Use of Lignite as a Low-Cost Material for Cadmium and Copper Removal from Aqueous Solutions: Assessment of Adsorption Characteristics and Exploration of Involved Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adsorbent Preparation and Characterization
2.2. Synthetic Heavy Metals Solutions Preparation and Analysis
2.3. Batch Adsorption Investigation
3. Results
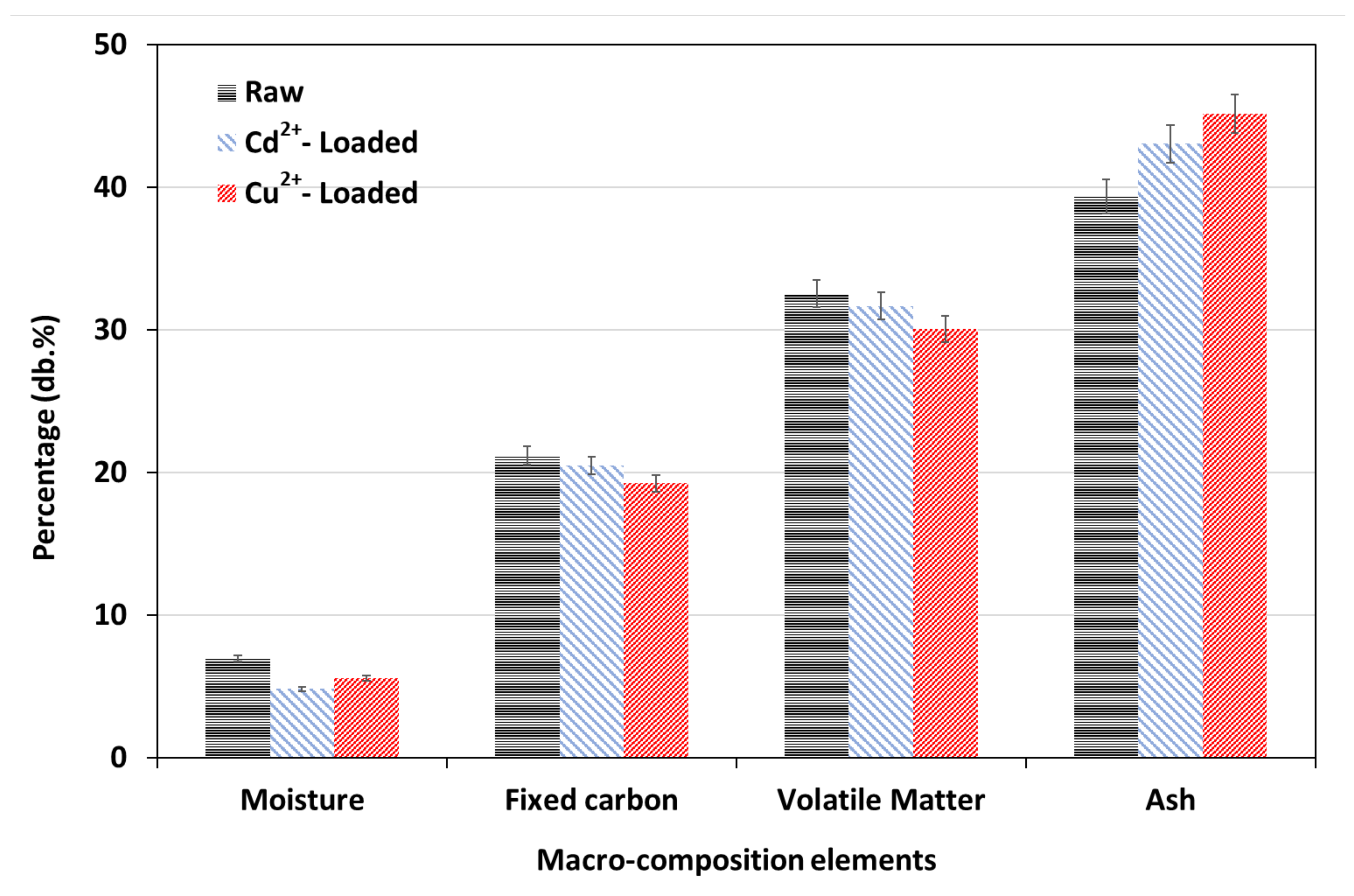
3.1. Lignite Characterization
3.2. Batch Adsorption Results
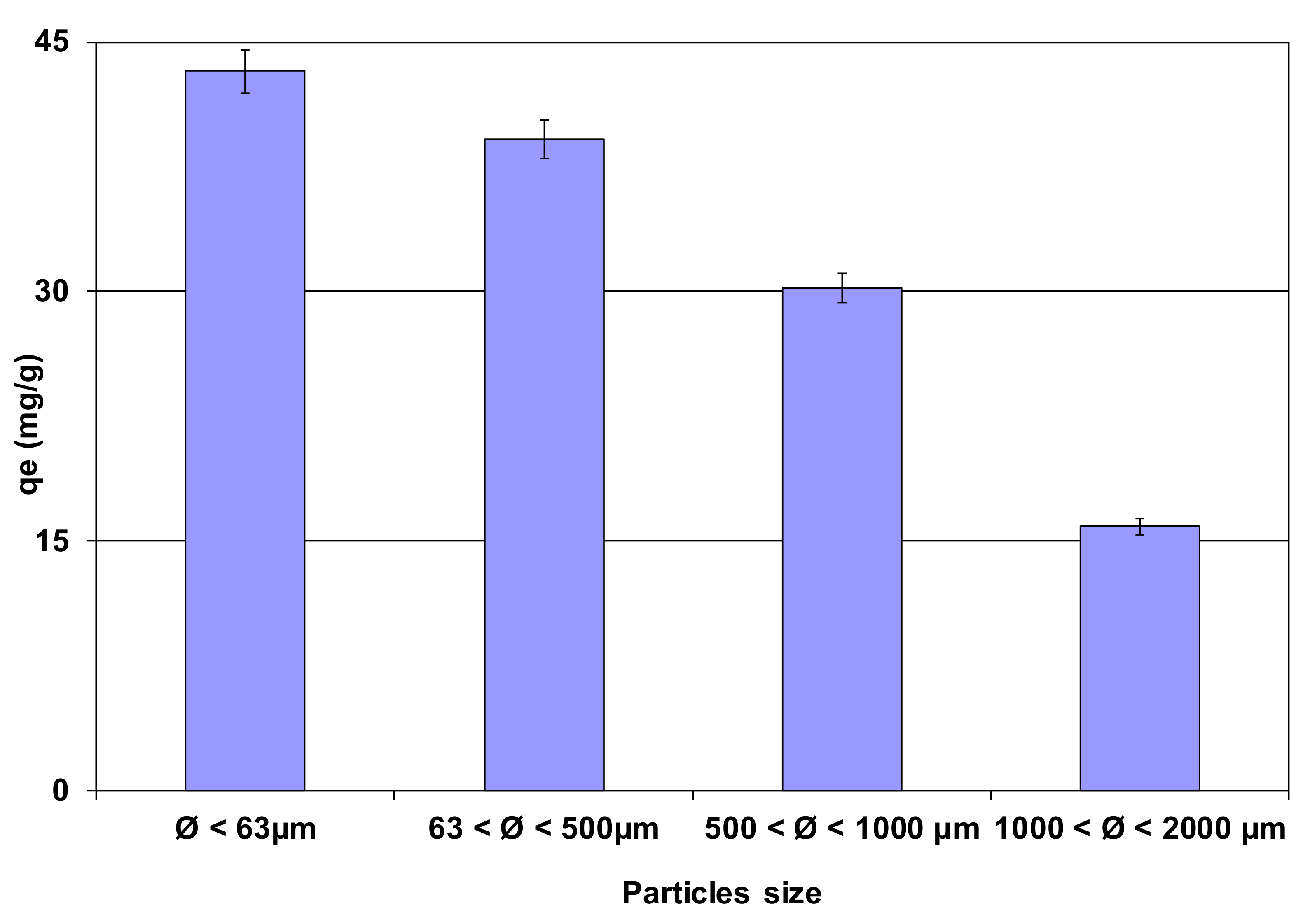
3.2.1. Effect of Lignite Granulometry
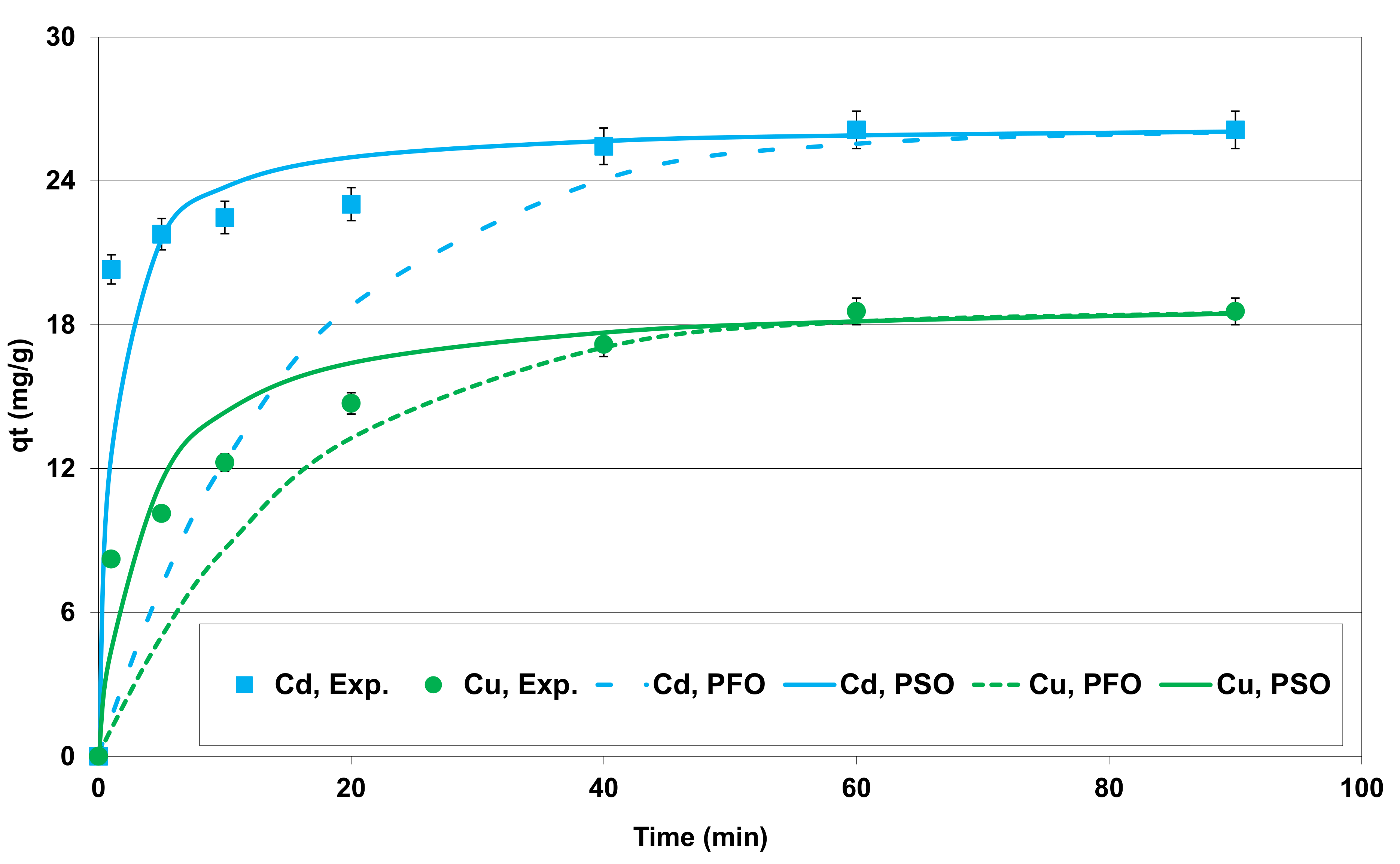
3.2.2. Effect of Contact Time—Kinetic Study
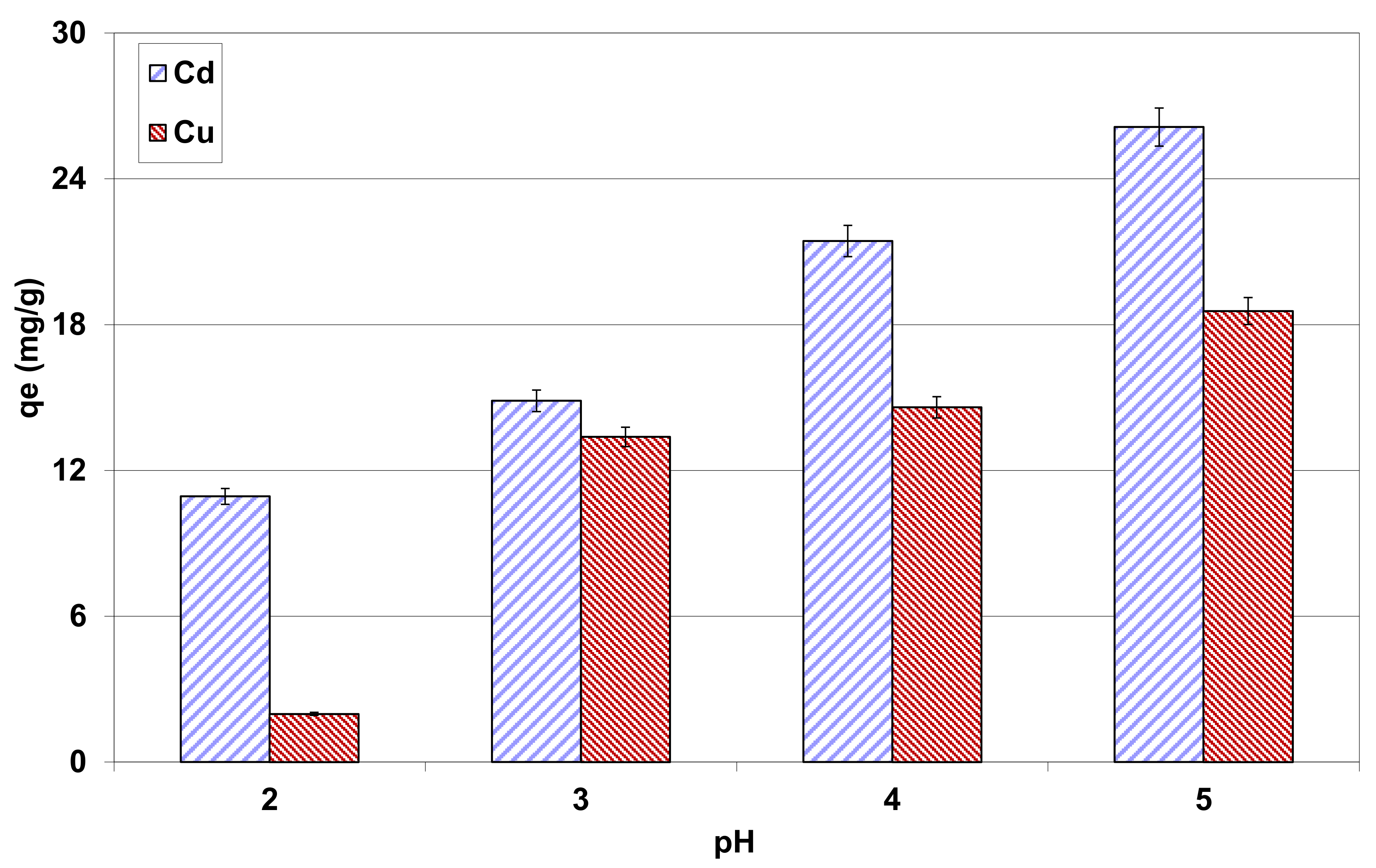
3.2.3. Effect of Initial Aqueous pH
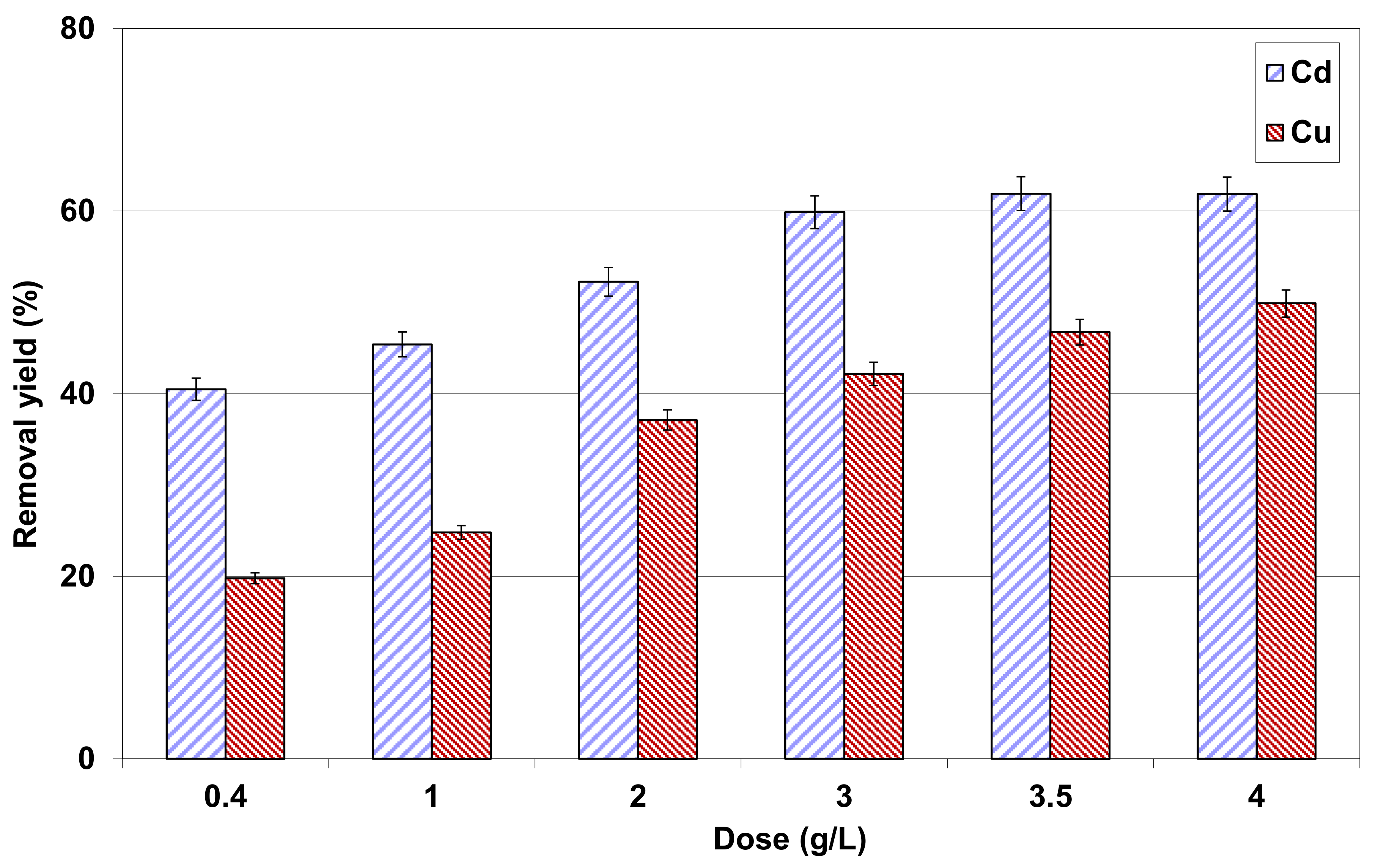
3.2.4. Effect of Lignite Dosage
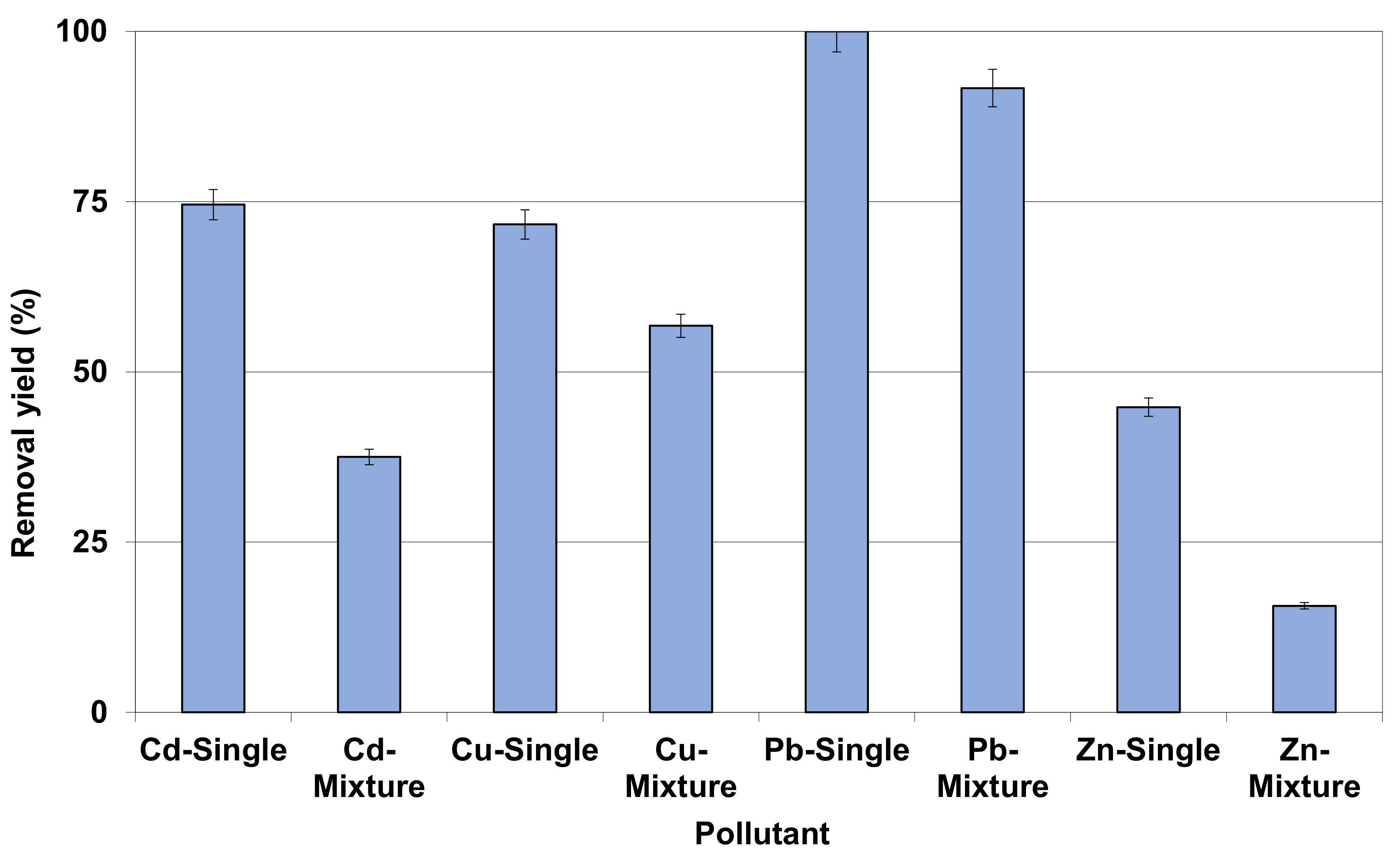
3.2.5. Competing Effect
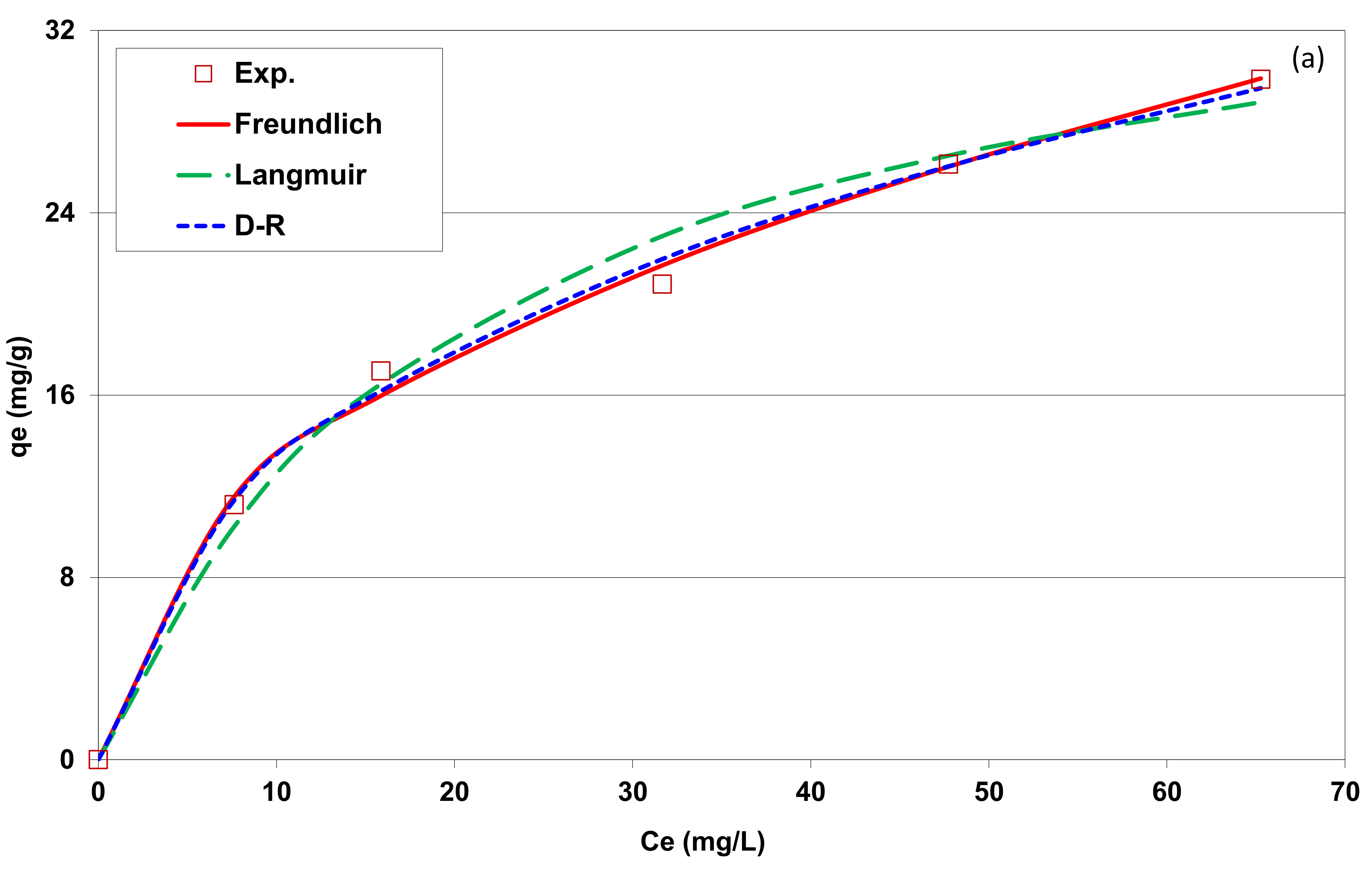
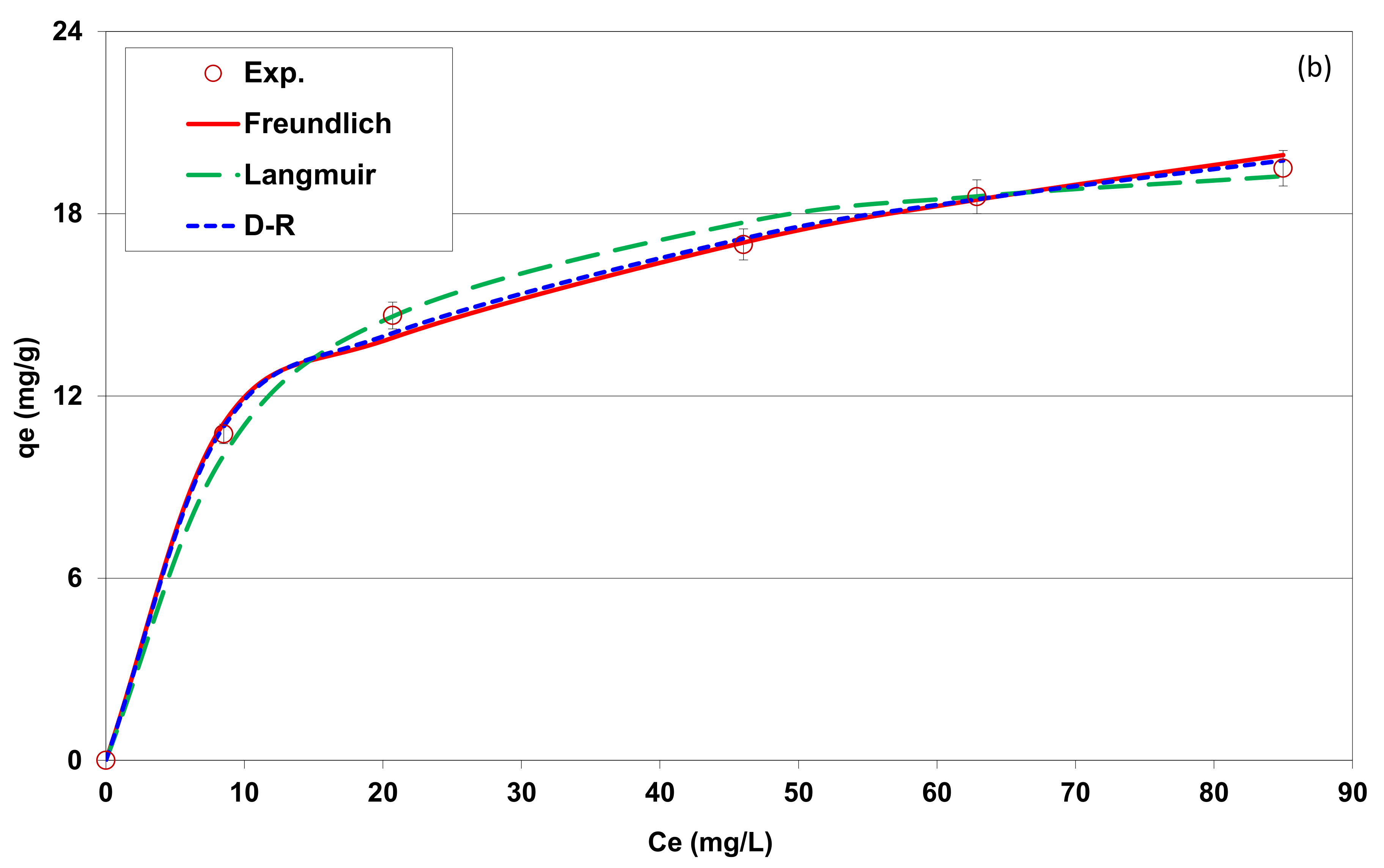
3.2.6. Isotherm Adsorption
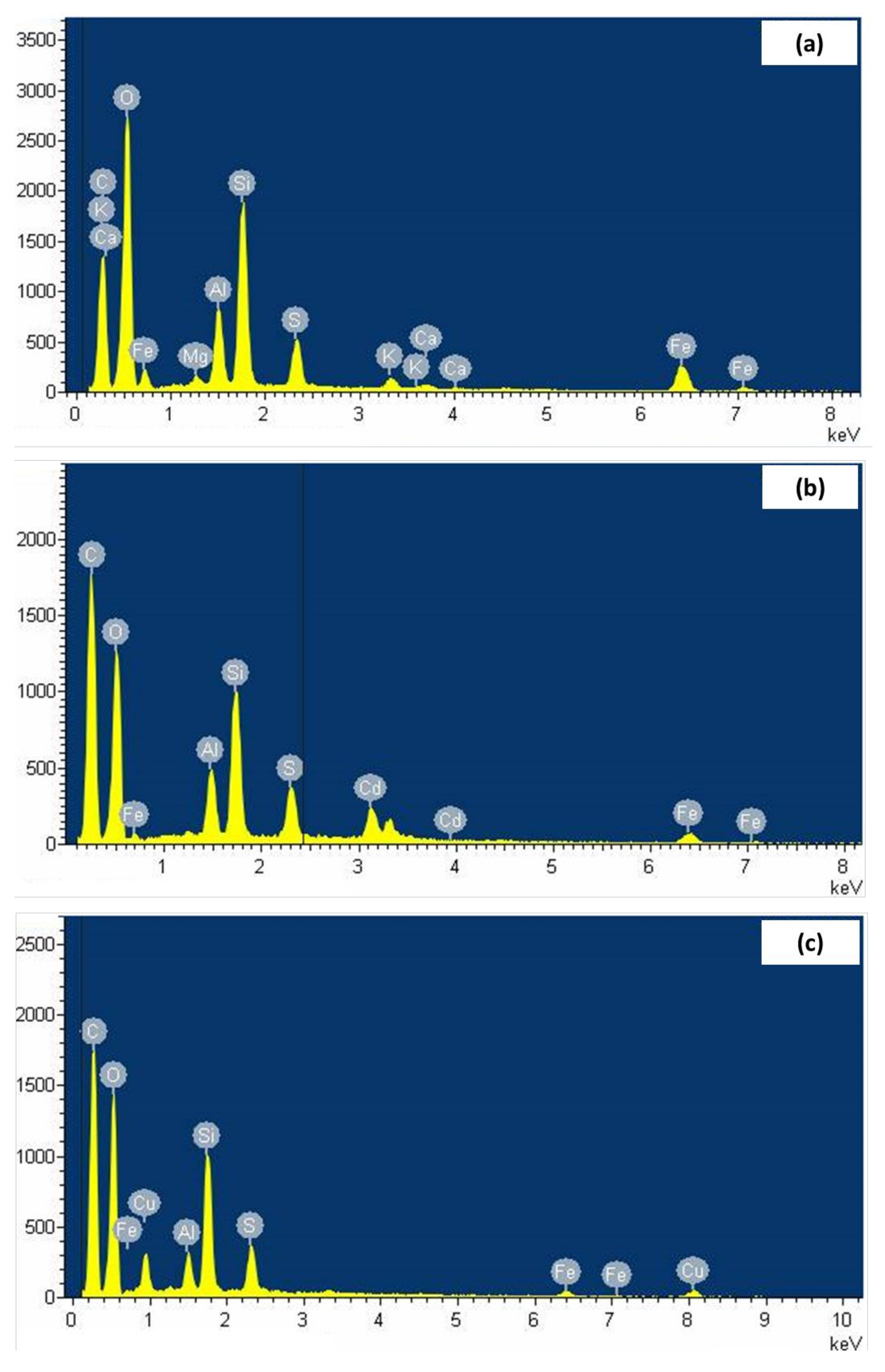
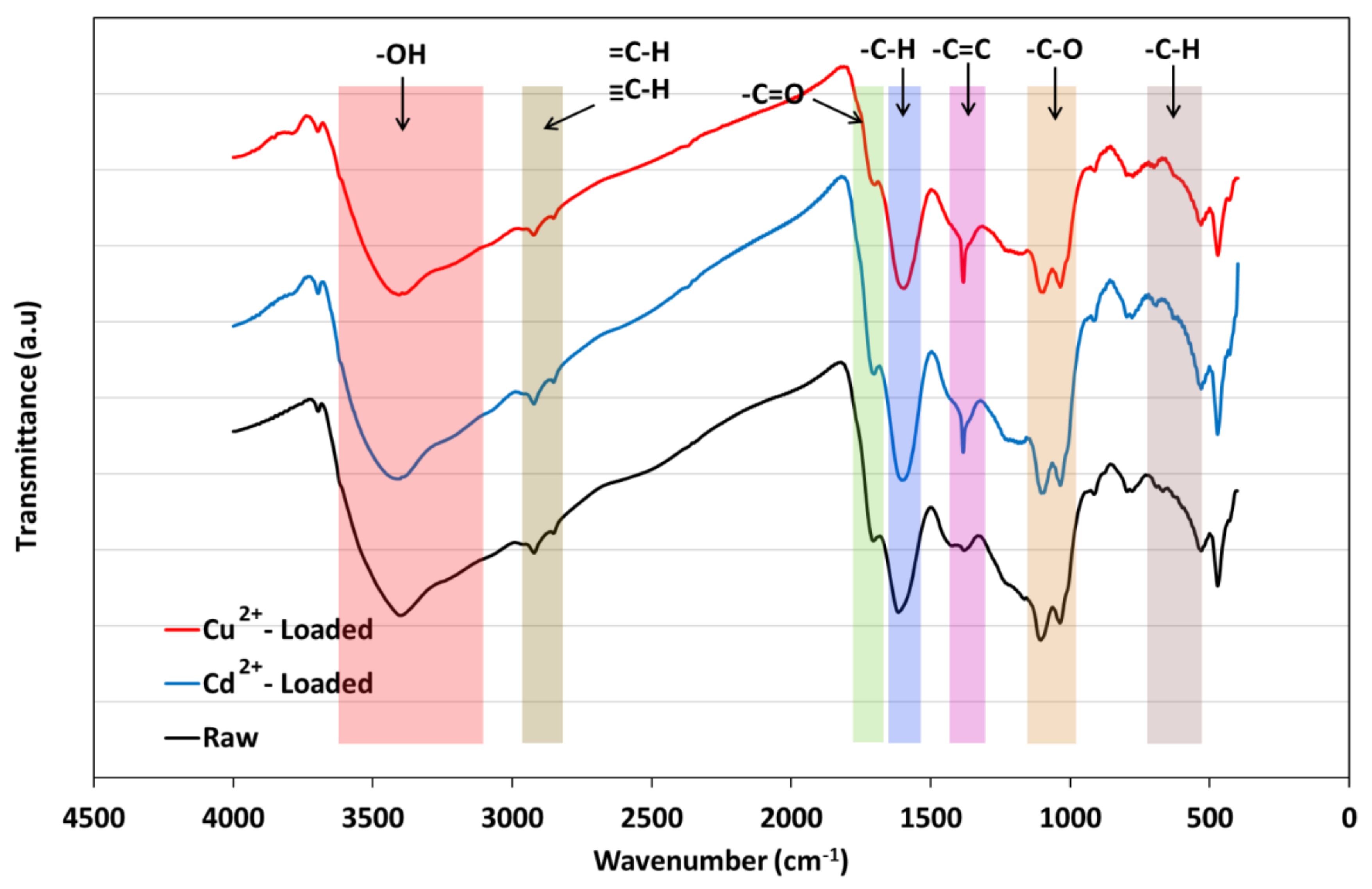
3.3. Raw and Metal-Loaded Lignite Characterization and Adsorption Mechanism Exploration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morais, S.; Costa, F.G.; Pereir, M.L. Heavy Metals and Human Health. In Environmental Health—Emerging Issues and Practice; IntechOpen: London, UK, 2012. [Google Scholar]
- Sharma, H.; Rawal, N.; Mathew, B.B. The Characteristics, Toxicity and Effects of Cadmium. Int. J. Nanotechnol. Nanosci. 2015, 3, 1–9. [Google Scholar]
- Araya, M.; Olivares, M.; Pizarro, F. Copper in human health. Int. J. Environ. Health 2007, 1, 608. [Google Scholar] [CrossRef]
- Abdullah, N.; Yusof, N.; Lau, W.; Jaafar, J.; Ismail, A. Recent trends of heavy metal removal from water/wastewater by membrane technologies. J. Ind. Eng. Chem. 2019, 76, 17–38. [Google Scholar] [CrossRef]
- Liu, Y.; Ke, X.; Zhu, H.; Chen, R.; Chen, X.; Zheng, X.; Jin, Y.; Van Der Bruggen, B.; Jin, Y. Treatment of raffinate generated via copper ore hydrometallurgical processing using a bipolar membrane electrodialysis system. Chem. Eng. J. 2020, 382, 122956. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, S.; Pan, S.-Y.; Zhu, S.; Yu, Y.; Zheng, H. Performance evaluation and optimization of flocculation process for removing heavy metal. Chem. Eng. J. 2020, 385, 123911. [Google Scholar] [CrossRef]
- Cui, Y.; Ge, Q.; Liu, X.-Y.; Chung, N.T.-S. Novel forward osmosis process to effectively remove heavy metal ions. J. Membr. Sci. 2014, 467, 188–194. [Google Scholar] [CrossRef]
