Investigation of Metal and Trace Elements of Cenospheres from Lignite High-Calcium Fly Ash (Thailand)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fly Ash and Cenospheres Separation
2.2. Determination of Metal Elements in Fly Ash and Cenospheres by Acid Digestion
2.3. Leaching Study of Cenospheres by TCLP Method
2.4. Material Characterization
3. Results
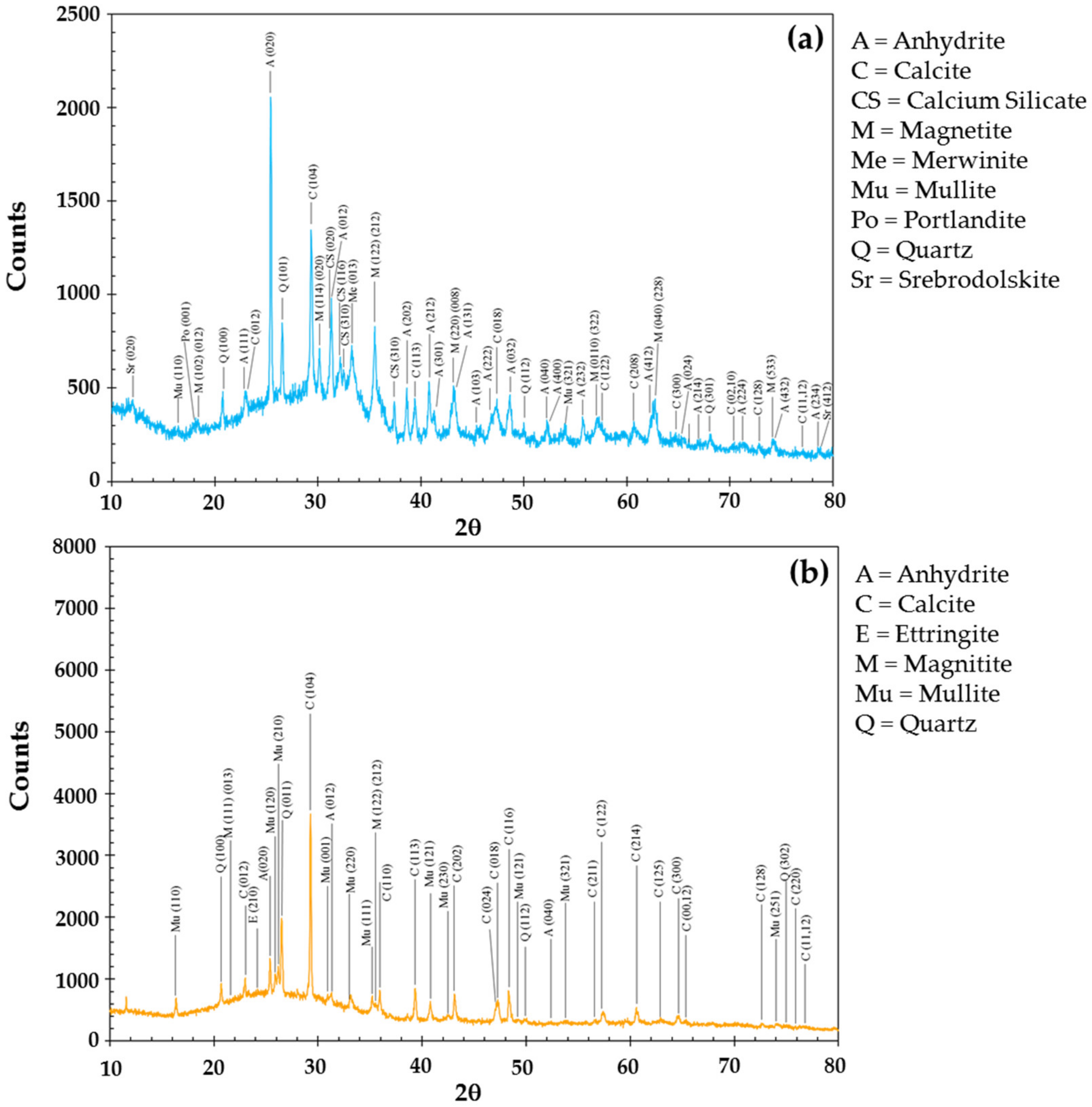
3.1. Physical, Chemical, Mineralogical Characterization of Fly Ash and Cenospheres
3.2. Acid Digestion of Fly Ash and Cenospheres
3.3. Leachability of Heavy Metal Elements from Cenospheres
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benito, Y.; Ruiz, M.; Cosmen, P.; Merino, J.L. Study of Leaches Obtained from the Disposal of Fly Ash from Pfbc and Afbc Processes. Chem. Eng. J. 2001, 84, 167–171. [Google Scholar] [CrossRef]
- Bouska, V.; Pesek, J. Quality Parameters of Lignite of the North Bohemian Basin in the Czech Republic in Comparison with the World Average Lignite. Int. J. Coal Geol. 1999, 40, 211–235. [Google Scholar] [CrossRef]
- Izquierdo, M.; Querol, X. Leaching Behaviour of Elements from Coal Combustion Fly Ash: An Overview. Int. J. Coal Geol. 2012, 94, 54–66. [Google Scholar] [CrossRef] [Green Version]
- Koukouzas, N.; Ketikidis, C.; Itskos, G. Heavy Metal Characterization of CFB-Derived Coal Fly Ash. Fuel Process. Technol. 2011, 92, 441–446. [Google Scholar] [CrossRef]
- Akar, G.; Polat, M.; Galecki, G.; Ipekoglu, U. Leaching Behavior of Seleted Trace Elements in Coal Fly Ash Samples from Yenikoy Coal-Fired Power Plants. Fuel Process. Technol. 2012, 104, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Popovic, A.; Djordjevic, D.; Polic, P. Trace and Major Element Pollution Originating from Coal Ash Suspension and Transport Processes. Environ. Int. 2001, 26, 251–255. [Google Scholar] [CrossRef]
- Fernandez-Turiel, J.L.; de Carvalho, W.; Cabanas, M.; Querol, X.; Lopes-Soler, A. Mobility of Heavy Metals from Coal Fly Ash. Environ. Geol. 1994, 23, 264–270. [Google Scholar] [CrossRef]
- Ugurlu, A. Leaching Characteristics of Fly Ash. Environ. Geol. 2004, 46, 890–895. [Google Scholar] [CrossRef]
