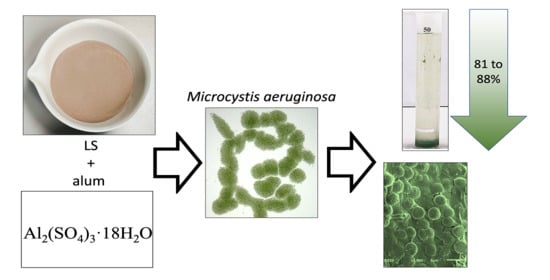
Low Dose Coagulant and Local Soil Ballast Effectively Remove Cyanobacteria (Microcystis) from Tropical Lake Water without Cell Damage
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Water Sampling
2.2. Coagulant and Ballast
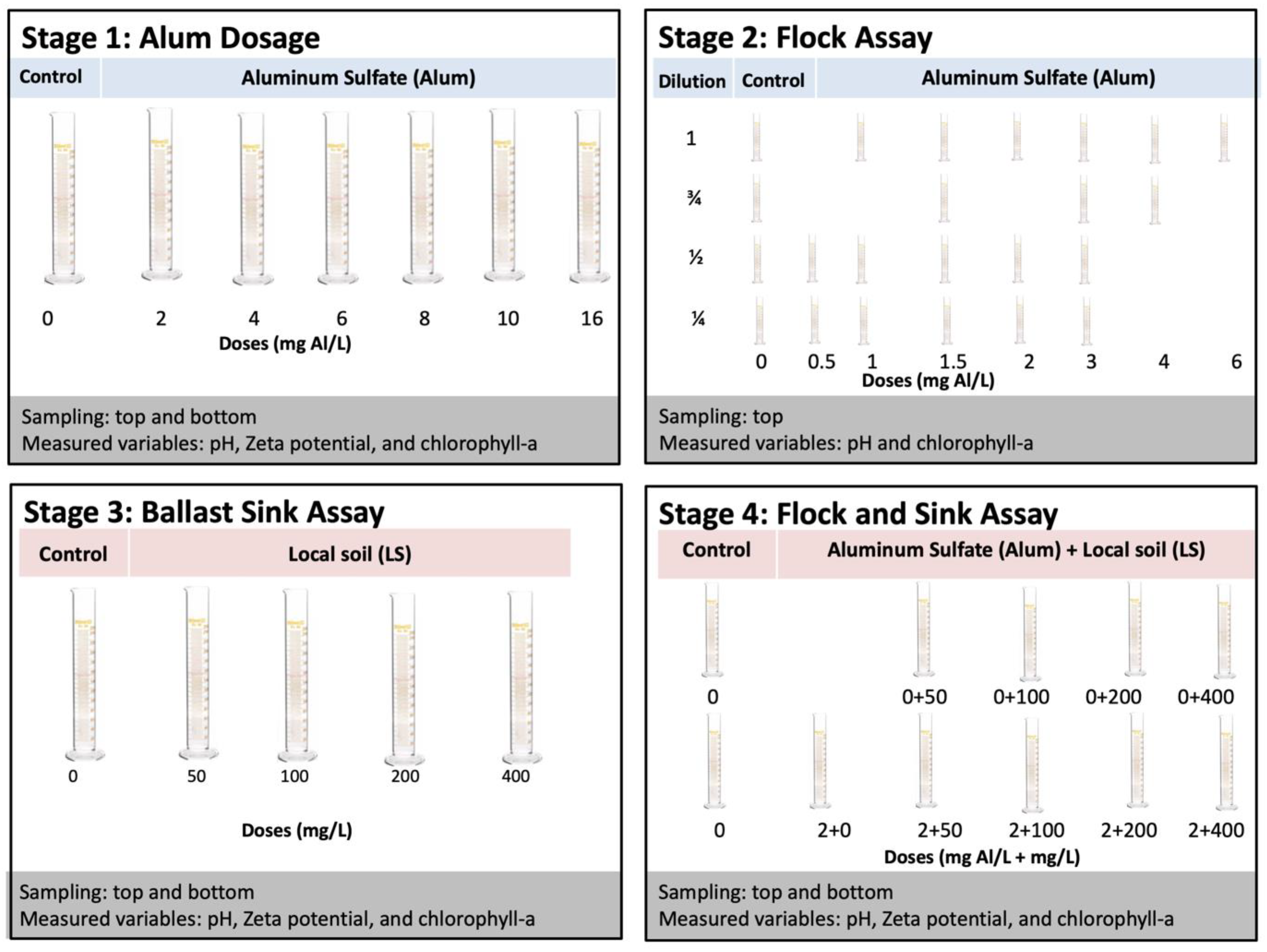
2.3. Experimental Procedures
2.4. Sample Processing and Laboratory Analysis
2.5. Statistical Analysis
3. Results
3.1. Characterization of Lake Water and Ballast
3.2. Effect of Coagulant Dose on Microcystis Floc Buoyancy and Cell Structure
3.3. Effect of Initial Cyanobacteria Concentration on Flocculation
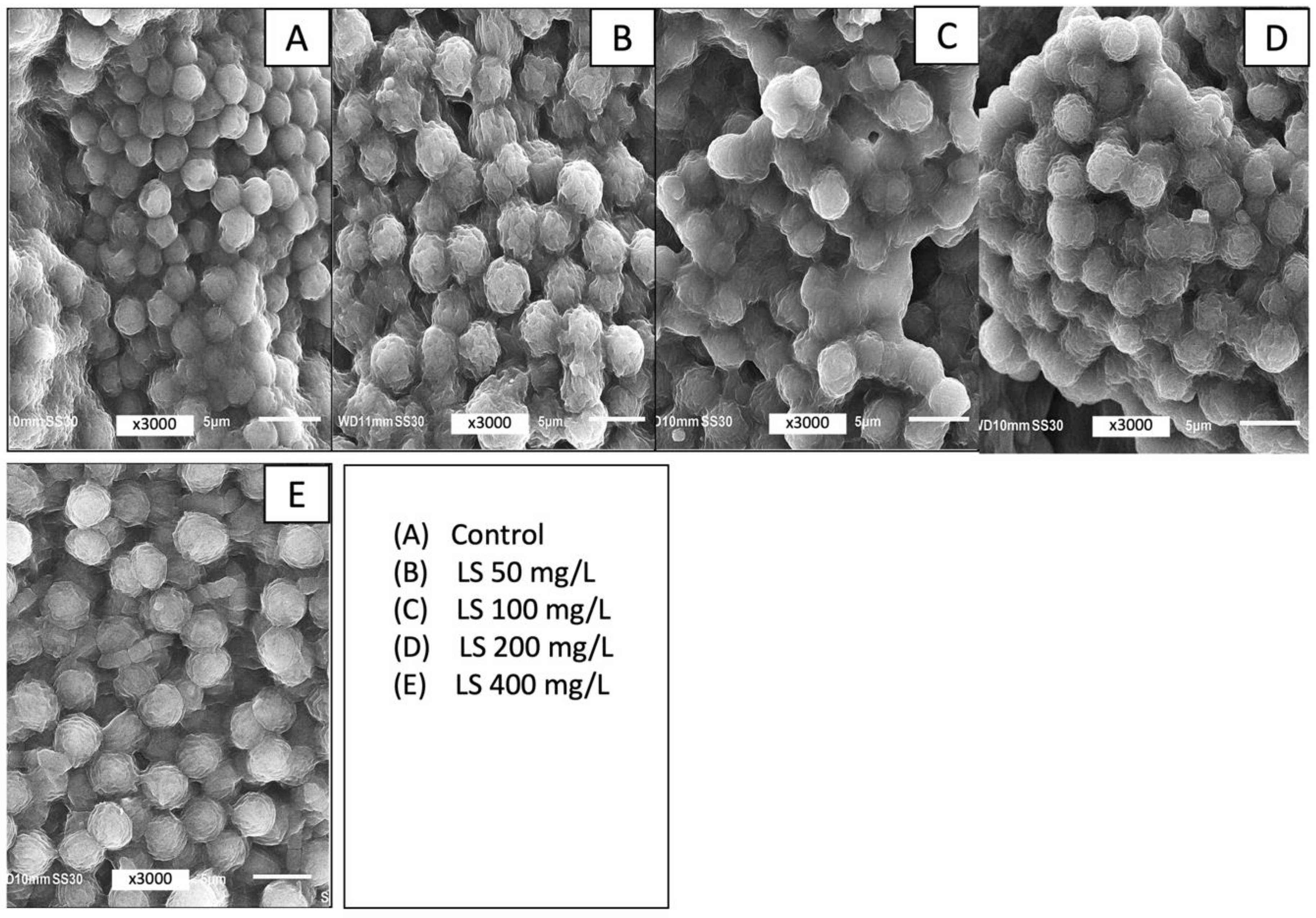
3.4. Effect of LS Ballast on Microcystsis
3.5. Removal Efficacy of LS Ballast Combined with Low-Dose Coagulant
4. Discussion
4.1. Mechanisms of Flock and Sink Technique for Microcystis
4.2. Considerations for Flock and Sink Applications in Fresh Waters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delkash, M.; Al-Faraj, F.A.M.; Scholz, M. Impacts of Anthropogenic Land Use Changes on Nutrient Concentrations in Surface Waterbodies: A Review. Clean Soil Air Water 2018, 46, 1–10. [Google Scholar] [CrossRef]
- Hobbie, S.E.; Finlay, J.C.; Janke, B.D.; Nidzgorski, D.A.; Millet, D.B.; Baker, L.A. Contrasting nitrogen and phosphorus budgets in urban watersheds and implications for managing urban water pollution. Proc. Natl. Acad. Sci. USA 2017, 114, 4177–4182. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.B.; von Gunten, U.; Wehrli, B. Global Water Pollution and Human Health. Annu. Rev. Environ. Resour. 2010, 35, 109–136. [Google Scholar] [CrossRef]
- Paerl, H.W. Mitigating Toxic Planktonic Cyanobacterial Blooms in Aquatic Ecosystems Facing Increasing Anthropogenic and Climatic Pressures. Toxins 2018, 10, 76. [Google Scholar] [CrossRef]
- Paerl, H.W.; Barnard, M.A. Mitigating the global expansion of harmful cyanobacterial blooms: Moving targets in a human- and climatically-altered world. Harmful Algae 2020, 96. [Google Scholar] [CrossRef]
- Jeppesen, E.; Kronvang, B.; Meerhoff, M.; Søndergaard, M.; Hansen, K.M.; Andersen, H.E.; Lauridsen, T.L.; Liboriussen, L.; Beklioglu, M.; Özen, A.; et al. Climate Change Effects on Runoff, Catchment Phosphorus Loading and Lake Ecological State, and Potential Adaptations. J. Environ. Qual. 2009, 38, 1930–1941. [Google Scholar] [CrossRef]
- Sinha, E.; Michalak, A.M.; Balaji, V. Eutrophication will increase during the 21st century as a result of precipitation changes. Science 2017, 357, 405–408. [Google Scholar] [CrossRef]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Muhetaer, G.; Asaeda, T.; Jayasanka, S.M.D.H.; Baniya, M.B.; Abeynayaka, H.D.L.; Rashid, M.H.; Yan, H. Effects of Light Intensity and Exposure Period on the Growth and Stress Responses of Two Cyanobacteria Species: Pseudanabaena galeata and Microcystis aeruginosa. Water 2020, 12, 407. [Google Scholar] [CrossRef]