- Duan, C.; Ma, T.; Wang, J.; Zhou, Y. Removal of heavy metals from aqueous solution using carbon-based adsorbents: A review. J. Water Process. Eng. 2020, 37, 101339. [Google Scholar] [CrossRef]
- Qin, H.; Hu, T.; Zhai, Y.; Lu, N.; Aliyeva, J. The improved methods of heavy metals removal by biosorbents: A review. Environ. Pollut. 2020, 258, 113777. [Google Scholar] [CrossRef]
- Shakoor, M.B.; Ali, S.; Rizwan, M.; Abbas, F.; Bibi, I.; Riaz, M.; Khalil, U.; Niazi, N.K.; Rinklebe, J. A review of biochar-based sorbents for separation of heavy metals from water. Int. J. Phytoremediation 2020, 22, 111–126. [Google Scholar] [CrossRef]
- Baby, R.; Saifullah, B.; Rehman, F.U.; Shaikh, R.I. Greener Method for the Removal of Toxic Metal Ions from the Wastewater by Application of Agricultural Waste as an Adsorbent. Water 2018, 10, 1316. [Google Scholar] [CrossRef] [Green Version]
- Cataldo, S.; Gianguzza, A.; Pettignano, A.; Piazzese, D.; Sammartano, S. Complex formation of copper(II) and cadmium(II) with pectin and polygalacturonic acid in aqueous solution. An ISE-H + and ISE-Me 2+ electrochemical study. Int. J. Electrochem. Sci. 2012, 7, 6722–6737. [Google Scholar]
- Zhong-Kai, Y.; Chen, Y.-D.; Yang, Z.-K.; Nagarajan, D.; Chang, J.-S.; Ren, N.-Q. High-efficiency removal of lead from wastewater by biochar derived from anaerobic digestion sludge. Bioresour. Technol. 2017, 246, 142–149. [Google Scholar] [CrossRef]
- Chouchene, A.; Jeguirim, M.; Trouvé, G. Biosorption performance, combustion behavior, and leaching characteristics of olive solid waste during the removal of copper and nickel from aqueous solutions. Clean Technol. Environ. Policy 2013, 16, 979–986. [Google Scholar] [CrossRef]
- Mlayah, A.; Jellali, S. Study of continuous lead removal from aqueous solutions by marble wastes: Efficiencies and mechanisms. Int. J. Environ. Sci. Technol. 2014, 12, 2965–2978. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Zhang, J.; Liu, J.; Sun, C.; Yan, Z. Adsorption characteristics of white pottery clay towards Pb(II), Cu(II), and Cd(II). Arab. J. Geosci. 2020, 13, 519. [Google Scholar] [CrossRef]
- Foroutan, R.; Mohammadi, R.; Farjadfard, S.; Esmaeili, H.; Saberi, M.; Sahebi, S.; Dobaradaran, S.; Ramavandi, B. Characteristics and performance of Cd, Ni, and Pb bio-adsorption using Callinectes sapidus biomass: Real wastewater treatment. Environ. Sci. Pollut. Res. 2019, 26, 6336–6347. [Google Scholar] [CrossRef]
