Temperature Affects the Time Required to Discern the Relationship between Primary Production and Export Production in the Ocean
Abstract
:1. Introduction
2. Food Web Model
3. Results
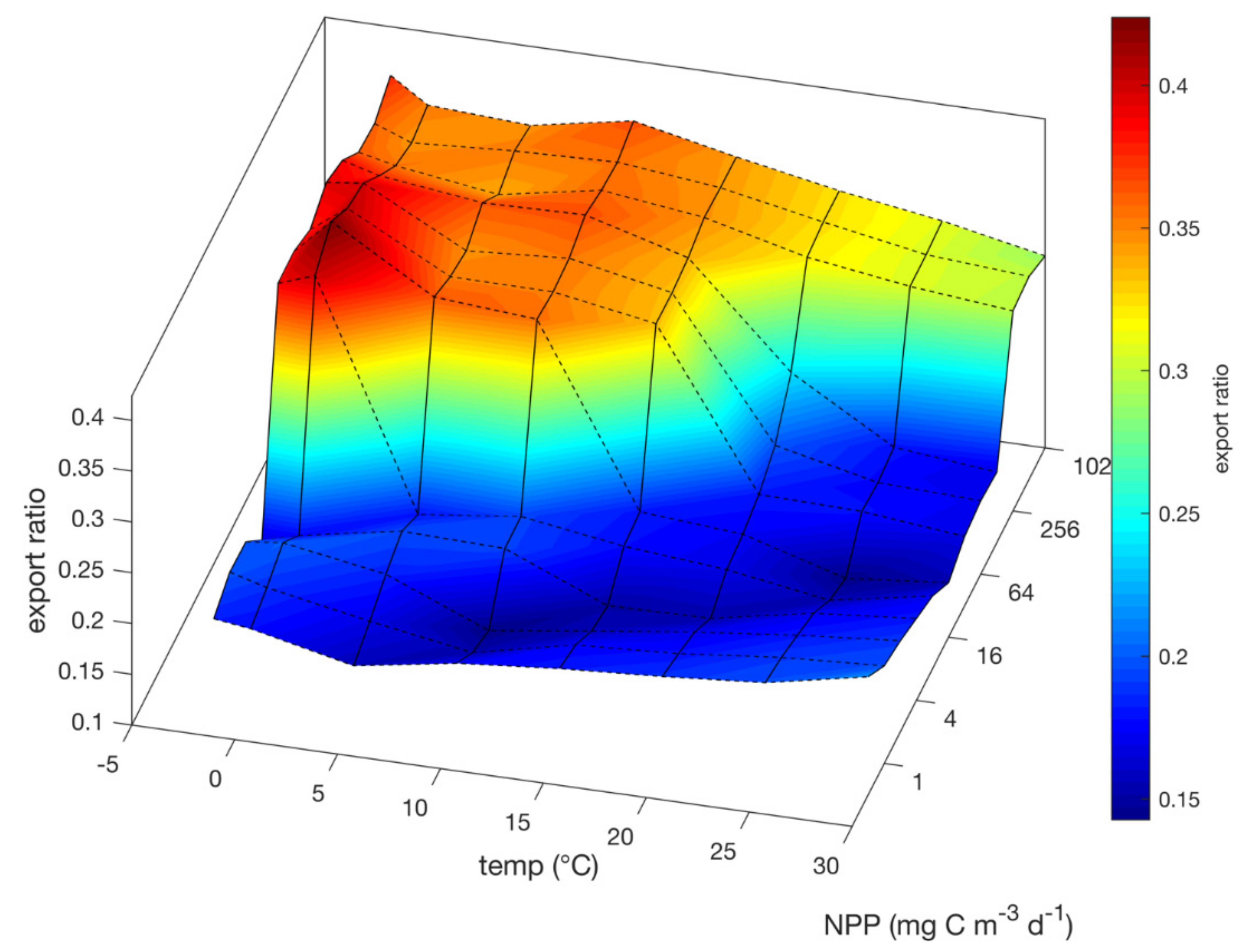
3.1. Steady State Behavior
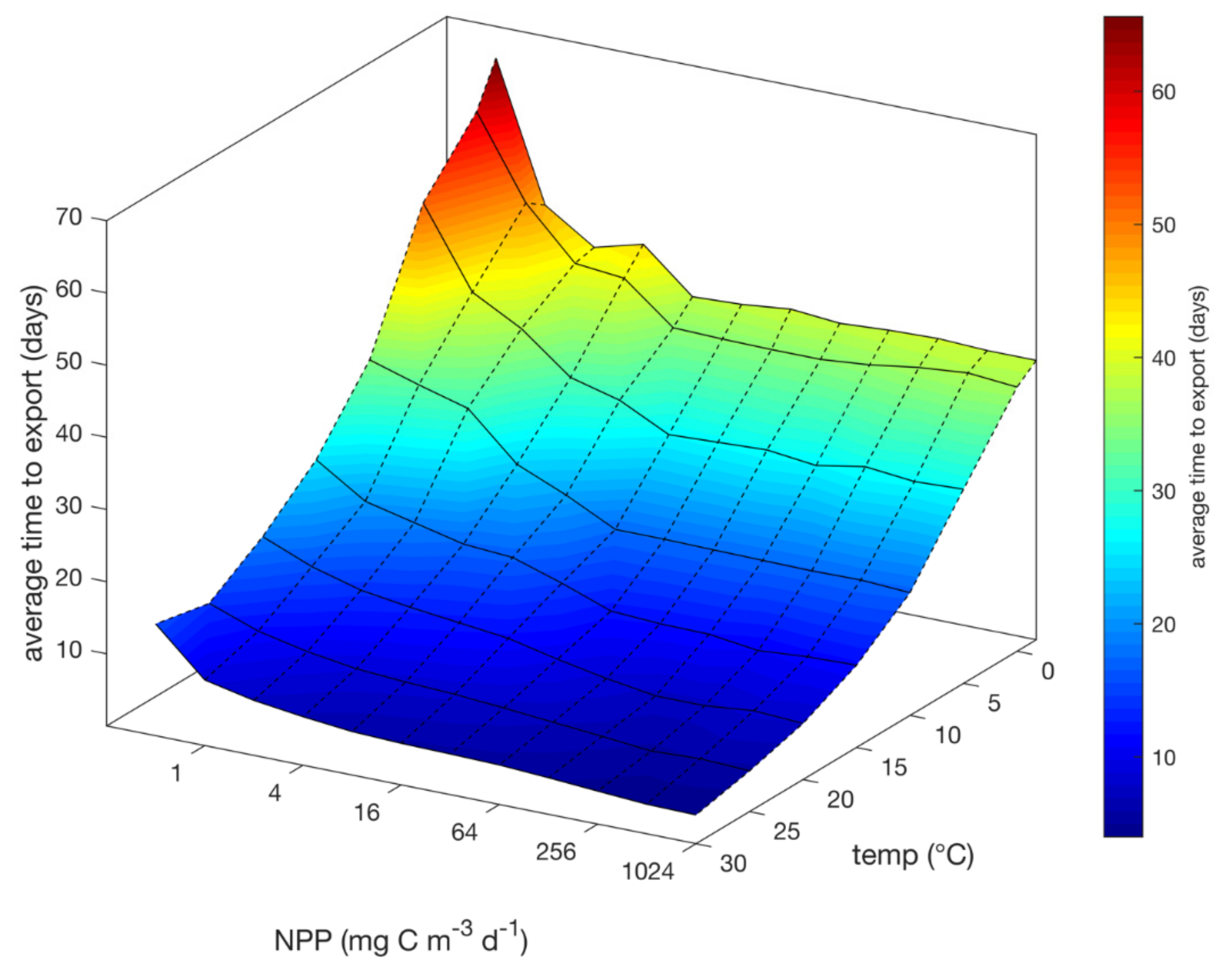
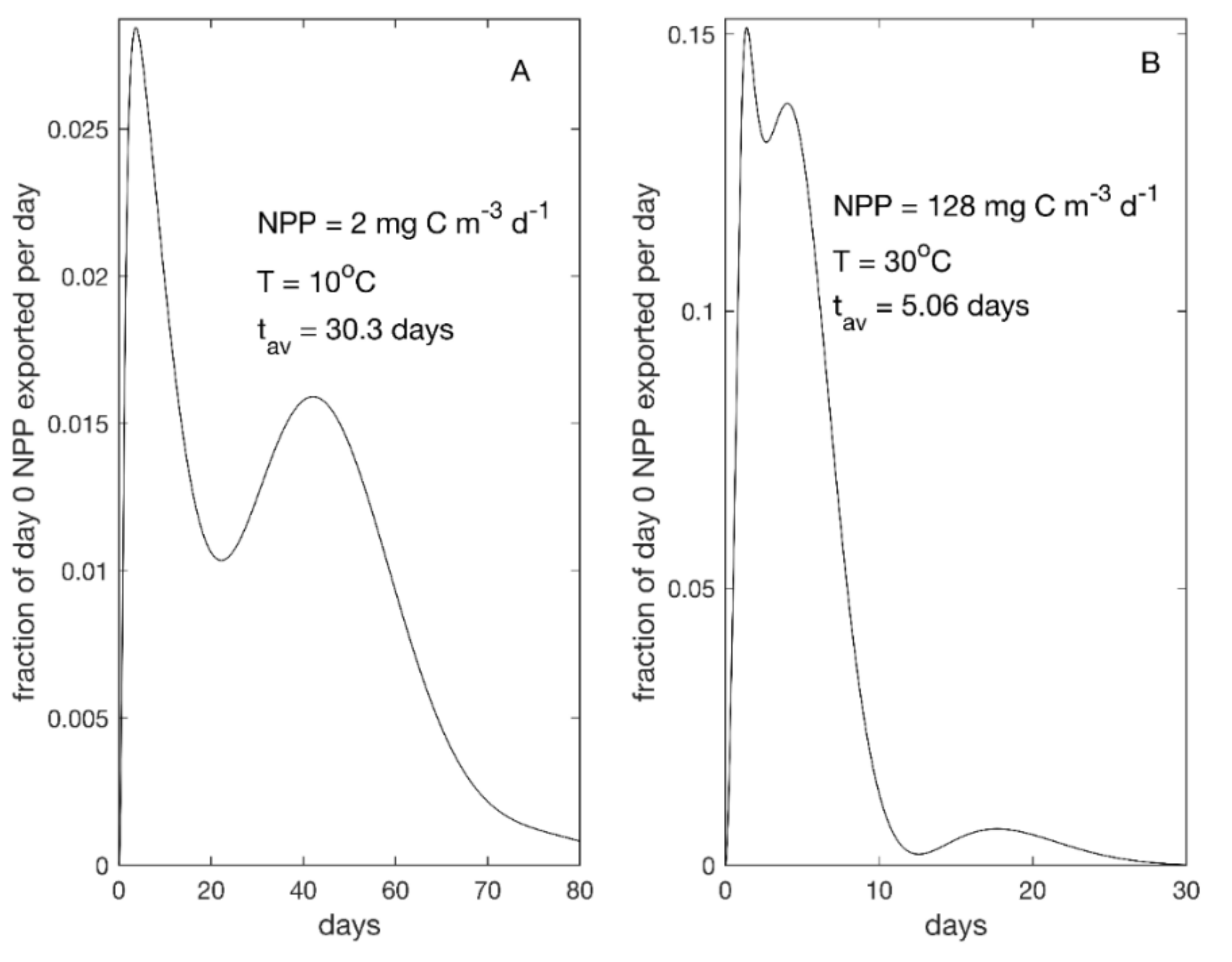
3.2. Time Lags
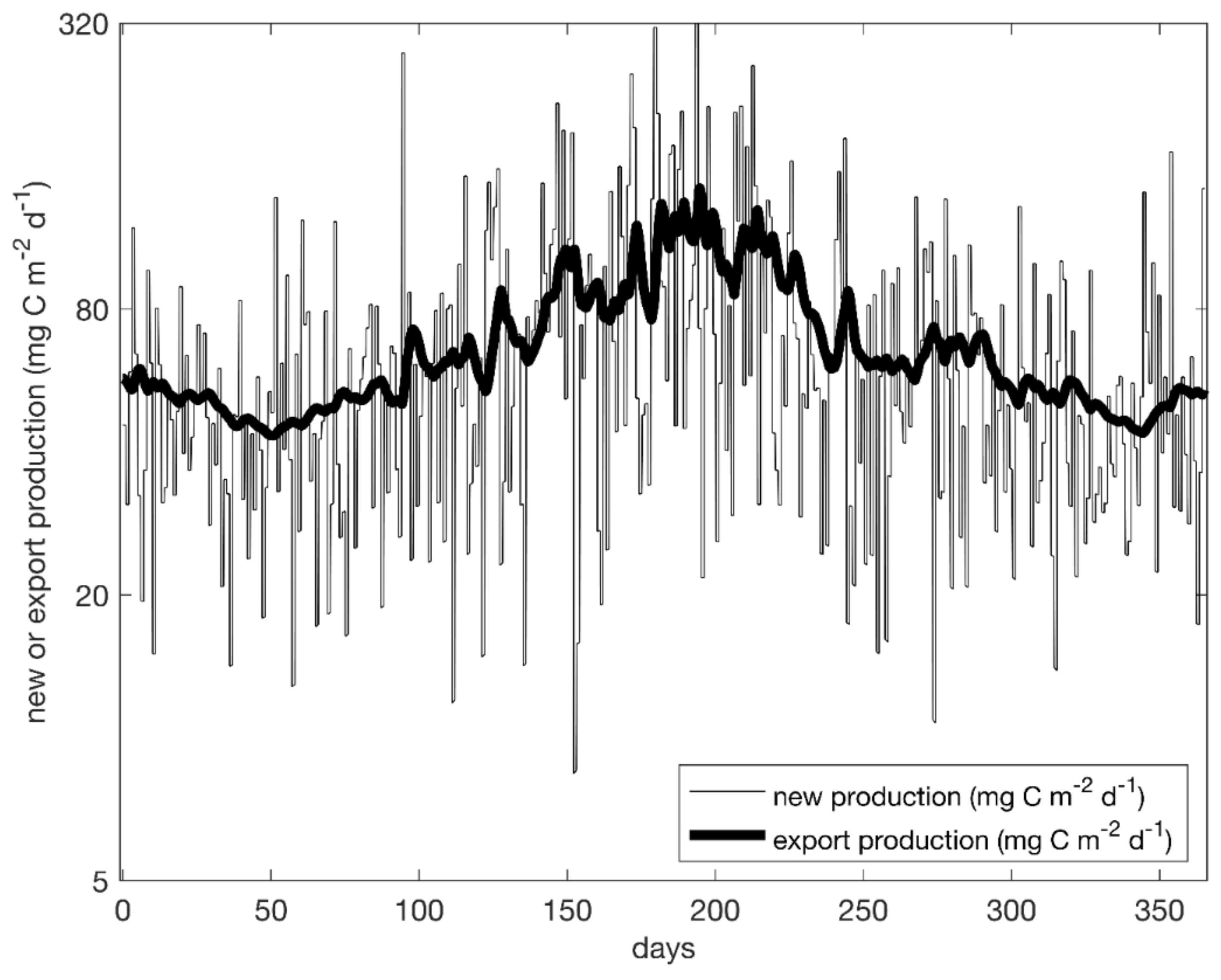
3.3. Resiliency and Response to Perturbations
4. Discussion
4.1. Sources of Error and Caveats
4.2. The Relationship between NPP and EP
4.3. Effects of Lateral Currents
4.4. Time Lags and Temperature
4.5. Steady State or Not
4.6. Response to Perturbations
5. Conclusions and Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarmiento, J.L.; Bender, M. Carbon biogeochemistry and climate change. Photosynth. Res. 1994, 39, 209–234. [Google Scholar] [CrossRef] [PubMed]
- Barone, B.; Nicholson, D.; Ferron, S.; Firing, E.; Karl, D.M. The estimation of gross oxygen production and community respiration from autonomous time-series measurements in the oligotrophic ocean. Limnol. Oceanogr. Methods 2019, 17, 650–664. [Google Scholar] [CrossRef] [Green Version]
- Karl, D.M.; Laws, E.A.; Morris, P.; Williams, P.J.L.; Emerson, S. Metabolic balance of the open sea. Nature 2003, 426, 32. [Google Scholar] [CrossRef] [PubMed]
- Bisson, K.M.; Siegel, D.A.; DeVries, T.; Cael, B.B.; Buesseler, K.O. How Data Set Characteristics Influence Ocean Carbon Export Models. Glob. Biogeochem. Cycles 2018, 32, 1312–1328. [Google Scholar] [CrossRef]
- Henson, S.A.; Yool, A.; Sanders, R. Variability in efficiency of particulate organic carbon export: A model study. Glob. Biogeochem. Cycles 2015, 29, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Buesseler, K.O.; Boyd, P.W. Shedding light on processes that control particle export and flux attenuation in the twilight zone of the open ocean. Limnol. Oceanogr. 2009, 54, 1210–1232. [Google Scholar] [CrossRef] [Green Version]
- Hansell, D.A. DOC in the Global Carbon Cycle. In Biogeochemistry of Marine Dissolved Organic Matter; Hansell, D.A., Carlson, C.A., Eds.; Academic Press: Amsterdam, The Netherlands, 2002; pp. 685–716. [Google Scholar]
- Kahler, P.; Oschlies, A.; Dietze, H.; Koeve, W. Oxygen, carbon, and nutrients in the oligotrophic eastern subtropical North Atlantic. Biogeosciences 2010, 7, 1143–1156. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.L.; Ruhl, H.A.; Huffard, C.L.; Messie, M.; Kahru, M. Episodic organic carbon fluxes from surface ocean to abyssal depths during long-term monitoring in NE Pacific. Proc. Natl. Acad. Sci. USA 2018, 115, 12235–12240. [Google Scholar] [CrossRef] [Green Version]