- Pan, G.; Yang, B.; Wang, D.; Chen, H.; Tian, B.; Zhang, M.; Yuan, X.; Chen, J. In-lake algal bloom removal and submerged vegetation restoration using modified local soils. Ecol. Eng. 2011, 37, 302–308. [Google Scholar] [CrossRef]
- Dodds, W.K.; Bouska, W.W.; Eitzmann, J.L.; Pilger, T.J.; Pitts, K.L.; Riley, A.J.; Schloesser, J.T.; Thornbrugh, D.J. Eutrophication of U.S. Freshwaters: Analysis of Potential Economic Damages. Environ. Sci. Technol. 2009, 43, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Díez-Quijada, L.; Prieto, A.I.; Guzmán-Guillén, R.; Jos, A.; Cameán, A.M. Occurrence and toxicity of microcystin congeners other than MC-LR and MC-RR: A review. Food Chem. Toxicol. 2019, 125, 106–132. [Google Scholar] [CrossRef] [PubMed]
- Pearson, L.; Mihali, T.; Moffitt, M.; Kellmann, R.; Neilan, B. On the chemistry, toxicology and genetics of the cyanobacterial toxins, microcystin, nodularin, saxitoxin and cylindrospermopsin. Mar. Drugs 2010, 8, 1650–1680. [Google Scholar] [CrossRef] [PubMed]
- Svirčev, Z.; Drobac, D.; Tokodi, N.; Mijović, B.; Codd, G.A.; Meriluoto, J. Toxicology of microcystins with reference to cases of human intoxications and epidemiological investigations of exposures to cyanobacteria and cyanotoxins. Arch. Toxicol. 2017, 91, 621–650. [Google Scholar] [CrossRef]
- Buratti, F.M.; Manganelli, M.; Vichi, S.; Stefanelli, M.; Scardala, S.; Testai, E.; Funari, E. Cyanotoxins: Producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch. Toxicol. 2017, 91, 1049–1130. [Google Scholar] [CrossRef]
- Carpenter, S.R. Phosphorus control is critical to mitigating eutrophication. Proc. Natl. Acad. Sci. USA 2008, 105, 11039–11040. [Google Scholar] [CrossRef]
- Schindler, D.W.; Kling, H.; Schmidt, R.V.; Prokopowich, J.; Frost, V.E.; Reid, R.A.; Capel, M. Eutrophication of Lake 227 by Addition of Phosphate and Nitrate: The Second, Third, and Fourth Years of Enrichment, 1970, 1971, and 1972. J. Fish. Res. Board Can. 1973, 30, 1415–1440. [Google Scholar] [CrossRef]
- Li, J.; Hansson, L.-A.; Persson, K. Nutrient Control to Prevent the Occurrence of Cyanobacterial Blooms in a Eutrophic Lake in Southern Sweden, Used for Drinking Water Supply. Water 2018, 10, 919. [Google Scholar] [CrossRef]
- Welch, E.B.; Cooke, G.D. Internal phosphorus loading in shallow lakes: Importance and control. Lake Reserv. Manag. 2005, 21, 209–217. [Google Scholar] [CrossRef]
- Huser, B.J.; Futter, M.; Lee, J.T.; Perniel, M. In-lake measures for phosphorus control: The most feasible and cost-effective solution for long-term management of water quality in urban lakes. Water Res. 2016, 97, 142–152. [Google Scholar] [CrossRef]
- Osgood, R.A. Inadequacy of best management practices for restoring eutrophic lakes in the United States: Guidance for policy and practice. Inland Waters 2017, 7, 401–407. [Google Scholar] [CrossRef]
- Lürling, M.; Mucci, M. Mitigating eutrophication nuisance: In-lake measures are becoming inevitable in eutrophic waters in the Netherlands. Hydrobiologia 2020. [Google Scholar] [CrossRef]