- Jellali, S.; Diamantopoulos, E.; Haddad, K.; Anane, M.; Durner, W.; Mlayah, A. Lead removal from aqueous solutions by raw sawdust and magnesium pretreated biochar: Experimental investigations and numerical modelling. J. Environ. Manag. 2016, 180, 439–449. [Google Scholar] [CrossRef]
- Puglla, E.P.; Guaya, D.; Tituana, C.; Osorio, F.; García-Ruiz, M.J. Biochar from Agricultural By-Products for the Removal of Lead and Cadmium from Drinking Water. Water 2020, 12, 2933. [Google Scholar] [CrossRef]
- Egirani, D.E.; Poyi, N.R.; Shehata, N. Preparation and characterization of powdered and granular activated carbon from Palmae biomass for cadmium removal. Int. J. Environ. Sci. Technol. 2020, 17, 2443–2454. [Google Scholar] [CrossRef]
- Vo, A.T.; Nguyen, V.P.; Ouakouak, A.; Nieva, A.; Doma, J.B.T.; Tran, H.N.; Chao, H.-P. Efficient Removal of Cr(VI) from Water by Biochar and Activated Carbon Prepared through Hydrothermal Carbonization and Pyrolysis: Adsorption-Coupled Reduction Mechanism. Water 2019, 11, 1164. [Google Scholar] [CrossRef] [Green Version]
- Ediger, V.Ş.; Berk, I.; Ersoy, M. An assessment of mining efficiency in Turkish lignite industry. Resour. Policy 2015, 45, 44–51. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Sprynskyy, M.; Świątkowski, A. Raw lignite as an effective low-cost adsorbent to remove phenol and chlorophenols from aqueous solutions. Sep. Sci. Technol. 2020, 55, 1741–1751. [Google Scholar] [CrossRef]
- Grajales-Mesa, S.J.; Malina, G. Pilot-Scale Evaluation of a Permeable Reactive Barrier with Compost and Brown Coal to Treat Groundwater Contaminated with Trichloroethylene. Water 2019, 11, 1922. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Gago-Ferrero, P.; Gao, Q.; Ahrens, L.; Blum, K.; Rostvall, A.; Björlenius, B.; Andersson, P.L.; Wiberg, K.; Haglund, P.; et al. Evaluation of five filter media in column experiment on the removal of selected organic micropollutants and phosphorus from household wastewater. J. Environ. Manag. 2019, 246, 920–928. [Google Scholar] [CrossRef]
- Nazari, M.A.; Mohaddes, F.; Pramanik, B.K.; Othman, M.; Muster, T.; Bhuiyan, M.A. Application of Victorian brown coal for removal of ammonium and organics from wastewater. Environ. Technol. 2018, 39, 1041–1051. [Google Scholar] [CrossRef]
- Zherebtsov, S.I.; Malyshenko, N.V.; Votolin, K.S.; Ismagilov, Z.R. Sorption of Metal Cations by Lignite and Humic Acids. Coke Chem. 2020, 63, 142–148. [Google Scholar] [CrossRef]
- Binabaj, M.A.; Nowee, S.M.; Ramezanian, N. Comparative study on adsorption of chromium(VI) from industrial wastewater onto nature-derived adsorbents (brown coal and zeolite). Int. J. Environ. Sci. Technol. 2017, 15, 1509–1520. [Google Scholar] [CrossRef]
