Digested Sludge Quality in Mesophilic, Thermophilic and Temperature-Phased Anaerobic Digestion Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up
2.2. Digestate Dewaterability
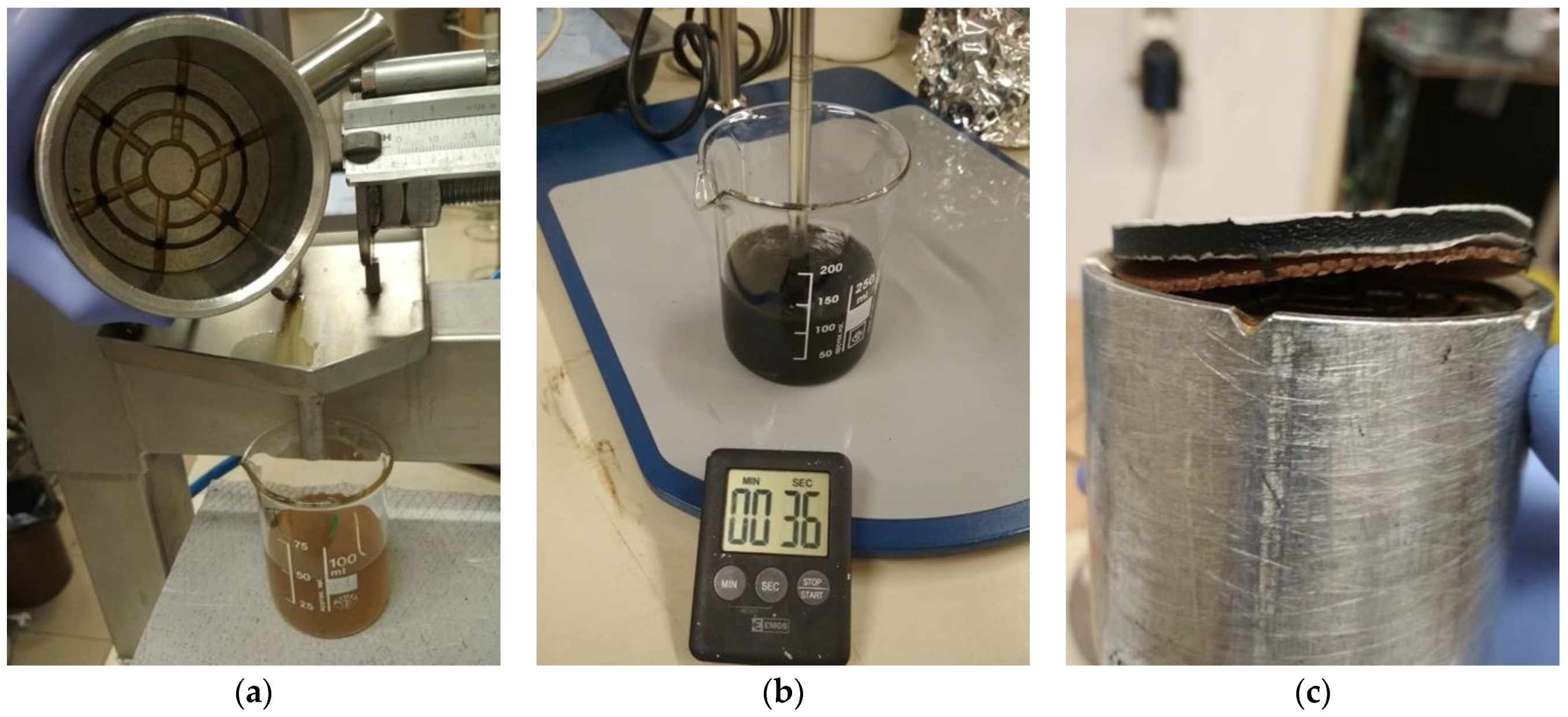
2.2.1. Centrifugation
2.2.2. Mechanical Pressing
2.3. Elemental Analysis and Lower Calorific Value
2.4. Pathogenic Bacteria Indicators
2.5. Analytical Methods
2.5.1. Biogas Production and Composition
2.5.2. Suspended Solids
2.5.3. Chemical Oxygen Demand
2.5.4. Temperature, pH and VFA Measurement
2.6. Statistics
3. Results and Discussion
3.1. Anaerobic Digestion Performance
3.2. Digestate Dewaterability
3.2.1. Centrifugation
3.2.2. Mechanical Pressing
3.3. Elemental Analysis and Lower Calorific Value
3.4. Hygienisation Efficiency Assessment
3.5. Comparison of Results
4. Conclusions
- Organic matter removal and methane production experimental data clearly showed that TPAD obtained the best results, followed by TAD and, finally, by MAD.
- Regarding the dewaterability, the results varied depending on the physical mechanism of the dewatering test. By centrifugation without flocculant addition, the highest dewaterability was obtained by TAD, which was 8.1% and 9.8% higher than TPAD and 14.3% and 21% higher than MAD during both HRTs (13.5 and 19.0 days, respectively). The mechanical pressing results showed the statistical insignificance among the AD systems.
- The calorific value of the sludge was reduced by 19.2% after TPAD at Phase A with an HRT of 19 days, which was the only statistically significant difference between TPAD and TAD/MAD. At Phase B with an HRT of 13.5, none of the AD systems showed any statistical difference in relation to the other ad systems.
