Exploring the Iron Oxide Functionalized Biobased Carbon-silica-polyethyleneimine Composites for Hexavalent Chromium Removal from Dilute Aqueous Solutions
Abstract
:1. Introduction
2. Experimental Methods
2.1. Chemical Reagents
2.2. Preparation of Adsorbents
- (i)
- Hydroxylation of AC
- (ii)
- Preparation of iron oxide modified AC (AC-Fe3O4)
- (iii)
- Preparation of AC-Fe3O4-SiO2
- (iv)
- Preparation of amino-modified adsorbent (AC-Fe3O4-SiO2-PEI)
2.3. Adsorption Experiments
2.4. Adsorption Isotherms
3. Results and Discussion
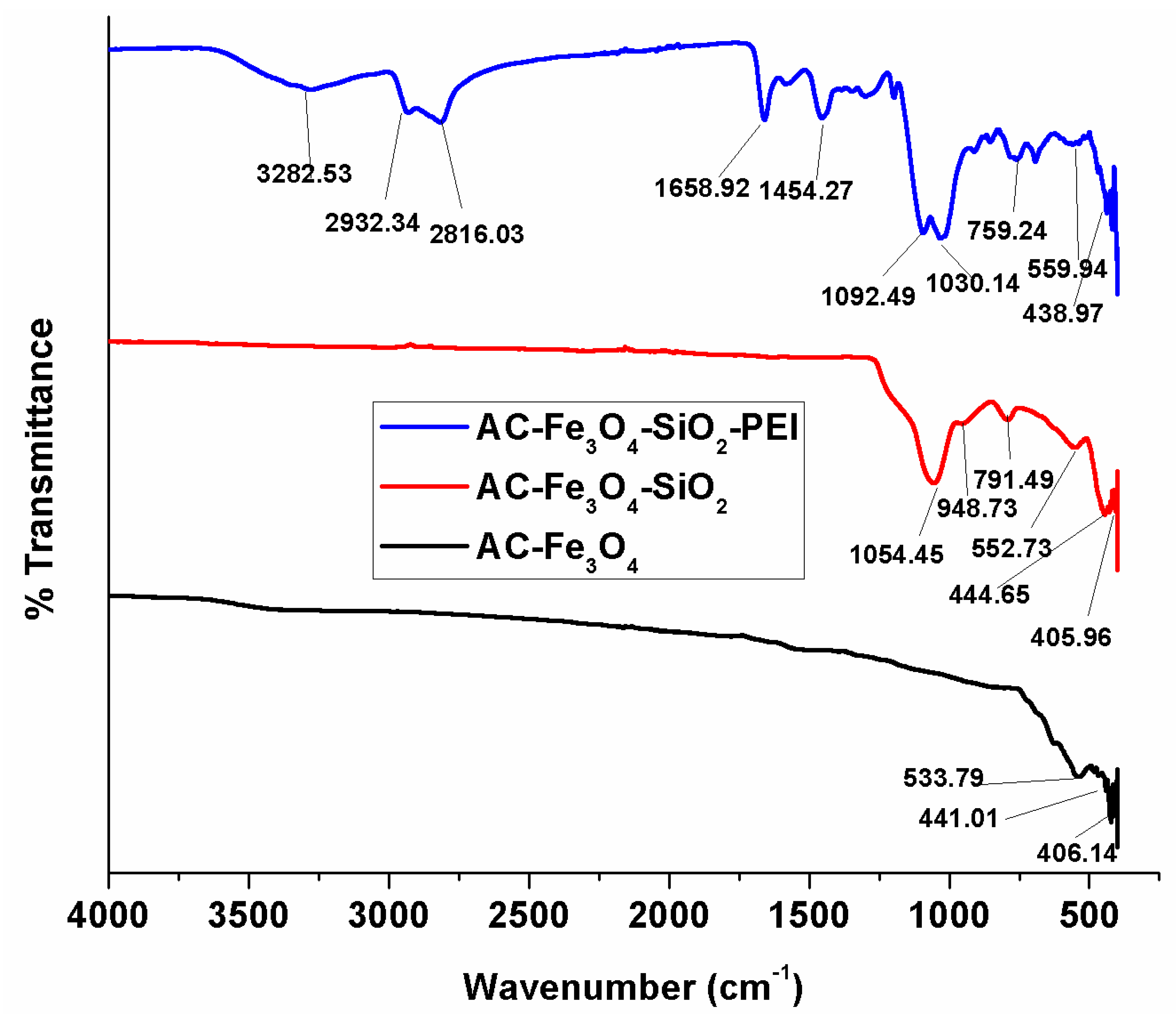
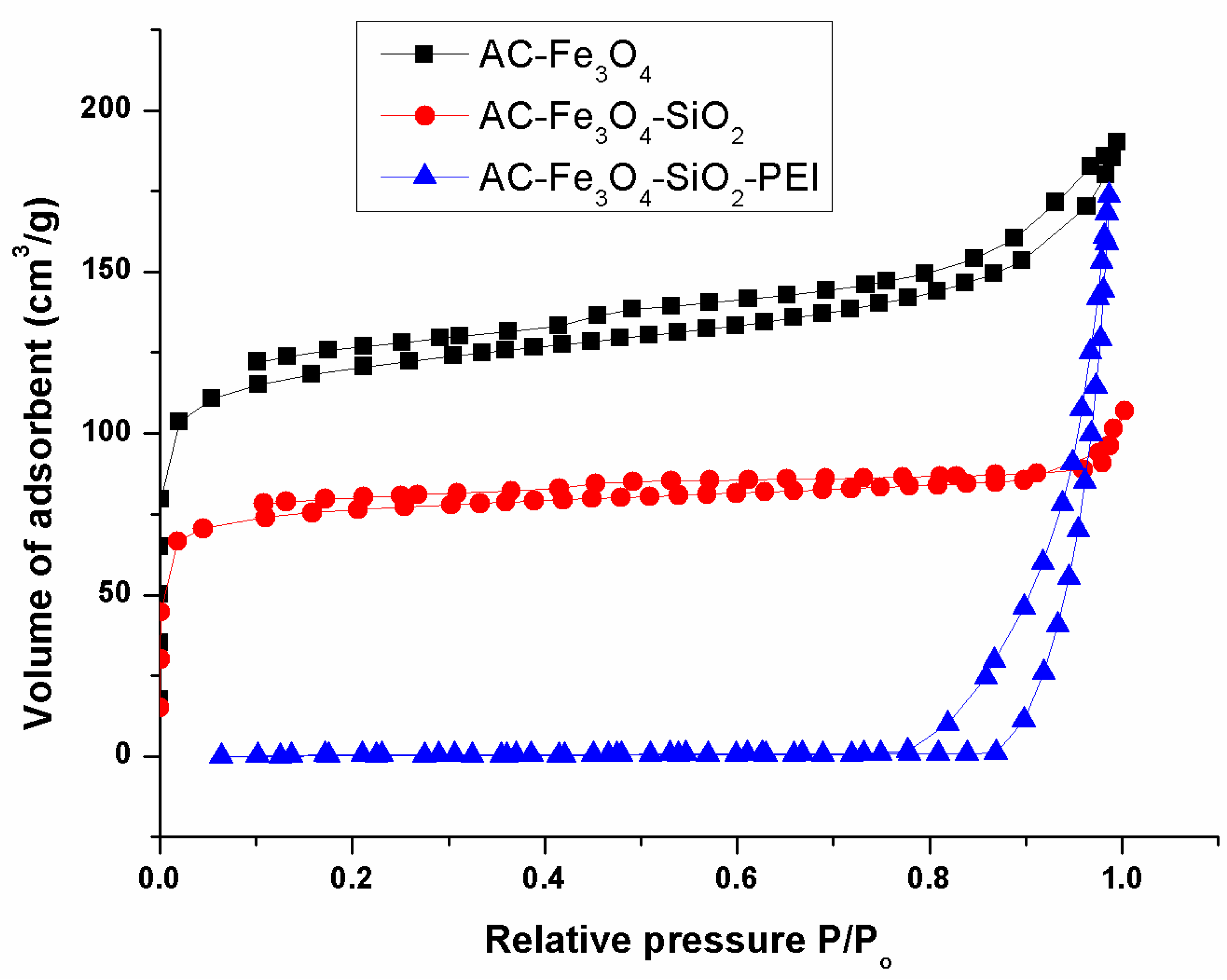
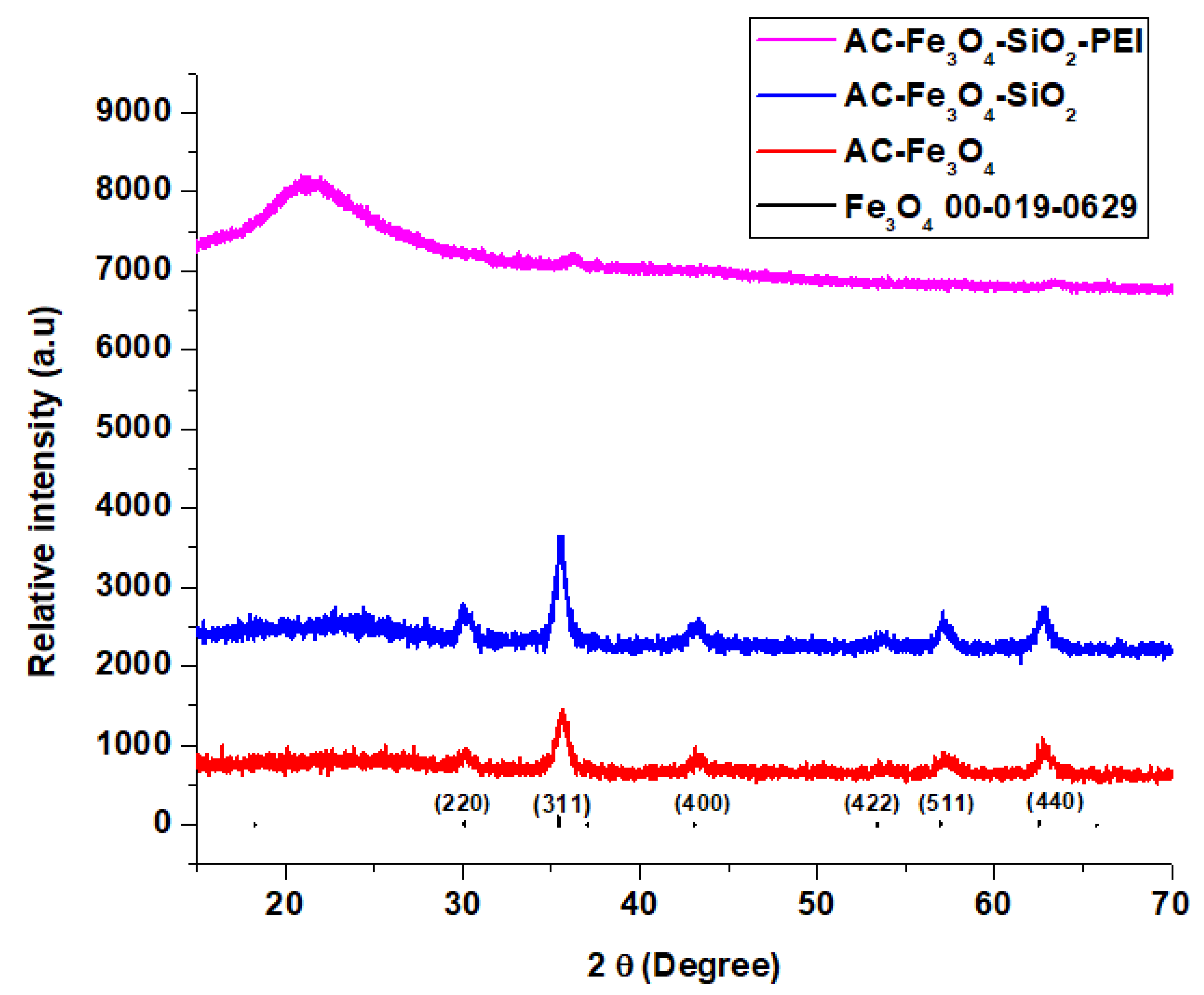
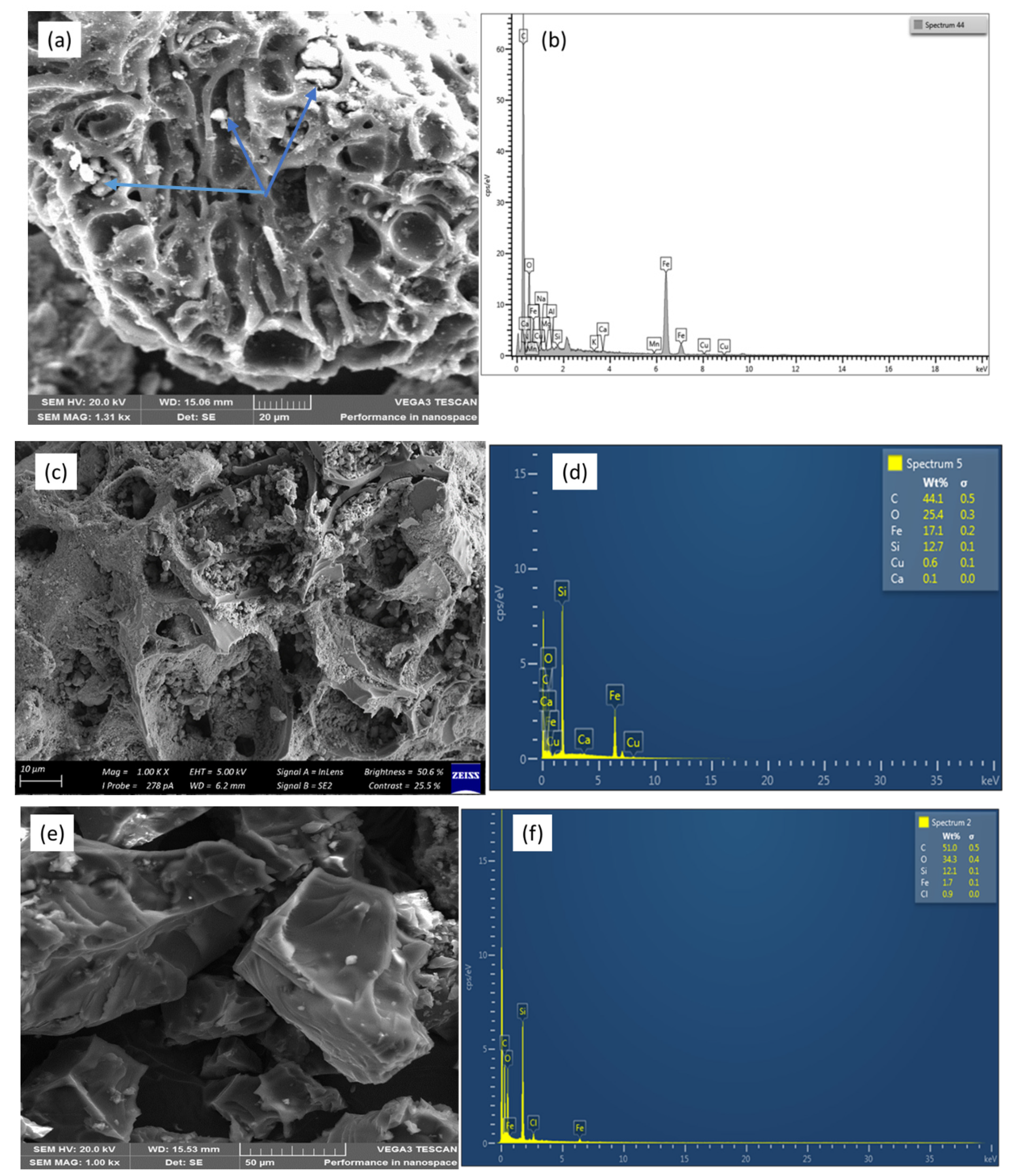
3.1. Adsorbent Characterization
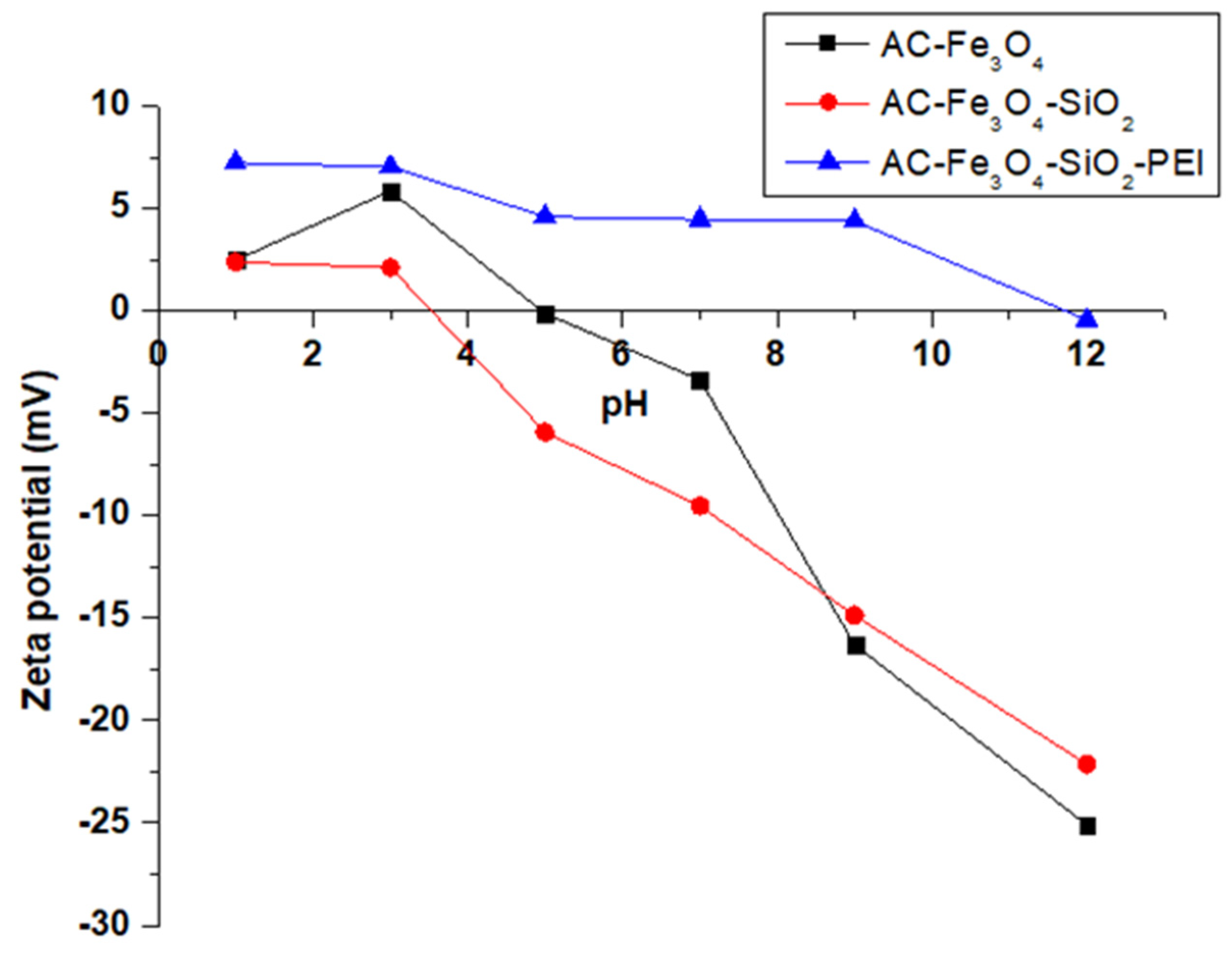
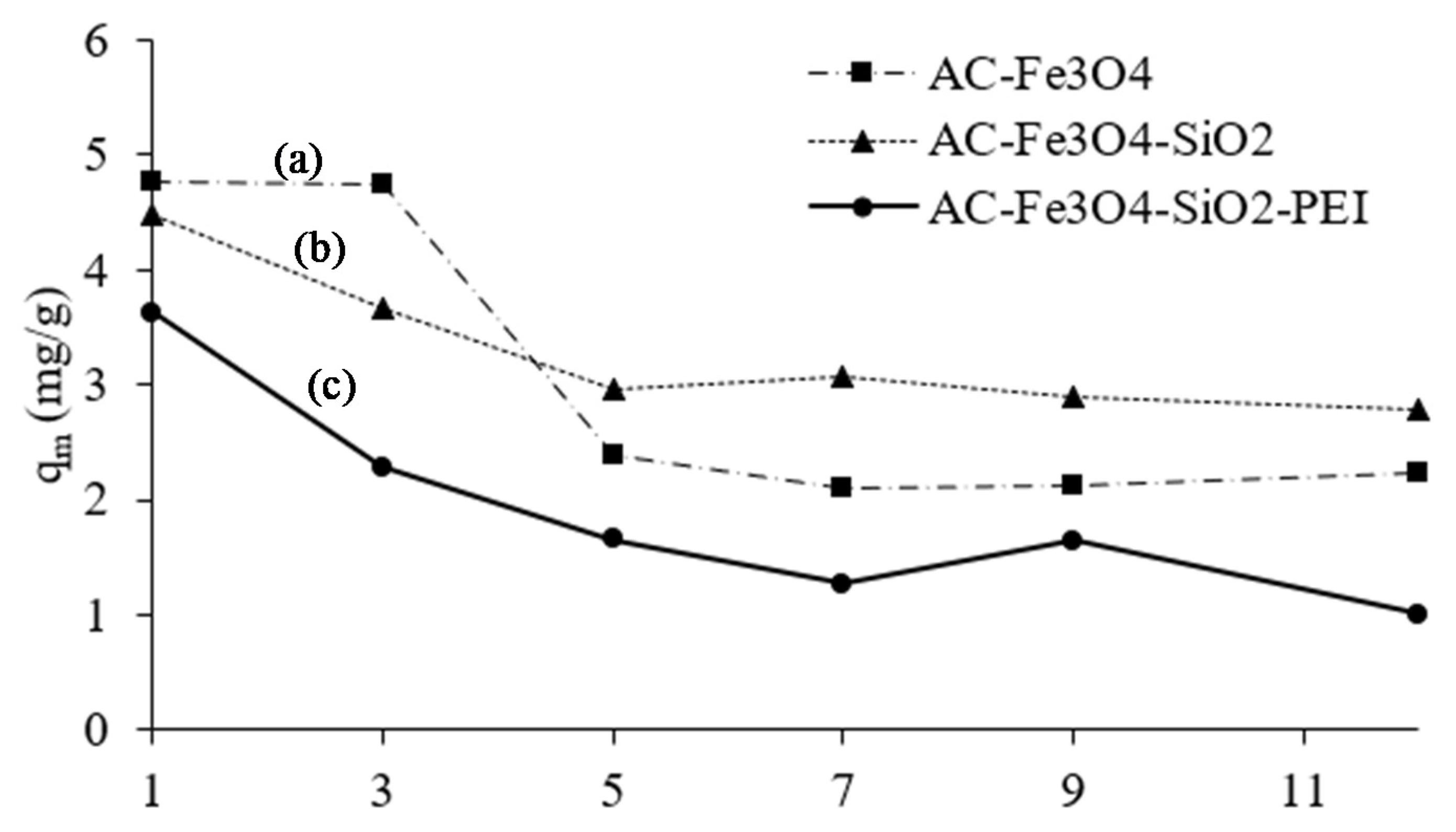
3.2. Influence of Solution pH on Adsorption of Cr(VI)
3.3. Adsorption Isotherms
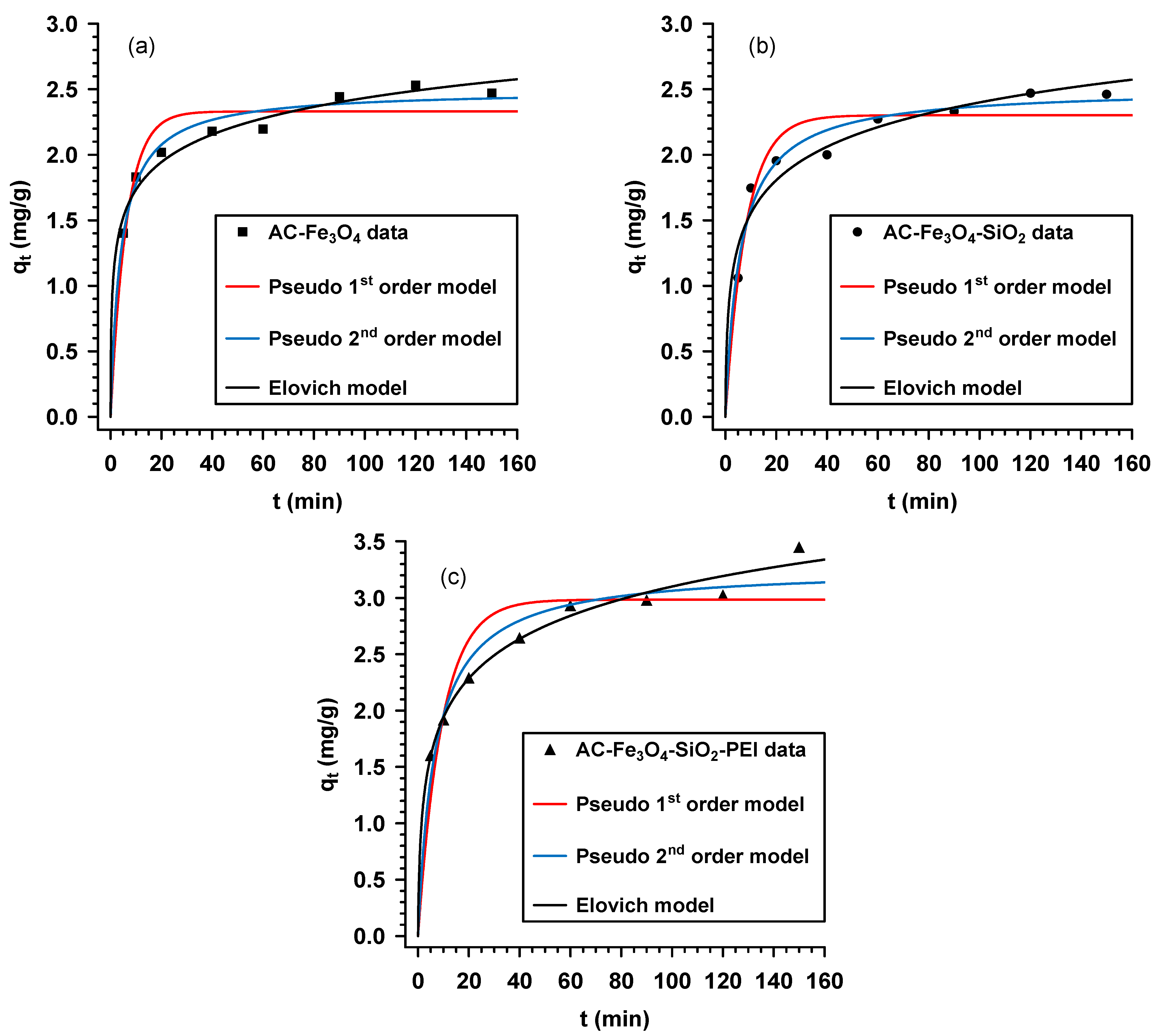
3.4. Adsorption Kinetics
3.5. Performance Comparison
3.6. Thermodynamic Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heiderscheidt, E.; Postila, H.; Leiviskä, T. Removal of metals from wastewaters by mineral and biomass-based sorbents applied in continuous-flow continuous stirred tank reactors followed by sedimentation. Sci. Total Environ. 2020, 700, 135079. [Google Scholar] [CrossRef]
- White, P.J.; Brown, P.H. Plant nutrition for sustainable development and global health. Ann. Bot. 2010, 105, 1073–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartlett, R.J. Chromium cycling in soils and water: Links, gaps, and methods. Environ. Health Perspect. 1991, 92, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Joseph, S.; Tsechansky, L.; Schreiter, I.J.; Schüth, C.; Taherysoosavi, S.; Mitchell, D.R.G.; Graber, E.R. Mechanistic evaluation of biochar potential for plant growth promotion and alleviation of chromium-induced phytotoxicity in Ficus elastica. Chemosphere 2020, 243, 125332. [Google Scholar] [CrossRef] [PubMed]
- Pap, S.; Bezanovic, V.; Radonic, J.; Babic, A.; Saric, S.; Adamovic, D.; Turk Sekulic, M. Synthesis of highly-efficient functionalized biochars from fruit industry waste biomass for the removal of chromium and lead. J. Mol. Liq. 2018, 268, 315–325. [Google Scholar] [CrossRef]