- Douglas, G.B.; Hamilton, D.P.; Robb, M.S.; Pan, G.; Spears, B.M.; Lurling, M. Guiding principles for the development and application of solid-phase phosphorus adsorbents for freshwater ecosystems. Aquat. Ecol. 2016, 50, 385–405. [Google Scholar] [CrossRef]
- Kelly Vargas, K.G.; Qi, Z. P immobilizing materials for lake internal loading control: A review towards future developments. Crit. Rev. Environ. Sci. Technol. 2019, 49, 518–552. [Google Scholar] [CrossRef]
- Dziga, D.; Maksylewicz, A.; Maroszek, M.; Marek, S. Combined treatment of toxic cyanobacteria Microcystis aeruginosa with hydrogen peroxide and microcystin biodegradation agents results in quick toxin elimination. Acta Biochim. Pol. 2018, 65, 133–140. [Google Scholar] [CrossRef]
- Peterson, S.A. Lake Restoration by Sediment Removal. JAWRA J. Am. Water Resour. Assoc. 1982, 18, 423–436. [Google Scholar] [CrossRef]
- Lürling, M.; van Oosterhout, F. Controlling eutrophication by combined bloom precipitation and sediment phosphorus inactivation. Water Res. 2013, 47, 6527–6537. [Google Scholar] [CrossRef]
- Noyma, N.P.; de Magalhaes, L.; Furtado, L.L.; Mucci, M.; van Oosterhout, F.; Huszar, V.L.M.; Marinho, M.M.; Lürling, M. Controlling cyanobacterial blooms through effective flocculation and sedimentation with combined use of flocculants and phosphorus adsorbing natural soil and modified clay. Water Res. 2016, 97, 26–38. [Google Scholar] [CrossRef]
- Miranda, M.; Noyma, N.P.; Pacheco, F.S.; de Magalhães, L.; Pinto, E.; Santos, S.; Soares, M.F.A.; Huszar, V.L.; Lürling, M.; Marinho, M.M. The efficiency of combined coagulant and ballast to remove harmful cyanobacterial blooms in a tropical shallow system. Harmful Algae 2017, 65, 27–39. [Google Scholar] [CrossRef]
- Noyma, N.P.; De Magalhães, L.; Miranda, M.; Mucci, M.; van Oosterhout, F.; Huszar, V.L.M.; Marinho, M.M.; Lima, E.R.A.; Lürling, M. Coagulant plus ballast technique provides a rapid mitigation of cyanobacterial nuisance. PLoS ONE 2017, 12. [Google Scholar] [CrossRef]
- Waajen, G.; van Oosterhout, F.; Douglas, G.; Lürling, M. Management of eutrophication in Lake De Kuil (The Netherlands) using combined flocculant—Lanthanum modified bentonite treatment. Water Res. 2016, 97, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Miao, X.; Bi, L.; Zhang, H.; Wang, L.; Wang, L.; Wang, Z.; Chen, J.; Ali, J.; Pan, M.; et al. Modified Local Soil (MLS) technology for harmful algal bloom control, sediment remediation, and ecological restoration. Water 2019, 11, 1123. [Google Scholar] [CrossRef]
- van Oosterhout, F.; Waajen, G.; Yasseri, S.; Manzi Marinho, M.; Pessoa Noyma, N.; Mucci, M.; Douglas, G.; Lürling, M. Lanthanum in Water, Sediment, Macrophytes and chironomid larvae following application of Lanthanum modified bentonite to lake Rauwbraken (The Netherlands). Sci. Total Environ. 2020, 706, 135188. [Google Scholar] [CrossRef]
- Lürling, M.; Kang, L.; Mucci, M.; van Oosterhout, F.; Noyma, N.P.; Miranda, M.; Huszar, V.L.M.; Waajen, G.; Marinho, M.M. Coagulation and precipitation of cyanobacterial blooms. Ecol. Eng. 2020, 158, 106032. [Google Scholar] [CrossRef]