- The deactivation of pathogens was proven for the TAD digestate regardless of the HRT, but not for the MAD digestate, while TPAD showed different results depending on the HRT. It seems that the HRT of the first stage of TPAD is crucial in relation to the TPAD digestate’s pathogenic safety. Hence, the possibility of using the TPAD digestate directly for agricultural purposes might still be a concern.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | anaerobic digestion |
| COD | chemical oxygen demand |
| EA | elemental analysis |
| GC | gas chromatography |
| HRT | hydraulic retention time |
| LCA | life cycle assessment |
| LCV | lower calorific value |
| MAD | mesophilic anaerobic digestion |
| OLR | organic loading rate |
| PE | people equivalent |
| SL-LCA | life cycle assessment of each AD system analysed separately as an AD system only with the functional unit of 1 m3 of produced methane |
| sCOD | soluble chemical oxygen demand |
| tCOD | total chemical oxygen demand |
| TAD | thermophilic anaerobic digestion |
| TPAD | temperature-phased anaerobic digestion |
| TPAD1 | the first stage (fermenter) of TPAD |
| TPAD2 | the second stage of TPAD |
| TS | total solids |
| TSS | total suspended solids |
| V | reactor working volume |
| VFA | volatile fatty acid |
| VS | volatile solids |
| VSS | volatile suspended solids |
| WAS | waste-activated sludge |
| WRRF | water resource recovery facility |
| WWTP | wastewater treatment plant |
| WWTP-LCA | life cycle assessment of each AD system analysed separately as a part of the whole WWTP with the functional unit of 1 m3 of treated wastewater |
References
- Zhao, G.; Garrido-Baserba, M.; Reifsnyder, S.; Xu, J.-C.; Rosso, D. Comparative energy and carbon footprint analysis of biosolids management strategies in water resource recovery facilities. Sci. Total Environ. 2019, 665, 762–773. [Google Scholar] [CrossRef]
- Lanko, I.; Flores, L.; Garfí, M.; Todt, V.; Posada, J.A.; Jenicek, P.; Ferrer, I. Life Cycle Assessment of the mesophilic, thermophilic, and temperature-phased anaerobic digestion of sewage sludge. Water 2020, 12, 3140. [Google Scholar] [CrossRef]
- Kominko, H.; Gorazda, K.; Wzorek, Z. Potentiality of sewage sludge-based organo-mineral fertilizer production in Poland considering nutrient value, heavy metal content and phytotoxicity for rapeseed crops. J. Environ. Manag. 2019, 248, 109283. [Google Scholar] [CrossRef] [PubMed]
- Pauline, A.L.; Joseph, K. Hydrothermal carbonization of oily sludge for solid fuel recovery – investigation of chemical characteristics and combustion behaviour. J. Anal. Appl. Pyrolysis 2021, 157, 105235. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Santanen, A.; Mäkelä, P.S.A. Recycling sludge on cropland as fertilizer – Advantages and risks. Resour. Conserv. Recycl. 2020, 155, 104647. [Google Scholar] [CrossRef]
- Chen, T.; Qiu, X.; Feng, H.; Yin, J.; Shen, D. Solid digestate disposal strategies to reduce the environmental impact and energy consumption of food waste-based biogas systems. Bioresour. Technol. 2021, 325, 124706. [Google Scholar] [CrossRef]
- Neczaj, E.; Grosser, A. 31—Biogas production by thermal hydrolysis and thermophilic anaerobic digestion of waste-activated sludge. Ind. Munic. Sludge 2019, 741–781. [Google Scholar] [CrossRef]
