guizzardi, S.; Cappelletti, B.; Iannazzo, S. A Large Prolonged Outbreak of Hepatitis A Associated with Consumption of Frozen Berries, Italy, 2013–2014. J. Med Microbiol. 2017, 66, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Severi, E.; Verhoef, L.; Thornton, L.; Guzman-Herrador, B.R.; Faber, M.; Sundqvist, L.; Rimhanen-Finne, R.; Roque-Afonso, A.M.; Ngui, S.L.; Allerberger, F. Large and Prolonged Food-Borne Multistate Hepatitis A Outbreak in Europe Associated with Consumption of Frozen Berries, 2013 to 2014. Eurosurveillance 2015, 20, 21192. [Google Scholar] [CrossRef] [PubMed]
- Romalde, J.L.; Rivadulla, E.; Varela, M.F.; Barja, J.L. An Overview of 20 Years of Studies on the Prevalence of Human Enteric Viruses in Shellfish from Galicia, Spain. J. Appl. Microbiol. 2018, 124, 943–957. [Google Scholar] [CrossRef]
- La Bella, G.; Martella, V.; Basanisi, M.G.; Nobili, G.; Terio, V.; La Salandra, G. Food-Borne Viruses in Shellfish: Investigation on Norovirus and HAV Presence in Apulia (SE Italy). Food Environ. Virol. 2017, 9, 179–186. [Google Scholar] [CrossRef]
- Polo, D.; Feal, X.; Romalde, J.L. Mathematical Model for Viral Depuration Kinetics in Shellfish: An Useful Tool to Estimate the Risk for the Consumers. Food Microbiol. 2015, 49, 220–225. [Google Scholar] [CrossRef]
- Amon, J.J.; Devasia, R.; Xia, G.; Nainan, O.V.; Hall, S.; Lawson, B.; Wolthuis, J.S.; MacDonald, P.D.; Shepard, C.W.; Williams, I.T. Molecular Epidemiology of Foodborne Hepatitis A Outbreaks in the United States, 2003. J. Infect. Dis. 2005, 192, 1323–1330. [Google Scholar] [CrossRef][Green Version]
- Janahi, E.M.; Mustafa, S.; Parkar, S.F.; Naser, H.A.; Eisa, Z.M. Detection of Enteric Viruses and Bacterial Indicators in a Sewage Treatment Center and Shallow Water Bay. Int. J. Environ. Res. Public Health 2020, 17, 6483. [Google Scholar] [CrossRef]
- Hamza, H.; Abd-Elshafy, D.N.; Fayed, S.A.; Bahgat, M.M.; El-Esnawy, N.A.; Abdel-Mobdy, E. Detection and Characterization of Hepatitis A Virus Circulating in Egypt. Arch. Virol. 2017, 162, 1921–1931. [Google Scholar] [CrossRef]
- Ouardani, I.; Turki, S.; Aouni, M.; Romalde, J.L. Detection and Molecular Characterization of Hepatitis A Virus from Tunisian Wastewater Treatment Plants with Different Secondary Treatments. Appl. Environ. Microbiol. 2016, 82, 3834–3845. [Google Scholar] [CrossRef]
- Beji-Hamza, A.; Khélifi-Gharbi, H.; Hassine-Zaafrane, M.; Della Libera, S.; Iaconelli, M.; Muscillo, M.; Petricca, S.; Ciccaglione, A.R.; Bruni, R.; Taffon, S. Qualitative and Quantitative Assessment of Hepatitis A Virus in Wastewaters in Tunisia. Food Environ. Virol. 2014, 6, 246–252. [Google Scholar] [CrossRef]
- Fumian, T.M.; Victoria, M.; Vieira, C.B.; Fioretti, J.M.; Rocha, M.S.; Prado, T.; Guimarães, F.R.; da Gama, N.P.; de Oliveira, J.M.; Mendes, A.C.O. Enteric Viruses’ Dissemination in a Private Reserve of Natural Heritage. Lett. Appl. Microbiol. 2018, 66, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Saïd, R.; Wolfaardt, M.; Taylor, M.B. Molecular Characterisation of Hepatitis A Virus Strains from Water Sources in South Africa. Water Sci. Technol. 2014, 69, 923–933. [Google Scholar] [CrossRef]
- Rachida, S.; Matsapola, P.N.; Wolfaardt, M.; Taylor, M.B. Genetic Characterization of a Novel Hepatitis A Virus Strain in Irrigation Water in South Africa. J. Med Virol. 2016, 88, 734–737. [Google Scholar] [CrossRef]
- Ahmad, T.; Arshad, N.; Adnan, F. Prevalence of Rotavirus, Adenovirus, Hepatitis A Virus and Enterovirus in Water Samples Collected from Different Region of Peshawar, Pakistan. Ann. Agric. Environ. Med. 2016, 23, 576–580. [Google Scholar] [CrossRef]
- Serres, G.D.; Cromeans, T.L.; Levesque, B.; Brassard, N.; Barthe, C.; Dionne, M.; Prud’homme, H.; Paradis, D.; Shapiro, C.N.; Nainan, O.V. Molecular Confirmation of Hepatitis A Virus from Well Water: Epidemiology and Public Health Implications. J. Infect. Dis. 1999, 179, 37–43. [Google Scholar] [CrossRef]
- de Souza, F.G.; da Silva, F.P.; Staggemeier, R.; Rigotto, C.; Spilki, F.R. Low Occurrence of Hepatitis A Virus in Water Samples from an Urban Area of Southern Brazil. Rev. Inst. Med. Trop. São Paulo 2018, 60, e69. [Google Scholar] [CrossRef]
- Iaconelli, M.; Purpari, G.; Della Libera, S.; Petricca, S.; Guercio, A.; Ciccaglione, A.R.; Bruni, R.; Taffon, S.; Equestre, M.; Fratini, M. Hepatitis A and E Viruses in Wastewaters, in River Waters, and in Bivalve Molluscs in Italy. Food Environ. Virol. 2015, 7, 316–324. [Google Scholar] [CrossRef]
- Truchado, P.; Garre, A.; Gil, M.I.; Simón-Andreu, P.J.; Sánchez, G.; Allende, A. Monitoring of Human Enteric Virus and Coliphages throughout Water Reuse System of Wastewater Treatment Plants to Irrigation Endpoint of Leafy Greens. Sci. Total. Environ. 2021, 782, 146837. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Chu, D.L.H.; Wong, M.M.L.; Qi, H.; Wu, R.S.S.; Kong, R.Y.C. Major Human Hepatitis A Virus Genotype in Hong Kong Marine Waters and Detection by Real-Time PCR. Mar. Pollut. Bull. 2011, 62, 2654–2658. [Google Scholar] [CrossRef] [PubMed]
- Schuster, C.J.; Aramini, J.J.; Ellis, A.G.; Marshall, B.J.; Robertson, W.J.; Medeiros, D.T.; Charron, D.F. Infectious Disease Outbreaks Related to Drinking Water in Canada, 1974–2001. Can. J. Public Health 2005, 96, 254–258. [Google Scholar] [CrossRef]
- Fernández-Correa, I.; Truchado, D.A.; Gomez-Lucia, E.; Doménech, A.; Pérez-Tris, J.; Schmidt-Chanasit, J.; Cadar, D.; Benítez, L. A Novel Group of Avian Astroviruses from Neotropical Passerine Birds Broaden the Diversity and Host Range of Astroviridae. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lulla, V.; Firth, A.E. A Hidden Gene in Astroviruses Encodes a Viroporin. Nat. Commun. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Toh, Y.; Harper, J.; Dryden, K.A.; Yeager, M.; Arias, C.F.; Méndez, E.; Tao, Y.J. Crystal Structure of the Human Astrovirus Capsid Protein. J. Virol. 2016, 90, 9008. [Google Scholar] [CrossRef]
- Fuentes, C.; Bosch, A.; Pintó, R.M.; Guix, S. Identification of Human Astrovirus Genome-Linked Protein (VPg) Essential for Virus Infectivity. J. Virol. 2012, 86, 10070. [Google Scholar] [CrossRef]
- Goodfellow, I. The Genome-Linked Protein VPg of Vertebrate Viruses—A Multifaceted Protein. Curr. Opin. Virol. 2011, 1, 355–362. [Google Scholar] [CrossRef]
- Vu, D.-L.; Sabrià, A.; Aregall, N.; Michl, K.; Rodriguez Garrido, V.; Goterris, L.; Bosch, A.; Pintó, R.M.; Guix, S. Novel Human Astroviruses: Prevalence and Association with Common Enteric Viruses in Undiagnosed Gastroenteritis Cases in Spain. Viruses 2019, 11, 585. [Google Scholar] [CrossRef]
- Hargest, V.; Davis, A.; Schultz-Cherry, S. Astroviridae. Elsevier 2019. [Google Scholar]
- Soares, C.C.; de Albuquerque, M.C.M.; Maranhão, A.G.; Rocha, L.N.; Ramírez, M.L.G.; Benati, F.J.; do Carmo Timenetsky, M.; Santos, N. Astrovirus Detection in Sporadic Cases of Diarrhea among Hospitalized and Non-Hospitalized Children in Rio De Janeiro, Brazil, from 1998 to 2004. J. Med Virol. 2008, 80, 113–117. [Google Scholar] [CrossRef]
- Espul, C.; Martínez, N.; Noel, J.S.; Cuello, H.; Abrile, C.; Grucci, S.; Glass, R.; Berke, T.; Matson, D.O. Prevalence and Characterization of Astroviruses in Argentinean Children with Acute Gastroenteritis. J. Med Virol. 2004, 72, 75–82. [Google Scholar] [CrossRef]