- Qiu, B.; Wang, Y.; Sun, D.; Wang, Q.; Zhang, X.; Weeks, B.L.; O’Connor, R.; Huang, X.; Wei, S.; Guo, Z. Cr(vi) removal by magnetic carbon nanocomposites derived from cellulose at different carbonization temperatures. J. Mater. Chem. A 2015, 3, 9817–9825. [Google Scholar] [CrossRef]
- Rai, M.K.; Shahi, G.; Meena, V.; Meena, R.; Chakraborty, S.; Singh, R.S.; Rai, B.N. Removal of hexavalent chromium Cr (VI) using activated carbon prepared from mango kernel activated with H3PO4. Resour. Technol. 2016, 2, S63–S70. [Google Scholar] [CrossRef]
- Corazza, M.Z.; Somera, B.F.; Segatelli, M.G.; Tarley, C.R.T. Grafting 3-mercaptopropyl trimethoxysilane on multi-walled carbon nanotubes surface for improving on-line cadmium(II) preconcentration from water samples. J. Hazard. Mater. 2012, 243, 326–333. [Google Scholar] [CrossRef]
- Kononova, O.N.; Bryuzgina, G.L.; Apchitaeva, O.V.; Kononov, Y.S. Ion exchange recovery of chromium (VI) and manganese (II) from aqueous solutions. Arab. J. Chem. 2019, 12, 2713–2720. [Google Scholar] [CrossRef]
- Mnif, A.; Bejaoui, I.; Mouelhi, M.; Hamrouni, B. Hexavalent Chromium Removal from Model Water and Car Shock Absorber Factory Effluent by Nanofiltration and Reverse Osmosis Membrane. Int. J. Anal. Chem. 2017, 2017, 7415708. [Google Scholar] [CrossRef]
- Rol, F.; Belgacem, M.N.; Gandini, A.; Bras, J. Recent advances in surface-modified cellulose nanofibrils. Prog. Polym. Sci. 2019, 88, 241–264. [Google Scholar] [CrossRef]
- Stylianou, S.; Simeonidis, K.; Mitrakas, M.; Zouboulis, A.; Ernst, M.; Katsoyiannis, I.A. Reductive precipitation and removal of Cr(VI) from groundwaters by pipe flocculation-microfiltration. Environ. Sci. Pollut. Res. 2018, 25, 12256–12262. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Yuan, X.; Huang, Z.; Wang, H.; Jiang, L.; Huang, J.; Tan, M.; Li, H. Activated biochar with iron-loading and its application in removing Cr (VI) from aqueous solution. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 579, 123642. [Google Scholar] [CrossRef]
- Miao, M.S.; Wang, Y.N.; Kong, Q.; Shu, L. Adsorption kinetics and optimum conditions for Cr(VI) removal by activated carbon prepared from luffa sponge. Desalin. Water Treat. 2016, 57, 7763–7772. [Google Scholar] [CrossRef]
- Gottipati, R.; Mishra, S. Preparation of microporous activated carbon from Aegle Marmelos fruit shell and its application in removal of chromium(VI) from aqueous phase. J. Ind. Eng. Chem. 2016, 36, 355–363. [Google Scholar] [CrossRef]
