Climate Change Effects on Fish Passability across a Rock Weir in a Mediterranean River
Abstract
1. Introduction
2. Materials and Methods
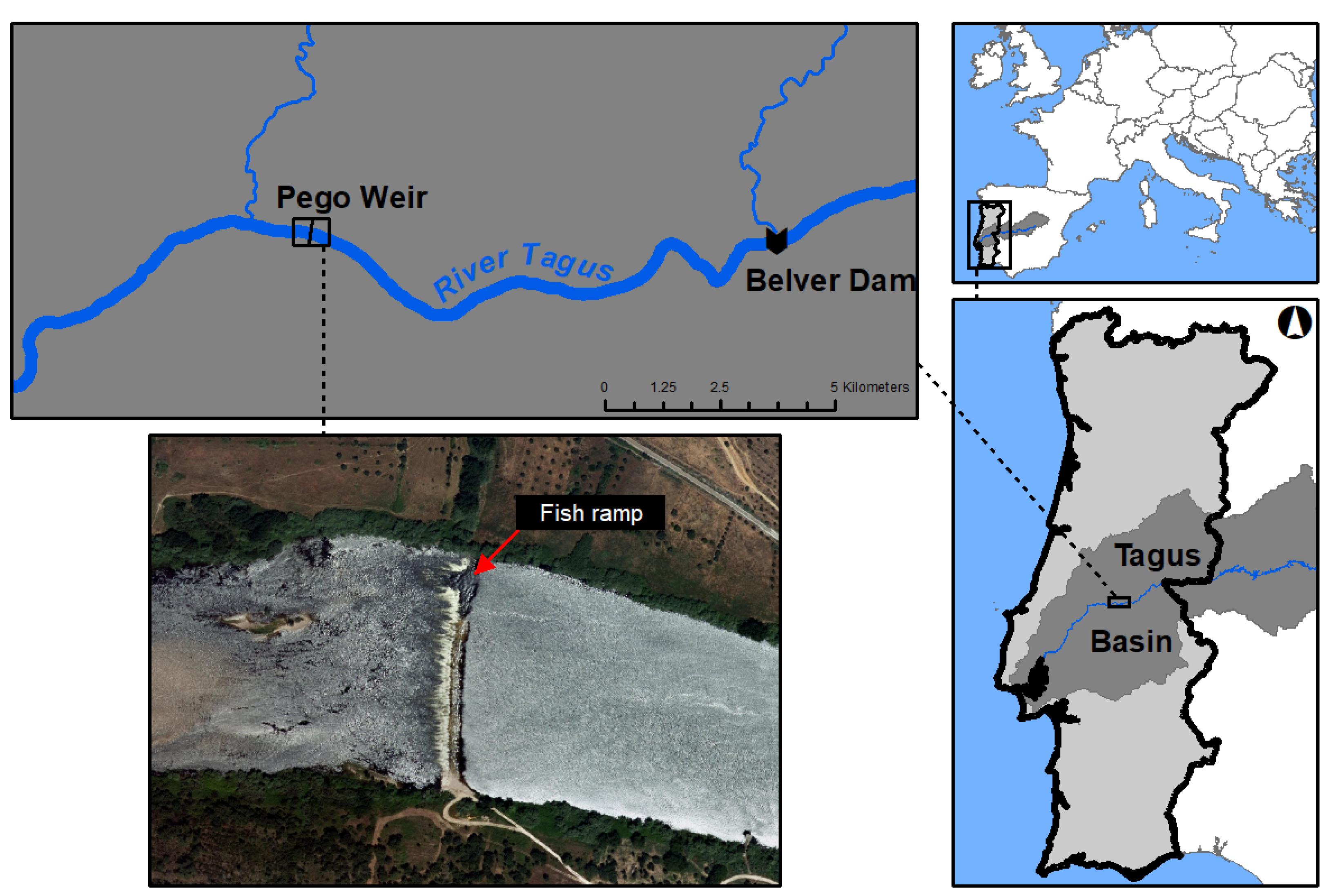
2.1. Study Area
2.2. Flow Data and Topographic Survey
2.3. Suitability Curves and Habitat Modelling
2.4. Data Analyses
3. Results
3.1. Flow under Future Climate Change Scenarios
3.2. Minimum Flow Assessment
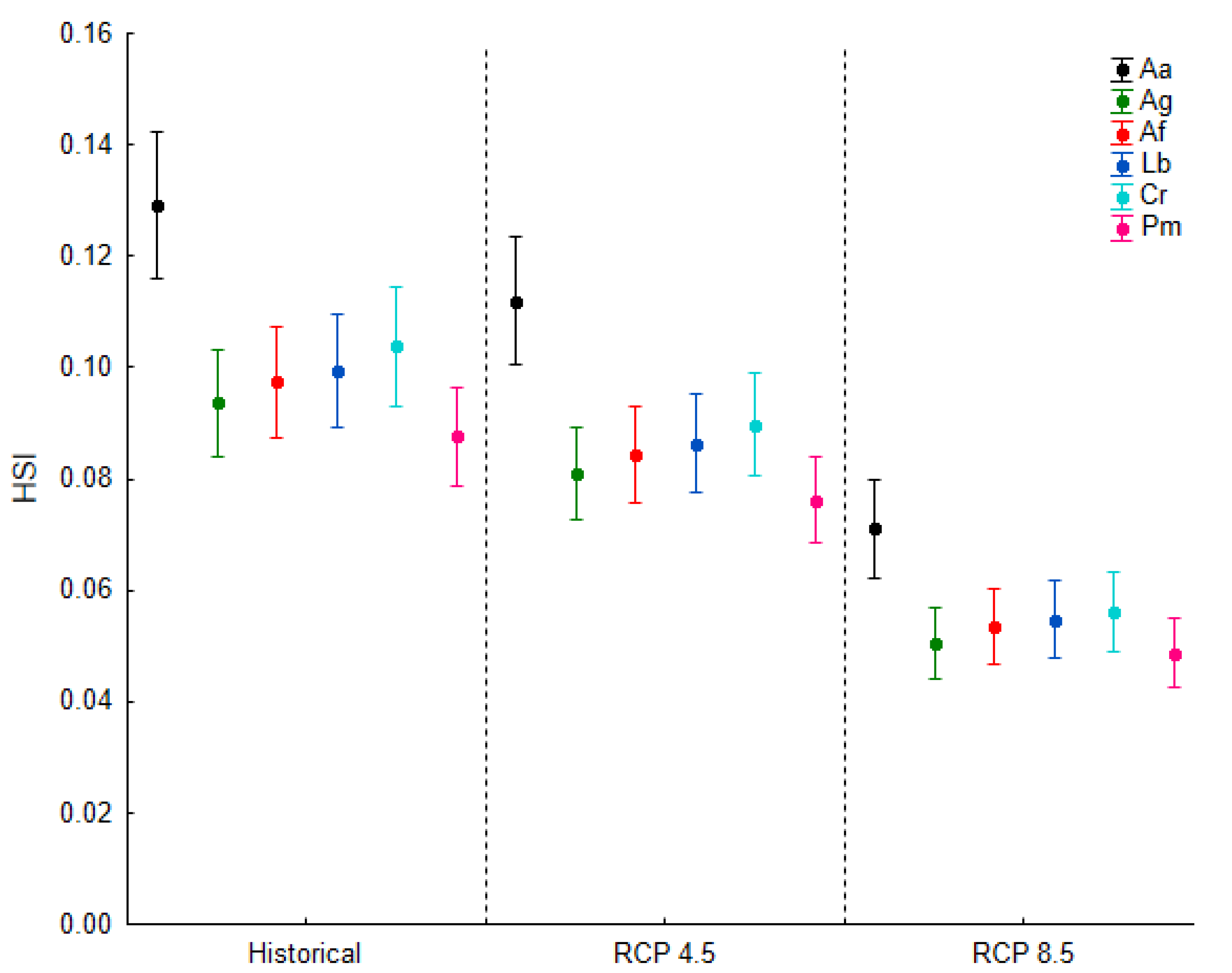
3.3. Passability across Different Climate Change Scenarios
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bruno, D.; Belmar, O.; Maire, A.; Morel, A.; Dumont, B.; Datry, T. Structural and functional responses of invertebrate communities to climate change and flow regulation in alpine catchments. Glob. Chang. Biol. 2019, 25, 1612–1628. [Google Scholar] [CrossRef]
- Capinha, C.; Larson, E.R.; Tricarico, E.; Olden, J.D.; Gherardi, F. Effects of Climate Change, Invasive Species, and Disease on the Distribution of Native European Crayfishes. Conserv. Biol. 2013, 27, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Green, F.B.; East, A.G.; Salice, C.J. Will temperature increases associated with climate change potentiate toxicity of environmentally relevant concentrations of chloride on larval green frogs (Lithobates clamitans)? Sci. Total. Environ. 2019, 682, 282–290. [Google Scholar] [CrossRef]
- Miara, A.; Vörösmarty, C.J.; Macknick, J.E.; Tidwell, V.C.; Fekete, B.; Corsi, F.; Newmark, R. Thermal pollution impacts on rivers and power supply in the Mississippi River watershed. Environ. Res. Lett. 2018, 13, 034033. [Google Scholar] [CrossRef]
- Aristi, I.; Arroita, M.; Larrañaga, A.; Ponsatí, L.; Sabater, S.; von Schiller, D.; Elosegi, A.; Acuña, V. Flow regulation by dams affects ecosystem metabolism in Mediterranean rivers. Freshw. Biol. 2014, 59, 1816–1829. [Google Scholar] [CrossRef]
- Schmutz, S.; Bakken, T.H.; Friedrich, T.; Greimel, F.; Harby, A.; Jungwirth, M.; Melcher, A.; Unfer, G.; Zeiringer, B. Response of Fish Communities to Hydrological and Morphological Alterations in Hydropeaking Rivers of Austria. River Res. Appl. 2014, 31, 919–930. [Google Scholar] [CrossRef]