- Ferrer, I.; Vazquez, F.; Font, X. Long term operation of a thermophilic anaerobic reactor: Process stability and efficiency at decreasing sludge retention time. Bioresour. Technol. 2010, 101, 2972–2980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markis, F.; Baudez, J.-C.; Parthasarathy, R.; Slatter, P.; Eshtiaghi, N. The apparent viscosity and yield stress of mixtures of primary and secondary sludge: Impact of volume fraction of secondary sludge and total solids concentration. Chem. Eng. J. 2016, 288, 577–587. [Google Scholar] [CrossRef]
- Liu, H.; Li, X.; Zhang, Z.; Nghiem, L.D.; Gao, L.; Wang, Q. Semi-continuous anaerobic digestion of secondary sludge with free ammonia pretreatment: Focusing on volatile solids destruction, dewaterability, pathogen removal and ist implications. Water Res. 2021, 202, 117481. [Google Scholar] [CrossRef]
- Jiang, X.; Lyu, Q.; Bi, L.; Xie, Y.; Ji, G.; Huan, C.; Xu, L.; Yan, Z. Improvement of sewage sludge anaerobic digestion through synergistic effect combined trace elements enhancer with enzyme pretreatment and microbial community response. Chemosphere 2022, 286, 131356. [Google Scholar] [CrossRef] [PubMed]
- Micolucci, F.; Gottardo, M.; Pavan, P.; Cavinato, C.; Bolzonella, D. Pilot scale comparison of single and double-stage thermophilic anaerobic digestion of food waste. J. Clean. Prod. 2018, 171, 1376–1385. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, S.; Yuan, H.; Zhou, Q.; Gu, G. Hydrolysis and acidification of waste activated sludge at different pHs. Water Res. 2007, 41, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Jing, Y.; Zhang, S.; Angelidaki, I.; Luo, G. Mesophilic and thermophilic alkaline fermentation of waste activated sludge for hydrogen production: Focusing on homoacetogenesis. Water Res. 2016, 102, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-L.; Wei, W.; Ni, B.-J. Enhanced methane production from anaerobic digestion of waste activated sludge through preliminary pretreatment using calcium hypochlorite. J. Environ. Manag. 2021, 295, 113346. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Shen, N.; Lu, C.; Chen, Y.; Guan, Y. Effective methane production from waste activated sludge in anaerobic digestion via formic acid pretreatment. Biomass Bioenergy 2021, 151, 106176. [Google Scholar] [CrossRef]
- Qasim, S.R. Wastewater Treatment Plants: Planning, Design, and Operation, 2nd ed.; Routledge: Boca Raton, FL, USA, 1999. [Google Scholar] [CrossRef]
- Lin, R.; Cheng, J.; Ding, L.; Murphy, J.D. Improved efficiency of anaerobic digestion through direct interspecies electron transfer at mesophilic and thermophilic temperature ranges. Chem. Eng. J. 2018, 350, 681–691. [Google Scholar] [CrossRef]
- Ruffino, B.; Campo, G.; Cerutti, A.; Scibilia, G.; Lorenzi, E.; Zanetti, M. Comparative analysis between a conventional and a temperature-phased anaerobic digestion system: Monitoring of the process, resources transformation and energy balance. Energy Convers. Manag. 2020, 223, 113463. [Google Scholar] [CrossRef]
- Li, X.; Guo, S.; Peng, Y.; He, Y.; Wang, S.; Li, L.; Yhao, M. Anaerobic digestion using ultrasound as pretreatment approach: Changes in waste activated sludge, anaerobic digestion performances and digestive microbial populations. Biochem. Eng. J. 2018, 139, 139–145. [Google Scholar] [CrossRef]
- Oles, J.; Dichtl, N.; Niehoff, H. Full scale experience of two stage thermophilic/mesophilic sludge digestion. Water Sci. Technol. 1997, 36, 449–456. [Google Scholar] [CrossRef]
- Qin, Y.; Higashimori, A.; Wu, L.-J.; Hojo, T.; Kubota, K.; Li, Y.-Y. Phase separation and microbial distribution in the hyperthermophilic-meso-type temperature-phased anaerobic digestion (TPAD) of waste activated sludge (WAS). Bioresour. Technol. 2017, 245, 401–410. [Google Scholar] [CrossRef]
