Solvothermal Synthesis of ZnO Nanoparticles for Photocatalytic Degradation of Methyl Orange and p-Nitrophenol
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Materials
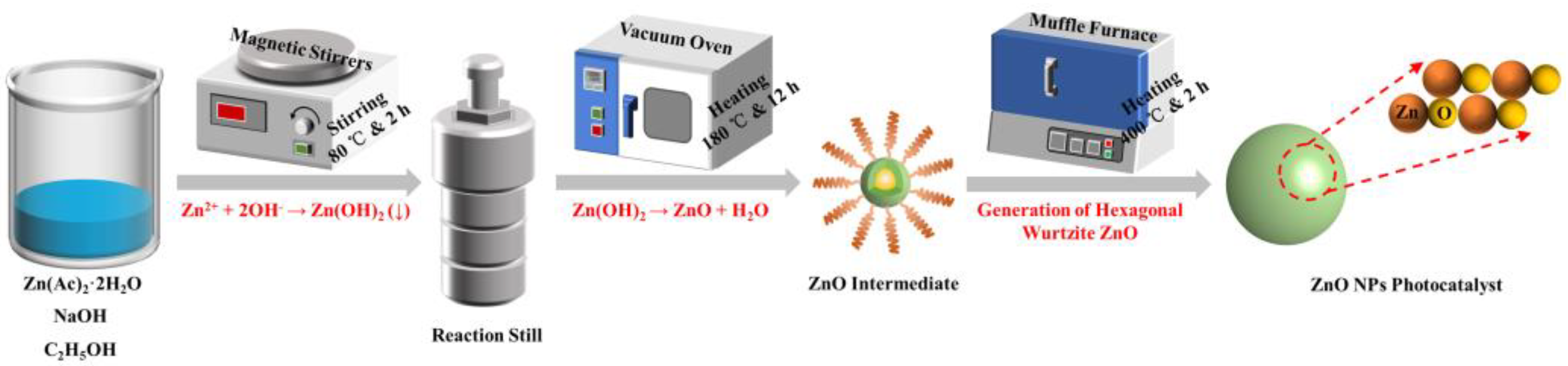
2.2. Preparation of ZnO NPs
2.3. Characterization of ZnO NPs
2.4. Photocatalytic Activity Test
3. Results and Discussion
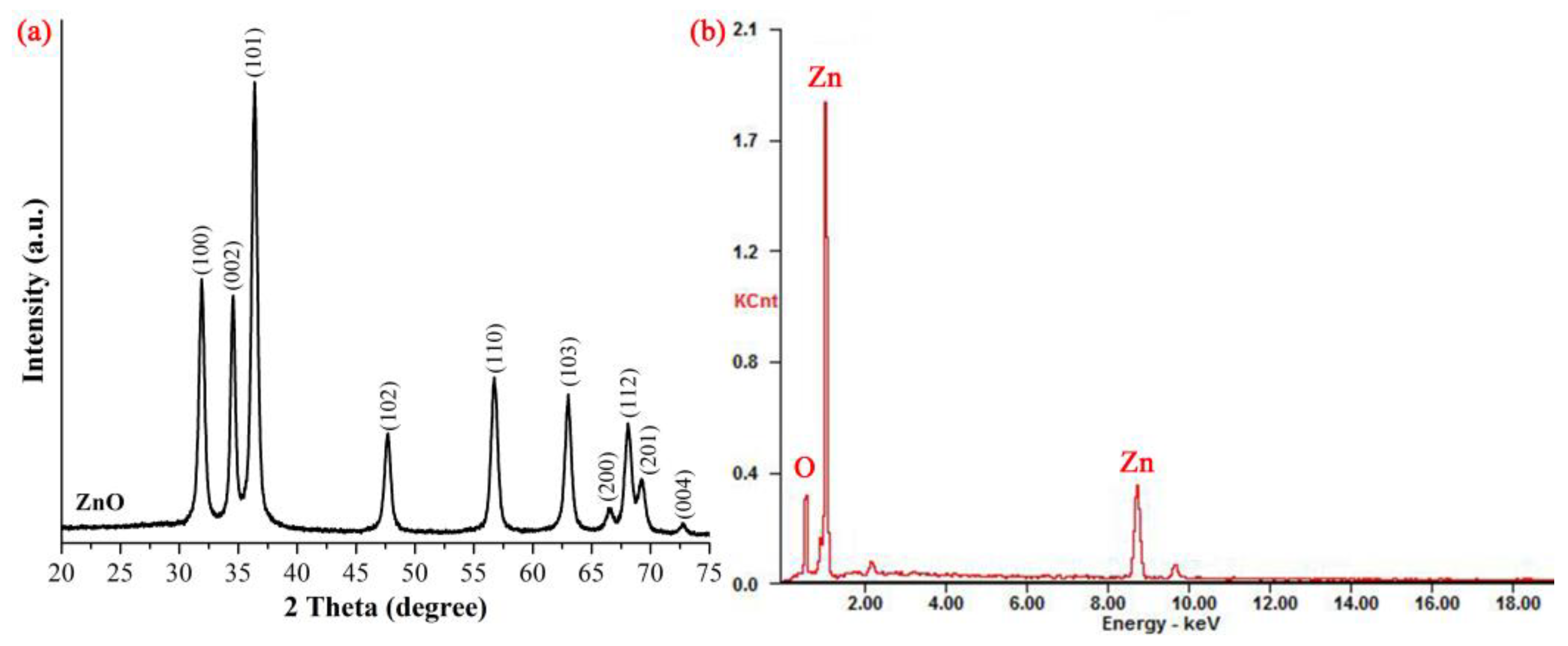
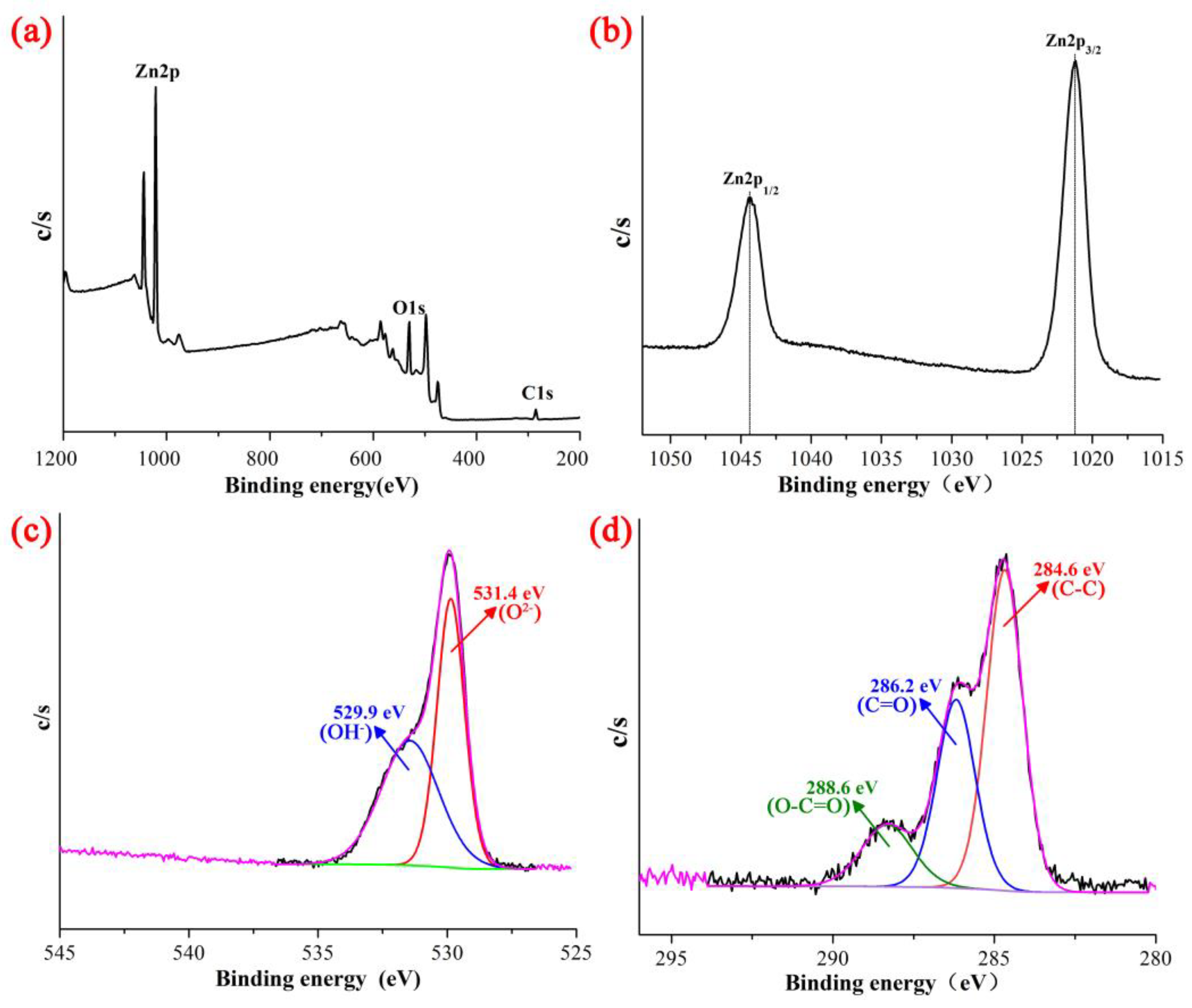
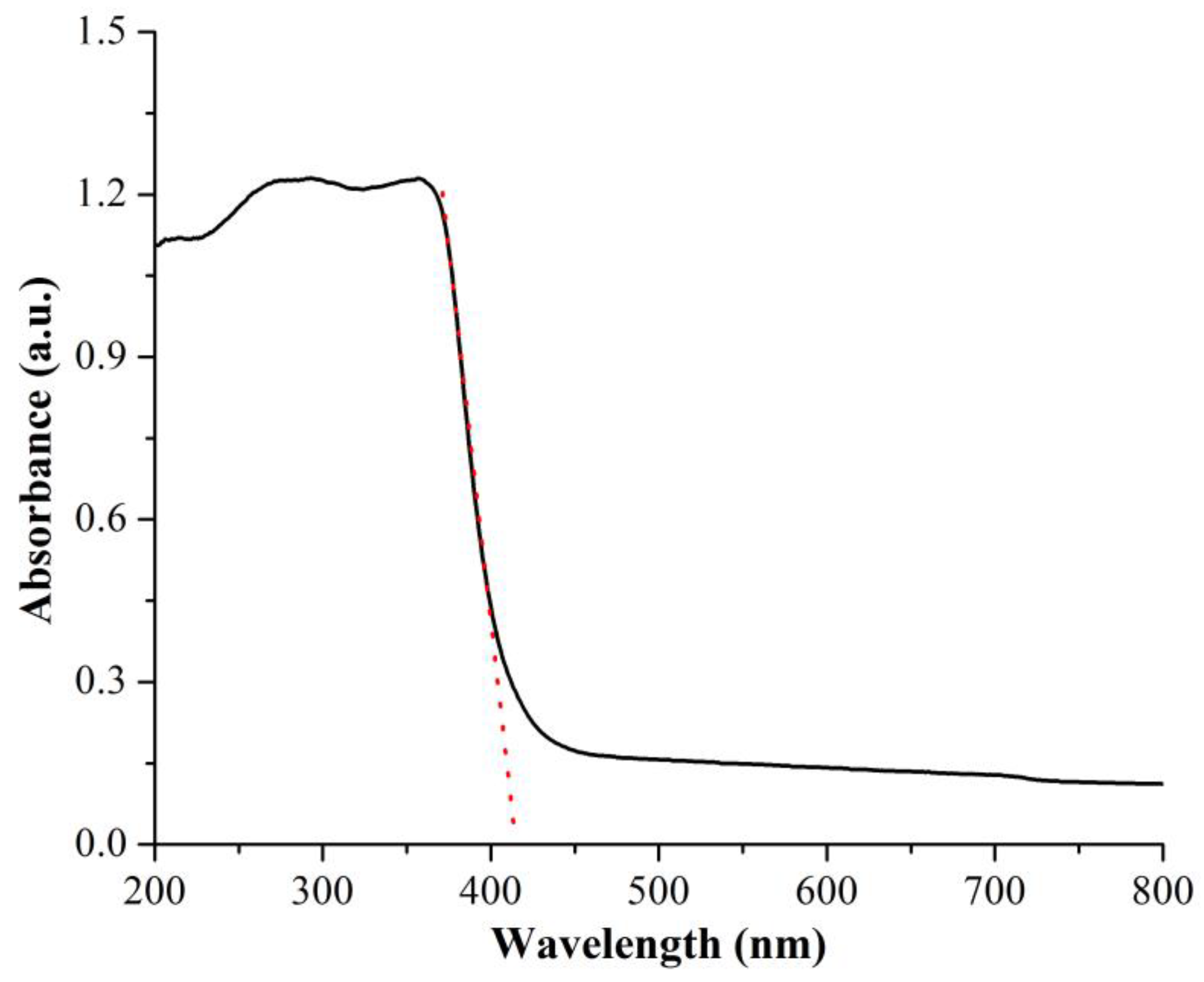
3.1. Characterization of ZnO NPs
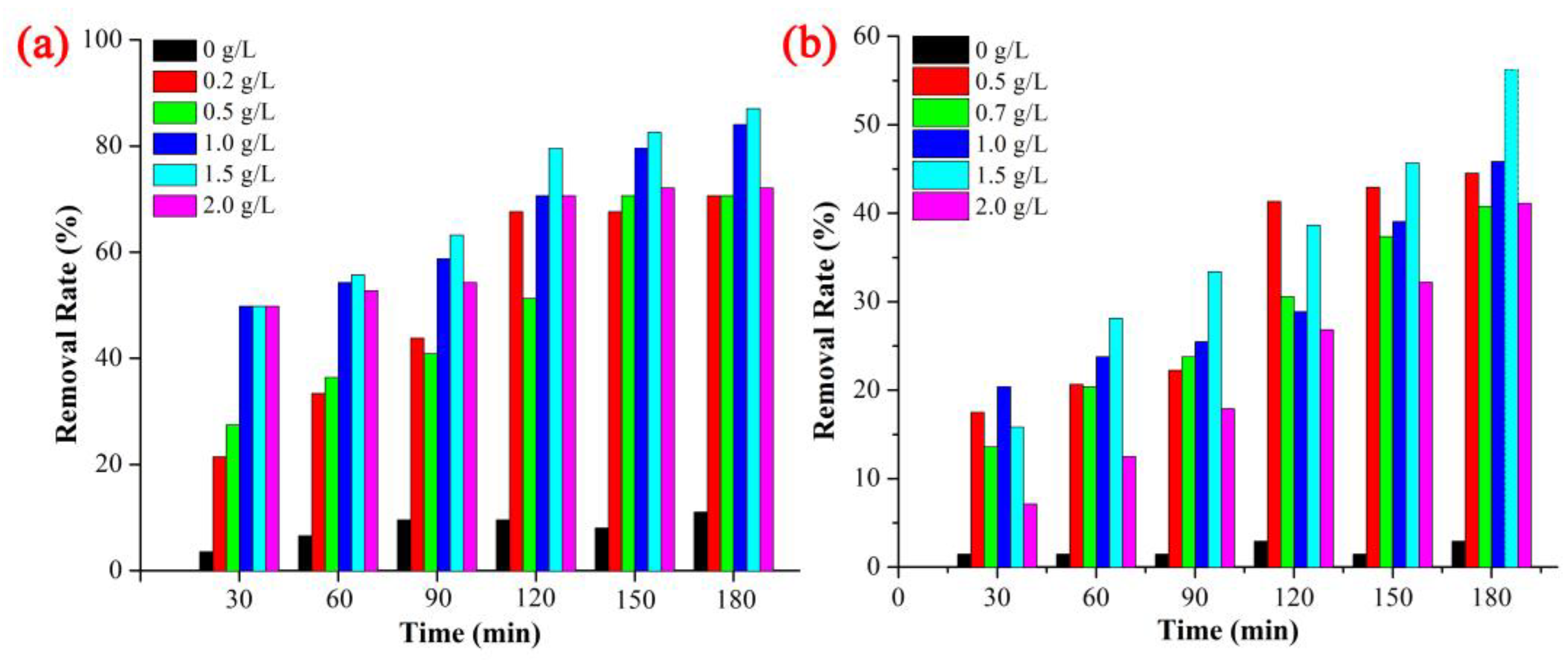
3.2. Photocatalytic Activity and Degradation Kinetics of ZnO NPs
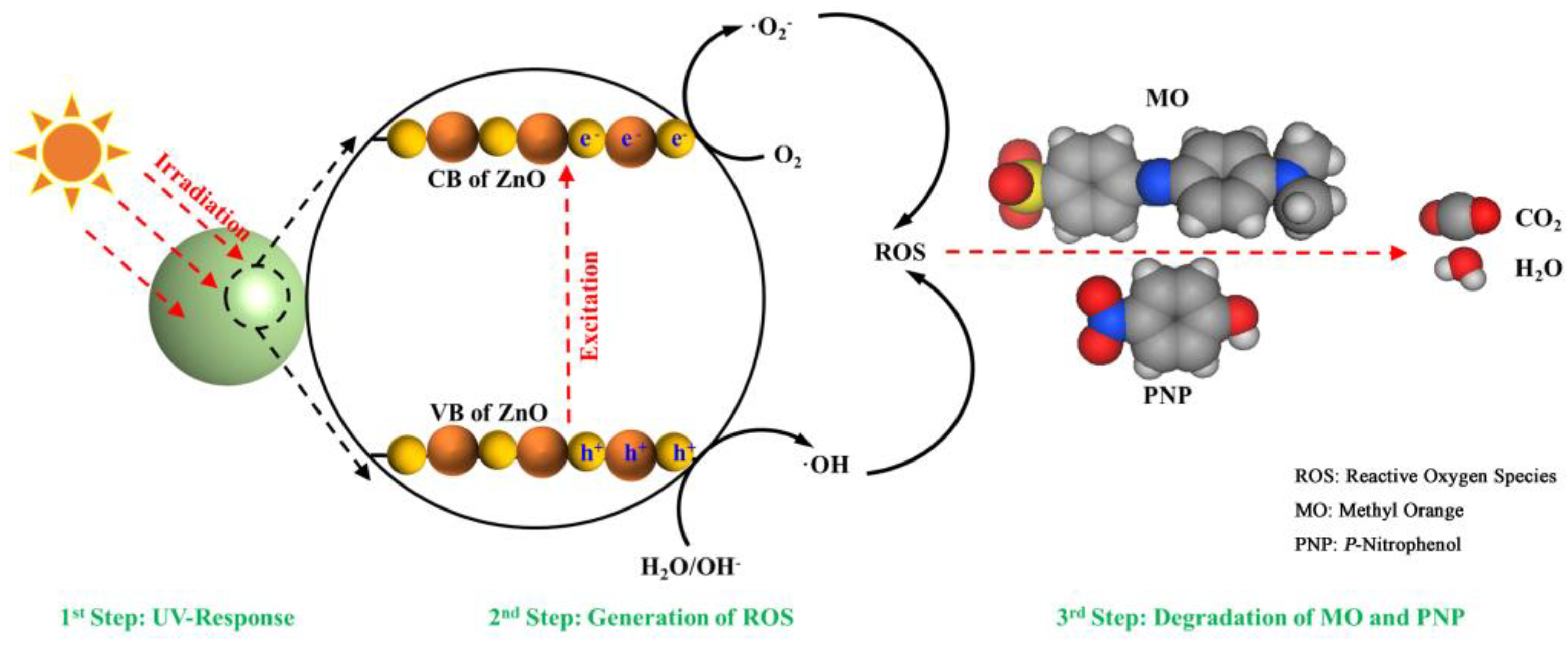
3.3. Photocatalytic Mechanism of ZnO NPs
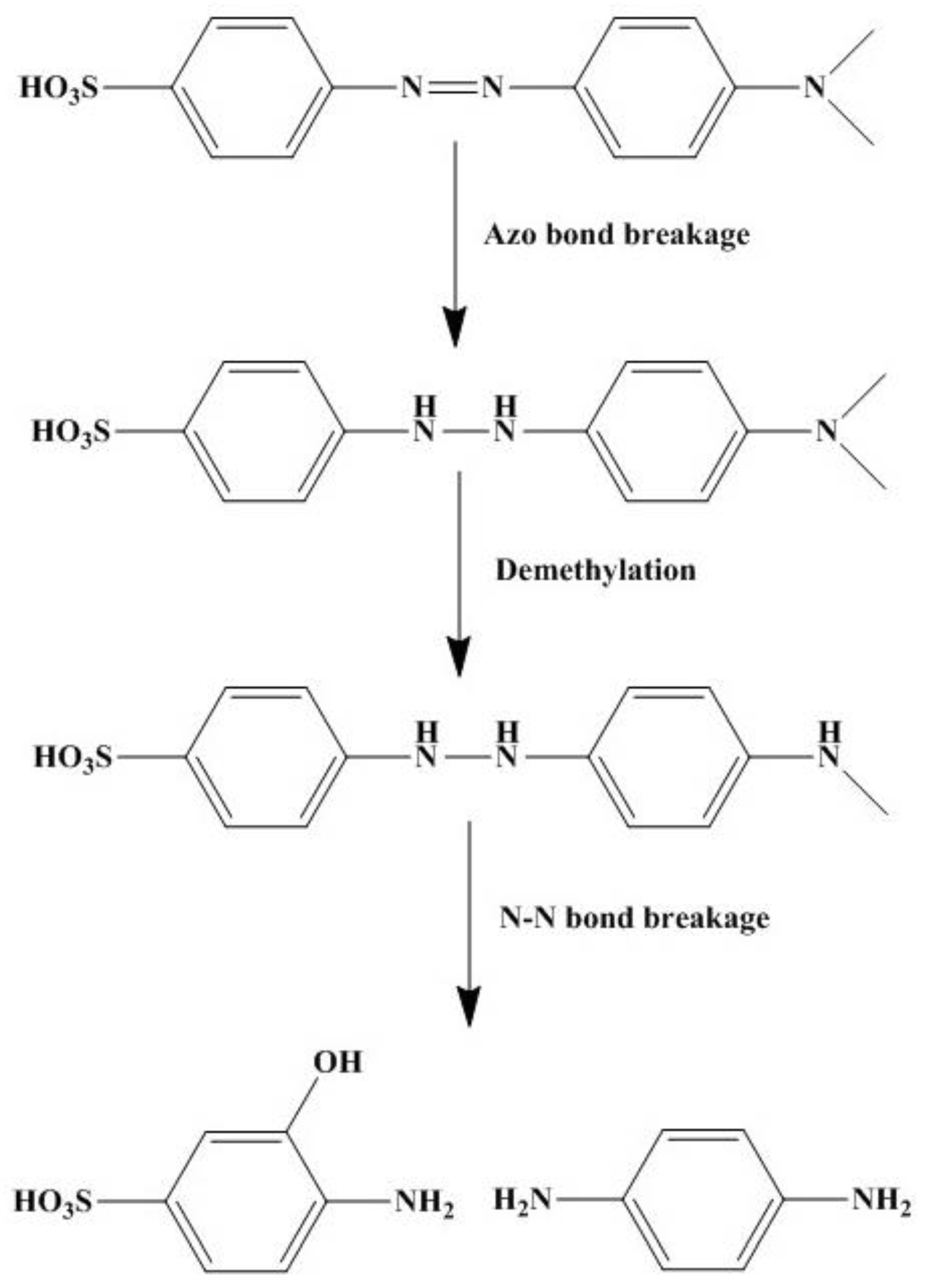
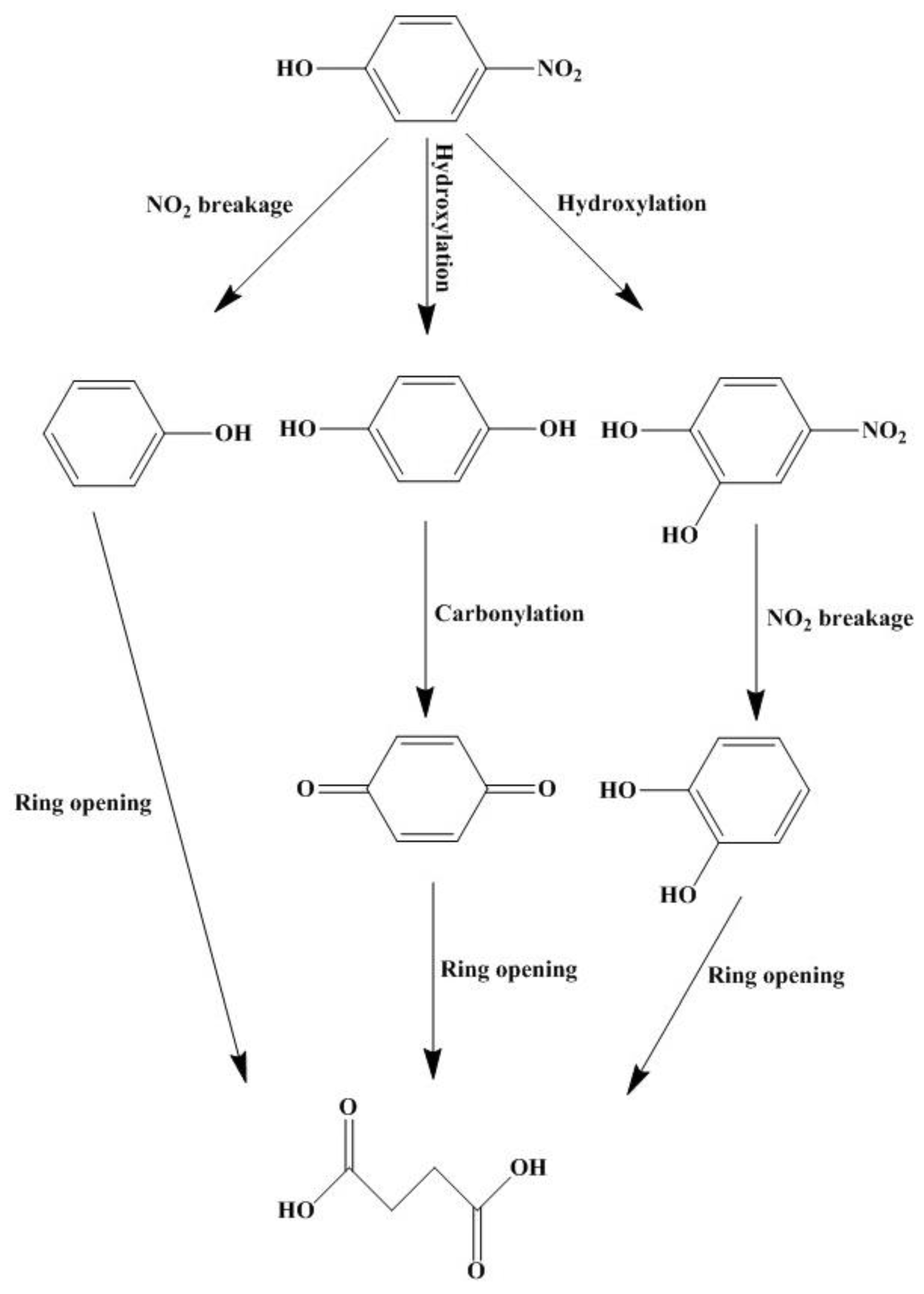
3.4. Degradation Pathways of MO and PNP
3.5. Environmental Impact
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Gole, J.L.; Stout, J.D.; Burda, C.; Lou, Y.B.; Chen, X.B. Highly efficient formation of visible light tunable TiO2-xNx photocatalysts and their transformation at the nanoscale. J. Cheminform. 2004, 108, 1230–1240. [Google Scholar]
- Mukhopadhyay, S.; Das, P.P.; Maity, S.; Ghosh, P.; Devi, P.S. Solution grown ZnO rods: Synthesis, characterization and defect mediated photocatalytic activity. Appl. Catal. B-Environ. 2015, 165, 128–138. [Google Scholar] [CrossRef]
- Dindar, B.; Icli, S. Unusual photoreactivity of zinc oxide irradiated by concentrated sunlight. J. Photochem Photobiol. A 2001, 140, 263–268. [Google Scholar] [CrossRef]
- Yeber, M.C.; Rodriguez, J.; Freer, J.; Baeza, J.; Duran, N.; Mansilla, H.D. Advanced oxidation of a pulp mill bleaching wastewater. Chemosphere 1999, 39, 1679–1688. [Google Scholar] [CrossRef]
- Song, K.Y.; Park, M.K.; Kwon, Y.T.; Lee, H.W.; Chung, W.J.; Lee, W.I. Preparation of transparent particulate MoO3/TiO2 and WO3/TiO2 films and their photocatalytic properties. Chem. Mater. 2001, 13, 2349–2355. [Google Scholar] [CrossRef]
