Biochemical Composition of Seston Reflecting the Physiological Status and Community Composition of Phytoplankton in a Temperate Coastal Embayment of Korea
Abstract
:1. Introduction
2. Materials and Methods
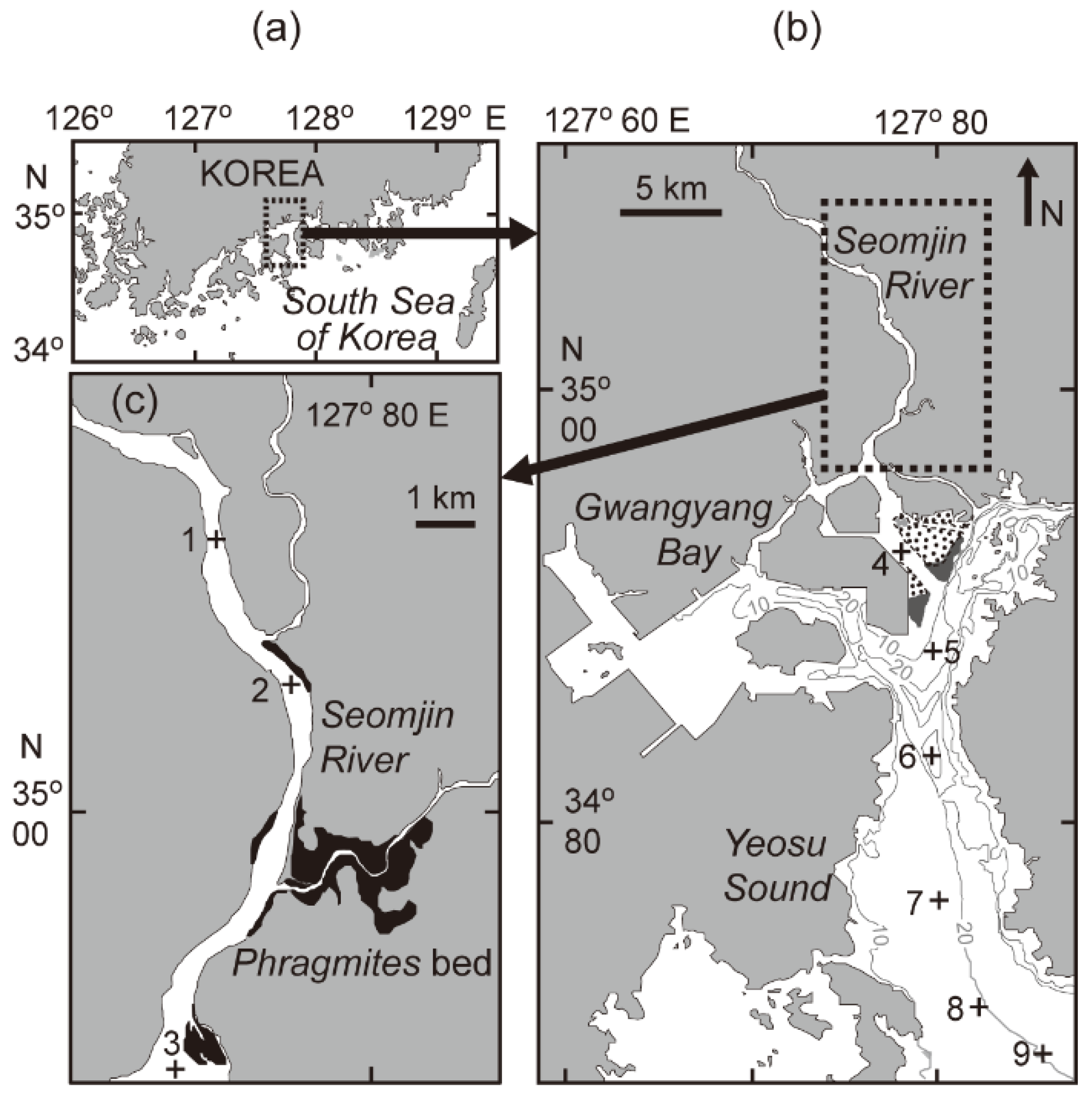
2.1. Study Site and Sample Collection
2.2. Sample Processing
2.3. Laboratory Analysis
2.3.1. Dissolved Inorganic Nutrients and SPM
2.3.2. Photosynthetic Pigments and Chemotaxonomic Composition of Phytoplankton
2.3.3. Primary Productivity of Phytoplankton
2.3.4. Biochemical Composition of Seston
2.3.5. Biopolymeric Carbon
2.3.6. POC, PN, δ13CPOM, and δ15NPOM
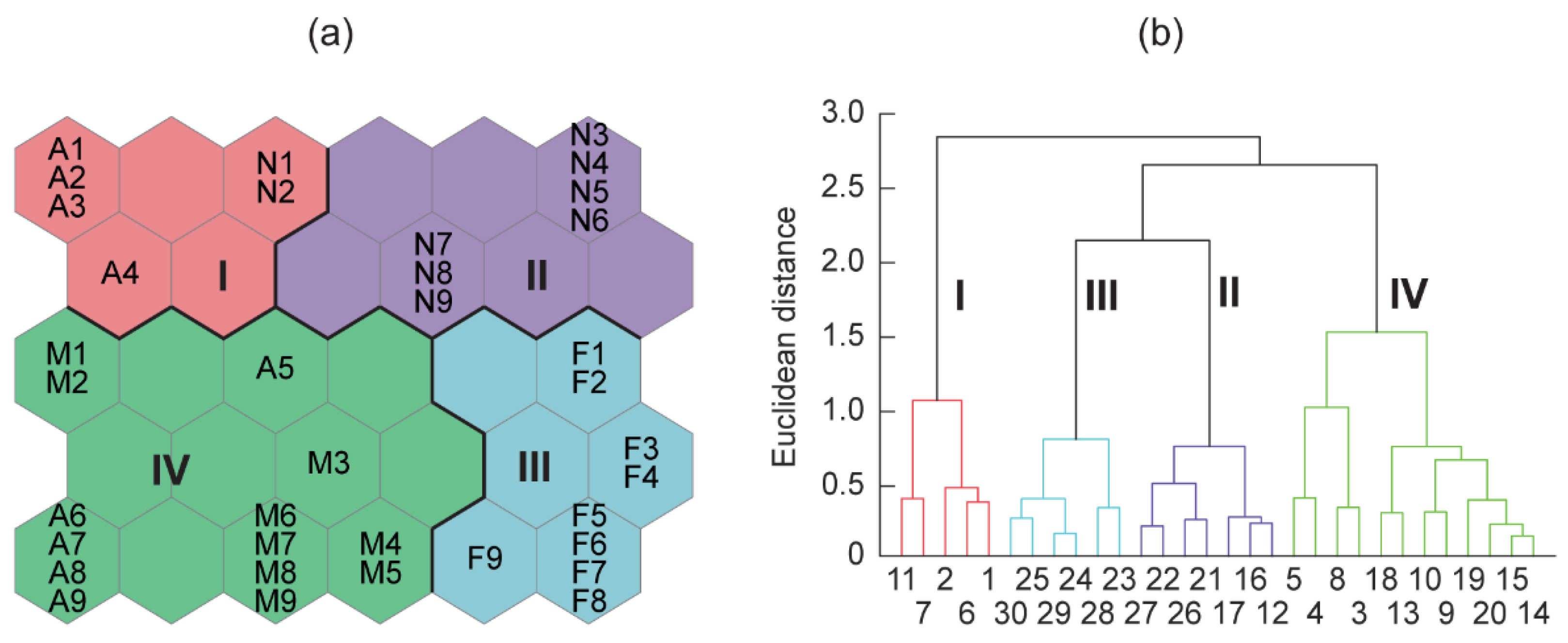
2.4. Data Analysis
3. Results
3.1. SPM Concentration and Composition
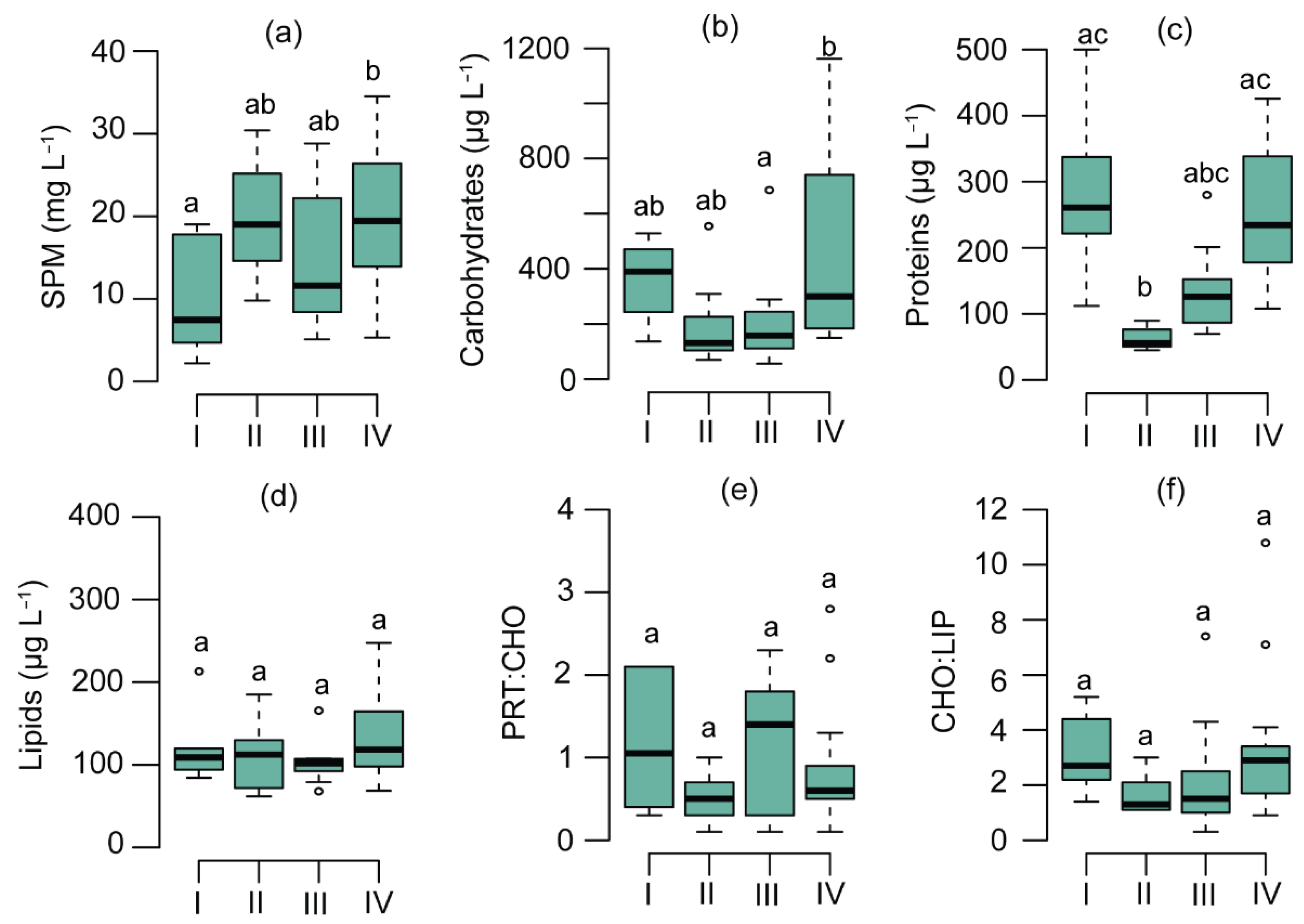
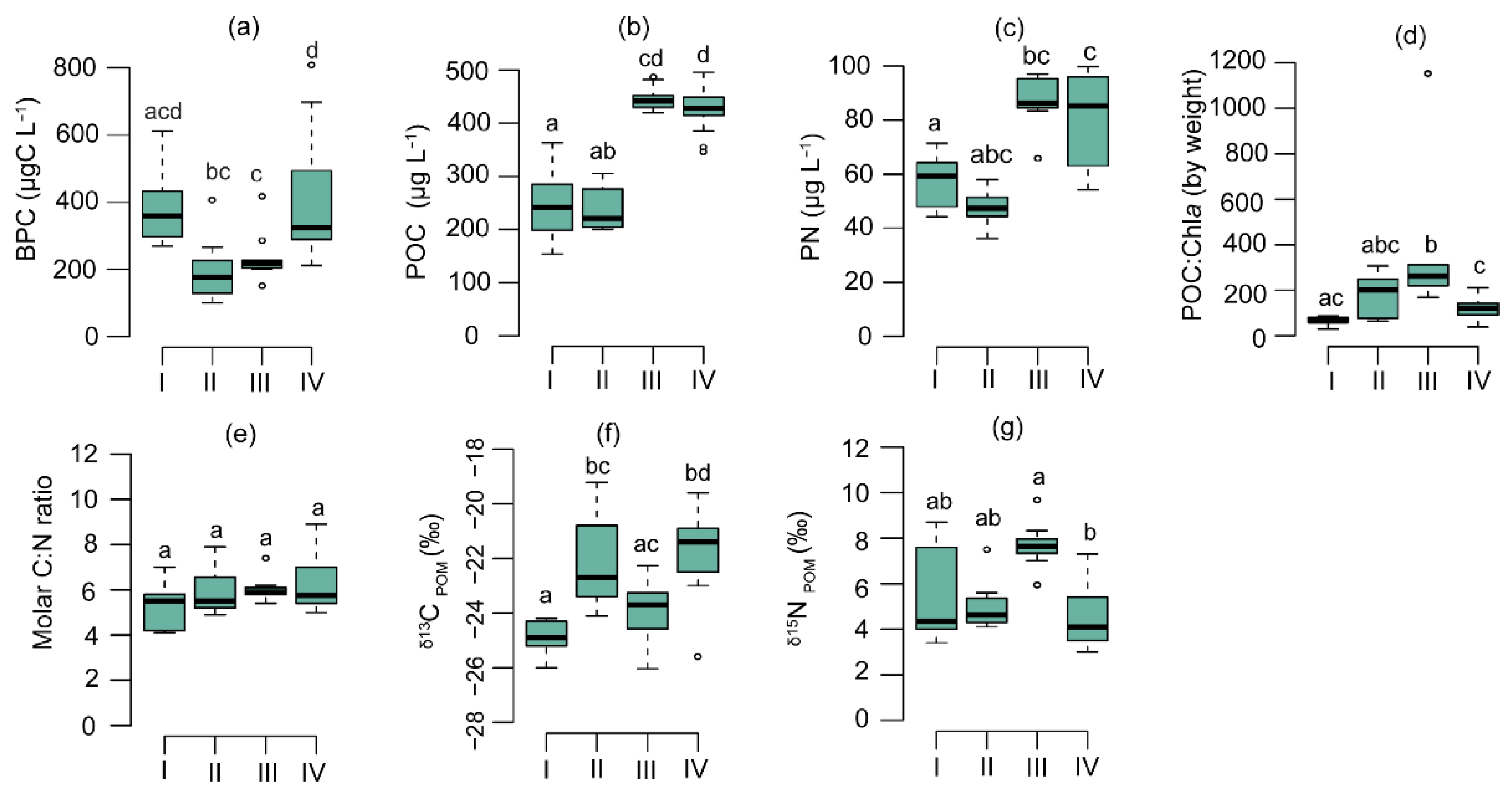
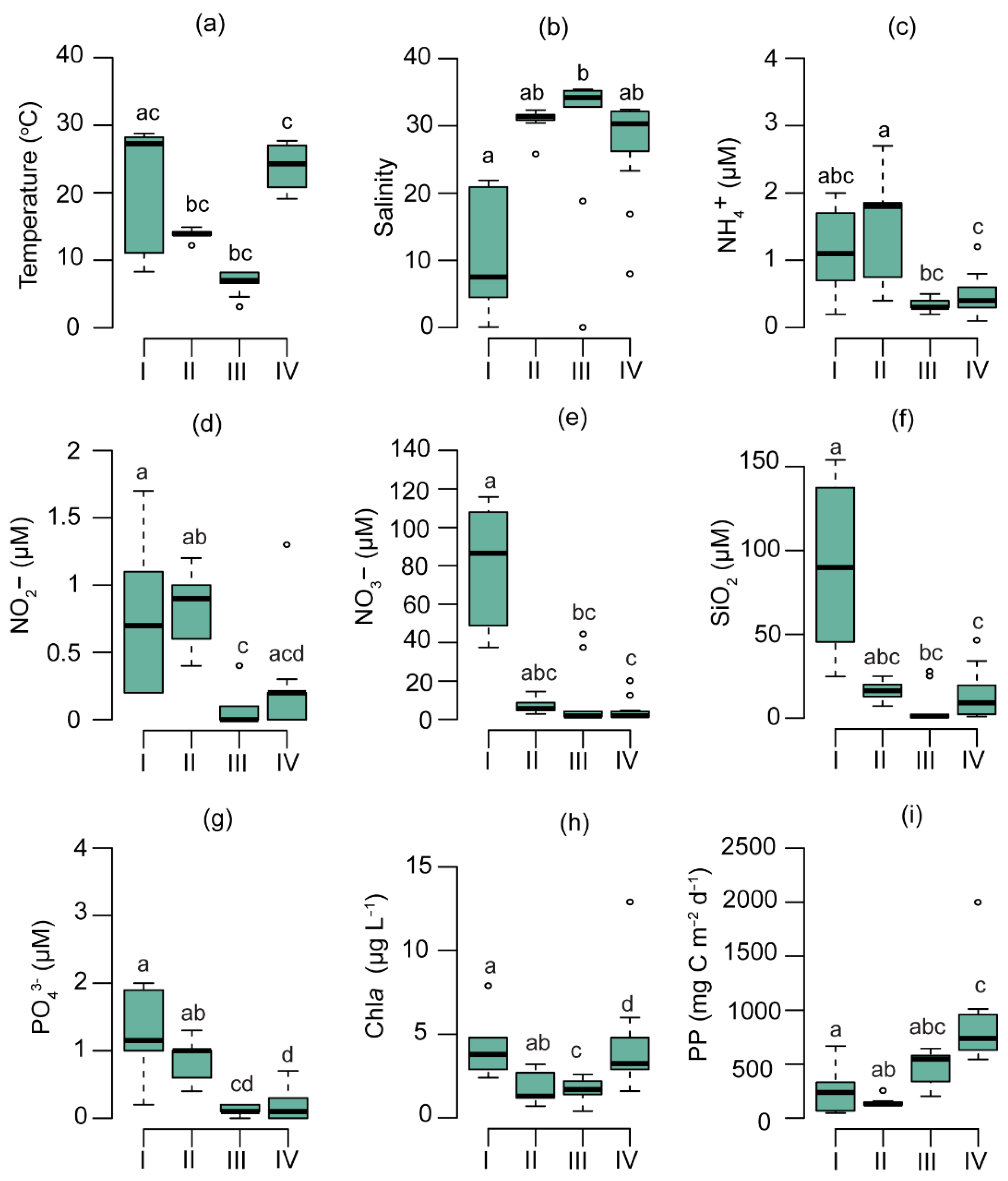
3.2. Physicochemical and Biological Parameters
3.3. Phytoplankton Community Composition
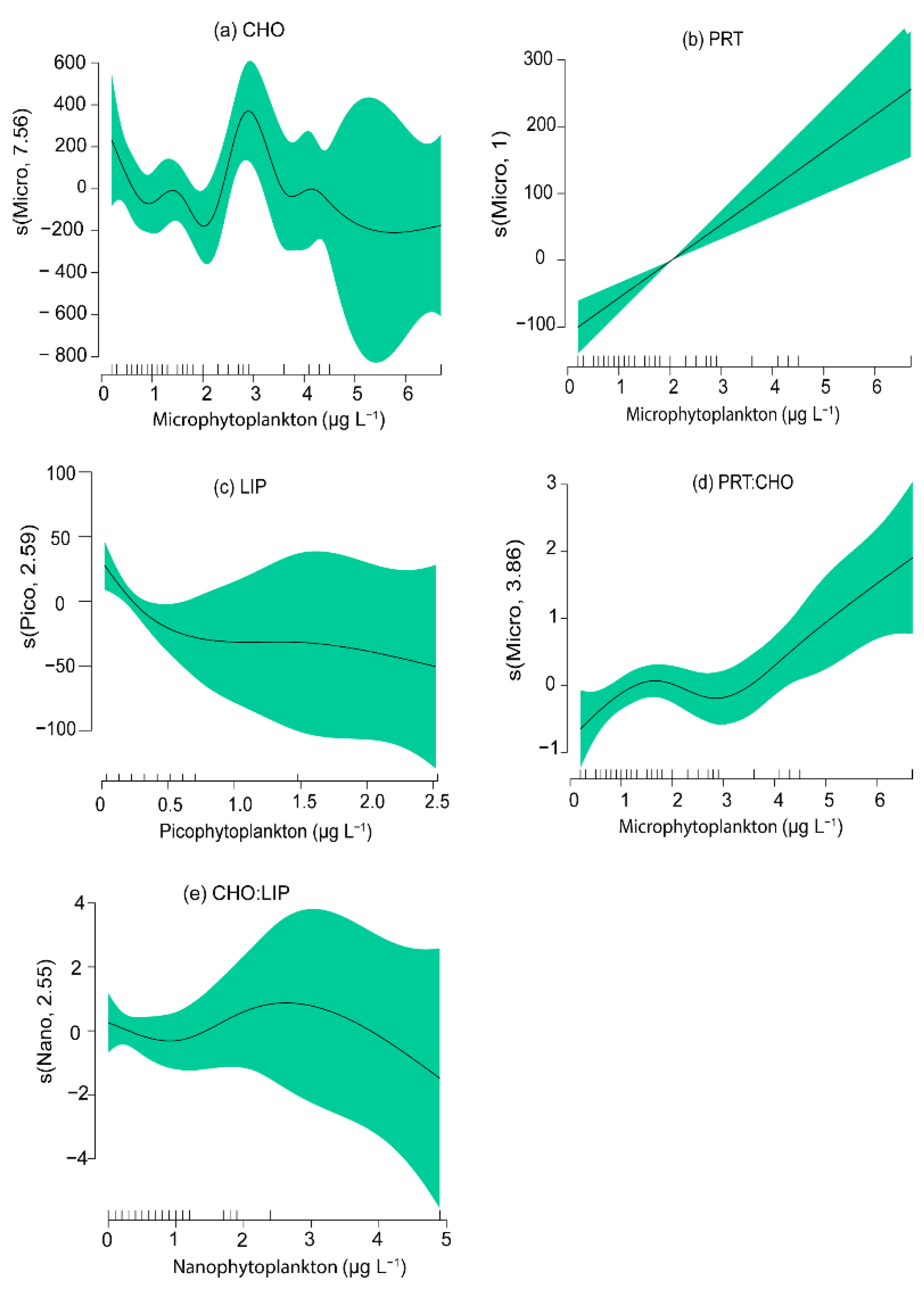
3.4. Relationships between Phytoplankton Groups and the Biochemical Composition of Seston
4. Discussion
4.1. Physiological Effects of Phytoplankton on POM Composition
4.2. Chemotaxonomic Effects of Phytoplankton on Seston Biochemical Composition
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elliott, M.; McLusky, D.S. The need for definitions in understanding estuaries. Estuar. Coast. Shelf Sci. 2002, 55, 815–827. [Google Scholar] [CrossRef] [Green Version]
- Baird, D.; Glade, J.M.; Ulanowicz, R.E. The comparative ecology of six marine ecosystems. Philos. Trans. R. Soc. Lond. 1991, 333, 15–29. [Google Scholar] [CrossRef]
- Canuel, E.A. Relations between river flow, primary production and fatty acid composition of particulate organic matter in San Francisco and Chesapeake Bays: A multivariate approach. Org. Geochem. 2001, 32, 563–583. [Google Scholar] [CrossRef]
- Attrill, M.J.; Rundle, S.D. Ecotone or ecocline: Ecological boundaries in estuaries. Estuar. Coast. Shelf Sci. 2002, 55, 929–936. [Google Scholar] [CrossRef]
- Kang, H.Y.; Kim, C.; Kim, D.; Lee, Y.J.; Park, H.J.; Kundu, G.K.; Kim, Y.K.; Bibi, R.; Jang, J.; Lee, K.H.; et al. Identifying patterns in the multitrophic community and food-web structure of a low-turbidity temperate estuarine bay. Sci. Rep. 2020, 10, 16637. [Google Scholar] [CrossRef]