- de Magalhães, L.; Noyma, N.P.; Furtado, L.L.; Mucci, M.; van Oosterhout, F.; Huszar, V.L.M.; Marinho, M.M.; Lürling, M. Efficacy of Coagulants and Ballast Compounds in Removal of Cyanobacteria (Microcystis) from Water of the Tropical Lagoon Jacarepaguá (Rio de Janeiro, Brazil). Estuar. Coast 2017, 40, 121–133. [Google Scholar] [CrossRef]
- de Lucena-Silva, D.; Molozzi, J.; Severiano, J.d.S.; Becker, V.; de Lucena Barbosa, J.E. Removal efficiency of phosphorus, cyanobacteria and cyanotoxins by the “flock & sink” mitigation technique in semi-arid eutrophic waters. Water Res. 2019, 159, 262–273. [Google Scholar] [CrossRef]
- Mucci, M.; Noyma, N.P.; de Magalhaes, L.; Miranda, M.; van Oosterhout, F.; Guedes, I.A.; Huszar, V.L.M.; Marinho, M.M.; Lurling, M. Chitosan as coagulant on cyanobacteria in lake restoration management may cause rapid cell lysis. Water Res. 2017, 118, 121–130. [Google Scholar] [CrossRef]
- Yu, Z.; Song, X.; Cao, X.; Liu, Y. Mitigation of harmful algal blooms using modified clays: Theory, mechanisms, and applications. Harmful Algae 2017, 69, 48–64. [Google Scholar] [CrossRef]
- Li, H.; Pan, G. Simultaneous Removal of Harmful Algal Blooms and Microcystins Using Microorganism- and Chitosan-Modified Local Soil. Environ. Sci. Technol. 2015, 49, 6249–6256. [Google Scholar] [CrossRef]
- Xu, F.; Zhu, W.; Xiao, M.; Li, M. Interspecific variation in extracellular polysaccharide content and colony formation of Microcystis spp. cultured under different light intensities and temperatures. J. Appl. Phycol. 2016, 28, 1533–1541. [Google Scholar] [CrossRef]
- Lürling, M.; Noyma, N.P.; de Magalhães, L.; Miranda, M.; Mucci, M.; van Oosterhout, F.; Huszar, V.L.M.; Marinho, M.M. Critical assessment of chitosan as coagulant to remove cyanobacteria. Harmful Algae 2017, 66, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Carlson, R.E. A trophic state index for lakes. Limnol. Oceanogr. 1977, 22, 361–369. [Google Scholar] [CrossRef]
- Komárek, J.; Kaštovský, J.; Mareš, J.; Johansen, J. Taxonomic classification of cyanoprokaryotes (cyanobacterial genera) 2014, using a polyphasic approach. Preslia 2014, 86, 295–335. [Google Scholar]
- Aguilera, A.; Gómez, E.B.; Kaštovský, J.; Echenique, R.O.; Salerno, G.L. The polyphasic analysis of two native Raphidiopsis isolates supports the unification of the genera Raphidiopsis and Cylindrospermopsis (Nostocales, Cyanobacteria). Phycologia 2018, 57, 130–146. [Google Scholar] [CrossRef]
- Ozaki, K.; Ito, E.; Tanabe, S.; Natsume, K.; Tsuji, K.; Harada, K. Electron Microscopic Study on Lysis of a Cyanobacterium Microcystis. J. Health Sci. 2009, 55, 578–585. [Google Scholar] [CrossRef]
- Reynolds, C.S.; Oliver, R.L.; Walsby, A.E. Cyanobacterial dominance: The role of buoyancy regulation in dynamic lake environments. N. Z. J. Mar. Freshw. Res. 1987, 21, 379–390. [Google Scholar] [CrossRef]