- Ma, D.; Li, A.; Zhang, L.; Wang, D.; Ji, G. Mechanical compression assisted conductive drying of thin-film dewatered sewage sludge: Process performance, heat and mass transfer behavior. Waste Manag. 2021, 126, 41–51. [Google Scholar] [CrossRef]
- Sakarika, M.; Stavropoulos, K.; Kopsahelis, A.; Koutra, E.; Zafiri, C.; Kornaros, M. Two-stage anaerobic digestion harnesses more energy from the co-digestion of end-of-life dairy products with agro-industrial waste compared to the single-stage process. Biochem. Eng. J. 2020, 153, 107404. [Google Scholar] [CrossRef]
- Nguyen, P.-D.; Truong Tran, N.-S.; Nguyen, T.-T.; Dang, B.-T.; Thi Le, M.-T.; Bui, X.-T.; Mukai, F.; Kobayashi, H.; Ngo, H.-H. Long-term operation of the pilot scale two-stage anaerobic digestion of municipal biowaste in Ho Chi Minh City. Sci. Total Environ. 2021, 766, 142562. [Google Scholar] [CrossRef]
- Shi, Z.; Zhao, R.; Wan, J.; Li, B.; Shen, Y.; Zhang, S.; Luo, G. Metagenomic analysis reveals the fate of antibiotic resistance genes in two-stage and one-stage anaerobic digestion of waste activated sludge. J. Hazard. Mater. 2021, 406, 124595. [Google Scholar] [CrossRef]
- Yuan, D.; Wang, Y.; Qian, X. Variations of internal structure and moisture distribution in activated sludge with stratified extracellular polymeric substances extraction. Water ResearcInternational Biodeterior. Biodegrad. 2017, 116, 1–9. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, Y.; Dong, B.; Dai, X. Characterizing the sludge moisture distribution during anaerobic digestion process through various approaches. Sci. Total Environ. 2019, 675, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Gao, B.; Ren, J.; Li, A.; Yang, H. Coagulation/flocculation in dewatering of sludge: A review. Water Res. 2018, 143, 608–631. [Google Scholar] [CrossRef]
- Abu-Orf, M.M.; Dentel, S.K. Effect of mixing on the rheological characteristics of conditioned sludge: Full-scale studies. Water Sci. Technol. 1997, 36, 51–60. [Google Scholar] [CrossRef]
- Svennevik, O.K.; Beck, G.; Rus, E.; Westereng, B.; Higgins, M.; Solheim, O.E.; Nilsen, P.J.; Horn, S.J. CNash-A novel parameter predicting cake solids of dewatered digestates. Water Res. 2019, 158, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Dai, X.; Chai, X. Critical review on dewatering of sewage sludge: Influential mechanism, conditioning technologies and implications to sludge re-utilizations. Water Res. 2020, 180, 115912. [Google Scholar] [CrossRef]
- Astals, S.; Venegas, C.; Peces, M.; Jofre, J.; Lucena, F.; Mata-Alvarez, J. Balancing hygienization and anaerobic digestion of raw sewage sludge. Water Res. 2012, 46, 6218–6227. [Google Scholar] [CrossRef] [PubMed]
- Nzihou, J.F.; Hamidou, S.; Bouda, M.; Koulidiati, J.; Segda, B.G. Using Dulong and Vandralek formulas to estimate the calorific heating value of a household waste model. Int. J. Sci. Eng. Res. 2014, 5, 1878–1883. [Google Scholar]
- Technical Committee CEN/TC 230 “Water Analysis”. ISO 6222: 1999 Water Quality—Enumeration of Culturable Micro-Organisms—Colony Count by Inoculation in a Nutrient Agar Culture Medium; ISO (The International Organization for Standardization): Geneva, Switzerland, 1999. [Google Scholar]
- Technická Normalizační Komise. ČSN 75 7835 (757835): 2009 Jakost Vod—Stanovení Termotolerantních Koliformních Bakterií a Escherichia coli [Water Quality—Enumeration of Thermotolerant Coliform Bacteria and Escherichia coli]; Úřad pro Technickou Normalizaci, Metrologii a Státní Zkušebnictví: Prague, Czech Republic, 2009. [Google Scholar]