- De Benedictis, P.; Schultz-Cherry, S.; Burnham, A.; Cattoli, G. Astrovirus Infections in Humans and Animals–Molecular Biology, Genetic Diversity, and Interspecies Transmissions. Infect. Genet. Evol. 2011, 11, 1529–1544. [Google Scholar] [CrossRef]
- Bosch, A.; Pintó, R.M.; Guix, S. Human Astroviruses. Clin. Microbiol. Rev. 2014, 27, 1048. [Google Scholar] [CrossRef]
- Vu, D.-L.; Bosch, A.; Pintó, R.M.; Guix, S. Epidemiology of Classic and Novel Human Astrovirus: Gastroenteritis and Beyond. Viruses 2017, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodriguez, F.J.; Vieille, G.; Turin, L.; Yildiz, S.; Tapparel, C.; Kaiser, L. Fecal Components Modulate Human Astrovirus Infectivity in Cells and Reconstituted Intestinal Tissues. Msphere 2019, 4. [Google Scholar] [CrossRef]
- Prevost, B.; Lucas, F.S.; Ambert-Balay, K.; Pothier, P.; Moulin, L.; Wurtzer, S. Deciphering the Diversities of Astroviruses and Noroviruses in Wastewater Treatment Plant Effluents by a High-Throughput Sequencing Method. Appl. Environ. Microbiol. 2015, 81, 7215. [Google Scholar] [CrossRef] [PubMed]
- Thongprachum, A.; Fujimoto, T.; Takanashi, S.; Saito, H.; Okitsu, S.; Shimizu, H.; Khamrin, P.; Maneekarn, N.; Hayakawa, S.; Ushijima, H. Detection of Nineteen Enteric Viruses in Raw Sewage in Japan. Infect. Genet. Evol. 2018, 63, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Van Zyl, W.B.; Zhou, N.A.; Wolfaardt, M.; Matsapola, P.N.; Ngwana, F.B.; Symonds, E.M.; Fagnant-Sperati, C.S.; Shirai, J.H.; Kossik, A.L.; Beck, N.K. Detection of Potentially Pathogenic Enteric Viruses in Environmental Samples from Kenya Using the Bag-Mediated Filtration System. Water Supply 2019, 19, 1668–1676. [Google Scholar] [CrossRef]
- Kiulia, N.M.; Netshikweta, R.; Page, N.A.; Van Zyl, W.B.; Kiraithe, M.M.; Nyachieo, A.; Mwenda, J.M.; Taylor, M.B. The Detection of Enteric Viruses in Selected Urban and Rural River Water and Sewage in Kenya, with Special Reference to Rotaviruses. J. Appl. Microbiol. 2010, 109, 818–828. [Google Scholar] [CrossRef]
- Victoria, M.; Tort, L.L.; García, M.; Lizasoain, A.; Maya, L.; Leite, J.P.G.; Miagostovich, M.P.; Cristina, J.; Colina, R. Assessment of Gastroenteric Viruses from Wastewater Directly Discharged into Uruguay River, Uruguay. Food Environ. Virol. 2014, 6, 116–124. [Google Scholar] [CrossRef]
- Taylor, M.B.; Cox, N.; Vrey, M.A.; Grabow, W.O.K. The Occurrence of Hepatitis A and Astroviruses in Selected River and Dam Waters in South Africa. Water Res. 2001, 35, 2653–2660. [Google Scholar] [CrossRef]
- Hata, A.; Kitajima, M.; Haramoto, E.; Lee, S.; Ihara, M.; Gerba, C.P.; Tanaka, H. Next-Generation Amplicon Sequencing Identifies Genetically Diverse Human Astroviruses, Including Recombinant Strains, in Environmental Waters. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Miagostovich, M.P.; Ferreira, F.F.; Guimarães, F.R.; Fumian, T.M.; Diniz-Mendes, L.; Luz, S.L.B.; Silva, L.A.; Leite, J.P.G. Molecular Detection and Characterization of Gastroenteritis Viruses Occurring Naturally in the Stream Waters of Manaus, Central Amazonia, Brazil. Appl. Environ. Microbiol. 2008, 74, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, B.; Ianiro, G.; Iaccarino, D.; D’Apice, F.; Ferraro, A.; Race, M.; Spasiano, D.; Esposito, E.; Monini, M.; Serra, F. A Potential Risk Assessment Tool to Monitor Pathogens Circulation in Coastal Waters. Environ. Res. 2021, 200, 111748. [Google Scholar] [CrossRef]
- Smith, A.; Reacher, M.; Smerdon, W.; Adak, G.K.; Nichols, G.; Chalmers, R.M. Outbreaks of Waterborne Infectious Intestinal Disease in England and Wales, 1992–2003. Epidemiol. Infect. 2006, 134, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- He, X.Q.; Cheng, L.; Zhang, D.Y.; Xie, X.M.; Wang, D.H.; Wang, Z. One-Year Monthly Survey of Rotavirus, Astrovirus and Norovirus in Three Sewage Treatment Plants in Beijing, China and Associated Health Risk Assessment. Water Sci. Technol. 2011, 63, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Prevost, B.; Lucas, F.S.; Goncalves, A.; Richard, F.; Moulin, L.; Wurtzer, S. Large Scale Survey of Enteric Viruses in River and Waste Water Underlines the Health Status of the Local Population. Environ. Int. 2015, 79, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, A.C.; Mazari-Hiriart, M.; Espinosa, R.; Maruri-Avidal, L.; Méndez, E.; Arias, C.F. Infectivity and Genome Persistence of Rotavirus and Astrovirus in Groundwater and Surface Water. Water Res. 2008, 42, 2618–2628. [Google Scholar] [CrossRef] [PubMed]
- Zannella, C.; Mosca, F.; Mariani, F.; Franci, G.; Folliero, V.; Galdiero, M.; Tiscar, P.G.; Galdiero, M. Microbial Diseases of Bivalve Mollusks: Infections, Immunology and Antimicrobial Defense. Mar. Drugs 2017, 15, 182. [Google Scholar] [CrossRef]
- Burge, C.A.; Closek, C.J.; Friedman, C.S.; Groner, M.L.; Jenkins, C.M.; Shore-Maggio, A.; Welsh, J.E. The Use of Filter-Feeders to Manage Disease in a Changing World. Integr. Comp. Biol. 2016, 56, 573–587. [Google Scholar] [CrossRef]
- Hernroth, B.E.; Conden-Hansson, A.-C.; Rehnstam-Holm, A.-S.; Girones, R.; Allard, A.K. Environmental Factors Influencing Human Viral Pathogens and Their Potential Indicator Organisms in the Blue Mussel, Mytilus Edulis: The First Scandinavian Report. Appl. Environ. Microbiol. 2002, 68, 4523. [Google Scholar] [CrossRef]
- Elbashir, S.; Parveen, S.; Schwarz, J.; Rippen, T.; Jahncke, M.; DePaola, A. Seafood Pathogens and Information on Antimicrobial Resistance: A Review. Food Microbiol. 2018, 70, 85–93. [Google Scholar] [CrossRef]
- Vasickova, P.; Pavlik, I.; Verani, M.; Carducci, A. Issues Concerning Survival of Viruses on Surfaces. Food Environ. Virol. 2010, 2, 24–34. [Google Scholar] [CrossRef]
- Polo, D.; Varela, M.F.; Romalde, J.L. Detection and Quantification of Hepatitis A Virus and Norovirus in Spanish Authorized Shellfish Harvesting Areas. Int. J. Food Microbiol. 2015, 193, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Shuval, H. Estimating the Global Burden of Thalassogenic Diseases: Human Infectious Diseases Caused by Wastewater Pollution of the Marine Environment. J. Water Health 2003, 1, 53–64. [Google Scholar] [CrossRef]
- Adegoke, A.A.; Amoah, I.D.; Stenström, T.A.; Verbyla, M.E.; Mihelcic, J.R. Epidemiological Evidence and Health Risks Associated With Agricultural Reuse of Partially Treated and Untreated Wastewater: A Review. Front. Public Health 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Bogler, A.; Packman, A.; Furman, A.; Gross, A.; Kushmaro, A.; Ronen, A.; Dagot, C.; Hill, C.; Vaizel-Ohayon, D.; Morgenroth, E. Rethinking Wastewater Risks and Monitoring in Light of the COVID-19 Pandemic. Nat. Sustain. 2020, 1–10. [Google Scholar] [CrossRef]
- Wurtzer, S.; Marechal, V.; Mouchel, J.M.; Maday, Y.; Teyssou, R.; Richard, E.; Almayrac, J.L.; Moulin, L. Evaluation of Lockdown Effect on SARS-CoV-2 Dynamics through Viral Genome Quantification in Waste Water, Greater Paris, France, 5 March to 23 April 2020. Eurosurveillance 2020, 25, 2000776. [Google Scholar] [CrossRef]
- Sharif, S.; Ikram, A.; Khurshid, A.; Salman, M.; Mehmood, N.; Arshad, Y.; Ahmed, J.; Safdar, R.M.; Rehman, L.; Mujtaba, G.; et al. Detection of SARs-CoV-2 in Wastewater Using the Existing Environmental Surveillance Network: A Potential Supplementary System for Monitoring COVID-19 Transmission. PLoS ONE 2021, 16, e0249568. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, G.; Iaconelli, M.; Mancini, P.; Ferraro, G.B.; Veneri, C.; Bonadonna, L.; Lucentini, L.; Suffredini, E. First Detection of SARS-CoV-2 in Untreated Wastewaters in Italy. Sci. Total. Environ. 2020, 736, 139652. [Google Scholar] [CrossRef]
