Iron, Phosphorus and Trace Elements in Mussels’ Shells, Water, and Bottom Sediments from the Severnaya Dvina and the Onega River Basins (Northwestern Russia)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Locations
2.2. Determination of the Taxonomic Position of Mollusks’ Individuals and Preparation Samples for Trace Elements’ Analysis
2.3. Analysis of the Trace Element Composition of Shells
2.4. Analysis of the Elemental Composition of Water Samples
2.5. Analysis of the Elemental Composition of Bottom Sediments Samples
2.6. Statistical Treatment of the Data
3. Results
3.1. Elemental Composition of Shells
3.2. Elemental Composition of Water and Sediments
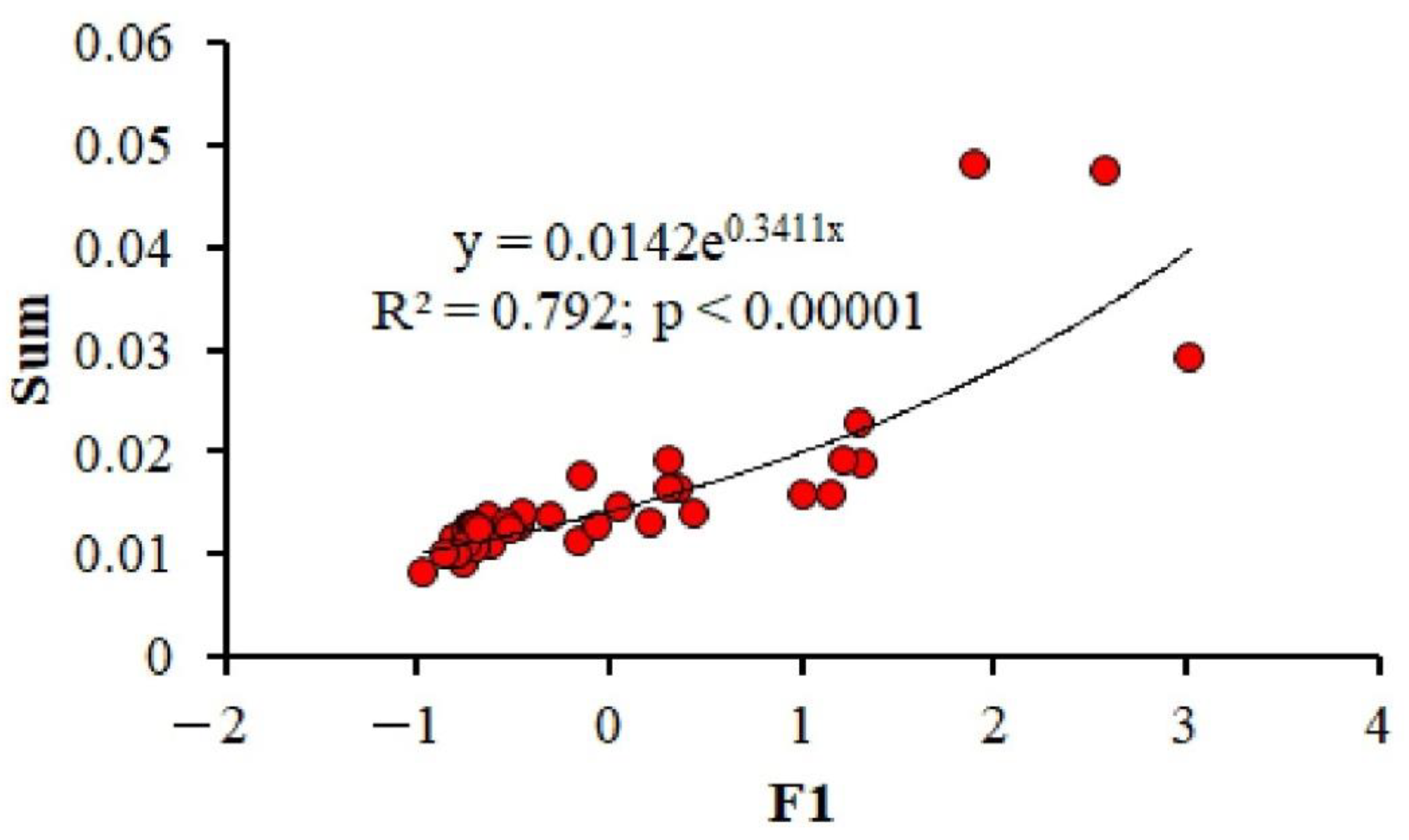
3.3. Multiparametric Statistics
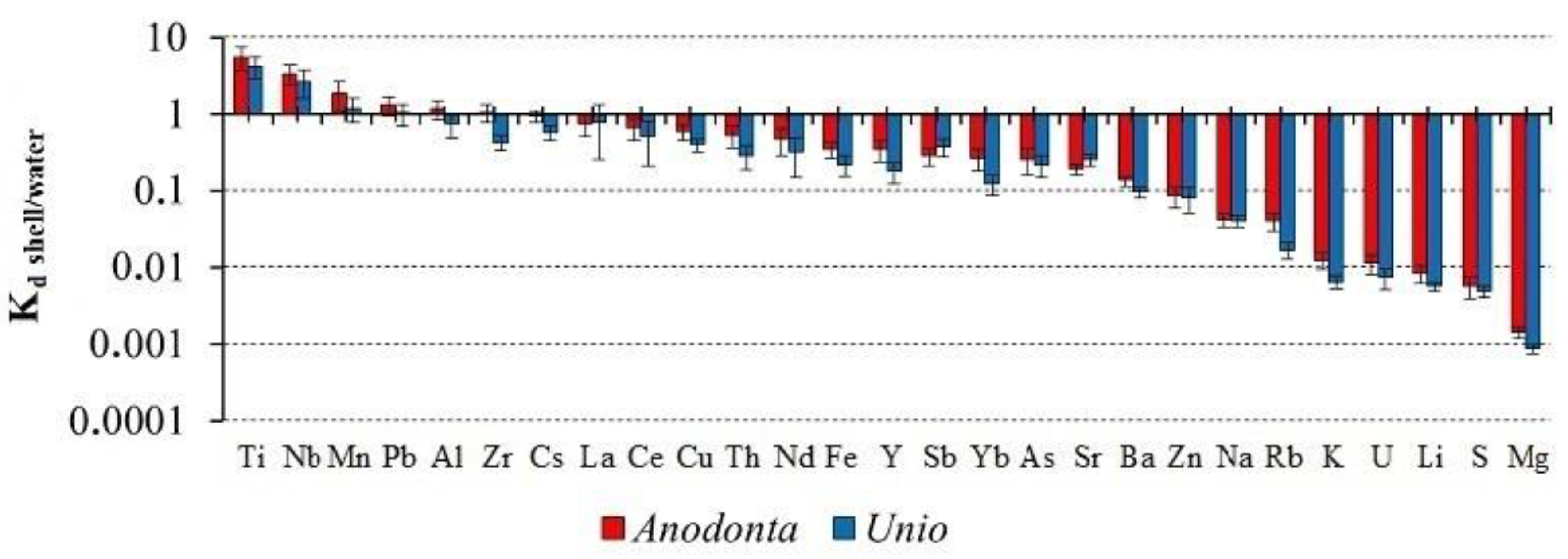
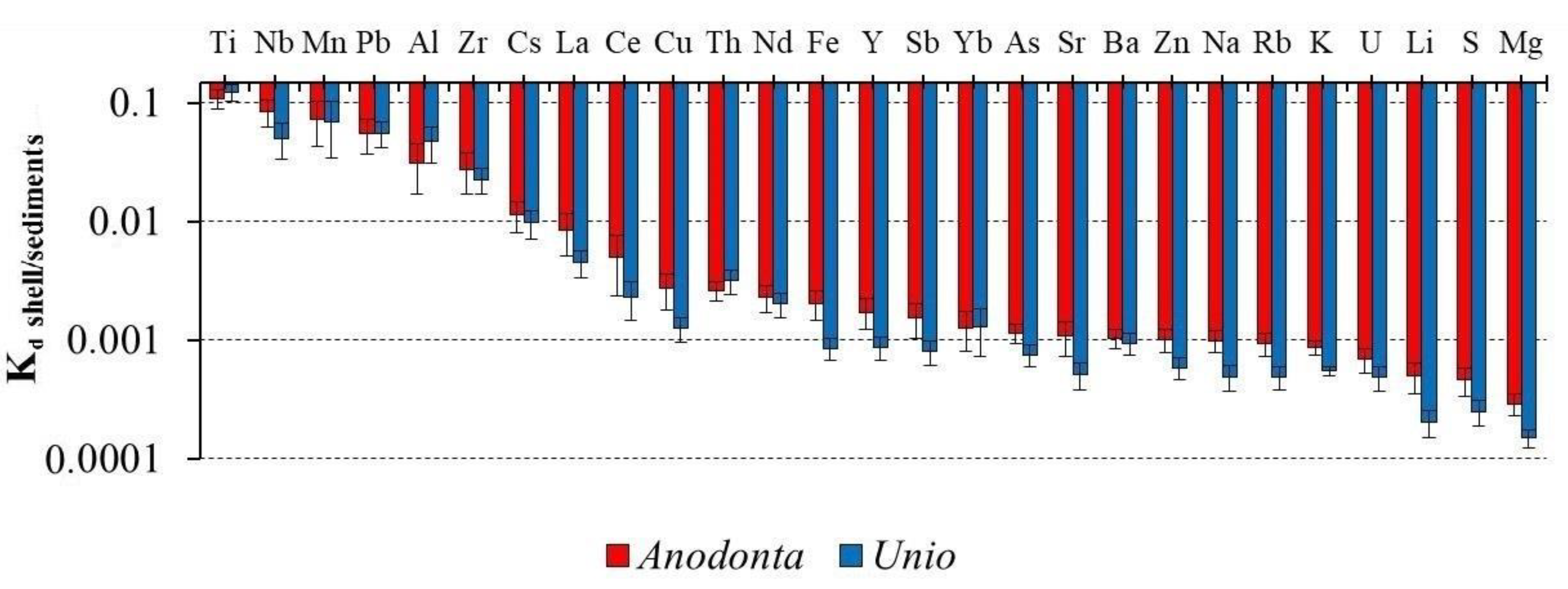
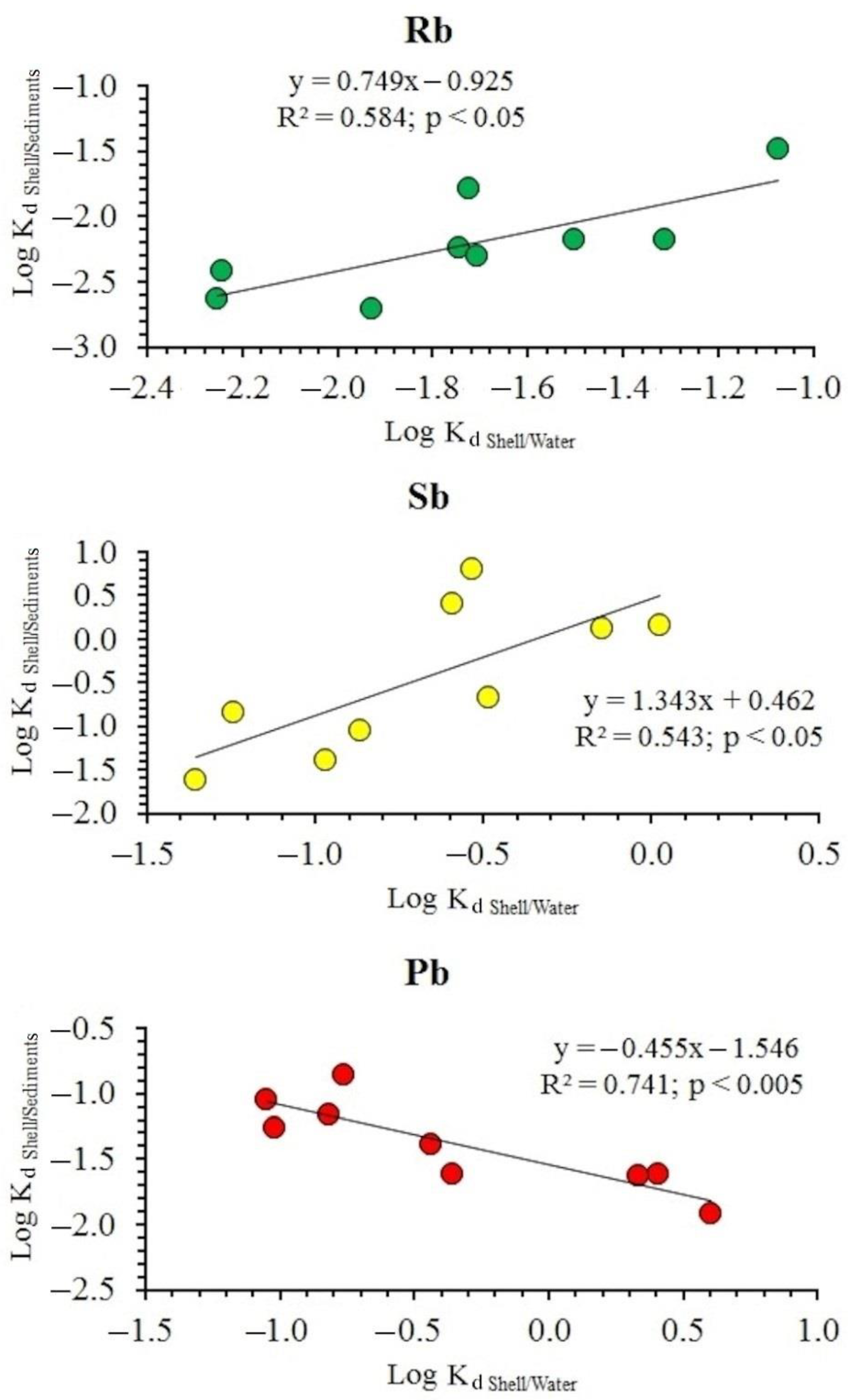
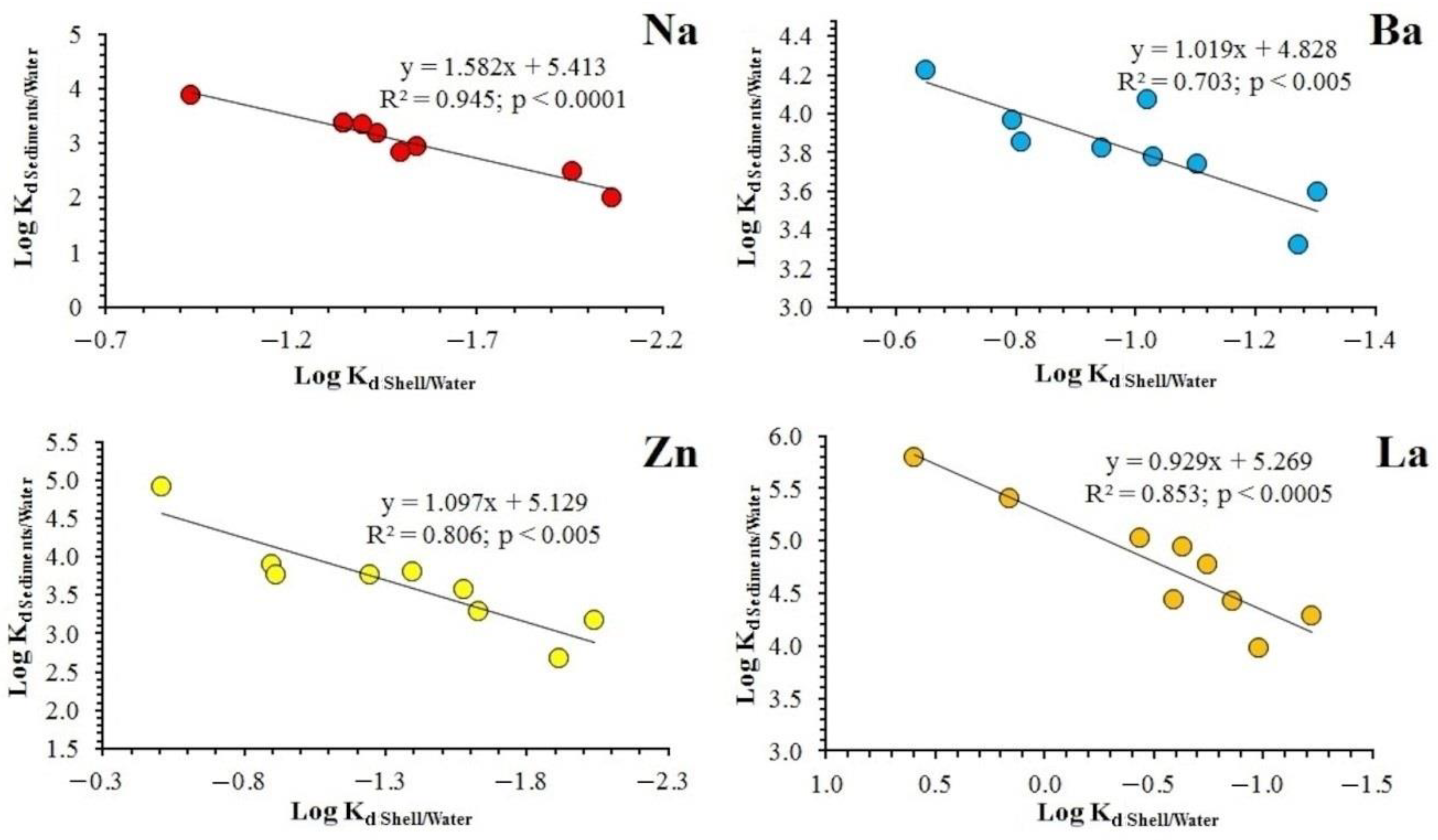
3.4. Element Distribution Coefficients between the Shell and the Environment
4. Discussion
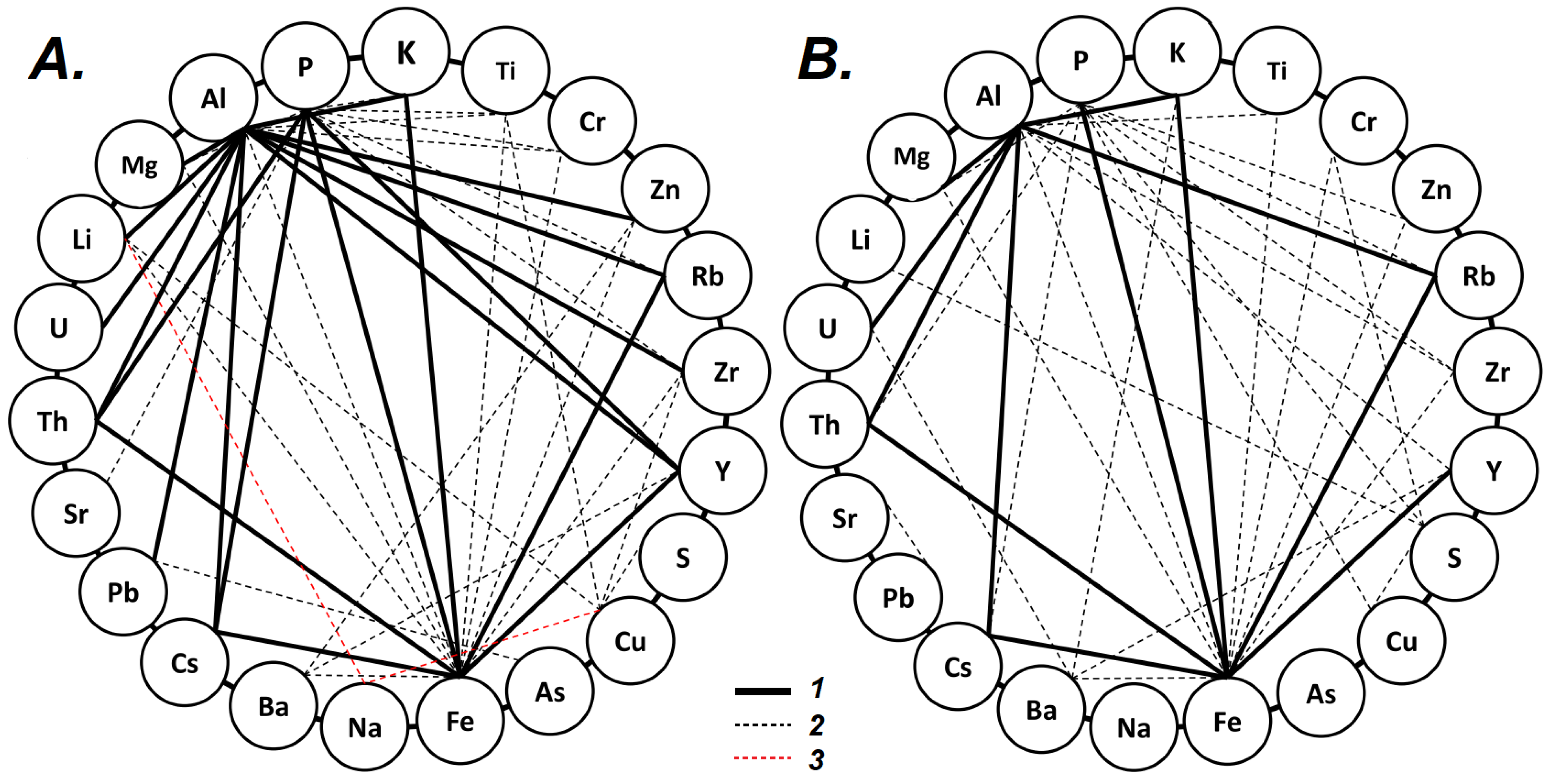
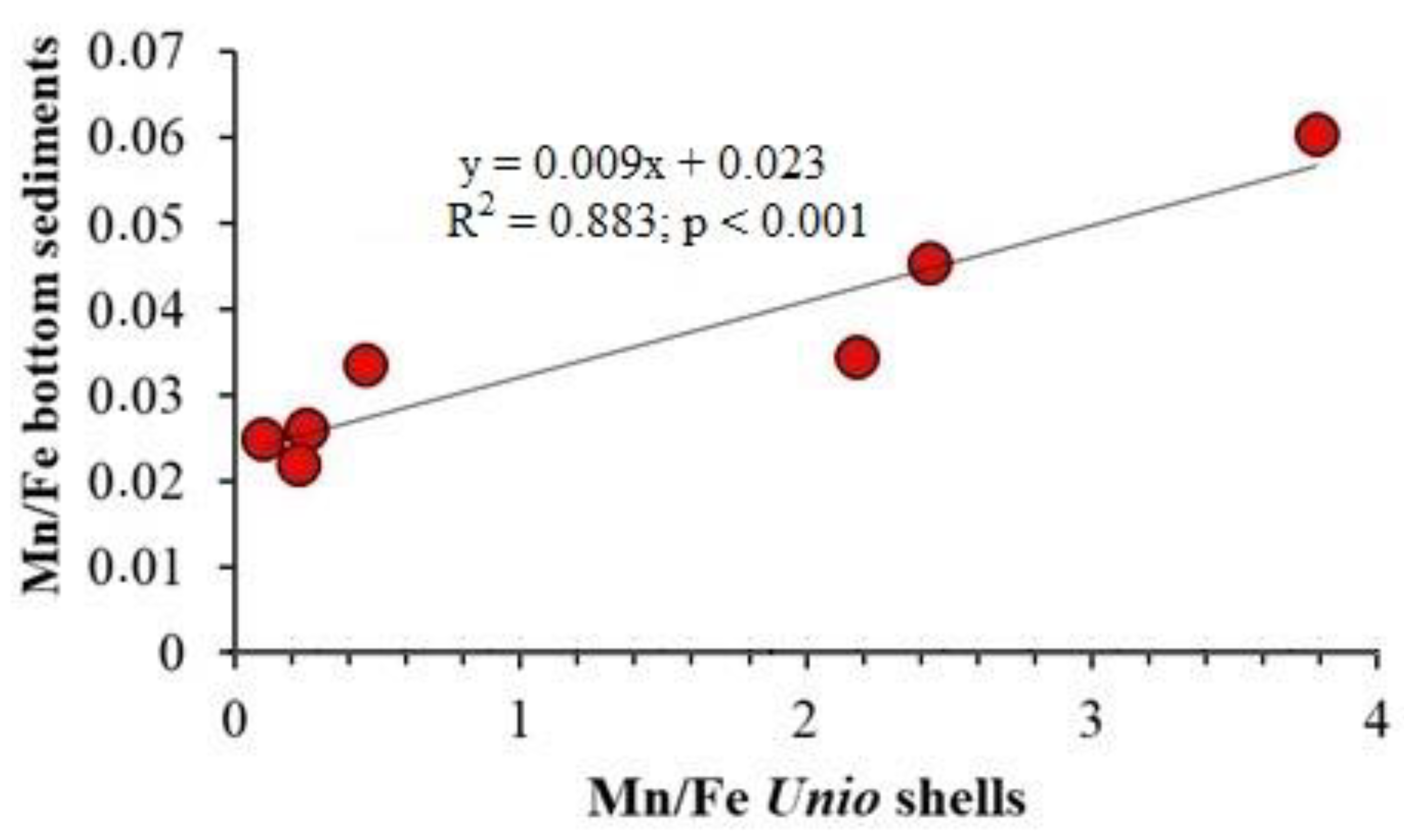
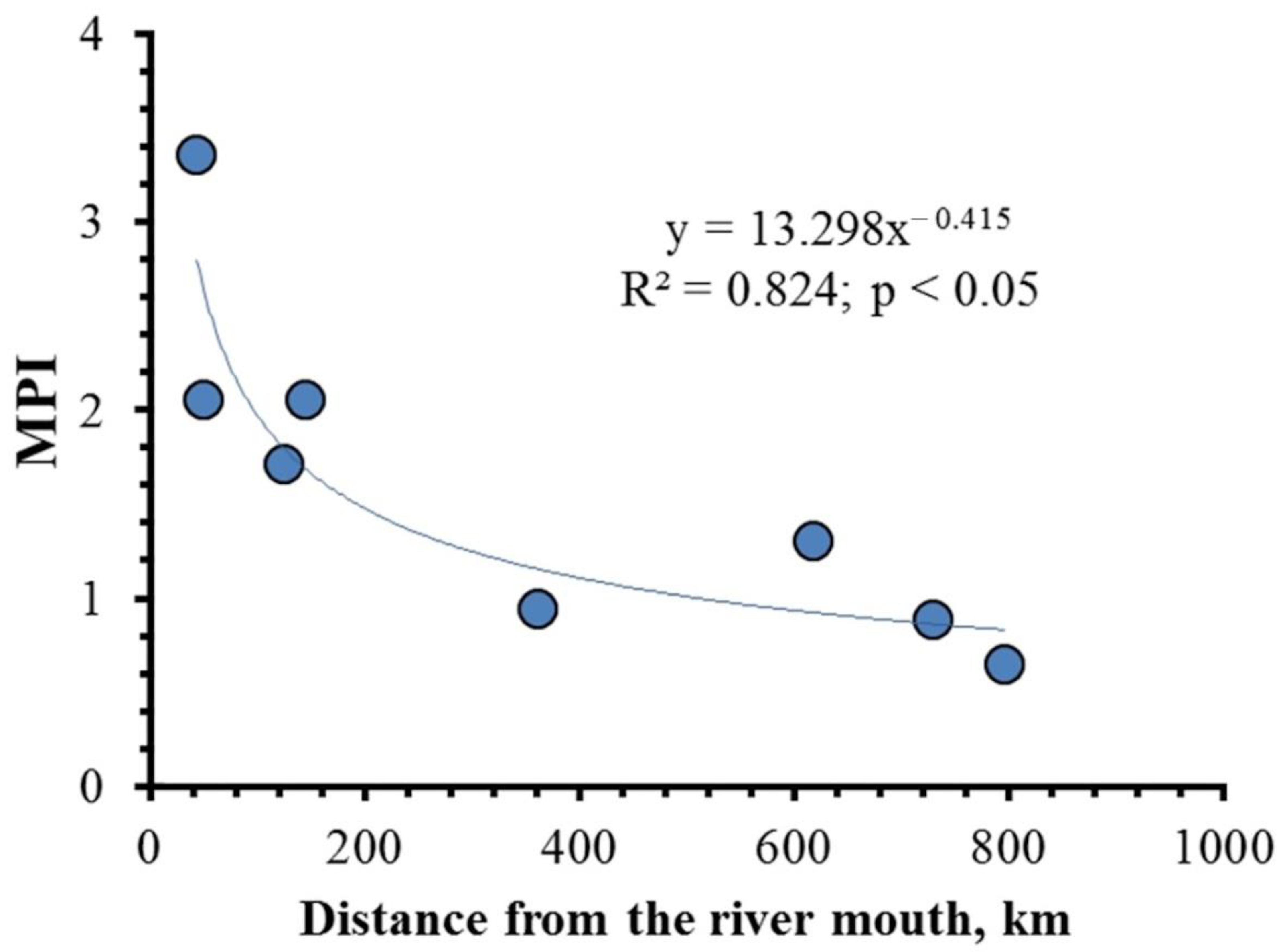
4.1. Element Inter-Correlations and Element Ratios as Indicators of Possible Mechanisms and Environmental Conditions
4.2. Relationship between the Content of Chemical Elements in Shells, Water and Sediments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schöne, B.R.; Dunca, E.; Mutvei, H.; Norlund, U. A 217-year record of summer air temperature reconstructed from freshwater pearl mussels (M. margaritifera, Sweden). Quat. Sci. Rev. 2004, 23, 1803–1816. [Google Scholar] [CrossRef]