- Technická Normalizační Komise. ČSN 75 7837 (757837): 2010 Jakost Vod—Stanovení Koliformních Bakterií v Nedesinfikovaných Vodách [Water Quality—Enumeration of Coliform Bacteria in Non-Disinficated Waters]; Úřad pro Technickou Normalizaci, Metrologii a Státní Zkušebnictví: Prague, Czech Republic, 2010. [Google Scholar]
- EU, C.D. 98/83/EC of Council of 3rd of November 1998 on the quality of water intended for human consumption. Off. J. Eur. Commun. 1998, L330, 32–54. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 22rd ed.; Rice, E.W., Baird, R.B., Eaton, A.D., Clesceri, L.S., Eds.; American Public Health Association (APHA): Washington, DC, USA; American Water Works Association (AWWA): Denver, FL, USA; Water Environment Federation (WEF): Chicago, IL, USA, 2012. [Google Scholar]
- Ge, H.; Jensen, P.D.; Batstone, D.J. Increased temperature in the thermophilic stage in temperature phased anaerobic digestion (TPAD) improves degradability of waste activated sludge. J. Hazard. Mater. 2020, 187, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rodríguez, J.; Pérez, M.; Romero, L.I. Semicontinuous Temperature-Phased Anaerobic Digestion (TPAD) of Organic Fraction of Municipal Solid Waste (OFMSW). Comparison with single-stage processes. Chem. Eng. J. 2016, 285, 409–416. [Google Scholar] [CrossRef]
- Amodeo, C.; Hattou, S.; Buffier, P.; Benbelkacem, H. Temperature phased anaerobic digestion (TPAD) of organic fraction of municipal solid waste (OFMSW) and digested sludge (DS): Effect of different hydrolysis conditions. Waste Manag. 2021, 126, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Hülsemann, B.; Wüst, D.; Illi, L.; Oechsner, H.; Kruse, A. Valorization of maize silage digestate from two-stage anaerobic digestion by hydrothermal carbonization. Energy Convers. Manag. 2020, 222, 113218. [Google Scholar] [CrossRef]
- Weimer, P.J. Manipulating ruminal fermentation: A microbial ecological perspective. J. Anim. Sci. 1998, 76, 3114–3122. [Google Scholar] [CrossRef]
- Xiao, B.; Qin, Y.; Zhang, W.; Wu, J.; Qiang, H.; Liu, J.; Li, Y.-Y. Temperature-phased anaerobic digestion of food waste: A comparison with single-stage digestions based on performance and energy balance. Bioresour. Technol. 2018, 249, 826–834. [Google Scholar] [CrossRef]
- Wu, L.-L.; Kobayashi, T.; Li, Y.-Y.; Xu, K.-Q. Comparison of single-stage and temperature-phased two-stage anaerobic digestion of oily food waste. Energy Convers. Manag. 2015, 106, 1174–1182. [Google Scholar] [CrossRef]
- Toutian, V.; Barjenbruch, M.; Loderer, C.; Remy, C. Pilot study of thermal alkaline pretreatment of waste activated sludge: Seasonal effects on anaerobic digestion and impact on dewaterability and refractory COD. Water Res. 2020, 182, 115910. [Google Scholar] [CrossRef]
- Cai, Y.; Zheng, Z.; Wang, X. Obstacles faced by methanogenic archaea originating from substrate-driven toxicants in anaerobic digestion. J. Hazard. Mater. 2021, 403, 123938. [Google Scholar] [CrossRef]
- Lloret, E.; Pastor, L.; Pradas, P.; Pascual, J.A. Semi full-scale thermophilic anaerobic digestion (TAnD) for advanced treatment of sewage sludge: Stabilization process and pathogen reduction. Chem. Eng. J. 2013, 232, 42–50. [Google Scholar] [CrossRef]
- Menon, U.; Suresh, N.; George, G.; Ealias, A.M.; Saravanakumar, M.P. A study on combined effect of Fenton and Free Nitrous Acid treatment on sludge dewaterability with ultrasonic assistance: Preliminary investigation on improved calorific value. Chem. Eng. J. 2020, 382, 123035. [Google Scholar] [CrossRef]