- Fuller, M.R.; Doyle, M.W.; Strayer, D.L. Causes and consequences of habitat fragmentation in river networks. Ann. New York Acad. Sci. 2015, 1355, 31–51. [Google Scholar] [CrossRef]
- van Vliet, M.T.H.; Franssen, W.H.P.; Yearsley, J.R.; Ludwig, F.; Haddeland, I.; Lettenmaier, D.P.; Kabat, P. Global river discharge and water temperature under climate change. Glob. Environ. Chang. 2013, 23, 450–464. [Google Scholar] [CrossRef]
- Dyer, F.; ElSawah, S.; Croke, B.; Griffiths, R.; Harrison, E.; Lucena-Moya, P.; Jakeman, A. The effects of climate change on ecologically-relevant flow regime and water quality attributes. Stoch. Environ. Res. Risk Assess. 2013, 28, 67–82. [Google Scholar] [CrossRef]
- Palmer, M.A.; Lettenmaier, D.P.; Poff, N.L.; Postel, S.L.; Richter, B.; Warner, R. Climate Change and River Ecosystems: Protection and Adaptation Options. Environ. Manag. 2009, 44, 1053–1068. [Google Scholar] [CrossRef]
- Plesiński, K.; Bylak, A.; Radecki-Pawlik, A.; Mikołajczyk, T.; Kukuła, K. Possibilities of fish passage through the block ramp: Model-based estimation of permeability. Sci. Total. Environ. 2018, 631–632, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Huntjens, P.; Pahl-Wostl, C.; Grin, J. Climate change adaptation in European river basins. Reg. Environ. Chang. 2010, 10, 263–284. [Google Scholar] [CrossRef]
- Lechner, A.; Keckeis, H.; Humphries, P. Patterns and processes in the drift of early developmental stages of fish in rivers: A review. Rev. Fish. Biol. Fish. 2016, 26, 471–489. [Google Scholar] [CrossRef]
- Lennox, R.J.; Crook, D.A.; Moyle, P.B.; Struthers, D.P.; Cooke, S.J. Toward a better understanding of freshwater fish responses to an increasingly drought-stricken world. Rev. Fish. Biol. Fish. 2019, 29, 71–92. [Google Scholar] [CrossRef]