- Zhao, T.-T.; Ge, W.-Z.; Yue, F.; Wang, Y.; Pedersen, C.M.; Zeng, F.-G.; Qiao, Y. Mechanism study of Cr(III) immobilization in the process of Cr(VI) removal by Huolinhe lignite. Fuel Process. Technol. 2016, 152, 375–380. [Google Scholar] [CrossRef]
- Pentari, D.; Perdikatsis, V.; Katsimicha, D.; Kanaki, A. Sorption properties of low calorific value Greek lignites: Removal of lead, cadmium, zinc and copper ions from aqueous solutions. J. Hazard. Mater. 2009, 168, 1017–1021. [Google Scholar] [CrossRef]
- Uçurum, M. A study of removal of Pb heavy metal ions from aqueous solution using lignite and a new cheap adsorbent (lignite washing plant tailings). Fuel 2009, 88, 1460–1465. [Google Scholar] [CrossRef]
- Pehlivan, E.; Arslan, G. Removal of metal ions using lignite in aqueous solution—Low cost biosorbents. Fuel Process. Technol. 2007, 88, 99–106. [Google Scholar] [CrossRef]
- Doskočil, L.; Pekař, M. Removal of metal ions from multi-component mixture using natural lignite. Fuel Process. Technol. 2012, 101, 29–34. [Google Scholar] [CrossRef]
- Pehlivan, E.; Richardson, A.; Zuman, P. Electrochemical Investigation of Binding of Heavy Metal Ions to Turkish Lignites. Electroanalysis 2004, 16, 1292–1298. [Google Scholar] [CrossRef]
- Azzaz, A.A.; Jellali, S.; Assadi, A.A.; Bousselmi, L. Chemical treatment of orange tree sawdust for a cationic dye enhancement removal from aqueous solutions: Kinetic, equilibrium and thermodynamic studies. Desalination Water Treat. 2015, 57, 22107–22119. [Google Scholar] [CrossRef]
- Azzaz, A.A.; Jeguirim, M.; Kinigopoulou, V.; Doulgeris, C.; Goddard, M.-L.; Jellali, S.; Ghimbeu, C.M. Olive mill wastewater: From a pollutant to green fuels, agricultural and water source and bio-fertilizer—Hydrothermal carbonization. Sci. Total. Environ. 2020, 733, 139314. [Google Scholar] [CrossRef] [PubMed]
- Haddad, K.; Jeguirim, M.; Jerbi, B.; Chouchene, A.; Dutournié, P.; Thevenin, N.; Ruidavets, L.; Jellali, S.; Limousy, L. Olive Mill Wastewater: From a Pollutant to Green Fuels, Agricultural Water Source and Biofertilizer. ACS Sustain. Chem. Eng. 2017, 5, 8988–8996. [Google Scholar] [CrossRef]
- Havelcová, M.; Mizera, J.; Sýkorová, I.; Pekař, M. Sorption of metal ions on lignite and the derived humic substances. J. Hazard. Mater. 2009, 161, 559–564. [Google Scholar] [CrossRef]