- Deacon, C.; Walsby, A.E. Gas vesicle formation in the dark, and in light of different irradiances, by the cyanobacterium Microcystis sp. Br. Phycol. J. 1990, 25, 133–139. [Google Scholar] [CrossRef]
- Oliver, R.L.; Walsby, A.E. Direct evidence for the role of light-mediated gas vesicle collapse in the buoyancy regulation of Anabaena flos-aquae (cyanobacteria)1. Limnol. Oceanogr. 1984, 29, 879–886. [Google Scholar] [CrossRef]
- Wallace, B.B.; Hamilton, D.P. The effect of variations in irradiance on buoyancy regulation in Microcystis aeruginosa. Limnol. Oceanogr. 1999, 44, 273–281. [Google Scholar] [CrossRef]
- Harke, M.J.; Steffen, M.M.; Gobler, C.J.; Otten, T.G.; Wilhelm, S.W.; Wood, S.A.; Paerl, H.W. A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 2016, 54, 4–20. [Google Scholar] [CrossRef]
- Li, M.; Nkrumah, P.N.; Xiao, M. Biochemical composition of Microcystis aeruginosa related to specific growth rate: Insight into the effects of abiotic factors. Inland Waters 2014, 4, 357–362. [Google Scholar] [CrossRef]
- Yang, Z.; Kong, F.; Shi, X.; Zhang, M.; Xing, P.; Cao, H. Changes in the morphology and polysaccharide content of microcystis aeruginosa (cyanobacteria) during flagellate grazing. J. Phycol. 2008, 44, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Burkert, U.; Hyenstrand, P.; Drakare, S.; Blomqvist, P. Effects of the mixotrophic flagellate Ochromonas sp. on colony formation in Microcystis aeruginosa. Aquat. Ecol. 2001, 35, 11–17. [Google Scholar] [CrossRef]
- Yang, Zhen; Kong, Fanxiang; Yang, Zhou; Zhang, Min; Yu, Yang; Qian, Shanqin Benefits and costs of the grazer-induced colony formation in Microcystis aeruginosa. Ann. Limnol. Int. J. Lim. 2009, 45, 203–208. [CrossRef]
- Wang, X.; Qin, B.; Gao, G.; Paerl, H.W. Nutrient enrichment and selective predation by zooplankton promote Microcystis (Cyanobacteria) bloom formation. J. Plankton Res. 2010, 32, 457–470. [Google Scholar] [CrossRef]
- Duan, Z.; Tan, X.; Parajuli, K.; Upadhyay, S.; Zhang, D.; Shu, X.; Liu, Q. Colony formation in two Microcystis morphotypes: Effects of temperature and nutrient availability. Harmful Algae 2018, 72, 14–24. [Google Scholar] [CrossRef]
- Sato, M.; Omori, K.; Datta, T.; Amano, Y.; Machida, M. Influence of Extracellular Polysaccharides and Calcium Ion on Colony Formation of Unicellular Microcystis aeruginosa. Environ. Eng. Sci. 2016, 34, 149–157. [Google Scholar] [CrossRef]
- Bi, X.; Zhang, S.; Dai, W.; Xing, K.; Yang, F. Effects of lead(II) on the extracellular polysaccharide (EPS) production and colony formation of cultured Microcystis aeruginosa. Water Sci. Technol. 2013, 67, 803–809. [Google Scholar] [CrossRef]
- Reynolds, C.S.; Jaworski, G.H.M.; Cmiech, H.A.; Leedale, G.F.; Lund, J.W.G. On the annual cycle of the blue-green alga Microcystis Aeruginosa Kütz. Emend. Elenkin. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1981, 293, 419–477. [Google Scholar] [CrossRef]