- Preston, C.M.; Durkin, C.A.; Yamahara, K.M. DNA metabarcoding reveals organisms contributing to particulate matter flux to abyssal depths in the North East Pacific ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 2020, 173, 17. [Google Scholar] [CrossRef]
- Carlson, C.A.; Hansell, D.A.; Nelson, N.B.; Siegel, D.A.; Smethie, W.M.; Khatiwala, S.; Meyers, M.M.; Halewood, E. Dissolved organic carbon export and subsequent remineralization in the mesopelagic and bathypelagic realms of the North Atlantic basin. Deep Sea Res. Part II Top. Stud. Oceanogr. 2010, 57, 1433–1445. [Google Scholar] [CrossRef]
- Carlson, C.A.; Hansell, D.A.; Peltzer, E.T.; Smith, W.O. Stocks and dynamics of dissolved and particulate organic matter in the southern Ross Sea, Antarctica. Deep Sea Res. Part II Top. Stud. Oceanogr. 2000, 47, 3201–3225. [Google Scholar] [CrossRef]
- Dugdale, R.C.; Goering, J.J. Uptake of new and regenerated forms of nitrogen in primary productivity. Limnol. Oceanogr. 1967, 12, 196–206. [Google Scholar] [CrossRef] [Green Version]
- Benitez-Nelson, C.; Buesseler, K.O.; Karl, D.M.; Andrews, J. A time-series study of particulate matter export in the North Pacific Subtropical Gyre based on super(234)Th: Super(238)U disequilibrium. Deep Sea Res. Part I Oceanogr. Res. Pap. 2001, 48, 2595–2611. [Google Scholar] [CrossRef]
- Stange, P.; Bach, L.T.; le Moigne, F.A.C.; Taucher, J.; Boxhammer, T.; Riebesell, U. Quantifying the time lag between organic matter production and export in the surface ocean: Implications for estimates of export efficiency. Geophys. Res. Lett. 2017, 44, 268–276. [Google Scholar] [CrossRef] [Green Version]
- Laws, E.A.; Maiti, K. The relationship between primary production and export production in the ocean: Effects of time lags and temporal variability. Deep Sea Res. Part I Oceanogr. Res. Pap. 2019, 148, 100–107. [Google Scholar] [CrossRef]
- Estapa, M.L.; Siegel, D.A.; Buesseler, K.O.; Stanley, R.H.R.; Lomas, M.W.; Nelson, N.B. Decoupling of net community and export production on submesoscales. Glob. Biogeochem. Cycles 2015, 29, 1266–1282. [Google Scholar] [CrossRef] [Green Version]
- May, R.M. Stability and Complexity in Model Ecosystems; Princeton University Press: Princeton, NJ, USA, 2001; 304p. [Google Scholar]
- Laws, E.A.; Falkowski, P.G.; Smith, W.O.; Ducklow, H.; McCarthy, J.J. Temperature effects on export production in the open ocean. Glob. Biogeochem. Cycles 2000, 14, 1231–1246. [Google Scholar] [CrossRef] [Green Version]