- Tian, Q.; Guo, B.; Nakama, S.; Sasaki, K. Distributions and Leaching Behaviors of Toxic Elements in Fly Ash. ACS Omega 2018, 3, 13055–13064. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G. A New Approach for the Classification of Coal Fly Ashes Based on Their Origin, Composition, Properties, and Behavior. Fuel 2007, 86, 1490–1512. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Menendez, R. Phase-Mineral and Chemical Compositon of Coal Fly Ashes as a Basis for Their Multicomponent Utilization. 4. Characterization of Heavy Concentrates and Improved Fly Ash Residues. Fuel 2005, 84, 973–991. [Google Scholar] [CrossRef]
- Sushil, S.; Batra, V.S. Analysis of Fly Ash Heavy Metal Content and Disposal in Three Thermal Power Plants in India. Fuel 2006, 85, 2676–2679. [Google Scholar] [CrossRef]
- Buema, G.; Harja, M.; Lupu, N.; Chiriac, H.; Forminte, L.; Ciobanu, G.; Bucur, D.; Bucur, R.D. Adsorption Performance of Modified Fly Ash for Copper Ion Removal from Aqueous Solution. Water 2021, 13, 207. [Google Scholar] [CrossRef]
- Haykiri-Acma, H.; Yaman, S.; Ozbek, N.; Kucukbayrak, S. Mobilization of Some Trace Elements from Ashes of Turkish Lignites in Rain Water. Fuel 2011, 90, 3447–3455. [Google Scholar] [CrossRef]
- El-Mogazi, D.; Lisk, D.J.; Weinstein, L.H. A Review of Physical, Chemical, and Biological Properties of Fly Ash and Effects on Agricultural Ecosystems. Sci. Total Environ. 1988, 74, 1–37. [Google Scholar] [CrossRef]
- Taylor, E.M.; Schuman, G.E. Fly Ash and Lime Amendment of Acidic Coal Spoil to Aid Revegetation. J. Environ. Qual. 1988, 17, 120–124. [Google Scholar] [CrossRef]
- Fomenko, E.V.; Anshits, N.N.; Solovyov, L.A.; Mikhaylova, O.A.; Anshits, A.G. Composition and Morphology of Fly Ash Cenospheres Produced from the Combustion of Kuznetsk Coal. Energy Fuels 2013, 27, 5440–5448. [Google Scholar] [CrossRef]
- Drozhzhin, V.S.; Shpirt, M.Y.; Danilin, L.D.; Kuvaev, M.D.; Pikulin, I.V.; Potemkin, G.A. Formation Processes and Main Properties of Hollow Aluminosilicate Microspheres in Fly Ash from Thermal Power Stations. Solid Fuel Chem. 2008, 42, 107–119. [Google Scholar] [CrossRef]
- Ranjbar, N.; Kuenzel, C. Cenospheres: A Review. Fuel 2017, 207, 1–12. [Google Scholar] [CrossRef]
- Danish, A.; Mosaberpanah, M.A. Formation Mechanism and Applications of Cenospheres: A Review. J. Mater. Sci. 2020, 55, 4539–4557. [Google Scholar] [CrossRef]