- Xiao, M.; Li, M.; Reynolds, C.S. Colony formation in the cyanobacterium Microcystis. Biol. Rev. 2018, 93, 1399–1420. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Yang, L.; Gan, L.; Guo, L.; Hu, Z.; Yuan, S.; Chen, Q.; Jiang, L. DO, pH, and Eh microprofiles in cyanobacterial granules from Lake Taihu under different environmental conditions. J. Appl. Phycol. 2014, 26, 1689–1699. [Google Scholar] [CrossRef]
- Spoof, L.; Jaakkola, S.; Važić, T.; Häggqvist, K.; Kirkkala, T.; Ventelä, A.-M.; Kirkkala, T.; Svirčev, Z.; Meriluoto, J. Elimination of cyanobacteria and microcystins in irrigation water—effects of hydrogen peroxide treatment. Environ. Sci. Pollut. Res. 2020, 27, 8638–8652. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.; Duan, J. Hydrolyzing metal salts as coagulants. Pure Appl. Chem. 2001, 73, 2017–2026. [Google Scholar] [CrossRef]
- Duan, J.; Gregory, J. Coagulation by hydrolysing metal salts. Adv. Colloid Interface Sci. 2003, 100–102, 475–502. [Google Scholar] [CrossRef]
- Gonzalez-Torres, A.; Putnam, J.; Jefferson, B.; Stuetz, R.M.; Henderson, R.K. Examination of the physical properties of Microcystis aeruginosa flocs produced on coagulation with metal salts. Water Res. 2014, 60, 197–209. [Google Scholar] [CrossRef]
- Xu, H.; Pei, H.; Xiao, H.; Jin, Y.; Li, X.; Hu, W.; Ma, C.; Sun, J.; Li, H. Behaviors of Microcystis aeruginosa cells during floc storage in drinking water treatment process. Sci. Rep. 2016, 6, 34943. [Google Scholar] [CrossRef]
- Yang, J.; Qiao, K.; Lv, J.; Liu, Q.; Nan, F.; Xie, S.; Feng, J. Isolation and Identification of Two Algae-Lysing Bacteria against Microcystis aeruginosa. Water 2020, 12, 2485. [Google Scholar] [CrossRef]
- Chow, C.W.K.; Drikas, M.; House, J.; Burch, M.D.; Velzeboer, R.M.A. The impact of conventional water treatment processes on cells of the cyanobacterium Microcystis aeruginosa. Water Res. 1999, 33, 3253–3262. [Google Scholar] [CrossRef]
- Drikas, M.; Chow, C.W.K.; House, J.; Burch, M.D. Using coagulation, flocculation, and settling to remove toxic cyanobacteria. J. Am. Water Works Assoc. 2001, 93, 100–111. [Google Scholar] [CrossRef]
- Han, J.; Jeon, B.-S.; Park, H.-D. Microcystin release and Microcystis cell damage mechanism by alum treatment with long-term and large dose as in-lake treatment. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2016, 51, 455–462. [Google Scholar] [CrossRef]
- Han, J.; Jeon, B.-S.; Futatsugi, N.; Park, H.-D. The effect of alum coagulation for in-lake treatment of toxic Microcystis and other cyanobacteria related organisms in microcosm experiments. Ecotoxicol. Environ. Saf. 2013, 96, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, D.P.; Salmaso, N.; Paerl, H.W. Mitigating harmful cyanobacterial blooms: Strategies for control of nitrogen and phosphorus loads. Aquat. Ecol. 2016, 50, 351–366. [Google Scholar] [CrossRef]
- Jilbert, T.; Couture, R.-M.; Huser, B.J.; Salonen, K. Preface: Restoration of eutrophic lakes: Current practices and future challenges. Hydrobiologia 2020. [Google Scholar] [CrossRef]