- Flaga, A. The aspects of sludge thermal utilization. In Conference Paper; Institute of Heat Engineering and Air Protection, Cracow University of Technology: Cracow, Poland, 2010. [Google Scholar]
- Hupfauf, S.; Winkler, A.; Wagner, A.O.; Podmirseg, S.M.; Insam, H. Biomethanation at 45 °C offers high process efficiency and supports hygienisation. Bioresour. Technol. 2020, 300, 122671. [Google Scholar] [CrossRef]
- Fu, B.; Wang, Y.; Liu, H.; Chen, Y.; Jiang, Q.; Liu, H. Balancing energy production and pathogen removal during temperature phased anaerobic digestion of sewage sludge. Fresenius Environ. Bull. 2014, 23, 1643–1649. [Google Scholar]
- Riau, V.; Angeles De La Rubia, M.; Perez, M. Temperature-phased anaerobic digestion (TPAD) to obtain class A biosolids: A semi-continuous study. Bioresour. Technol. 2010, 101, 2706–2712. [Google Scholar] [CrossRef] [PubMed]
- Nabaterega, R.; Kumar, V.; Khoei, S.; Eskicioglu, C. A review on two-stage anaerobic digestion options for optimizing municipal wastewater sludge treatment process. J. Environ. Chem. Eng. 2021, 9, 105502. [Google Scholar] [CrossRef]
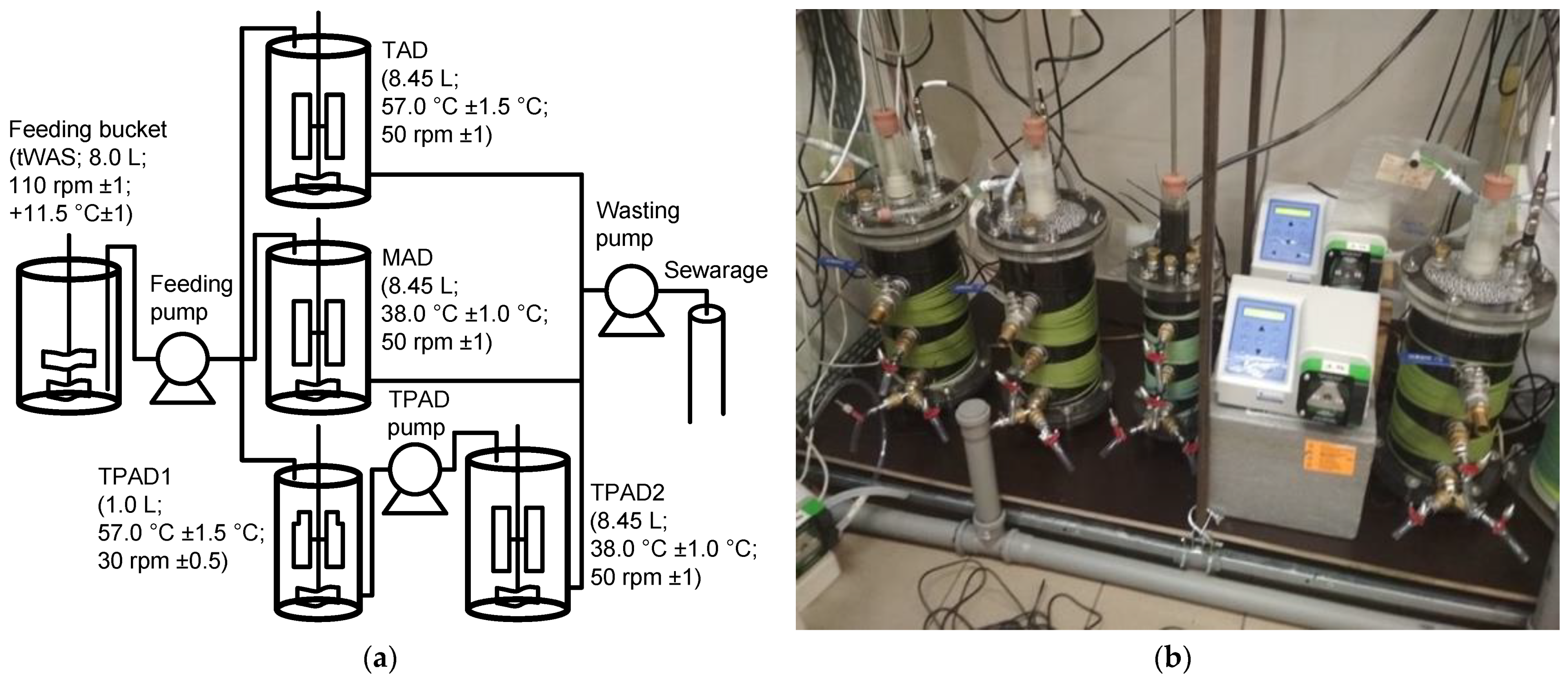


| Type of Reactor | Abbreviation | Phase A, Lasting 5 Months | Phase B, Lasting 3 Months | Phase A and Phase B | ||
|---|---|---|---|---|---|---|
| HRT, Days | Temperature Range, °C | HRT, Days | Temperature Range, °C | Mixing Speed, rpm ** | ||
| Single-stage, thermophilic | TAD | 19.0 | 57 ± 1.5 °C | 13.5 | 57 ± 1.0 °C * | 50 ± 1 |
| Single-stage, mesophilic | MAD | 19.0 | 38 ± 1.5 °C | 13.5 | 38 ± 1.0 °C * | 50 ± 1 |
| Double-stage, thermophilic, the first stage | TPAD1 | 2.0 | 57 ± 1.5 °C | 2.0 | 57 ± 1.0 °C * | 30 ± 1 |
| Double-stage, mesophilic, the second stage | TPAD2 | 17.0 | 38 ± 1.5 °C | 11.5 | 38 ± 1.0 °C * | 50 ± 1 |
| Phase | Parameter | TAD | MAD | TPAD1 | TPAD2 | Substrate |
|---|---|---|---|---|---|---|
| Phase A, at HRT = 19.0 days | Digestate VS, g/L | 30.8 ± 1.7 | 31.3 ± 2.6 | 35.4 ± 4.1 | 28.6 ± 1.0 | 42.3 ± 4.1 |
| VS removal, % | 27.2 | 26.0 | 16.3 | 32.4 | - | |
| Digestate VSS, g/L | 21.0 ± 1.8 | 25.2 ± 2.6 | 28.0 ± 3.0 | 22.2 ± 1.7 | 37.5 ± 3.4 | |
| VSS removal, % | 44.0 | 32.8 | 25.3 | 40.8 | - | |
| Phase B, at HRT = 13.5 days | Digestate VS, g/L | 32.6 ± 0.9 | 32.0 ± 0.7 | 35.6 ± 1.0 | 30.1 ± 0.5 | 46.5 ± 0.6 |
| VS removal, % | 22.9 | 24.3 | 15.8 | 28.8 | - | |
| Digestate VSS, g/L | 21.6 ± 1.2 | 23.7 ± 0.7 | 27.6 ± 0.6 | 20.8 ± 0.8 | 42.2 ± 2.1 | |
| VSS removal, % | 42.9 | 36.8 | 26.4 | 44.5 | - |
| Phases | VFAs, mgCOD/L | |||
|---|---|---|---|---|
| TAD | MAD | TPAD1 | TPAD2 | |
| Phase A, HRT = 19.0 days | 3858.3 ± 973.1 | 519.1 ± 184.5 | 7451.8 ± 1777.7 | 522.4 ± 145.4 |
| Phase B, HRT = 13.5 days | 4821.1 ± 195.0 | 328.9 ± 43.2 | 7809.7 ± 534.0 | 366.8 ± 17.9 |
| Phase | Methane Production, mL/g CODadded | ||||
|---|---|---|---|---|---|
| TAD | MAD | TPAD1 | TPAD2 | TPAD | |
| Phase A, at HRT = 19 days | 169.4 ± 9.2 | 156.0 ± 9.1 | 46.1 ± 4.0 | 186.9 ± 10.7 | 233.1 ± 12.0 |
| Phase B, at HRT = 13.5 days | 116.5 ± 12.0 | 133.9 ± 26.4 | 40.2 ± 8.1 | 132.0 ± 9.8 | 172.3 ± 11.6 |
| Phase | Biogas Content, % | |||||||
|---|---|---|---|---|---|---|---|---|
| TAD | MAD | TPAD1 | TPAD2 | |||||
| CH4 | CO2 | CH4 | CO2 | CH4 | CO2 | CH4 | CO2 | |
| Phase A, at HRT = 19 days | 61.7 ± 4.8 | 34.7 ± 5.0 | 66.1 ± 1.8 | 30.2 ± 3.6 | 58.9 ± 12.8 | 33.2 ± 8.4 | 70.9 ± 2.7 | 24.7 ± 2.7 |
| Phase B, at HRT = 13.5 days | 61.7 ± 1.7 | 33.5 ± 2.3 | 64.2 ± 2.1 | 29.9 ± 1.6 | 53.4 ± 4.7 | 39.8 ± 5.0 | 71.4 ± 1.3 | 23.8 ± 0.9 |
| Phase | Dewaterability Coefficient, % | ||