- Yoriya, S.; Tepsri, P. Separation Process and Microstructure-Chemical Composition Relationship of Cenospheres from Lignite Fly Ash Produced from Coal-Fired Power Plant in Thailand. Appl. Sci. 2020, 10, 5512. [Google Scholar] [CrossRef]
- Yoriya, S.; Tepsri, P. Crystal Growth on Cenospheres from High-Calcium Fly Ash. Crystals 2021, 11, 919. [Google Scholar] [CrossRef]
- Anshits, N.N.; Fedorchak, M.A.; Sharonova, O.M.; Kirik, N.P.; Shishkina, N.N.; Zhizhaev, A.M.; Anshits, A.G. Structure-Composition Relationship of Platelike Ferrospheres in Calcium-Rich Power Plant Ash. Inorg. Mater. 2018, 54, 466–472. [Google Scholar] [CrossRef]
- Tiwari, M.; Shukla, S.P.; Mohan, D.; Bhargava, D.S.; Kisku, G.C. Modified Cenospheres as an Adsorbent for the Removal of Disperse Dyes. Adv. Environ. Chem. 2015, 349254. [Google Scholar] [CrossRef]
- ASTM C618-12a, Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete; ASTM International: West Conshohocken, PA, USA, 2012.
- Yoriya, S.; Intana, T.; Tepsri, P. Separation of Cenospheres from Lignite Fly Ash Using Acetone-Water Mixture. Appl. Sci. 2019, 9, 3792. [Google Scholar] [CrossRef] [Green Version]
- United State Environmental Protection Agency. Method 3051a: Microwave Assissted Acid Digestion of Sediments, Sludges, Soils, and Oils; United State Environmental Protection Agency: Washington, DC, USA, 2007.
- Neupane, G.; Donahoe, R. Leachability of Elements in Alkaline and Acidic Coal Fly Ash Samples During Batch and Column Leaching Tests. Fuel 2013, 104, 758–770. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Test Method 1311, Toxicity Characteristic Leaching Procedure. In Index to EPA Test Methods; EPA Report #SW-846, Chapter 8.4; Nelson, P., Ed.; US EPA New England Region 1 Library: Boston, MA, USA, 2003. [Google Scholar]
- Sarode, D.B.; Jadhav, R.N.; Khatik, V.A.; Ingle, S.T.; Attarde, S.B. Extraction and Leaching of Heavy Metals from Thermal Power Plant Fly Ash and Its Admixtures. Pol. J. Environ. Stud. 2010, 19, 1325–1330. [Google Scholar]
- United States Environment Protection Agency (U.S.EPA). Test Methods for Evaluating Solid Waste, SW846, 3rd ed.; EPA: Washington, DC, USA, 2003.
