Growth and Nutrient Uptake Characteristics of Heterosigma akashiwo (Raphidophyceae) under Nitrogen and Phosphorus Concentrations in the East China Sea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultures
2.2. Experimental Setup
2.3. Sampling and Measurement
2.4. Data and Statistical Analysis
3. Results
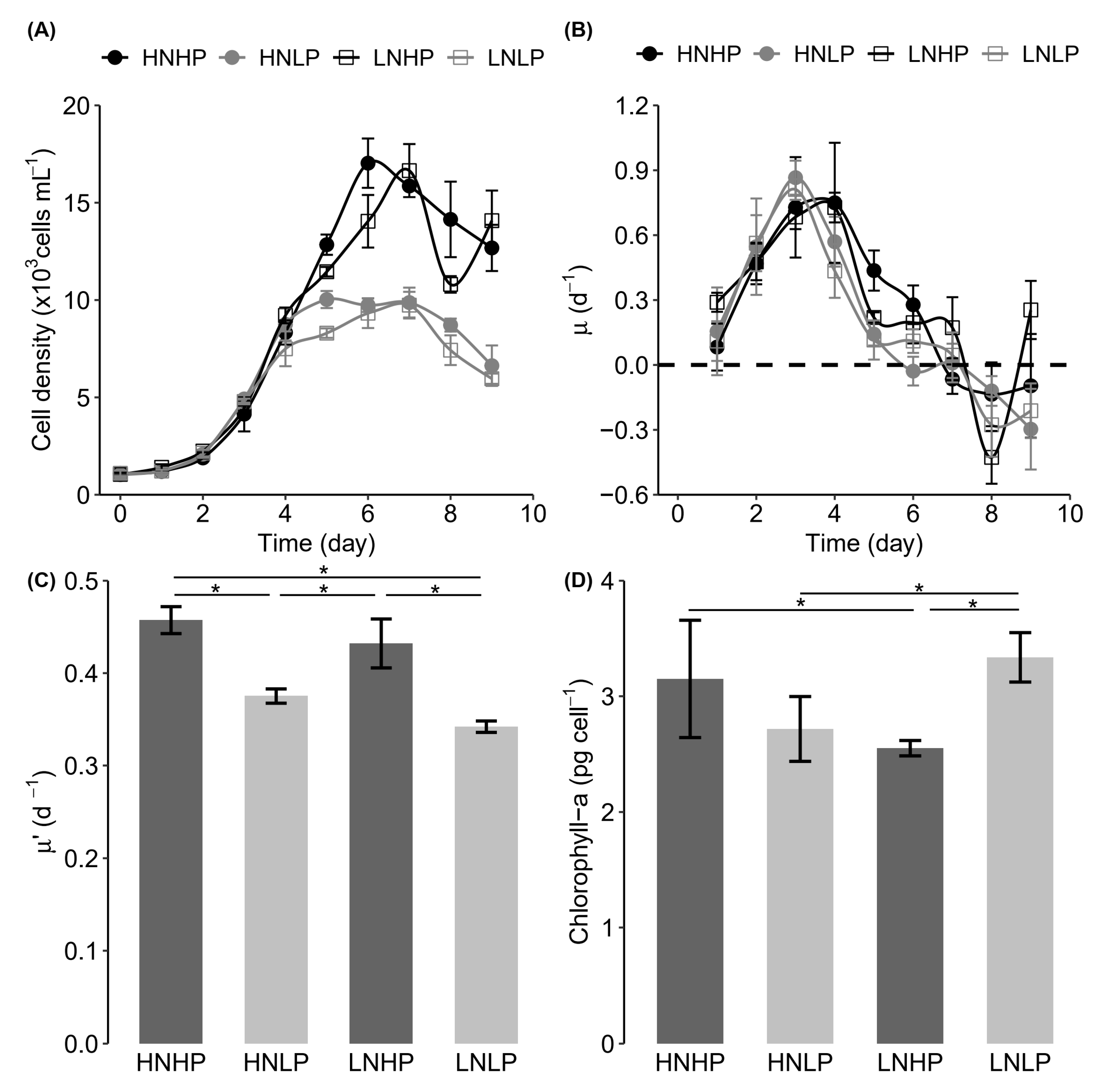
3.1. Growth Response
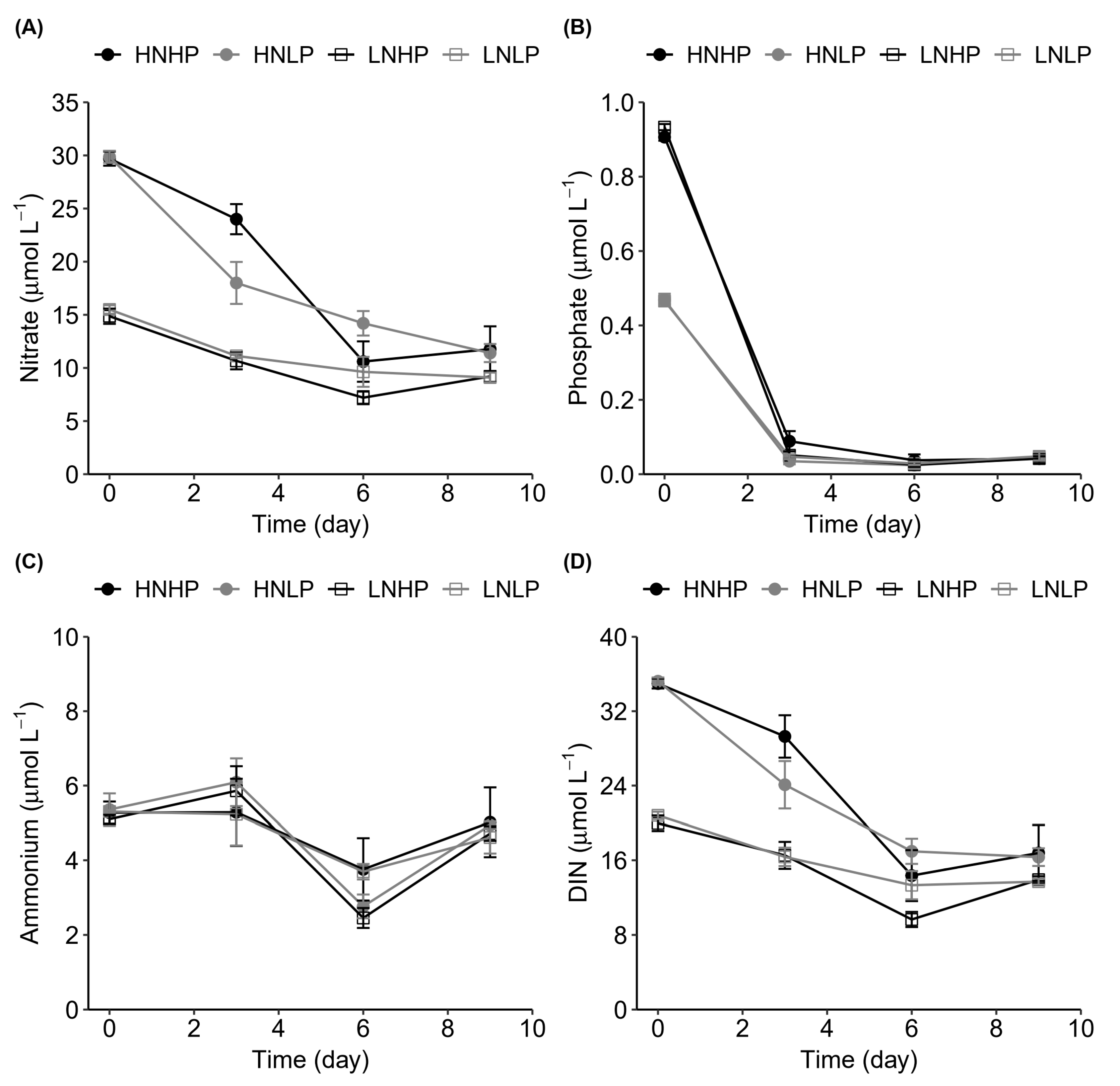
3.2. Nutrient Variation
4. Discussion
4.1. Heterosigma Akashiwo Growth Response to Nutrient Limitation
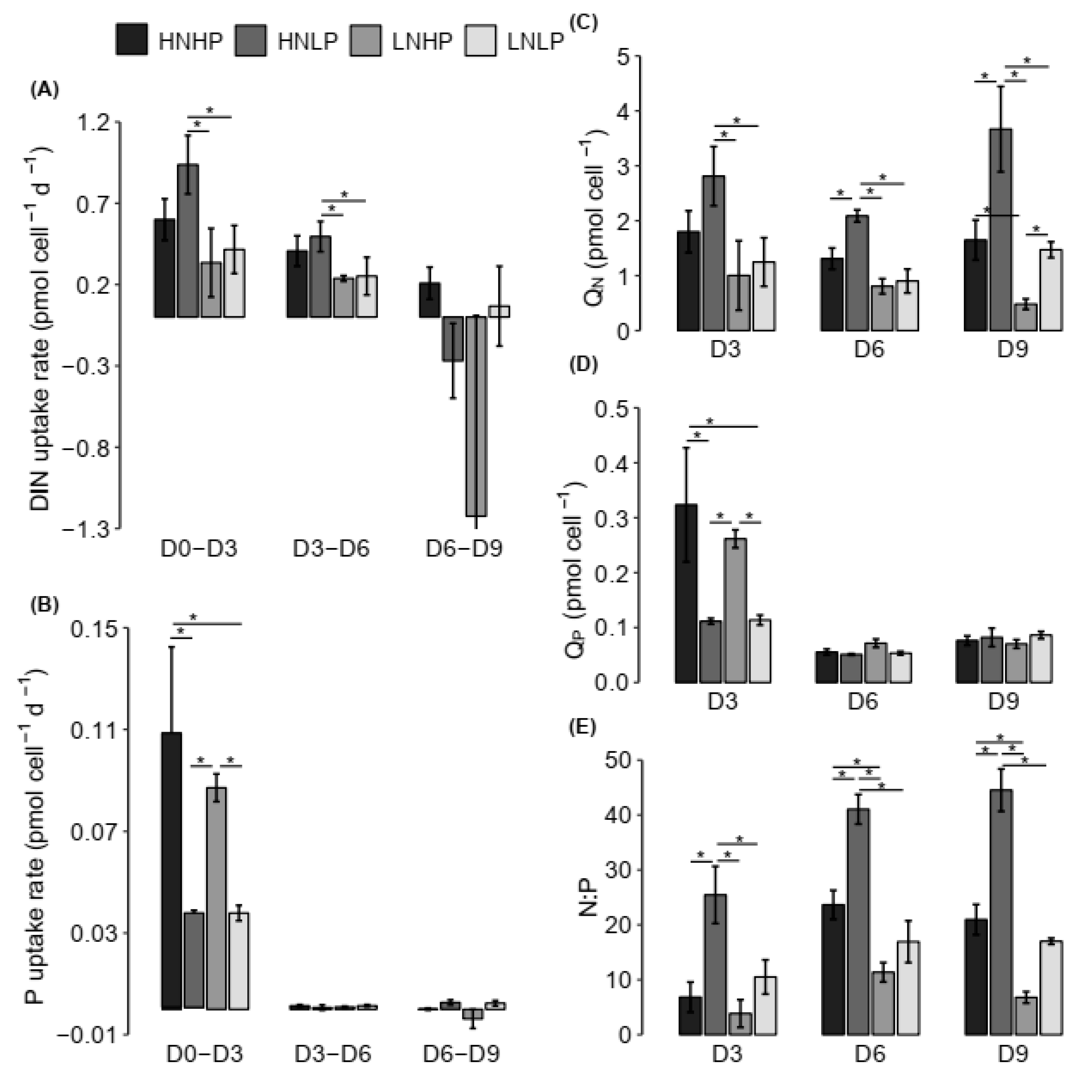
4.2. Nutrient Uptake Dynamics
4.3. Stoichiometry of H. akashiwo
4.4. Applicability of Low-Nutrient Setup
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sha, J.; Xiong, H.; Li, C.; Lu, Z.; Zhang, J.; Zhong, H.; Zhang, W.; Yan, B. Harmful algal blooms and their eco-environmental indication. Chemosphere 2021, 274, 129912. [Google Scholar] [CrossRef] [PubMed]
- Grattan, L.M.; Holobaugh, S.; Morris, J.G. Harmful algal blooms and public health. Harmful Algae 2016, 57, 2–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, M.L.; Karlson, B.; Wulff, A.; Kudela, R.; Trick, C.; Asnaghi, V.; Berdalet, E.; Cochlan, W.; Davidson, K.; De Rijcke, M.; et al. Future HAB science: Directions and challenges in a changing climate. Harmful Algae 2020, 91, 101632. [Google Scholar] [CrossRef] [PubMed]
- Kok, J.W.K.; Yeo, D.C.J.; Leong, S.C.Y. Growth and physiological responses of a tropical toxic marine microalga Heterosigma akashiwo (Heterokontophyta: Raphidophyceae) from Singapore waters to varying nitrogen sources and light conditions. Ocean Sci. J. 2015, 50, 491–508. [Google Scholar] [CrossRef]
- Mehdizadeh Allaf, M.; Trick, C.G. Multiple-stressor design-of-experiment (DOE) and one-factor-at-a-time (OFAT) observations defining Heterosigma akashiwo growth and cell permeability. J. Appl. Phycol. 2019, 31, 3515–3526. [Google Scholar] [CrossRef]