- Cid, N.; Bonada, N.; Carlson, S.; Grantham, T.; Gasith, A.; Resh, V. High Variability Is a Defining Component of Mediterranean-Climate Rivers and Their Biota. Water 2017, 9, 52. [Google Scholar] [CrossRef]
- García-Vega, A.; Sanz-Ronda, F.J.; Fernandes Celestino, L.; Makrakis, S.; Leunda, P.M. Potamodromous brown trout movements in the North of the Iberian Peninsula: Modelling past, present and future based on continuous fishway monitoring. Sci. Total. Environ. 2018, 640–641, 1521–1536. [Google Scholar] [CrossRef]
- Bae, M.-J.; Merciai, R.; Benejam, L.; Sabater, S.; García-Berthou, E. Small Weirs, Big Effects: Disruption of Water Temperature Regimes with Hydrological Alteration in a Mediterranean Stream. River Res. Appl. 2015, 32, 309–319. [Google Scholar] [CrossRef]
- Belletti, B.; Garcia de Leaniz, C.; Jones, J.; Bizzi, S.; Börger, L.; Segura, G.; Castelletti, A.; van de Bund, W.; Aarestrup, K.; Barry, J.; et al. More than one million barriers fragment Europe’s rivers. Nature 2020, 588, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Ordeix, M.; Pou-Rovira, Q.; Sellarès, N.; Munné, A.; Bardina, M.; Casamitjana, A. Fish pass assessment in the rivers of Catalonia (NE Iberian Peninsula). A case study of weirs associated with hydropower plants and gauging stations. Limnetica 2011, 29, 405–426. [Google Scholar]
- Amaral, S.D.; Branco, P.; da Silva, A.T.; Katopodis, C.; Viseu, T.; Ferreira, M.T.; Pinheiro, A.N.; Santos, J.M. Upstream passage of potamodromous cyprinids over small weirs: The influence of key-hydraulic parameters. J. Ecohydraulics 2016, 1, 79–89. [Google Scholar] [CrossRef]
- Cortes, R.; Peredo, A.; Terêncio, D.; Sanches Fernandes, L.; Moura, J.; Jesus, J.; Magalhães, M.; Ferreira, P.; Pacheco, F. Undamming the Douro River Catchment: A Stepwise Approach for Prioritizing Dam Removal. Water 2019, 11, 693. [Google Scholar] [CrossRef]
- Reiser, D.W.; Huang, C.-M.; Beck, S.; Gagner, M.; Jeanes, E. Defining Flow Windows for Upstream Passage of Adult Anadromous Salmonids at Cascades and Falls. Trans. Am. Fish. Soc. 2016, 135, 668–679. [Google Scholar] [CrossRef]