- Cai, W.; Li, Z.; Wei, J.; Liu, Y. Synthesis of peanut shell based magnetic activated carbon with excellent adsorption performance towards electroplating wastewater. Chem. Eng. Res. Des. 2018, 140, 23–32. [Google Scholar] [CrossRef]
- Hlungwane, L.; Viljoen, E.L.; Pakade, V.E. Macadamia nutshells-derived activated carbon and attapulgite clay combination for synergistic removal of Cr(VI) and Cr(III). Adsorpt. Sci. Technol. 2018, 36, 713–731. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Q.; Huang, Y.; Huang, L.; Hu, L.; Xiong, D.; Zhong, H. Efficient removal of hexavalent chromium in a wide pH range by composite of SiO2 supported nano ferrous oxalate. Chem. Eng. J. 2020, 383, 123209. [Google Scholar] [CrossRef]
- Zeng, Q.; Huang, Y.; Wang, H.; Huang, L.; Hu, L.; Zhong, H. A novel composite of almandine supported humboldtine nanospheres, in situ synthesized from natural almandine, possesses high removal efficiency of Cr (Ⅵ) over a wide pH range. J. Hazard. Mater. 2020, 383, 121199. [Google Scholar] [CrossRef]
- Ta, T.K.H.; Trinh, M.T.; Long, N.V.; Nguyen, T.T.M.; Nguyen, T.L.T.; Thuoc, T.L.; Phan, B.T.; Mott, D.; Maenosono, S.; Tran-Van, H.; et al. Synthesis and surface functionalization of Fe3O4-SiO2 core-shell nanoparticles with 3-glycidoxypropyltrimethoxysilane and 1,1′-carbonyldiimidazole for bio-applications. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 504, 376–383. [Google Scholar] [CrossRef]
- Shi, S.; Yang, J.; Liang, S.; Li, M.; Gan, Q.; Xiao, K.; Hu, J. Enhanced Cr(VI) removal from acidic solutions using biochar modified by Fe3O4@SiO2-NH2 particles. Sci. Total Environ. 2018, 628–629, 499–508. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, Z.; Zeng, G.; Liu, Y.; Shao, B.; Li, Z.; Liu, Y.; Zhang, W.; He, Q. Polyaniline-based adsorbents for removal of hexavalent chromium from aqueous solution: A mini review. Environ. Sci. Pollut. Res. 2018, 25, 6158–6174. [Google Scholar] [CrossRef]