- Chen, H.M.; Chen, C.K.; Chang, Y.C.; Tsai, C.W.; Liu, R.S.; Hu, S.F.; Chang, W.S.; Chen, K.H. Quantum dot monolayer sensitized ZnO nanowire-array photoelectrodes: True efficiency for water splitting. Angew. Chem. Int. Ed. 2010, 49, 5966–5969. [Google Scholar] [CrossRef]
- Chu, H.O.; Wang, Q.; Shi, Y.J.; Song, S.G.; Liu, W.G.; Zhou, S.; Gibson, D.; Alajlani, Y.; Li, C. Structural, optical properties and optical modelling of hydrothermal chemical growth derived ZnO nanowires. T. Nonferr. Metal. Soc. 2020, 30, 191–199. [Google Scholar] [CrossRef]
- Baskoutas, S. Zinc oxide nanostructures: Synthesis and characterization. Materials 2018, 11, 873. [Google Scholar] [CrossRef] [Green Version]
- Luevano-Hipolito, E.; Martinez-de la Cruz, A.; Cuellar, E.L. Performance of ZnO synthesized by sol-gel as photocatalyst in the photooxidation reaction of NO. Environ. Sci. Pollut. Res. 2016, 24, 6361–6371. [Google Scholar] [CrossRef]
- Yu, W.L.; Zhang, J.F.; Peng, T.Y. New insight into the enhanced photocatalytic activity of N-, C- and S-doped ZnO photocatalysts. Appl. Catal. B-Environ. 2016, 181, 220–227. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Hu, Z.B.; Tong, M.X.; Zhang, Q.L.; Tong, C.Q. Preparation and photocatalytic performance of bamboo-charcoal-supported nano-ZnO composites. Mater. Sci. 2018, 24, 49–52. [Google Scholar] [CrossRef] [Green Version]
- Umar, A.; Chauhan, M.S.; Chauhan, S.; Kumar, R.; Kumar, G.; Al-Sayari, S.A.; Hwang, S.W.; Al-Hajry, A. Large-scale synthesis of ZnO balls made of fluffy thin nanosheets by simple solution process: Structural, optical and photocatalytic properties. J. Colloid. Interf. Sci. 2011, 363, 521–528. [Google Scholar] [CrossRef]
- Cheng, P.F.; Wang, Y.L.; Xu, L.P.; Sun, P.; Su, Z.S.; Jin, F.M.; Liu, F.M.; Sun, Y.F.; Lu, G.Y. High specific surface area urchin-like hierarchical ZnO-TiO2 architectures: Hydrothermal synthesis and photocatalytic properties. Mater. Lett. 2016, 175, 52–55. [Google Scholar] [CrossRef]