- Shaw, E.A.; Lange, E.; Shucksmith, J.D.; Lerner, D.N. Importance of partial barriers and temporal variation in flow when modelling connectivity in fragmented river systems. Ecol. Eng. 2016, 91, 515–528. [Google Scholar] [CrossRef]
- Santos, J.M.; Rivaes, R.; Boavida, I.; Branco, P. Structural microhabitat use by endemic cyprinids in a Mediterranean-type river: Implications for restoration practices. Aquat. Conserv. Mar. Freshw. Ecosyst. 2017, 28, 26–36. [Google Scholar] [CrossRef]
- Fonseca, A.R.; Santos, J.A. Predicting hydrologic flows under climate change: The Tâmega Basin as an analog for the Mediterranean region. Sci. Total. Environ. 2017, 668, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Lobanova, A.; Koch, H.; Liersch, S.; Hattermann, F.F.; Krysanova, V. Impacts of changing climate on the hydrology and hydropower production of the Tagus River basin. Hydrol. Process. 2016, 30, 5039–5052. [Google Scholar] [CrossRef]
- Sellami, H.; Benabdallah, S.; La Jeunesse, I.; Vanclooster, M. Quantifying hydrological responses of small Mediterranean catchments under climate change projections. Sci. Total. Environ. 2016, 543, 924–936. [Google Scholar] [CrossRef] [PubMed]
- Boavida, I.; Jesus, J.B.; Pereira, V.; Santos, C.; Lopes, M.; Cortes, R.M.V. Fulfilling spawning flow requirements for potamodromous cyprinids in a restored river segment. Sci. Total. Environ. 2018, 635, 567–575. [Google Scholar] [CrossRef]
- Best, J. Anthropogenic stresses on the world’s big rivers. Nat. Geosci. 2018, 12, 7–21. [Google Scholar] [CrossRef]
- Schwartz, J. Use of Ecohydraulic-Based Mesohabitat Classification and Fish Species Traits for Stream Restoration Design. Water 2016, 8, 520. [Google Scholar] [CrossRef]
- Spurgeon, J.; Pegg, M.; Parasiewicz, P.; Rogers, J. River-Wide Habitat Availability for Fish Habitat Guilds: Implications for In-Stream Flow Protection. Water 2019, 11, 1132. [Google Scholar] [CrossRef]