- Medema, G.; Heijnen, L.; Elsinga, G.; Italiaander, R.; Brouwer, A. Presence of SARS-Coronavirus-2 RNA in Sewage and Correlation with Reported COVID-19 Prevalence in the Early Stage of the Epidemic in the Netherlands. Environ. Sci. Technol. Lett. 2020, 7, 511–516. [Google Scholar] [CrossRef]
- Rimoldi, S.G.; Stefani, F.; Gigantiello, A.; Polesello, S.; Comandatore, F.; Mileto, D.; Maresca, M.; Longobardi, C.; Mancon, A.; Romeri, F. Presence and Infectivity of SARS-CoV-2 Virus in Wastewaters and Rivers. Sci. Total. Environ. 2020, 744, 140911. [Google Scholar] [CrossRef]
- Ahmed, W.; Angel, N.; Edson, J.; Bibby, K.; Bivins, A.; O’Brien, J.W.; Choi, P.M.; Kitajima, M.; Simpson, S.L.; Li, J. First Confirmed Detection of SARS-CoV-2 in Untreated Wastewater in Australia: A Proof of Concept for the Wastewater Surveillance of COVID-19 in the Community. Sci. Total. Environ. 2020, 728, 138764. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhang, J.; Xiao, A.; Gu, X.; Lee, W.L.; Armas, F.; Kauffman, K.; Hanage, W.; Matus, M.; Ghaeli, N. SARS-CoV-2 Titers in Wastewater Are Higher than Expected from Clinically Confirmed Cases. Msystems 2020, 5, e00614-20. [Google Scholar] [CrossRef] [PubMed]
- Nemudryi, A.; Nemudraia, A.; Wiegand, T.; Surya, K.; Buyukyoruk, M.; Cicha, C.; Vanderwood, K.K.; Wilkinson, R.; Wiedenheft, B. Temporal Detection and Phylogenetic Assessment of SARS-CoV-2 in Municipal Wastewater. Cell Rep. Med. 2020, 1, 100098. [Google Scholar] [CrossRef] [PubMed]
- Kocamemi, B.A.; Kurt, H.; Sait, A.; Sarac, F.; Saatci, A.M.; Pakdemirli, B. SARS-CoV-2 Detection in Istanbul Wastewater Treatment Plant Sludges. MedRxiv 2020. [Google Scholar]
- Randazzo, W.; Truchado, P.; Cuevas-Ferrando, E.; Simón, P.; Allende, A.; Sánchez, G. SARS-CoV-2 RNA in Wastewater Anticipated COVID-19 Occurrence in a Low Prevalence Area. Water Res. 2020, 181, 115942. [Google Scholar] [CrossRef]
- Haramoto, E.; Malla, B.; Thakali, O.; Kitajima, M. First Environmental Surveillance for the Presence of SARS-CoV-2 RNA in Wastewater and River Water in Japan. Sci. Total. Environ. 2020, 737, 140405. [Google Scholar] [CrossRef]
- Kumar, M.; Patel, A.K.; Shah, A.V.; Raval, J.; Rajpara, N.; Joshi, M.; Joshi, C.G. First Proof of the Capability of Wastewater Surveillance for COVID-19 in India through Detection of Genetic Material of SARS-CoV-2. Sci. Total. Environ. 2020, 746, 141326. [Google Scholar] [CrossRef]
- Guerrero-Latorre, L.; Ballesteros, I.; Villacrés-Granda, I.; Granda, M.G.; Freire-Paspuel, B.; Ríos-Touma, B. SARS-CoV-2 in River Water: Implications in Low Sanitation Countries. Sci. Total. Environ. 2020, 743, 140832. [Google Scholar] [CrossRef] [PubMed]
- Westhaus, S.; Weber, F.-A.; Schiwy, S.; Linnemann, V.; Brinkmann, M.; Widera, M.; Greve, C.; Janke, A.; Hollert, H.; Wintgens, T. Detection of SARS-CoV-2 in Raw and Treated Wastewater in Germany–Suitability for COVID-19 Surveillance and Potential Transmission Risks. Sci. Total. Environ. 2021, 751, 141750. [Google Scholar] [CrossRef]
- Rodríguez-Lázaro, D.; Cook, N.; Ruggeri, F.M.; Sellwood, J.; Nasser, A.; Nascimento, M.S.J.; D’Agostino, M.; Santos, R.; Saiz, J.C.; Rzeżutka, A. Virus Hazards from Food, Water and Other Contaminated Environments. FEMS Microbiol. Rev. 2012, 36, 786–814. [Google Scholar] [CrossRef]
- Simmons, F.J.; Xagoraraki, I. Release of Infectious Human Enteric Viruses by Full-Scale Wastewater Utilities. Water Res. 2011, 45, 3590–3598. [Google Scholar] [CrossRef] [PubMed]
- Barrella, K.M.; Garrafa, P.; Monezi, T.A.; Hársi, C.M.; Salvi, C.; Violante, P.A.B.C.; Mehnert, D.U. Longitudinal Study on Occurrence of Adenoviruses and Hepatitis A Virus in Raw Domestic Sewage in the City of Limeira, São Paulo. Braz. J. Microbiol. 2009, 40, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Masachessi, G.; Pisano, M.B.; Prez, V.E.; Martínez, L.C.; Michelena, J.F.; Martínez-Wassaf, M.; Giordano, M.O.; Isa, M.B.; Pavan, J.V.; Welter, A. Enteric Viruses in Surface Waters from Argentina: Molecular and Viable-Virus Detection. Appl. Environ. Microbiol. 2018, 84, e02327-17. [Google Scholar] [CrossRef] [PubMed]
- Sherchan, S.P.; Shahin, S.; Ward, L.M.; Tandukar, S.; Aw, T.G.; Schmitz, B.; Ahmed, W.; Kitajima, M. First Detection of SARS-CoV-2 RNA in Wastewater in North America: A Study in Louisiana, USA. Sci. Total. Environ. 2020, 743, 140621. [Google Scholar] [CrossRef] [PubMed]
- Bonadonna, L.; La Rosa, G. A Review and Update on Waterborne Viral Diseases Associated with Swimming Pools. Int. J. Environ. Res. Public Health 2019, 16, 166. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Chen, F.; Lin, Y.; Zhu, H.; Yuan, F.; Kuang, D.; Jia, Z.; Yuan, Z. Microbial Indicators and Their Use for Monitoring Drinking Water Quality—A Review. Sustainability 2020, 12, 2249. [Google Scholar] [CrossRef]
- Bitton, G. Wastewater Microbiology; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Xagoraraki, I.; Yin, Z.; Svambayev, Z. Fate of Viruses in Water Systems. J. Environ. Eng. 2014, 140, 04014020. [Google Scholar] [CrossRef]
- Jurzik, L.; Hamza, I.A.; Puchert, W.; Überla, K.; Wilhelm, M. Chemical and Microbiological Parameters as Possible Indicators for Human Enteric Viruses in Surface Water. Int. J. Hyg. Environ. Health 2010, 213, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Payyappat, S.; Cassidy, M.; Besley, C. Enhanced Insights from Human and Animal Host-Associated Molecular Marker Genes in a Freshwater Lake Receiving Wet Weather Overflows. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Malla, B.; Makise, K.; Nakaya, K.; Mochizuki, T.; Yamada, T.; Haramoto, E. Evaluation of Human-and Animal-Specific Viral Markers and Application of Crassphage, Pepper Mild Mottle Virus, and Tobacco Mosaic Virus as Potential Fecal Pollution Markers to River Water in Japan. Food Environ. Virol. 2019, 11, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Crank, K.; Petersen, S.; Bibby, K. Quantitative Microbial Risk Assessment of Swimming in Sewage Impacted Waters Using CrAssphage and Pepper Mild Mottle Virus in a Customizable Model. Environ. Sci. Technol. Lett. 2019, 6, 571–577. [Google Scholar] [CrossRef]
- Boehm, A.B.; Graham, K.E.; Jennings, W.C. Can We Swim yet? Systematic Review, Meta-Analysis, and Risk Assessment of Aging Sewage in Surface Waters. Environ. Sci. Technol. 2018, 52, 9634–9645. [Google Scholar] [CrossRef]
- Symonds, E.M.; Young, S.; Verbyla, M.E.; McQuaig-Ulrich, S.M.; Ross, E.; Jiménez, J.A.; Harwood, V.J.; Breitbart, M. Microbial Source Tracking in Shellfish Harvesting Waters in the Gulf of Nicoya, Costa Rica. Water Res. 2017, 111, 177–184. [Google Scholar] [CrossRef]
- Law, I.B. Rouse Hill-Australia’n9s First Full Scale Domestic Non-Potable Reuse Application. Water Sci. Technol. 1996, 33, 71–78. [Google Scholar] [CrossRef]
- Kayaalp, N.M. Regulatory Framework in South Australia and Reclaimed Water Reuse Options and Possibilities. Desalination 1996, 106, 317–322. [Google Scholar] [CrossRef]
- EPA. Guidelines for Water Reuse; EPA/625/R-04/108; US Environmental Protection Agency: Washington, DC, USA, 2004.