- Steele, J. The Structure of Marine Ecosystems; Harvard University Press: Cambridge, MA, USA, 1974. [Google Scholar]
- Eppley, R.W. Temperature and phytoplankton growth in the sea. Fish. Bull. 1972, 70, 1063–1085. [Google Scholar]
- Laws, E.A.; Redalie, D.G.; Haas, L.W.; Bienfang, P.K.; Eppley, R.W.; Harrison, W.G.; Karl, D.M.; Marra, J. High phytoplankton growth and production rates in oligotrophic Hawaiian coastal waters. Limnol. Oceanogr. 1984, 29, 1161–1169. [Google Scholar] [CrossRef]
- Laws, E.A.; DiTullio, G.R.; Redalje, D.G. High phytoplankton growth and production rates in the North Pacific subtropical gyre. Limnol. Oceanogr. 1987, 32, 905–918. [Google Scholar] [CrossRef]
- Latasa, M.; Landry, M.R.; Schlueter, L.; Bidigare, R.R. Pigment-specific growth and grazing rates of phytoplankton in the central equatorial Pacific. Limnol. Oceanogr. 1997, 42, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Landry, M.R.; Constantinou, J.; Latasa, M.; Brown, S.L.; Bidigare, R.R.; Ondrusek, M.E. Biological response to iron fertilization in the eastern equatorial Pacific (IronEx II). 3. Dynamics of phytoplankton growth and microzooplankton grazing. Mar. Ecol. Prog. Ser. 2000, 201, 57–72. [Google Scholar] [CrossRef] [Green Version]
- White, P.A.; Kalff, J.; Rasmussen, J.B.; Gasol, J.M. The effect of temperature and algal biomass on bacterial production and specific growth rate in freshwater and marine habitats. Microb. Ecol. 1991, 21, 99–118. [Google Scholar] [CrossRef]
- Hobbie, J.E.; Cole, J.J. Response of a detrital food web to eutrophication. Bull. Mar. Sci. 1984, 35, 357–363. [Google Scholar]
- Huntley, M.E.; Lopez, M.D.G. Temperature-dependent production of marine copepods: A global synthesis. Am. Nat. 1992, 140, 201–242. [Google Scholar] [CrossRef]
- Caperon, J.; Meyer, J. Nitrogen-limited growth of marine phytoplankton—I. Changes in population characteristics with steady-state growth rate. Deep. Sea Res. Oceanogr. Abstr. 1972, 19, 601–618. [Google Scholar] [CrossRef]
- Sunda, W.G.; Hardison, D.R. Ammonium uptake and growth limitation in marine phytoplankton. Limnol. Oceanogr. 2007, 52, 2496–2506. [Google Scholar] [CrossRef]
- Eppley, R.W.; Peterson, B.J. Particulate organic matter flux and planktonic new production in the deep ocean. Nature 1979, 282, 677–680. [Google Scholar] [CrossRef]
- Downs, J.N. Export Production in Oceanic Systems: Information from Phaeopigment Carbon and Nitrogen Analysis, in Oceanography. Ph.D. Thesis, University of Washington, Seattle, WA, USA, 1989. [Google Scholar]