- Schöne, B.R.; Page, N.A.; Rodland, D.L.; Fiebig, J.; Baier, S.; Helama, S.O.; Oschmann, W. ENSO-coupled precipitation records (1959–2004) based on shells of freshwater bivalve mollusks (Margaritifera falcata) from British Columbia. Int. J. Earth Sci. 2007, 96, 525–540. [Google Scholar] [CrossRef]
- Van der Putten, E.; Dehairs, F.; Keppens, E.; Baeyens, W. High resolution distribution of trace elements in the calcite shell layer of modern Mytilus edulis: Environmental and biological controls. Geochim. Cosmochim. Acta. 2000, 64, 997–1011. [Google Scholar] [CrossRef]
- Roditi, H.; Fisher, N.; Sañudo-Wilhelmy, S. Uptake of dissolved organic carbon and trace elements by zebra mussels. Nature 2000, 407, 78–80. [Google Scholar] [CrossRef]
- Dunca, E.; Mutvei, H.; Schöne, B.R. Freshwater bivalves tell of past climates: But how clearly do shells from polluted rivers speak? Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 228, 43–57. [Google Scholar] [CrossRef]
- Bolotov, I.N.; Pokrovsky, O.S.; Auda, Y.; Bespalaya, J.V.; Vikhrev, I.V.; Gofarov, M.Y.; Lyubas, A.A.; Viers, J.; Zouiten, C. Trace element composition of freshwater pearl mussels Margaritifera spp. across Eurasia: Testing the effect of species and geographic location. Chem. Geol. 2015, 402, 125–139. [Google Scholar] [CrossRef]