- Harmelin-Vivien, M.; Loizeau, V.; Mellon, C.; Beker, B.; Arlhac, D.; Bodiguel, X.; Ferraton, R.; Hermand, X.; Philippon, C.S.P. Comparison of C and N stable isotope ratios between surface particulate organic matter and microphytoplankton in the Gulf of Lions (NW Mediterranean). Cont. Shelf Res. 2008, 28, 1911–1919. [Google Scholar] [CrossRef] [Green Version]
- Hecky, R.E.; Kilham, P. Nutrient limitation of phytoplankton in freshwater and marine environments: A review of recent evidence on the effects of enrichment. Limnol. Oceanogr. 1988, 33, 790–822. [Google Scholar] [CrossRef] [Green Version]
- McLusky, D.S. The Estuarine Ecosystem; Chapman and Hall: New York, NY, USA, 1989. [Google Scholar]
- Bouillon, S.; Gillikin, D.P.; Connolly, R.M. Use of stable isotopes to understand food webs and ecosystem functioning in estuaries. In Treatise on Estuarine and Coastal Science; Wolanski, E., McLusky, D.S., Eds.; Academic Press: Waltham, MA, USA, 2011; pp. 143–173. [Google Scholar] [CrossRef]
- Bianchi, T.S. Estuarine chemistry. In Estuarine Ecology; Day, J.W., Crump, B.C., Kemp, W.M., Yanez-Arancibia, A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 39–83. [Google Scholar]
- Fichez, R.; Dennis, P.; Fontaine, M.F.; Jickells, T.C. Isotopic and biochemical composition of particulate organic matter in a shallow water estuary (Great Ouse, North Sea, England). Mar. Chem. 1993, 43, 263–276. [Google Scholar] [CrossRef]
- Chaparro, O.R.; Segura, C.J.; Montiel, Y.A.; Thompson, R.J.; Navarro, J.M. Variations in the quantity and composition of seston from an estuary in southern Chile on different temporal scales. Estuar. Coast. Shelf Sci. 2008, 76, 845–860. [Google Scholar] [CrossRef]
- Cloern, J.E. Turbidity as a control on phytoplankton biomass and productivity in estuaries. Cont. Shelf Res. 1987, 7, 1367–1381. [Google Scholar] [CrossRef]
- Gameiro, C.; Zwolinski, J.; Brotas, V. Light control on phytoplankton production in a shallow and turbid estuarine system. Hydrobiologia 2011, 669, 249–263. [Google Scholar] [CrossRef]
- Thompson, P.A.; Guo, M.; Harrison, P.J. Effects of variation in temperature. I. On the biochemical composition of eight species of marine phytoplankton. J. Phycol. 1992, 28, 481–488. [Google Scholar] [CrossRef]