- Murray, J.W.; Downs, J.; Strom, S.; Wei, C.-L.; Jannasch, H.W. Nutrient assimilation, export production, and 234Th scavenging in the eastern equatorial Pacific. Deep Sea Res. Part A. Oceanogr. Res. Pap. 1989, 36, 1471–1489. [Google Scholar] [CrossRef]
- Redfield, A.C.; Ketchum, B.H.; Richard, F.A. The Influence of Organisms on the Composition of Seawater. In The Sea; Hill, M.N., Ed.; Interscience: New York, NY, USA, 1963; pp. 27–77. [Google Scholar]
- Dunne, J.P.; Armstrong, R.A.; Gnanadesikan, A.; Sarmiento, J.L. Empirical and mechanistic models for the particle export ratio. Glob. Biogeochem. Cycles 2005, 19, 16. [Google Scholar] [CrossRef]
- Knauer, G.A.; Redalje, D.G.; Harrison, W.G.; Karl, D.M. New production at the VERTEX time-series site. Deep Sea Res. Part A Oceanogr. Res. Pap. 1990, 37, 1121–1134. [Google Scholar] [CrossRef]
- Buesseler, K.O.; Benitez-Nelson, C.R.; Moran, S.B.; Burd, A.; Charette, M.; Cochran, J.K.; Coppola, L.; Fisher, N.S.; Fowler, S.W.; Gardner, W.D.; et al. An assessment of particulate organic carbon to thorium-234 ratios in the ocean and their impact on the application of Th-234 as a POC flux proxy. Mar. Chem. 2006, 100, 213–233. [Google Scholar] [CrossRef] [Green Version]
- Grabowski, E.; Letelier, R.M.; Laws, E.A.; Karl, D.M. Coupling carbon and energy fluxes in the North Pacific Subtropical Gyre. Nat. Commun. 2019, 10, 9. [Google Scholar] [CrossRef]
- Christian, J.R.; Karl, D.M. Microbial community structure at the U.S.-Joint Global Ocean Flux study station ALOHA: Inverse methods for estimating biochemical indicator ratios. J. Geophys. Res. Ocean. 1994, 99, 14269–14276. [Google Scholar] [CrossRef]
- Goldman, J.C.; Caron, D.A.; Dennett, M.R. Regulation of gross growth efficiency and ammonium regeneration in bacteria by substrae C:N ratio. Limnol. Oceanogr. 1987, 32, 1239–1252. [Google Scholar] [CrossRef]
- Linley, E.A.S.; Newell, R.C. Estimates of Bacterial Growth Yields Based on Plant Detritus. Bull. Mar. Sci. 1984, 35, 409–425. [Google Scholar]
- Kirchman, D.L.; Suzuki, Y.; Garside, C.; Ducklow, H.W. High Turnover Rates of Dissolved Organic-Carbon During a Spring Phytoplankton Bloom. Nature 1991, 352, 612–614. [Google Scholar] [CrossRef]
- Bjornsen, P.K. Bacterioplankton Growth-Yield in Continuous Seawater Cultures. Mar. Ecol. Prog. Ser. 1986, 30, 191–196. [Google Scholar] [CrossRef]
- Tranvik, L.J. Availability of Dissolved Organic-Carbon for Planktonic Bacteria in Oligotrophic Lakes of Differing Humic Content. Microb. Ecol. 1988, 16, 311–322. [Google Scholar] [CrossRef]