- Lyubas, A.A.; Kabakov, M.B.; Kriauciunas, V.V.; Obada, T.F.; Nicoara, I.N.; Tomilova, A.A. Freshwater mollusks from Neogene-Quaternary Dniester and Prut riverine deposits as indicator paleoenvironments: Chemical composition of shells and its palaeoecological interpretation. Arct. Environ. Res. 2019, 19, 35–42. [Google Scholar] [CrossRef]
- Van Plantinga, A.A.; Grossman, E.L. Trace elements in mussel shells from the Brazos River, Texas: Environmental and biological control. Biogeosci. Discuss. 2019, 1–27. [Google Scholar] [CrossRef]
- Aljahdali, M.O.; Alhassan, A.B. Spatial variation of metallic contamination and its ecological risk in sediment and freshwater mollusk: Melanoides tuberculata (Müller, 1774) (Gastropoda: Thiaridae). Water 2020, 12, 206. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Walliser, E.O.; Mertz-Kraus, R.; Schöne, B.R. Unionid shells (Hyriopsis cumingii) record manganese cycling at the sediment–water interface in a shallow eutrophic lake in China (Lake Taihu). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 484, 97–108. [Google Scholar] [CrossRef]
- Naeher, S.; Gilli, A.; North, R.P.; Hamann, Y.; Schubert, C.J. Tracing bottom water oxygenation with sedimentary Mn/Fe ratios in Lake Zurich, Switzerland. Chem. Geol. 2013, 352, 125–133. [Google Scholar] [CrossRef]