- Zhang, P.; Li, B.B.; Zhao, Z.B.; Yu, C.; Hu, C.; Wu, S.J.; Qiu, J.S. Furfural-induced hydrothermal synthesis of ZnO@C gemel hexagonal microrods with enhanced photocatalytic activity and stability. ACS Appl. Mater. Interfaces 2014, 6, 8560–8566. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, H.; Ahmadpour, A.; Bamoharram, F.F.; Zebarjad, S.M.; Heravi, M.M.; Tayari, F. Controllable one-step synthesis of ZnO nanostructures using molybdophosphoric acid. Chem. Pap. 2013, 68, 516–524. [Google Scholar] [CrossRef]
- Chen, X.B.; Shen, S.H.; Guo, L.J.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef]
- Tian, L.; Yang, X.F.; Lu, P.; Williams, I.D.; Wang, C.H.; Ou, S.Y.; Liang, C.L.; Wu, M.M. Hollow single-crystal spinel nanocubes: The case of zinc cobalt oxide grown by a unique kirkendall effect. Inorg. Chem. 2008, 47, 5522–5524. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Salleh, W.N.W.; Jaafar, J.; Ismail, A.F. Structural characterization of N-doped anatase-rutile mixed phase TiO2 nanorods assembled microspheres synthesized by simple sol-gel method. J. Sol-Gel. Sci. Technol. 2015, 74, 513–520. [Google Scholar] [CrossRef] [Green Version]
- Akir, S.; Hamdi, A.; Addad, A.; Coffinier, Y.; Boukherroub, R.; Omrani, A.D. Facile synthesis of carbon-ZnO nanocomposite with enhanced visible light photocatalytic performance. Appl. Surf. Sci. 2017, 400, 461–470. [Google Scholar] [CrossRef]
- Osman, H.; Su, Z.; Ma, X.L.; Liu, S.S.; Liu, X.Y.; Abduwayit, D. Synthesis of ZnO/C nanocomposites with enhanced visible light photocatalytic activity. Ceram. Int. 2016, 42, 10237–10241. [Google Scholar] [CrossRef]
- Yang, C.X.; Wang, X.N.; Ji, Y.J.; Ma, T.; Zhang, F.; Wang, Y.Q.; Ci, M.W.; Chen, D.T.; Jiang, A.X.; Wang, W.L. Photocatalytic degradation of methylene blue with ZnO@C nanocomposites: Kinetics, mechanism, and the inhibition effect on monoamine oxidase A and B. Nanoimpact 2019, 15, 100174. [Google Scholar] [CrossRef]