- ASTM C7348-13, Standard Test Methods for Loss on Ignition (LOI) of Solid Combustion Residues; ASTM International: West Conshohocken, PA, USA, 2013.
- Anshits, N.; Mikhailova, O.; Salanov, A.; Anshits, A. Chemical Composition and Structure of the Shell of Fly Ash Non-Perforated Cenospheres Produced from the Combustion of the Kuznetsk Coal (Russia). Fuel 2010, 89, 1849–1862. [Google Scholar] [CrossRef]
- Blissett, R.S.; Rowson, N.A. A Review of the Multi-Component Utilization of Coal Fly Ash. Fuel 2012, 97, 1. [Google Scholar] [CrossRef]
- Zheng, S.; Yao, Y.; Zhang, S.; Liu, Y.; Yang, J. Insights into Multifractal Characterization of Coals by Mercury Intrusion Porosimetry. Energies 2019, 12, 4743. [Google Scholar] [CrossRef] [Green Version]
- Kutchko, B.G.; Kim, A.G. Fly Ash Characterization by SEM–EDS. Fuel 2006, 85, 2537–2544. [Google Scholar] [CrossRef]
- Brouwers, H.J.H.; Eijk, R.J.V. Chemical Reaction of Fly Ash. In Proceedings of the 11th International Congress on the Chemistry of Cement (ICCC), ‘Cements’s Contribution to the Development in the 21st Century’, the Cement and Concrete Institute of South Africa, Durban, South Africa, 11–16 May 2003; Owens, G., Grieve, G., Eds.; Document Transformation Technologies: Durban, South Africa, 2003; pp. 791–800. [Google Scholar]
- Linton, R.W.; Loh, A.; Natusch, D.F.; Evans, J.C.A.; Williams, P. Surface Predominance of Trace Elements in Airborne Particles. Science 1976, 191, 852–854. [Google Scholar] [CrossRef]
- Saikia, N.; Kato, S.; Kojima, T. Compositions and Leaching Behaviors of Combustion Residues. Fuel 2006, 85, 264–271. [Google Scholar] [CrossRef]
- Manz, O.E. Coal Fly Ash: A Retrospective and Future Look. Fuel 1999, 78, 133–136. [Google Scholar] [CrossRef]
- Diamon, S. On the Glass Present in Low-Ca and High-Ca Fly Ash. Cem. Concr. Res. 1983, 13, 459–464. [Google Scholar] [CrossRef]
- Sokol, E.; Maksimova, N.; Volkova, N.; Nigmatulina, E.; Frenkel, A. Hollow Silicate Microspheres from Fly Ashes of the Chelyabinsk Brown Coals (South Urals, Russia). Fuel Process. Technol. 2000, 67, 35–52. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Menendez, R.; Diaz-Somoano, M.; Martinez-Tarazona, M.R. Phase-Mineral and Chemical Composition of Coal Fly Ashes as a Basis for Their Multicomponent Utilization. 2. Characterization of Ceramic Cenosphere and Salt Concentrates. Fuel 2004, 83, 585–603. [Google Scholar] [CrossRef]
- Kim, A.G.; Kazonich, G. The Silicate-Non-Silicate Distribution of Metals in Fly Ash and Its Effect on Solubility. Fuel 2004, 83, 2285–2292. [Google Scholar] [CrossRef]
- Ram, L.C.; Masto, R.E. An Appraisal of the Potential Use of Fly Ash for Reclaiming Coal Mine Spoil. J. Environ. Manag. 2010, 91, 603–617. [Google Scholar] [CrossRef]
- Żyrkowski, M.; Neto, R.C.; Santos, L.F.; Witkowski, K. Characterization of Fly-Ash Cenospheres from Coal-Fired Power Plant Unit. Fuel 2016, 174, 49–53. [Google Scholar] [CrossRef]
- Jegadeesan, G.; Al-Abed, S.R.; Pinto, P. Influence of Trace Metal Distribution on Its Leachability from Coal Fly Ash. Fuel 2008, 87, 1887–1893. [Google Scholar] [CrossRef]