- WHO. World Health Organization Guidelines for the safe use of wastewater and excreta in agriculture and aquaculture. In Guidelines for the Safe Use of Wastewater and Excreta in Agriculture and Aquaculture; WHO: Geneva, Switzerland, 1989; pp. 7–187. [Google Scholar]
- Elbana, T.A.; Bakr, N.; Elbana, M. Reuse of treated wastewater in Egypt: Challenges and opportunities. In Unconventional Water Resources and Agriculture in Egypt; Springer: Berlin, Germany, 2017; pp. 429–453. [Google Scholar]
- Ouda, O.K. Treated Wastewater Use in Saudi Arabia: Challenges and Initiatives. Int. J. Water Resour. Dev. 2016, 32, 799–809. [Google Scholar] [CrossRef]
- Kalavrouziotis, I.K.; Kokkinos, P.; Oron, G.; Fatone, F.; Bolzonella, D.; Vatyliotou, M.; Fatta-Kassinos, D.; Koukoulakis, P.H.; Varnavas, S.P. Current Status in Wastewater Treatment, Reuse and Research in Some Mediterranean Countries. Desalination Water Treat. 2015, 53, 2015–2030. [Google Scholar] [CrossRef]
- EPA. Guidelines for Water Reuse; EPA/600/R-12/618; EPA: Washington, DC, USA, 2012.
- Pedrero, F.; Kalavrouziotis, I.; Alarcón, J.J.; Koukoulakis, P.; Asano, T. Use of Treated Municipal Wastewater in Irrigated Agriculture—Review of Some Practices in Spain and Greece. Agric. Water Manag. 2010, 97, 1233–1241. [Google Scholar] [CrossRef]
- Elbana, T.A.; Ramadan, M.A.; Gaber, H.M.; Bahnassy, M.H.; Kishk, F.M.; Selim, H.M. Heavy Metals Accumulation and Spatial Distribution in Long Term Wastewater Irrigated Soils. J. Environ. Chem. Eng. 2013, 1, 925–933. [Google Scholar] [CrossRef]
- Ouda, O.K. Impacts of Agricultural Policy on Irrigation Water Demand: A Case Study of Saudi Arabia. Int. J. Water Resour. Dev. 2014, 30, 282–292. [Google Scholar] [CrossRef]
- Van der Hoek, J.P.; de Fooij, H.; Struker, A. Wastewater as a Resource: Strategies to Recover Resources from Amsterdam’s Wastewater. Resour. Conserv. Recycl. 2016, 113, 53–64. [Google Scholar] [CrossRef]
- Lefebvre, O. Beyond NEWater: An Insight into Singapore’s Water Reuse Prospects. Curr. Opin. Environ. Sci. Health 2018, 2, 26–31. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Merayo, N.; Prinsen, P.; Luque, R.; Blanco, A.; Zhao, M. A Review on Greywater Reuse: Quality, Risks, Barriers and Global Scenarios. Rev. Environ. Sci. Bio/Technol. 2019, 18, 77–99. [Google Scholar] [CrossRef]
- Oh, K.S.; Leong, J.Y.C.; Poh, P.E.; Chong, M.N.; Von Lau, E. A Review of Greywater Recycling Related Issues: Challenges and Future Prospects in Malaysia. J. Clean. Prod. 2018, 171, 17–29. [Google Scholar] [CrossRef]
- Abrantes, S.; Silva, F.; Albuquerque, A. Technical Solutions for Water Reuse in a Social and Cultural Center. In Proceedings of the 5th Water Efficiency Conference 2018, Aveiro, Portugal, 5–7 September 2018. [Google Scholar]
- Jumat, M.R.; Hasan, N.A.; Subramanian, P.; Heberling, C.; Colwell, R.R.; Hong, P.-Y. Membrane Bioreactor-Based Wastewater Treatment Plant in Saudi Arabia: Reduction of Viral Diversity, Load, and Infectious Capacity. Water 2017, 9, 534. [Google Scholar] [CrossRef]
- Capocelli, M.; Prisciandaro, M.; Piemonte, V.; Barba, D. A Technical-Economical Approach to Promote the Water Treatment & Reuse Processes. J. Clean. Prod. 2019, 207, 85–96. [Google Scholar]
- Avni, N.; Eben-Chaime, M.; Oron, G. Optimizing Desalinated Sea Water Blending with Other Sources to Meet Magnesium Requirements for Potable and Irrigation Waters. Water Res. 2013, 47, 2164–2176. [Google Scholar] [CrossRef] [PubMed]
- Ghermandi, A.; Minich, T. Analysis of Farmers’ Attitude toward Irrigation with Desalinated Brackish Water in Israel’s Arava Valley. Desalin. Water Treat. 2017, 76, 328–331. [Google Scholar] [CrossRef]
- Al-Seekh, S.H.; Mohammad, A.G. The Effect of Water Harvesting Techniques on Runoff, Sedimentation, and Soil Properties. Environ. Manag. 2009, 44, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Van Lier, J.B.; Huibers, F.P. From Unplanned to Planned Agricultural Use: Making an Asset out of Wastewater. Irrig. Drain. Syst. 2010, 24, 143–152. [Google Scholar] [CrossRef]
- Chien, S.-S.; Hong, D.-L.; Lin, P.-H. Ideological and Volume Politics behind Cloud Water Resource Governance–Weather Modification in China. Geoforum 2017, 85, 225–233. [Google Scholar] [CrossRef]
- Molinos-Senante, M.; Hernandez-Sancho, F.; SalA–Garrido, R. Tariffs and Cost Recovery in Water Reuse. Water Resour. Manag. 2013, 27, 1797–1808. [Google Scholar] [CrossRef]
- AATSE. Water Recycling in Australia; Australian Academy of Technological Sciences and Engineering: Melbourne, VIC, Australia, 2004. [Google Scholar]
- Iglesias, R.; Ortega, E.; Batanero, G.; Quintas, L. Water Reuse in Spain: Data Overview and Costs Estimation of Suitable Treatment Trains. Desalination 2010, 263, 1–10. [Google Scholar] [CrossRef]
- WSAA. WSAA Facts 2005. Pricing for Recycled Water; Occasional Paper No. 12; Water Services Association of Australia: Melbourne, VIC, Australia, 2005.
- Huang, X.; Zhao, Z.; Hernandez, D.; Jiang, S.C. Near Real-Time Flow Cytometry Monitoring of Bacterial and Viral Removal Efficiencies during Water Reclamation Processes. Water 2016, 8, 464. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R.; Weil, R.R. The Nature and Properties of Soils; Prentice Hall: Upper Saddle River, NJ, USA, 2008; Volume 13. [Google Scholar]
- Sparks, D.L. Environmental Soil Chemistry; Elsevier: Amsterdam, The Netherlands, 2003; ISBN 978-0-08-049480-7. [Google Scholar]
- Bloom, P. Soil pH and pH Buffering. In Handbook of Soil Science; CRC Press: Boca Raton, FL, USA, 2000; pp. B333–B352. [Google Scholar]
- Barker, S.F.; O’Toole, J.; Sinclair, M.I.; Leder, K.; Malawaraarachchi, M.; Hamilton, A.J. A Probabilistic Model of Norovirus Disease Burden Associated with Greywater Irrigation of Home-Produced Lettuce in Melbourne, Australia. Water Res. 2013, 47, 1421–1432. [Google Scholar] [CrossRef]
- Rice, J.; Wutich, A.; Westerhoff, P. Assessment of de Facto Wastewater Reuse across the US: Trends between 1980 and 2008. Environ. Sci. Technol. 2013, 47, 11099–11105. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.; Westerhoff, P. Spatial and Temporal Variation in de Facto Wastewater Reuse in Drinking Water Systems across the USA. Environ. Sci. Technol. 2015, 49, 982–989. [Google Scholar] [CrossRef]
- National Research Council. Water Reuse: Potential for Expanding the Nation’s Water Supply through Reuse of Municipal Wastewater; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Lim, K.-Y.; Wu, Y.; Jiang, S.C. Assessment of Cryptosporidium and Norovirus Risk Associated with de Facto Wastewater Reuse in Trinity River, Texas. Microb. Risk Anal. 2017, 5, 15–24. [Google Scholar] [CrossRef]
- Soller, J.A.; Eftim, S.E.; Nappier, S.P. Comparison of Predicted Microbiological Human Health Risks Associated with de Facto, Indirect, and Direct Potable Water Reuse. Environ. Sci. Technol. 2019, 53, 13382–13389. [Google Scholar] [CrossRef]
- Milly, P.C.; Dunne, K.A.; Vecchia, A.V. Global Pattern of Trends in Streamflow and Water Availability in a Changing Climate. Nature 2005, 438, 347–350. [Google Scholar] [CrossRef]
- WHO. Water Safety Plans. Available online: https://www.euro.who.int/en/health-topics/environment-and-health/water-and-sanitation/water-safety-plans (accessed on 1 June 2021).