- Iqbal, T.; Zahra, S.K.; Khan, M.A.R.; Shafique, M.; Raza, S.R.A.; Andleeb, S. Microplasma-assisted electrochemical synthesis of ZnO nanostructures for photocatalytic and antibacterial applications. Phys. Scripta 2021, 96, 125801. [Google Scholar] [CrossRef]
- Lopez-Munoz, M.J.; Revilla, A.; Alcalde, G. Brookite TiO2-based materials: Synthesis and photocatalytic performance in oxidation of methyl orange and As(III) in aqueous suspensions. Catal. Today 2015, 240, 138–145. [Google Scholar] [CrossRef]
- Pinna, M.; Binda, G.; Altomare, M.; Marelli, M.; Dossi, C.; Monticelli, D.; Spanu, D.; Recchia, S. Biochar nanoparticles over TiO2 nanotube arrays: A green Co-catalyst to boost the photocatalytic degradation of organic pollutants. Catalysts 2021, 11, 1048. [Google Scholar] [CrossRef]
- Liu, S.; Su, Z.L.; Liu, Y.; Yi, L.Y.; Chen, Z.L.; Liu, Z.Z. Mechanism and purification effect of photocatalytic wastewater treatment using graphene oxide-doped titanium dioxide composite nanomaterials. Water-Sui. 2021, 13, 1915. [Google Scholar] [CrossRef]
- Wang, Z.C.; Hou, Q.Y.; Guan, Y.Q.; Sha, S.L.; Chen, M.X. Influence of (Li/Na/K) doping and point defect (VAl, Hi) on the magnetic and photocatalytic performance of AlN: A first-principles study. Mater. Chem. Phys. 2021, 268, 124706. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sust. Energ. Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Ochiai, T.; Fujishima, A. Photoelectrochemical properties of TiO2 photocatalyst and its applications for environmental purification. J. Photochem. Photobiol. C 2012, 13, 247–262. [Google Scholar] [CrossRef]