- Healey, F.P.; Hendzel, L.L. Physiological indicators of nutrient deficiency in lake phytoplankton. J. Fish. Res. Board Can. 1980, 37, 442–453. [Google Scholar] [CrossRef]
- Danovaro, R.; Dell’Anno, A.; Pusceddu, A.; Marrale, D.; Croce, N.D.; Fabiano, M.; Tselepides, A. Biochemical composition of pico-, nano- and micro-particulate organic matter and bacterioplankton biomass in the oligotrophic Cretan Sea (NE Mediterranean). Prog. Oceanogr. 2000, 46, 279–310. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, H.J.; Whitledge, T.E. High incorporation of carbon into proteins by the phytoplankton of the Bering Strait and Chukchi Sea. Cont. Shelf Res. 2009, 29, 1689–1696. [Google Scholar] [CrossRef]
- Finkel, Z.V. Light absorption and size scaling of light limited metabolism in marine diatoms. Limnol. Oceanogr. 2001, 46, 86–94. [Google Scholar] [CrossRef] [Green Version]
- Finkel, Z.V.; Beardall, J.; Flynn, K.J.; Quigg, A.; Rees, T.A.V.; Raven, J.A. Phytoplankton in a changing world: Cell size and elemental stoichiometry. J. Plankton Res. 2010, 32, 119–137. [Google Scholar] [CrossRef] [Green Version]
- Marañón, E. Cell size as a key determinant of phytoplankton metabolism and community structure. Ann. Rev. Mar. Sci. 2015, 7, 241–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marañón, E.; Cermeño, P.; Latasa, M.; Tadonléké, R.D. Temperature, resources, and phytoplankton size structure in the ocean. Limnol. Oceanogr. 2012, 57, 1266–1278. [Google Scholar] [CrossRef]
- Finkel, Z.V.; Follows, M.J.; Liefer, J.D.; Brown, C.M.; Benner, I.; Irwin, A.J. Phylogenetic diversity in the macromolecular composition of microalgae. PLoS ONE 2016, 11, e0155977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Lee, D.; Kang, J.J.; Joo, H.T.; Lee, J.H.; Lee, H.W.; Ahn, S.H.; Kang, C.K.; Lee, S.H. The effects of different environmental factors on the biochemical composition of particulate organic matter in Gwangyang Bay, South Korea. Biogeosciences 2017, 14, 1903–1917. [Google Scholar] [CrossRef] [Green Version]
- Whyte, J.N.C. Biochemical composition and energy content of six species of phytoplankton used in mariculture of bivalves. Aquaculture 1987, 60, 231–241. [Google Scholar] [CrossRef]