- Middelboe, M.; Sondergaard, M. Bacterioplankton Growth Yield: Seasonal-Variations and Coupling to Substrate Lability And Beta-Glucosidase Activity. Appl. Environ. Microbiol. 1993, 59, 3916–3921. [Google Scholar] [CrossRef] [Green Version]
- Carlson, C.A.; Ducklow, H.W. Growth of bacterioplankton and consumption of dissolved organic carbon in the Sargasso Sea. Aquat. Microb. Ecol. 1996, 10, 69–85. [Google Scholar] [CrossRef] [Green Version]
- Giorgio, P.A.; Cole, J.J. Bacterial growth efficiency in natural aquatic systems. Annu. Rev. Ecol. Syst. 1998, 29, 503–541. [Google Scholar] [CrossRef] [Green Version]
- Buesseler, K.O.; Boyd, P.W.; Black, E.E.; Siegel, D.A. Metrics that matter for assessing the ocean biological carbon pump. Proc. Natl. Acad. Sci. USA 2020, 117, 9679–9687. [Google Scholar] [CrossRef] [Green Version]
- Laws, E.A.; Letelier, R.M.; Karl, D.M. Estimating the compensation irradiance in the ocean: The importance of accounting for non-photosynthetic uptake of inorganic carbon. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2014, 93, 35–40. [Google Scholar] [CrossRef]
- Buesseler, K.O. The decoupling of production and particulate export in the surface ocean. Glob. Biogeochem. Cycles 1998, 12, 297–310. [Google Scholar] [CrossRef]
- Karl, D.M.; Christian, J.R.; Dore, J.E.; Hebel, D.V.; Letelier, R.M.; Tupas, L.M.; Winn, C.D. Seasonal and interannual variability in primary production and particle flux at Station ALOHA. Deep Sea Res. Part II Top. Stud. Oceanogr. 1996, 43, 539–568. [Google Scholar] [CrossRef]
- Michaels, A.F.; Knap, A.H. Overview of the US JGOFS Bermuda Atlantic Time-series Study and the Hydrostation S program. Deep Sea Res. Part II Top. Stud. Oceanogr. 1996, 43, 157–198. [Google Scholar] [CrossRef]
- Henson, S.; Le Moigne, F.; Giering, S. Drivers of Carbon Export Efficiency in the Global Ocean. Glob. Biogeochem. Cycles 2019, 33, 891–903. [Google Scholar] [CrossRef]
- Armstrong, R.A.; Lee, C.; Hedges, J.I.; Honjo, S.; Wakeham, S.G. A new, mechanistic model for organic carbon fluxes in the ocean based on the quantitative association of POC with ballast minerals. Deep Sea Res. Part II Top. Stud. Oceanogr. 2002, 49, 219–236. [Google Scholar] [CrossRef]
- Feng, Y.; Hare, C.E.; Leblanc, K.; Rose, J.M.; Zhang, Y.; DiTullio, G.R.; Lee, P.A.; Wilhelm, S.W.; Rowe, J.M.; Sun, J.; et al. Effects of increased pCO2 and temperature on the North Atlantic spring bloom. I. The phytoplankton community and biogeochemical response. Mar. Ecol. Prog. Ser. 2009, 388, 13–25. [Google Scholar] [CrossRef]