- Bryan, J.J. Hazard Analysis and Critical Control Points and Their Application to the Drinking Water Treatment Process. In Proceedings of the AWWA Water Quality Technology Conference, Miami, FL, USA, 7–11 November 1993; pp. 169–176. [Google Scholar]
- Havelaar, A.H. Application of HACCP to Drinking Water Supply. Food Control 1994, 5, 145–152. [Google Scholar] [CrossRef]
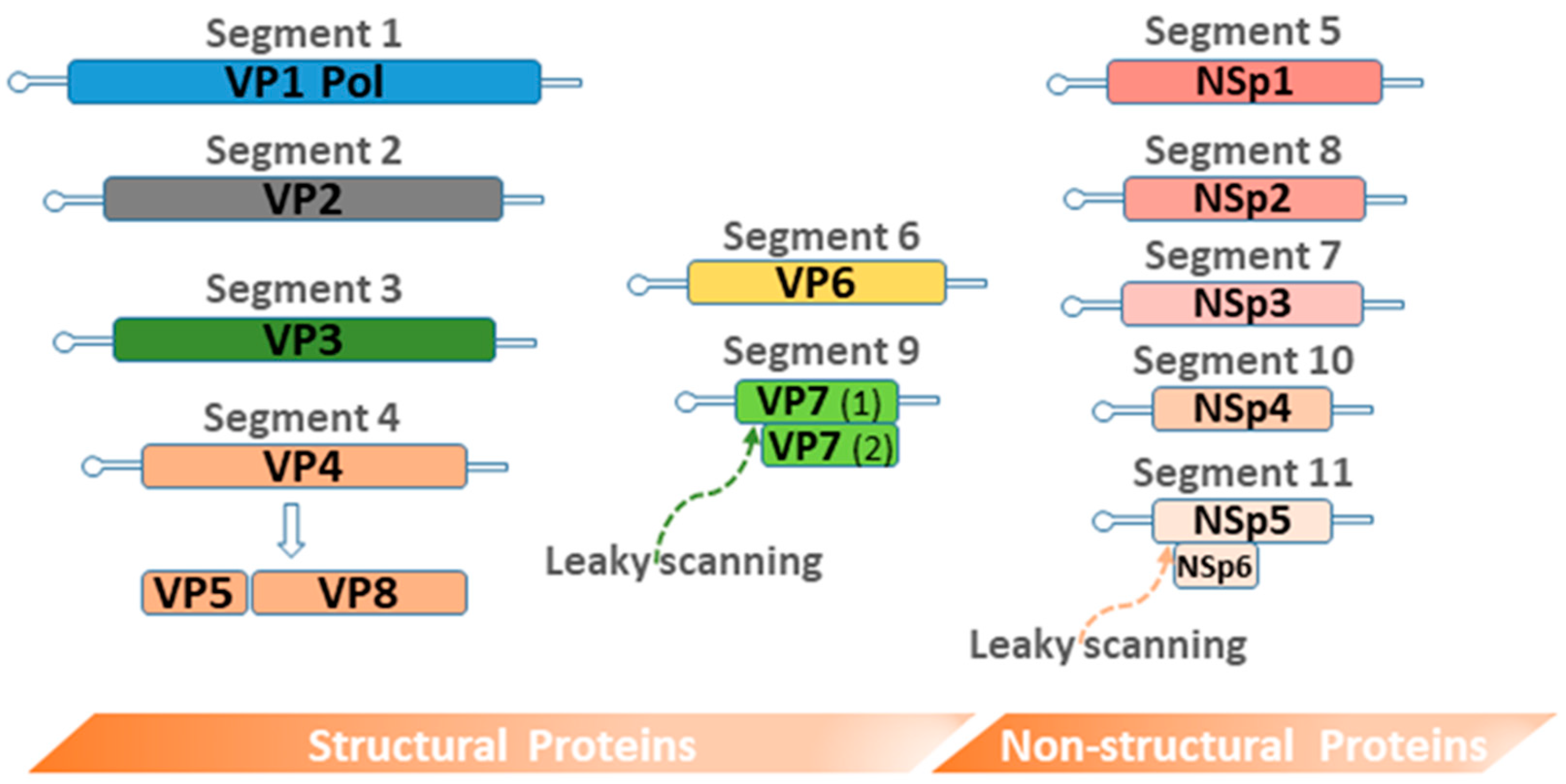
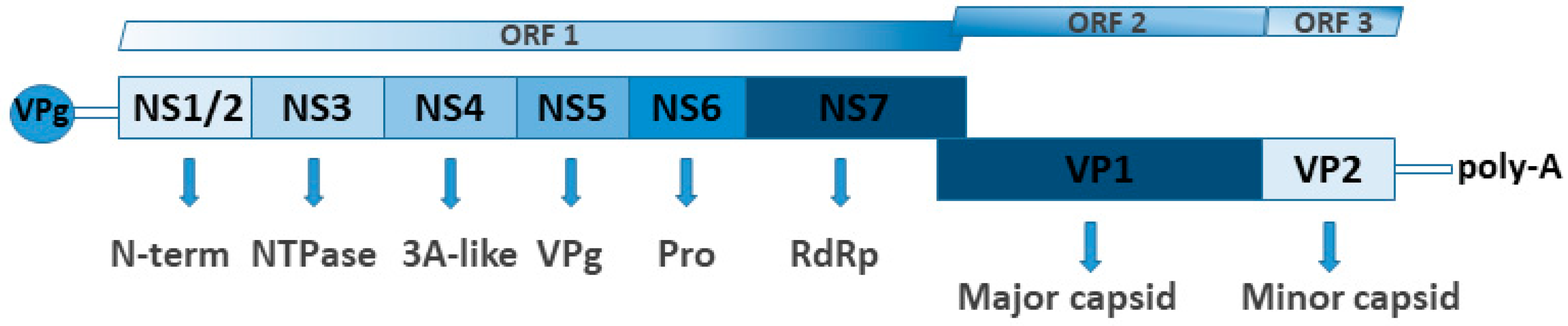
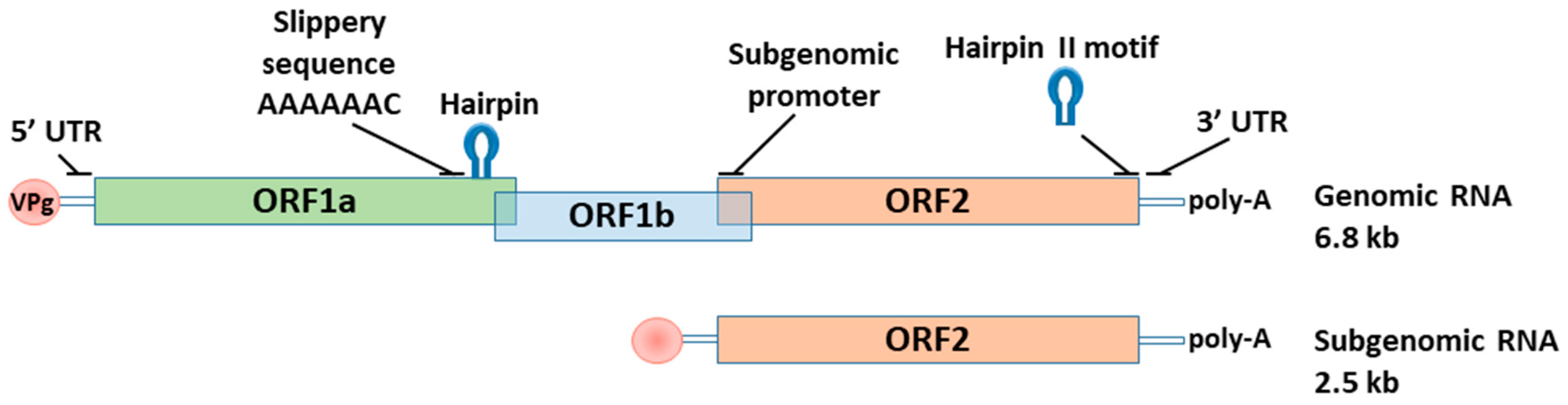
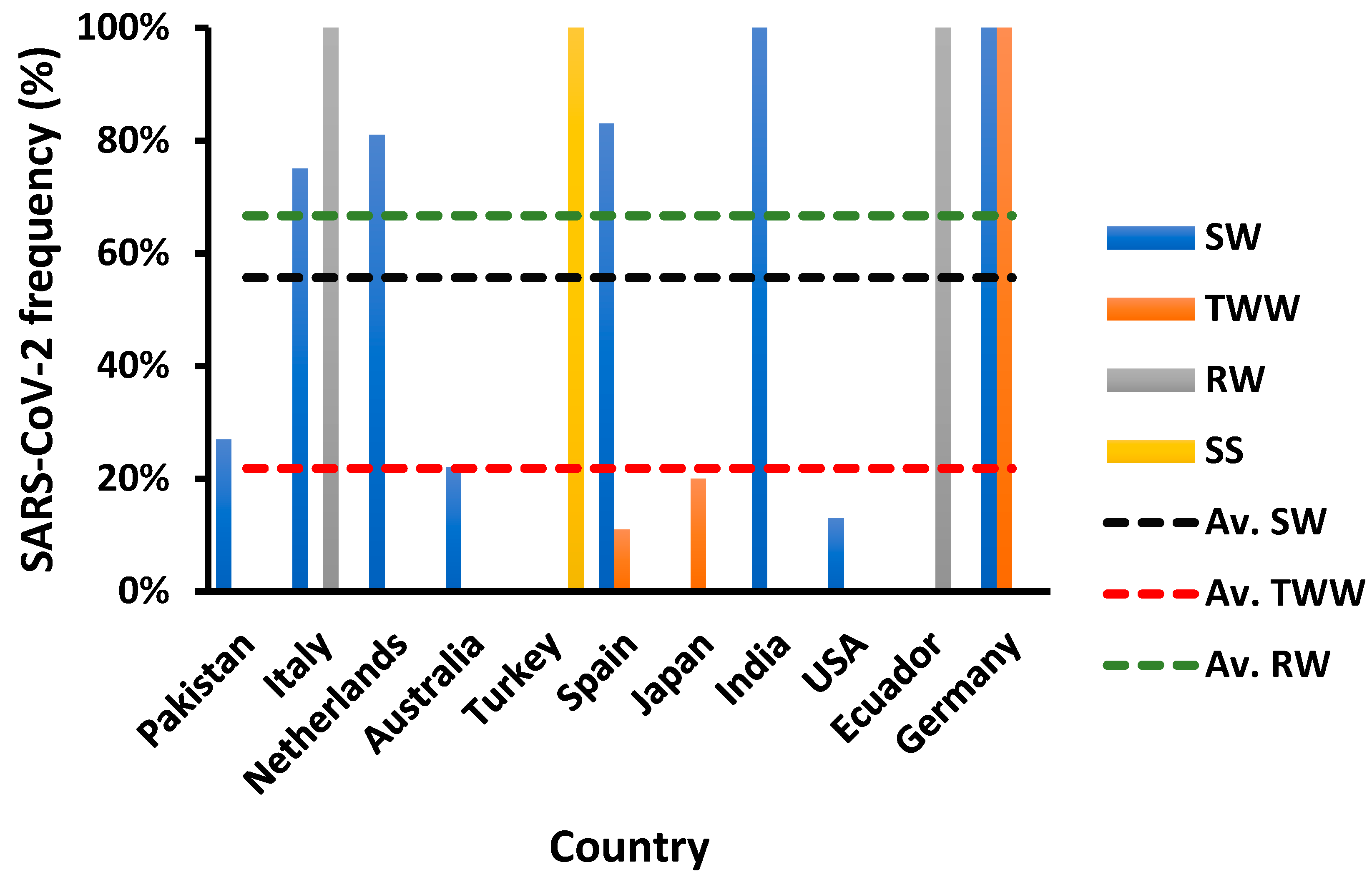
| Country | Water Source | Frequency (% Positive) | Study Period | Concentration | Species (Serotype) | Assessment Tools | Reference |
|---|---|---|---|---|---|---|---|
| Saudi Arabia | Treated effluents | 44.44–61.11% | April 2018–March 2019 | - | F (41) | PCR and direct sequencing | [72] |
| Lakes | 75–77.78% | ||||||
| Wastewater landfill | 83.33% | ||||||
| Irrigation water | 52.78% | ||||||
| Egypt | Raw sewage water | 84.4% | January–December 2017 | 4.3 × 105–8.7 × 106 GC/mL | - | Conventional PCR and Real-Time PCR | [73] |
| Treated effluents | 50% | 1.22 × 104–3.7 × 106 GC/mL | |||||
| Irrigation water (Nile River) | 62.5% | September–December 2017 | 1.5 × 107 GC/L a | - | Real-Time PCR and amplicon cloning | [74] | |
| Japan | Raw sewage water | 100% | July 2003–June 2004 | 320 PCR units/mL a | - | TaqMan PCR and quantification by the MPN (Most probable number method | [75] |
| Secondary treated water ** | 99% | 7 PCR units/mL a | |||||
| Treated effluents *** | 100% | ||||||
| Tunisia | Raw sewage water | 0.4% | January 2003–April 2007 | - | F (41) | PCR and direct sequencing | [76] |
| Treated effluents | 0% | - | |||||
| Uruguay | River | 18% | June 2015–May 2016 | 1.5 × 104 GC/L a | B (3) | Real-Time PCR, cell culture, ICC-qPCR, nested PCR and amplicon sequencing | [77] |
| Underground water | 0.7% | November 2013–September 2014 | - | - | Nested PCR | [78] | |
| South Africa | River | 30.56% | August 2010–July 2011 | 8.49 × 104 GC/L b | C (1, 2, 5 & 6) and F (41) | Real-Time PCR and Multiplex PCR (Serotype-specific) | [79] |
| Treated effluents | 64% | September 2012–August 2013 | 2.37 × 105 GC/L b | C (2) and F (41) | Real-Time PCR and serotype-specific PCR | [80] | |
| Pakistan | Tap water | 20% | - | - | - | PCR | [81] |
| USA | Raw sewage water | 100% | August 2005–August 2006 | 1.15 × 106 viruses/L | F (41) and A (12) | Real-Time PCR, Molecular cloning and sequencing | [67] |
| Combined sewer overflows | 100% | February–June 2008 | 5.35 × 105 viruses/L | - | |||
| Primary treated water | - | August 2005–August 2006 | 1.12 × 106 viruses/L | F (41) and A (12) | |||
| Secondary treated water | - | 2 × 104 viruses/L | - | ||||
| Tertiary treated water | - | 8.3 × 104 viruses/L | - | ||||
| Surface water | 30% | 2006–2007 | 7.76 × 103 viruses/L | - | |||
| Raw sewage water | 100% | Fall 2007 | - | - | Nested PCR and sequencing | [82] | |
| Treated effluents | 25% | - | - | ||||
| Brazil | Recreational water (Lagoons and beaches) | 93.1% c | March 2015–July 2016 | 109 GC/L b | C | qPCR, ICC-qPCR, Nested PCR and sequencing | [83] |
| Italy | Raw sewage water | 96% | May–September 2007 | 9.8 × 108 GC/mL | - | TaqMan real-time PCR | [84] |
| Treated effluent | 76% | 4.9 × 108 GC/mL | |||||
| Raw sewage water | 60% | January–December 2013 | - | F (41) | Nested PCR, Sanger sequencing and Next generation sequencing | [85] | |
| UK | Raw sewage water | 90% | March 2016 and August 2016–August 2017 | 6 × 105 GC/L b | - | SYBR Green qPCR | [86] |
| Treated effluent | 87% | 103 GC/L b | |||||
| Surface water | 88% | 105 GC/L b | |||||
| Netherlands | Drinking water influents **** | 54% | 2012 | 2.5 Log10 GC/L a | F(40, 41) | Real-Time PCR and mpnPCR | [87] |
| China | Recreational water (Swimming pool) | 40% | May 2013 | - | E (4) | Conventional PCR, sequencing and cell culture | [88] |
| Canada | River upstream | 50% c | June 2012–May 2013 | 2.66 Log10 GE * copies/L a | - | qPCR and ICC-qPCR | [89] |
| River downstream | 92% c | 4.55 Log10 GE copies/L | |||||
| France | River | 100% | January–June 2016 | 3.6 × 103 GC/L a | F (41) | MPN assay, ddPCR (digital droplet PCR) and ICC-qPCR | [90] |
| Sweden | Raw sewage water | 100% | November–December 2015 | 9.07 × 104 GC/mL a | F (41) | Nested PCR, Library construction, Ion-Torrent sequencing and qPCR | [91] |
| Conventionally treated water | 1.06 × 103 GC/mL a | ||||||
| Ozone treated water | 8.25 × 103 GC/mL a | ||||||
| Raw sewage water | 100% | January–May 2013 | 3.3 × 105 virus/L b | - | Real-Time PCR and sequencing | [92] |
| Country | Water Source | Frequency (% Positive) | Study Period | Concentration | Dominant Genotypes | Assessment Tools | Reference |
|---|---|---|---|---|---|---|---|
| Saudi Arabia | Treated effluent | 5.56–13.89% | April 2018–March 2019 | - | G2 | RT-PCR and Sanger sequencing | [22] |
| Lakes | 27.78–30.56% | - | G2 | ||||
| Wastewater landfill | 63.89% | - | G2 | ||||
| Irrigation water | 5.56 | - | G2 | ||||
| Dams | 13.33% | February–April 2017 | - | - | RT-PCR | [110] | |
| Japan | Drinking water effluent | 86% a–95% b | June 2017–August 2018 | 5.5 b–6.3 a log10 GC/L | - | RT-PCR, semi-nested PCR and direct sequencing | [111] |
| Egypt | Nile river | 18.75 % | June 2016–May 2017 | - | G1P(8) and G1P(4) | RT-PCR and Multiplex semi-nested PCR | [112] |
| Irrigation water (Nile river) | 50% | September–December 2017 | 2.7 × 105 GC/L d | - | RT-PCR, amplicon cloning and qPCR | [74] | |