- Huber, M.; Badenberg, S.C.; Wulff, M.; Drewes, J.E.; Helmreich, B. Evaluation of Factors Influencing Lab-Scale Studies to Determine Heavy Metal Removal by Six Sorbents for Stormwater Treatment. Water 2016, 8, 62. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.S.; Korving, L.; Keesman, K.J.; Van Loosdrecht, M.C.; Witkamp, G.-J. Effect of pore size distribution and particle size of porous metal oxides on phosphate adsorption capacity and kinetics. Chem. Eng. J. 2019, 358, 160–169. [Google Scholar] [CrossRef]
- Pn, I.; Cp, U. Overview on the Effect of Particle Size on the Performance of Wood Based Adsorbent. J. Biosens. Bioelectron. 2016, 7, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Jellali, S.; Wahab, M.A.; Anane, M.; Riahi, K.; Jedidi, N. Biosorption characteristics of ammonium from aqueous solutions onto Posidonia oceanica (L.) fibers. Desalination 2011, 270, 40–49. [Google Scholar] [CrossRef]
- Pentari, D.; Vamvouka, D. Cadmium Removal from Aqueous Solutions Using Nano-Iron Doped Lignite. IOP Conf. Ser. Earth Environ. Sci. 2019, 221, 012135. [Google Scholar] [CrossRef]
- Shrestha, S.; Son, G.; Lee, S.H. Kinetic Parameters and Mechanism of Zn (II) Adsorption on Lignite and Coconut Shell–Based Activated Carbon Fiber. J. Hazard. Toxic Radioact. Waste 2014, 18, 1–8. [Google Scholar] [CrossRef]
- Pardo-Botello, R.; Fernández-González, C.; Pinilla-Gil, E.; Cuerda-Correa, E.M.; Gómez-Serrano, V. Adsorption kinetics of zinc in multicomponent ionic systems. J. Colloid Interface Sci. 2004, 277, 292–298. [Google Scholar] [CrossRef]
- Jellali, S.; Wahab, M.A.; Ben Hassine, R.; Hamzaoui, A.H.; Bousselmi, L. Adsorption characteristics of phosphorus from aqueous solutions onto phosphate mine wastes. Chem. Eng. J. 2011, 169, 157–165. [Google Scholar] [CrossRef]
- Haddad, K.; Jellali, S.; Jaouadi, S.; Benltifa, M.; Mlayah, A.; Hamzaoui, A.H. Raw and treated marble wastes reuse as low cost materials for phosphorus removal from aqueous solutions: Efficiencies and mechanisms. Comptes Rendus Chim. 2015, 18, 75–87. [Google Scholar] [CrossRef]
- Haddad, K.; Jellali, S.; Jeguirim, M.; Trabelsi, A.B.H.; Limousy, L. Investigations on phosphorus recovery from aqueous solutions by biochars derived from magnesium-pretreated cypress sawdust. J. Environ. Manag. 2018, 216, 305–314. [Google Scholar] [CrossRef]
- Gürses, A.; Hassani, A.; Kıranşan, M.; Açışlı, Ö.; Karaca, S. Removal of methylene blue from aqueous solution using by untreated lignite as potential low-cost adsorbent: Kinetic, thermodynamic and equilibrium approach. J. Water Process. Eng. 2014, 2, 10–21. [Google Scholar] [CrossRef]