- NOAA Fisheries NWFSC PARR. The Ecophysiology and Toxicity of Heterosigma akashiwo in Puget Sound: A Living Laboratory Ecosystem Approach 2016. Available online: https://www.webapps.nwfsc.noaa.gov/apex/parrdata/inventory/projects/project/581 (accessed on 15 May 2021).
- Kamiyama, T. Change in the microzooplankton community during decay of a Heterosigma akashiwo bloom. J. Oceanogr. 1995, 51, 279–287. [Google Scholar] [CrossRef]
- Lemley, D.A.; Adams, J.B.; Rishworth, G.M.; Purdie, D.A. Harmful algal blooms of Heterosigma akashiwo and environmental features regulate Mesodinium cf. rubrum abundance in eutrophic conditions. Harmful Algae 2020, 100, 101943. [Google Scholar] [CrossRef]
- Jansson, A.; Klais-Peets, R.; Griniené, E.; Rubene, G.; Semenova, A.; Lewandowska, A.; Engström-Öst, J. Functional shifts in estuarine zooplankton in response to climate variability. Ecol. Evol. 2020, 10, 11591–11606. [Google Scholar] [CrossRef]
- Shikata, T.; Yoshikawa, S.; Matsubara, T.; Tanoue, W.; Yamasaki, Y.; Shimasaki, Y.; Ma-tsuyama, Y.; Oshima, Y.; Jenkinson, I.R.; Honjo, T. Growth dynamics of Heterosigma akashiwo (Raphidophyceae) in Hakata Bay, Japan. Eur. J. Phycol. 2008, 43, 395–411. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, F.; Whereat, E.; Coyne, K.J.; Hutchins, D.A. Bottom-up controls on a mixed-species HAB assemblage: A comparison of sympatric Chattonella subsalsa and Heterosigma akashiwo (Raphidophyceae) isolates from the Delaware Inland Bays, USA. Harmful Algae 2006, 5, 310–320. [Google Scholar] [CrossRef]
- Strom, S.L.; Harvey, E.L.; Fredrickson, K.A.; Menden-Deuer, S. Broad Salinity Tolerance as a Refuge from Predation in the Harmful Raphidophyte Alga Heterosigma akashiwo (Raphidophyceae). J. Phycol. 2013, 49, 20–31. [Google Scholar] [CrossRef]