- Passow, U.; Alldredge, A.L.; Logan, B.E. The role of particulate carbohydrate exudates in the flocculation of diatom blooms. Deep Sea Res. Part I Oceanogr. Res. Pap. 1994, 41, 335–357. [Google Scholar] [CrossRef]
- Passow, U.; Shipe, R.F.; Murray, A.; Pak, D.K.; Brzezinski, M.A.; Alldredge, A.L. The origin of transparent exopolymer particles (TEP) and their role in the sedimentation of particulate matter. Cont. Shelf Res. 2001, 21, 327–346. [Google Scholar] [CrossRef] [Green Version]
- Ebersbach, F.; Trull, T.W. Sinking particle properties from polyacrylamide gels during the KErguelen Ocean and Plateau compared Study (KEOPS): Zooplankton control of carbon export in an area of persistent natural iron inputs in the Southern Ocean. Limnol. Oceanogr. 2008, 53, 212–224. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.L.; Sherman, A.D.; Huffard, C.L.; McGill, P.R.; Henthorn, R.; Von Thun, S.; Ruhl, H.A.; Kahru, M.; Ohman, M.D. Large salp bloom export from the upper ocean and benthic community response in the abyssal northeast Pacific: Day to week resolution. Limnol. Oceanogr. 2014, 59, 745–757. [Google Scholar] [CrossRef]
- Moore, J.K.; Doney, S.C.; Lindsay, K. Upper ocean ecosystem dynamics and iron cycling in a global three-dimensional model. Glob. Biogeochem. Cycles 2004, 18, 21. [Google Scholar] [CrossRef]
- Ducklow, H.W.; Erickson, M.; Kelly, J.; Montes-Hugo, M.; Ribic, C.A.; Smith, R.C.; Stammerjohn, S.E.; Karl, D.M. Particle export from the upper ocean over the continental shelf of the west Antarctic Peninsula: A long-term record, 1992–2007. Deep Sea Res. Part II Top. Stud. Oceanogr. 2008, 55, 2118–2131. [Google Scholar] [CrossRef]
- Siegel, D.A.; Deuser, W.G. Trajectories of sinking particles in the Sargasso Sea: Modeling of statistical funnels above deep-ocean sediment traps. Deep Sea Res. Part I Oceanogr. Res. Pap. 1997, 44, 1519–1541. [Google Scholar] [CrossRef]
- Buesseler, K.O.; Pike, S.; Maiti, K.; Lamborg, C.H.; Siegel, D.A.; Trull, T.W. Thorium-234 as a tracer of spatial, temporal and vertical variability in particle flux in the North Pacific. Deep Sea Res. Part I Oceanogr. Res. Pap. 2009, 56, 1143–1167. [Google Scholar] [CrossRef]
- Boyd, P.W.; Jickells, T.; Law, C.S.; Blain, S.; Boyle, E.A.; Buesseler, K.O.; Coale, K.H.; Cullen, J.J.; De Baar, H.J.; Follows, M.; et al. Mesoscale iron enrichment experiments 1993–2005: Synthesis and future directions. Science 2007, 315, 612–617. [Google Scholar] [CrossRef] [Green Version]