- Allen, S.J.; Brown, P.A. Isotherm analyses for single component and multi-component metal sorption onto lignite. J. Chem. Technol. Biotechnol. 1995, 62, 17–24. [Google Scholar] [CrossRef]
- Karabulut, S.; Karabakan, A.; Denizli, A.; Yürüm, Y. Batch removal of copper(II) and zinc(II) from aqueous solutions with low-rank Turkish coals. Sep. Purif. Technol. 2000, 18, 177–184. [Google Scholar] [CrossRef]
- Sun, H.; He, X.; Wang, Y.; Cannon, F.S.; Wen, H.; Li, X. Nitric acid-anionic surfactant modified activated carbon to enhance cadmium(II) removal from wastewater: Preparation conditions and physicochemical properties. Water Sci. Technol. 2018, 78, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Pekař, M.; Klučáková, M. Comparison of Copper Sorption on Lignite and on Soils of Different Types and Their Humic Acids. Environ. Eng. Sci. 2008, 25, 1123–1128. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Datta, S.; Dhanjal, D.S.; Sharma, K.; Samuel, J.; Singh, J. Current advancement and future prospect of biosorbents for bioremediation. Sci. Total. Environ. 2020, 709, 135895. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Asthana, A.; Singh, A.K.; Jain, B.; Susan, A.B.H. Adsorption of heavy metal ions by various low-cost adsorbents: A review. Int. J. Environ. Anal. Chem. 2020, 1–38. [Google Scholar] [CrossRef]
- Leyva-Ramos, R.; Bernal-Jacome, L.; Acosta-Rodriguez, I. Adsorption of cadmium(II) from aqueous solution on natural and oxidized corncob. Sep. Purif. Technol. 2005, 45, 41–49. [Google Scholar] [CrossRef]
- Krishnani, K.K.; Meng, X.; Christodoulatos, C.; Boddu, V.M. Biosorption mechanism of nine different heavy metals onto biomatrix from rice husk. J. Hazard. Mater. 2008, 153, 1222–1234. [Google Scholar] [CrossRef] [PubMed]
- Demessie, B.; Sahle-Demessie, E.; Sorial, G.A. Cleaning Water Contaminated With Heavy Metal Ions Using Pyrolyzed Biochar Adsorbents. Sep. Sci. Technol. 2015, 50, 2448–2457. [Google Scholar] [CrossRef]
- Villaescusa, I.; Fiol, N.; Martı́nez, M.; Miralles, N.; Poch, J.; Serarols, J. Removal of copper and nickel ions from aqueous solutions by grape stalks wastes. Water Res. 2004, 38, 992–1002. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Chen, Z.; Cheng, F. Factors controlling adsorption of recalcitrant organic contaminant from bio-treated coking wastewater using lignite activated coke and coal tar-derived activated carbon. J. Chem. Technol. Biotechnol. 2017, 93, 112–120. [Google Scholar] [CrossRef]