- Zeng, Q.; Hu, Y.; Yang, Y.; Hu, L.; Zhong, H.; He, Z. Cell envelop is the key site for Cr (Ⅵ) reduction by Oceanobacillus oncorhynchi W4, a newly isolated Cr (Ⅵ) reducing bacterium. J. Hazard. Mater. 2019, 368, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Wang, S.; Vincent, T.; Desbrieres, J.; Faur, C.; Guibal, E. New highly-percolating alginate-PEI membranes for efficient recovery of chromium from aqueous solutions. Carbohydr. Polym. 2019, 225, 115177. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Z.; Chen, H.; Kai, C.; Jiang, M.; Wang, Q.; Zhou, Z. Polyethylenimine functionalized Fe 3 O 4 /steam-exploded rice straw composite as an efficient adsorbent for Cr(VI) removal. Appl. Surf. Sci. 2018, 440, 1277–1285. [Google Scholar] [CrossRef]
- Moyo, M.; Modise, S.J.; Pakade, V.E. Palladium nanoparticles dispersed on functionalized macadamia nutshell biomass for formic acid-mediated removal of chromium(VI) from aqueous solution. Sci. Total Environ. 2020, 743, 140614. [Google Scholar] [CrossRef]
- Tangtubtim, S.; Saikrasun, S. Adsorption behavior of polyethyleneimine-carbamate linked pineapple leaf fiber for Cr(VI) removal. Appl. Surf. Sci. 2019, 467–468, 596–607. [Google Scholar] [CrossRef]
- Geng, J.; Yin, Y.; Liang, Q.; Zhu, Z.; Luo, H. Polyethyleneimine cross-linked graphene oxide for removing hazardous hexavalent chromium: Adsorption performance and mechanism. Chem. Eng. J. 2019, 361, 1497–1510. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, J.; Li, Y.; Wei, H.P.; Li, X.S.; Zhang, X.H.; Chen, S.M.; Chen, X.Q. Synthesis of polyethyleneimine capped carbon dots for preconcentration and slurry sampling analysis of trace chromium in environmental water samples. Talanta 2015, 134, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Fatehi, M.H.; Shayegan, J.; Zabihi, M.; Goodarznia, I. Functionalized magnetic nanoparticles supported on activated carbon for adsorption of Pb(II) and Cr(VI) ions from saline solutions. J. Environ. Chem. Eng. 2017, 5, 1754–1762. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Lee, J.H.; Chung, J.W.; Kwak, S.Y. Hydrophilic and positively charged polyethylenimine-functionalized mesoporous magnetic clusters for highly efficient removal of Pb(II) and Cr(VI) from wastewater. J. Environ. Manag. 2018, 206, 740–748. [Google Scholar] [CrossRef]
- Liang, X.; Fan, X.; Li, R.; Li, S.; Shen, S.; Hu, D. Efficient removal of Cr(VI) from water by quaternized chitin/branched polyethylenimine biosorbent with hierarchical pore structure. Bioresour. Technol. 2018, 250, 178–184. [Google Scholar] [CrossRef]