- Ravera, O.; Cenci, R.; Beone, G.M.; Dantas, M.; Lodigiani, P. Trace element concentrations in freshwater mussels and macrophytes as related to those in their environment. J. Limnol. 2003, 62, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, T.; Suzuki, M.; Komoto, Y.; Shirai, K.; Yamazaki, A. Daily and annual shell growth in a long-lived freshwater bivalve as a proxy for winter snowpack. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2021, 569, 110346. [Google Scholar] [CrossRef]
- Zhadin, V.I. Mollusks of fresh and brackish waters of the USSR. Keys Fauna USSR Publ. Zool. Inst. USSR Acad. Sci. 1952, 46, 1–376. (In Russian) [Google Scholar]
- Klishko, O.K.; Lopes-Lima, M.; Bogan, A.E.; Matafonov, D.V.; Froufe, E. Morphological and molecular analyses of Anodontinae species (Bivalvia, Unionidae) of Lake Baikal and Transbaikalia. PLoS ONE 2018, 13, e0194944. [Google Scholar] [CrossRef]
- Bolotov, I.N.; Kondakov, A.V.; Konopleva, E.S.; Vikhrev, I.V.; Aksenova, O.V.; Aksenov, A.S.; Bespalaya, Y.V.; Borovskoy, A.V.; Danilov, P.P.; Dvoryankin, G.A.; et al. Integrative taxonomy, biogeography and conservation of freshwater mussels (Unionidae) in Russia. Sci. Rep. 2020, 10, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Konopleva, E.S.; Bolotov, I.N.; Vikhrev, I.V.; Gofarov, M.Y.; Kondakov, A.V. An integrative approach underscores the taxonomic status of Lamellidens exolescens, a freshwater mussel from the Oriental tropics (Bivalvia: Unionidae). System. Biodivers. 2017, 15, 204–217. [Google Scholar] [CrossRef]