- Pargoletti, E.; Pifferi, V.; Falciola, L.; Facchinetti, G.; Depaolini, A.R.; Davoli, E.; Marelli, M.; Cappelletti, G. A detailed investigation of MnO2 nanorods to be grown onto activated carbon. High efficiency towards aqueous methyl orange adsorption/degradation. Appl. Surf. Sci. 2019, 472, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.M.; Chen, L.; Wang, H.L.; Feng, T.Z.; Yan, Z.C. Degradation of methyl orange by atmospheric DBD plasma: Analysis of the degradation effects and degradation path. J. Electrostat. 2012, 70, 43–47. [Google Scholar] [CrossRef]
- Dai, Q.Z.; Lei, L.C.; Zhang, X.W. Enhanced degradation of organic wastewater containing p-nitrophenol by a novel wet electrocatalytic oxidation process: Parameter optimization and degradation mechanism. Sep. Purif. Technol. 2008, 61, 123–129. [Google Scholar] [CrossRef]
- Alharthi, M.N.; Ismail, I.; Bellucci, S.; Khdary, N.H.; Salam, M.A. Biosynthesis microwave-assisted of zinc oxide nanoparticles with ziziphus jujuba leaves extract: Characterization and photocatalytic application. Nanomater. Basel 2021, 11, 1682. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Zhu, H.; Wang, H.; Yu, H. Preparation of Ag-sensitized ZnO and its photocatalytic performance under simulated solar light. Korean J. Chem. Eng. 2007, 24, 1022–1026. [Google Scholar] [CrossRef]
- Chen, C.C.; Liu, J.F.; Liu, P.; Yu, B.H. Investigation of photocatalytic degradation of methyl orange by using nano-sized ZnO catalysts. Adv. Chem. Eng. Sci. 2011, 1, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Feng, Q.; Li, S.Y.; Ma, W.H.; Fan, H.J.; Wan, X.H.; Lei, Y.; Chen, Z.J.; Yang, J.; Qin, B. Synthesis and characterization of Fe3O4/ZnO-GO nanocomposites with improved photocatalytic degradation methyl orange under visible light irradiation. J. Alloys Compd. 2018, 737, 197–206. [Google Scholar] [CrossRef]
- Zhang, W.P.; Xiao, X.Y.; Li, Y.; Zeng, X.Y.; Zheng, L.L.; Wan, C.X. Liquid-exfoliation of layered MoS2 for enhancing photocatalytic activity of TiO2/g-C3N4 photocatalyst and DFT study. Appl. Surf. Sci. 2016, 389, 496–506. [Google Scholar] [CrossRef]