- Yin, C.Y.; Aroua, M.K.; Daud, W.M.A.W. Impregnation of palm shell activated carbon with polyethyleneimine and its effects on Cd2+ adsorption. Colloids Surfaces A Physicochem. Eng. Asp. 2007, 307, 128–136. [Google Scholar] [CrossRef]
- Zhong, D.; Zhang, Y.; Wang, L.; Chen, J.; Jiang, Y.; Tsang, D.C.W.; Zhao, Z.; Ren, S.; Liu, Z.; Crittenden, J.C. Mechanistic insights into adsorption and reduction of hexavalent chromium from water using magnetic biochar composite: Key roles of Fe3O4 and persistent free radicals. Environ. Pollut. 2018, 243, 1302–1309. [Google Scholar] [CrossRef]
- Lesaoana, M.; Mlaba, R.P.V.; Mtunzi, F.M.; Klink, M.J.; Edijike, P.; Pakade, V.E. Influence of inorganic acid modification on Cr(VI) adsorption performance and the physicochemical properties of activated carbon. S. Afr. J. Chem. Eng. 2019, 28. [Google Scholar] [CrossRef]
- Li, B.; Zhang, L.; Yin, W.; Lv, S.; Li, P.; Zheng, X.; Wu, J. Effective immobilization of hexavalent chromium from drinking water by nano-FeOOH coating activated carbon: Adsorption and reduction. J. Environ. Manag. 2021, 277, 111386. [Google Scholar] [CrossRef]
- Zhang, T.; Yue, X.; Zhang, K.; Li, J.; Zhu, C.; Zhang, L.; Chen, W. Selective adsorption of CuSO4 from mixed sulfate solutions by Cu(II) ion-imprinted polymers containing salicylaldoximes, ammonium cations, and tertiary amino groups. Mater. Des. 2016, 107, 372–377. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, R.; Zhu, Y.; Liu, J.; Zhu, L.; Ma, L.; Chen, M. Adsorption of polyhydroxy fullerene on polyethylenimine-modified montmorillonite. Appl. Clay Sci. 2016, 132–133, 412–418. [Google Scholar] [CrossRef]
- Nchoe, O.B.; Ntuli, T.D.; Klink, M.J.; Mtunzi, F.M.; Pakade, V.E. A comparative study of acid, base and Fenton-like reagent treated biomass for Cr(VI) sequestration from aqueous solutions. Water Environ. Res. 2020, 93, 370–383. [Google Scholar] [CrossRef]
- Yan, Y.; An, Q.; Xiao, Z.; Zheng, W.; Zhai, S. Flexible core-shell/bead-like alginate@PEI with exceptional adsorption capacity, recycling performance toward batch and column sorption of Cr(VI). Chem. Eng. J. 2017, 313, 475–486. [Google Scholar] [CrossRef]