|---|---|---|---|
| TAD | MAD | TPAD2 | |
| Phase A, at HRT = 19 days | 16.1 ± 0.9 | 13.8 ± 0.7 | 14.8 ± 0.7 |
| Phase B, at HRT = 13.5 days | 17.4 ± 0.9 | 13.6 ± 0.6 | 15.7 ± 0.7 |
| Phase | Parameter | TAD | MAD | TPAD2 |
|---|---|---|---|---|
| Phase A, at HRT = 19.0 days | TS of sludge cake, % | 25.0 ± 1.0 | 26.1 ± 3.8 | 25.6 ± 1.7 |
| polymer dose, g/kgTS | 35.0 | 35.0 | 35.0 | |
| Phase B, at HRT = 13.5 days | TS of sludge cake, % | 30.8 ± 4.2 | 31.4 ± 2.4 | 28.7 ± 4.3 |
| polymer dose, g/kgTS | 30.0 | 30.0 | 32.5 |
| Phase | Element, % | TAD | MAD | TPAD1 | TPAD2 | Substrate |
|---|---|---|---|---|---|---|
| Phase A, at HRT = 19.0 days | N | 3.87 ± 0.05 | 4.23 ± 0.21 | 4.31 ± 0.05 | 3.83 ± 0.22 | 5.37 ± 0.13 |
| C | 25.70 ± 0.27 | 25.56 ± 0.25 | 27.32 ± 0.70 | 24.60 ± 0.19 | 30.26 ± 0.50 | |
| H | 4.39 ± 0.14 | 4.36 ± 0.12 | 4.65 ± 0.11 | 4.29 ± 0.06 | 4.97 ± 0.19 | |
| S | 0.85 ± 0.02 | 0.84 ± 0.01 | 0.84 ± 0.01 | 0.88 ± 0.04 | 0.77 ± 0.01 | |
| O | 65.20 ± 0.48 | 65.01 ± 0.59 | 62.88 ± 0.87 | 66.41 ± 0.51 | 58.64 ± 0.82 | |
| Phase B, at HRT = 13.5 days | N | 4.50 ± 0.06 | 5.18 ± 0.17 | 5.06 ± 0.07 | 4.61 ± 0.07 | 6.50 ± 0.03 |
| C | 30.75 ± 0.53 | 30.45 ± 0.34 | 32.58 ± 0.07 | 29.80 ± 0.59 | 35.28 ± 0.30 | |
| H | 4.77 ± 0.21 | 4.74 ± 0.18 | 4.95 ± 0.23 | 4.63 ± 0.27 | 5.31 ± 0.20 | |
| S | 0.92 ± 0.03 | 0.98 ± 0.01 | 0.90 ± 0.03 | 0.99 ± 0.01 | 0.81 ± 0.01 | |
| O | 59.06 ± 0.79 | 58.66 ± 0.28 | 56.52 ± 0.35 | 59.97 ± 0.80 | 52.10 ± 0.50 |
| Phase | TAD | MAD | TPAD1 | TPAD2 | Substrate | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| LCV, kJ/kg | Loss in LCV, % | LCV, kJ/kg | Loss in LCV, % | LCV, kJ/kg | Loss in LCV, % | LCV, kJ/kg | Loss in LCV, % | LCV, kJ/kg | Loss in LCV, % | |
| Phase A, at HRT = 19.0 days | 9157 ± 76 | 15.4 | 9111 ± 73 | 15.8 | 9742 ± 251 | 10.0 | 8750 ± 63 | 19.2 | 10,827 ± 155 | - |
| Phase B, at HRT = 13.5 days | 11,127 ± 97 | 13.0 | 10,949 ± 137 | 14.3 | 11,715 ± 29 | 8.4 | 10,793 ± 89 | 15.6 | 12,783 ± 8.9 | - |
| Phase | TAD | MAD | TPAD | Substrate | ||||
|---|---|---|---|---|---|---|---|---|
| Cake Solids as TSS, % | Correl. Coef. | Cake Solids as TSS, % | Correl. Coef. | Cake Solids as TSS, % | Correl. Coef. | Cake Solids as TSS, % | Correl. Coef. | |
| Phase A, at HRT = 19.0 days | 26.5 | 1.06 | 23.8 | 0.91 | 25.6 | 1.00 | 20.4 | - |
| Phase B, at HRT = 13.5 days | 23.6 | 0.77 | 20.6 | 0.66 | 22.9 | 0.80 | 16.0 | - |
| Phase | Microbiological Parameter | WAS from the Feeding Bucket, Stored at +11.5 °C | TAD | MAD | TPAD1 | TPAD2 |
|---|---|---|---|---|---|---|
| Phase A, at HRT = 19.0 days | Microorganisms cultivated at 22 °C, CFU/g * | 2.5 × 106 | 2.1 × 104 | 6.2 × 104 | 3.7 × 104 | 1.3 × 105 |
| Microorganisms cultivated at 36 °C, CFU/g * | 1.2 × 106 | 1.4 × 104 | 7.4 × 104 | 3.3 × 104 | 9.1 × 104 | |
| COLI, CFU/g * | 8.2 × 104 | <1 | 299 | <1 | 38 | |
| E. coli, (CFU/g * | 4.9 × 104 | <1 | 155 | <1 | <1 | |
| CLO, CFU/g * | 2.3 × 104 | 2.5 × 103 | 1.3 × 104 | 1.5 × 104 | 4.8 × 103 | |
| Phase B, at HRT = 13.5 days | Microorganisms cultivated at 22 °C, CFU/g * | 2.8 × 107 | 1.4 × 105 | 1.2 × 106 | 4.0 × 105 | 1.2 × 106 |
| Microorganisms cultivated at 36 °C, CFU/g * | 1.2 × 107 | 2.2 × 105 | 9.8 × 105 | 9.7 × 105 | 1.7 × 106 | |
| COLI, CFU/g * | 3.7 × 104 | <1 | 38 | <1 | <1 | |
| E. coli, (CFU/g * | 2.0 × 103 | <1 | 20 | <1 | <1 | |
| CLO, CFU/g * | 5.0 × 104 | 1.4 × 103 | 1.8 × 104 | 9.2 × 103 | 4.9 × 103 |
| Phase | Parameter | TAD | MAD | TPAD1 | TPAD2 | TPAD |
|---|---|---|---|---|---|---|
| Phase A, at HRT = 19.0 days | VS removal, % | + | - | ND | ++ | ND |