- Bibi, R.; Kang, H.Y.; Kim, D.; Jang, J.; Kundu, G.K.; Kim, Y.K.; Kang, C.K. Dominance of autochthonous phytoplankton-derived particulate organic matter in a low-turbidity temperate estuarine embayment, Gwangyang Bay, Korea. Front. Mar. Sci. 2020, 7, 580260. [Google Scholar] [CrossRef]
- Shaha, D.C.; Cho, Y.K.; Kim, T.W.; Valle-Levinson, A. Spatio-temporal variation of flushing time in the Sumjin River estuary. Terr. Atmos. Ocean. Sci. 2012, 23, 119–130. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.; Kang, Y.H.; Kim, J.K.; Kang, H.Y.; Kang, C.K. Year-to-year variation of phytoplankton blooms in an anthropogenically impacted and complex estuary: A novel paradigm for river discharge influence. Mar. Pollut. Bull. 2020, 161, 111756. [Google Scholar] [CrossRef]
- Kwon, K.Y.; Moon, C.H.; Yang, H.S. Behavior of nutrient along the salinity gradients in the Seomjin River Estuary. J. Korean Fish. Soc. 2001, 34, 199–206. [Google Scholar]
- Kwon, K.Y.; Moon, C.H.; Lee, J.S.; Yang, S.R.; Park, M.O.; Lee, P.Y. Estuarine behavior and flux of nutrients in the Seomjin River estuary. Sea J. Korean Soc. Oceanogr. 2004, 9, 153–163. [Google Scholar]
- Kim, C.; Kang, H.Y.; Lee, Y.J.; Yun, S.G.; Kang, C.K. Isotopic variation of macrobenthic invertebrates and their sources of organic matters along an estuarine gradient. Estuar. Coast. 2020, 43, 496–511. [Google Scholar] [CrossRef]
- Liénart, C.; Savoye, N.; Bozec, Y.; Breton, E.; Conan, P.; David, V.; Feunteun, E.; Grangeré, K.; Kerhervé, P.; Lebreton, B.; et al. Dynamics of particulate organic matter composition in coastal systems: A spatio-temporal study at multi-systems scale. Prog. Oceanogr. 2017, 156, 221–239. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.J.; Ro, Y.J.; Jung, K.Y.; Park, K.S. Numerical modeling of circulation characteristics in the Kwangyang Bay estuarine system. J. Korean Soc. Coast. Ocean Eng. 2014, 26, 253–266. [Google Scholar] [CrossRef]
- Kwon, K.Y.; Moon, C.H.; Kang, C.K.; Kim, Y.N. Distribution of particulate organic matters along the salinity gradients in the Seomjin River estuary. J. Korean Fish. Soc. 2002, 25, 86–96. [Google Scholar] [CrossRef]