- Zhao, D.; Gao, X.; Wu, C.; Xie, R.; Feng, S.; Chen, C. Facile preparation of amino functionalized graphene oxide decorated with Fe3O4 nanoparticles for the adsorption of Cr(VI). Appl. Surf. Sci. 2016, 384, 1–9. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, B.; Xin, J.; Sun, P.; Wu, D. Adsorption behavior and mechanism of Cr(VI) by modified biochar derived from Enteromorpha prolifera. Ecotoxicol. Environ. Saf. 2018, 164, 440–447. [Google Scholar] [CrossRef]
- Chang, J.; Wang, H.; Zhang, J.; Xue, Q.; Chen, H. New insight into adsorption and reduction of hexavalent chromium by magnetite: Multi-step reaction mechanism and kinetic model developing. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 611, 125784. [Google Scholar] [CrossRef]
- Nkutha, C.S.; Diagboya, P.N.; Mtunzi, F.M.; Dikio, E.D. Application of eco-friendly multifunctional porous graphene oxide for adsorptive sequestration of chromium in aqueous solution. Water Environ. Res. 2020, 92, 1070–1079. [Google Scholar] [CrossRef]
- Pakade, V.E.; Maremeni, L.C.; Ntuli, T.D.; Tavengwa, N.T. Application of quaternized activated carbon derived from macadamia nutshells for the removal of hexavalent chromium from aqueous solutions. S. Afr. J. Chem. 2016, 69, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Niazi, L.; Lashanizadegan, A.; Sharififard, H. Chestnut oak shells activated carbon: Preparation, characterization and application for Cr (VI) removal from dilute aqueous solutions. J. Clean. Prod. 2018, 185, 554–561. [Google Scholar] [CrossRef]
- Qhubu, M.C.; Mgidlana, L.G.; Madikizela, L.M.; Pakade, V.E. Preparation, characterization and application of activated clay biochar composite for removal of Cr(VI) in water: Isotherms, kinetics and thermodynamics. Mater. Chem. Phys. 2021, 260, 124165. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, N.; Feng, C.; Li, M.; Gao, Y. Chromium removal using a magnetic corncob biochar/polypyrrole composite by adsorption combined with reduction: Reaction pathway and contribution degree. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 556, 201–209. [Google Scholar] [CrossRef]
- Zhang, X.; Lv, L.; Qin, Y.; Xu, M.; Jia, X.; Chen, Z. Removal of aqueous Cr(VI) by a magnetic biochar derived from Melia azedarach wood. Bioresour. Technol. 2018, 256, 1–10. [Google Scholar] [CrossRef]