- Konopleva, E.S.; Pfeiffer, J.M.; Vikhrev, I.V.; Kondakov, A.V.; Gofarov, M.Y.; Aksenova, O.V.; Lunn, Z.; Chan, N.; Bolotov, I.N. A new genus and two new species of freshwater mussels (Unionidae) from western Indochina. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Karandashev, V.K.; Turanov, A.N.; Orlova, T.A.; Lezhnev, A.E.; Nosenko, S.V.; Zolotareva, N.I.; Moskvina, I.R. Application of mass-spectrometry with inductively coupled plasma to elemental analysis of environmental objects. Ind. Lab. Diagn. Mater. 2007, 73, 12–22. [Google Scholar]
- Karandashev, V.K.; Leikin, A.Y.; Khvostikov, V.A.; Kutseva, N.K.; Pirogova, S.V. Water analysis by inductively coupled plasma mass spectrometry. Inorg. Mater. 2016, 52, 1391–1404. [Google Scholar] [CrossRef]
- Usero, J.; González, E.; Regalado, L.; Gracia, I. Trace metals in the bivalve mollusc Chamelea gallina from the Atlantic coast of Southern Spain. Baseline 1996, 32, 305–310. [Google Scholar] [CrossRef]
- Sedeño-Díaz, J.E.; López-López, E.; Mendoza-Martínez, E.; Rodríguez-Romero, A.J.; Morales-García, S.S. Distribution coefficient and metal pollution index in water and sediments: Proposal of a new index for ecological risk assessment of metals. Water 2020, 12, 29. [Google Scholar] [CrossRef] [Green Version]
- Gural’-Sverdlova, N.V.; Gural’, R.I. Mollusks of the Unionidae Family in the Funds of the State Museum of Natural History of the National Academy of Sciences of Ukraine, Their Conchological Variability and Diagnostic Features. 2015. Available online: http://www.pip-mollusca.org/page/epubl/unionidae.php (accessed on 25 March 2021).