| Tunisia | Raw sewage water | 21.2% | January 2003–April 2007 | - | GxP(8) and GxP(4) | RT-PCR and direct sequencing | [76] |
| Treated effluent | 10.8% | ||||||
| Uruguay | River watersheds | 41 % c | June 2015–May 2016 | 1.3 × 105 GC/L d | - | RT-PCR and Real-Time PCR | [105] |
| Underground water | 32 % | November 2013–September 2014 | 1.72 × 103 GC/L a | RT-PCR and qPCR | [78] | ||
| South Africa | Treated effluents | 41.7% e | September 2012–August 2013 | 5.2 × 103–1.2 × 105 GC/L f | - | RT-PCR and Real-Time PCR | [113] |
| Pakistan | Treated effluents | 5% | February–July 2014 | - | - | Enzyme-linked immunosorbent assay (ELISA) | [114] |
| Drinking water | 5% | ||||||
| Surface water (River and dam water) | 23% | November 2014–February 2015 and April 2015–July 2015 | - | G3 and G9 | RT-PCR, Nested-PCR and sequencing | [115] | |
| USA | Treated effluents | 83% | August 2011–July 2012 | 2.8 × 106 GC/L f | - | RT-PCR and qPCR | [116] |
| Brazil | Raw sewage water | 100% | August 2009–July 2010 | 2.40 × 105–1.16 × 107 GC/L e | G2P(4) and G2P(6) | RT-PCR, qPCR, Nested PCR and amplicon sequencing | [117] |
| Treated effluents | 71% | 1.35 × 103–1.64 × 105 GC/L e | |||||
| Italy | Raw sewage water | 60.4% | 2010–2011 | - | G1P(8) and G2P(4) | RT-PCR, Nested PCR and amplicon sequencing | [118] |
| Netherlands | Drinking water | 48% | 1999–2002 | 2.2 × 103 PDU/liter f | - | RT-PCR, and molecular cloning | [119] |
| China | Surface water (Rivers) | 75-83.33% | September 2014–August 2015 | - | - | (RT-)qPCR and sequencing | [120] |
| Treated effluent | 100% | ||||||
| Raw sewage water | 91.67% | ||||||
| Tap water | 91.67% | ||||||
| Canada | Surface water (Rivers) | 37% g–75% h | June 2012–May 2013 | 4.5 log10 GE * copies/L f | G1 | (RT-)qPCR, cell culture and ICC-qPCR (integrated cell culture with qPCR) and sequencing | [89] |
| Country | Water Source | Frequency (% Positive) | Study Period | Concentration | Genogroups (Genotype) | Assessment Tools | Reference |
|---|---|---|---|---|---|---|---|
| Saudi Arabia | Raw sewage water | 19% | January 2009–February 2010 | - | - | One step RT-PCR | [170] |
| Egypt | Raw sewage water | 25% | April 2017–March 2018 | - | GI ‡ and GII | Semi-nested RT-PCR | [171] |
| River | 0% *–16.6% ** | ||||||
| Irrigation water | 31.25% | September–December 2017 | 3.5 × 103 GC/L a | GI | One step RT-PCR, Amplicon cloning and Real-Time PCR | [74] | |
| Urban sewage water | 33.3% | October 2017–September 2018 | - | GI ‡ and GII | RT-PCR and Semi- nested RT-PCR | [172] | |
| Treated effluents | 25% | GI and GII | |||||
| Japan | Raw sewage water | - | October 2006- December 2007 | - | GI(8) ***, GI(4), GII(4) ‡, GII(6) and GII(13) | RT-PCR and DNA Sequencing | [173] |
| Raw sewage water | 50% | March 2005–February 2006 | 6.9 × 104 GC/L b | GIV | RT-PCR and TaqMan-based real-time PCR | [174] | |
| Treated effluents | 25% | 4.8 × 103 GC/L b | |||||
| River water | 31% | April 2003–March 2004 | 1.5 × 104 GC/L b | ||||
| Tunisia | Raw sewage water | 2.8% | January 2003–April 2007 | - | GI(2), GI(5), GI(9) and GII(4) ‡ | RT-PCR, Second-round typing PCR and amplicon sequencing | [76] |
| Treated effluents | 1.6% | ||||||
| South Africa | Raw sewage water | 72.2% | April 2015–March 2016 | 6.0 × 105 GC/L b | GI(4) ‡, GII(2) ‡ and GII(17) ‡ | Real-time reverse transcription-PCR, semi-nested RT-PCR, conventional PCR, amplicon cloning and clone sequencing | [175] |
| Treated effluents | 83.3% | 6.8 × 106 GC/L b | |||||
| Rivers | 62.9% | 2008–2010 | 2.37 × 105 GC/L b | GI(5) ‡, GI(4) ‡, GI(3), GII(6) ‡ and GII(4) ‡ | one-step real-time RT-PCR, two-step real-time RT–PCR, conventional PCR, semi-nested PCR, amplicon cloning and clone sequencing | [176] | |
| India | Tap water ư | 16.67% | June–July 2015 and April–October 2017 | 1.9 × 104 GC/L †, b and 8.0 × 104 GC/L ¥, b | GI and GII | RT-PCR, ddPCR (singleplex and multiplex probe-based assays) | [177] |
| USA | Untreated graywater | 6% | December–April, June, July | 2.5 log10 GC/L | GII | two-step RT-qPCR and duplexed RT-ddPCR | [178] |
| Combined wastewater | 39% †–96% ¥ | 4.0 log10 GC/L †,b and 7.9 log10 GC/L ¥,b | GI and GII ‡ | ||||
| Mexico | Recreational water (karst aquifer) | 40% ¥–50% † | - | 1.6 × 103 GC/L †,b and 2.9 × 102 GC/L ¥,b | GI(2) and GII(17) ‡ | RT-qPCR, Nested PCR, amplicon cloning, and Sanger sequencing | [179] |
| Norway | Raw sewage water | 100% | February 2008–February 2009 | 6.1 log10 GC/L †, b and 6.3 log10 GC/L ¥, b | GI and GII | RT-PCR, two genogroup-specific monoplex PCR and direct sequencing | [180] |
| Treated effluents | 95% c | 5.65 log10 GC/L †, b 5.75 GC/L ¥, b | |||||
| Brazil | Raw sewage water | 38.5% †–96.1% ¥ | May 2013–May 2014 | 6.2 log10 GC/L †, b–7.3 log10 GC/L ¥, b | GI, GII.4 ‡, GII.17, GII.5, GII.2, GII.3 and GII.1 | RT-PCR, qPCR and Sanger sequencing | [181] |
| Primary effluent | 40.4% †–96.1% ¥ | ||||||
| Final effluent | 1.9% †–5.8% ¥ | ||||||
| Italy | Treated urban wastewater stream | 30% | May–September 2018 | 13 GC/L b | GI(4) and GII ‡ | Real-time RT-qPCR and Qualitative nested (RT)-PCR | [182] |
| Recreational (bathing) water | 25% | 3.2 GC/L b | |||||
| Netherlands | Raw sewage water | 100% | November 1998–April 1999 | 8.5 × 106 PDU §/liter b | GI(2), GII(1), GII(2), GII(3), GII(4) ‡ and GII(7) | RT-PCR, Southern blotting, amplicon cloning and sequencing | [183] |
| Treated effluents | 2.7 × 105 PDU/liter b | ||||||