- Zamyadi, A.; Greenstein, K.E.; Glover, C.M.; Adams, C.; Rosenfeldt, E.; Wert, E.C. Impact of Hydrogen Peroxide and Copper Sulfate on the Delayed Release of Microcystin. Water 2020, 12, 1105. [Google Scholar] [CrossRef]
- Chen, X.; Yang, X.; Yang, L.; Xiao, B.; Wu, X.; Wang, J.; Wan, H. An effective pathway for the removal of microcystin LR via anoxic biodegradation in lake sediments. Water Res. 2010, 44, 1884–1892. [Google Scholar] [CrossRef]
- Grützmacher, G.; Wessel, G.; Klitzke, S.; Chorus, I. Microcystin Elimination during Sediment Contact. Environ. Sci. Technol. 2010, 44, 657–662. [Google Scholar] [CrossRef]
- Pei, H.-Y.; Ma, C.-X.; Hu, W.-R.; Sun, F. The behaviors of Microcystis aeruginosa cells and extracellular microcystins during chitosan flocculation and flocs storage processes. Bioresour. Technol. 2014, 151, 314–322. [Google Scholar] [CrossRef]
- Shen, X.; Yuan, N.; Wang, C. The bioturbation effect of the snail Bellamya aeruginosa on phosphorus immobilisation by drinking water treatment residue in sediment: A long-term continuous flow test. J. Environ. Manag. 2020, 266, 110579. [Google Scholar] [CrossRef]
- Huser, B.J.; Bajer, P.G.; Chizinski, C.; Sorensen, P.W. Effects of common carp (Cyprinus carpio) on sediment mixing depth and mobile phosphorus mass in the active sediment layer of a shallow lake. Hydrobiologia 2016, 763, 23–33. [Google Scholar] [CrossRef]
- Oliver, R.L.; Thomas, R.H.; Reynolds, C.S.; Walsby, A.E. The Sedimentation of Buoyant Microcystis Colonies Caused by Precipitation with an Iron-Containing Colloid. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1985, 223, 511–528. [Google Scholar]
- Gulati, R.D.; Dionisio Pires, L.M.; Van Donk, E. Lake restoration studies: Failures, bottlenecks and prospects of new ecotechnological measures. Limnologica 2008, 38, 233–247. [Google Scholar] [CrossRef]
- Huser, B.J.; Egemose, S.; Harper, H.; Hupfer, M.; Jensen, H.; Pilgrim, K.M.; Reitzel, K.; Rydin, E.; Futter, M. Longevity and effectiveness of aluminum addition to reduce sediment phosphorus release and restore lake water quality. Water Res. 2016, 97, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Kuster, A.C.; Kuster, A.T.; Huser, B.J. A comparison of aluminum dosing methods for reducing sediment phosphorus release in lakes. J. Environ. Manag. 2020, 261, 110195. [Google Scholar] [CrossRef]
- Wang, C.; Gao, S.; Pei, Y.; Zhao, Y. Use of drinking water treatment residuals to control the internal phosphorus loading from lake sediments: Laboratory scale investigation. Chem. Eng. J. 2013, 225, 93–99. [Google Scholar] [CrossRef]
- Makris, K.C.; Harris, W.G.; O’Conno, G.A.; Obreza, T.A. Phosphorus Immobilization in Micropores of Drinking-Water Treatment Residuals: Implications for Long-Term Stability. Environ. Sci. Technol. 2004, 38, 6590–6596. [Google Scholar] [CrossRef]


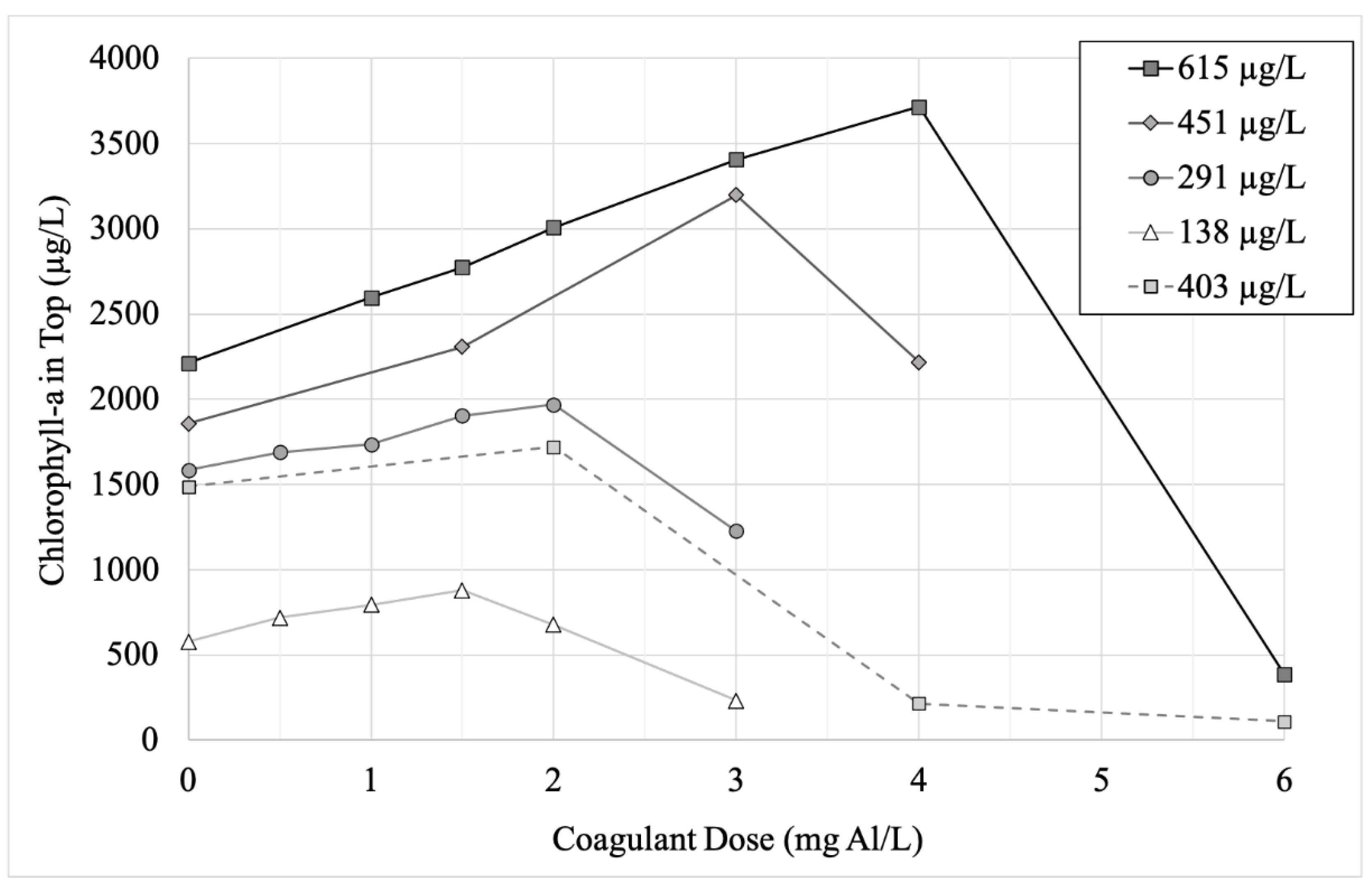
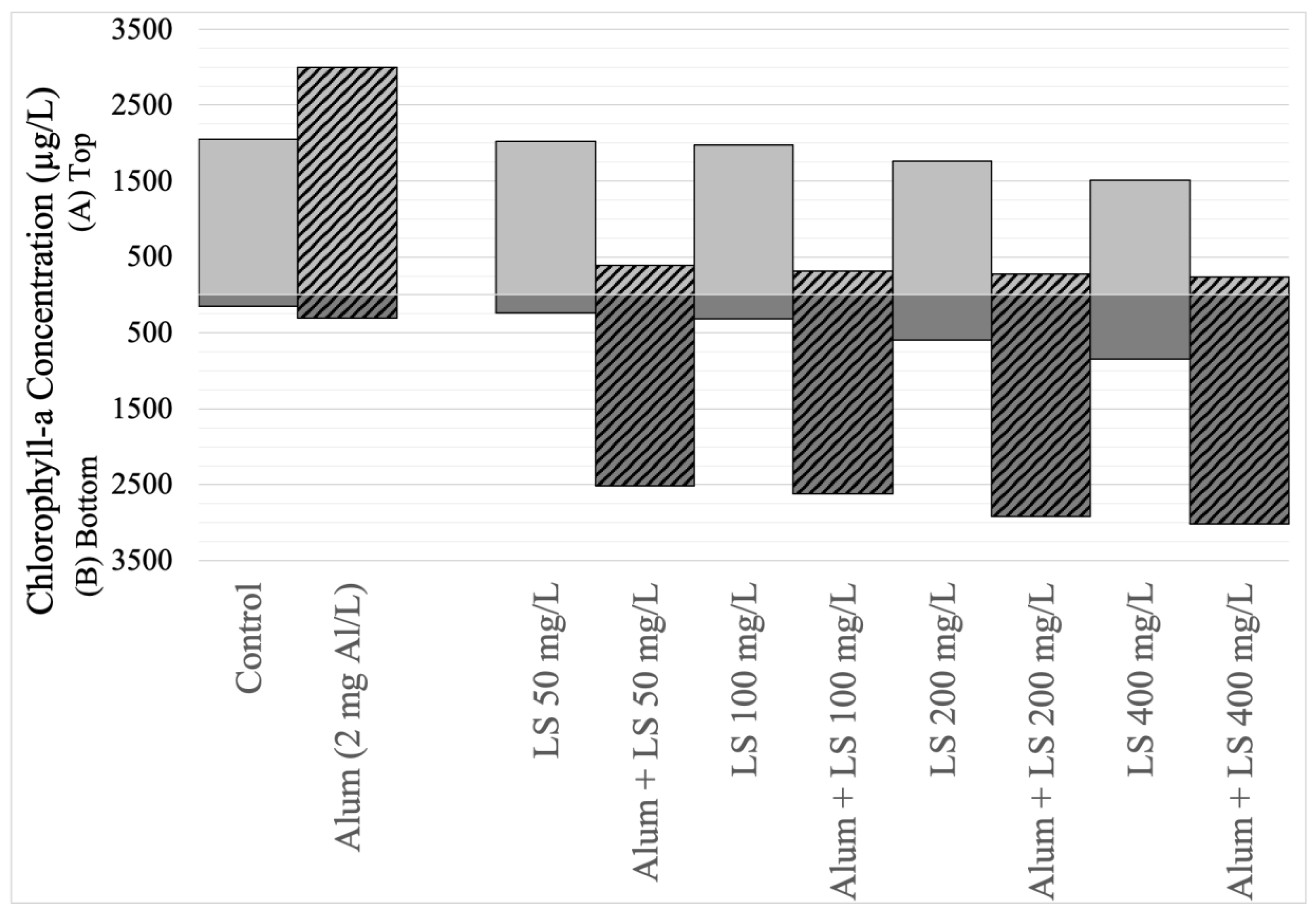
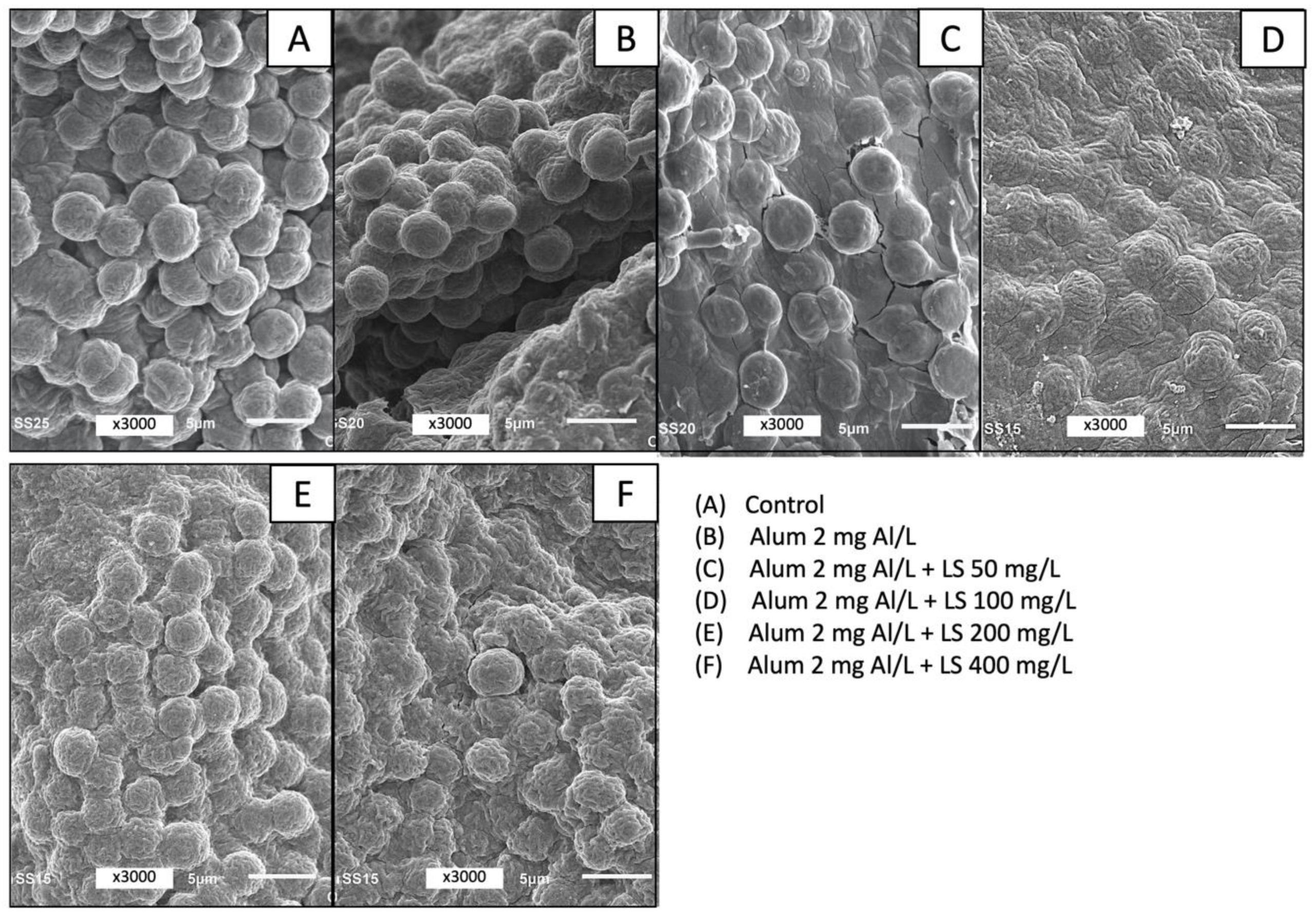