- Butrón, A.; Madariaga, I.; Orive, E. Tolerance to high irradiance levels as a determinant of the bloom-forming Heterosigma akashiwo success in estuarine waters in summer. Estuar. Coast. Shelf Sci. 2012, 107, 141–149. [Google Scholar] [CrossRef]
- Watanabe, M.; Kohata, K.; Kunugi, M. Phosphate accumulation and metabolism by Heterosigma akashiwo (Raphidophyceae) during diel vertical migration in a stratified microcosm. J. Phycol. 1988, 24, 22–28. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, Y. Growth and alkaline phosphatase activity of Chattonella marina and Heterosigma akashiwo in response to phosphorus limitation. J Envitron. Sci.-China 2015, 28, 1–7. [Google Scholar] [CrossRef]
- Herndon, J.; Cochlan, W.P. Nitrogen utilization by the raphidophyte Heterosigma akashiwo: Growth and uptake kinetics in laboratory cultures. Harmful Algae 2007, 6, 260–270. [Google Scholar] [CrossRef]
- Baek, S.H.; Lee, M.; Kim, Y. Spring phytoplankton community response to an episodic windstorm event in oligotrophic waters offshore from the Ulleungdo and Dokdo islands, Korea. J. Sea Res. 2018, 132, 1–14. [Google Scholar] [CrossRef]
- Ye, L.A.; Zhang, H.B.; Fei, Y.J.; Liu, L.; Li, D.L. Nutrient distributions in the East China Sea and changes over the last 25 years. Appl. Ecol. Environ. Res. 2020, 18, 973–985. [Google Scholar] [CrossRef]
- Fu, F.X.; Place, A.R.; Garcia, N.S.; Hutchins, D.A. CO2 and phosphate availability control the toxicity of the harmful bloom dinoflagellate Karlodinium veneficum. Aquat. Microb. Ecol. 2010, 59, 55–65. [Google Scholar] [CrossRef]