- Larraza, I.; López-Gónzalez, M.; Corrales, T.; Marcelo, G. Hybrid materials: Magnetite-Polyethylenimine-Montmorillonite, as magnetic adsorbents for Cr(VI) water treatment. J. Colloid Interface Sci. 2012, 385, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.C.; Hosseini-Bandegharaei, A.; Moreno-Piraján, J.C.; Anastopoulos, I. A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calculation of thermodynamic parameters of adsorption. J. Mol. Liq. 2019, 273, 425–434. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, M.; Li, H.; Ge, H.; Bian, Z. The enhanced photoreduction of Cr(VI) to Cr(III) using carbon dots coupled TiO2 mesocrystals. Appl. Catal. B Environ. 2018, 226, 213–219. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.J.; Chao, H.P. Thermodynamic parameters of cadmium adsorption onto orange peel calculated from various methods: A comparison study. J. Environ. Chem. Eng. 2016, 4, 2671–2682. [Google Scholar] [CrossRef]
- Hokkanen, S.; Bhatnagar, A.; Repo, E.; Lou, S.; Sillanpää, M. Calcium hydroxyapatite microfibrillated cellulose composite as a potential adsorbent for the removal of Cr(VI) from aqueous solution. Chem. Eng. J. 2016, 283, 445–452. [Google Scholar] [CrossRef]
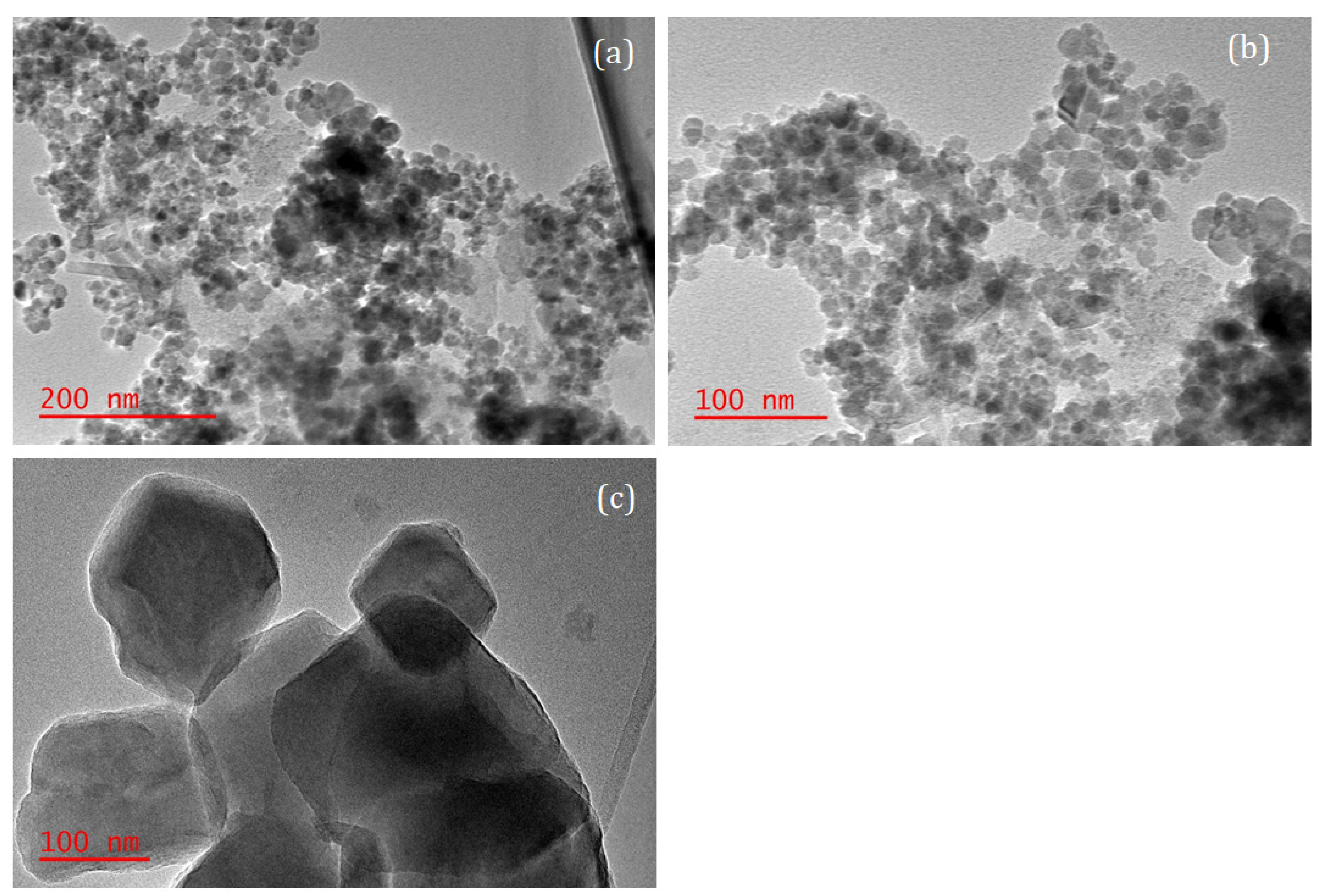


| Adsorbents | Elemental Analysis | Surface Characterization | ||||||
|---|---|---|---|---|---|---|---|---|
| %C | %H | %N | %S | %R * | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) | |
| AC | 83.27 | 1.53 | 0.50 | - | 14.70 | 546.00 | 0.354 | 2.59 |
| AC-Fe3O4 | 44.03 | 1.41 | 0.25 | <0.1 | 55.69 | 386.89 | 0.294 | 7.85 |
| AC-Fe3O4-SiO2 | 68.30 | 1.79 | 0.44 | <0.1 | 29.47 | 234.51 | 0.149 | 5.75 |
| AC-Fe3O4-SiO2-PEI | 38.50 | 6.71 | 3.00 | - | 57.75 | 0.15 | 0.267 | 26.48 |
| Isotherms | Parameters | AC-Fe3O4 | AC-Fe3O4-SiO2 | AC-Fe3O4-SiO2-PEI |
|---|---|---|---|---|
| Langmuir isotherm | qmL (mg/g) | 5.43 | 4.67 | 8.00 |
| b (L/mg) | 7.07 | 3.16 | 0.80 | |
| R2 | 0.828 | 0.837 | 0.921 | |
| RSE | 0.172 | 0.163 | 0.079 | |
| Freundlich isotherm | n | 3.55 | 2.99 | 2.00 |
| KF (mg/g)(mg/L)−1/n | 4.23 | 3.07 | 3.46 | |
| R2 | 0.737 | 0.778 | 0.957 | |
| RSE | 0.263 | 0.222 | 0.043 | |
| Dubinin-Radushkevich isotherm | qmDR (mg/g) | 5.23 | 4.22 | 4.72 |
| KDR (mol2/kJ2) | 0.02 | 0.04 | 0.03 | |
| R2 | 0.840 | 0.850 | 0.852 | |
| RSE | 0.160 | 0.150 | 0.148 |
| Model | Parameters | AC-Fe3O4 | AC-Fe3O4-SiO2 | AC-Fe3O4-SiO2-PEI |
|---|---|---|---|---|
| Pseudo-first order | qt (mg/g) | 2.33 | 2.30 | 2.98 |
| k1 (1/min) | 0.16 | 0.12 | 0.11 | |
| R2 | 0.829 | 0.889 | 0.794 | |