| Experimental Stage | Water Sampling Date | Mean Chlorophyll-a (Standard Deviation) in µg/L (n = 3) | Cell Count (Percentage of Total) in Cells/mL by Genus | |
|---|---|---|---|---|
| Microcystis | Cylindrospermopsis | |||
| Stage 1 | May 2020 | 403 (26.7) | 2.62 × 106 (99.7%) | 9.16 × 104 (0.3%) |
| Stage 2 | June 2020 | 615 (14.2) | 4.21 × 106 (99.9%) | 8.33 × 102 (0.1%) |
| Stage 3 | July 2020 | 243 (5.4) | 2.66 × 106 (99.9%) | 3.33 × 103 (0.1%) |
| Stage 4 | August 2020 | 485 (21.5) | 2.92 × 106 (99.8%) | 5.83 × 103 (0.2%) |
| Alum Dose (mg Al/L) | pH | Chlorophyll-a (µg/L) | Zeta Potential (mV) | ||||
|---|---|---|---|---|---|---|---|
| Top | Bottom | % Change in Top 1 | Top | Bottom | Weighted Average | ||
| 0 | 8.32 (0.05) | 1488 (81) | 117 (5.6) | n/a | −10.07 (1.3) | −8.59 (0.9) | −9.96 |
| 2 | 7.34 (0.04) | 1720 (88) | 140 (18.8) | 16% | −9.85 (2.1) | −8.03 (1.2) | −9.72 |
| 4 | 6.97 (0.01) | 214 (26) | 1186 (21.4) | −86% | −9.08 (1.4) | −8.94 (1.3) | −8.96 |
| 6 | 6.72 (0.01) | 110 (16) | 1950 (42.1) | −93% | −8.16 (0.5) | −7.04 (1.0) | −7.10 |
| 8 | 6.49 (0.03) | 73 (11) | 2023 (52.1) | −95% | −8.82 (0.9 | −5.29 (2.7) | −5.41 |
| 10 | 6.30 (0.01) | 22 (4) | 2143 (84.4) | −99% | −7.92 (0.9) | −4.96 (0.9) | −4.99 |
| 16 | 5.23 (0.23) | 10 (3) | 2214 (86.6) | −99% | −1.86 (0.8) | 0.71 (0.2) | 0.70 |
| LS Dose (mg/L) | pH | Chlorophyll-a (µg/L) | Zeta Potential (mV) | ||||
|---|---|---|---|---|---|---|---|
| Top | Bottom | % Change in Top 1 | Top | Bottom | Weighted Average | ||
| 0 | 8.54 (0.04) | 769 (30) | 114 (0) | −10.4 (1.0) | −10.6 (1.8) | −10.4 | |
| 50 | 8.59 (0.01) | 765 (30) | 139 (21) | −1% | −11.4 (1.7) | −10.3 (1.0) | −11.2 |
| 100 | 8.61 (0.00) | 748 (17) | 173 (10) | −3% | −12.6 (1.3) | −10.5 (0.6) | −12.2 |
| 200 | 8.6 (0.02) | 654 (10) | 222 (21) | −15% | −11.7 (1.2) | −10.2 (1.5) | −11.3 |
| 400 | 8.54 (0.06) | 611 (51) | 279 (2) | −21% | −12.3 (0.8) | −10.4 (1.3) | −11.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thongdam, S.; Kuster, A.C.; Huser, B.J.; Kuster, A.T. Low Dose Coagulant and Local Soil Ballast Effectively Remove Cyanobacteria (Microcystis) from Tropical Lake Water without Cell Damage. Water 2021, 13, 111. https://doi.org/10.3390/w13020111
Thongdam S, Kuster AC, Huser BJ, Kuster AT. Low Dose Coagulant and Local Soil Ballast Effectively Remove Cyanobacteria (Microcystis) from Tropical Lake Water without Cell Damage. Water. 2021; 13(2):111. https://doi.org/10.3390/w13020111
Chicago/Turabian StyleThongdam, Somjate, Anthony C. Kuster, Brian J. Huser, and Anootnara T. Kuster. 2021. "Low Dose Coagulant and Local Soil Ballast Effectively Remove Cyanobacteria (Microcystis) from Tropical Lake Water without Cell Damage" Water 13, no. 2: 111. https://doi.org/10.3390/w13020111
APA StyleThongdam, S., Kuster, A. C., Huser, B. J., & Kuster, A. T. (2021). Low Dose Coagulant and Local Soil Ballast Effectively Remove Cyanobacteria (Microcystis) from Tropical Lake Water without Cell Damage. Water, 13(2), 111. https://doi.org/10.3390/w13020111