- Lu, L.L.; Shan, R.; Shi, Y.Y.; Wang, S.X.; Yuan, H.R. A novel TiO2/biochar composite catalysts for photocatalytic degradation of methyl orange. Chemosphere 2019, 222, 391–398. [Google Scholar] [CrossRef]
- Abu Hanif, M.; Akter, J.; Islam, M.A.; Sapkota, K.P.; Hahn, J.R. Visible-light-driven enhanced photocatalytic performance using cadmium-doping of tungsten (VI) oxide and nanocomposite formation with graphitic carbon nitride disks. Appl. Surf. Sci. 2021, 565, 150541. [Google Scholar] [CrossRef]
- Tsuji, M.; Matsuda, K.; Tanaka, M.; Kuboyama, S.; Uto, K.; Wada, N.; Kawazumi, H.; Tsuji, T.; Ago, H.; Hayashi, J. Enhanced photocatalytic degradation of methyl orange by Au/TiO2 nanoparticles under neutral and acidic solutions. Chemistryselect 2018, 3, 1432–1438. [Google Scholar] [CrossRef]
- Kadam, V.V.; Shanmugam, S.D.; Ettiyappan, J.P.; Balakrishnan, R.M. Photocatalytic degradation of p-nitrophenol using biologically synthesized ZnO nanoparticles. Environ. Sci. Pollut. Res. 2021, 28, 12119–12130. [Google Scholar] [CrossRef] [PubMed]
- Gangarajula, Y.; Kedharnath, R.; Gopal, B. Investigation of photocatalytic activity of pure strontium hydroxyapatite and its Ti-substituted and TiO2 loaded forms. Appl. Catal. A-Gen. 2015, 506, 100–108. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, F.Q.; Huang, Z.J.; Chen, H.; Zhuang, Z.F.; Pan, Z.Z.; Long, J.F.; Gu, F.L. Photochemical fabrication of SnO2 dense layers on reduced graphene oxide sheets for application in photocatalytic degradation of p-Nitrophenol. Appl. Catal. B-Environ. 2017, 215, 8–17. [Google Scholar] [CrossRef]
- Lakshmi, K.; Kadirvelu, K.; Mohan, P.S. Photo-decontamination of p-nitrophenol using reusable lanthanum doped ZnO electrospun nanofiber catalyst. J. Mater. Sci.-Mater. Electron. 2018, 29, 12109–12117. [Google Scholar] [CrossRef]