- Wegscheider, B.; Linnansaari, T.; Ndong, M.; Haralampides, K.; St-Hilaire, A.; Schneider, M.; Curry, R.A. Fish habitat modelling in large rivers: Combining expert opinion and hydrodynamic modellingto inform river management. J. Ecohydraulics 2021, 1–19. [Google Scholar] [CrossRef]
- Jowett, I.G.; Duncan, M.J. Effectiveness of 1D and 2D hydraulic models for instream habitat analysis in a braided river. Ecol. Eng. 2012, 48, 92–100. [Google Scholar] [CrossRef]
- Baki, A.B.M.; Zhu, D.Z.; Rajaratnam, N. Flow Simulation in a Rock-Ramp Fish Pass. J. Hydraul. Eng. 2016, 142, 04016031. [Google Scholar] [CrossRef]
- Tran, T.D.; Chorda, J.; Laurens, P.; Cassan, L. Modelling nature-like fishway flow around unsubmerged obstacles using a 2D shallow water model. Environ. Fluid Mech. 2015, 16, 413–428. [Google Scholar] [CrossRef]
- Boavida, I.; Santos, J.M.; Pinheiro, A.N.; Ferreira, M.T. Fish habitat availability simulations using different morphological variables. Limnetica 2011, 30, 393–404. [Google Scholar] [CrossRef]
- Rivaes, R.; Boavida, I.; Santos, J.M.; Pinheiro, A.N.; Ferreira, T. Importance of considering riparian vegetation requirements for the long-term efficiency of environmental flows in aquatic microhabitats. Hydrol. Earth Syst. Sci. 2017, 21, 5763–5780. [Google Scholar] [CrossRef]
- Warszawski, L.; Frieler, K.; Huber, V.; Piontek, F.; Serdeczny, O.; Schewe, J. The Inter-Sectoral Impact Model Intercomparison Project (ISI–MIP): Project framework. Proc. Natl. Acad. Sci. USA 2013, 111, 3228–3232. [Google Scholar] [CrossRef]
- Fazelpoor, K.; Martínez-Fernández, V.; García de Jalón, D. Exploring the hydromorphological response to human pressure in Tagus River (1946–2014) by complementary diagnosis. CATENA 2021, 198, 105052. [Google Scholar] [CrossRef]
- APA–Agência Portuguesa do Ambiente, I.P. SNIRH–Sistema Nacional de Informação em Recursos Hídricos; Ministério do Ambiente e Transição Energética: Lisboa, Portugal, 2021. Available online: https://snirh.apambiente.pt/ (accessed on 1 March 2021).