| RSE | 0.171 | 0.111 | 0.206 | |
| Pseudo-second order | qt (mg/g) | 2.50 | 2.51 | 3.27 |
| k2 ((g/(mg min)) | 0.10 | 0.07 | 0.05 | |
| R2 | 0.949 | 0.948 | 0.925 | |
| RSE | 0.051 | 0.052 | 0.075 | |
| Elovich | α (mg/(g min)) | 8.98 | 2.41 | 2.25 |
| β (mg/g) | 3.28 | 2.70 | 1.96 | |
| R2 | 0.953 | 0.914 | 0.978 | |
| RSE | 0.047 | 0.086 | 0.022 |
| Adsorbent | pH | Co (mg/L) | qmL (mg/g) | Reference |
|---|---|---|---|---|
| Magnetic biochar | 3 | 100 | 8.35 | [35] |
| MBC/PPy | 3 | 10 | 19.23 | [49] |
| Magnetic biochar (MMABC) | 3 | 10 | 25.27 | [50] |
| Fe3O4-PEI800-MNT | 1–9 | 10–25 | 8.77 | [51] |
| Fe3O4-PEI25000-MNT | 1–9 | 10–25 | 7.69 | [51] |
| AC-Fe3O4 | 3 | 5 | 5.43 | This study |
| AC-Fe3O4-SiO2 | 1 | 5 | 4.67 | This study |
| AC-Fe3O4-SiO2-PEI | 1 | 5 | 8.00 | This study |
| Adsorbent | T (K) | b (L/mg) | ln (Koc) | ΔGo (kJ/mol) | |TΔSo| (kJ/mol) | ΔHo (kJ/mol) | ΔSo (J/(mol K)) |
|---|---|---|---|---|---|---|---|
| AC-Fe3O4 | 298 | 20.85 | 14.71 | −25.43 | 160.03 | 133.45 | 537.00 |
| 308 | 5.51 | 13.38 | −34.25 | 165.40 | |||
| 318 | 7.07 | 13.68 | −36.17 | 170.77 | |||
| AC-Fe3O4-SiO2 | 298 | 5.44 | 13.36 | −23.11 | 160.62 | 136.09 | 539.00 |
| 308 | 3.10 | 12.80 | −32.78 | 166.01 | |||
| 318 | 3.16 | 12.82 | −33.89 | 171.40 | |||
| AC-Fe3O4-SiO2-PEI | 298 | 0.15 | 9.77 | −16.90 | 881.63 | 871.45 | 2958.50 |
| 308 | 0.25 | 10.28 | −26.33 | 911.22 | |||
| 318 | 0.80 | 28.77 | −76.07 | 940.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qhubu, M.C.; Nomngongo, P.N.; Pakade, V.E. Exploring the Iron Oxide Functionalized Biobased Carbon-silica-polyethyleneimine Composites for Hexavalent Chromium Removal from Dilute Aqueous Solutions. Water 2021, 13, 3081. https://doi.org/10.3390/w13213081
Qhubu MC, Nomngongo PN, Pakade VE. Exploring the Iron Oxide Functionalized Biobased Carbon-silica-polyethyleneimine Composites for Hexavalent Chromium Removal from Dilute Aqueous Solutions. Water. 2021; 13(21):3081. https://doi.org/10.3390/w13213081
Chicago/Turabian StyleQhubu, Mpho Cynthia, Philiswa Nosizo Nomngongo, and Vusumzi Emmanuel Pakade. 2021. "Exploring the Iron Oxide Functionalized Biobased Carbon-silica-polyethyleneimine Composites for Hexavalent Chromium Removal from Dilute Aqueous Solutions" Water 13, no. 21: 3081. https://doi.org/10.3390/w13213081