- Nieuwenhuize, J.; Maas, Y.E.M.; Middelburg, J.J. Rapid analysis of organic carbon and nitrogen in particulate materials. Mar. Chem. 1994, 45, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Murphy, J.; Riley, J.P.A. Modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta. 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Helder, W.; de Vries, R.T.P. An automatic phenol-hypochlorite method for the determination of ammonia in sea and brackish water. Neth. J. Sea Res. 1979, 13, 154–160. [Google Scholar] [CrossRef]
- Hansen, H.P.; Grasshoff, K. Automated chemical analysis. In Methods of Seawater Analysis; Grasshoff, K., Kremling, K., Ehrhardt, M., Eds.; Verlag Chemie: Weinheim, Germany, 1983; pp. 347–379. [Google Scholar]
- Zapata, M.; Rodríguez, F.; Garrido, J.L. Separation of chlorophylls and carotenoids from marine phytoplankton: A new HPLC method using a reversed phase C8 column and pyridine containing mobile phases. Mar. Ecol. Progr. Ser. 2000, 195, 29–45. [Google Scholar] [CrossRef] [Green Version]
- Park, H.J.; Choy, E.J.; Kang, C.K. Spatial and temporal variations of microphytobenthos on the common reed Phragmites australis bed in a marine protected area of Yeoja Bay, Korea. Wetlands 2013, 33, 737–745. [Google Scholar] [CrossRef]
- Jeffrey, S.W. Application of pigment methods to oceanography. In Phytoplankton Pigments in Oceanography: Guidelines to Modern Methods; Jeffery, S.W., Mantoura, R.F.C., Wright, S.W., Eds.; UNESCO Publishing: Paris, France, 1997; pp. 127–166. [Google Scholar]
- Lewitus, A.J.; White, D.L.; Tymowski, R.G.; Geesey, M.E.; Hymel, S.N.; Noble, P.A. Adapting the CHEMTAX method for assessing phytoplankton taxonomic composition in southeastern US estuaries. Estuaries 2005, 1, 160–172. [Google Scholar] [CrossRef]
- Lee, Y.W.; Park, M.O.; Kim, Y.S.; Kim, S.S.; Kang, C.K. Application of photosynthetic pigment analysis using a HPLC and CHEMTAX program to studies of phytoplankton community composition. J. Korean Soc. Oceanogr. 2011, 16, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Hama, T.; Miyazaki, T.; Ogawa, Y.; Iwakuma, M.; Takahashi, M.; Otsuki, A.; Ichimura, S. Measurement of photosynthetic production of a marine phytoplankton population using a sTable 13C isotope. Mar. Biol. 1983, 73, 31–36. [Google Scholar] [CrossRef]