- Adolf, J.E.; Bachvaroff, T.R.; Place, A.R. Environmental modulation of karlotoxin levels in strains of the cosmopolitan dinoflagellate Karlodinium veneficum (Dinophyceae). J. Phycol. 2009, 45, 176–192. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Niu, X.; Feng, X.; Goncalves, R.J.; Guan, W. Effects of ocean acidification and phosphate limitation on physiology and toxicity of the dinoflagellate Karenia mikimotoi. Harmful Algae 2019, 87, 101621. [Google Scholar] [CrossRef]
- Guan, W.; Li, P. Dependency of UVR-induced photoinhibition on atomic ratio of N to P in the dinoflagellate Karenia mikimotoi. Mar. Biol. 2017, 164, 31. [Google Scholar] [CrossRef]
- Lema, K.A.; Latimier, M.; Nézan, É.; Fauchot, J.; Le Gac, M. Inter and intra-specific growth and domoic acid production in relation to nutrient ratios and concentrations in Pseu-do-nitzschia: Phosphate an important factor. Harmful Algae 2017, 64, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, H.; Sai, K. Simulating the vertical dynamics of phosphate and their effects on the growth of harmful algae. Estuar. Coast. Shelf Sci. 2015, 164, 425–432. [Google Scholar] [CrossRef]
- Ji, N.; Lin, L.; Li, L.; Yu, L.; Zhang, Y.; Luo, H.; Li, M.; Shi, X.; Wang, D.; Lin, S. Meta-transcriptome analysis reveals environmental and diel regulation of a Heterosigma akashiwo (raphidophyceae) bloom. Environ. Microbiol. 2018, 20, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Maslukah, L.; Zainuri, M.; Wirasatriya, A.; Salma, U. Spatial Distribution of Chlorophyll-a and Its Relationship with Dissolved Inorganic Phosphate Influenced by Rivers in the North Coast of Java. J. Ecol. Eng. 2019, 20, 18–25. [Google Scholar] [CrossRef]
- Guisande, C.; Frangopulos, M.; Maneiro, I.; Vergara, A.R.; Riveiro, I. Ecological advantages of toxin production by the dinoflagellate Alexandrium minutum under phosphorus limitation. Mar. Ecol. Prog. Ser. 2002, 225, 169–176. [Google Scholar] [CrossRef]