- Zhang, N.; Shen, Y. One-step pyrolysis of lignin and polyvinyl chloride for synthesis of porous carbon and its application for toluene sorption. Bioresour. Technol. 2019, 284, 325–332. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, Y. Particle size effect on pore structure characteristics of lignite determined via low-temperature nitrogen adsorption. J. Nat. Gas Sci. Eng. 2020, 84, 103633. [Google Scholar] [CrossRef]
- Kanca, A. Investigation on pyrolysis and combustion characteristics of low quality lignite, cotton waste, and their blends by TGA-FTIR. Fuel 2020, 263, 116517. [Google Scholar] [CrossRef]
- Qi, Y.; Hoadley, A.F.; Chaffee, A.L.; Garnier, G. Characterisation of lignite as an industrial adsorbent. Fuel 2011, 90, 1567–1574. [Google Scholar] [CrossRef]
- Azzaz, A.A.; Jellali, S.; Bengharez, Z.; Bousselmi, L.; Akrout, H. Investigations on a dye desorption from modified biomass by using a low-cost eluent: Hysteresis and mechanisms exploration. Int. J. Environ. Sci. Technol. 2019, 16, 7393–7408. [Google Scholar] [CrossRef]

| Cadmium | Copper | |
|---|---|---|
| qe,exp (mg g−1) | 26.1 | 18.6 |
| Pseudo-first-order-model (PFO) | ||
| k1 (min−1) | 0.063 | 0.063 |
| R2 | 0.533 | 0.883 |
| APE (%) | 28.9 | 22.5 |
| Pseudo-second-order-model (PSO) | ||
| qe,theo (mg g−1) | 26.4 | 19.2 |
| K2 (g mg−1 min−1) | 0.034 | 0.016 |
| R2 | 0.894 | 0.932 |
| APE (%) | 6.9 | 11.7 |
| Diffusion model | ||
| Df (×10−14 m2 s−1) | 4.68 | 2.04 |
| R2 | 0.750 | 0.844 |
| Dip (×10−14 m2 s−1) | 2.26 | 2.16 |
| R2 | 0.936 | 0.999 |
| Metal | Cd | Cu |
|---|---|---|
| Freundlich model | ||
| Freundlich constant: KF | 4.7 | 6.4 |
| Freundlich constant: n | 2.3 | 3.9 |
| Determination coefficient: R2 | 0.991 | 0.983 |
| Average percentage error: APE (%) | 2.8 | 2.3 |
| Langmuir model | ||
| Langmuir’s maximum adsorption capacity; qm (mg g−1) | 38.0 | 21.4 |
| Langmuir constant: KL | 0.048 | 0.104 |
| Determination coefficient: R2 | 0.979 | 0.957 |
| APE (%) | 5.4 | 2.5 |
| D-R model | ||
| DR’s maximum adsorption capacity; qm (mg g−1) | 124.8 | 40.5 |
| E (kJ mol−1) | 10.7 | 13.4 |
| Determination coefficient: R2 | 0.990 | 0.990 |
| APE (%) | 2.3 | 1.5 |
| Material | BET Surface Area (m2 g−1) | Adsorption Conditions | Cd Langmuir Adsorption Capacity (mg g−1) | Cu Langmuir Adsorption Capacity (mg g−1) | Reference |
|---|---|---|---|---|---|
| Lignite, Beypazarı, Turkey | 2.56 | C0 = 0.00015–0.0015 M; pH = 4; D = 10 g L−1; t = 1 h ; T = 20 °C | - | 1.62 | [51] |
| Ilgin lignite, Konya, Turkey | 2.06 | C0 = 0.0025–0.025 M; pH = 4.5; D = 4 g L−1; t = 120 min; T = 20 °C | - | 17.8 | [32] |