| Surface water (Rivers) | 100% | 4.6 × 104 PDU/liter b | GI(2),GI(4), GII(3), GII(4) ‡ and GII(7) | ||||
| China | Drinking (barreled) water | 45.5% d | February 2014 | - | GII | RT-PCR | [184] |
| Tap water (Secondary Water Supply System) | 50% | May 2017 | - | GII(17) | RT-PCR, Targeted gene (RdRP) sequencing | [185] | |
| Canada | River upstream | 50% d | June 2012–May 2013 | 3.24 log10 GE copies/L | - | Two-step RT-qPCR | [89] |
| River downstream | 75% d | 4.43 log10 GE copies/L | |||||
| France | River | 100% | January–June 2016 | 6.1 × 102 GC/L a, †–3.7 × 103 GC/L a,¥ | GI and GII | ddPCR | [90] |
| Sweden | Raw sewage water | 100% | November–December 2015 | 4.3 × 104 GC/mLb, †–6.5 × 104 GC/mLb,¥ | GI and GII | RT-PCR, nested PCR, library construction, Ion-torrent sequencing and qPCR | [91] |
| Conventionally treated effluents | 3.9 × 102 GC/mLb | GII | |||||
| Ozone treated effluents | 33.3% | 61 GC/mL | GII | ||||
| Raw sewage water | 100% | January–May 2013 | 3.5 × 103 virus particle/L b, †–3.2 × 105 virus particle/L b,¥ | GI and GII | RT-PCR and qPCR | [92] |
| Country | Water Source | Frequency (% Positive) | Study Period | Concentration | Genotypes-Subgenotype | Assessment Tools | Reference |
|---|---|---|---|---|---|---|---|
| Bahrain | Raw sewage water | 12.5% | January–February and May–June | - | - | RT-PCR | [224] |
| Tertiary treated effluents | 0% | ||||||
| Bay downstream water (effluent discharge) | 0% | ||||||
| Egypt | Irrigation water | 34.4% | September–December 2017 | 1.2 × 104 GC/L a | - | One-step RT-PCR and Real-Time PCR | [74] |
| Raw sewage water | 97.4% | 2014 | - | IB | RT-PCR, cell culture and direct sequencing | [225] | |
| Treated effluents | 47% | ||||||
| Tunisia | Raw sewage water | 66.9% | December 2009–December 2010 | 2.7 × 103 GC/mL | IA ‡ and IB- | One-step real-time RT-qPCR, semi nested RT-PCR and sequencing | [226] |
| Treated effluents | 40.7% | 6.0 × 103 GC/mL | |||||
| Raw sewage water | 68.3% | 2007–2008 | 3.5 × 105 GC/L a | IA ‡ and IB | RT-PCR, nested RT-PCR, sequencing, qPCR | [227] | |
| Treated effluents | 64.7% | 2.5 × 105 GC/L a | |||||
| Uruguay | Surface water (rivers and logoon) | 13.95% | 2009–2012 | 3.7 × 103 GC/L | - | qualitative PCR and TaqMan-based qPCR | [228] |
| Drinking water | 0% | - | |||||
| Raw sewage water | 2.3% | ||||||
| Treated effluents | 0% | ||||||
| South Africa | Surface water (Rivers and dams) | 76% | August 2010–December 2011 Υ, and January 2012–August 2012 ¥ | - | IB | One-step RT-qPCR, nested PCR, amplicon cloning and sequencing | [229] |
| Treated effluents | 37% | ||||||
| Irrigation water | 73% | January, March and May 2013 | 2.37 × 105 GC/L b | V | real-time RT-PCR, molecular cloning and sequencing | [230] | |
| Pakistan | Surface and subsurface water | 12.63% | June 2014–May 2015 | - | - | RT-PCR | [231] |
| USA | Well water | 60% | September 1995–December 1995 | - | 1A | IC (immunocapture)-RT-PCR and amplicon sequencing | [232] |
| Brazil | River water | 1.19% | 2012–2014 | 1.5 × 104 GC/L | - | RT-TaqMan probe-mediated qPCR and ICC-RT-qPCR | [233] |
| Recreational water (Lagoons and beaches) | 0% | March 2015–July 2016 | - | - | RT-PCR | [83] | |
| Italy | Raw sewage water | 28.16% | 2015–2018 | - | IA ‡ and IB | RT-nested-PCR and amplicon sequencing | [199] |
| Coastal discharge water | 3.97% | ||||||
| Seawater | 12.8% | ||||||
| Raw sewage water | 33.3% | January–December 2013 | - | IA and IB ‡ | nested RT-PCR and sequencing | [234] | |
| Treated effluents | 14.3% | IA ‡ and IB | |||||
| River water | 7.4% | IB | |||||
| Spain | Raw sewage water | 1.75% | 2019 | - | - | Real-time RT-PCR | [235] |
| Tertiary-treated effluent | 0.35% | ||||||
| Hong Kong | Seawater § | 57.14% | 2011 | 1.028 × 103 particle/L b | IB | RT-PCR, amplicon cloning, sequencing and TaqMan real-time PCR | [236] |
| Canada | Drinking water | 10% | 1974–2001 | - | - | ◊ | [237] |
| France | Raw sewage water | 59.3% | October 2014–October 2015 | - | IIIA and IA | RT-PCR and sanger sequencing | [23] |
| Treated effluents | 19.2% | - | |||||
| Sweden | Raw sewage water | 100% | January–May 2013 | 1.4 × 104 virus/L b | - | Two-step real-time RT-qPCR | [92] |
| Country | Water Source | Frequency (% Positive) | Study Period | Concentration | Genogroups-Genotypes | Assessment Tools | Reference |
|---|---|---|---|---|---|---|---|
| Egypt | Raw sewage water | 40.2% | April 2017–March 2018 | - | A and B | Semi-nested RT-PCR | [171] |
| River | 8.3% *–25% ** | ||||||
| Urban sewage water | 58.3% | October 2017–September 2018 | - | B ‡ and A | Semi-nested RT-PCR | [172] | |
| Treated effluent | 33.3% | A and B | |||||
| Japan | Raw Sewage water | 91.67% | July 2015–June 2016 | - | A(1), MLB1 ‡ and MLB2 | RT-PCR and sequencing | [252] |
| Kenya | Raw sewage water | 98% d | April 2015–April 2016 | 30.8 ∫ | - | Real-time RT-PCR | [253] |
| Urban river | 60% b | May 2007–February 2008 | - | - | Qualitative singleplex real-time RT-PCR | [254] | |
| Rural river | 41.7% | ||||||
| Urban sewage water | 87.5% b | ||||||
| Uruguay | Raw sewage water | 45% | March 2011–February 2012 | 4.3 × 107 GC/L a | - | Qualitative RT-PCR and qPCR | [255] |
| South Africa | River water | 21.6% | June 1997–May 1998 | - | - | Cell culture, RT-PCR, dot-blot Hybridisation assay | [256] |
| Dam water | 5.9% | ||||||
| Nepal | Ground water | 8.1% | August 2009–May 2011 | - | A(1,2,4,5 and 8) ‡ and MLB | RT- semi-nested PCR and Next-generation amplicon sequencing | [257] |