- Forest, A.; Wassmann, P.; Slagstad, D.; Bauerfeind, E.; Nothig, E.M.; Klages, M. Relationships between primary production and vertical particle export at the Atlantic-Arctic boundary (Fram Strait, HAUSGARTEN). Polar Biol. 2010, 33, 1733–1746. [Google Scholar] [CrossRef]
- Alkire, M.B.; D’Asaro, E.; Lee, C.; Perry, M.J.; Gray, A.; Cetinić, I.; Briggs, N.; Rehm, E.; Kallin, E.; Kaiser, J.; et al. Estimates of net community production and export using high-resolution, Lagrangian measurements of O2, NO3−, and POC through the evolution of a spring diatom bloom in the North Atlantic. Deep Sea Res. Part I Oceanogr. Res. Pap. 2012, 64, 157–174. [Google Scholar] [CrossRef]
- Giering, S.L.C.; Sanders, R.; Martin, A.P.; Henson, S.A.; Riley, J.S.; Marsay, C.M.; Johns, D.G. Particle flux in the oceans: Challenging the steady state assumption. Glob. Biogeochem. Cycles 2017, 31, 159–171. [Google Scholar] [CrossRef] [Green Version]
- Emerson, S.; Stump, C. Net biological oxygen production in the ocean-II: Remote in situ measurements of O2 and N2 in subarctic pacific surface waters. Deep Sea Res. Part I Oceanogr. Res. Pap. 2010, 57, 1255–1265. [Google Scholar] [CrossRef]
- Juranek, L.W.; Quay, P.D.; Feely, R.A.; Lockwood, D.; Karl, D.M.; Church, M.J. Biological production in the NE Pacific and its influence on air-sea CO2 flux: Evidence from dissolved oxygen isotopes and O2/Ar. J. Geophys. Res. Ocean. 2012, 117, 23. [Google Scholar] [CrossRef] [Green Version]
- Quay, P.; Stutsman, J. Surface layer carbon budget for the subtropical N. Pacific: δ13C constraints at station ALOHA. Deep Sea Res. Part I Oceanogr. Res. Pap. 2003, 50, 1045–1061. [Google Scholar] [CrossRef]
- Yang, B.; Emerson, S.R.; Quay, P.D. The Subtropical Ocean’s Biological Carbon Pump Determined From O2 and DIC/(DIC)-C-13 Tracers. Geophys. Res. Lett. 2019, 46, 5361–5368. [Google Scholar] [CrossRef]
- Chacko, N. Chlorophyll bloom in response to tropical cyclone Hudhud in the Bay of Bengal: Bio-Argo subsurface observations. Deep Sea Res. Part I Oceanogr. Res. Pap. 2017, 124, 66–72. [Google Scholar] [CrossRef]
- Mignot, A.; Claustre, H.; Uitz, J.; Poteau, A.; D’Ortenzio, F.; Xing, X.G. Understanding the seasonal dynamics of phytoplankton biomass and the deep chlorophyll maximum in oligotrophic environments: A Bio-Argo float investigation. Glob. Biogeochem. Cycles 2014, 28, 856–876. [Google Scholar] [CrossRef]