| Beysehir lignite, Konya, Turkey | 2.96 | - | 18.9 | ||
| Tyul’gansk lignite, South Ural Basin Russia | - | C0 = 0.1 M; pH = 4.1; D = 40 g L−1; t = 5 days; T = 25 °C | - | 27.3 | [27] |
| Tisul’sk lignite, Kansko-Achinsk Basin, Russia | - | - | 27.3 | ||
| Lignite, South Moravian deposit, Mikulcice, Czech Republic | - | C0 = 0.001–0.2 M; pH = 4–5; D = 20 g L−1; t = 24 h; T = 25 °C | - | 82.0 | [53] |
| Lignite activated carbon (LAC), China | 158.11 | C0 = 0.00015–0.0015 M; pH = 7.2; D = 1 g L−1; t = 24 h ; T = 25 °C | 6.8 | - | [52] |
| Lignite activated carbon, treated with sodium dodecyl benzene sulfonate (SAC) | 118.2 | 26.6 | - | ||
| HNO3-treated lignite activated carbon (NAC) | 185.07 | 22.8 | - | ||
| HNO3-treated lignite activated carbon, post treated with sodium dodecyl benzene sulfonate (NSAC) | 131.4 | 44.2 | - | ||
| Lignite, Drama, northern Greece | 154 | C0 = 0.00015–0.015 M; pH = 4.5; D = 10 g L−1; t = 12 h; T = 25 °C | 25.5 | - | [43] |
| Same lignite doped with Nano-scale-zero-valent-iron particles | 109 | 34.7 | - | ||
| Lignite, Macedonia and Thrace, Greece | 4.2 | C0 = 0.00015–0.015 M; pH = 4–5; D = 10 g L−1; t = 45 min; T = 25 °C | 51.5 | 42.7 | [30] |
| Lignite, Cap Bon, Tunisia | 11.2 | C0 = 0.00047–0.00197 M; D = 2 g L−1; pH = 5; T = 20 ± 2 °C | 38.0 | 21.0 | Current study |
| Functional Group | –OH | C=O | C–H | –C=C | –C–O | –C–H |
|---|---|---|---|---|---|---|
| Raw | 3400 | 1705 | 1614 | Absent | 1105 | 794 |
| Cd2+—loaded lignite | 3408 | 1701 | 1600 | 1382 | 1101 | 794 |
| Cu2+—loaded lignite | 3404 | 1699 | 1593 | 1380 | 1103 | 794 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jellali, S.; Azzaz, A.A.; Jeguirim, M.; Hamdi, H.; Mlayah, A. Use of Lignite as a Low-Cost Material for Cadmium and Copper Removal from Aqueous Solutions: Assessment of Adsorption Characteristics and Exploration of Involved Mechanisms. Water 2021, 13, 164. https://doi.org/10.3390/w13020164
Jellali S, Azzaz AA, Jeguirim M, Hamdi H, Mlayah A. Use of Lignite as a Low-Cost Material for Cadmium and Copper Removal from Aqueous Solutions: Assessment of Adsorption Characteristics and Exploration of Involved Mechanisms. Water. 2021; 13(2):164. https://doi.org/10.3390/w13020164
Chicago/Turabian StyleJellali, Salah, Ahmed Amine Azzaz, Mejdi Jeguirim, Helmi Hamdi, and Ammar Mlayah. 2021. "Use of Lignite as a Low-Cost Material for Cadmium and Copper Removal from Aqueous Solutions: Assessment of Adsorption Characteristics and Exploration of Involved Mechanisms" Water 13, no. 2: 164. https://doi.org/10.3390/w13020164