- Kanda, J.; Saino, T.; Hattori, A. Nitrogen uptake by natural populations of phytoplankton and primary production in the Pacific Ocean: Regional variability of uptake capacity. Limnol. Oceanogr. 1985, 30, 987–999. [Google Scholar] [CrossRef]
- Fan, C.; Glibert, P.M. Effects of light on nitrogen and carbon uptake during a Prorocentrum minimum bloom. Harmful Algae. 2005, 4, 629–641. [Google Scholar] [CrossRef]
- Kwak, J.H.; Lee, S.H.; Hwang, J.; Suh, Y.S.; Park, H.; Chang, K.I.; Kim, K.R.; Kang, C.K. Summer primary productivity and phytoplankton community composition driven by different hydrographic structures in the East/Japan Sea and the Western Subarctic Pacific. J. Geophys. Res. Oceans. 2014, 11, 4505–4519. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Boil. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Marsh, J.B.; Weinstein, D.B. Simple charring method for determination of lipids. J. Lipid Res. 1966, 7, 574–576. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Fabiano, M.; Danovaro, R. Composition of organic matter in sediments facing a river estuary (Tyrrhenian Sea): Relationship with bacteria and microphytobenthic biomass. Hydrobiology 1994, 277, 71–84. [Google Scholar] [CrossRef]
- Fabiano, M.; Danovaro, R.; Fraschetti, S. A three-year time series of elemental and biochemical composition of organic matter in subtidal sandy sediments of the Ligurian sea (North western Mediterranean). Cont. Shelf Res. 1995, 15, 1453–1469. [Google Scholar] [CrossRef]
- Kohonen, T. Self-Organizing Maps, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Park, Y.S.; Céréghino, R.; Compin, A.; Lek, S. Applications of artificial neural networks for patterning and predicting aquatic insect species richness in running waters. Ecol. Model. 2003, 160, 265–280. [Google Scholar] [CrossRef]
- Vesanto, J.; Alhoniemi, E. Clustering of the self-organizing map. IEEE Trans. Neural Netw. 2000, 11, 586–600. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Hastie, T.J.; Tibshirani, R.J. Generalized Additive Models; Chapman and Hall/CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Wood, S. Package ‘mgcv’. R Package Version 1.8-36. 2021. Available online: https://CRAN.R-project.org/package=mgcv (accessed on 15 March 2021).