| River water | 100% | A, B, MLB and VA | |||||
| USA | Raw sewage water | 75% b | August 2011 and July 2012 | - | A ‡, B, MLB and VA | RT- semi-nested PCR and Next-generation amplicon sequencing | [257] |
| Treated effluent | 63% b | ||||||
| Brazil | Surface water (basins) | 15.4% b | August 2004–June 2005 | - | A(1) | RT-PCR, nested PCR and amplicon sequencing | [258] |
| Italy | Seawater (receiving treated water) | 9% | February 2019–August 2020 | 1 × 102 GC/L | A(1) | Real-time (RT) qPCR, OneStep RT-PCR, nested or semi-nested PCR and sequencing | [259] |
| Seawater (receiving non-treated water) | 7% | ||||||
| Seawater (receiving rain drain and raw water) | 4% | ||||||
| Seawater receiving mixed waters | 11% | ||||||
| UK | Water supplies | 1% | 1992–2003 | - | - | ‡ | [260] |
| China | Sewage treatment plant water | 6.3% | November 2006–October 2007 | - | - | RT-nested PCR | [261] |
| Canada | River upstream | 42% b | June 2012–May 2013 | 2.52 Log10 GE § copies/L a | - | Two-step RT-qPCR | [89] |
| River downstream | 92% b | 4.1 Log10 GE § copies/L a | |||||
| France | River water | 36% | May 2013–May 2014 | 103 GC/L a | - | Real-time RT-qPCR | [262] |
| Tributaries water | 16% | 103 GC/L a | |||||
| Treated effluents | 84% | 104 GC/L a | |||||
| Sweden | Raw sewage water | 100% | November–December 2015 | 1.1 × 106 GC/mL a | A(4) | RT-nested PCR, Library construction, NGS sequencing and qPCR | [91] |
| Conventionally treated effluents | 33.3% | 1.8 × 102 GC/mL | |||||
| Ozone treated effluents | 0% | - | |||||
| Raw sewage water | 100% | January–May 2013 | 4.6 × 105 virus/L c | - | Two-step real-time RT-qPCR | [92] |
| Water Source | Virus | Genotype | Infectivity Rate | Number of Samples | Infection Risk (IR) ◊ | Detection Method | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| IR/person | Infection Risk Reduction | ||||||||
| Period (days) | RA (Log10 or fold) | ||||||||
| Recreational water | HAdV | C | 1 IVP/90% of sites * | 159 (144 [9 sites] and 15 [3 sites]) | - | Integrated cell culture-qPCR assay | [83] | ||
| Raw sewage water | HAV | IIIA and IA | 64.3% (9/14) ** | 14 | - | Cell culture | [23] | ||
| Treated effluents | 100% (1/1) ** | 1 | |||||||
| Drinking water (40 mJ/cm2 UV-treated) | HAdV | F(40, 41) | 1/1700 (5.88 × 10−4) *** | 35 | 10−4/year § | - | 5 Ұ | Integrated cell culture PCR | [87] |
| Drinking water (73 mJ/cm2 UV-treated) | 6.5 Ұ | ||||||||
| Groundwater | RV | - | - | 35 | - | 90 | 100 ¥ | Immunoperoxidase focus infectious assay | [263] |
| 150 | 1000 ¥ | ||||||||
| ~213 | |||||||||
| Surface water | 35 | 30 | 40 ¥ | ||||||
| 150 | 1500 ¥ | ||||||||
| 180 | 10,000 ¥ | ||||||||
| Ground water | AstV | - | - | 20 | - | 30 | 10 ¥ | ||
| 2.0 × 10−3 ‡ | 60–120 | 2000 ¥ | |||||||
| Surface water | 5 | 10−4 ‡ | 30 | 10,000 ¥ | |||||
| Pre-DIS influent | HAdV and EV | - | 100% † | 30 | - | - | - | Cell culture | [288] |
| Post-DIS influent | 4.2 Ұ,a | ||||||||
| Raw sewage water | HAdV | F | 64% (32/50) ** | 50 | - | - | - | Cell culture | [289] |
| Dam water | EV | 64.6% (31/48)–66.7% (44/66) ** | 114 (48 samples at Jan.–Dec. 2012 and 66 samples at Oct. 2013–Oct. 2015) | - | - | - | Cytopathic effect detection and Direct immunofluorescence assay | [290] | |
| Usage | Guideline Category | Allowed Limit | Average Limit | Reference |
|---|---|---|---|---|
| Irrigation (unrestricted) | Enteric viruses | <2 Virus/50 L | - | [302] |
| Toilet flushing, primary contact recreation, food aquaculture and car wash | Total coliform | <10 cfu/100 mL | - | [303] |
| Irrigation (unrestricted) | Total coliform | <10 cfu/100 mL | - | [304] |
| Gardens with access, ponds parks, crops and secondary contact recreation | Total coliform | <100 cfu/100 mL | - | [305] |
| Gardens and parks with no public access during irrigation and passive recreation | Total coliform | <1000 cfu/100 mL | - | |
| Non-food crops, Pasture and fodder irrigation | Total coliform | <10,000 cfu/100 mL | - | |
| Food crops | Total coliform | <23 cfu/100 mL | 2.2 cfu/100 mL | [304] |
| Non-food crops | Total coliform | <240 cfu/100 mL | 23 cfu/100 mL | |
| Recreational reuse (unrestricted) | Total coliform | <240 cfu/100 mL | 23 cfu/100 mL | |
| Irrigation (unrestricted) | Fecal coliform | <1000 cfu/100 mL | - | [305] |
| Food crops | Fecal coliform | <400 cfu/100 mL | 200 cfu/100 mL | [304] |
| Non-food crops | Fecal coliform | <400 cfu/100 mL | 200 cfu/100 mL | |
| Recreational reuse (unrestricted) | Fecal coliform | <23 cfu/100 mL | 2.2 cfu/100 mL | |
| Irrigation (unrestricted) | Fecal coliform | <1 cfu/100 mL | - | [302] |
| Irrigation (unrestricted) | Nematode egg | <2 egg/L | - | [305] |
| Irrigation (unrestricted) | (Oo)cysts | <1 cyst/50 L | - | [302] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nour, I.; Hanif, A.; Ryan, M.; Eifan, S. Insights into Gastrointestinal Virome: Etiology and Public Exposure. Water 2021, 13, 2794. https://doi.org/10.3390/w13192794
Nour I, Hanif A, Ryan M, Eifan S. Insights into Gastrointestinal Virome: Etiology and Public Exposure. Water. 2021; 13(19):2794. https://doi.org/10.3390/w13192794
Chicago/Turabian StyleNour, Islam, Atif Hanif, Martin Ryan, and Saleh Eifan. 2021. "Insights into Gastrointestinal Virome: Etiology and Public Exposure" Water 13, no. 19: 2794. https://doi.org/10.3390/w13192794
APA StyleNour, I., Hanif, A., Ryan, M., & Eifan, S. (2021). Insights into Gastrointestinal Virome: Etiology and Public Exposure. Water, 13(19), 2794. https://doi.org/10.3390/w13192794