- Spears, D.A.; Martinez-Tarazona, M.R. Trace Elements in Combustion Residues from a Uk Power Station. Fuel 2004, 83, 2265–2270. [Google Scholar] [CrossRef]
- Goodarzi, F.; Huggins, F.F.; Sanei, H. Assessment of Elements, Speciation of as, Cr, Ni and Emitted Hg for a Canadian Power Plant Burning Bituminous Coal. Int. J. Coal Geol. 2008, 74, 1–12. [Google Scholar] [CrossRef]
- Papaefthymiou, H.; Gouseti, O. Natural Radioactivity and Associated Ratiation Hazards in Building Materials Used in Peloponnese, Greece. Radiat. Meas. 2008, 43, 1453–1457. [Google Scholar] [CrossRef]
- Iyer, R. The Surface Chemistry of Leaching Coal Fly Ash. J. Hazard. Mater. 2002, B93, 321–329. [Google Scholar] [CrossRef]
- Huggins, F.E.; Senior, C.L.; Chu, P.; Ladwig, K.; Huffman, G.P. Selenium and Arsenic Speciation in Fly Ash from Full-Scale Coal-Burning Utility Plants. Environ. Sci. Technol. 2007, 41, 3284–3289. [Google Scholar] [CrossRef]
- Meij, R.; Vredenbregt, L.H.J.; Wingkel, H.T. The Fate and Behavior of Mercury in Coal-Fired Power Plants. J. Air Waste Manag. Assoc. 2002, 52, 912–917. [Google Scholar] [CrossRef] [Green Version]
- Kim, A.G.; Kazonich, G.; Dahlberg, M. Relative Solubility of Cations in Class F Fly Ash. Environ. Sci. Technol. 2003, 37, 4507–4511. [Google Scholar] [CrossRef]
- Pandey, V.; Ray, M.; Kumar, V. Assessment of Water-Quality Parameters of Groundwater Contaminated by Fly Ash Leachate near Koradi Thermal Power Plant, Nagpur. Environ. Sci. Pollut. Res. 2020, 27, 27422–27434. [Google Scholar] [CrossRef] [PubMed]
- Warren, C.J.; Dudas, M.J. Leaching Behavior of Selected Trace Elements in Chemically Weathered Alkaline Fly Ash. Sci. Total Environ. 1988, 76, 229–246. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Tang, Y.; Shi, H.; Ladwig, K. Leaching Characteristics of Arsenic and Selenium from Coal Fly Ash: Role of Calcium. Energy Fuels 2009, 23, 2959–2966. [Google Scholar] [CrossRef]
- Carlson, C.L.; Adriano, D.C. Environmental Impacts of Coal Combustion Residues. J. Environ. Qual. 1993, 22, 227–247. [Google Scholar] [CrossRef]
- Wang, W.; Qin, Y.; Song, D.; Wang, K. Column Leaching of Coal and Its Combustion Residues, Shizuishan, China. Int. J. Coal Geol. 2008, 75, 81–87. [Google Scholar] [CrossRef]
- Zandi, M.; Russell, N.V. Design of a Leaching Test Framework for Coal Fly Ash Accounting for Environmental Conditions. Environ. Monit. Assess. 2007, 131, 509–526. [Google Scholar] [CrossRef]
- Wilberley, L.J.; Wall, T.F. Alkali Ash Reactions and Deposit Silicate Formation in Pulverised Coal Boilers, Experimental Aspects of Sodium Silicate Formation and Formation of Deposit. Fuel 1982, 61, 94–99. [Google Scholar]
- Querol, X.; Moreno, N.; Umana, J.C.; Alastuey, A.; Hernandez, E.; Lopez-Soler, A.; Plana, F. Synthesis of Zeolites from Coal Fly Ash: An Overview. Fuel 2002, 50, 413–423. [Google Scholar] [CrossRef]
- Notification of the National Environment Board, No. 25, B.E. 2547 (2004), Soil Quality Standard; Pollution Control Department: Bangkok, Thailand, 2004.