- Ahn, S.H.; Whitledge, T.E.; Stockwell, D.A.; Lee, J.H.; Lee, H.; Lee, S.H. The biochemical composition of phytoplankton in the Laptev and East Siberian seas during the summer of 2013. Polar Biol. 2019, 42, 133–148. [Google Scholar] [CrossRef]
- Kang, J.J.; Joo, H.; Lee, J.H.; Lee, J.H.; Lee, H.W.; Lee, D.; Kang, C.K.; Yun, M.S.; Lee, S.H. Comparison of biochemical compositions of phytoplankton during spring and fall seasons in the northern East/Japan Sea. Deep Sea Res. II 2017, 143, 73–81. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J.H.; Kang, J.J.; Lee, J.H.; Lee, H.W.; Kang, C.K.; Lee, S.H. River discharge effects on the contribution of small-sized phytoplankton to the total biochemical composition of POM in the Gwangyang Bay, Korea. Estuar. Coast. Shelf Sci. 2019, 226, 106293. [Google Scholar] [CrossRef]
- Finkel, Z.V.; Quigg, A.S.; Raven, J.A.; Reinfelder, O.E.; Schofield; Falkowski, P.G. Irradiance and the elemental stoichiometry of marine phytoplankton. Limnol. Oceanogr. 2006, 51, 2690–2701. [Google Scholar] [CrossRef] [Green Version]
- Cloern, J.E.; Jassby, A.D.; Schraga, T.S.; Nejad, E.; Martin, C. Ecosystem variability along the estuarine salinity gradient: Examples from longterm study of San Francisco Bay. Limnol. Oceanogr. 2017, 62, 272–291. [Google Scholar] [CrossRef] [Green Version]
- Hernando, M.; Schloss, I.; Malanga, G.; Almandoz, G.; Ferreyra, G.; Aguiar, M.; Puntarulo, S. Effects of salinity changes on coastal Antarctic phytoplankton physiology and assemblage composition. J. Exp. Mar. Biol. Ecol. 2015, 466, 110–119. [Google Scholar] [CrossRef]
- Jang, P.K.; Lee, W.J.; Jang, M.C.; Lee, J.D.; Lee, W.J.; Chang, M.; Hwang, K.C.; Shin, K. Spatial and temporal distribution of inorganic nutrients and factors controlling their distributions in Gwangyang Bay. Ocean Polar Res. 2005, 27, 359–379. [Google Scholar] [CrossRef] [Green Version]
- Kilham, S.S.; Kreeger, D.A.; Goulden, C.E.; Lynn, S.G. Effects of nutrient limitation on biochemical constituents of Ankistrodesmus falcatus. Freshw. Biol. 1997, 38, 591–596. [Google Scholar] [CrossRef]
- Litchman, E.; Klausmeier, C.A.; Schofield, O.M.; Falkowski, P.G. The role of functional traits and trade-offs in structuring phytoplankton communities: Scaling from cellular to ecosystem level. Ecol. Lett. 2007, 10, 1170–1181. [Google Scholar] [CrossRef]
- Fabiano, M.; Povero, P.; Danovaro, R. Distribution and composition of particulate organic matter in the Ross sea (Antarctica). Polar Biol. 1993, 13, 525–533. [Google Scholar] [CrossRef]
- Rhee, G.Y.; Gotham, I.J. The effect of environmental factors on phytoplankton growth: Temperature and the interactions of temperature with nutrient limitation. Limnol. Oceanogr. 1981, 26, 635–648. [Google Scholar] [CrossRef]
- Gasparovic, B.; Frka, S.; Koch, B.P.; Zhu, Z.Y.; Bracher, A.; Lechtenfeld, O.J.; Neogi, S.B.; Lara, R.J.; Kattner, G. Factors influencing particulate lipid production in the East Atlantic Ocean. Deep-Sea Res. I 2014, 89, 56–67. [Google Scholar] [CrossRef] [Green Version]
- Arin, L.; Morán, X.A.G.; Estrada, M. Phytoplankton size-distribution and growth rates in the Alboran Sea (SW Mediterranean): Short-term variability related to mesoscale hydrodynamics. J. Plankton Res. 2002, 24, 1019–1033. [Google Scholar] [CrossRef] [Green Version]
- Putland, J.N.; Iverson, R.L. Phytoplankton biomass in a subtropical estuary: Distribution, size composition, and carbon:chlorophyll ratios. Estuar. Coast. 2007, 30, 878–885. [Google Scholar] [CrossRef]
- Garcia, N.S.; Sexton, J.; Riggins, T.; Brown, J.; Lomas, M.W.; Martiny, A.C. High variability in cellular stoichiometry of carbon, nitrogen, and phosphorus within classes of marine eukaryotic phytoplankton under sufficient nutrient conditions. Front. Microbio. 2018, 9, 543. [Google Scholar] [CrossRef] [Green Version]
- Pusceddu, A.; Bianchelli, S.; Gambi, C.; Danovaro, R. Assessment of benthic tropic status of marine coastal ecosystems: Significance of meiofaunal rare taxa. Estuar. Coast. Shelf Sci. 2011, 93, 420–430. [Google Scholar] [CrossRef]
- Danovaro, R.; Fabiano, M. Seasonal changes in quality and quantity of food available for benthic suspension—Feeders in the Golfo Marconi (North–Western Mediterranean). Estuar. Coast. Shelf Sci. 1997, 44, 726–733. [Google Scholar] [CrossRef]
- Gillooly, J.F.; Brown, J.H.; West, G.B.; Savage, V.M.; Charnov, E.L. Effects of size and temperature on metabolic rate. Science 2001, 293, 2248–2251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liebezeit, G. Particulate carbohydrate in relation to phytoplankton in the euphotic zone of the Bransfield Strait. Polar Biol. 1984, 2, 225–228. [Google Scholar] [CrossRef]
- D’souza, F.; Bhosle, N.B. Variation in the composition of carbohydrates in the Dona Paula Bay (west of India) during May/June 1998. Oceanol. Acta 2001, 24, 221–237. [Google Scholar] [CrossRef] [Green Version]
- Cermeño, P.; Estévez-Blanco, P.; Marañón, E.; Fernóndez, E. Maximum photosynthetic efficiency of size-fractionated phytoplankton assessed by 14C uptake and fast repetition rate fluorometry. Limnol. Oceanogr. 2005, 50, 1438–1446. [Google Scholar] [CrossRef] [Green Version]
- Maranón, E.; Cermeño, P.; Latasa, M.; Tadonléké, R.D. Resource supply alone explains the variability of marine phytoplankton size structure. Limnol. Oceanogr. 2015, 60, 1848–1854. [Google Scholar] [CrossRef] [Green Version]
- Justić, D.; Rabalais, N.N.; Turner, R.E.; Dortch, Q. Changes in nutrient structure of river-dominated coastal water: Stoichiometric nutrient balance and its consequences. Estuar. Coast. Shelf Sci. 1995, 40, 339–356. [Google Scholar] [CrossRef]
- Baek, S.H.; Kim, D.; Son, M.; Yun, S.M.; Kim, Y.O. Seasonal distribution of phytoplankton assemblages and nutrient-enriched bioassays as indicators of nutrient limitation of phytoplankton growth in Gwangyang Bay, Korea. Estuar. Coast. Shelf Sci. 2015, 163, 265–278. [Google Scholar] [CrossRef]
- Brooks, P.D.; Haas, P.A.; Huth, A.K. Seasonal variability in the concentration and flux of organic matter and inorganic nitrogen in a semiarid catchment, San Pedro River, Arizona. J. Geophys. Res. 2007, 112, G03S04. [Google Scholar] [CrossRef] [Green Version]
- Lewandowska, A.M.; Breithaupt, P.; Hillebrand, H.; Hoppe, H.G.; Jürgens, K.; Sommer, U. Responses of primary productivity to increased temperature and phytoplankton diversity. J. Sea Res. 2012, 72, 87–93. [Google Scholar] [CrossRef]
- Liefer, J.D.; Garg, A.; Fyfe, M.H.; Irwin, A.J.; Benner, I.; Brown, C.M.; Follows, M.J.; Omta, A.W.; Finkel, Z.V. The Macromolecular Basis of Phytoplankton C:N:P Under Nitrogen Starvation. Front. Microbiol. 2019, 10, 763. [Google Scholar] [CrossRef] [Green Version]
- Bhosle, N.B.; Wagh, A.B. Particulate carbohydrates in the Arabian Sea. Oceanol. Acta. 1989, 12, 57–63. Available online: http://drs.nio.org/drs/handle/2264/7254 (accessed on 10 June 2021).