- Moreno, A.R.; Martiny, A.C. Ecological Stoichiometry of Ocean Plankton. Annu. Rev. Mar. Sci. 2018, 10, 43–69. [Google Scholar] [CrossRef]
- Dyhrman, S.T.; Chappell, P.D.; Haley, S.T.; Moffett, J.W.; Orchard, E.D.; Waterbury, J.B.; Webb, E.A. Phosphonate utilization by the globally important marine diazotroph Trichodesmium. Nature 2006, 439, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Ou, L.; Hong, H.; Luo, H.; Wang, D. Bioavailability of dissolved organic phosphorus compounds to typical harmful dinoflagellate Prorocentrum donghaiense Lu. Mar. Pollut. Bull. 2005, 51, 838–844. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, Y.; Kang, W. Utilization of dissolved organic phosphorus by different groups of phytoplankton taxa. Harmful Algae 2011, 12, 113–118. [Google Scholar] [CrossRef]
- Watanabe, M.; Takamatsu, T.; Kohata, K.; Kunugi, M.; Kawashima, M.; Koyama, M. Luxury phosphate uptake and variation of intracellular metal concentrations in Heterosigma akashiwo (Raphidophyceae). J. Phycol. 1989, 25, 428–436. [Google Scholar] [CrossRef]
- Pitta, P.; Nejstgaard, J.C.; Tsagaraki, T.M.; Zervoudaki, S.; Egge, J.K.; Frangoulis, C.; Lagaria, A.; Magiopoulos, I.; Psarra, S.; Sandaa, R.; et al. Confirming the “Rapid phosphorus transfer from microorganisms to mesozooplankton in the Eastern Mediterranean Sea” scenario through a mesocosm experiment. J. Plankton Res. 2016, 38, 502–521. [Google Scholar] [CrossRef]
- Hall, S.R. Stoichiometrically Explicit Food Webs: Feedbacks between Resource Supply, Elemental Constraints, and Species Diversity. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 503–528. [Google Scholar] [CrossRef] [Green Version]