- Ferreira, M.T.; Santos, J.M.; Rivaes, R. Avaliação da Transponibilidade do Travessão da Central Termoelétrica do Pego Para Espécies Piscícolas. Relatório Final; Instituto Superior de Agronomia: Lisboa, Portugal; Universidade de Lisboa: Lisboa, Portugal, 2014. [Google Scholar]
- Hempel, S.; Frieler, K.; Warszawski, L.; Schewe, J.; Piontek, F. A trend-preserving bias correction–the ISI-MIP approach. Earth Syst. Dyn. 2013, 4, 219–236. [Google Scholar] [CrossRef]
- Steffler, P.; Blackburn, J. River2D, Two-Dimensional Depth Averaged Model. of River Hydrodynamics and Fish. In Habitat, Introduction to Depth Averaged Modeling and User’s Manual; University of Alberta: Edmonton, AB, Canada, 2002. [Google Scholar]
- Amaral, S.D.; Quaresma, A.L.; Branco, P.; Romão, F.; Katopodis, C.; Ferreira, M.T.; Pinheiro, A.N.; Santos, J.M. Assessment of Retrofitted Ramped Weirs to Improve Passage of Potamodromous Fish. Water 2019, 11, 2441. [Google Scholar] [CrossRef]
- Solà, C.; Ordeix, M.; Pou-Rovira, Q.; Sellarès, N.; Queralt, A.; Bardina, M.; Casamitjana, A.; Munné, A. Longitudinal connectivity in hydromorphological quality assessments of rivers. The ICF index: A river connectivity index and its application to Catalan rivers. Limnetica 2011, 30, 273–292. [Google Scholar] [CrossRef]
- Adamczyk, M.; Parasiewicz, P.; Vezza, P.; Prus, P.; De Cesare, G. Empirical Validation of MesoHABSIM Models Developed with Different Habitat Suitability Criteria for Bullhead Cottus gobio L. as an Indicator Species. Water 2019, 11, 726. [Google Scholar] [CrossRef]
- Parasiewicz, P.; Prus, P.; Theodoropoulos, C.; Alfredsen, K.; Adamczyk, M.; Comoglio, C.; Vezza, P. Environmental Flows Determination and Monitoring with Hydraulic Habitat Models—Pushing the Boundaries of Habitat Models Application. Water 2019, 11, 1950. [Google Scholar] [CrossRef]
- Boavida, I.; Dias, V.; Ferreira, M.T.; Santos, J.M. Univariate functions versus fuzzy logic: Implications for fish habitat modeling. Ecol. Eng. 2014, 71, 533–538. [Google Scholar] [CrossRef]
- Mouton, A.M.; De Baets, B.; Goethals, P.L.M. Data-driven fuzzy habitat models: Impact of performance criteria and opportunities for ecohydraulics. In Ecohydraulics: An Integrated Approach; Maddock, I., Harby, A., Kemp, P., Wood, P., Eds.; John Wiley & Sons Ltd: Chichester, UK, 2013; pp. 93–107. [Google Scholar]
- Parasiewicz, P.; Rogers, J.N.; Vezza, P.; Gortazar, J.; Seager, T.; Pegg, M.; Wiśniewolski, W.; Comoglio, C. Applications of the MesoHABSIM simulation model. In Ecohydraulics: An Integrated Approach; Maddock, I., Harby, A., Kemp, P., Wood, P., Eds.; John Wiley & Sons Ltd: Chichester, UK, 2013; pp. 109–125. [Google Scholar]
- Belaud, A.; Carette, A.; Cassou-Leins, F.; Cassou-Leins, J.J. Choix des sites de fraie par la grand alose (Alosa alosa L.) en Moyenne Garonne. Bull. Français de La Pêche et de La Piscic. 2001, 362–363, 869–880. [Google Scholar] [CrossRef]
- Caswell, P.A.; Aprahamian, M.W. Use of River Habitat Survey to determine the spawning habitat characteristics of twaite shad (Alosa fallax fallax). Bull. Français de La Pêche et de La Piscic. 2001, 362–363, 919–929. [Google Scholar] [CrossRef]
- Maitland, P.S. Ecology of the river, brook and sea lamprey. In Conserving Natura 2000 Rivers Ecology Series No. 5; English Nature: Peterborough, UK, 2003. [Google Scholar]
- Pereira, E.; Quintella, B.R.; Mateus, C.S.; Alexandre, C.M.; Belo, A.F.; Telhado, A.; Quadrado, M.F.; Almeida, P.R. Performance of a Vertical-Slot Fish Pass for the Sea Lamprey Petromyzon marinus L. and Habitat Recolonization. River Res. Appl. 2016, 33, 16–26. [Google Scholar] [CrossRef]