- Takacs, I.; Murthy, S.; Smith, S.; McGrath, M. Chemical phosphorus removal to extremely low levels: Experience of two plants in the Washington, DC area. Water Sci. Technol. 2006, 53, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-T.; Hesterberg, D. Phosphate Bonding on Noncrystalline Al/Fe-Hydroxide Coprecipitates. Environ. Sci. Technol. 2011, 45, 6283–6289. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.Y.; Hsu, L.C.; Chan, Y.T.; Cho, Y.L.; Tsao, F.Y.; Tzou, Y.M.; Hsieh, Y.C.; Liu, Y.T. Phosphate removal in relation to structural development of humic acid-iron coprecipitates. Sci. Rep. 2018, 8, 10363. [Google Scholar] [CrossRef]
- Dzhamalov, R.G.; Mironenko, A.A.; Myagkova, K.G.; Reshetnyak, O.S.; Safronova, T.I. Space–time analysis of hydrochemical composition and pollution of water in the Severnaya Dvina Basin. Water Resour. 2019, 46, 188–198. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Viers, J.; Shirokova, L.S.; Shevchenko, V.P.; Fillipov, A.S.; Dupre, B. Dissolved, suspended, and colloidal fluxes of organic carbon, major and trace elements in Severnaya Dvina River and its tributary. Chem. Geol. 2010, 273, 136–149. [Google Scholar] [CrossRef]
- Malov, A.I. The use of the geological benchmarks to assess the residence time of groundwater in the aquifer using uranium isotopes on the example of the Northern Dvina basin. Lithol. Mineral Resour. 2013, 48, 254–265. [Google Scholar] [CrossRef]
- Chupakov, A.V.; Pokrovsky, O.S.; Moreva, O.Y.; Shirokova, L.S.; Neverova, N.V.; Chupakova, A.A.; Kotova, E.I.; Vorobyeva, T.Y. High resolution multi-annual riverine fluxes of organic carbon, nutrient and trace element from the largest European Arctic river, Severnaya Dvina. Chem. Geol. 2020, 538, 119491. [Google Scholar] [CrossRef]
- Kokryatskaya, N.M.; Volkov, I.I.; Demidova, T.P.; Murzina, T.S. Sulfur compounds in bottom sediments of fresh-water basins (mouth of the Severnaya Dvina River and Rybinsk Water Reservoir). Lithol. Miner. Resour. 2003, 38, 552–563. [Google Scholar] [CrossRef]
- Usero, J.; Gonzalez-Regalado, E.; Gracia, I. Trace metals in the bivalve molluscs Ruditapes decussatus and Ruditapes philippinarum from the Atlantic coast of southern Spain. Environ. Int. 1997, 23, 291–298. [Google Scholar] [CrossRef]
- Stankovic, S.; Jovic, M.; Mihajlovic, M.L.; Joksimović, D.; Tanaskovski, B. Metal pollution determined by pollution indices for sea grass P. oceanica and surface sediments. Arch. Biol. Sci. 2015, 67, 91–101. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Shirokova, L.S.; Viers, J.; Gordeev, V.V.; Shevchenko, V.P.; Chupakov, A.V.; Vorobieva, T.Y.; Candaudap, F.; Causserand, C.; Lanzanova, A.; et al. Fate of colloids during estuarine mixing in the Arctic. Ocean Sci. 2014, 10, 107–125. [Google Scholar] [CrossRef] [Green Version]
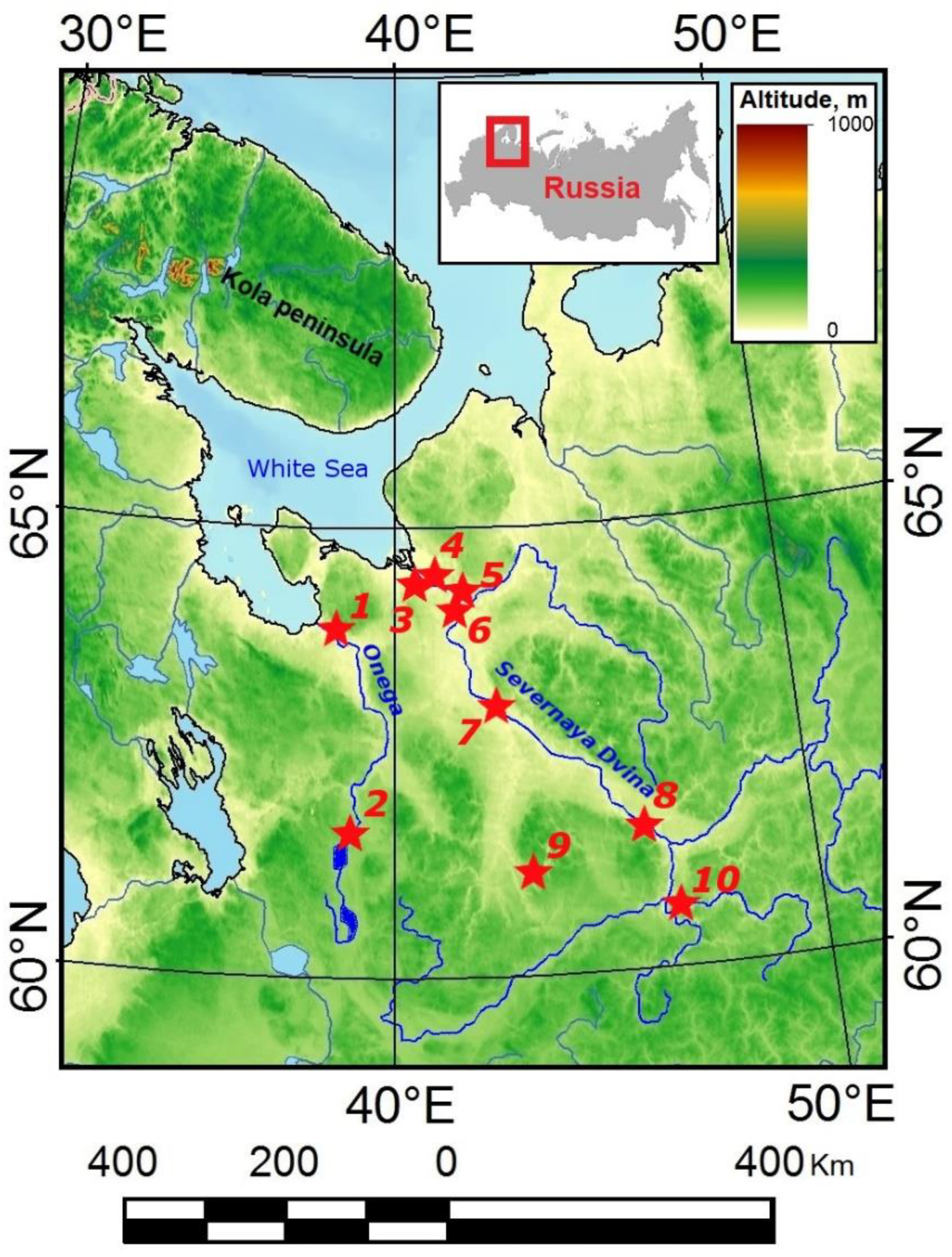


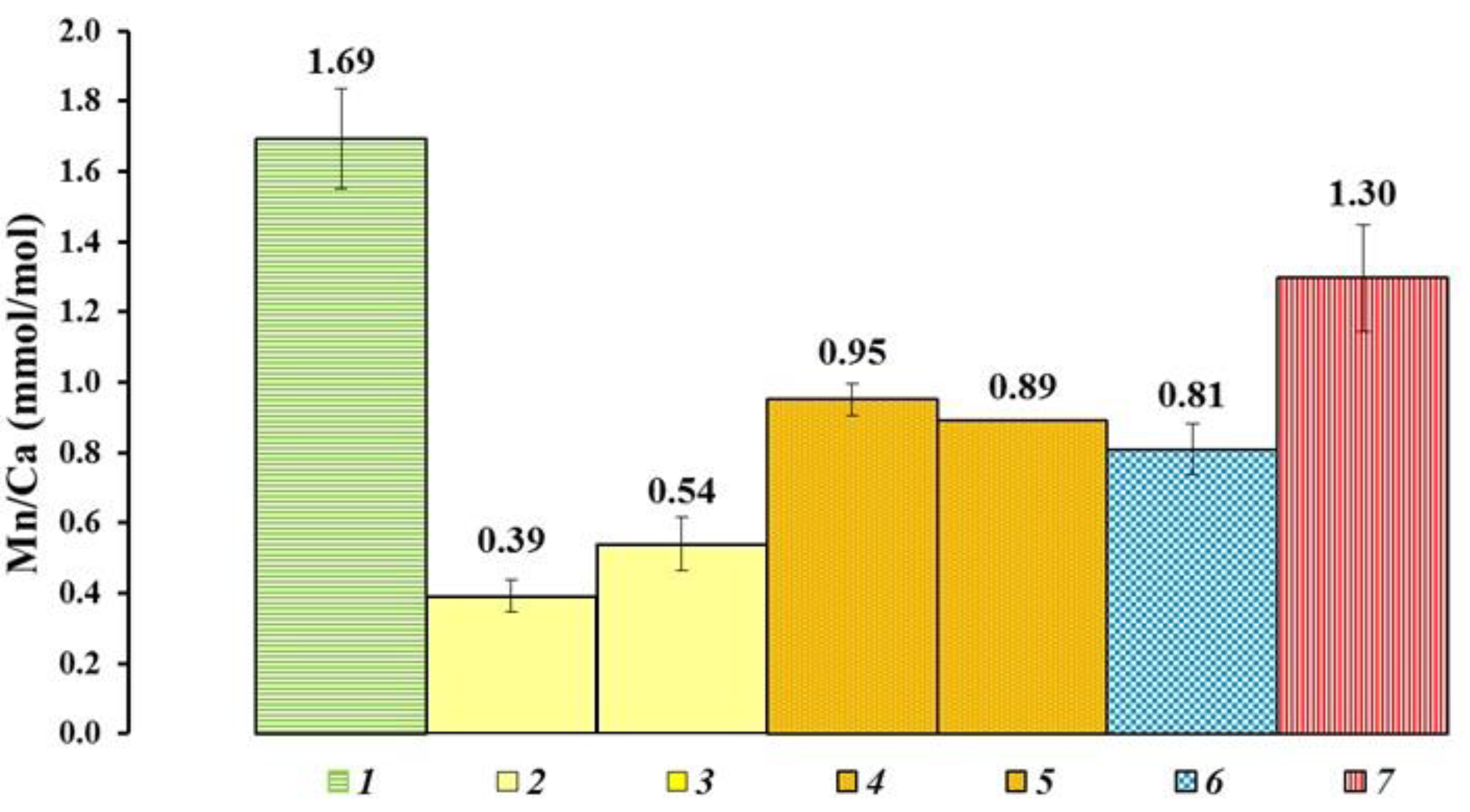
| № | Locality | River Basin | Coordinates | Bottom Sediments and Short Site Description | Number of Samples |
|---|---|---|---|---|---|
| 1 | Porog village, right bank before the rifts | Onega River | 63°49′44.5″ N 38°28′32.3″ E | Silted area of the bottom before the river bend, bottom sediments—sandy loam | Shells—4 Water—1 Bottom Sediment—1 |
| 2 | Kuftyrikha boundary near Kargopol town | Onega River | 61°32′46.9″ N 38°59′54.4″ E | Coarse sand, depth 1–1.5 m, slight current, bottom sediments—sand with stones. | Shells—4 Water—1 Bottom Sediment—1 |
| 3 | Perkhachevsky settlement, Isakogorka anabranch | Severnaya Dvina River | 64°27′55.2″ N 40°29′22.2″ E | Silty bottom, smell of hydrogen sulfide | Shells—4 Water—1 Bottom Sediment—1 |