- Klausmeier, C.A.; Litchman, E.; Daufresne, T.; Levin, S.A. Optimal nitrogen-to-phosphorus stoichiometry of phytoplankton. Nature 2004, 429, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, Y.; Noman, M.A.; Thangaraj, S.; Sun, J. Physiological Changes and Elemental Ratio of Scrippsiella trochoidea and Heterosigma akashiwo in Different Growth Phase. Water 2021, 13, 132. [Google Scholar] [CrossRef]
- Jin, J.; Liu, S.M.; Ren, J.L. Phosphorus utilization by phytoplankton in the Yellow Sea during spring bloom: Cell surface adsorption and intracellular accumulation. Mar. Chem. 2021, 231, 103935. [Google Scholar] [CrossRef]
- Geider, R.; La Roche, J. Redfield revisited: Variability of C:N:P in marine microalgae and its biochemical basis. Eur. J. Phycol. 2002, 37, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Karpinets, T.V.; Greenwood, D.J.; Sams, C.E.; Ammons, J.T. RNA:protein ratio of the unicellular organism as a characteristic of phosphorous and nitrogen stoichiometry and of the cellular requirement of ribosomes for protein synthesis. BMC Biol. 2006, 4, 30. [Google Scholar] [CrossRef]
- Loladze, I.; Elser, J.J. The origins of the Redfield nitrogen-to-phosphorus ratio are in a homoeostatic protein-to-rRNA ratio. Ecol. Lett. 2011, 14, 244–250. [Google Scholar] [CrossRef]
- Lewitus, A.J.; Schmidt, L.B.; Mason, L.J.; Kempton, J.W.; Wilde, S.B.; Wolny, J.L.; Williams, B.J.; Hayes, K.C.; Hymel, S.N.; Keppler, C.J.; et al. Harmful Algal Blooms in South Carolina Residential and Golf Course Ponds. Popul. Environ. 2003, 24, 387–413. [Google Scholar] [CrossRef]
- Lewitus, A.J.; Brock, L.M.; Burke, M.K.; DeMattio, K.A.; Wilde, S.B. Lagoonal stormwater detention ponds as promoters of harmful algal blooms and eutrophication along the South Carolina coast. Harmful Algae 2008, 8, 60–65. [Google Scholar] [CrossRef]