| VFA concentration, mgCOD/L | + | ++ | - | ++ | ND | |
| Methane production, mL/gCODadded | ++ | ++ | - | ++ | +++ | |
| Dewaterability coefficient, % | ++ | - | ND | + | ND | |
| Polymer dose, g/kgTS | - | - | ND | - | ND | |
| LCV, kJ/kg | + | + | ND | - | ND | |
| Cake solids as TSS, % | ++ | - | ND | + | ND | |
| Microorganisms cultivated at 22 °C, CFU/g | +++ | + | ++ | - | ND | |
| Microorganisms cultivated at 36 °C, CFU/g | +++ | + | ++ | - | ND | |
| COLI, CFU/g | + | - | + | + | ND | |
| E. coli, CFU/g | + | - | + | + | ND | |
| CLO, CFU/g | + | - | - | + | ND | |
| WWTP-LCA * | + | - | ND | ND | ++ | |
| SL-LCA ** | ++ | + | ND | ND | 0 | |
| Phase B, at HRT = 13.5 days | VS removal, % | - | - | ND | + | ND |
| VFA concentration, mgCOD/L | + | ++ | - | ++ | ND | |
| Methane production, mL/gCODadded | ++ | ++ | - | ++ | +++ | |
| Dewaterability coefficient, % | ++ | - | ND | + | ND | |
| Polymer dose, g/kgTS | - | - | ND | + | ND | |
| LCV, kJ/kg | + | + | ND | - | ND | |
| Cake solids as TSS, % | ++ | - | ND | + | ND | |
| Microorganisms cultivated at 22 °C, CFU/g | ++ | - | + | - | ND | |
| Microorganisms cultivated at 36 °C, CFU/g | ++ | + | + | - | ND | |
| COLI, CFU/g | - | - | - | - | ND | |
| E. coli, CFU/g | - | - | - | - | ND | |
| CLO, CFU/g | +++ | - | + | ++ | - |
| Phase | Parameter | TAD | MAD | TPAD |
|---|---|---|---|---|
| Phases A and B | Degradation efficiency and methane production | 2 | 3 | 1 |
| Process stability (VFA content) | 2 | 1 | 1 | |
| Digestate quality (dewaterability) | 1 | 3 | 2 | |
| Final disposal as a fuel (LCV) | 1 | 1 | 2 | |
| Final disposal as a fertilizer (pathogen safety) | 1 | 3 | 2 | |
| 5-group average value | 1.40 | 2.20 | 1.60 | |
| Phase A | Environmental burden | 1 | 3 | 2 |
| 6-group average value | 1.33 | 2.33 | 1.67 |
| Phase | Parameter | TAD | MAD | TPAD | Other Source | |
|---|---|---|---|---|---|---|
| Phases A and B | Degradation efficiency as VS decrease, % | Study | 22.9–27.2 | 26.0–32.8 | 28.8–32.4 | - |
| Other sources | - | 24–34 | 38–48 | [40] | ||
| Specific methane production, mL/gVSdded | Study | 168–244 * | 189–220 * | 314–413 * | - | |
| Other sources | - | 111–185 140 | 370 360 | [40] [19] | ||
| Process stability as VFA content, mgCOD/L | Study | 3.9–4.8 | 0.3–0.5 | 0.4–0.5 | - | |
| Other sources | 0.87 ** | 0.16 ** | 0.31 ** | [45] | ||
| Energetic value as LCV loss, kJ/kg | Study | 13.0–15.4 | 14.3–15.8 | 15.6–19.2 | - | |
| Other sources | 16.24 ** | 16.74 ** | 16.59 ** | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanko, I.; Hejnic, J.; Říhová-Ambrožová, J.; Ferrer, I.; Jenicek, P. Digested Sludge Quality in Mesophilic, Thermophilic and Temperature-Phased Anaerobic Digestion Systems. Water 2021, 13, 2839. https://doi.org/10.3390/w13202839
Lanko I, Hejnic J, Říhová-Ambrožová J, Ferrer I, Jenicek P. Digested Sludge Quality in Mesophilic, Thermophilic and Temperature-Phased Anaerobic Digestion Systems. Water. 2021; 13(20):2839. https://doi.org/10.3390/w13202839
Chicago/Turabian StyleLanko, Iryna, Jakub Hejnic, Jana Říhová-Ambrožová, Ivet Ferrer, and Pavel Jenicek. 2021. "Digested Sludge Quality in Mesophilic, Thermophilic and Temperature-Phased Anaerobic Digestion Systems" Water 13, no. 20: 2839. https://doi.org/10.3390/w13202839
APA StyleLanko, I., Hejnic, J., Říhová-Ambrožová, J., Ferrer, I., & Jenicek, P. (2021). Digested Sludge Quality in Mesophilic, Thermophilic and Temperature-Phased Anaerobic Digestion Systems. Water, 13(20), 2839. https://doi.org/10.3390/w13202839