- Meij, R.; Winkel, H. Health Aspects of Coal Fly Ash. In Proceedings of the International Ash Utilizatin Symposium, Lexington, KY, USA, 22–24 October 2001; pp. 21–27. [Google Scholar]
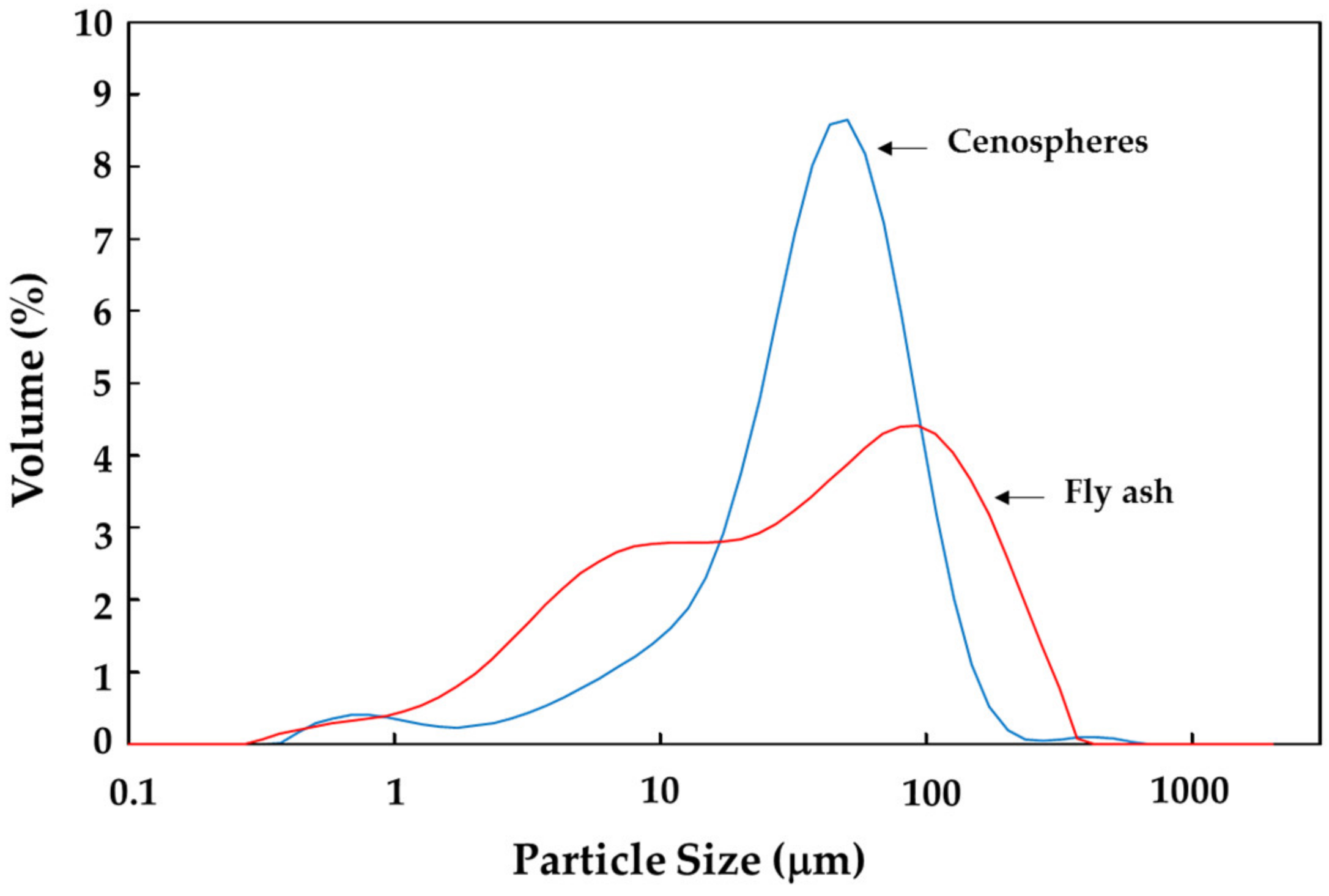

| Sample | Density (g/cm3) | Particle Size (μm) | Size Distribution (Wt.%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D (4,3) | dv10 | dv50 | dv90 | <1 µm | 1–10 µm | 10–50 µm | 50–100 µm | 100–250 µm | 250–500 µm | ||
| Fly ash | 2.47 | 55.97 | 3.28 | 31.86 | 146.81 | 2.16 | 25.88 | 32.68 | 19.43 | 18.34 | 1.51 |
| Cenospheres | 1.03 | 44.47 | 8.30 | 37.42 | 84.92 | 2.20 | 9.58 | 54.52 | 27.87 | 5.41 | 0.42 |
| Item | Composition (Wt.%) | |||||||
|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | SO3 | K2O | TiO2 | MnO | |
| Fly ash | ||||||||
| 30.52 ± 0.10 | 13.04 ± 0.10 | 15.67 ± 0.23 | 28.90 ± 0.39 | 9.02 ± 0.06 | 2.21 ± 0.02 | 0.50 ± 0.00 | 0.14 ± 0.00 | |
| Cenospheres (initial) | ||||||||
| 48.68 ± 0.33 | 23.24 ± 0.06 | 9.49 ± 0.06 | 9.52 ± 0.06 | 3.62 ± 0.82 | 4.68 ± 0.00 | 0.70 ± 0.00 | 0.60 ± 0.74 | |
| Cenospheres after being leached by TCLP method | ||||||||
| 53.12 ± 0.25 | 25.14 ± 0.13 | 8.73 ± 0.20 | 7.08 ± 0.10 | 0.34 ± 0.04 | 4.73 ± 0.04 | 0.80 ± 0.01 | 0.06 ± 0.00 | |
| Sample | Concentration (mg/kg) | |||||||
|---|---|---|---|---|---|---|---|---|
| Mg | Al | Zn | Pb | Cd | Cr | Cu | ||
| Fly ash | Average | 11,163.66 | 18,255.81 | 217.11 | 33.37 | 4.83 | 65.05 | 40.15 |
| SD | 376.91 | 455.01 | 1.64 | 0.58 | 0.05 | 2.18 | 0.86 | |