- Xing, X.G.; Morel, A.; Claustre, H.; Antoine, D.; D’Ortenzio, F.; Poteau, A.; Mignot, A. Combined processing and mutual interpretation of radiometry and fluorimetry from autonomous profiling Bio-Argo floats: Chlorophyll a retrieval. J. Geophys. Res. Ocean. 2011, 116, 14. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; Falkowski, P.G. Photosynthetic rates derived from satellite-based chlorophyll concentration. Limnol. Oceanogr. 1997, 42, 1–20. [Google Scholar] [CrossRef]
- Sonnerup, R.E.; Mecking, S.; Bullister, J.L.; Warner, M.J. Transit time distributions and oxygen utilization rates from chlorofluorocarbons and sulfur hexafluoride in the Southeast Pacific Ocean. J. Geophys. Res. Ocean. 2015, 120, 3761–3776. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Roberts, D.C.; Masson-Delmotte, V.; Zhai, P.; Tignor, M.; Poloczanska, E.; Weyer, N.M. (Eds.) IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2019; p. 755. [Google Scholar]




| Parameter | Value |
|---|---|
| q2S | 0.70 |
| q2L | 0.70 |
| q3 | 0.20 |
| q4 | 0.20 |
| q5 | 0.30 |
| q6 | 0.35 |
| qB | 0.15 |
| r2S | 0.00 |
| r2L | 0.85 |
| r3 | 0.3 |
| r4 | 0.3 |
| r5 | 0.3 |
| r6 | 0.5 |
| A2S | (1.2/q2S)e0.0633(T − 25) |
| A2L | (1.2/q2L)e0.0633(T − 25) |
| A3 | (2.4/q3)e0.1(T − 25) |
| A4 | (2.4/q4)e0.1(T − 25) |
| A5 | 0.5e0.1(T − 25) |
| A6 | 0.5e0.1(T − 25) |
| AB | (1.2/qB)e0.0633(T − 25) |
| P2S | 7.5 nM |
| P2L | 75 nM |
| PB | 7.5 nM |
| Observed | Predicted by Model | |
|---|---|---|
| NPP (mg C m−2 d−1) | 415 | |
| Temperature (°C) | 24.64 | |
| EP (mg C m−2 d−1) | 55.1 | 66.4 |
| ef ratio | 0.13 | 0.16 |
| Small phytoplankton carbon (mg C m−3) | 3.63 | |
| Large phytoplankton carbon (mg C m−3) | 1.64 | |
| Chlorophyll a (mg m−3) | 0.109 | |
| Phytoplankton C/chl a | 50 | 48.3 a |
| Heterotrophic bacterial carbon (mg C m−3) | 4 | 4.8 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laws, E.; Maiti, K. Temperature Affects the Time Required to Discern the Relationship between Primary Production and Export Production in the Ocean. Water 2021, 13, 3085. https://doi.org/10.3390/w13213085
Laws E, Maiti K. Temperature Affects the Time Required to Discern the Relationship between Primary Production and Export Production in the Ocean. Water. 2021; 13(21):3085. https://doi.org/10.3390/w13213085
Chicago/Turabian StyleLaws, Edward, and Kanchan Maiti. 2021. "Temperature Affects the Time Required to Discern the Relationship between Primary Production and Export Production in the Ocean" Water 13, no. 21: 3085. https://doi.org/10.3390/w13213085
APA StyleLaws, E., & Maiti, K. (2021). Temperature Affects the Time Required to Discern the Relationship between Primary Production and Export Production in the Ocean. Water, 13(21), 3085. https://doi.org/10.3390/w13213085