| 4 | Krasnoflotsky island, beach in the southwest of the island | Severnaya Dvina River | 64°30′07″ N 40°37′02″ E | Willow beach, bottom sediments—medium-grained sand | Shells—4 Water—1 Bottom Sediment—1 |
| 5 | Kholmogory village | Severnaya Dvina River | 64°14′39.3″ N 41°36′29.9″ E | The bottom is silted in places. The depth increases rapidly near the coast, bottom sediments—sand and silt | Shells—4 Water—1 Bottom Sediment—1 |
| 6 | Marilovo village | Severnaya Dvina River | 64°08′29.0″ N 41°45′47.0″ E | Sandy coast along the village, bottom sediments—rocky-sandy bottom with clay | Shells—4 Water—0 Bottom Sediment—0 |
| 7 | Bereznik village, western side of the beach | Severnaya Dvina River | 62°50′38.0″ N 42°48′01.1″ E | Beach, bottom sediments—fine-grained sand | Shells—3 Water—1 Bottom Sediment – 1 |
| 8 | Krasnoborsk village, west of Zeleny Island | Severnaya Dvina River | 61°34′58.0″ N 45°50′42.8″ E | Beach northwest of the village, bottom sediments—medium-grained sand | Shells—4 Water—1 Bottom Sediment—1 |
| 9 | Shangaly village Ust’ya river, beach at the suspension bridge | Severnaya Dvina River | 61°07′52.3″ N 43°20′46.0″ E | Depth 1.2–1.5 m, straight section of the river, beach, bottom sediments—medium-grained sand pebbles stones. | Shells—4 Water—1 Bottom Sediment—1 |
| 10 | Yug river, beach in the village Ust-Alekseevo, | Severnaya Dvina River | 60°27′54.1″ N 46°30′21.9″ E | The flow rate is low, bottom sediments—bottom sediments—medium-grained sand. | Shells—4 Water—1 Bottom Sediment—1 |
| Element | Anodonta, ppm Mean ± SE (n = 18) | Unio, ppm Mean ± SE (n = 21) | Statistically Significant Differences | ||
|---|---|---|---|---|---|
| by Genera | by Basins | by Localities | |||
| Li | 0.28 ± 0.04 | 0.23 ± 0.02 | × | ✓ | ✓ |
| Na | 2340 ± 30 | 2300 ± 27 | × | ✓ | ✓ |
| Mg | 138 ± 18 | 87.6 ± 9.03 | ✓ | × | ✓ |
| Al | 304 ± 47 | 174 ± 30 | ✓ | × | ✓ |
| P | 246 ± 89 | 171 ± 62 | × | ✓ | ✓ |
| K | 129 ± 22 | 76.8 ± 12.1 | × | × | ✓ |
| Ti | 44 ± 6.9 | 31.1 ± 3.62 | × | × | ✓ |
| Cr | 1.89 ± 0.3 | 1.15 ± 0.19 | × | × | × |
| Mn | 680 ± 79 | 423 ± 38 | ✓ | × | ✓ |
| Fe | 1580 ± 653 | 1030 ± 493 | × | × | ✓ |
| Cu | 3.32 ± 0.28 | 2.93 ± 0.23 | × | × | × |
| Zn | 3.47 ± 0.60 | 3.26 ± 0.53 | × | × | ✓ |
| As | 2.49 ± 0.81 | 2.92 ± 1.36 | × | × | × |
| Rb | 0.46 ± 0.10 | 0.22 ± 0.05 | × | × | ✓ |
| Sr | 635 ± 48 | 713 ± 68 | × | ✓ | ✓ |
| Zr | 1.86 ± 0.41 | 0.99 ± 0.19 | × | × | ✓ |
| Y | 0.35 ± 0.08 | 0.22 ± 0.06 | × | ✓ | ✓ |
| Nb | 0.095 ± 0.019 | 0.063 ± 0.012 | × | × | ✓ |
| S | 314 ± 36 | 301 ± 25 | × | × | ✓ |
| Sb | 0.12 ± 0.02 | 0.14 ± 0.02 | × | × | ✓ |
| Cs | 0.022 ± 0.005 | 0.012 ± 0.002 | × | × | ✓ |
| Ba | 64.6 ± 10.1 | 43.4 ± 5.3 | ✓ | ✓ | ✓ |
| La | 0.65 ± 0.14 | 0.45 ± 0.11 | × | ✓ | ✓ |
| Ce | 1.2 ± 0.23 | 0.76 ± 0.18 | × | ✓ | ✓ |
| Pr | 0.14 ± 0.03 | 0.04 ± 0.02 | × | ✓ | ✓ |
| Nd | 0.48 ± 0.1 | 0.31 ± 0.07 | × | ✓ | ✓ |
| Sm | 0.094 ± 0.019 | 0.058 ± 0.013 | × | ✓ | ✓ |
| Eu | 0.018 ± 0.004 | 0.012 ± 0.003 | × | ✓ | ✓ |
| Gd | 0.077 ± 0.018 | 0.047 ± 0.013 | × | ✓ | ✓ |
| Dy | 0.069 ± 0.015 | 0.044 ± 0.009 | × | ✓ | ✓ |
| Er | 0.032 ± 0.007 | 0.019 ± 0.005 | × | ✓ | ✓ |
| Yb | 0.03 ± 0.007 | 0.016 ± 0.004 | × | ✓ | ✓ |
| Pb | 0.49 ± 0.03 | 0.46 ± 0.04 | × | × | × |
| Th | 0.062 ± 0.011 | 0.039 ± 0.011 | × | × | ✓ |
| U | 0.030 ± 0.005 | 0.019 ± 0.004 | ✓ | × | ✓ |
| Element | C, ppm Mean ± SE (n = 9) | Element | C, ppm Mean ± SE (n = 9) |
|---|---|---|---|
| B | 42.0 ± 11.7 | Zr | 0.226 ± 0.036 |
| Na | 12000 ± 4340 | Nb | 0.005 ± 0.001 |
| Mg | 11300 ± 1590 | Mo | 0.53 ± 0.12 |