- Bracken, F.S.A.; Rooney, S.M.; Kelly-Quinn, M.; King, J.J.; Carlsson, J. Identifying spawning sites and other critical habitat in lotic systems using eDNA “snapshots”: A case study using the sea lamprey Petromyzon marinus L. Ecol. Evol. 2018, 9, 553–567. [Google Scholar] [CrossRef]
- Mitchell, C.P. Swimming performances of some native freshwater fishes. New Zealand J. Mar. Freshw. Res. 1989, 23, 181–187. [Google Scholar] [CrossRef]
- Vagner, M.; Lefrançois, C.; Ferrari, R.S.; Satta, A.; Domenici, P. The effect of acute hypoxia on swimming stamina at optimal swimming speed in flathead grey mullet Mugil cephalus. Mar. Biol. 2008, 155, 183–190. [Google Scholar] [CrossRef]
- Lamouroux, N.; Capra, H.; Pouilly, M.; Souchon, Y. Fish habitat preferences in large streams of southern France. Freshw. Biol. 1999, 42, 673–687. [Google Scholar] [CrossRef]
- Arai, T.; Kotake, A.; McCarthy, T.K. Habitat use by the European eel Anguilla anguilla in Irish waters. Estuar. Coast. Shelf Sci. 2006, 67, 569–578. [Google Scholar] [CrossRef]
- Daverat, F.; Limburg, K.; Thibault, I.; Shiao, J.; Dodson, J.; Caron, F.; Tzeng, W.; Iizuka, Y.; Wickström, H. Phenotypic plasticity of habitat use by three temperate eel species, Anguilla anguilla, A. japonica and A. rostrata. Mar. Ecol. Prog. Ser. 2006, 308, 231–241. [Google Scholar] [CrossRef]
- Riley, W.D.; Walker, A.M.; Bendall, B.; Ives, M.J. Movements of the European eel (Anguilla anguilla) in a chalk stream. Ecol. Freshw. Fish. 2011, 20, 628–635. [Google Scholar] [CrossRef]
- Johnson, J.H.; Nack, C.C. Habitat use of American eel (Anguilla rostrata) in a tributary of the Hudson River, New York. J. Appl. Ichthyol. 2013, 29, 1073–1079. [Google Scholar] [CrossRef]
- Alexandre, C.M.; Quintella, B.R.; Ferreira, A.F.; Romão, F.A.; Almeida, P.R. Swimming performance and ecomorphology of the Iberian barbel Luciobarbus bocagei (Steindachner, 1864) on permanent and temporary rivers. Ecol. Freshw. Fish 2013, 23, 244–258. [Google Scholar] [CrossRef]
- Mateus, C.S.; Quintella, B.R.; Almeida, P.R. The critical swimming speed of Iberian barbel Barbus bocagei in relation to size and sex. J. Fish Biol. 2008, 73, 1783–1789. [Google Scholar] [CrossRef]
- Santos, J.M.; Silva, A.; Katopodis, C.; Pinheiro, P.; Pinheiro, A.; Bochechas, J.; Ferreira, M.T. Ecohydraulics of pool-type fishways: Getting past the barriers. Ecol. Eng. 2012, 48, 38–50. [Google Scholar] [CrossRef]
- Sanz-Ronda, F.J.; Ruiz-Legazpi, J.; Bravo-Córdoba, F.J.; Makrakis, S.; Castro-Santos, T. Sprinting performance of two Iberian fish: Luciobarbus bocagei and Pseudochondrostoma duriense in an open channel flume. Ecol. Eng. 2015, 83, 61–70. [Google Scholar] [CrossRef]
- Sanz-Ronda, F.J.; Bravo-Córdoba, F.J.; Fuentes-Pérez, J.F.; Castro-Santos, T. Ascent ability of brown trout, Salmo trutta, and two Iberian cyprinids–Iberian barbel, Luciobarbus bocagei, and northern straight-mouth nase, Pseudochondrostoma duriense–in a vertical slot fishway. Knowl. Manag. Aquat. Ecosyst. 2016, 417, 10. [Google Scholar] [CrossRef]
- Theuerkauf, S.J.; Lipcius, R.N. Quantitative Validation of a Habitat Suitability Index for Oyster Restoration. Front. Mar. Sci. 2016, 3. [Google Scholar] [CrossRef]
- R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, version 4.1.0; R Core Team: Vienna, Austria, 2021.
- Statistica (Data Analysis Software System); Version 10; StatSoft, Inc.: Hamburg, Germany, 2011.