- Sicko-Goad, L.M.; Schelske, C.L.; Stoermer, E.F. Estimation of intracellular carbon and silica content of diatoms from natural assemblages using morphometric techniques. Limnol. Oceanogr. 1984, 29, 1170–1178. [Google Scholar] [CrossRef]
- Granum, E.; Kirkvold, S.; Myklestad, S.M. Cellular and extracellular production of carbohydrates and amino acids by the marine diatom Skeletonema costatum: Diel variations and effects of N depletion. Mar. Ecol. Prog. Ser. 2002, 242, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Wetz, M.S.; Wheeler, P.A. Release of dissolved organic matter by coastal diatoms. Limnol. Oceanogr. 2007, 52, 798–807. [Google Scholar] [CrossRef]
- Bergkvist, J.; Thor, P.; Jakobsen, H.H.; Wängberg, S.A.; Selander, E. Grazer-induced chain length plasticity reduces grazing risk in a marine diatom. Limnol. Oceanogr. 2012, 57, 318–324. [Google Scholar] [CrossRef]
- Chen, M.; Kim, D.; Liu, H.; Kang, C.K. Variability in copepod trophic levels and feeding selectivity based on stable isotope analysis in Gwangyang Bay of the southern coast of the Korean peninsula. Biogeosciences 2018, 15, 2055–2073. [Google Scholar] [CrossRef] [Green Version]
- Martin, G.J.O.; Hill, D.R.A.; Olmstead, I.L.D.; Bergamin, A.; Shears, M.J.; Dias, D.A.; Kentish, S.E.; Scales, P.J.; Botté, C.Y.; Callahan, D.L. Lipid profile remodeling in response to nitrogen deprivation in the microalgae Chlorella sp. (Trebouxiophyceae) and Nannochloropsis sp. (Eustigmatophyceae). PLoS ONE 2014, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Irwin, A.J.; Finkel, Z.V.; Schofield, O.M.; Falkowski, P.G. Scaling-up from nutrient physiology to the size-structure of phytoplankton communities. J. Plankton Res. 2006, 5, 459–471. [Google Scholar] [CrossRef] [Green Version]
- Cardol, P.; Bailleul, B.; Rappaport, F.; Derelle, E.; Béal, D.; Breyton, C.; Bailey, S.; Wollman, F.A.; Grossman, A.; Moreau, H.; et al. An original adaptation of photosynthesis in the marine green alga Ostreococcus. Proc. Natl. Acad. Sci. USA 2008, 105, 7881–7886. [Google Scholar] [CrossRef] [Green Version]
- Six, C.; Sherrard, R.; Lionard, M.; Roy, S.; Campbell, D.A. Photosystem II and pigment dynamics among ecotypes of the green alga Ostreococcus. Plant Physiol. 2009, 151, 379–390. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, S.; Barry, A.N.; Pieris, S.; Sayre, R.T. Comparative energetics and kinetics of autotrophic lipid and starch metabolism in chlorophytic microalgae: Implications for biomass and biofuel production. Biotechnol. Biofuels. 2013, 6, 150. [Google Scholar] [CrossRef] [Green Version]
- Lacour, T.; Sciandra, A.; Talec, A.; Mayzaud, P.; Bernard, O. Diel variations of carbohydrate and neutral lipids in nitrogen-sufficient and nitrogen-starved cyclostat cultures of Isochrysis sp. J. Phycol. 2012, 48, 966–975. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bibi, R.; Kang, H.Y.; Kim, D.; Jang, J.; Kim, C.; Kundu, G.K.; Kang, C.-K. Biochemical Composition of Seston Reflecting the Physiological Status and Community Composition of Phytoplankton in a Temperate Coastal Embayment of Korea. Water 2021, 13, 3221. https://doi.org/10.3390/w13223221
Bibi R, Kang HY, Kim D, Jang J, Kim C, Kundu GK, Kang C-K. Biochemical Composition of Seston Reflecting the Physiological Status and Community Composition of Phytoplankton in a Temperate Coastal Embayment of Korea. Water. 2021; 13(22):3221. https://doi.org/10.3390/w13223221
Chicago/Turabian StyleBibi, Riaz, Hee Yoon Kang, Dongyoung Kim, Jaebin Jang, Changseong Kim, Goutam K. Kundu, and Chang-Keun Kang. 2021. "Biochemical Composition of Seston Reflecting the Physiological Status and Community Composition of Phytoplankton in a Temperate Coastal Embayment of Korea" Water 13, no. 22: 3221. https://doi.org/10.3390/w13223221
APA StyleBibi, R., Kang, H. Y., Kim, D., Jang, J., Kim, C., Kundu, G. K., & Kang, C.-K. (2021). Biochemical Composition of Seston Reflecting the Physiological Status and Community Composition of Phytoplankton in a Temperate Coastal Embayment of Korea. Water, 13(22), 3221. https://doi.org/10.3390/w13223221