- Sun, J.W.; Xu, J.S.; Grafmueller, A.; Huang, X.; Liedel, C.; Algara-Siller, G.; Willinger, M.; Yang, C.; Fu, Y.S.; Wang, X.; et al. Self-assembled carbon nitride for photocatalytic hydrogen evolution and degradation of p-nitrophenol. Appl. Catal. B-Environ. 2017, 205, 1–10. [Google Scholar] [CrossRef]
- Abazari, R.; Mahjoub, A.R.; Salehi, G. Preparation of amine functionalized g-C3N4@H/SMOF NCs with visible light photocatalytic characteristic for 4-nitrophenol degradation from aqueous solution. J. Hazard. Mater. 2019, 365, 921–931. [Google Scholar] [CrossRef]


| ZnO NPs | At% from EDS | At% from XPS |
|---|---|---|
| Zn | 56.74 | 41.28 |
| O | 43.26 | 39.16 |
| C | 19.55 | |
| Zn/O atomic ratio | 1.31 | 1.05 |
| Pollutant | ZnO NPs Dose (g/L) | Pseudo-First-Order | Pseudo-Second-Order | ||
|---|---|---|---|---|---|
| k1′app (min−1) | R2 | k2′app ((mg/L)−1·min−1) | R2 | ||
| MO | 0 | 0.0005 | 0.8131 | 0.00003 | 0.8129 |
| 0.2 | 0.0077 | 0.9454 | 0.0010 | 0.8935 | |
| 0.5 | 0.0070 | 0.9444 | 0.0009 | 0.9028 | |
| 1.0 | 0.0082 | 0.9688 | 0.0017 | 0.9268 | |
| 1.5 | 0.0128 | 0.8966 | 0.0049 | 0.6219 | |
| 2.0 | 0.0096 | 0.8109 | 0.0026 | 0.5965 | |
| PNP | 0 | 0.0001 | 0.6120 | 0.000006 | 0.6114 |
| 0.5 | 0.0033 | 0.9218 | 0.0003 | 0.9206 | |
| 0.7 | 0.0028 | 0.9838 | 0.0002 | 0.9889 | |
| 1.0 | 0.0029 | 0.9230 | 0.0002 | 0.9206 | |
| 1.5 | 0.0042 | 0.9788 | 0.0004 | 0.9534 | |
| 2.0 | 0.0029 | 0.9804 | 0.0002 | 0.9575 | |
| Catalysts | Preparation | Light | Time | Photocatalytic Activity | References |
|---|---|---|---|---|---|
| ZnO NPs | solvothermal | UV | 180 min | 92% | This work |
| ZnO balls | biosynthesis | solar irradiation | 100 min | 100% | [33] |
| leaf-like ZnO | microwave heating | 1000 W, Xe lamp | 60 min | 69.5% | [34] |
| pseudo-spherical ZnO | precipitation | 300 W, mercury lamp | 120 min | 50% | [35] |
| ZnO/bamboo charcoal | precipitation | UV | 40 min | 92.3% | [12] |
| Fe3O4/ZnO-GO | chemical | 300 W, Xe lamp | 30 min | 93% | [36] |
| TiO2/g-C3N4/MoS2 | solvothermal | Xe lamp | 60 min | 96.5% | [37] |