| Al | 70.9 ± 19.1 | Cd | 0.018 ± 0.008 |
| Si | 2180 ± 321 | Sb | 0.07 ± 0.02 |
| S | 10200 ± 2590 | Cs | 0.005 ± 0.001 |
| K | 1320 ± 277 | La | 0.208 ± 0.064 |
| Ca | 42000 ± 5410 | Ce | 0.437 ± 0.15 |
| Ti | 2.12 ± 0.54 | Pr | 0.057 ± 0.017 |
| V | 0.904 ± 0.063 | Nd | 0.234 ± 0.068 |
| Mn | 190 ± 84.9 | Sm | 0.047 ± 0.014 |
| Fe | 449 ± 104 | Eu | 0.0089 ± 0.0025 |
| Co | 0.15 ± 0.04 | Gd | 0.046 ± 0.012 |
| Ni | 0.503 ± 0.118 | Tb | 0.0064 ± 0.0018 |
| Cu | 0.933 ± 0.121 | Dy | 0.0346 ± 0.0090 |
| Zn | 10.3 ± 3.4 | Ho | 0.0069 ± 0.0016 |
| As | 1.3 ± 0.2 | Er | 0.0173 ± 0.0043 |
| Br | 34 ± 11 | Tm | 0.0022 ± 0.0006 |
| Sr | 448 ± 111 | Yb | 0.0142 ± 0.0033 |
| Ba | 59 ± 11 | Lu | 0.0021 ± 0.0005 |
| Pb | 0.192 ± 0.053 | Hf | 0.0055 ± 0.0010 |
| Li | 4.8 ± 0.9 | W | 0.0068 ± 0.0018 |
| Be | 0.009 ± 0.002 | Tl | 0.0029 ± 0.0003 |
| Rb | 1.21 ± 0.15 | Th | 0.0215 ± 0.0043 |
| Y | 0.176 ± 0.047 | U | 0.426 ± 0.122 |
| Element | C, ppm Mean ± SE (n = 9) | Element | C, ppm Mean ± SE (n = 9) |
|---|---|---|---|
| Na | 9130 ± 1520 | Sn | 0.349 ± 0.104 |
| Al | 30200 ± 4260 | Cs | 0.702 ± 0.214 |
| P | 231 ± 51 | Ba | 337 ± 34 |
| S | 1620 ± 1450 | La | 9.79 ± 1.88 |
| K | 8720 ± 910 | Ce | 21.3 ± 3.98 |
| Ca | 16200 ± 665 | Pr | 2.31 ± 0.43 |
| Ti | 1120 ± 257 | Nd | 8.84 ± 1.63 |
| Mn | 362 ± 87 | Sm | 1.64 ± 0.32 |
| Fe | 12100 ± 2980 | Eu | 0.392 ± 0.064 |
| Li | 13.1 ± 6.03 | Gd | 1.45 ± 0.28 |
| Be | 0.786 ± 0.182 | Tb | 0.198 ± 0.041 |
| Sc | 4.00 ± 0.94 | Dy | 1.25 ± 0.25 |
| V | 25.1 ± 6.3 | Ho | 0.234 ± 0.047 |
| Cr | 28.9 ± 5.3 | Er | 0.733 ± 0.160 |
| Co | 4.98 ± 1.12 | Tm | 0.105 ± 0.022 |
| Ni | 16.0 ± 4.3 | Yb | 0.759 ± 0.148 |
| Cu | 9.42 ± 5.22 | Lu | 0.106 ± 0.021 |
| Zn | 34.4 ± 9.1 | Hf | 1.23 ± 0.22 |
| Ga | 5.84 ± 0.83 | Ta | 0.334 ± 0.094 |
| As | 2.71 ± 0.63 | W | 0.298 ± 0.13 |
| Rb | 27.8 ± 2.89 | Tl | 0.135 ± 0.012 |
| Sr | 388 ± 267 | Pb | 8.03 ± 1.59 |
| Y | 6.76 ± 1.52 | Bi | 0.03 ± 0.01 |
| Zr | 49.8 ± 8.8 | Th | 1.99 ± 0.53 |
| Nb | 4.6 ± 1.2 | U | 0.79 ± 0.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyubas, A.A.; Tomilova, A.A.; Chupakov, A.V.; Vikhrev, I.V.; Travina, O.V.; Orlov, A.S.; Zubrii, N.A.; Kondakov, A.V.; Bolotov, I.N.; Pokrovsky, O.S. Iron, Phosphorus and Trace Elements in Mussels’ Shells, Water, and Bottom Sediments from the Severnaya Dvina and the Onega River Basins (Northwestern Russia). Water 2021, 13, 3227. https://doi.org/10.3390/w13223227
Lyubas AA, Tomilova AA, Chupakov AV, Vikhrev IV, Travina OV, Orlov AS, Zubrii NA, Kondakov AV, Bolotov IN, Pokrovsky OS. Iron, Phosphorus and Trace Elements in Mussels’ Shells, Water, and Bottom Sediments from the Severnaya Dvina and the Onega River Basins (Northwestern Russia). Water. 2021; 13(22):3227. https://doi.org/10.3390/w13223227
Chicago/Turabian StyleLyubas, Artem A., Alena A. Tomilova, Artem V. Chupakov, Ilya V. Vikhrev, Oksana V. Travina, Alexander S. Orlov, Natalia A. Zubrii, Alexander V. Kondakov, Ivan N. Bolotov, and Oleg S. Pokrovsky. 2021. "Iron, Phosphorus and Trace Elements in Mussels’ Shells, Water, and Bottom Sediments from the Severnaya Dvina and the Onega River Basins (Northwestern Russia)" Water 13, no. 22: 3227. https://doi.org/10.3390/w13223227
APA StyleLyubas, A. A., Tomilova, A. A., Chupakov, A. V., Vikhrev, I. V., Travina, O. V., Orlov, A. S., Zubrii, N. A., Kondakov, A. V., Bolotov, I. N., & Pokrovsky, O. S. (2021). Iron, Phosphorus and Trace Elements in Mussels’ Shells, Water, and Bottom Sediments from the Severnaya Dvina and the Onega River Basins (Northwestern Russia). Water, 13(22), 3227. https://doi.org/10.3390/w13223227