- Segurado, P.; Branco, P.; Jauch, E.; Neves, R.; Ferreira, M.T. Sensitivity of river fishes to climate change: The role of hydrological stressors on habitat range shifts. Sci. Total. Environ. 2016, 562, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Arnell, N.W.; Gosling, S.N. The impacts of climate change on river flow regimes at the global scale. J. Hydrol. 2013, 486, 351–364. [Google Scholar] [CrossRef]
- Branco, P.; Santos, J.M.; Amaral, S.; Romão, F.; Pinheiro, A.N.; Ferreira, M.T. Potamodromous fish movements under multiple stressors: Connectivity reduction and oxygen depletion. Sci. Total. Environ. 2016, 572, 520–525. [Google Scholar] [CrossRef]
- Jaeger, K.L.; Olden, J.D.; Pelland, N.A. Climate change poised to threaten hydrologic connectivity and endemic fishes in dryland streams. Proc. Natl. Acad. Sci. USA 2014, 111, 13894–13899. [Google Scholar] [CrossRef]
- Raabe, J.K.; Hightower, J.E.; Ellis, T.A.; Facendola, J.J. Evaluation of Fish Passage at a Nature-Like Rock Ramp Fishway on a Large Coastal River. Trans. Am. Fish. Soc. 2019, 148, 798–816. [Google Scholar] [CrossRef]
- Foulds, W.L.; Lucas, M.C. Extreme inefficiency of two conventional, technical fishways used by European river lamprey (Lampetra fluviatilis). Ecol. Eng. 2013, 58, 423–433. [Google Scholar] [CrossRef]
- Muraoka, K.; Nakanishi, S.; Kayaba, Y. Boulder arrangement on a rocky ramp fishway based on the swimming behavior of fish. Limnologica 2017, 62, 188–193. [Google Scholar] [CrossRef]
- Castro-Santos, T.; Sanz-Ronda, F.J.; Ruiz-Legazpi, J. Breaking the speed limit–comparative sprinting performance of brook trout (Salvelinus fontinalis) and brown trout (Salmo trutta). Can. J. Fish. Aquat. Sci. 2013, 70, 280–293. [Google Scholar] [CrossRef]
- Kemp, P.S.; Russon, I.J.; Vowles, A.S.; Lucas, M.C. The influence of discharge and temperature on the ability of upstream migrant adult river lamprey (Lampetra fluviatilis) to pass experimental overshot and undershot weirs. River Res. Appl. 2011, 27, 488–498. [Google Scholar] [CrossRef]
- Samia, Y.; Lutscher, F.; Hastings, A. Connectivity, passability and heterogeneity interact to determine fish population persistence in river networks. J. R. Soc. Interface 2015, 12, 20150435. [Google Scholar] [CrossRef] [PubMed][Green Version]


| Common Name | Scientific Name | References |
|---|---|---|
| Allis shad | Alosa alosa | [51,52] |
| Twait shad | Alosa fallax | [51,53] |
| Sea lamprey | Petromizon marinus | [51,54,55,56] |
| Thinlip grey mullet | Chelon ramada | [51,57,58] |
| European eel | Anguilla anguilla | [51,58,59,60,61,62] |
| Iberian barbel | Luciobarbus bocagei | [51,63,64,65,66,67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mameri, D.; Rivaes, R.; Ferreira, M.T.; Schmutz, S.; Santos, J.M. Climate Change Effects on Fish Passability across a Rock Weir in a Mediterranean River. Water 2021, 13, 2758. https://doi.org/10.3390/w13192758
Mameri D, Rivaes R, Ferreira MT, Schmutz S, Santos JM. Climate Change Effects on Fish Passability across a Rock Weir in a Mediterranean River. Water. 2021; 13(19):2758. https://doi.org/10.3390/w13192758
Chicago/Turabian StyleMameri, Daniel, Rui Rivaes, Maria Teresa Ferreira, Stefan Schmutz, and José Maria Santos. 2021. "Climate Change Effects on Fish Passability across a Rock Weir in a Mediterranean River" Water 13, no. 19: 2758. https://doi.org/10.3390/w13192758
APA StyleMameri, D., Rivaes, R., Ferreira, M. T., Schmutz, S., & Santos, J. M. (2021). Climate Change Effects on Fish Passability across a Rock Weir in a Mediterranean River. Water, 13(19), 2758. https://doi.org/10.3390/w13192758