| TiO2/biochar composite | hydrolysis | 500 W, Hg lamp | 160 min | 96.88% | [38] |
| Cd-doped WO3@g-C3N4 | metal-doped | 300 W, Xe lamp | 80 min | 42.90% | [39] |
| Au/TiO2 nanoparticles | microwave-polyol | UV | 20 min | 96% | [40] |
| Catalysts | Preparation | Light | Time | Photocatalytic Activity | References |
|---|---|---|---|---|---|
| ZnO NPs | solvothermal | UV | 180 min | 56.2% | This work |
| ZnO | biologically | UV | 480 min | 82.5% | [41] |
| TiO2-hydroxyapatite | reflux method | UV | 660 min | 100% | [42] |
| SnO2-rGO | photochemical | UV | 80 min | 95.6% | [43] |
| La-ZnO | hydrothermal | UV | 150 min | 92% | [44] |
| Self-assembled carbon nitride | template-free | 500 W, Hg lamp | 80 min | 90% | [45] |
| g-C3N4@H/SMOF NCs | sonochemical | Visible light | 120 min | 75% | [46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yang, C.; Liu, Y.; Fan, Y.; Dang, F.; Qiu, Y.; Zhou, H.; Wang, W.; Liu, Y. Solvothermal Synthesis of ZnO Nanoparticles for Photocatalytic Degradation of Methyl Orange and p-Nitrophenol. Water 2021, 13, 3224. https://doi.org/10.3390/w13223224
Wang Y, Yang C, Liu Y, Fan Y, Dang F, Qiu Y, Zhou H, Wang W, Liu Y. Solvothermal Synthesis of ZnO Nanoparticles for Photocatalytic Degradation of Methyl Orange and p-Nitrophenol. Water. 2021; 13(22):3224. https://doi.org/10.3390/w13223224
Chicago/Turabian StyleWang, Ying, Chuanxi Yang, Yonglin Liu, Yuqi Fan, Feng Dang, Yang Qiu, Huimin Zhou, Weiliang Wang, and Yuzhen Liu. 2021. "Solvothermal Synthesis of ZnO Nanoparticles for Photocatalytic Degradation of Methyl Orange and p-Nitrophenol" Water 13, no. 22: 3224. https://doi.org/10.3390/w13223224
APA StyleWang, Y., Yang, C., Liu, Y., Fan, Y., Dang, F., Qiu, Y., Zhou, H., Wang, W., & Liu, Y. (2021). Solvothermal Synthesis of ZnO Nanoparticles for Photocatalytic Degradation of Methyl Orange and p-Nitrophenol. Water, 13(22), 3224. https://doi.org/10.3390/w13223224