| %RSD | 3.3800 | 2.4900 | 0.760 | 1.8 | 1 | 3.35 | 2.1 | |
| Cenospheres | Average | 4341.47 | 20,818.32 | 68.67 | 30.48 | 2.69 | 38.11 | 27.41 |
| SD | 64.65 | 334.06 | 0.92 | 0.73 | 0.04 | 0.87 | 0.42 | |
| %RSD | 1.490 | 1.6000 | 1.4 | 2.4 | 1 | 2.3 | 1.5 | |
| LOQ | 0.00300 | 0.003 | 0.003 | 0.003 | 0.028 | 0.005 | 0.003 | |
| Analyte | Concentration (mg/kg) | %Leached | Soil Quality Criteria (mg/kg) | ||
|---|---|---|---|---|---|
| Acid Digestion | TCLP Test | Residential Area/ Agriculture | Non-Residential Area/ Agriculture | ||
| Pb | 30.48 | 17 | 55.77 | <400 | <750 |
| Cd | 2.69 | 0.21 | 7.81 | <37 | <810 |
| Cr | 38.11 | 23.80 | 62.45 | <300 | <640 |
| Cu | 27.41 | 30.60 | 111.64 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoriya, S.; Tepsri, P. Investigation of Metal and Trace Elements of Cenospheres from Lignite High-Calcium Fly Ash (Thailand). Water 2021, 13, 2935. https://doi.org/10.3390/w13202935
Yoriya S, Tepsri P. Investigation of Metal and Trace Elements of Cenospheres from Lignite High-Calcium Fly Ash (Thailand). Water. 2021; 13(20):2935. https://doi.org/10.3390/w13202935
Chicago/Turabian StyleYoriya, Sorachon, and Phattarathicha Tepsri. 2021. "Investigation of Metal and Trace Elements of Cenospheres from Lignite High-Calcium Fly Ash (Thailand)" Water 13, no. 20: 2935. https://doi.org/10.3390/w13202935
APA StyleYoriya, S., & Tepsri, P. (2021). Investigation of Metal and Trace Elements of Cenospheres from Lignite High-Calcium Fly Ash (Thailand). Water, 13(20), 2935. https://doi.org/10.3390/w13202935