- Jeong, H.J.; Yoo, Y.D.; Lim, A.S.; Kim, T.; Lee, K.; Kang, C.K. Raphidophyte red tides in Korean waters. Harmful Algae 2013, 30, S41–S52. [Google Scholar] [CrossRef]
- Moon, J.; Lee, K.; Lim, W.; Lee, E.; Dai, M.; Choi, Y.; Han, I.; Shin, K.; Kim, J.; Chae, J. Anthropogenic nitrogen is changing the East China and Yellow seas from being N deficient to being P deficient. Limnol. Oceanogr. 2020, 66, 914–924. [Google Scholar] [CrossRef]
| Scenarios | Concentration of Nitrate Nitrogen (N) | Concentration of Phosphate (P) |
|---|---|---|
| HNHP | 30 μmol L−1 (HN) | 1 μmol L−1 (HP) |
| HNLP | 30 μmol L−1 (HN) | 0.5 μmol L−1 (LP) |
| LNHP | 15 μmol L−1 (LN) | 1 μmol L−1 (HP) |
| LNLP | 15 μmol L−1 (LN) | 0.5 μmol L−1 (LP) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, A.; Bellerby, R.G.J.; Wang, Y.; Li, X. Growth and Nutrient Uptake Characteristics of Heterosigma akashiwo (Raphidophyceae) under Nitrogen and Phosphorus Concentrations in the East China Sea. Water 2021, 13, 3166. https://doi.org/10.3390/w13223166
Yang A, Bellerby RGJ, Wang Y, Li X. Growth and Nutrient Uptake Characteristics of Heterosigma akashiwo (Raphidophyceae) under Nitrogen and Phosphorus Concentrations in the East China Sea. Water. 2021; 13(22):3166. https://doi.org/10.3390/w13223166
Chicago/Turabian StyleYang, Anqiang, Richard G. J. Bellerby, Yanna Wang, and Xiaoshuang Li. 2021. "Growth and Nutrient Uptake Characteristics of Heterosigma akashiwo (Raphidophyceae) under Nitrogen and Phosphorus Concentrations in the East China Sea" Water 13, no. 22: 3166. https://doi.org/10.3390/w13223166
APA StyleYang, A., Bellerby, R. G. J., Wang, Y., & Li, X. (2021). Growth and Nutrient Uptake Characteristics of Heterosigma akashiwo (Raphidophyceae) under Nitrogen and Phosphorus Concentrations in the East China Sea. Water, 13(22), 3166. https://doi.org/10.3390/w13223166





